Abstract
Objective
The objective of this study was to use daily data collected via a smartphone app for characterization of patient-reported and symptom-based (using an a priori definition) flares in an adult idiopathic inflammatory myopathy (IIM) cohort.
Methods
UK adults with an IIM answered patient-reported outcome measurements (PROMs) daily via a smartphone app during a 91-day study. Daily symptom PROMs addressed global activity, overall pain, myalgia, fatigue, and weakness (on a 0–100 visual analogue scale). Patient-reported flares were recorded via a weekly app question. Symptom-based flares were defined via an a priori definition related to increase in daily symptom data from the previous 4-day mean.
Results
Twenty participants (65% female) participated. Patient-reported flares occurred on a median of 5 weeks (IQR 3, 7) per participant, out of a possible 13. The mean of each symptom score was significantly higher in flare weeks, compared with non-flare weeks (e.g. mean flare week myalgia score 34/100, vs 21/100 during non-flare week, t test P-value <0.01). Fatigue accounted for the most symptom-based flares [incidence-rate 23/100 person-days (95% CI 19, 27)], and myalgia the fewest [incidence rate 13/100 person-days (95% CI 11, 16)]. Symptom-based flares typically resolved after 3 days, although fatigue-predominant flares lasted 2 days. The majority (69%) of patient-reported flare weeks coincided with at least one symptom-based flare.
Conclusions
IIM flares are frequent and associated with increased symptom scores. This study has demonstrated the ability to identify and characterize patient-reported and symptom-based flares (based on an a priori definition), using daily app-collected data.
Keywords: myositis, muscle disease, muscle, mobile health, epidemiology
Rheumatology key messages.
Daily symptom data can provide novel insights into idiopathic inflammatory myopathy flares.
Patient-reported idiopathic inflammatory myopathy flares occur frequently and are associated with increased symptom scores.
Future clinical care and research could utilize daily symptom data for enhanced insights.
Introduction
Idiopathic inflammatory myopathy (IIM) is a chronic multisystem inflammatory condition [1, 2]. Muscle inflammation, termed myositis, is the most common IIM clinical manifestation. Symptoms associated with myositis include weakness, fatigue, and muscle pain (myalgia).
The concept of an IIM flare is widely used, but interpretation varies between patients, clinicians, and researchers. No consensus definition of an IIM flare exists but could provide a novel aid for clinical management and research. Research into patient-reported IIM flares remains limited. Detailed investigation into the characteristics (e.g. frequency, relationship with relevant symptoms) of patient-reported IIM flares is a logical first step for future development of a consensus definition.
Research in other areas, including OA [3] and asthma [4], have used frequently collected data to elucidate detailed characteristics of patient-reported flares. The digital healthcare revolution has led to increased use of digital technologies in clinical care and research [5, 6]. Increasing personal smartphone ownership [7, 8] has made collection of daily symptom information using patient-reported outcome measurements (PROMs) a reality via tailor-made smartphone apps. This capability has been demonstrated in a number of conditions, including RA [9], schizophrenia [10], and Parkinson’s disease [11].
High-frequency longitudinal patient-reported data related to IIM flare occurrence collected via a smartphone-based app could allow: 1) quantification of frequency of patient-reported IIM flares and 2) investigation of relationships between patient-reported IIM flares and relevant symptoms.
Qualitative research indicates IIM flares are characterized by brief (i.e. 2–3-day) acute increases in symptom severity, including myalgia, weakness, and fatigue, compared with a baseline over recent days [12]. Daily patient-reported symptom data could potentially allow objective identification of such symptom-based flares using an a priori definition, similar to that of Parry et al. in OA [3]. Investigating concurrence of patient-reported and symptom-based flares could provide further insights. Development and validation of such a symptom-based flare definition could provide a novel method of real-time flare detection using daily patient-reported symptom data.
This study therefore aimed to:
quantify how often patient-reported IIM flares occur
compare daily symptom scores between patient-reported flare weeks and non-flare weeks
characterise incidence and profile of symptom-based IIM flares, based on an a priori definition
explore concordance (i.e. synchronicity) between patient-reported and symptom-based flares.
Methods
The Myositis Physical Activity Device (MyoPAD) Study designed and tested a smartphone app and accelerometer sensor–based system and aimed to develop a method for daily/continuous data collection applicable for use in IIM research and clinical care.
Domains that the app questions should address were identified via a focus group comprised of five adult participants with IIM (disease duration range 2–14 years; 4:1 female:male; 2 DM, 1 PM, 1 immune-mediated necrotizing myopathy). Participants reported weakness, fatigue, myalgia (muscle-specific pain), and overall pain (i.e. non-muscle-specific pain) were associated with their flares. Daily, weekly, and monthly question sets were created by the study team (see Supplementary Table S1, available at Rheumatology online). Only responses from daily symptom and weekly flare occurrence questions were included in the analysis in this article (see Table 1). The daily question panel addressed weakness, fatigue, myalgia, overall pain, and global activity. Symptom scores could be answered on a 0–100 horizontal visual analogue scale (VAS). Daily symptom questions were available to answer every day of the 91-day study period. The weekly question panel included a binary flare occurrence question, which was available to answer for a single day every 7 days only. All questions on any given day had to be completed to allow submission, and omission of even a single question would result in non-submission. Question responses were remotely transferred to a secure cloud-based server. Questions and app functionality were beta-tested and approved by three participants with IIM. The app software was developed by a collaborating industry partner, ZiteLab ApS. The MyoPAD app was available to the study participants for download from Apple App Store and Google Play.
Table 1.
Symptom Score Questions available via the MyoPAD app and included in the analysis in this article
| Domain | Question stem | Answer scale | Answer anchors |
|---|---|---|---|
| Daily questions | |||
| Global activity | Considering all of the ways it affects you, how active do you feel your myositis is today? | VAS | Not active (0) |
| Very active (100) | |||
| Fatigue | How much fatigue do you feel today? | VAS | No fatigue (0) |
| Very severe fatigue (100) | |||
| Weakness | How weak do you feel today? | VAS | No weakness (0) |
| Very severe weakness (100) | |||
| Myalgia | What is your level of pain due to myositis today? | VAS | No pain (0) |
| Extreme pain (100) | |||
| Pain | What is your overall level of pain today? | VAS | No pain (0) |
| Extreme pain (100) | |||
| Weekly question | |||
| Flare occurrence | Have you experienced a flare of myositis in the last 7 days? | Dichotomous | Yes; no |
Details of all questions included in the MyoPAD are displayed in Supplementary Table S1, available at Rheumatology online. VAS: visual analogue scale.
Participants were recruited via the neuromuscular clinic at Salford Royal Hospital (UK) between August 2019 and February 2020. All participants provided written informed consent. Participants were invited to join the study if they were aged over 18 years, had a physician-verified IIM diagnosis via the International Myositis Classification Criteria Project [13] or European Neuromuscular Centre criteria [14], owned their own smartphone, and had regular access to their own Wi-Fi connection. Participants unable to walk or enter information via a smartphone touchscreen were excluded. Participants with IBM or CTD-related IIM were excluded to facilitate identification of flares due to IIM only and limit interaction with other rheumatological conditions. The study did not have the capacity to translate the materials into other languages; therefore, potential participants unable to speak English and/or understand English verbal explanations were ineligible.
Participants were invited to take part in a 91-day study and to download the MyoPAD app. Verbal and paper-based instructions for completing symptom questions were provided. A member of the study team was available for technical support. Participants did not receive automated reminders or push-factor notifications for question completion.
Analysis
Two definitions of flare occurrence were used in analysis: 1) patient-reported flares and 2) symptom-based flares.
Patient-reported flare definition
Patient-reported flare occurrence data was derived from weekly app questions (Table 1). Only responses actively answered were included in analysis, i.e. an omitted weekly flare question was not counted as absence of a patient-reported flare. The week (7 days) prior to the weekly flare question was deemed to be a flare week if a flare was reported. The week prior to the weekly question was deemed to be a non-flare week if a flare was reported as being absent.
Patient-reported flare data analysis
Analysis aimed to quantify the frequency of patient-reported flares and to investigate their relationships with symptom scores.
First, the number of patient-reported flare weeks, non-flare weeks, and missing entries for flare weeks were counted across the cohort throughout the study period. Second, the mean score across the cohort was calculated throughout the whole study period for each symptom (myalgia, weakness, overall pain, fatigue, global activity). Third, the mean magnitude of day-to-day change (i.e. negative differences were multiplied by –1) across the cohort was calculated throughout the whole study period for each symptom. The mean score and mean magnitude of day-to-day change were calculated for each participant week prior to each scheduled patient-reported flare question and stratified according to occurrence/non-occurrence of patient-reported flares, as well as for weeks for which weekly patient-reported flare data was missing. Weeks were excluded from analysis if fewer than 4 days of daily symptom data had been completed. Variables were compared between flare and non-flare weeks using the independent two-group t test. Multi-level mixed-effects logistic regression modelling was used to quantify the relationships between flare occurrence and mean score and mean magnitude of day-to-day change for each individual symptom and they were reported as odds ratios, adjusted for age and gender. Modelling was carried out individually for each mean weekly symptom score and each mean weekly magnitude of day-to-day change.
Symptom-based flare definition
The symptom-based flare definition was based on identifying acute increases in symptoms (global activity, fatigue, weakness, myalgia, overall pain) compared with a participant’s recent trend. This definition was based on previous qualitative work, in which participants with an IIM identified flares that were characterized by brief (i.e. 2–3-day) acute increases in symptoms, including myalgia, weakness, and fatigue, compared with their recent baseline [12].
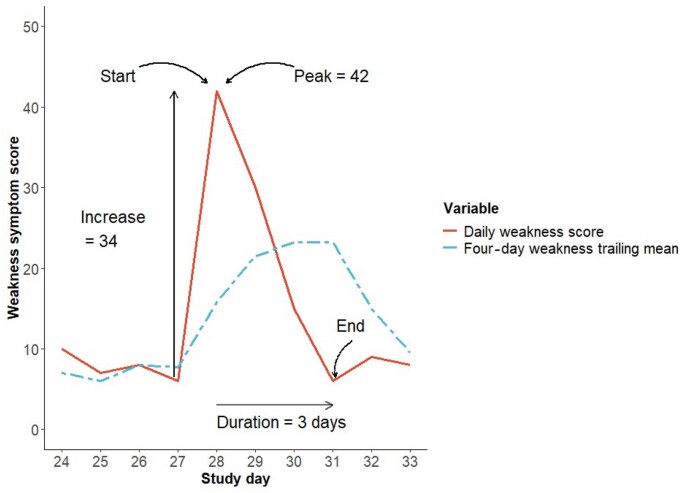
The following definitions relating to symptom-based flares were used (see Fig. 1):
Fig. 1.
Example of a single flare of weakness using the symptom-based definition for a single participant with identified parameters displayed
Four-day trailing mean
The 4-day trailing mean (i.e. mean score over previous 4 days) was calculated per day for each symptom score for each individual participant. Symptom scores for each of the previous 4 days were required in order to calculate the 4-day trailing mean; therefore, missing symptom scores precluded calculation.
Eligible person-day
An eligible person-day was defined as a day on which a flare could begin and needed to fulfil the following:
the preceding 4 days of symptom data had been completed, thus allowing calculation of a 4-day trailing mean
it was outside the period of another symptom-based flare—i.e. the days between the start and end of a symptom-based flare were not eligible person-days
Start of symptom-based flare
A symptom-based flare was defined as starting on an eligible person-day on which the symptom score increased to >10 points higher than the 4-day trailing mean. No definitions of the minimum clinically important difference for each daily symptom in IIM have been made, so the 10-point threshold was based on minimum clinically important differences for global activity, pain, and fatigue identified in studies of other rheumatological conditions, including RA, AS and SLE [15–19]. A symptom-based flare could not begin until a previous flare had ended.
End of symptom-based flare
A flare was deemed to have ended on the day when the symptom score returned to the level of or lower than the pre-flare 4-day trailing mean. The final day in a time period of contiguous symptom data was defined as the final flare day for flares that did not resolve according to our definition.
Peak of symptom-based flare
The flare peak was identified as the highest symptom score occurring between the flare start and end.
Magnitude of symptom score increase
The symptom increase was calculated as the difference between the peak symptom score and that on the day preceding the flare start.
Duration of symptom-based flare
The flare duration was calculated as the number of days between the flare start and end.
Symptom-based flare analysis
Symptom-based flares for each of the five variables (i.e. global activity, fatigue, weakness, myalgia, and overall pain) were identified separately for each participant throughout the study period, and the following metrics were reported:
total number of symptom-based flares across the cohort
incidence rate per 100 eligible person-days across the cohort
median magnitude of the symptom score increase
median score of the peak for each flare
median duration of the symptom-based flare.
Assessment of concordance between patient-reported and symptom-based flares
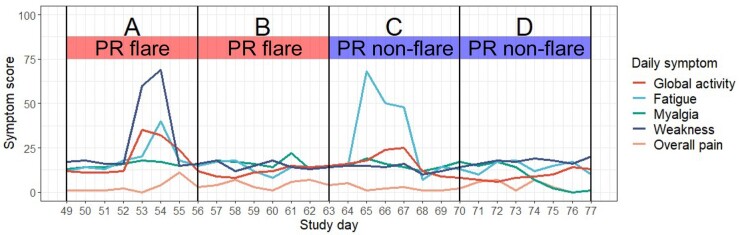
Concordance (i.e. synchronicity) of patient-reported and symptom-based flares was ascertained by calculating the proportion of patient-reported flare weeks coinciding with any day throughout a symptom-based flare. See Fig. 2 for an illustration of patient-reported flares, daily symptoms and thus symptom-based flares, and the concordance between the two definitions of flares.
Fig. 2.
Illustration of concordance between patient-reported and symptom-based flares
Panel A—patient-reported flare week with symptom-based flare (weakness) identification; Panel B—patient-reported flare week without symptom-based flare identification; Panel C—patient-reported non-flare week with symptom-based flare (fatigue) identified; Panel D—patient-reported non-flare week without identification of symptom-based flare. PR: patient-reported.
All analysis was carried out using the statistical programme R [20].
Ethical approval
The Greater Manchester Central Research Ethics Committee approved the study (ref. 18/NW/0676).
Results
Twenty participants took part [13 (65%) female]. The median age of the cohort was 50 years (IQR 43, 56), and the median IIM disease duration was 3 years (IQR 2, 5; range 1–26 years). Eleven (55%) had DM, five (25%) PM, three (15%) immune-mediated necrotizing myopathy, and one (5%) anti-synthetase syndrome. Cohort-wide disease activity measurements at baseline included: median physician global VAS 4/10 (IQR 2, 6), median patient global VAS 3/10 (IQR 2, 5), median manual muscle test (MMT26) 252/260 (IQR 244, 256), median HAQ score 0.9/3 (IQR 0.6, 1.2), and median global extramuscular involvement VAS 2.5/10 (IQR 2.0, 3.5).
Data was collected on a total of 1562 individual participant days, which was 86% of a potential total of 1820. A total of 248 weekly flare question answers were submitted throughout the 91-day study period, 95% of a potential total of 260 (13 weekly questions for each of the 20 participants). A total of 7810 daily symptom scores were submitted throughout the 91-day study period, 86% of a potential total of 9100 (five daily symptom questions over a 91-day period for each of the 20 participants).
Patient-reported flare analysis
Twelve (5% of 260) weeks were excluded from patient-reported flare analysis due to non-completion of the weekly flare question, and a further 15 (6% of 260) were excluded due to fewer than 4 days of completed symptom data, resulting in a total of 233 study weeks (90% of 260) being included in the analysis.
A total of 78 (33% of 233 eligible responses) flare weeks and 155 (67% of 233 responses) non-flare weeks were reported. Flares were reported on a median of 5 weeks (IQR 3, 7) per participant throughout the study period, out of a potential maximum of 13.
The mean of each symptom score and the mean magnitude of day-to-day change is displayed in Table 2. The mean of each symptom score was significantly higher in flare weeks, compared with non-flare weeks. The magnitude of the day-to-day change was also higher during flare weeks, compared with non-flare weeks; however, this difference was only significant for global activity and weakness.
Table 2.
Mean score and magnitude of day-to-day variation for each symptom score, separated by patient-reported flare weeks and non-flare weeks
| Variable | Whole study period | Flare weeks | Non-flare weeks | t-test P-valuea | Modelling resultsb |
|||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | ||||||
| Mean (s.d.) symptom score 7 days prior to flare reporting | Global activity | 32.6 (21.0) | 38.5 (19.8) | 29.6 (21.0) | <0.01 | 1.09 | 1.03, 1.14 | <0.01 |
| Fatigue | 37.2 (21.5) | 43.0 (19.5) | 34.4 (21.9) | <0.01 | 1.05 | 1.02, 1.09 | <0.01 | |
| Weakness | 33.2 (22.7) | 38.1 (20.0) | 30.7 (23.7) | 0.01 | 1.10 | 1.03, 1.17 | <0.01 | |
| Myalgia | 25.5 (18.3) | 33.9 (21.9) | 21.4 (14.5) | <0.01 | 1.08 | 1.03, 1.12 | <0.01 | |
| Overall pain | 28.3 (19.2) | 35.4 (21.1) | 24.7 (17.2) | <0.01 | 1.09 | 1.04, 1.14 | <0.01 | |
| Mean (s.d.) magnitude of day-to-day change 7 days prior to flare reporting | Global activity | 8.2 (5.9) | 9.3 (5.6) | 7.6 (6.0) | 0.03 | 1.07 | 1.00, 1.14 | 0.05 |
| Fatigue | 11.0 (7.2) | 11.8 (6.8) | 10.6 (7.3) | 0.22 | 1.02 | 0.96, 1.08 | 0.52 | |
| Weakness | 8.7 (5.9) | 10.1 (5.9) | 8.0 (5.8) | 0.01 | 1.10 | 1.02, 1.19 | 0.01 | |
| Myalgia | 7.8 (5.8) | 8.7 (6.3) | 7.3 (5.5) | 0.09 | 1.06 | 1.06, 1.07 | <0.01 | |
| Overall pain | 8.6 (6.1) | 9.3 (6.5) | 8.2 (5.9) | 0.22 | 1.04 | 0.97, 1.10 | 0.27 | |
Variables were compared between flare and non-flare weeks using the independent two-group t test.
Modelling results calculated via multi-level mixed effects logistic regression modelling, adjusted for age and gender. OR: odds ratio.
The mean symptom scores in the 12 weeks in which the patient-reported flare question was unanswered were similar to those of the 248 that were answered (see Supplementary Table S2 for details, available at Rheumatology online). The mean magnitude of day-to-day change was higher for global activity, myalgia, and overall pain and lower for weakness in weeks in which the patient-reported flare question was unanswered, compared with weeks in which it was answered.
Patient-reported flares were significantly associated with higher scores for all symptoms in multi-level mixed-effects logistic regression analysis. Higher magnitude of day-to-day change of weakness and myalgia was significantly associated with patient-reported flares.
Symptom-based flare analysis
A total of 968 eligible person-days (62% of 1562 days of available daily symptom data) across the cohort were available for symptom-based flare identification. The 594 non-eligible person-days occurred on 80 distinct time periods across the cohort, which had a median duration of 4 days (IQR 4, 7). The first 4 days (totalling 80 days across the cohort) of each participant’s 91-day study duration were non-eligible for symptom-based flare identification due to the requirement of comparison of daily symptom data to the 4-day trailing mean.
A total of 535 individual symptom-based flares were identified, and they began on 269 individual eligible person-days. One hundred and forty (52%) symptom-based flares occurred without co-occurrence of another symptom-based flare and 129 (48%) identified symptom-based flares occurred concurrently with at least one other.
Table 3 displays the number of identified symptom-based flares and their characteristics, including mean scores, number of identified flares, median score of flare peak, and duration until flare resolution. Fatigue symptom–based flares occurred most commonly, had highest score increases on the first day of a flare, and highest peak scores. Myalgia flares were the least common and had the lowest peak scores. Flares typically resolved after 3 days; however, fatigue flares were shorter in duration, lasting 2 days on average. All symptom-based flares resolved within the study period.
Table 3.
Profiles of symptom-based flares
| Median number of flare events per participant (IQR) | Total number of flare events across cohort (%a) | Incidence rate/100 person-days (95% CI)b | Median (IQR) symptom 4-day trailing mean on first day of flare | Median (IQR) magnitude of symptom score increase on first day of flare | Median (IQR) score of peak of flare | Median (IQR) flare duration/days | |
|---|---|---|---|---|---|---|---|
| Global activity | 5.5 (2.8, 8.0) | 107 (20.0) | 18.8 (15.4, 22.7) | 31.0 (22.4, 43.8) | 15.8 (12.0, 21.8) | 52.0 (38.0, 71.3) | 3.0 (2.0, 4.0) |
| Fatigue | 6.0 (3.8, 9.3) | 128 (24.9) | 23.0 (19.2, 27.3) | 36.8 (25.8, 52.6) | 18.6 (13.2, 27.2) | 64.0 (51.0, 76.0) | 2.0 (2.0, 4.0) |
| Weakness | 4.5 (2.0, 8.0) | 107 (20.0) | 17.8 (14.6, 21.5) | 36.8 (25.8, 46.4) | 17.5 (12.8, 30.5) | 62.0 (46.5, 77.5) | 3.0 (2.0, 4.0) |
| Myalgia | 3.0 (1.8, 6.3) | 87 (16.3) | 13.2 (10.5, 16.2) | 25.8 (20.9, 40.6) | 17.3 (13.6, 24.0) | 47.0 (36.0, 66.0) | 3.0 (2.0, 4.0) |
| Overall pain | 5.0 (1.8, 8.3) | 106 (19.8) | 17.3 (14.2, 20.9) | 28.4 (21.1, 42.6) | 17.3 (13.3, 23.2) | 49.0 (36.5, 69.0) | 3.0 (2.0, 4.0) |
Calculated as percentage of all symptom-based flares identified.
Denominator was number of eligible person-days (i.e. available 4-day trailing mean and resolution of previous flare). IQR: interquartile range.
Concordance of patient-reported and symptom-based flares
Table 4 displays the number of patient-reported flares that did/did not occur alongside symptom-based flares. Out of 78 patient-reported flare weeks, 54 (69%) coincided with at least one symptom-based flare of any of the five symptoms. Of 155 patient-reported non-flare weeks, 61 (39%) coincided with no symptom-based flare.
Table 4.
Numbers of symptom-based flares that did and did not occur during a patient-reported flare week
| Symptom-based flare | Patient-reported flare |
Row total | ||
|---|---|---|---|---|
| Reported | Not reported | |||
| Total | Occurred | 54 (23.2) | 94 (40.3) | 148 (63.5) |
| Did not occur | 24 (10.3) | 61 (26.2) | 85 (36.5) | |
| Column total | 78 (33.5) | 155 (66.5) | 233 | |
Each number is expressed as a whole number (percentage). The denominator for the calculations was 233, which is the total number of eligible weeks in which patient-reported flare questions were answered.
Discussion
We utilized app-collected daily symptom and weekly flare data to investigate the characteristics of IIM flares.
On average, patient-reported flares occurred once every 3 weeks per participant throughout the 3-month study period. Patient-reported flares were associated with increased symptom scores (global activity, fatigue, weakness, myalgia, overall pain) and increased day-to-day variation of weakness and myalgia. Associations between day-to-day symptom variation and flares or increased disease activity in IIM has previously been reported in qualitative studies [21–24]. Our findings, therefore, provide further evidence of the role symptoms play in patient-reported flares and indicate the importance of symptom variation measurement alongside absolute scores.
A high frequency of symptom-based flares, based on an a priori definition, was identified, occurring once every 7–11 days per participant throughout the study period. Symptom-based flares were typically of short duration (2–3 days). Fatigue flares occurred most frequently, and myalgia flares least so. Previous research has highlighted the prominent role fatigue plays in IIM [22–25], and qualitative analysis of interviews carried out in the MyoPAD study highlighted the short duration of IIM flares, and fatigue as a predominant symptom [12].
The complex relationship between patient-reported flares and acute increases in symptoms, represented by symptom-based flares, is indicated by the concordance analysis results. Around two-thirds of patient-reported flares coincided with at least one symptom-based flare. There was greater mismatch in patient-reported non-flare weeks, most coincided with at least one symptom-based flare. There are a number of potential explanations for this. First, the symptom-based flare a priori definition may have been too sensitive, therefore, identifying symptom increases that patients do not deem a flare. Second, an identified symptom-based flare may have occurred on a minority of days within the 7-day period prior to the patient-reported flare questions; for example, a 2-day fatigue-based flare may have occurred on the first and second day of a week, but the participant may have deemed a flare to not have occurred during that week due to low symptom scores on days 3–7. Third, symptoms or disease manifestations not measured in this study may have occurred in patient-reported flare weeks in which a symptom-based flare was not identified. Finally, patient-reported IIM flares may not be wholly characterized by sudden increases in symptoms and are likely more complex than clinicians or researchers may have appreciated. Functional limitation, inability to perform specific tasks, or other unmeasured symptoms may account for these patient-reported flares.
Relation to previous research
Evidence concerning the frequency of patient-reported IIM flares and associated symptoms is limited. Studies have not defined flares according to patient-focused qualities, such as symptoms or functional impact; the majority of previous studies considering IIM flares have used definitions based on treatment escalation [26–28]. In accordance with our results, Christopher-Stine et al. reported patient-reported IIM flares to be associated with increased symptom scores [29]. A consensus definition of IIM flare incorporating daily symptom data could be used in both clinical and research settings. Daily symptom data collection in larger international IIM cohorts over longer study periods with frequent, repeated objective disease activity and disease damage assessment could facilitate flare definition development and provide insights into clinical utility/translation. Detailed qualitative data will also be key to IIM flare definition development. It may be appropriate to follow a similar approach to that taken by OMERACT, who have developed consensus definitions of flares in a number of conditions. For example, both qualitative and quantitative data were utilized in the recent development of a definition of RA flare, involving clinicians, patients, and researchers [30–32].
Limitations
A number of limitations exist. First, app domains were codesigned with a patient focus group not necessarily representative of the wider IIM population with regards to IIM subtype and disease duration; future studies could consider distinct question sets for individual IIM subtypes and stages of disease to allow for more specific characterization of flare in these subgroups. Second, we analysed data from a small UK adult IIM cohort not necessarily representative of the wider IIM population, thus potentially limiting external validity. Participants with higher symptom levels or more frequent flares may have been motivated to volunteer for recruitment; future research should aim to recruit a cohort representative of the wider IIM population, specifically in terms of disease activity and disease damage. Third, information relating to the impact of flares upon clinical care (i.e. urgent physician assessments, medication change) was not collected, and participants were not asked to provide details about why they reported a flare. Availability of such details may have facilitated insights into the clinical utility of remote daily symptom data collection and provided further contextualization of flares for which no symptom-based flare coincided. Fourth, the threshold of an increase of at least 10/100 symptom points used in the definition of symptom-based flares may have been too lenient and resulted in false identification of irrelevant symptom variations. Future research on IIM flare definition, as described above, could include identification of the minimum clinically important difference specific to IIM-related symptoms. Finally, this study did not collect repeated IIM disease activity data throughout the study period, thus precluding the ability to identify associations between DASs, patient-reported flares, and symptom-based flares; identifying such associations in future studies will provide valuable information on the utility of daily symptom data and potential impact on clinical care. The benefit of clinicians and patients being able to view daily symptom data generated in the period since a previous consultation could be assessed in future studies, thus clarifying its clinical utility and added value alongside traditional disease activity measurements. Self-reported flare is a useful measure when examining time trends within an individual in clinical settings; however, each patient’s interpretation of what a flare means might be different, and is thus potentially less useful when compared across a population.
Clinical and research relevance
Remote daily monitoring could aid detection of defined IIM flares in clinical and research settings. Remote detection of flare-associated symptom patterns may be used to alert a clinician, allowing them to instigate/escalate treatment. Efficacy assessment in IIM clinical trials could be enhanced by remote daily monitoring and flare detection.
Conclusion
Remote daily symptom data collection via a smartphone-based app has identified a high frequency of patient-reported flares (every 3 weeks) and symptom-based flares (every 7–11 days), based on an a priori definition. Patient-reported flares were associated with increased symptoms (global activity, fatigue, weakness, myalgia, overall pain) and greater day-to-day variation (weakness and myalgia only). This study highlights the role daily symptom data could play in future research to develop consensus IIM flare definitions and assisting in clinical decision making. Opportunities posed by the digital healthcare revolution and smartphone app-based daily symptom data collection make remote flare detection a plausible future capability.
Supplementary Material
Acknowledgements
The authors would like to thank all patient participants who contributed data for analysis in this study. A.G.S.O., H.C. and W.G.D. formulated the study design and funding applications. A.G.S.O. coordinated the study, recruited all study participants, and analysed data. N.S.K. provided the app infrastructure and support. The authors would like to acknowledge Mikkel Kramme Abildtoft for constructing the MyoPAD app and providing technical support throughout the study. A.G.S.O. led the manuscript preparation, which was edited and amended by W.G.D. and H.C. N.S.K. provided additional amendments to the manuscript. All authors approved the final manuscript.
Contributor Information
Alexander G S Oldroyd, NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust; Centre for Musculoskeletal Research, University of Manchester, Manchester Academic Health Science Centre; Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester, Manchester; Department of Rheumatology, Salford Royal Hospital, Salford, UK.
Niels Steen Krogh, ZiteLab, Copenhagen, Denmark.
William G Dixon, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester, Manchester; Department of Rheumatology, Salford Royal Hospital, Salford, UK.
Hector Chinoy, NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust; Centre for Musculoskeletal Research, University of Manchester, Manchester Academic Health Science Centre; Department of Rheumatology, Salford Royal Hospital, Salford, UK.
Funding: This report includes independent research supported by the National Institute for Health Research Manchester Biomedical Research Centre Funding Scheme. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. This work was supported by funding via a research fellowship from The Myositis Association (to A.G.S.O.); Versus Arthritis (Award No. 21993 to A.G.S.O.); and the National Institute for Health Research Manchester Biomedical Research Centre (to A.G.S.O., W.G.D., and H.C.).
Disclosure statement: H.C. works in a unit that has received funding from Eli Lilly, MedImmune, and Novartis. H.C. has received educational funding support from Corbus Pharmaceuticals. H.C. has received honoraria from Argenx, Abbvie, AstraZeneca, Janssen, Orphazyme, UCB, Biogen, and Novartis. W.G.D. has received consultancy fees from Abbvie and Google, unrelated to this work. N.S.K. is CEO of ZiteLab ApS. All other authors have declared no conflicts of interest.
Data availability statement
Data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Oldroyd A, Lilleker J, Chinoy H.. Idiopathic inflammatory myopathies – a guide to subtypes, diagnostic approach and treatment. Clin Med 2017;17:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chinoy H, Lilleker JB. Polymyositis and dermatomyositis. In: Watts RA, Conaghan PG, Denton C et al., ed. Oxford textbook of rheumatology. UK: Oxford University Press, 2013: 1009–20. http://www.oxfordmedicine.com/view/10.1093/med/9780199642489.001.0001/med-9780199642489-chapter-124 (30 March 2022, date last accessed). [Google Scholar]
- 3. Parry E, Ogollah R, Peat G.. ‘Acute flare-ups’ in patients with, or at high risk of, knee osteoarthritis: a daily diary study with case–crossover analysis. Osteoarthr Cartil 2019;27:1124–8. [DOI] [PubMed] [Google Scholar]
- 4. Ducharme FM, Jensen ME, Mendelson MJ. et al. Asthma Flare-up Diary for Young Children to monitor the severity of exacerbations. J Allergy Clin Immunol 2016;137:744–9.e6. [DOI] [PubMed] [Google Scholar]
- 5. Mathews SC, Mcshea MJ, Hanley CL. et al. Digital health: a path to validation. NPJ Digit Med 2019;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kataria S, Ravindran V.. Digital health: a new dimension in rheumatology patient care. Rheumatol Int 2018;38:1949–57. [DOI] [PubMed] [Google Scholar]
- 7. Pew Research Center. Mobile Fact Sheet. 2018. https://www.pewresearch.org/internet/fact-sheet/mobile/ (30 March 2022, date last accessed).
- 8. Ofcom. The Communications Market Report 2018. 2018. https://www.ofcom.org.uk/__data/assets/pdf_file/0022/117256/CMR-2018-narrative-report.pdf (30 March 2022, date last accessed).
- 9. Austin L, Sharp CA, van der Veer SN. et al. Providing ‘the bigger picture’: benefits and feasibility of integrating remote monitoring from smartphones into the electronic health record: findings from the Remote Monitoring of Rheumatoid Arthritis (REMORA) study. Rheumatology 2020;59:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisner E, Drake RJ, Berry N. et al. Development and long-term acceptability of ExPRESS, a mobile phone app to monitor basic symptoms and early signs of psychosis relapse. JMIR mHealth uHealth 2019;7:e11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daeschler M, Elm J, Klintworth E. et al. Clinician-Input Study (CIS-PD): how the Fox Wearable Companion Application can influence treatment and care in Parkinson’s disease (P3.048). Neurology 2018;90(Suppl 15). [Google Scholar]
- 12. Oldroyd A, Dixon W, Chinoy H, Howells K.. Patient insights on living with idiopathic inflammatory myopathy and the limitations of disease activity measurement methods – a qualitative study. BMC Rheumatol 2020;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lundberg IE, Tjärnlund A, Bottai M. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allenbach Y, Mammen AL, Benveniste O. et al. 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord 2018;28:87–99. [DOI] [PubMed] [Google Scholar]
- 15. Curtis JR, Yang S, Chen L. et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2015;67:1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colangelo KJ, Pope JE, Peschken C.. The minimally important difference for patient reported outcomes in systemic lupus erythematosus including the HAQ-DI, pain, fatigue, and SF-36. J Rheumatol 2009;36:2231–7. [DOI] [PubMed] [Google Scholar]
- 17. Khanna D, Pope JE, Khanna PP. et al. The minimally important difference for the fatigue visual analog scale in patients with rheumatoid arthritis followed in an academic clinical practice. J Rheumatol 2008;35:2339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitchen H, Hansen B, Abetz L, Højbjerre L, Strandberg-Larsen M.. Patient-reported outcome measures for rheumatoid arthritis: minimal important differences review. Arthritis Rheumatol 2013;65. [Google Scholar]
- 19. Tubach F, Ravaud P, Martin-Mola E. et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multina. Arthritis Care Res (Hoboken) 2012;64:1699–707. [DOI] [PubMed] [Google Scholar]
- 20. R Core Team. R: A language and environment for statistical computing. 2014. http://www.r-project.org/ (30 March 2022, date last accessed).
- 21. Alexanderson H, Grande MD, Bingham CO. et al. Patient-reported outcomes and adult patients’ disease experience in the idiopathic inflammatory myopathies. Report from the OMERACT 11 myositis special interest group. J Rheumatol 2014;41:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JK, Mecoli CA, Alexanderson H. et al. Advancing the development of patient-reported outcomes for adult myositis at OMERACT 2016: an international Delphi study. J Rheumatol 2017;44:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regardt M, Basharat P, Christopher-Stine L. et al. Patients’ experience of myositis and further validation of a myositis-specific patient reported outcome measure – establishing core domains and expanding patient input on clinical assessment in myositis. Report from OMERACT 12. J Rheumatol 2015;42:2492–5. [DOI] [PubMed] [Google Scholar]
- 24. Mecoli CA, Park JK, Alexanderson H. et al. Perceptions of patients, caregivers, and healthcare providers of idiopathic inflammatory myopathies: an international OMERACT study. J Rheumatol 2019;46:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albrecht K, Huscher D, Callhoff J. et al. Trends in idiopathic inflammatory myopathies: cross-sectional data from the German National Database. Rheumatol Int 2020;40:1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landon-Cardinal O, Devilliers H, Chavarot N. et al. Responsiveness to change of 5-point MRC scale, endurance and functional evaluation for assessing myositis in daily clinical practice. J Neuromuscul Dis 2019;6:99–107. [DOI] [PubMed] [Google Scholar]
- 27. Saygin D, Oddis CV, Marder G. et al. Follow-up results of myositis patients treated with H. P. Acthar gel. Rheumatology 2020;59:2976–81. [DOI] [PubMed] [Google Scholar]
- 28. Mamyrova G, Rider LG, Ehrlich A. et al. Environmental factors associated with disease flare in juvenile and adult dermatomyositis. Rheumatology (Oxford) 2017;56:1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christopher-Stine L, Wan GJ, Kelly W. et al. Patient-reported dermatomyositis and polymyositis flare symptoms are associated with disability, productivity loss, and health care resource use. J Manag Care Spec Pharm 2020;26:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bingham CO, Alten R, Bartlett SJ. et al. Identifying preliminary domains to detect and measure rheumatoid arthritis flares: report of the OMERACT 10 RA Flare Workshop. J Rheumatol 2011;38:1751–8. [DOI] [PubMed] [Google Scholar]
- 31. Bykerk VP, Bingham CO, Choy EH. et al. Identifying flares in rheumatoid arthritis: reliability and construct validation of the OMERACT RA Flare Core Domain Set. RMD Open 2016;2:e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bartlett SJ, Hewlett S, Bingham CO. et al. ; OMERACT RA Flare Working Group. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis 2012;71:1855–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying this article will be shared on reasonable request to the corresponding author.