Abstract
Purpose
South Asians face a high burden of type 2 diabetes (T2D). We systematically summarized current research on the efficacy, cultural relevance, and research gaps of nutrition interventions that could be used for treatment in this population.
Findings
We identified 18 articles published since 2010. Dietary pattern interventions have focused on low-glycemic index (GI) solutions and consistently reported improvement in glycemic management. Trials of nutrition education and counselling had diverse approaches, with those utilizing more intensive interventions generally eliciting better glycemic outcomes. Many studies developed interventions with cultural relevance by including traditional foods, providing materials in the local language, and acknowledging important food-related customs. These adaptations were seen in South Asian countries as well as Western countries hosting immigrants.
Summary
Data from South Asian countries support low-GI and intensive counselling approaches for the treatment of T2D. Given the high prevalence of T2D in these populous countries, approaches that can reach large numbers of people are needed. In Western countries, more emphasis on providing culturally relevant nutrition therapy is needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13668-022-00446-9.
Keywords: Type 2 diabetes, Nutrition therapy, Nutrition education, Nutrition counseling, Cultural relevance, South Asia, India, Pakistan, Sri Lanka, Bangladesh, Immigrant
Introduction
Nearly 122 million people in the region of South Asia had diagnosed with diabetes in 2021. This represents a prevalence of 8.7%, 9.6%, 10.4%, 11.3%, 14.2%, and 30.8% in Nepal, India, Bhutan, Sri Lanka, Bangladesh, and Pakistan, respectively [1] and is a crushing health burden individually and for society. Millions more South Asians who have immigrated to other countries are at high risk of developing T2D. South Asians living in Canada have 3–5 times higher risk of diabetes than white people [2], with prevalence ranging from 16.5–26.8% [3]. Similar high risk is documented in the USA [4] and the UK [5]. This may be related, in part, to the adoption of new dietary patterns with low nutritional quality. South Asians living in Alberta, Canada, consume 30% of calories from foods high in sodium, sugar, and saturated fat [6].
Together with pharmacotherapy, nutrition therapy is a fundamental component of diabetes care. Although aggressive pharmaceutical management is recommended because of higher glycated hemoglobin (HbA1C) in South Asians at diagnosis [7], the cost of newer generation antiglycemic agents is a barrier in South Asian countries [8]. A recent consensus document highlights the lack of consistent delivery of medical nutrition therapy in South Asian countries [9]. The majority of people with diabetes are not prescribed specific diabetes diets [10]. In Western countries, South Asian immigrants experience barriers related to language and culturally tailored T2D therapy [11] and the majority do not meet treatment targets for HbA1C, blood pressure, or lipids [12]. A recent systematic review found 4 behavioral interventions incorporating culturally relevant content conducted in the UK and the Netherlands. Overall, they did not lower HbA1C. Methods to integrate culturally congruent care were lacking as was the necessity to consider the heterogeneity of South Asian culture [13]. The efficacy of diabetes interventions in South Asian populations needs to be documented to identify promising strategies for more widespread implementation. Sohal et al. [11] found that many barriers and facilitators to improved diabetes care were similar whether the South Asians lived in India or Western countries, highlighting the enduring importance of culture.
The purpose of this systematic review is to evaluate the efficacy to improve glycemic outcomes in recent medical nutrition therapy interventions carried out in South Asia, North America, or Europe. The primary research question was: In South Asian people with T2D, living in their home countries or immigrants to North America/Europe, what nutrition interventions improve health outcomes (glycemia, lipidemia, blood pressure, anthropometrics) or nutrition behaviors or knowledge? Additional research questions included:
What measures were taken to make these interventions culturally relevant to the target audience?
What are the gaps in research that could improve nutrition intervention strategies?
Methods
Eligibility Criteria
Eligible articles were original studies focused on people diagnosed with T2D, an outcome of glycemic management and/or nutrition behavior and a nutrition intervention conducted in India, Pakistan, Sri Lanka, Bhutan, Bangladesh, Nepal, or in a North American or European country specifically for immigrants from a South Asian country. We included case–control, quasi-experimental and randomized controlled trials (RCT). Secondary outcomes were blood pressure, lipid panel, and body weight or body mass index (BMI). Exclusion criteria were as follows: studies published before 2010, not English language, and studies focused on a single food, nutrient, or supplement. Abstracts, letters, editorials, general or systematic reviews and meta-analyses, practice guidelines, and study protocols were excluded.
Information Sources and Search Strategy
Six databases (CINAHL, Medline, Web of Science, Cochrane, Psych Info, Proquest Dissertations) were searched in October 2021 by JT and the output was uploaded into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). The MEDLINE search strategy, which documents the keywords used, is provided in Supplemental Table 1.
Selection Process
Duplicate studies were removed automatically by the Covidence software. Title and abstract screening was conducted by DNF and CBC. Potentially eligible articles were retrieved, and full-text review was conducted by DNF and CBC.
Data Collection Process
Data were extracted independently by DNF and FBS. Data included study title, authors, year and DOI; the study objective and population (country, urban/rural, sample size and gender, age range; study design, and details of the dietary intervention. Results were recorded in Excel and included clinical outcomes of glycemic management and cardiovascular risk factors, behavioral outcomes of dietary intake, self-efficacy, nutrition knowledge; cultural relevance of the intervention, barriers, and facilitators to uptake (by patients or by the health system).
Effect Measures and Synthesis
Where possible, quantitative data such as glycemic or nutritional outcomes were reported as intergroup mean differences for RCT or changes from baseline for quasi-experimental designs ± standard deviation or confidence intervals. A narrative synthesis was conducted and organized to address each research question.
Results
Study Selection
The study selection details are shown in Supplemental Fig. 1. Eighteen articles met the inclusion criteria.
Study Characteristics (Table 1)
Table 1.
Study characteristics by study design
| Study | Country | Sample size, age | Design | Intervention /control group | Duration | Intervention and cultural relevance |
|---|---|---|---|---|---|---|
| Non-randomized trials with or without a control group | ||||||
| Pande et al. [14] | India (urban) |
N = 20 Age 35–58y |
Case–control | Both T2D (n = 10) and healthy group (n = 10) received 1 meal/day | Acute | Provision of low-GI mixed meals using traditional Indian foods and spices in a medical setting. |
| Pande et al. [15] | India (urban) |
N = 15 Age 42–58 y |
Quasi-experimental, pre-post |
Provided meals and snacks No control group |
4 wk | Provision of low-GI mixed meals and snacks using traditional Indian foods and spices to cook at home. |
| Jain et al. [12] | India (urban) |
N = 180 Mean age 47 y |
Quasi-experimental, pre-post |
Education No control group |
3 mon | In-person education supported with a booklet describing the principles of diabetes management. |
| Singh et al. [16] | India (urban) |
N = 120 Age 35–65 y |
Quasi-experimental, pre-post |
Education No-intervention control |
3 mon | In-person nutrition education from dietitians. Type of PA recommended to participants not reported. |
| Krishnan et al. [13] | India (urban) |
N = 134 Mean age 50 y |
Quasi-experimental pre-post |
Counselling– I – attended 1 session, no follow-up II – attended dietary counselling with periodic follow-up III – attended dietary and exercise counselling with periodic follow-up No control group |
3 and 6 mon | In-person counselling to participant and caregiver/family, home visits and telephone counselling for those unable to attend in person. Type of physical activity recommended to participants not reported. |
| Bairy et al. [11] | India (urban) |
N = 101 Median age 55 y |
Quasi-experimental, pre-post |
Provision of meals + education as part of an integrated naturopathy and yoga program No control group |
3 mon | Meals were low-GI, plant-based, low in salt, oil, sugar. Also engaged in yoga, mild aerobic activity (walking, swimming, boat-pedalling in river), rest, “therapeutic fasting” (1–3 d) + 2 lectures/d on naturopathy, personal development + 1:1 physician counselling + 1-h cooking classes daily. Based on traditional practices. Patients were admitted to a naturopathy hospital for 15- or 30-d treatment. |
| Kumari et al. [17] |
India (urban) |
N = 202 Mean age 53 y |
Quasi-experimental |
Holistic lifestyle intervention Usual care controls |
3, 6, and 12 mon | Interdisciplinary team provided monthly counselling for 6 mon in local language. Supported with pictures, videos, 1:1, and group sessions. Included diet, physical activity (brisk walking, yoga), tobacco cessation, stress management, self-management adherence. |
| St. John [18] | UK | N = 34 | Quasi-experimental pre-post |
Education No control group |
2 mon | In-person education supported with a booklet developed for Pakistani immigrants to teach carbohydrate content of traditional foods. |
| Randomized controlled trials | ||||||
| Johansen et al. [19] | Norway |
N = 198 Age 26–63 y |
RCT |
Education No-intervention control |
7 mon | In-person, 2-h group sessions on managing blood glucose with diet and exercise (walking). Used culturally adapted resources, translated written materials, focused on traditional foods. |
| Raberg Kjollesdal [20] | Norway |
N = 82 Age 28–62 y |
RCT |
Education No-intervention control |
7 mon | Same as Johansen et al. [19] |
| Myers et al. [21] | India |
N = 239 Age 25–69 y |
Cluster RCT |
Education and self-management support Usual care controls |
6 and 12 mon | Dietitians received standardized training prior to implementation. Education provided in person by dietitian based on evidence-based nutrition practice guidelines (EBNPG) (Diabetes in India Nutrition Guidelines), motivational interviewing, goal setting, self-monitoring. Participants received handouts including meal plans, exchange lists. |
| Pavithran et al. [22] | India |
N = 40 Age 35–65 y |
RCT |
Nutrition intervention Controls maintain usual diet |
24 wk | Educational in-person interview focused on consuming low-GI whole grain cereals using traditional foods, periodically reinforced by a dietitian. |
| Pavithran et al. [23] | India |
N = 80 Age 35–65 y |
RCT |
Nutrition intervention Controls maintain usual diet |
24 wk | Educational interview focused on consuming low-GI traditional foods, periodically reinforced by a dietitian. |
| Yasmin et al. [24] | Bangladesh |
N = 320 Age 30–85 y |
RCT |
Interactive voice calls and telephone-based physician access Usual care controls |
6 mon | Regular personalized advice regarding diet, physical activity, medications, clinic visits. Call service provided physician contact 24/7. |
| Devi [25] | India (urban) |
N = 340 Age 20–80 y |
Pre-post, with randomization of participants |
I—Education with self-learning module, paper-based II – Education with a powerpoint presentation III – usual care control group |
6 mon |
I—Diet and nutrition self-learning module available in Hindi and English (participant choice) + in-person 90-min discussion in the participant’s home. II – The same materials provided via laptop with an educator present, and DVD and handout for viewing independently at the participant’s home. |
| Islam et al. [26] | Bangladesh |
N = 200 Mean age 48 y |
RCT |
Text messages Usual care controls |
6 mon | Daily text messages (20 out of 90 related to diet) over 6 mon, guided by behavior change theory and diabetes guidelines (Bangladesh + UK and ADA). |
| Thadchanamoorthy et al. [27] | Sri Lanka |
N = 135 Mean age 58 y |
RCT |
I – usual care controls II – general diabetes management education III – nutrition education |
3 and 6 mon |
II – Standard diet based on Dietary Guidelines and Nutrition Therapy for Specific Diseases, Sri Lanka; 4 sessions. III—Nutrition education focused on low carbohydrate diet; 4 sessions. |
| Varadaraj et al. [28] | India |
N = 98 Age 18 + y |
RCT |
Education comparing 2 intensities Usual care control |
3 and 6 mon | In-person nutrition education provided by a dietitian either once monthly or every 3 mon after an initial session (also provided to the usual care control). |
ADA American Diabetes Association, DVD digital video disk, GI glycemic index, PA physical activity, RCT randomized controlled trial, UK United Kingdom
Country
Ten studies (eliciting 12 articles) were conducted in India [14–17, 21–28], 2 in Bangladesh [18, 20], and 1 study in each of Sri Lanka [19], the UK [29], and Norway (2 articles) [30, 31].
Design
Seven studies were quasi-experimental [15, 16, 22–26, 29], 9 (10 articles) were RCT [14, 16–21, 27, 28, 30] and 1 was a case–control study [26].
Sample Size, Participant Age, and Study Duration
The trial size ranged from 15 to 340 participants and most were of middle-aged adults. One pilot study was an acute evaluation of low-GI foods. All other trials ranged from 4 weeks to 12 months.
Effects of Nutrition Interventions on Health Outcomes
Low Glycemic Index Dietary Interventions
Five studies focused on intervening with a low-GI diet (Table 1) with results reported in Table 2 [17, 21, 23, 24, 26].
Table 2.
Study outcomes by study design
| Study | Biological outcomes | Behavioral outcomes | Barriers |
|---|---|---|---|
| Pande et al. [14] |
GI range of 7 meals 28–45 Acute BG AUC response: Healthy group – 960–1719 mg/dl × h vs Diabetes group—1677–1959 mg/dl × h |
Sensory and satiety responses similar between healthy and diabetes groups |
Low patient motivation Lack of access to low GI foods |
| Pande et al. [15] |
After 4 wk vs baseline: FBG decreased (− 36 mg/dl) (p < 0.001) HbA1C decreased (− 0.9%) (p < 0.001) TG decreased (− 80 mg%) (p < 0.001) TC decreased (− 39 mg%) (p < 0.001) HDL-C increased (+ 6.8 mg%) (p < 0.001) LDL-C decreased (− 25.8 mg%) (p < 0.001) |
Patients indicated low acceptability of foods, low motivation | |
| Jain et al. [12] |
After 3 mon vs baseline: FBG decreased in people diagnosed with diabetes for ≥ 5 y (− 52 mg/dL) (p < 0.001) and ≥ 10 y (− 58 mg/dL) (p < 0.001) |
– | Low motivation, which was mitigated by individual counseling and education |
| Singh et al. [16] |
After 3 mon vs baseline: Weight and BMI decreased in all 3 intervention groups Improvements in body composition (upper arm circumference, triceps skinfold thickness) after intervention |
Increased nutrition knowledge, attitudes, and practices after the intervention. PA results not reported | – |
| Krishan et al. [13] |
After 3 mon vs baseline: FBG (mg/dL): Group I + 4; Group II − 24; Group III -14 PPG (mg/dL): Group I + 4; Group II − 11; Group III -8.3 SBP (mmHg): Group I − 4; Group II + 1; Group III -1.5 DBP (mmHg): Group I + 1; Group II + 0.7; Group III -0.7 BMI (kg/m2): Group I + 0.2; Group II -0.2; Group III − 0.8 After 6 mon vs baseline: HbA1C (%): Group I 0; Group II + 0.3; Group III − 0.2 FBG (mg/dL): Group I − 6.3; Group II -21; Group III − 16 PPG (mg/dL): Group I − 10; Group II -10; Group III − 19 TG (mg/dL): Group I − 1; Group II + 18; Group III − 19 TC (mg/dL): Group I − 5; Group II -14; Group III − 15 LDL-C (mg/dL): Group I − 7; Group II -12; Group III − 14 HDL-C (mg/dL): Group 1 0; Group II -2; Group III − 0.1 SBP (mmHg): Group I − 2; Group II -0.4; Group III − 1 DBP (mmHg): Group I − 0.5; Group II -1; Group III − 0.4 BMI (kg/m2): Group I − 0.6: Group II -0.1; Group III − 0.3 |
Noted frequent consumption of rice, pulse grains, egg, sheep, and chicken; milk and buttermilk, and sunflower oil by all groups. Higher consumption of leafy green vegetables by Groups II and III. PA results not reported | Patients reported difficulty adhering to diet during festivals; reported various taboos related to vegetable consumption. These issues were addressed in counselling sessions |
| Bairy et al. [11] |
After 3 months vs baseline: HbA1C − 0.9% overall; decrease was greater (− 1.7%) with excellent compared with poor compliance (− 0.4%) FBG (mg/dL) decreased from 149 to 109 PPG (mg/dL) decreased from 188 to 152 |
Increased compliance to diet, increased knowledge of Integrated Naturopathy and yoga-recommended (low-GI) diet, meal spacing, meal preparation methods |
Patients reported low motivation and cost as obstacles; increased follow-up support would be beneficial HCP reported lack of time and capacity to provide lifestyle interventions; indicated some responsibilities could be delegated |
| Kumari et al. [17] |
After 3 mon vs baseline in intervention (I) and control (C): HbA1C (%): I − 0.5, C − 0.2 (p < 0.001 between groups) After 6 mon from baseline: HbA1C (%): I − 0.9, C − 0.2 (p < 0.001 between groups) After 12 mon from baseline: HbA1C (%): I − 0.9, C + 0.1 (p < 0.001 between groups) Significant benefit (p < 0.001) of the intervention also seen for TG, TC, LDL-C, HDL-C, SBP at 12 mon relative to baseline. No effect of body weight between groups over follow-up period |
Self-report of diet: Intervention group improved overall diet, intakes of fibrous foods, reduced sugar-rich and junk foods Self report of PA: Intervention improved adherence to recommendations |
Adherence tapered off by 12 months, recommend continued counselling. Patient motivation was a barrier |
| St. John [18] | – | Increased knowledge, understanding, and carbohydrate estimating ability for traditional and commonly eaten foods; awareness of carbohydrate’s impact on blood glucose | Used simple messages and pictures for people with low literacy |
| Raberg Kjollesdal et al. [20] | – |
76% reported personal dietary changes 68% reported dietary habits of their families changed No PA results reported |
About half of women reported difficulty in changing dietary habits of self and family; barrier was likes/dislikes of family members, especially children |
| Johansen et al. [19] | – | Intervention increased the stage of change for sugar (p = 0.01), fat (p < 0.001) but vegetables and pulse grains NS. Women in intervention reported dietary changes for self and their families | See above |
| Myers et al. [21] |
After 6 mon vs baseline in intervention (I) and control (C): HbA1C (%): I − 1.7; C − 1.3 (NS between groups) TG (mg/dL): I − 87; C − 3 (p < 0.01) NS intergroup differences for TC, LDL-C, HDL-C BMI (kg/m2): I − 0.8; C − 0.3 (NS) After 12 mon vs baseline in intervention (I) and control (C): HbA1C (%): I − 1.0; C − 1.0 (NS between groups) TG (mg/dL): I − 74; C − 4 (p < 0.01) BMI (kg/m2): I − 0.95; C − 0.5 (NS) NS intergroup differences for TC, LDL-C, HDL-C |
Noted reductions in excessive carbohydrate intake, increase in fiber intake, better carbohydrate spacing in at least 5 participants; noted improved physical activity, nutrition knowledge, energy intake, self-management practices, adherence to recommendations, food choices, involuntary weight gain, carbohydrate quality, fluid intake | Dietitian-reported barriers included complexity of nutrition recommendations, inconsistency with Indian systems, norms and practices, time required to teach patients, low familiarity of dietitians with teaching lifestyle modifications. Enhanced support for dietitians (technical, expertise) could mitigate difficulties |
| Pavithran et al. [23] |
After 24 wk vs baseline in intervention (I) and control (C): HbA1C (%): I − 1.0; C + 0.1 (p < 0.001 intergroup difference) BMI (kg/m2): I − 0.75; C + 0.07 (p = 0.007) TC, TG, HDL-C, LDL-C and VLDL all were NS |
– | Patients wanted familiar foods to increase compliance and acceptability |
| Yasmin et al. [24] |
After 6 mon vs baseline in intervention (I) and control (C): FBG (mmol/L): I − 1.9 (p < 0.001); C − 0.4 (NS) 2-h PPG (mmol/l): I − 2.5 (p < 0.001); C − 0.9 (p = 0.035) |
Intervention – adherence to recommended carbohydrate (p < 0.001), total energy (p < 0.001), and fruit (p < 0.001) improved but protein and fat did not (NS) Control group – NS changes in diet NS difference in medication adherence in both groups. PA volume (h/wk) was unchanged in both groups although intervention increased PA frequency |
Patients reported low motivation, high cost were barriers. Patients could benefit from subsidies, more counselling |
| Pavithran et al. [22] |
After 24 wk vs baseline in intervention (I) and control (C): HbA1C (%): I − 0.9; C + 0.1 (p = 0.003 intergroup difference) TG (mg/dL): I − 27; control + 22 (p = 0.025 between groups) BMI, SBP, DBP, TC (NS) |
After 24 wk vs baseline: Macronutrients as % energy intake Carbohydrate (%): I 61.6, C 65.9 (p < 0.001 between groups) Fat (%): I 24.4, C 21.1 (p = 0.003) Protein—NS |
|
| Devi [25] |
After 6 mon vs baseline: Meeting blood glucose targets: SLM increased from 52 to 95% of participants (p < 0.01); PPP increased from 42 to 98% of participants (p < 0.01); control increased from 49 to 54% (NS) Body weight maintenance target: SLM increased from 4.7% to 32% (p < 0.01); PPP increased from 4.7% to 40% (p < 0.01); control increased from 6.5% to 10.6% (NS) |
SLM and PPP increased overall nutrition knowledge similarly – SLM increased score from 6.3 to 25.1 (p < 0.001); PPP increased score from 6.1 to 28.0 (p < 0.001); control increased score from 6.0 to 6.8 (NS) Knowledge of GI of foods increased in both SLM and PPP (with PPP superior for some foods) |
|
| Islam et al. [26] |
After 6 mon: HbA1C (intergroup difference: − 0.66%, 95% CI: − 0.97, − 0.35)* |
Adjusted mean difference between groups – Vegetables – NS Fruit – NS Sugar beverages – decreased in both groups (NS) Sugar in tea—NS |
|
| Thadchanamoorthy et al. [27] |
After 3 mon vs baseline: HbA1C: Control − 0.31%, standard diet + 0.03%, LCD: + 1.07% (p < 0.001) BMI: Control + 0.21 kg/m2, standard diet + 0.04 kg/m2, LCD − 1.17 kg/m2 (p = 0.007) SBP, DBP, TC, TG – NS After 6 months vs baseline: SBP: Control + 9.2 mmHg, Standard diet + 1.6 mmHg, LCD − 5.8 mmHg (p = 0.001) DBP: Control + 2.4 mmHg, standard diet 0.0 mmHg, LCD + 5.4 mmHg (p = 0.022) All other outcomes NS |
– | No benefit of standard diet based on Sri Lankan guidelines |
| Varadaraj et al. [28] |
Results at baseline, 3 and 6 months: HbA1C: Intensive 7.6%, 7.0%, 6.4% (p < 0.001), intermittent 7.7%, 7%, 7.5% (p < 0.001), control 7.2%, 7.5%, 7.9% (p < 0.001) BMI: intensive 27.1, 26.6, 26.0 kg/m2 (p < 0.001), intermittent 27.5, 27.0, 27.4 kg/m2 (p < 0.001), control 26.7, 27.0, 27.5 kg/m2 (p < 0.001) |
– | – |
AUC area under the curve, BG blood glucose, BMI body mass index, C control group, DBP diastolic blood pressure, FBG fasting blood glucose, GI glycemic index, HbA1C glycosylated hemoglobin, HDL-C high-density lipoprotein cholesterol, I intervention group, LCD low carbohydrate diet, LDL-C low-density lipoprotein cholesterol, NS not significant, PA physical activity, PPG post-prandial glucose, PPP powerpoint presentation intervention, SBP systolic blood pressure, SLM self-learning module intervention, TC total cholesterol, TG triglyceride
*These data appeared in a letter, which was excluded by our search strategy. Shariful Islam SM, Niessen LW, Ferrari U, et al. Effects of Mobile Phone SMS to Improve Glycemic Control Among Patients With Type 2 Diabetes in Bangladesh: A Prospective, Parallel-Group, Randomized Controlled Trial. Diabetes Care 2015;38:e112-3
Glycemic Outcomes
In 2 RCT of 40 and 80 T2D participants that were well-matched at baseline [17, 21], people in the intervention arm were advised to include low-GI whole grain cereals and traditional foods and compared to usual-diet controls for 24 weeks, with periodic support from a dietitian. In both trials, the low-GI group significantly reduced FBG and PPG. Compared with controls, low-GI participants exhibited a significant lowering of HbA1C (− 0.9%). In a pilot case–control trial, researchers developed Indian-style low-GI foods that enabled blood glucose to return to baseline within 2 h of consumption in people with T2D [26]. They then reported that consuming low-GI meals and snacks for 4 weeks in T2D participants lowered HbA1C by − 0.9% compared to baseline [24]; however, this could not be conclusively attributed to the intervention as the study duration was < 12 weeks. FBG was improved [24]. Another non-randomized study used diet, naturopathy and yoga in participants admitted to a naturopathy hospital for 15–30 days. They were provided plant-based low-GI meals, performed yoga and other exercises, received comprehensive education, and one-on-one counselling with their physician daily [23]. At 3-month follow-up, participants showed significant declines in HbA1C, FBG, and PPG compared with baseline; however, 55% of those enrolled were not followed up. A dose–response relationship between compliance to dietary practices and HbA1C was observed with mean reductions of 0.4%, 1.1%, and 1.7% among those with poor, moderate, and excellent compliance to dietary practices, respectively. A majority of participants (65%) achieved a reduction in HbA1C with 19% stopping medication [23]. However, given the comprehensive lifestyle intervention, biological benefits cannot be fully attributed to the low-GI diet.
Lipid and Blood Pressure Management
Evidence from the above studies also indicates some beneficial effects of a low-GI diet on lipid profile (Table 2). An RCT of 80 participants found a sustained significant reduction in triglyceride (TG) compared with controls [21] although the smaller (n = 40) trial did not [17]. No effect on blood pressure (BP) was detected [21]. In a single-arm intervention, a low-GI diet elicited significant improvement in TG, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) compared with baseline [24].
Anthropometrics
One RCT (n = 40) [17] reported a significant reduction in BMI in the low-GI group but this was not replicated in the larger trial [21].
Summary
Overall, the 4 studies of a low-GI diet lasting 4 weeks to 6 months found significant reductions in HbA1C or other glycemic outcomes [17, 21, 23, 24]. Other cardiovascular risk factors were inconsistently measured, precluding conclusions. While 2 were RCT, they were conducted by the same group and had a total enrolment of 120 participants [17, 21].
Education and Counselling Interventions
Thirteen articles focused on nutrition education or counselling as the primary intervention (Table 1) with the outcomes reported in Table 2. Group education sessions were the primary intervention in 4 articles (3 studies) [19, 25, 30, 31] whereas individual counselling methods were employed in 5 interventions [14–16, 22, 27]. Two articles investigated the use of mobile phone-based interventions [18, 20] while 1 study each focused on self-learning modules [28] and the development of educational materials [29].
Glycemic Outcomes
Nine articles reported on glycemic management in participants. In an RCT employing individual nutrition counselling, the HbA1C of participants receiving education on low carbohydrate diet for 3 months was not different from controls at 3- or 6-month follow-up despite excellent retention (84–96%) in the intervention. However, no measure of compliance was reported [19]. An RCT with a pre-post evaluation of self-learning modules versus educator-led instruction supported by PowerPoint presentations reported that the proportion of individuals meeting blood sugar targets increased similarly in both groups after 6 months [28]. In a single-arm, 3-month trial, individual counseling on diet, exercise, and medication elicited improvement in FBG in participants compared with baseline but no information on dietary intake was provided [22].
The intensity (frequency) of educational intervention was compared in a 3-arm trial [27]. In one group, participants received intensive medical nutrition therapy from dietitians once per month for 6 months and experienced a sustained decrease in HbA1C up to 6 months. Participants who received intermittent nutritional therapy (2 sessions over 6 months) had improvements in HbA1C only at 3 months, with benefits attenuating by 6 months. Conversely, a control group taken from a different clinic receiving usual care recorded an increase in HbA1C [27].
The importance of providing comprehensive education (nutrition plus other health behavior information) was unclear in a pre-post study in which one-third of participants received periodic, ongoing, in-person counselling on diet and physical activity. Although declines in FBG and PPG relative to baseline were reported, HbA1C was unchanged. Participants receiving periodic ongoing counselling on diet alone showed improvements only in FPG [16]. A control group receiving only 1 education session had similar HbA1C values at 6 months as the intervention groups [16].
Approaches combining both intensive counselling and comprehensive education on diabetes management were explored in 2 RCTs. An intensive individual counseling intervention led by dietitians and grounded in evidence-based Indian nutrition practice guidelines (EBNPG) included education, motivational interviewing, goal setting, and self-monitoring but did not improve HbA1C compared with controls [14]. In another, monthly sessions for 6 months led by content experts from an urban hospital covering diet, physical activity, and other self-management practices resulted in an intergroup difference in HbA1C favoring the intervention up to 1 year of follow-up [15].
Technology was used to deliver content in 2 RCTs. The first examined comprehensive messaging on various topics including nutrition (20 out of 90 messages in total) as well as physical activity and medications delivered via text messaging and resulted in greater improvement in HbA1C among intervention than controls at 6 months [18]. Another 6-month RCT employing both text messaging and interactive voice calls, plus telephone-based access to a physician 7 days/week, 24 h/day showed improvements in FBG and PPG in the intervention group whereas in the control group only PPG was lower than baseline. However, the dropout was high, possibly due to low motivation or high cost of intervention-recommended foods [20].
Lipid and Blood Pressure Management
Among participants in an RCT who received intermittent nutritional therapy, improvement in lipids was observed only at 3 months. However, more intensive nutritional therapy showed decreases in total cholesterol and LDL-C up to 6 months [27]. One RCT intervening with either general diabetes management or nutrition education focused on a low-carbohydrate diet did not report significant intergroup differences in lipid profile. However, BP decreased in the intervention group [19]. Periodic intensive counselling on diet and exercise elicited significant decreases in total cholesterol and LDL-C compared to baseline but less intensive interventions did not [16]. Participants in a single-arm trial of a holistic lifestyle modification experienced significant reduction in TG, LDL-C, and total cholesterol, an increase in HDL-C, and reduced systolic BP [15]. Similarly, an improved lipid profile was also observed among participants who followed EBNPG recommendations [14].
Anthropometrics
Participants in a low-carbohydrate diet health education RCT had a significant reduction in BMI compared with controls [19]. In another RCT [14, 15], no intergroup differences were found whereas Varadaraj et al. [27] reported decreases in BMI compared with baseline in both more- and less-intense intervention groups but an increase in BMI in controls. In a non-randomized trial, significant improvements were observed in BMI in participants who received individual and group nutrition education but only men were included [25]. Similar results were observed in participants receiving periodic intensive counselling on either diet alone or diet and exercise [16]. However, these results might not be generalizable to the broader population, as most enrollees were of moderate-high socioeconomic status.
Summary
Four out of 9 studies of 3–6 months duration reported improvements in glycemic outcomes and 1 RCT intervention yielded sustained benefit at 12 months. Successful interventions tended to have a higher frequency of sessions along with comprehensive education encompassing dietary and other health behaviors. Where benefits on lipid or blood pressure outcomes were reported, it was usually in higher intensity interventions. Of the 6 trials that measured BMI, 2 randomized and 2 non-randomized interventions found a benefit.
Effects of Nutrition Interventions on Behavior Outcomes
Fifteen studies assessed behavior change outcomes in participants as described in Table 1 and reported in Table 2. Most behaviors were assessed subjectively using participant self-report and included parameters such as nutrition knowledge, dietary intake (some of which was objectively measured using validated questionnaires), dietary compliance, stage of change for certain behaviors, or diabetes self-management practices.
Low-GI Dietary Interventions with Educational Support
In an RCT with objectively measured dietary intake, the intervention was low-GI foods based on local, traditional whole grains with support provided by dietitians, who evaluated dietary compliance using repeated 24-h dietary recall. Compliance and acceptability of the low-GI diet were higher than the usual diet. The low-GI group consumed significantly less carbohydrate and fat as % energy at the trial conclusion [21]. A holistic diet and yoga program held in a naturopathy hospital included a variety of strategies to improve dietary intake, twice daily yoga practice plus optional physical activities and diabetes management skills in addition to the provision of low GI meals, meditation, and a variety of education sessions throughout the study period [23]. Overall, 80% of patients showed moderate to excellent dietary compliance and increased knowledge of the educational topics presented, meal preparation, and spacing. However, self-reported better adherence to yoga practice was not associated with favorable glycemic outcomes [23]. To assess the sense of satisfaction with low-GI versions of traditional Indian mixed meals, sensory evaluation of the low-GI meals with healthy and T2D participants was conducted. Satiety and hedonic ratings for flavor, appearance, texture, taste, and acceptability were similar between low-GI and original foods [26]. The low-GI meals were prescribed to T2D participants in a follow-up study for 4 weeks with good dietary compliance by 13 of 15 participants [24].
Culturally Adapted Dietary Education
An RCT of Norwegian-Pakistani women [31] demonstrated that culturally adapted group nutrition education sessions changed the participants’ intention to eat healthier. Women in the intervention group received 6 education sessions over 6–8 months. There was a significant shift from the pre-action (pre-contemplation, contemplation, and preparation) stages to the action stage in the intervention group for intentions to consume a healthier diet (reduced sugar and white flour, healthier fats, increased vegetables, fruits, and legumes). At the follow-up, all food intake variables, except fruit intake, were improved in the intervention group compared with the control group [31].
Summary
Compliance to dietary recommendations was assessed by a variety of techniques. The trials utilizing traditional foods with low GI or a focus on healthier eating patterns were generally successful in improving dietary outcomes. Other behaviors, such as physical activity, were inconsistently reported.
Barriers to Uptake of Interventions and Culturally Responsive Mitigation Strategies
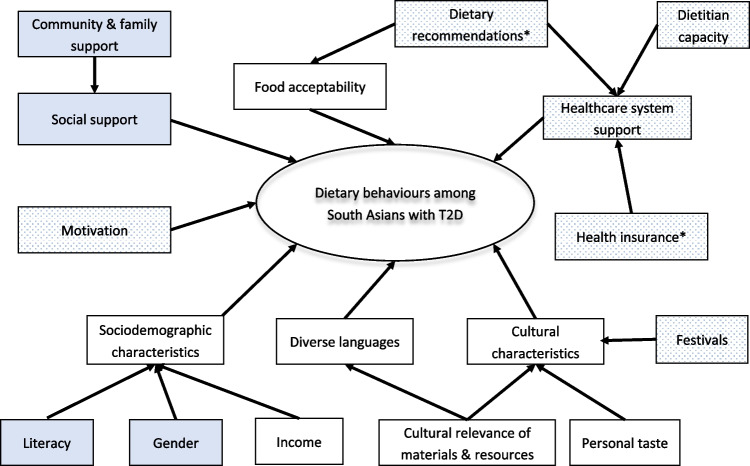
Here, we present results for 5 of the most prominent barriers and culturally relevant solutions. Figure 1 depicts factors that influence dietary behaviors in the study population, and summarizes which were most important in people with T2D in their home country vs immigrants to North America/Europe. The conceptual model is adapted from Zeng et al. [32].
Fig. 1.
Factors influencing dietary behaviors in South Asians living in their home country or immigrants to North America or Europe. Legend—clear boxes depict factors identified both in South Asia and in immigrant settings; stippled boxes depict factors only identified in South Asia; blue boxes depict factors identified only in immigrant settings. As indicated, there is considerable overlap in the barriers and challenges faced by South Asian people with T2D, independent of where they live. Mentioned in other literature; not based on an exhaustive interview.
Adapted from the conceptual model of Zeng et al. [32] originally developed for Chinese immigrants. https://doi.org/10.3390/ijerph110706727
Diverse Languages
Several languages and dialects are spoken among people in South Asia. Five studies created educational resources, text messages, or conducted counselling in local South Asian languages. In an RCT implementing EBNPG, participants’ local dietitians provided individualized counselling in 1 of 11 Indian languages across India, focusing on dietary recommendations and behavioral strategies such as goal setting. Outcomes up to 1 year included reduced energy and carbohydrate quantity and quality and improved nutrition knowledge, increased physical activity, self-management practices, and adherence to recommendations [14]. Another intervention based on holistic lifestyle practices in a naturopathy clinic provided the intervention in the local language, supported by pictures, videos, and interviews and discussions with individuals or groups. Self-reported diet of the intervention group indicated increased intake of fiber while decreasing sugary and junk foods [15]. In a study comparing the effectiveness of education delivery by self-learning modules and educator-led PowerPoint presentations, the content was available in both English and Hindi. Other translated resources such as handouts and DVDs were also provided to participants, with both self-learning and educator-led interventions yielding increased nutrition knowledge [28].
Low Literacy
In Pakistani women living in Norway, the authors identified that the immigrant women may have lower literacy, education, and poorer language skills than natives of the host country, creating barriers to accessing healthcare services and health information [31]. Also, some women were reluctant to go out alone or speak to healthcare staff of the opposite sex without an accompanying family member. Culturally adapted and translated audiovisual materials were used in the education program. Dietary assessment was conducted in Urdu and/or Punjabi using a culturally adapted Food Frequency Questionnaire [31]. The outcomes included participants advancing through the stages of change for reducing sugar and fat intake, although intentions to increase vegetable and pulse grain intake were not increased [30].
The authors of a UK-based study created an educational resource for ethnic minority communities including Gujarati and Pakistani people. To address language and literacy barriers, it included simple messages, culturally relevant information in the form of photographs of foods commonly eaten in the selected communities, alongside their carbohydrate content. This enabled participants to understand the carbohydrate content of traditional foods and how they might affect their blood glucose [29]. In a pilot trial, participants reported increased knowledge and understanding of carbohydrate counting for traditional and commonly eaten foods while gaining an improved understanding of the impact of carbohydrates on blood glucose [29].
Personal Preferences and Family Influences
Participants in a 6-month counselling intervention indicated that adherence to the prescribed dietary recommendations was most difficult during festivals and special occasions when the consumption of certain traditional foods is essential. To manage this situation, study participants were encouraged to substitute low-fat snacks and sweets prepared with artificial sweeteners [16].
In a study among Pakistani immigrants in Norway, preferences of the children and husbands were identified as a significant barrier to dietary changes, as were social expectations to consume sweets and soft drinks [30]. Barriers limiting the intake of fruits and vegetables included high prices, taste and appearance of the food, lower quality and freshness, and decreased availability of certain foods compared to Pakistan. To mitigate the barriers, the intervention was based on the Pakistani lifestyle in Pakistan and in Norway. Acknowledging the women’s roles as mothers, wives, and as providers of food for their family [31] was successfully navigated, as the women reported improved dietary habits of themselves and their families [30].
Some participants’ diets were influenced by taboos regarding fruit and vegetable consumption. For example, “Gourd vegetables and citrus fruits were avoided during rainy season as they were believed to be cold producing foods. Many of the subjects did not consume any fruit as they believed fruits were not permitted for subjects with diabetes” [16]. Diet counselling encouraged increased intake of vegetables and allaying misconceptions about fruit. In addition, participants following a non-vegetarian diet were encouraged to include more vegetable dishes. Overall, the intake of leafy green vegetables increased after the counselling intervention [16].
Responsive Programming to Increase Uptake
In 1 study [16], family members and caregivers were invited to participate in the counselling sessions. When participants were unable to attend in person, home visits or telephone calls were provided. In the Norwegian study of Pakistani women, walking groups were created as part of the study and, to encourage participation, childcare was provided and stroller-friendly walking paths were identified [30].
Traditional Foods and Exercise
Prescriptions for a dietary recommendation that are culturally unfamiliar may be daunting to participants and prevent sustained adherence. To improve adherence to and acceptability of the dietary recommendations some studies used local, traditional foods and recipes in their recommendations. In a 24-week RCT, participants in the intervention arm were provided low-GI, local, traditional whole grains such as red rice puttu (steamed rice flour logs), whole wheat flour, barley, and rolled oats, while the control arm followed usual dietary practice [21]. Compliance to dietary recommendations was excellent in the intervention group versus the control group, but could not be attributed solely to the adoption of traditional recipes because of the meticulous phone follow-up by dietitians with the intervention group. Pande et. al. [26] reported that the use of modified meals and snacks prepared with Indian foods was associated with improved satiety, acceptance, and compliance to the recommendations in 13 out of 15 participants. Others reported that 80% of participants were compliant with dietary intake recommendations based on low-GI plant-based local foods. In some interventions, traditional yoga exercises and Pranayama (breathing exercises) to improve physical activity were utilized [15, 23] while others recommended walking [23, 31]. Other studies did not report the type of physical activity recommended, nor outcomes [16, 20].
Discussion
Medical nutrition therapy is a cornerstone of the management of T2D and, when successfully implemented, is proven to improve health outcomes and prevent comorbidities. The current literature provides consistent evidence that low-GI diets are efficacious in the management of hyperglycemia in South Asians in RCT [17, 21] and single-arm trials [23, 24]. Low-GI diets can be made culturally relevant by the inclusion of familiar whole grains, pulses, vegetables, and fruits, and the pattern is adaptable to both vegetarian and omnivorous diets. One limitation might be South Asians’ preference for refined carbohydrates including white rice and rice-based products that are consumed several times daily (i.e., food acceptability in Fig. 1) and can contribute two-thirds of the daily caloric needs [33]. However, alternative traditional whole grains, millets, and whole wheat meal preparations are well received [17]. Adapting cooking methods to reduce the available starch content may also work [26]; for example, rice cooked in excess water drained after cooking has a GI of 19, compared to white rice (GI 72) and parboiled rice (GI 57). The high GI of rice is primarily due to its low content of amylose (a resistant starch), which improves its softness and palatability [34] but also enables more rapid digestion, resulting in high blood sugar following consumption [35]. The GI of rice is also affected by other properties of starch and its interactions with other components during processing and cooking [36]. Innovative technology and research are needed to fully understand the GI properties of rice and create new palatable rice varieties with low-GI content [34].
Interventions of nutrition education and counselling sessions had mixed results on glycemic control. As expected, interventions that were more intensive (more frequent visits) and/or comprehensive had sustained improvements in HbA1C for 6–12 months [15, 27] as shown elsewhere [37]. Less intense or shorter interventions were less effective [19, 27]. Consistent with these findings in South Asian countries, a systematic review and meta-analysis of 4 nutrition education interventions conducted in low/middle-income countries reported significant benefits on HbA1C or fasting glucose at 3 but not 6 months [38]. Globally, trials including at least 10 contact hours of education produced the greatest HbA1C reduction [39]. However, participants receiving intensive counselling and education sessions may benefit from changes in dietary intake behaviors that improve adherence to the dietary recommendations [31] that may reduce the risk of complications if there is sufficient follow-up. A meta-analysis of combined dietary and physical activity interventions for type 2 diabetes reports that efficacy is generally not sustained beyond 6 months [40].
Given the substantial number of people in South Asia who have T2D [1], individual counselling will not reach everyone who needs treatment. We found no studies of public health measures to educate and raise awareness about healthier diets for T2D. Most South Asian countries have a limited capacity of government-funded hospitals to serve large populations, thus medical treatment is mostly obtained through privatized health care providers for which there is a limited medical insurance coverage (Fig. 1, healthcare system support) [41]. We see an immediate need to develop and implement large-scale public education initiatives to reach those diagnosed with T2D also applicable to those who are undiagnosed or have pre-diabetes. The use of technology offers a promising solution for addressing several barriers. The increase in web connectivity and 5G services [42], the use of cellular/smartphones and technological advances in both urban and rural areas make technology-based services a viable solution. These services are cost-effective, convenient, and can reach large audiences with a small time commitment [43]. Two articles found that the use of technology improved glycemic control and evoked positive behavior changes in South Asian people with T2D [18, 20].
We also highlighted several approaches to reducing barriers related to cultural relevance (Fig. 1, diverse languages, cultural characteristics, social support) such as the use of local foods, creating educational materials in local languages, tailoring interventions to support the lifestyle needs of families, offering supportive services such as home visits, and inclusion of family members [14, 16–21, 27, 28, 30]. Offering services like these and others to make interventions culturally relevant and meaningful to participants is likely to increase participation and compliance to program requirements. In Western countries, assessing the degree of acculturation can improve the tailoring of healthcare interventions for immigrant populations [44, 45]. Given recent adaptations to healthcare necessitated by the COVID-19 pandemic, future interventions may be able to make greater use of tele- or video-conferencing or social media [46].
Limitations and Gaps
Many studies were small, lacked control groups, or did not provide intergroup statistical analysis but several well-done RCT have been published recently. Single-arm trial outcomes need to be interpreted with caution in terms of the magnitude of results and generalizability. Many studies relied on self-report of dietary intakes (not using standardized questionnaires) and other behaviors. Low GI is the only dietary pattern reported; we were unable to find studies examining other dietary patterns. Since there are regional variations in dietary patterns within South Asian countries, other interventions such as healthy vegetarian diets, lacto-ovo vegetarian diet, pescatarian diet, or Mediterranean diet need could be adapted for segments of this heterogeneous population. One of the biggest challenges faced is the huge population affected by T2D, which also makes it a limitation because the healthcare systems of most South Asian countries are not equipped to offer universal diabetes self-management, education, and support initiatives. Few studies reported barriers from the healthcare system perspective, although lack of dietitians’ and other experts’ availability to deliver lifestyle interventions was mentioned [14, 23]. Possibly, low-GI diet messaging could be integrated into public health initiatives and broadly promoted to reach large populations. Moreover, all of the trials reported were conducted in urban settings. Approaches to support people living in rural areas need to be developed.
Conclusions
Studies from South Asian countries support approaches using low-GI dietary pattern adapted to use local foods, along with intensive counselling approaches for the treatment of T2D. Practical, public health approaches are needed in order to provide access to care because of the high prevalence of T2D in South Asian countries. In Western countries, more research is required on best practices for providing culturally relevant nutrition therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Work in the laboratory of CBC is funded by Dairy Farmers of Canada, Alberta Health Services, Alberta Agriculture and Forestry, Alberta Canola. DNF received a stipend from the Alberta Diabetes Institute.
Data Availability
Data are available upon reasonable request to the corresponding author.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Diabetes and Obesity
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatheema B. Subhan, Email: fsubhan@cpp.edu
Dineli N. Fernando, Email: wfernand@ualberta.ca
Jessica Thorlakson, Email: jthorlak@ualberta.ca.
Catherine B. Chan, Email: cbchan@ualberta.ca
References
- 1.International Diabetes Federation (2021) Diabetes Atlas. https://diabetesatlas.org/atlas/tenth-edition/. Accessed 4 September 2022.
- 2.Sohal PS. Prevention and management of diabetes in South Asians. Can J Diabetes. 2008;32(3):206–210. doi: 10.1016/S1499-2671(08)23011-X. [DOI] [Google Scholar]
- 3.Banerjee AT, Shah BR. Differences in prevalence of diabetes among immigrants to Canada from South Asian countries. Diabet Med. 2018;35(7):937–943. doi: 10.1111/dme.13647. [DOI] [PubMed] [Google Scholar]
- 4.Lee JW, Brancati F, Yeh H-C. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care. 2011;34(2):353–357. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanif W, Susaria R. Diabetes and cardiovascular risk in UK South Asians: an overview. Br J Cardiol. 2018;25:S8–S13. doi: 10.5837/bjc.2018.s08. [DOI] [Google Scholar]
- 6.Subhan FB, Chan CB. Diet quality and risk factors for cardiovascular disease among South Asians in Alberta. Appl Physiol Nutr Metab. 2019;44(8):886–893. doi: 10.1139/apnm-2018-0868. [DOI] [PubMed] [Google Scholar]
- 7.Hanif W, Ali SN, Bellary S, Patel V, Farooqi A, Karamat MA, Saeed M, Sivaprasad S, Patel K, Khunti K. Pharmacological management of South Asians with type 2 diabetes: consensus recommendations from the South Asian Health Foundation. Diabet Med. 2021;38(4):e14497. doi: 10.1111/dme.14497. [DOI] [PubMed] [Google Scholar]
- 8.Misra A, Ramchandran A, Jayawardena R, Shrivastava U, Snehalatha C. Diabetes in South Asians. Diabet Med. 2014;31(10):1153–1162. doi: 10.1111/dme.12540. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor N, Sahay R, Kalra S, Bajaj S, Dasgupta A, Shrestha D, Dhakal G, Tiwaskar M, Sahay M, Somasundaram N, Reddy R, Bhattacharya S, Reddy VB, Viswanathan V, Krishnan D, Baruah M, Das AK. Consensus on medical nutrition therapy for diabesity (CoMeND) in adults: a South Asian perspective. Diabetes Metab Syndr Obes. 2021;14:1703–1728. doi: 10.2147/DMSO.S278928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasturia S, Ali MK, Narayan VKM, Tandon N, Shivashankar R, Garg V, Kapoor D, Mohanasundaram A, Mohan D, Kadir MM, Prabhakaran D, Mohan V, Jaacks L. Diets for South Asians with diabetes: recommendations, adherence, and outcomes. Asia Pac J Clin Nutr. 2018;27(4):823–831. doi: 10.6133/apjcn.112017.03. [DOI] [PubMed] [Google Scholar]
- 11.Sohal T, Sohal P, King-Shier KM, Khan NA. Barriers and facilitators for type-2 diabetes management in South Asians: a systematic review. PLoS ONE. 2015;10(9):e0136202. doi: 10.1371/journal.pone.0136202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhurji N, Javer J, Gasevic D, Khan NA. Improving management of type 2 diabetes in South Asian patients: a systematic review of intervention studies. BMJ Open. 2016;6(4):e008986. doi: 10.1136/bmjopen-2015-008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navodia N, Wahoush O, Tang T, Yost J, Ibrahim SM, Sherifali D. Culturally tailored self-management interventions for South Asians with type 2 diabetes: a systematic review. Can J Diabetes. 2019;43:445–452. doi: 10.1016/j.jcjd.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Myers EF, Trostler N, Varsha V, Voet H. Insights from the Diabetes in India Nutrition Guidelines Study: adopting innovations using a knowledge transfer model. Top Clin Nutr. 2017;32(1):69–86. doi: 10.1097/TIN.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumari G, Singh V, Jhingan AK, Chhajer B, Dahiya S. Effectiveness of lifestyle modification counseling on glycemic control in type 2 diabetes mellitus patients. Curr Res Nutr Food Sci. 2018;6(1):70–82. doi: 10.12944/CRNFSJ.6.1.07. [DOI] [Google Scholar]
- 16.Krishnan D, Gururajan R, Hafez-Baig A, Kondalsamy-Chennakesavan S, Wickramasinghe N, Gururajan R. The impact of diet counselling on type 2 diabetes mellitus: an Indian case study. J Diabetes Metab. 2015;6(10):1000610. doi: 10.4172/2155-6156.1000610. [DOI] [Google Scholar]
- 17.Pavithran N, Kumar H, Menon AS, Pillai GK, Sundaram KR, Ojo O. The effect of a low GI diet on truncal fat mass and glycated hemoglobin in South Indians with type 2 diabetes-a single centre randomized prospective study. Nutrients. 2020;12:179. 10.3390/nu12010179. [DOI] [PMC free article] [PubMed]
- 18.Islam SMS, George ES, Maddison R. Effectiveness of a mobile phone text messaging intervention on dietary behaviour in patients with type 2 diabetes: a post-hoc analysis of a randomised controlled trial. Mhealth. 2021;7:10. doi: 10.21037/mhealth-2020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thadchanamoorthy S, Gnanaselvam K, Somasuriyam K, Ibrahim MS. Dietary intervention for glycaemic control among patients with type 2 diabetes mellitus at the medical clinic, teaching hospital, Batticaloa, Sri Lanka. J Res Med Dent Sci. 2021;9(8):343–350. [Google Scholar]
- 20.Yasmin F, Nahar N, Banu B, Ali L, Sauerborn R, Souares A. The influence of mobile phone-based health reminders on patient adherence to medications and healthy lifestyle recommendations for effective management of diabetes type 2: a randomized control trial in Dhaka. Bangladesh BMC Health Serv Res. 2020;20(1):520. doi: 10.1186/s12913-020-05387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavithran N, Kumar H, Menon AS, Pillai GK, Sundaram KR, Ojo O. South Indian cuisine with low glycemic index ingredients reduces cardiovascular risk factors in subjects with type 2 diabetes. Int J Environ Res Public Health 17. 2020;17:6232. 10.3390/ijerph17176232. [DOI] [PMC free article] [PubMed]
- 22.Jain B, Kuvera D. Effect of nutritional counselling on glucose level of middle aged non-insulin dependent diabetics. Asian J Home Sci. 2012;7(2):472–478. [Google Scholar]
- 23.Bairy S, Kumar AM, Raju M, Achanta S, Naik B, Tripathy JP, Zachariah R. Is adjunctive naturopathy associated with improved glycaemic control and a reduction in need for medications among type 2 diabetes patients? A prospective cohort study from India. BMC Complement Altern Med. 2016;16(1):290. doi: 10.1186/s12906-016-1264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pande A, Krishnamoorthy G, Moulick ND. Hypoglycaemic and hypolipidaemic effects of low GI and medium GL Indian diets in type 2 diabetics for a period of 4 weeks: a prospective study. Int J Food Sci Nutr. 2012;63(6):649–658. doi: 10.3109/09637486.2011.649247. [DOI] [PubMed] [Google Scholar]
- 25.Singh U, Kochhar A. Impact of nutrition education on anthropometric and knowledge, attitudes and practices (KAP) score on nutritional status of non-insulin dependent diabetics. J Hum Ecol. 2013;41(2):157–163. doi: 10.1080/09709274.2013.11906563. [DOI] [Google Scholar]
- 26.Pande A, Krishnamoorthy G, Moulick N. Effect of redesigned Indian mixed meals on blood glucose and insulin levels in normal versus type 2 diabetic subjects–a comparative study. Int J Food Sci Nutr. 2011;62(8):881–892. doi: 10.3109/09637486.2011.591368. [DOI] [PubMed] [Google Scholar]
- 27.Varadaraj G, Sangeetha B, Nithiya DR, Dixit PS. Effectiveness of medical nutritional therapy in the management of type 2 diabetes mellitus. J Assoc Physicians India. 2021;69:1–11. [PubMed] [Google Scholar]
- 28.Devi R. Comparison of two educational approaches on knowledge regarding diet among type 2 diabetes patients in East Delhi. Indian J Publ Health Res Devel. 2021;12(2):312–321. [Google Scholar]
- 29.St. John J, on behalf of the World Foods team The journey towards building engaging dietary resources for BAME communities. Diabetes Prim Care. 2019;21:13–16. [Google Scholar]
- 30.RabergKjollesdal MK, Telle Hjellset V, Bjorge B, Holmboe-Ottesen G, Wandel M. Barriers to healthy eating among Norwegian-Pakistani women participating in a culturally adapted intervention. Scand J Public Health. 2010;38(5 Suppl):52–59. doi: 10.1177/1403494810378923. [DOI] [PubMed] [Google Scholar]
- 31.Johansen KS, Bjorge B, Hjellset VT, Holmboe-Ottesen G, Raberg M, Wandel M. Changes in food habits and motivation for healthy eating among Pakistani women living in Norway: results from the InnovaDiab-DEPLAN study. Publ Health Nutr. 2010;13(6):858–867. doi: 10.1017/S1368980009992047. [DOI] [PubMed] [Google Scholar]
- 32.Zeng B, Sun W, Gary RA, Li C, Liu T. Towards a conceptual model of diabetes self-management among Chinese immigrants in the United States. Int J Environ Res Public Health. 2014;11:6727–6742. doi: 10.3390/ijerph110706727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radhika G, Sathya RM, Ganesan A, Saroja R, Vijayalakshmi P, Sudha V, Mohan V. Dietary profile of urban adult population in South India in the context of chronic disease epidemiology (CURES-68) Public Health Nutr. 2011;14(4):591–598. doi: 10.1017/S136898001000203X. [DOI] [PubMed] [Google Scholar]
- 34.Huang M, Hu L-Q. Low glycemic index: the next target for rice production in China? J Integr Agric. 2021;20(6):1727–1729. doi: 10.1016/s2095-3119(20)63299-3. [DOI] [Google Scholar]
- 35.Ohtsubo K, Nakamura S, Maeda S, Kobayashi A, Yamazaki A, Watanabe S. Possibility of diabetes prevention by high-amylose rice and super hard rice. J Diabetes Obes. 2016;3(1):1–7. doi: 10.15436/2376-0949.16.728. [DOI] [Google Scholar]
- 36.Kaur B, Ranawana V, Henry J. The glycemic index of rice and rice products: a review, and table of GI values. Crit Rev Food Sci Nutr. 2016;56(2):215–236. doi: 10.1080/10408398.2012.717976. [DOI] [PubMed] [Google Scholar]
- 37.Pimentel GD, Portero-McLellan KC, Oliveira EP, Spada AP, Oshiiwa M, Zemdegs JC, Barbalho SM. Long-term nutrition education reduces several risk factors for type 2 diabetes mellitus in Brazilians with impaired glucose tolerance. Nutr Res. 2010;30(3):186–190. doi: 10.1016/j.nutres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Guilbert E, Perry R, Whitmarsh A, Sauchelli S. Short-term effectiveness of nutrition therapy to treat type 2 diabetes in low-income and middle-income countries: systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2022;12(3):e056108. doi: 10.1136/bmjopen-2021-056108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillay J, Armstrong MJ, Butalia S, Donovan LE, Sigal RJ, Vandermeer B, Chordiya P, Dhakal S, Hartling L, Nuspl M, Featherstone R, Dryden DM. Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta-analysis. Ann Intern Med. 2015;163(11):848–860. doi: 10.7326/M15-1400. [DOI] [PubMed] [Google Scholar]
- 40.Cradock KA, OLaighin G, Finucane F, Gainforth HL, Quinlan LR, Martin Ginis KA. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2017;14(1):18. doi: 10.1186/s12966-016-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tikkanen R, Osborn R, Mossialos E, Djordjevic A, Wharton GA. International health care system profiles: India. International Commonwealth Fund. 2020. https://www.commonwealthfund.org/sites/default/files/2020-12/2020_IntlOverview_INDIA.pdf. Accessed 4 September 2022.
- 42.Corner S. The state of 5G in South Asia 2022, country-by-country guide. NetworkWorld. 2022. https://www.networkworld.com/article/3652228/the-state-of-5g-in-south-asia-2022-country-by-country-guide.html. Accessed 4 September 2022.
- 43.Li D. 5G and intelligence medicine-how the next generation of wireless technology will reconstruct healthcare? Precis Clin Med. 2019;2(4):205–208. doi: 10.1093/pcmedi/pbz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng F, Zhang A, Chan CB. Acculturation, diet acceptability, and diabetes management among Chinese in North America. Front Endocrinol (Lausanne) 2013;4(Aug 27):108. 10.3389/fendo.2013.00108. [DOI] [PMC free article] [PubMed]
- 45.Deng F, Chan CB. Nutrition interventions for type 2 diabetes in Chinese populations: a scoping review. J Immigr Minor Health. 2019;21(6):1416–1431. doi: 10.1007/s10903-018-0845-z. [DOI] [PubMed] [Google Scholar]
- 46.Leong CM, Lee T-I, Chien Y-M, Kuo L-N, Kuo Y-F, Chen H-S. Social media-delivered patient education to enhance self-management and attitudes of patients with type 2 diabetes during the COVID-19 pandemic: randomized controlled trial. J Med Internet Res. 2022;24(3):e31449. doi: 10.2196/31449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the corresponding author.