Abstract
Purpose
The study aims to evaluate the usefulness of lutein/trypan blue vital dye for the staining of corneal tissues and endothelium–Descemet membrane (EDM) for Descemet membrane endothelial keratoplasty (DMEK).
Methods
Sixteen human corneal tissues (Eye Bank, Rome, Italy) were used. Corneal endothelium was tested at 25 s (T0), 1 min (T1), 2 min (T2), and 4 min (T4) from dye addition. Staining intensity and cell counting were compared. Stripped EDM was analyzed for selected apoptotic (AP, caspases, BCL2, BAX) and differentiation (VEGF-A, TGF-β1RI, SMAD3/7, SMA) targets and changes in target expression. Protein extracts were analyzed through SDS-PAGE/IB.
Results
Although trypan blue staining produced the same color intensity of lutein/trypan blue dye in half the time, lutein/trypan blue reached a good and adequate color intensity at T4, which persisted even on excised and washed EDM grafts. Lutein/trypan blue-stained EDM showed a reduced number of blue-stained cells and AP immunoreactivity was significantly reduced in the same samples. An increased BCL2 transcript and a reduced BAX transcript were detected in lutein/trypan blue-stained EDM. No significant changes were observed for the main effector caspases (3/9) upon both treatments and the target genes representative of endothelial cell trans-differentiation (TGF-β1RI, SMAD3/7, SMA). A trend in vascular endothelial growth factor (VEGF-A) regulation was observed in lutein/trypan blue-treated EDM grafts.
Conclusion
Obtained results suggest that lutein/trypan blue dye deserves attention in the DMEK field and support the potential routine use of this dye as a valid alternative to trypan blue for all procedures devoted to the assessment of endothelial cell viability and visualization of EDM graft before DMEK grafting.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00417-022-05909-x.
Keywords: Vital dyes, Corneal endothelial cells, EDM, DMEK, Keratoplasty, Eye bank, Toxicity
Introduction
Descemet membrane endothelial keratoplasty (DMEK) is the gold standard procedure for treating corneal endothelial diseases [1, 2]. The preparation of pre-stripped DMEK grafts requires the staining of the endothelium–Descemet membrane (EDM) [3].
The trypan blue vital dye is routinely used to allow good visualization of corneal endothelium for quick excision, vital cell assessment, and easy intracameral unfolding EDM for positioning [4, 5]. Unfortunately, some studies displayed that trypan blue dye can exert toxic effects on corneal cells and retinal pigment epithelium, forcing the definition of appropriate dosage and exposure times to reduce cytotoxic effects [6, 7]. Since endothelial cell loss can occur (over 30%) after DMEK surgery, even in a standardized procedure, new vital dyes deserve attention in the DMEK field [8, 9].
Phacodyne™ and Phaco Lutein™ (Alfa Instruments s.r.l., Italy) are vital intraocular dyes consisting of 1% soluble lutein, a major component of the macular pigment, in combination with 0.04% trypan blue (hereafter termed lutein/trypan blue dye). Lutein/trypan blue dye was tested as a good, useful, and safe dye during capsulorhexis in phacoemulsification. No signs of toxicity or side effects were detected during follow-up (30 days) [10]. This protective effect was associated with the properties of lutein, as suggested by clinical, histological, and electroretinographic analyses performed to evaluate the safety profile of solutions containing lutein in rabbit eyes after intravitreal injections, implying good protection of vitreoretinal interphase and underlined retina [11].
Since lutein-based dyes showed a good safety profile and optimized outcomes in some ophthalmic surgery, this pilot study aimed to test lutein-based dye performance to prepare EDM grafts before DMEK surgery. Both lutein/trypan blue dye and the trypan blue dye were tested in parallel and compared by means of staining, immunofluorescent (acidic phosphatases), and molecular (caspases 3/9, BCL2, BAX, VEGF-A, TGF-β1RI, SMAD3/7, SMA) target expression.
Materials and methods
Reagents and ethics
The lutein/trypan blue dye (1% lutein in combination with 0.04% trypan blue; Phacodyne™ and Phaco Lutein™; Alfa Instruments s.r.l., Italy) and the trypan blue (0.1%) dye were used for these comparative studies.
All tissue procedures were carried out according to standardized operating procedures belonging to the Eye Bank protocols, in line with the Declaration of Helsinki and the ARVO statements for management of ocular biosamples. Written informed consent was collected from donors in life in order to use corneal tissues for transplantation and/or research purposes.
Specimens: tissue dissection and subgrouping
A total of 16 age- and sex-matched corneoscleral tissues (38% females/62% males; mean age 65 ± 5 years; age range: 47–79 years) were obtained within 24 h from death, and tissue handling was carried out under sterile conditions. Human tissues were preserved in tissue-C medium (Alchimia, Ponte San Nicolò, Padova, Italy) at 2–8 °C for up to 7 days or at 31 °C for up to 30 days [12, 13]. Tissues were deemed unsuitable for transplantation because of insufficient endothelial density (<1500 cells/mm2), severe endothelial polymorphism or severe endothelial dystrophy, and stromal alteration, in line with proper exclusion criteria [14].
Light microscopy analysis, digital acquisitions, and cell counting
Whole corneas (eight samples/four time points) were placed upside-down on Petri dishes under the stereomicroscope (STEMI 2000-c; Carl Zeiss, Oberkochen, Germany). Specimens were equally divided between the lutein/trypan blue and trypan blue analyses. Dye drops were gently added, and the blue staining intensity was registered at each time point under a stereomicroscope (SZW1000 Nikon, Tokyo, Japan), according to a standard grading scale for T0 (25 s), T1 (1 min), T2 (2 min), and T4 (4 min) [15]. T0 was defined as control (25 s) and used for single ratio calculations. After that, blue-stained nuclei were counted from dye addition for each time point. Optical fields (10 × 10 mm2) were used for counting the number of blue cells. Cell mortality was assessed on flat-mounted EDM by counting the number of blue nuclei at each time point under a direct light microscope (Eclipse TE200; Nikon). Optic fields (areas; 5 central squares, 1 mm2) were selected to analyze the most central portion of each sample by using a 10 × 10 ocular grid (×100 magnification). Blue cell ratios (mean ± SD) were produced for each time point with respect to T0 value (cell mortality).
Acidic phosphatase staining on stripped EDM
Corneal tissues (four specimens) were stained at T4, subjected to the whole EDM stripping, and graded EDM for color intensity under a stereomicroscope equipped with optic fibers (Nikon). Over time, the persistence of lutein/trypan blue staining was assessed by placing free-floating EDM in agitation (physiological solution for 15 min).
Excised EDM was post-fixed in buffered formaldehyde, placed on glass slides, and equilibrated in 20 mM phosphate-buffered saline (PBS). Flat-mounted EDMs were probed with anti-acid phosphatase (AP) antibody (1:1000 in PBS; ab58688, Abcam, Cambridge, UK) for 1 h at room temperature. After gentle washing in 0.05% Tween 20-PBS, slides were covered with FC-conjugated secondary antibodies (1:400 in PBS; Vector Laboratories Inc., CA, USA) for 30 min at room temperature. Finally, slides were mounted in an aqueous mounting medium (Vectamount; Vector) containing propidium iodide (PI; 1:1000 in PBS; Sigma-Aldrich, PA, USA) for nuclear staining. Images were acquired using the epifluorescent direct microscope (Ni-Eclipse; Nikon, Tokyo, Japan) equipped with a UV lamp, digital camera (Axiocam 208 color), and ZEN 3.1 (free available blue edition) acquisition software (both from Carl Zeiss, Jena, Germany). Single specific acquisitions were carried out at ×40 objectives and merged according to a standard procedure (8-tiff format). Mean fluorescence intensities (MFI) of stained areas (1000 × 1000 square) were calculated for comparison between subgroups.
Biomolecular analysis on stripped EDM
Corneal tissues (four specimens) were stained at T4 and subjected to whole EDM stripping. Peeled-off EDM was processed according to the TRIfast technique (EuroClone SpA, Milan, Italy) to extract total RNA and denatured proteins simultaneously, as below described in detail.
Real-time PCR and REST analysis
Total RNA was quantified spectrophotometrically (N1000 Spectrophotometer; NanoDrop; Celbio, Milan, Italy) after rehydration in DEPC-treated Milli-Q water. cDNA synthesis was carried out using the GoScript kit (Promega, Madison, WI, USA) in a programmable thermocycler (LifePro Thermal Cycler; EuroClone) in the presence of 50 ng total RNA (template), random primers, and dNTPs. Amplification procedures were performed in a real-time thermocycler (ECO-48 Illumina, San Diego, CA, USA) using the SYBR Green PCR mixture (Applied Biosystems, CA, USA) and cDNA (3 μL for target or /1 μL for referring genes) as template and primers. Supplementary Table 1 shows the primer set as designed one-intron spanning (Primer3 free-software) and synthesized by Eurofins Genomics GmbH (Ebersberg, Germany). The amplification profile comprised one cycle of 95 °C/5-min initial denaturation followed by 35 cycles at 95 °C/15 s (denaturation), 59 °C/5 s (annealing), and 72 °C/15 s (elongation), followed by melting analysis. Normalized RNAs were used for amplification, and cycle threshold (Ct) values from good melting curves were utilized for the calculation of fold-changes (FC; 2log expression) with the REST software (www.gene-quantification.de). The relative gene expression for each target was obtained with respect to referring genes (H3 and/or GAPDH). Fold-changes inside the range [−2 to +2] were not considered different for statistical analysis.
SDS-PAGE analysis and IntDen analysis
Total protein contents were A280 quantified (NanoDrop), and normalized samples (15 μg/lane) were separated by electrophoresis on 12% SDS-polyacrylamide gel under reducing conditions (130 V/frontline; Miniprotean apparatus; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Subsequently, the separated bands were transferred from SDS-PAGE to Hy-Bond membranes (GE Healthcare, Buckinghamshire, UK) under semi-dry conditions (10 V/55 min; semi-dry condition, transblotting apparatus; Bio-Rad) in the presence of 48 mM Tris-HCl (pH 6.8), 39 mM glycine, 0.0037% SDS, and 20% methanol solution. Membranes were stained with Sypro blot stain assay (Invitrogen, MA, USA) for image acquisition in a B-BOX Blue Light LED epi-Illuminator (SMOBIO Technology Inc., Hsinchu City, Taiwan). Specific immunoblotting was performed to verify Integrin (anti-Integrin β1/ITGB1 antibody [A-4]: predicted/observed band size at 138/100 kDa; 1:1000; sc-374429; Santa Cruz Biotech, Santa Cruz, CA), F-actin (anti-F-actin antibody, [4E3.adl], ab130935; predicted/observed band size at 42/37 kDa; 1:1000; Abcam, Cambridge, UK), and VEGF (anti-VEGF 165A antibody [6B7]; ab69479; predicted/observed band size at 23/20 kDa; 1:1000) proteins at related size separation. Bands were quantified with the ImageJ available at the imagej.nih.gov/ij, and data for graphical representation were provided as Integrated Density values (IntDen; mean ± SD).
Statistical analysis
All data were provided as mean ± SD or mean ± standard error from the mean (SEM). Prism 9 software (GraphPad Software Inc., CA, USA) was used for statistical analysis and graphical representation. After the normality check (Shapiro–Wilk test), the parametric ANOVA coupled Tukey-Kramer post hoc comparison was used to estimate differences between groups after the normality check (Shapiro–Wilk test). p < 0.05 or p < 0.01 was considered statistically significant.
Results
This ex vivo pilot study was conducted to compare lutein/trypan blue and trypan blue dyes’ staining performance in terms of staining intensity and cell viability (number, apoptosis, and differentiation).
Lutein/trypan blue and trypan blue staining are comparable
A pilot study was carried out on whole corneal specimens for both dyes in parallel before EDM excision and specific analysis to define the best staining protocol. As shown in Fig. 1, a time-dependent increase of blue staining was monitored for both dyes showing a staining intensity directly proportional to exposure times (trypan blue vs. lutein/trypan blue; Fig. 1A–C vs. D–F). The quantification showed that at each time point the trypan blue had a one-degree higher blue staining than lutein/trypan blue one (Fig. 1C vs. F). Lutein/trypan blue dye induced a grade 3 color intensity at T4-processed as observed after EDM excision (Fig. 1G, H). Of interest, grade 3 lutein/trypan blue intensity of excited-EDM persisted after extensive washing in physiological solution for up to 15 min (Fig. 1I). The color-grid scale used for scoring is shown (Fig. 1J).
Fig. 1.
Corneal endothelial staining. Representative images of whole upside-down corneas stained with trypan blue (A–C) and lutein/trypan blue (D–F) dyes according to timelines (T1, T2, T4). Representative free-floating lutein/trypan blue-stained EDM (T4; G), folded EDM (T4; H), and flat-mounted EDMs after washing (T4; I). Magnification: ×200. Staining intensity was graded according to the color grid palette (J): grade 1 (hexadecimal (HEX)#E0E0FF), grade 2 (HEX#AFB1FE), grade 3 (HEX#898CFD), grade 4 (HEX#4F53FC), and grade 5 (HEX#0000E0). K Graphical representation of blue-stained cells counted in whole corneal endothelia from each group (lutein/trypan blue, white bar and trypan blue, gray bar) at 1 min (T1), 2 min (T2), and 4 min (T4), with respect to 25 s (T0; control). **p < 0.01, lutein/trypan blue vs. trypan blue at T4 (intergroup analysis)
Endothelial mortality was assessed on whole stained corneal specimens placed upside-down on Petri dishes to allow endothelial cell counting at increasing time points (T0–T4). The results are shown in Fig. 1K. The counting of blue-stained nuclei per optic field showed that cell mortality was significantly higher after trypan blue staining with respect to T0 (25 s, control) and depending on time exposure (T0 vs. T1: p < 0.05; T0 vs. T2 and T4: p < 0.01; see Supplementary Fig. 1). Lutein/trypan blue dye showed a comparable number of blue-stained cells at all times examined (T1, T2, T4 vs. T0; p > 0.05). The number of blue-stained cells at T4 was significantly reduced in the presence of lutein/trypan blue compared to trypan blue (p < 0.05; Fig. 1K). No statistically significant difference in cell mortality was detected intragroup for lutein/trypan blue dye, at all examined times.
Lutein/trypan blue displayed lower AP immunoreactivity with respect to trypan blue
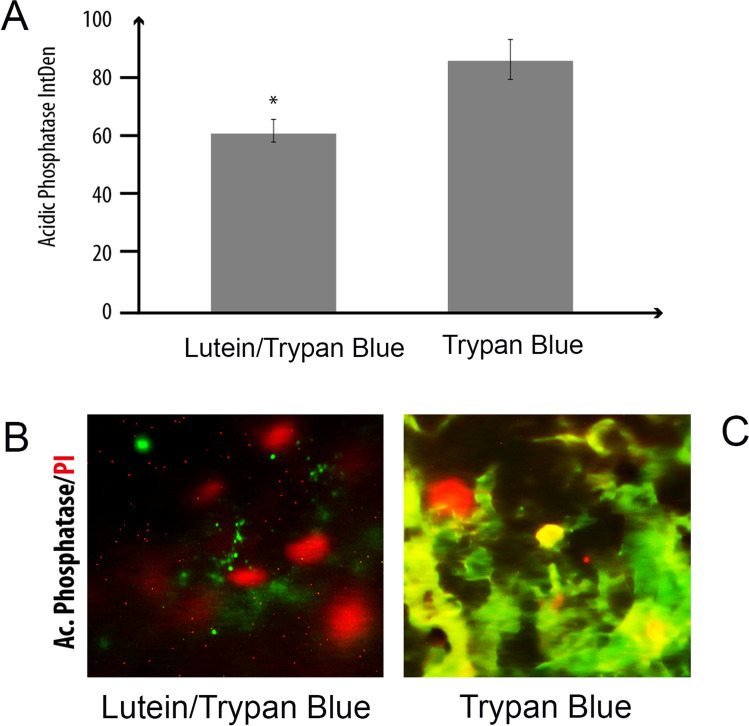
As lutein/trypan blue dye displayed good staining of endothelium at T4 (Fig. 1I), this time point was selected for the subsequent immunofluorescent analyses. Epifluorescent analysis carried out on flat-mounted stained and prefixed-EDM showed a lower AP immunoreactivity in lutein/trypan blue samples with respect to trypan blue ones over a nuclear counterstaining (p < 0.05; Fig. 2A–C). Images in the figure are representative of a marginal zone, showing cells characterized by an elongated morphological appearance. The densitometric analysis (IntDen) was carried out on single-channel images (fluorescent channel; 8-tiff) (Fig. 2A).
Fig. 2.
Acidic phosphatase immunoreactivity. Flat-mounted EDMs were processed for immunofluorescent analysis of acidic phosphatases (AP/green). Graphical representation of AP Integrated Density (IntDen) expression from specific quantification on single-channel images (ImageJ). Difference between groups was *p < 0.05 (ANOVA analysis; A). Representative digital images of green AP immunoreactive cells over a red nuclear staining (PI/red) in EDM exposed to lutein/trypan blue (B) and trypan blue (C). Note the higher immunoreactivity in trypan blue-stained EDM. Magnification: ×400
Lutein/trypan blue dye did not influence the expression of apoptotic transcripts
Next, we checked for transcript expression of some genes involved in the apoptotic pathway (caspases 3/9 and BCL2/BAX). As shown in Fig. 3A, no significant changes between groups were observed for caspase 3 and 9 subtypes. Interestingly, the analysis of BCL2 (anti-apoptotic target) and BAX (pro-apoptotic target) resulted in a low BCL2/BAX ratio in trypan blue group, as it shifted toward the apoptotic activation. Noteworthy, a high BCL2/BAX ratio was detected in lutein/trypan blue group due to the BCL2 upregulation with respect to BAX, which was significantly downregulated (p < 0.05).
Fig. 3.
Real-time PCR analysis of lutein/trypan blue and trypan blue-stained extracts. Real-time PCR analysis specific for apoptotic (A) and EndMT (B) patterns performed on stained-EDM extracts. A Bar diagram showing the caspase 3/9 and BCL2/BAX target gene expression. Note the inverted BCL2/BAX ratio in lutein/trypan blue-stained vs. trypan blue ones. B Bar diagram showing the specific amplification of VEGF-A, TGF-β1RI, SMAD3–7, and SMA transcripts in lutein/trypan blue and trypan blue-stained EDM. Note the absence of specific amplification for VEGFR targets with respect to the huge deregulation of EndMT ones. Statistical significance is indicated by asterisk (*) in bar graphs: ***p < 0.001
Lutein/trypan did not influence proper endothelial cell parameters
Some targets belonging to the endothelial-to-mesenchymal transformation (EndMT) were also investigated in lutein/trypan blue and trypan blue groups. REST analysis showed no significant changes in both groups for TGF-β1/SMAD3–7 and SMA transcript expression (Fig. 3B). Target gene expression was highly deregulated upon both treatments. Unchanged VEGF mRNA expression was also observed in the lutein/trypan blue group compared to the trypan blue one, even if a trend for regulation can be observed in lutein/trypan blue group.
Lutein/trypan blue displayed no changes in total protein profile
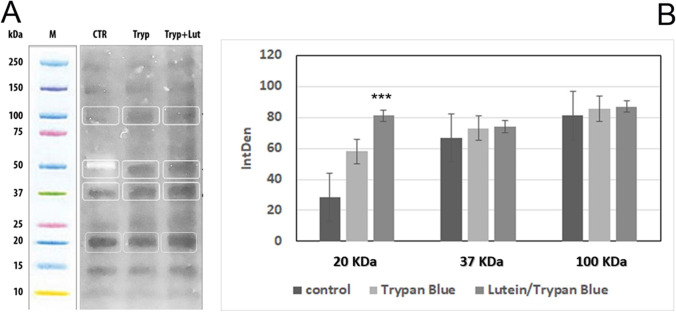
To characterize the total protein profile of stained EDM, preliminary SDS-PAGE analyses were carried out on protein extracts. As shown in Fig. 4A, a similar total protein expression and separation were observed for both groups. Although upon further immunoblotting, no IntDen changes were detected for 100-kDa (Integrin) and 37-kDa (F-actin) protein fractions between groups, a trend to an increase for 20-kDa (VEGF-A) proteins was observed for lutein/trypan blue extracts, as compared to trypan blue and control ones (p < 0.001; Fig. 4B).
Fig. 4.
SDS-PAGE analysis. A EDM specimens were extracted in lysis buffer under reducing conditions and equal protein amounts were separated according to their molecular weights. B Specific immunolabeled 20-kDa (VEGF), 37-kDa (F-actin), and 100-kDa (Integrin β1) observed band sizes from total protein fractions were subjected to densitometric analysis (Integrated Density (IntDen); ImageJ). Related IntDen analysis showed a trend to an increase for VEGF (***p < 0.001) in lutein/trypan blue-stained EDM protein extracts. CRT: control; tryp + lut: lutein/trypan blue; tryp: trypan
Discussion
In the present study, we compared the staining properties of lutein/trypan blue and trypan blue vital dyes in order to provide data to characterize the use of lutein/trypan blue vital dye for pre-surgery EDM preparation.
Since endothelial cell mortality might be a limitation for DMEK transplantation, corneal endothelial cell viability assessment by trypan blue dye represents an obligatory phase and a useful tool for EDM grafts [16]. Trypan blue also allows a good visualization and positioning of blue-stained EDM during DMEK [12, 13]. Increasing concentrations (0.1–0.4%), as well as appropriate time exposures (up to 7 min) for trypan blue staining, have been tested to visualize tissue better and allow cell counting [12, 16]. Although standardized, this trypan blue procedure is not completely “free from cytotoxic effects,” so the search for alternative vital dyes with higher performance and lower cellular toxicity is still ongoing [12, 13].
New-generation vital dyes composed of 0.04% trypan blue in solution with 1% lutein are now available for ophthalmic surgery (Phacodyne™, Phaco Lutein™, manufactured by Alfa Instruments s.r.l., Italy) [10].
Herein, we investigated, discussed, and implemented the literature data on lutein/trypan blue dye specifically for EDM preparation before DMEK. Although trypan blue dye produced the same staining intensity as lutein/trypan blue dye at half the time, the lutein/trypan blue protocol was also effective at 4 min. Lutein/trypan blue staining persisted even if excited EDM tissues were extensively washed before DMEK grafting. This finding represents a great value as DMEK surgery involves the manipulation of EDM-stained grafts inside the anterior chamber for at least 7 min, the mean time required for the unrolling of the flap and its correct positioning in contact with the recipient’s cornea [17, 18].
Endothelial cell mortality can represent a gap in the DMEK procedure. Herein, the stain intensity analysis showed that even if trypan blue dye displayed a one-degree higher score than lutein/trypan blue dye at all exposure times, the number of blue-stained cells displayed by lutein/trypan blue dye was lower if compared to those observed by trypan blue at the same exposure times and this reduction was statistically significant at T4 (p < 0.05). The reduced endothelial cell mortality highlights the safety profile of lutein/trypan blue dye combination. Of interest, the reduced immunoreactivity for AP in stripped EDM further supports it. This latter observation is noteworthy as AP is a protein involved in the early stages of apoptosis, representing initial cell membrane perturbation [19].
The absence of transcript regulation specific for caspase 3/9 (few apoptotic effector genes) in both groups supports the absence of any apoptotic event for both combined and single dyes [19]. More interesting, lutein/trypan blue RNA extracts displayed downregulation of BAX and upregulation of BCL2 transcripts, supporting the presence of some protective anti-apoptotic mechanisms for lutein exerted in the lutein/trypan blue combination, as previously reported [20]. Indeed, although still controversial, BCL2 gene has been associated with an anti-apoptotic action, as suggested by different studies on the crosstalk between autophagy and apoptosis [21, 22]. Noteworthy, lutein has been described as protective for both macula and the entire retina, most probably due to its ability to work as a “scavenger” in preventing the formation of free radicals (reactive oxygen species) [15]. Furthermore, the lutein’s property to absorb light at 446 nm (blue region) might appear of great utility also during EDM manipulation, as the blue light was found to trigger reactive oxygen species generation at least in retinal tissue (mainly by photoreceptors) [23].
Any tissue (including cornea) can respond to vital dyes depending on intrinsic characteristics and/or the type of iatrogenic insult (cadaveric sampling/storage), so the possibility that cells can undergo further stimulation cannot be excluded for different donor specimens [24]. A panel of previously reported targets associated with exogenous-driven differentiation of endothelial cells (EndMT) was further investigated at the molecular level [24–27]. The findings on the absence of any upregulation of TGF-β1RI, SMAD3/7, and SMA imply that both lutein/trypan blue and trypan blue do not affect endothelial cell function or trigger the expression of other molecules/factors related to endothelial differentiation [24]. Although investigated mainly in the proangiogenic and inflammatory process, the VEGF/VEGFR represents an essential pathway for the functional activity of corneal endothelial cells, acting primarily as a survival factor at physiological levels [28, 29]. Our biomolecular data suggests that lutein/trypan blue does not affect this pathway showing data comparable to trypan blue dye. Since corneal specimens selected for EDM excision were in accordance with exclusion criteria, we cannot exclude tissue/cell changes due to other growth factors’ imbalance in storage media.
The main limitation of this study is the small number of human corneoscleral specimens to test ex vivo for both vital dyes (further reduced by the COVID-19 pandemic) and the missing data from in vivo models to verify lutein/trypan blue-stained EDM grafts at follow-up.
Nevertheless, taken together, our findings are aligned with literature data and prospect the lutein/trypan blue use as a valid alternative to trypan blue to better counteract endothelial cell mortality, which represents a limitation for the DMEK procedure [30]. These promising results need further in vitro investigation before considering full validation of this staining procedure as a propaedeutic for DMEK surgical practice.
Supplementary Information
Time-point cell mortality (PNG 79 kb)
(DOCX 14 kb)
Acknowledgements
The authors would like to thank Dr. Anna Rita Blanco (Medical Liaison, Alfa Intes) for her technical assistance and scientific support. Editorial and graphical assistance was provided by Simonetta Papa, PhD; Massimiliano Pianta; and Aashni Shah (Polistudium SRL, Milan, Italy). This editorial assistance was supported by Alfa Instruments s.r.l. Alfa Instruments s.r.l. was not involved in the study design, collection, analysis, or interpretation of data, in the writing of this article or in the decision to submit it for publication. The study was partially supported by the Italian Ministry of Health (RC 2765948). Bijorn Omar Balzamino, Graziana Esposito and Alessandra Micera are grateful to Fondazione Roma (Italy) for continuous support.
Author contribution
Study conception and design: Rossella Colabelli Gisoldi, Alessandra Micera, and Augusto Pocobelli; data collection: Gemma Lodato and Bijorn Omar Balzamino; experiments/statistics: all; data interpretation: all; manuscript drafting: Rossella Colabelli Gisoldi and Alessandra Micera; manuscript editing: all; and approval to submit: all.
Data availability
Data can be made available upon request.
Declarations
Ethics approval
This experimental study (Jan–Dec 2020), involving human specimens not suitable for transplantation, was approved by Università Tor Vergata (G. L.). Donors gave the appropriate written consent in life to donate for corneal tissue transplantation or research purposes in case of unsuitability for transplantation.
Consent to participate
NA.
Consent for publication
NA.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295–310. doi: 10.1016/j.ophtha.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Cursiefen C. Descemet membrane endothelial keratoplasty: the taming of the shrew. JAMA Ophthalmol. 2013;131:88–89. doi: 10.1001/jamaophthalmol.2013.609. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann B, Schaub F, Cursiefen C. Treatment of corneal endothelial disorders by DMEK and UT-DSAEK: indications, complications, results and follow-up [in German] Ophthalmologe. 2016;113:196–203. doi: 10.1007/s00347-016-0221-0. [DOI] [PubMed] [Google Scholar]
- 4.Menassa N, Pagano L, Gadhvi KA, Coco G, Kaye SB, Levis HJ, Romano V. Free-floating DMEK in the host anterior chamber: surgical management. Cornea. 2020;39(11):1453–1456. doi: 10.1097/ICO.0000000000002380. [DOI] [PubMed] [Google Scholar]
- 5.Fogla R, Thazethaeveetil IR. A novel technique for donor insertion and unfolding in Descemet membrane endothelial keratoplasty. Cornea. 2021;40(8):1073–1078. doi: 10.1097/ICO.0000000000002698. [DOI] [PubMed] [Google Scholar]
- 6.Rezai KA, Farrokh-Siar L, Gasyna EM, et al. Trypan blue induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol. 2004;138:492–495. doi: 10.1016/j.ajo.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Hisatomi T, Enaida H, Matsumoto H, et al. Staining ability and biocompatibility of brilliant blue G: preclinical study of brilliant blue G as an adjunct for capsular staining. Arch Ophthalmol. 2006;124:514–519. doi: 10.1001/archopht.124.4.514. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann BO, Laaser K, Cursiefen C, et al. A method to confirm correct orientation of Descemet membrane during Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2010;149:922–925.e2. doi: 10.1016/j.ajo.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Kruse FE, Laaser K, Cursiefen C, et al. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea. 2011;30:580–587. doi: 10.1097/ICO.0b013e3182000e2e. [DOI] [PubMed] [Google Scholar]
- 10.Vianna LM, Cohen MJ, Muccioli C, et al. Efficacy of a lutein-based dye (Phacodyne™) for visualizing anterior capsulorhexis during cataract surgery by phacoemulsification. Arq Bras Oftalmol. 2014;77(3):173–177. doi: 10.5935/0004-2749.20140044. [DOI] [PubMed] [Google Scholar]
- 11.Furlani BA, Barroso L, Sousa-Martins D, et al. Lutein and zeaxanthin toxicity with and without brilliant blue in rabbits. J Ocul Pharmacol Ther. 2014;30(7):559–566. doi: 10.1089/jop.2013.0171. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Fong AG, Cho H, Zhang C, Gritz DC, Mian G, Herzlich AA, Gore P, Morganti A, Chuck RS. Protocol for vital dye staining of corneal endothelial cells. Cornea. 2012;31(12):1476–1479. doi: 10.1097/ICO.0b013e31824d0dda. [DOI] [PubMed] [Google Scholar]
- 13.van Dooren BT, Beekhuis WH, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol. 2004;122(5):736–742. doi: 10.1001/archopht.122.5.736. [DOI] [PubMed] [Google Scholar]
- 14.Lo HM, Tsai YJ, Du WY, et al. A naturally occurring carotenoid, lutein, reduces PDGF and H2O2 signaling and compromised migration in cultured vascular smooth muscle cells. J Biomed Sci. 2012;19:18. doi: 10.1186/1423-0127-19-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sujak A, Gabrielska J, Grudziński W, et al. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys. 1999;371(2):301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- 16.Singh G, Böhnke M, von-Domarus D, Draeger J, Lindstrom RL, Doughman DJ (1985-1986) Vital staining of corneal endothelium. Cornea. 4(2):80-91 [PubMed]
- 17.Majmudar PA, Johnson L. Enhancing DMEK success by identifying optimal levels of trypan blue dye application to donor corneal tissue. Cornea. 2017;36(2):217–221. doi: 10.1097/ICO.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 18.Ling JJ, Kyrillos R, Burckart KA, Aldrich BT, Skeie JM, Schmidt GA, Conwell C, Ramirez T, Reed CR, Zimmerman MB, Greiner MA, Li JY. Optimizing visualization of Descemet membrane endothelial keratoplasty tissue: assessing the impact of trypan blue exposure on stain duration and corneal endothelial cell function. Cornea. 2021;40(3):292–298. doi: 10.1097/ICO.0000000000002440. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278(3):403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 20.Siebelmann S, Matthaei M, Hörster R, Cursiefen C, Bachmann B. Lutein and brilliant blue-based dye for donor preparation and transplantation in Descemet membrane endothelial keratoplasty. Cornea. 2017;36(4):440–444. doi: 10.1097/ICO.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Yao S. JNK-Bcl-2/Bcl-xL-Bax/Bak pathway mediates the crosstalk between matrine-induced autophagy and apoptosis via interplay with Beclin 1. Int J Mol Sci. 2015;16(10):25744–25758. doi: 10.3390/ijms161025744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu SJ, Chen JL, Bee YS, et al. Differential autophagic effects of vital dyes in retinal pigment epithelial ARPE-19 and photoreceptor 661W cells. PLoS One. 2017;12(3):e0174736. doi: 10.1371/journal.pone.0174736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JE, Dennison J. The photobiology of lutein and zeaxanthin in the eye. J Ophthalmol. 2015;2015:687173. doi: 10.1155/2015/687173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khodapasand E, Jafarzadeh N, Farrokhi F, et al. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Solbes AS, Youker K. Epithelial to mesenchymal transition (EMT) and endothelial to mesenchymal transition (EndMT): role and implications in kidney fibrosis. Results Probl Cell Differ. 2017;60:345–372. doi: 10.1007/978-3-319-51436-9_13. [DOI] [PubMed] [Google Scholar]
- 26.Díez M, Musri MM, Ferrer E, Barberà JA, Peinado VI. Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGFbetaRI. Cardiovasc Res. 2010;88(3):502–511. doi: 10.1093/cvr/cvq236. [DOI] [PubMed] [Google Scholar]
- 27.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94(7):910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 28.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24(2):188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41(9):2514–2522. [PubMed] [Google Scholar]
- 30.Yoon YC, Byun YS, Kim P, Ha MJ, Whang WJ, Na KS, Kim EC, Kim HS, Hwang HS. Quantitative analysis of cornea endothelial cell damage from enucleation, corneal buttoning, and storage in donor corneas using trypan blue dye staining. Medicine. 2022;101(36):e30430. doi: 10.1097/MD.0000000000030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-point cell mortality (PNG 79 kb)
(DOCX 14 kb)
Data Availability Statement
Data can be made available upon request.