To the Editor:
Corticosteroids were established as the standard first line systemic treatment of chronic graft-versus-host disease (cGVHD) more than four decades ago [1]. Later attempts to improve the outcomes by using corticosteroids in combination with other immunosuppressive treatments demonstrated no improvement in survival compared to corticosteroids alone [2]. On the other hand, long-term administration of corticosteroids increases the risk of life-threatening infectious complications, leads to Cushing syndrome, bone loss, diabetes, avascular necrosis, myodystrophy, dyslipidemia, hypertension, suppressed growth in children, cataract, glaucoma and chronic adrenal insufficiency. All these complications create a significant healthcare burden on transplant centers and local providers [3]. Furthermore it was demonstrated that less than a half of patients have complete resolution of all cGVHD manifestations after systemic treatment, but rather either reduce or completely discontinue systemic immunosuppressive therapy with topically manageable residual cGVHD symptoms [4]. These two later factors have driven transplantation physicians to explore immunosuppressive approaches with reduced number of complications compared to corticosteroids. These alterations in clinical practice were further encouraged by introduction of new GVHD prophylaxis regimens, including ones without calcineurin inhibitors [5], and effective second-line options for cGVHD treatment that allow tapering and discontinuation of corticosteroids [6, 7]. Nonetheless, no prospective or large retrospective studies are published using these strategies in the first line systemic treatment of moderate and severe cGVHD by National Cacner Institute definitions. Thus a survey was conducted (Supplementary text S1) among all member centers of European Society for Blood and Marrow Transplantation (EBMT) performing allogeneic stem cell transplantation (HSCT) evaluating if steroid-free regimens are used in clinical practice. Statistical methods are presented in supplementary text S2.
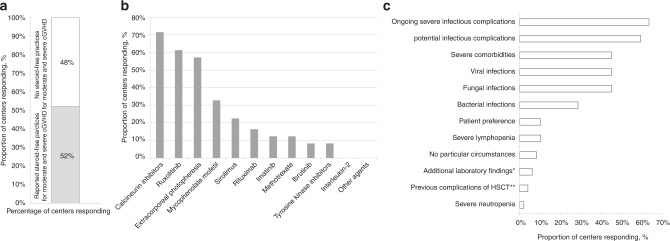
Among 327 allogeneic EBMT centers, 102 (31%) centers from 27 countries responded to the questionnaire and 53 (51.9%) confirmed using steroid-free first line treatments in moderate and severe cGVHD (Fig. 1a). There was no association between using this approach and transplant center activity (p = 0.95) or JACIE accreditation status (p = 0.48). Among centers using steroid-free treatments, 75% use them in rare clinical situations, 19% in specific clinical situations and 6% for majority of patients. Most commonly used treatments included calcineurin inhibitors (CNIs) (71.4%), ruxolitinib (61.2%) and extracorporeal photopheresis (57.1%). A lower proportion of centers reported using mycophenolate mofetil (MMF) (32.7%), sirolimus (22.5%), rituximab (16.3%), imatinib (12.2%), methotrexate (12.2%), ibrutinib (8.2%) and other tyrosine kinase inhibitors (8.2%). No other steroid-free approaches were reported (Fig. 1b). The following approaches were reported by the centers as second line when remaining within steroid-free strategy: ruxolitinib (44.9%), extracorporeal photopheresis (14.3%) and calcineurin inhibitors (14.3%). Other regimens were also rarely used as a second choice of treatment (Supplementary Tables S1, S2).
Fig. 1. Key information from the survey.
a Percentage of centers reporting use of steroid-free first line treatment of moderate and severe cGVHD. b Percentage of centers reporting specific steroid-free first line treatments. More than one answer was possible. c Percentage of centers reporting clinical indications for steroid-free first line treatments. More than one answer was possible. *Low lymphocyte count, abnormal glucose levels; ** steroid myopathy, osteonecrosis.
The majority of centers (59.2%) reported that steroid-free treatment was used only in a minority of cGVHD patients (defined by questionnaire as <10%), while 12 centers use this strategy in 10–20% of patients, 4 centers in 20–30%, 3 centers in 30–50% and only one in the majority of patients. The choice of steroid-free regimen was rarely dictated by underlying disease. Only 3 centers prefer to use this approach in myelofibrosis and one in non-hodgkin lymphomas. The rest reported that the decision is driven by other clinical factors, predominantly either ongoing severe infectious complications (63.3%) or concern for potential infectious complications (59.2%). Among infectious complications the responders highlighted the significance of fungal (44.9%), viral infections (44.9%) and to a lesser extent bacterial infections (28.6%) when choosing steroid-free approach. Another significant factor to avoid corticosteroids was presence of severe comorbidities (44.9%), including diabetes, severe osteoporosis, avascular osteonecrosis, preexisting myopathy, sarcopenia, poor performance status, obesity and metabolic syndrome, hypertension and uncontrolled psychiatric disorder. Several centers reported that neutropenia (2.0%), lymphopenia (10.2%) and patient preference (10.2%) might drive the choice of treatment (Fig. 1c). Underlying malignancy status was considered as additional factor when choosing steroid-free treatment by 53.1% of centers. Previous hematological relapse (8.16%), minimal residual disease (10.2%) or both (34.7%) were considered when choosing steroid-free approach. Also 22.45% considered mixed chimerism affecting their judgment regarding cGVHD steroid-free treatment. On the contrary, nor risk of COVID-19 (93.9%), neither active COVID-19 infection (87.8%) significantly affected clinical decision towards steroid-free regimens. The respondents commented that usually they postpone treatment until clearance of COVID-19.
Manifestations of cGVHD were also considered when choosing steroid-free first line treatment. The majority of centers use this approach in patients with moderate disease (65.3%), 30.6% in both moderate and severe, and 4.08% in certain forms of severe cGVHD. Centers preferably use steroid-free treatment in presence of skin involvement without scleroderma (63.3%) or with scleroderma (49.0%), involvement of oral mucosa (46.9%), eyes (40.8%), liver (32.7%), gastrointestinal (30.6%), joints (26.5%), lungs (20.4%) and genitalia (16.3%), which reflects the usual organ involvement in moderate and severe cGVHD. On the other hand, in a number of centers lung (66.0%), gastrointestinal (46.8%) and hepatic (25.5%) cGVHD is considered a contraindication to steroid-free approach. The other organ manifestations of cGVHD were considered a contraindication by smaller number of centers (Supplementary Table S3).
The majority of centers used National Institute of Health criteria to assess response (59.6%), however a significant proportion defined “clinical improvement” as a sign of response (40.4%). Major criteria for changing therapy were no response by 4 weeks (in 57.1% off centers), by 8 weeks (in 18.37%), 12 weeks (in 10.2%) and 24 weeks (in one center). Only 20.4% of centers indicated absence of complete response by 6–24 months as a sign of treatment failure. On the other hand, the majority of respondents indicated that progressive disease at any time point is an indication for different therapy. Addition of either standard-dose (≥1 mg/kg, 36.7%), or low-dose corticosteroids (<1 mg/kg, 46.9%) was indicated as the most common practice in case of first line failure. Only 16.3% remain within steroid-free strategy after first line failure and use ECP, combination of ECP and ruxolitinib, combination of rituximab, ECP and TKIs, combinations with MMF, methotrexate and CNIs. Majority of centers (87.8%) continue the initial steroid-free regimen while adding second line treatment.
The survey identified that more than half of the centers do use steroid-free regimens for cGVHD outside of clinical recommendations. Emergence of these practices are likely related to the changing landscape of either GVHD prophylaxis [5] and second-line GVHD treatment [6]. We identified that most commonly used steroid-free treatments involve CNIs, ECP and ruxolitinib. These methods were never studied in the randomized studies against corticosteroids, but all of them demonstrated steroid-sparing potential and allowed to discontinue steroids completely in a proportion of patients [7–9].
We observed that steroid-free practice is typically applied to less than 20% of patients with certain comorbidities and previous complications of HSCT. These results are in line with a recent multicenter study from the USA where 18% of patients were treated with steroid-free first line regimen [10]. Given the significant number of patients already treated with this approach our study warrants boosting research within the EBMT community both in the retrospective and prospective settings and define the optimal strategy for specific chronic GVHD manifestations. On the other hand, the study raises a concern about the extent of steroid-free treatment practices without existing evidence on outcomes and understanding of a clinical benefit to patients. Currently the response rate with this approach is unknown and might be relatively low. Twenty eight centers that responded to the survey agreed to participate in a retrospective analysis, which will identify safety and efficacy of first line steroid-free approach in moderate and severe chronic GVHD. The results of the present survey can provide guidance for further studies.
Supplementary information
Acknowledgements
We thank all the centers that showed interest in this problem and responded to the survey.
Author contributions
IM: conceptualization, methodology, project administration, writing - original draft, visualization; PA: investigation, data curation; MB: methodology, validation; CP: dormal analysis; GB: methodology, writing - review & editing; CK: methodology, writing - review & editing; HS: methodology, writing – review & editing; OP: methodology, writing – review & editing; ZP: methodology, writing – review & editing, supervision.
Data availability
For primary data please contact EBMT study office, pascale.ambron@ebmt.org.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-022-01881-6.
References
- 1.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–76. doi: 10.1182/blood.V57.2.267.267. [DOI] [PubMed] [Google Scholar]
- 2.Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–15. doi: 10.1182/blood-2014-08-551994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuji S, Byrne M, Nagler A, Mohty M, Savani BN. How we can mitigate the side effects associated with systemic glucocorticoid after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56:1248–56. doi: 10.1038/s41409-020-01205-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Nguyen TD, Onstad L, Bar M, Krakow EF, Salit RB, et al. Success of immunosuppressive treatments in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2018;24:555–62. doi: 10.1016/j.bbmt.2017.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanakry CG, Bolaños-Meade J, Kasamon YL, Zahurak M, Durakovic N, Furlong T, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood. 2017;129:1389–93. doi: 10.1182/blood-2016-09-737825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. REACH3 investigators. ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–38. doi: 10.1056/NEJMoa2033122. [DOI] [PubMed] [Google Scholar]
- 7.Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278–89. doi: 10.1182/blood.2021012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Jagasia M, Scheid C, Socié G, Ayuk FA, Tischer J, Donato ML, et al. Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019;3:2218–29. doi: 10.1182/bloodadvances.2019000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pidala J, Onstad L, Martin PJ, Hamilton BK, Cutler C, Kitko CL, et al. Initial therapy for chronic graft-versus-host disease: analysis of practice variation and failure-free survival. Blood Adv. 2021;5:4549–59. doi: 10.1182/bloodadvances.2021005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For primary data please contact EBMT study office, pascale.ambron@ebmt.org.