Abstract
Background
There is a paucity of knowledge about the effects of COronaVIrus Disease-19 (COVID-19) on long-term frailty development or progression over time.
Aim
This study aims to assess transitions in frailty status in older adults who survived hospitalization for COVID-19.
Methods
This is a longitudinal panel study. A multidisciplinary outpatient follow-up service was established since summer 2020, for the evaluation of individuals discharged alive, after hospitalization due to COVID-19. Frailty status was assessed in-hospital and at follow-up using the clinical frailty scale (CFS). Main patients’ characteristics, including health, functional, cognitive, and psychological status were collected.
Results
A total of 177 patients aged 65 years and older were evaluated until June 2022. They were predominantly male, with a median age of 70 (Q1–Q3 67–75) years and a median body mass index of 27.5 (Q1–Q3 24.9–30.6) kg/m2 at hospital admission. The median follow-up time was 6.3 (Q1–Q3 3.7–10.9) months. Sixty-one patients (34.5%) scored worse at CFS follow-up compared to hospital admission, and twenty-two patients (12.4%) became frail.
Discussion and conclusion
This study shows that one out of three older patients previously hospitalized for COVID-19 had an unfavorable transition in CFS score during a median follow-up of nearly 6 months. Specific interventions to prevent frailty development or progression should be considered for patients at risk. Further studies are required to confirm our findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-022-02308-4.
Keywords: Frailty, Geriatric assessment, Older, COVID-19
Background
Studies have shown that COronaVIrus Disease-19 (COVID-19) can have prolonged effects over several months, a condition termed long-COVID syndrome [1]. Long-COVID syndrome may involve almost all body systems and encompasses several symptoms, such as fatigue, breathlessness, tachycardia, cognitive deficits, dysautonomia, depression, and hair loss [1].
Frailty is an age-related clinical syndrome, with decline in physiological capacity of several organ systems, characterized by increased susceptibility to sudden, disproportionate functional decline following stressor events [2]. There is wide consensus that addressing frailty is the best method to detect heterogeneity in risk of adverse outcomes among people of the same chronological age [2].
COVID-19 has disproportionately affected frail older people, with high rates of mortality and symptom persistence in the long term [3–5]. No studies, however, have assessed the long-term effect of COVID-19 on the transition in frailty syndrome over time after hospitalization.
This study aims to fill this gap, assessing a cohort of older patients who were hospitalized for COVID-19 during three pandemic waves (March–June 2020; September–December 2020; February–May 2021).
Methods
Patients and setting
This study was conducted in a 700-bed urban hospital of the Northern Italy, serving a population of about 870,000 inhabitants. During the first three pandemic waves, most acute wards of that hospital were transformed into COVID-19 wards to respond to the massive demand of infected people for hospitalization. All patients referred to the hospital were admitted directly to the acute wards after an initial evaluation by an emergency room physician and an infectious disease specialist. There were no criteria based on age for the admission to the acute geriatric ward or other medical wards. Shortly after the beginning of the first pandemic wave (March 2020), the Hospital Physician Directors' Board held daily meetings to monitor the situation and reorganize the patients’ care. It was during one of these meetings that they decided to establish a post-acute outpatient service for COVID-19 survivors, which opened in summer 2020.
The inclusion criteria for being admitted to the service were: (1) a previous hospitalization for COVID-19; (2) a negative real-time reverse transcriptase-polymerase chain reaction for SARS-CoV-2 at the follow-up visit, and (3) an informed consent signed by the patient.
This study was approved by the Monza-Brianza Ethics Committee (Clinical.Trial.gov Identifier: NCT04424992).
Multidisciplinary assessment
The follow-up consisted in a multidisciplinary team assessment, including specialists in infectious, respiratory, cardiac, hematology, intensive care, and geriatrics, respectively. Each assessment was carried out sequentially on the same day by the specialists, to avoid waste of time for the patient. Follow-up timing, originally set between 3 and 6 months from hospital discharge, has been delayed up to 11 months, due to the subsequent intermittent pandemic waves. All the enrolled patients already had the following data registered at hospital’s ward admission: socio-demographic data (gender, age, and cohabitation), body mass index (BMI), presence of the principal acute or chronic diseases (hypertension, ischemic heart disease, congestive heart failure, peripheral vascular disease, stroke or transient ischemic attack, diabetes, depression, osteoporosis, osteoarthritis, pulmonary diseases, renal failure, liver disease, hyperthyroidism or hypothyroidism, visual impairment, hearing impairment, dementia, solid neoplasm, hematological neoplasm, peptic ulcer, anemia, and rheumatological disease) and Charlson comorbidity index (CCI) [6].
Presence of frailty was assessed twice (at hospital admission and at follow-up visit), with the clinical frailty scale (CFS) [7], a nine-point scale based on the assessment of mobility, energy, physical activity, and function. A score of 1 indicates a very fit person, whereas 9 corresponds to a terminally ill person. The CFS has been widely used in intensive care units (ICUs) and in geriatric and medical wards with COVID-19 patients [5, 8]. At hospital admission, the CFS was ranked by an intensivist (for patients admitted to ICU) or a geriatrician (for patients admitted to medical wards), collecting information from patients or relatives. All the raters (either intensivists or geriatricians) have been previously trained in the use of CFS. The score was obtained after having gathered the patient’s clinical information, including autonomy in the basic and instrumental activities of daily living, cognitive and psychological status, and the presence of diseases, with reference to 2 weeks before hospital admission. At follow-up, patients also underwent the Montreal Cognitive Assessment (MoCA) [9] and the Short Physical Performance Battery (SPPB) [10] to better evaluate global cognitive and functional status; the CFS was ranked again by an intensivist or geriatrician according to the ward of admission whereas the SPPB and the MoCA scores were ranked by a geriatrician previously trained in their use.
Statistical analyses
Patient characteristics are described as median and first-third quartiles (Q1–Q3) for continuous variables and number (percentages) for categorical variables. Longitudinal transitions in CFS score from hospital admission to follow-up are presented as an alluvial plot. A multiple linear regression model was used to evaluate the association between CFS at follow-up and baseline characteristics: age, sex, CCI, and maximum ventilatory support during hospitalization, adjusting for CFS at baseline and follow-up time. Statistical analyses were performed with R 4.1.2 (http://www.R-roject.org).
Results
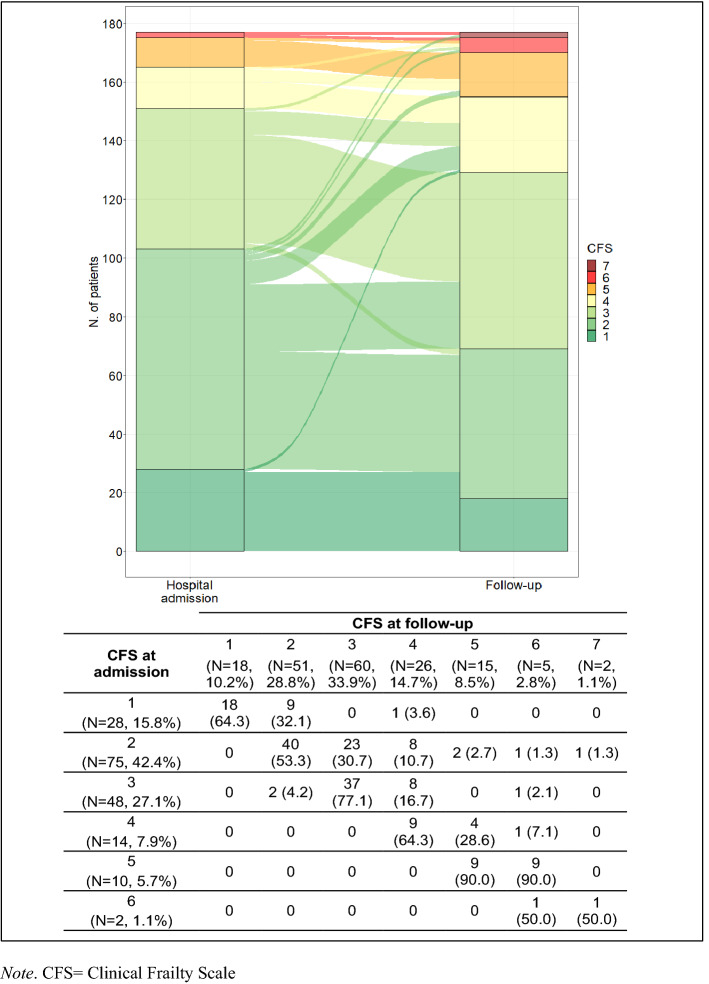
Between August 2020 and June 2022, 468 older (≥ 65 years) patients who were hospitalized with COVID-19 during the first 3 pandemic waves, underwent a complete geriatric assessment both at the hospital’s admissions and at the post-acute outpatient service. The demographic and clinical characteristics of our sample (N = 177) at hospital admission are shown in Table 1. The median age was 70 (Q1–Q3 67–75) years, patients were predominantly male (N = 118, 66.7%) and married or living maritally (N = 123, 74.1%). At baseline, most patients were either overweight or obese (median BMI 27.5, Q1–Q3 24.9–30.6), in good health (median CCI 3, Q1–Q3 2–4), and fit: in fact, 28 (15.8%) patients scored 1 on the CFS, 75 (42.4%) scored 2, and 48 (27.1%) scored 3. Only 26 patients scored 4 or more. The main diseases were hypertension (N = 97, 58.4%) and diabetes without complications (N = 31, 18.7%) while only one patient had a history of dementia. Most patients (79.8%) had bilateral pneumonia. The median length of hospitalization was 21 (Q1–Q3 14–34) days, and 38 patients (25.9%) were admitted to the ICU with a median length of stay of 14 (Q1–Q3 9–28) days. Figure 1 shows the alluvial plot of the longitudinal transitions in CFS score from hospitalization to follow-up (median follow-up time = 6.3, Q1–Q3 3.7–10.9 months), along with the numbers of each transition. A substantial number of patients scored worse (61 out of 177, 34.5%) at follow-up compared to hospital admission, and 22 patients with a CFS ≤ 3 at hospital admission became frail (CFS ≥ 4) at follow-up (12.4%). COVID-19-associated symptoms or signs reported by the patients who became newly frail at follow-up were motor deficits at discharge, and sleep and mood disorders at the follow-up (Supplementary Table S1). Female sex [95% CI (0.632; 0.133)] and tracheotomy/ECMO/intubation ([95% CI (0.429; 1.296)] were positively associated with higher CFS at follow-up, after adjusting for CFS at hospital admission, time of follow-up, and CCI (Table 2).
Table 1.
Demographic and clinical characteristics of the study population at hospital admission
| Variable | N = 177 |
|---|---|
| Age (years), median (Q1–Q3) | 70 (67–75) |
| Gender (males), N (%) | 118 (66.7) |
| Living situation, N (%) | |
| Alone/caregiver | 17 (10.4) |
| With family members | 145 (88.4) |
| Nursing home | 2 (1.2) |
| Married/partner | 123 (74.1) |
| Schooling, N (%) | |
| ≤ 8 years | 96 (57.1) |
| 9–13 years | 45 (26.8) |
| > 13 years | 27 (16.1) |
| BMI (kg/m2), median (Q1–Q3) | 27.5 (24.9–30.6) |
| Chest radiography, N (%) (available in N = 139 subjects) | |
| Bilateral pneumonia | 111 (79.8) |
| Unilateral pneumonia | 19 (13.7) |
| Acute respiratory distress syndrome, N (%) | 63 (48.5) |
| Mild | 9 (18.0) |
| Moderate | 20 (40.0) |
| Severe | 21 (42.0) |
| Maximum ventilatory support during stay, N (%) | |
| Low flow O2 | 26 (17.8) |
| CPAP | 85 (58.2) |
| Tracheotomy/ECMO/intubation | 35 (24.0) |
| Maximum CRP [mg/dL], median (Q1–Q3) | 10.5 (6.0–15.9) |
| Comorbidities, N (%) (available in N = 166 subjects) | |
| Hypertension | 97 (58.4) |
| Myocardial infarction | 12 (7.2) |
| Congestive heart failure | 3 (1.8) |
| Peripheral vascular disease | 11 (6.6) |
| Stroke/transient ischemic attack | 3 (1.8) |
| Diabetes mellitus, uncomplicated | 31 (18.7) |
| Diabetes mellitus, end-organ damage | 7 (4.2) |
| Depression | 8 (4.8) |
| Osteoporosis | 3 (1.8) |
| Osteoarthritis | 5 (3.0) |
| COPD | 9 (5.4) |
| Renal failure | 8 (4.8) |
| Liver disease, mild | 5 (3.0) |
| Liver disease, moderate to severe | 4 (2.5) |
| Hyperthyroidism or hypothyroidism | 12 (7.2) |
| Visual impairment | 1 (0.6) |
| Hearing impairment | 2 (1.2) |
| Dementia | 1 (0.6) |
| Solid neoplasm | 11 (6.6) |
| Hematological neoplasm | 1 (0.6) |
| Peptic ulcer | 2 (1.2) |
| Anemia | 5 (3.0) |
| Rheumatological disease | 5 (3.0) |
| Charlson comorbidity index, median (Q1–Q3) | 3 (2–4) |
| CFS, N (%) | |
| 1—Very fit | 28 (15.8) |
| 2—Fit | 75 (42.4) |
| 3—Managing well | 48 (27.1) |
| 4—Living with very mild frailty | 14 (7.9) |
| 5—Living with mild frailty | 10 (5.6) |
| 6—Living with moderate frailty | 2 (1.1) |
| Delirium, N (%) | 6 (4.1) |
| Length of hospital stay (days), median (Q1–Q3) | 21 (14–34) |
| Admitted to ICU, N (%) (available in N = 147 subjects) | 38 (25.9) |
| Length of ICU stay (days), median (Q1–Q3) | 14 (9–28) |
Q quartile, BMI body mass index, CPAP continuous positive airway pressure therapy, ECMO extracorporeal membrane oxygenation, CRP C-reactive protein, COPD chronic obstructive pulmonary disease, CFS clinical frailty scale, ICU intensive care unit
Fig. 1.
Alluvial plot and numbers of the longitudinal transitions in clinical frailty scale (CFS) score from hospital admission to follow-up. Note: CFS clinical frailty scale
Table 2.
Multiple linear regression model on CFS at follow-up by CFS at baseline, follow-up time, age, sex, CCI, and maximum ventilatory support during hospitalization
| Coefficient estimate | 95% confidence interval | p value | |
|---|---|---|---|
| Intercept | 0.756 | (− 1.006; 2.518) | 0.398 |
| CFS at admission | 0.903 | (0.782; 1.025) | < 0.001 |
| Follow-up time | 0.000 | (− 0.001; 0.001) | 0.925 |
| Age | − 0.004 | (− 0.029; 0.020) | 0.730 |
| Males | − 0.382 | (− 0.632; − 0.133) | 0.003 |
| CCI | 0.025 | (− 0.026; 0.075) | 0.333 |
| Maximum ventilatory support during hospitalization | |||
| Low flow O2 | Reference | – | – |
| CPAP | 0.230 | (− 0.122; 0.581) | 0.199 |
| Tracheotomy/ECMO/intubation | 0.863 | (0.429; 1.296) | < 0.001 |
CFS clinical frailty scale, CCI Charlson comorbidity index, CPAP continuous positive airway pressure, ECMO extracorporeal membrane oxygenation
Supplementary Table S2 shows the MoCA and SPPB score at follow-up, corrected for age and level of education. MOCA score was abnormal (MoCA < 20) in 19 patients (12.8%) and borderline (MoCA 20–25) in 66 (44.6%) while 7 (4%) patients had a SPPB score suggesting overt disability (0–2), 15 (8.6%) moderate physical frailty, and 39 (22.3%) mild physical frailty.
Discussion
This study shows that one out of three older patients previously hospitalized for COVID-19 had an unfavorable transition in CFS score from hospitalization to follow-up, and about one out of eight patients became mildly or overtly frail. To the best of our knowledge, this is the first study evaluating the transition in frailty status among patients previously hospitalized for COVID-19, using a clinically based assessment.
A recent systematic review and meta-analysis found 16 studies focusing on frailty transitions in community-dweller adults [11]. All these studies categorized frailty according to the Fried’s criteria and the average follow-up period was 3.9 years. The pooled rates of frailty transitioning in this review were 4.5% (95% CI 3.2–6.1%) and 18.2% (95% CI 14.9–21.7%) among robust and pre-frail individuals, respectively, in a population that was on average older than ours [11]. Moreover, a study by Ahmad et al. [12] included a population which was more similar to ours with regard to the cohort's median age (70 years, comparable to our study), the overall prevalence of frailty at baseline (9.4% vs. 14.7% in our study), and the follow-up period (12 months vs. 6 months in our study). Again, frailty was evaluated in the Ahmad’s study according to the Fried’s criteria, while with the 9-item CFS in our study. Ahmad et al. found that 22.9% of participants worsened their frailty status at 12 months versus 34.5% in our study, and the observed 12-month frailty transitions from robust to frail and from pre-frail to frail were 2.9% and 8.9%, respectively [12].
Only one study, as far as we know, examined the transitioning to frailty during the COVID-19 pandemic [13]. Shinohara et al. explored frailty transition during three COVID-19 waves (from May to July 2020, from November 2020 to January 2021, and from May to July 2021) in 1953 community-dwelling older people (≥ 65 years) living in Japan, using the frailty screening index (FSI) [13]. Among 706 respondents, they found a 9.8% increase in frailty transition, which is slightly lower than ours [13]. Based on these findings, we can hypothesize that the proportion of frailty transitioning in our study is worse than in pre-COVID-19 general population. However, given the dynamic nature of frailty, we cannot exclude that some individuals who were labeled as frails at 6-month follow-up might have reverted to non-frail status in the following months.
A decline in immune function and the proinflammatory state are key components of frailty in COVID-19 patients [14]. A recent review suggests that hyper-inflammatory status, typical of SARS-CoV-2 infection, may exacerbate the immunosenescence process, promote endothelial damage, and lead to myofibrillar breakdown and muscle degradation through mitochondrial dysfunction and autophagy [15]. Therefore, it might be hypothesized that COVID-19 can induce frailty through an acute dysregulation of the immune response and the development of deep changes in patient’s body composition. The aftermath of these immunological phenomena, augmented by anosmia, ageusia, reduced food intake, and the lack of physical activity imposed by the national lockdown, may have further accelerated individual’s catabolism [15] and muscle wasting. Moreover, the association between ICU admission and frailty after COVID-19 is in line with these assumptions since ICU-acquired weakness is a well known and common neuromuscular complication of critical illnesses [16].
Our findings are in accordance with Shinohara’s study [13] and with another study showing that sarcopenia affected nearly one in five young and older COVID-19 survivors, being higher in patients with a longer hospital stay and lower in patients who were more physically active and had higher levels of serum albumin [17].
It is of interest that nearly 35% of patients had a follow-up SPPB score indicative of either disability or physical frailty and that 12% of patients had MoCA score indicative of cognitive impairment. There might be a relationship between COVID-19 and these findings, but the lack of data regarding the SPPB and MoCA scores at hospital admission prevents conclusions from being drawn about the timeframe for impairment onset.
Holistic management of COVID-19 patients appears essential to minimize the deleterious effect of this disease, preventing long-COVID-19 syndrome and the development or progression of frailty. To this aim, specific interventions including physical activity programs and/or nutritional support should be started as soon as possible in patients at risk.
A limitation of this study concerns the lack of a comparison group that prevents us to assess if transitions to frailty for non-COVID hospital admissions are substantially different from COVID group. However, this would have required a specific study design and fundings that we have not. Another limitation concerns the method of assessing CFS, which was obviously different from baseline to follow-up: CFS assessment on hospital admission was mainly based on telephonic interview, given the pandemic context, and this might be a source of bias, as we cannot exclude that grading of frailty levels may have differed between the two observations. A third limitation is that CFS have been ranked either by both intensivists and geriatricians, which may have partially biased the study results. However, it should be considered that all raters were familiar with the use of CFS, as this tool has been used in our hospital for many years, and the CFS inter-rater reliability is generally very good [18].
It should also be considered that the only method used to capture frailty was CFS. Indeed, at the time of the study, the electronic medical records of our hospital did not include information about the patient’s functional status and other domains required to retrospectively build up a frailty index according to the Rockwood’s approach. Furthermore, it was impossible to assess frailty according to the Fried’s phenotype in the pandemic context, since it requires the use of specific devices (such as handgrip strength) and pencil-and-paper tests which were not feasible in a work environment burdened by stress and high contagiousness. Lack of details on the level of physical activity and dietary changes occurring during the study period, may also be considered a limitation. Lastly, the sample size in this single-center study is relatively small and includes a quite selected population, since adhesion to the program was on a voluntary basis.
In conclusion, this study suggests that long-COVID may have influenced the transition in frailty status after a median follow-up of 6 months in a cohort of older patients who survived hospitalization for COVID-19. Knowledge in this field may be critical to promote specific interventions and prevent frailty development or progression for individuals at risk. Further studies are required to confirm our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Table S1: COVID-19 associated symptoms or signs reported by the patients at discharge and at follow-up, according to the presence of new-onset frailty at follow-up. Table S2: Montreal Cognitive Assessment (MoCA) and Short Physical Performance Battery scores at follow-up assessment. Table S3: Full list of the members of the STORM Long-COVID Team. (DOCX 23 KB)
Acknowledgements
We are grateful to Drs. Silvia Mori for study support, and to Dr. Fiona Ecarnot and Dr. Kimberly Alison Katte for English editing.
Author contributions
MCF: data collection, study design, interpretation of data, draft and critical revision of the manuscript, final approval. CZ: data collection, final approval; ET: data analysis and interpretation, critical revision of the manuscript, final approval. PR: data analysis and interpretation, critical revision of the manuscript, final approval. ER: data analysis and interpretation, critical revision of the manuscript, final approval. FL: study design, interpretation, critical revision of the manuscript, final approval. GF: study design, interpretation, critical revision of the manuscript, final approval. NS: study design, interpretation, critical revision of the manuscript, final approval. ML: study design, interpretation, critical revision of the manuscript, final approval. MGS: study design, interpretation, critical revision of the manuscript, final approval. PB: study design, critical revision of the manuscript, final approval. GB: conceptualization, study design, interpretation of data, draft and critical revision of the manuscript, final approval.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
All authors declare no conflict of interest.
Ethical standards
The study was compliant with the declaration of Helsinki and no identifiable personal data were used for this study.
Informed consent
Informed consent for participation in clinical studies was obtained from all patients.
Ethical approval
The study protocol was approved by the hospital Institutional Review Board.
Statement of human and animal rights
This study was approved by the Monza-Brianza Ethics Committee (Clinical.Trial.gov Identifier: NCT04424992).
Footnotes
Paolo Bonfanti for the STORM Long-COVID Team.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4.Ward H, Flower B, Garcia PJ, et al. Global surveillance, research, and collaboration needed to improve understanding and management of long COVID. Lancet. 2021;398:2057–2059. doi: 10.1016/S0140-6736(21)02444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebora P, Focà E, Salvatori A, et al. The effect of frailty on in-hospital and medium-term mortality of patients with COronaVIrus Disease-19: the FRACOVID study. Panminerva Med. 2021 doi: 10.23736/S0031-0808.21.04506-7. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shears M, Takaoka A, Rochwerg B, et al. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. 2018;45:197–203. doi: 10.1016/j.jcrc.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Seeman TE, Tinetti ME, et al. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994;6:410–419. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- 11.Kojima G, Taniguchi Y, Iliffe S, et al. Transitions between frailty states among community-dwelling older people: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:81–88. doi: 10.1016/j.arr.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad NS, Hairi NN, Said MA, et al. Prevalence, transitions and factors predicting transition between frailty states among rural community-dwelling older adults in Malaysia. PLoS ONE. 2018;5:e0206445. doi: 10.1371/journal.pone.0206445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara T, Saida K, Tanaka S, et al. Transition to frailty in older Japanese people during the coronavirus disease 2019 pandemic: a prospective cohort study. Arch Gerontol Geriatr. 2022;98:104562. doi: 10.1016/j.archger.2021.104562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vellas C, Delobel P, de Souto BP, et al. Covid-19, virology and geroscience: a perspective. J Nutr Health Aging. 2020;24:685–691. doi: 10.1007/s12603-020-1416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piotrowicz K, Gąsowski J, Michel JP, et al. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33:2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piva S, Fagoni N, Latronico N (2019) Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res 8:1000. 10.12688/f1000research.17376.1 [DOI] [PMC free article] [PubMed]
- 17.Martone AM, Tosato M, Ciciarello F et al, Gemelli Against COVID-19 Post-Acute Care Team (2022) Sarcopenia as potential biological substrate of long COVID-19 syndrome: prevalence, clinical features, and risk factors. J Cachexia Sarcopenia Muscle. 10.1002/jcsm.12931 [DOI] [PMC free article] [PubMed]
- 18.Lo AX, Heinemann AW, Gray E, et al. Inter-rater reliability of clinical frailty scores for older patients in the emergency department. Acad Emerg Med. 2021;2021:110–113. doi: 10.1111/acem.13953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: COVID-19 associated symptoms or signs reported by the patients at discharge and at follow-up, according to the presence of new-onset frailty at follow-up. Table S2: Montreal Cognitive Assessment (MoCA) and Short Physical Performance Battery scores at follow-up assessment. Table S3: Full list of the members of the STORM Long-COVID Team. (DOCX 23 KB)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.