Abstract
Background and aims
Many patients who undergo bariatric surgery will experience weight regain and effective strategies are needed to help these patients. A dilated gastrojejunal anastomosis (GJA) has been associated with weight recidivism after Roux-en-Y gastric bypass surgery (RYGB). Endoscopic transoral outlet reduction (TORe) with a full thickness endoscopic suturing device (Overstitch, Apollo Endosurgery, Austin, TX) is a minimally invasive therapeutic option. The primary aim of this project was to examine the safety and long-term efficacy data from three bariatric surgery centers and to conduct a systematic review and meta-analysis of the existing literature.
Methods
Patients who underwent TORe with the Overstitch device from Jan 2013 to Nov 2016 at 3 participating bariatric surgery centers were included in the multicenter analysis. For the systematic review and meta-analysis, a comprehensive search of multiple English databases was conducted. Random effects model was used.
Results
130 consecutive patients across three centers underwent TORe with an endolumenal suturing device. These patients (mean age 47; mean BMI 36.8) had experienced 24.6% weight regain from nadir weight after RYGB. Average weight lost at 6, 12, and 18 months after TORe was 9.31 ± 6.7 kg (N = 84), 7.75 ± 8.4 kg (N = 70), 8 ± 8.8 kg (N = 46) (p < 0.01 for all three time points), respectively. The meta-analysis included 330 patients. The pooled weight lost at 12 months was 8.4 kg (95% CI 6.5–10.3) with no significant heterogeneity across included studies (p = 0.07). Overall, 14% of patients experienced nausea, 18% had pain and 8% required a repeat EGD. No serious adverse events reported.
Conclusion
When implemented as part of a multidisciplinary intervention, TORe using endolumenal suturing is safe, reproducible, and effective approach to manage weight recidivism after RYGB and should be utilized early in the management algorithm of these patients.
Keywords: Endoscopic, Bariatric surgery, Revision
Obesity is becoming a global health concern. In the U.S, over two thirds of the population is considered to be overweight or obese [1]. While nonsurgical methods have had modest success, metabolic surgery has been the most successful in the long-term [2–4]. With Laparoscopic Roux-en-Y (RYGB), patients can expect to lose around 60–80% of their excess weight at one year [5, 6]. High resolution rates of obesity related comorbidities and improved mortality have also been reported [7]. However, as longitudinal long-term prospective data are becoming available, recidivism both in terms of weight regain and return of obesity-related comorbidities is becoming a relevant issue for up to a third of the patients after RYGB. [7–9].
While factors leading to weight regain are complex and include behavioral and genetic mechanisms, anatomically a dilated (>10 mm) gastrojejunal anastomosis (GJA) has been shown to be a major and an independent predictor or weight regain [10–13]. Surgical revision entails open reduction of a dilated gastric pouch and a redo of the GJA. Revision surgery, however, is technical challenging and invasive requiring longer procedural times and hospital stay compared to the original RYGB. Furthermore, it is associated with significant morbidity and limited efficacy [14–16].
Endoscopic transoral outlet reduction (TORe) is a therapeutic option for management of weight regain after RYGB that can easily reduce the GJA aperture using a commercially available full thickness endoscopic suturing device (Overstitch, Apollo Endosurgery, Austin, TX). Compared to the surgical approach, TORe is a minimally invasive and repeatable technique [17]. Currently, only few specialized centers have reported their initial experience with TORe using endolumenal suturing. Data regarding the long-term safety and efficacy, reproducibility, and generalizability of the technique are still limited.
The aims of this study were to report the experience with TORe using endolumenal suturing from three bariatric surgery (two in North American and one in South America) centers and conduct a systematic review and meta-analysis of the existing literature to summarize the reproducibility and generalizability of the technique for patients that have experienced weight regain after having bariatric surgery.
Methods
Multicenter international study
A retrospective analysis of prospectively collected databases from three different centers (Mayo Clinic, Rochester, MN, USA; The University of Texas Health Science Center at Houston, Houston, TX, USA; and Bariatric Endoscopy Center, São Paulo, SP, Brazil) was performed. All consecutive patients who underwent TORe using endoscopic endolumenal suturing (OverStitch, Apollo Endosurgery, Austin, TX) for weight regain in the period of January 2012 through November 30th 2016 were included in the analysis. Patients were assessed in the clinic prior to the procedure where their RYGB history, weight nadir, and current weight were recorded. Periprocedural details such as GJA diameter pre and post-procedure, gastric pouch size, and presence of a gastrogastric fistula were collected. Patients were also evaluated and referred for behavioral interventions as part of a multidisciplinary approach for weight regain at this juncture.
Procedures were performed under general anesthesia with endotracheal intubation. Routine upper endoscopy was first completed to evaluate the diameter of the anastomosis and the length of the gastric pouch. Tissue at the rim of the anastomosis was then ablated using argon plasma coagulation. Interrupted or figure of eight stitches were placed transmurally at the anastomosis using the suturing device mounted on a double-channel endoscope (GIF-2T160 or 180; Olympus America, Central Valley, Pennsylvania, USA). The final GJA and pouch sizes were measured before instrument withdrawal. The procedure was considered technically successful when the anastomosis diameter was reduced to <10 mm (Fig. 1). All patients were given a course of oral antibiotics, oral antiemetics as needed. Post-procedure, the diet consisted of 2 weeks of liquid protein shakes, followed by 2 weeks of puréed diet, and then transitioning to a regular diet. The post-procedural diet was designed to provide 1000–1200 calories per day, delivering 70 g of protein. In addition, patients were encouraged to drink 56 oz of non-caloric fluids per day and take a daily chewable multivitamin. All patients were counseled to follow a standardized healthy lifestyle modification program, although this was not monitored or enforced during the duration of the study.
Fig. 1.
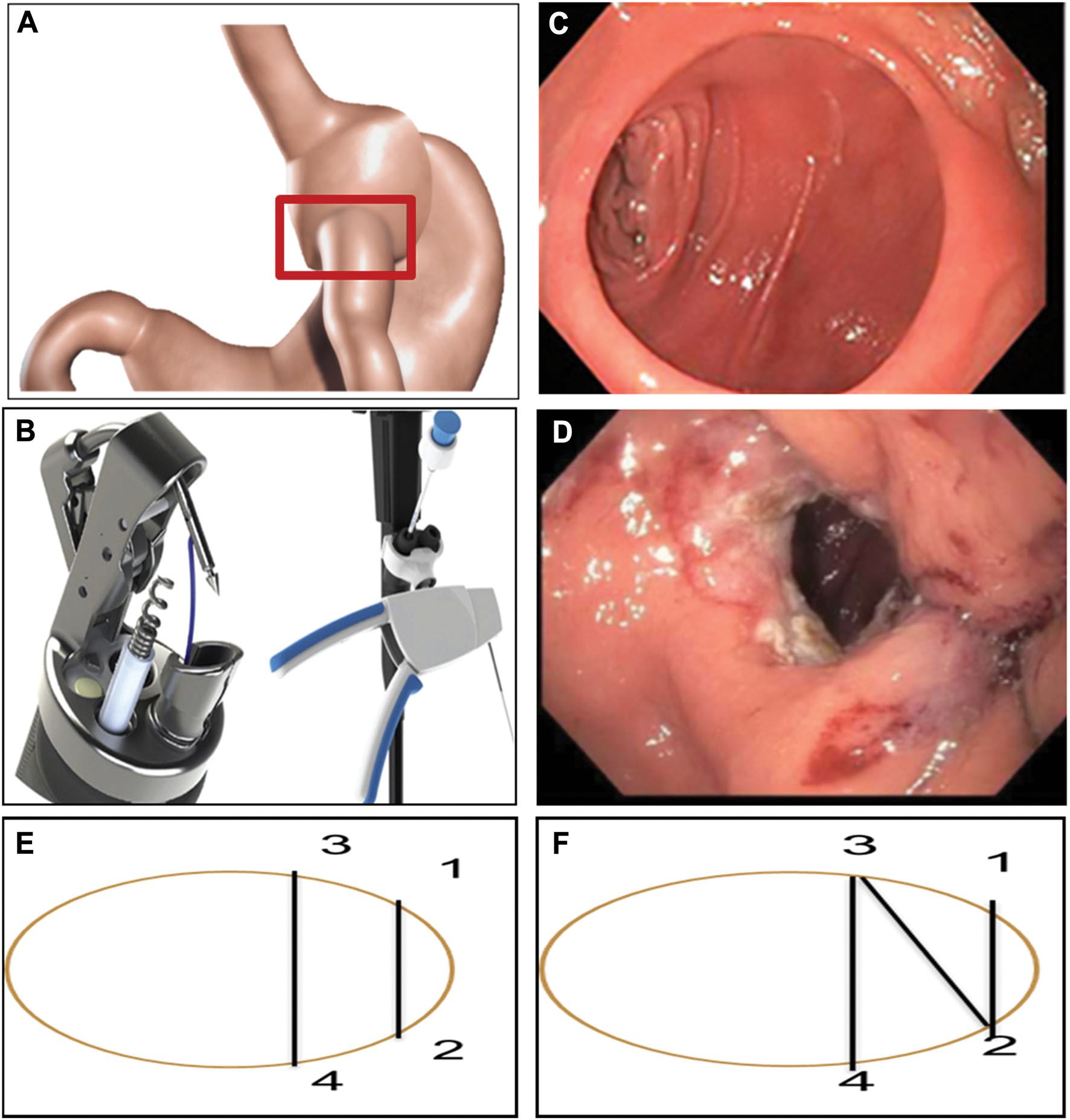
A Gastrojejunal anastomosis (GJA). B Overstitch device. C Dilated GJA. D GJA after TORe. E Interrupted suture technique. F Figure eight suturing technique
A retrospective chart review was then performed to extract post-procedure weight measurements at 6, 12 and >18 months. Percentage excess body weight loss (%EWL) was calculated by using BMI 25 kg/m2 as ideal body weight. Weight regain arrest was defined as maintaining or losing weight at follow-up compared to the weight prior to TORe. This study was approved by the IRB board at each institution.
Systematic review and meta-analysis
Data sources and search strategies
A comprehensive search of several English-language databases from 1990 to December 1st, 2016 was conducted. The databases included Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study’s principle investigator. Controlled vocabulary supplemented with keywords was used to search for studies of endoscopic suturing for revision of gastric bypass surgery for treatment of weight regain.
Study selection and data extraction
Two independent reviewers reviewed the search strategy results. When a disagreement occurred, a third reviewer was consulted to reach a consensus. Duplicate citations were removed and manuscript titles were reviewed for eligibility in the study. Only human trials published as full articles in a peer-reviewed English journal evaluating the use of the Overstitch Endoscopic suturing device for TORe were included in the meta-analysis. Studies had to report baseline demographical data, procedural details, and their 6 and 12 weight loss outcomes to be included in the analysis. Authors were contacted if further data were needed to include their study in the meta-analysis. We then combined the results of our international multicenter experience with the studies identified by the systematic review using meta-analysis to assess the reproducibility and generalizability of the techniques (Fig. 2). Quality of included studies was critically appraised using the Newcastle-Ottawa Quality Assessment Scale for cohort studies [18]. Two independent reviewers performed data extraction from the final selected citations. A third a reviewer was again included if any uncertainty arose.
Fig. 2.
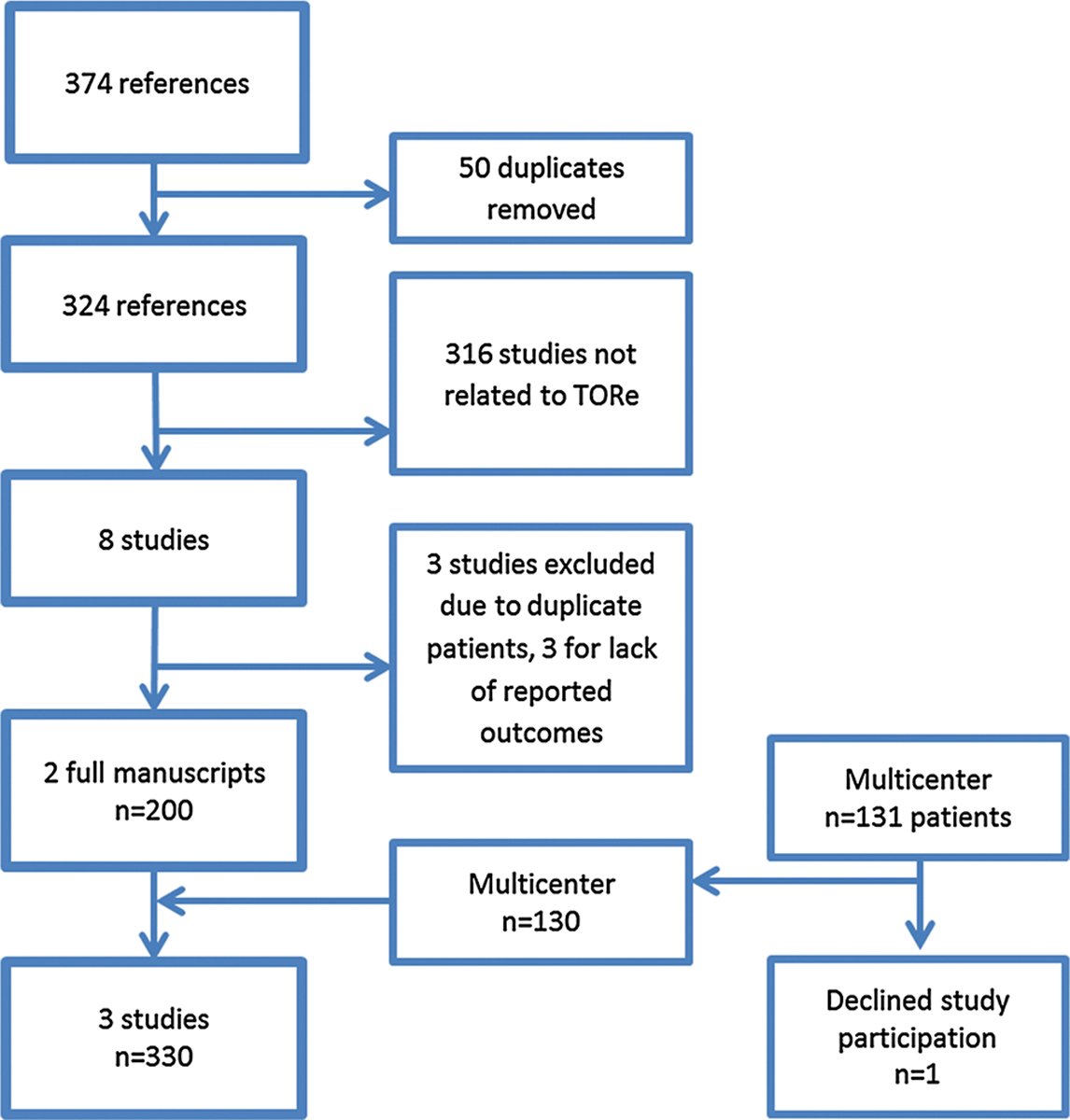
Study selection flow diagram
Statistical analysis
For our multicenter retrospective study, continuous variables were described by their means and standard deviations. Categorical variables were described in frequencies. A paired t test was used to compare baseline measurement with subsequent 6, 12, and >18 month to assess for statistically significant weight loss outcomes. Analyses were performed using JMP Pro 10.0 (SAS Institute, Cary, NC).
Given the small amount of studies and high heterogeneity, a random effects model was used for the meta-analysis. Heterogeneity was evaluated by means of the I-squared statistic. An I2 > 50% was considered to indicate high heterogeneity. Forest plots were used to evaluate and depict the overall effect size. A funnel plot was used to assess for publication bias. The comprehensive meta-analysis software (Comprehensive Meta-Analysis, Version 2.2; Biostat Inc, Englewood, NJ) was used for this analysis.
Results
Multicenter results: weight loss and adverse events
A total of 130 cases were performed across three institutions [Minnesota (n = 50), Texas (n = 42), São Paulo (n = 38)] from January 2012–December 2016. Average age was 47.12 ± 8.55 years and 88% were female. Patients were an average 8.4 ± 4.78 years from RYGB, with an initial %EWL of 70 ± 16 from initial surgery. At the time of endoscopic intervention, BMI was 36.8 ± 6.84, with average weight regain from nadir 24.6 ± 16.6 kg and average percent weight regained at 38.8%. Average prerevision stoma diameter was 28 ± 4.74 mm. Post-procedure stoma diameter was 8.3 ± 1.42 with a median of 3 [3–4] sutures placed. Average weight lost at 6, 12, and >18 months was 9.31 ± 6.7 kg (N = 84), 7.75 ± 8.4 kg (N = 70), 8 ± 8.8 kg (N = 46) 9 (p < 0.001 for all three time points), respectively. Percent total and excess weight loss at 12 months was 6 ± 7 kg and 20.2 ± 10%, respectively. The proportion of patients achieving ≥5% total body weight loss at 12 months was 67.6%. Overall, over 75% of patients experienced weight gain arrest or stabilization at 6 and 12 months. Post-procedure complications included nausea (n = 18) and abdominal pain (n = 23) all managed with oral medications. One patient experienced a superficial esophageal tear during overtube removal closed with 4 through the scope clips. No other serious adverse events reported.
Repeat EGD was performed in 11 patients (8%) to evaluate persistent symptoms after TORe with 5 patients (4%) undergoing balloon dilation to dilate a narrowed anastomosis.
Systematic review and meta-analysis
Our literature search strategy yielded 374 citations. After removing duplicates, 324 citations remained. Review of abstract titles and manuscripts narrowed the number of citations to 8. Further full text review of the 8 citations revealed duplicate number of patients from prior published results and the list was narrowed to 5. Three studies were then removed due to lack of demographical and comparable outcome measures that were unable to be obtained after contacting study authors. Only full manuscripts were incorporated in the study. With our multicenter experience, this brought the total number of studies to three, including 330 unique TORe cases using the Overstitch device (Fig. 2). All included studies were of similar moderate quality and received 5 out 9 stares on the Newcastle-Ottawa Scale (Table 1). All studies were observational without a comparator group.
Table 1.
Summary of critical appraisal of included studies using the Newcastle-Ottawa quality assessment scale for cohort studies
| Study ID | Selection (max 4 stars) | Comparability (max 2 stars) | Outcomes (max 3 stars) |
|---|---|---|---|
|
| |||
| Kumar and Thompson [19] | *** | - | ** |
| Patel et al. [20] | *** | - | ** |
| Vargas et al. 2017 (This study) | *** | - | ** |
The number of * reflects the number of stars each reference was given as part of their appraisal process with the least amount being none and highest amount attainable found in each column
Baseline demographics, procedural details, and weight loss outcomes are summarized in Table 2. Using a random effects model, the pooled absolute weight loss at 6, 12, and 18–24 months was 9.5 kg (95% CI 7.9–11.1), 8.4 kg (95% CI 6.5–10.3), 8.4 kg (95% CI 5.9–10.9), respectively (Fig. 3). Heterogeneity was acceptable across all time points (6 month: Q = 3.9 I2 = 49 p = 0.14; 12 month: Q = 5.1 I2= 60.9 p = 0.07; 24 months: Q = 0.22, I2 = 0 p = 0.63) (Fig. 4). Funnel plot did not reveal evidence of publication bias although limited by the small number of studies (Fig. 4).
Table 2.
Summary demographics and outcomes by each study when available
| Our cohort (N = 130) | Patel 2016 (N = 50) | Kumar 2016 (N = 150) | |
|---|---|---|---|
|
| |||
| Age (years) | 47.12 ± 8.55 | 50.9 ± 10.89 | 51.2 ± 9.97 |
| BMI (kg/m2) | 36.8 ± 6.84 | 41.4 ± 9.5 | 40.1 ± 8.57 |
| Years from RYGB | 8.4 ± 4.78 | 9.6 ± 3.3 | 8.6 ± 3.67 |
| Weight regain (kg) | 24.6 ± 16.6 | 23.9 ± 12.7 | 35.3 |
| % weight regain | 38.8% | 39.7% | 49.7% |
| Pre GJ stoma (mm) | 28 ± 4.74 | 29.6 ± 6.3 | 24.1 ± 7.34 |
| Final GJ stoma (mm) | 8.3 ± 1.42 | 6 ± 2.2 | 9 ± 2.44 |
| Weight gain arrest 6 months (%) | 78% (n = 84) | 97% (n = 50) | 100% (n = 144) |
| Weight gain arrest 12 months (%) | 77% (n = 70) | 77% (n = 50) | 100% (n = 109) |
| Weight loss 6 months (Kg) | 9.31 ± 6.7 (n = 84) | 7.5 ± 8.62 (n = 31) | 10.6 ± 8.4 (n = 144) |
| Weight loss 12 months (Kg) | 7.75 ± 8.4 (n = 70) | 5.83 ± 11(n = 30) | 10.5 ± 12.5 (n = 109) |
| Weight loss 18–24 months (Kg) | 8 ± 8.8 (n = 46) | N/A | 9 ± 1.7 (n = 63) |
| % EWL at 12 months | 20.2 ± 10 | 11 ± 21 | 24.9 ± 27 |
| % TWL at 12 months | 6 ± 7.0 kg | N/A | 9.5 ± 0.9 kg |
| Adverse events | Nausea 14% | Nausea 14% | N/A |
| Pain 18% | Pain 4% | ||
| Esophageal tear requiring endoscopic clipping <1% | |||
| Balloon dilation of narrowed GJA after TORe (5%) | |||
Fig. 3.
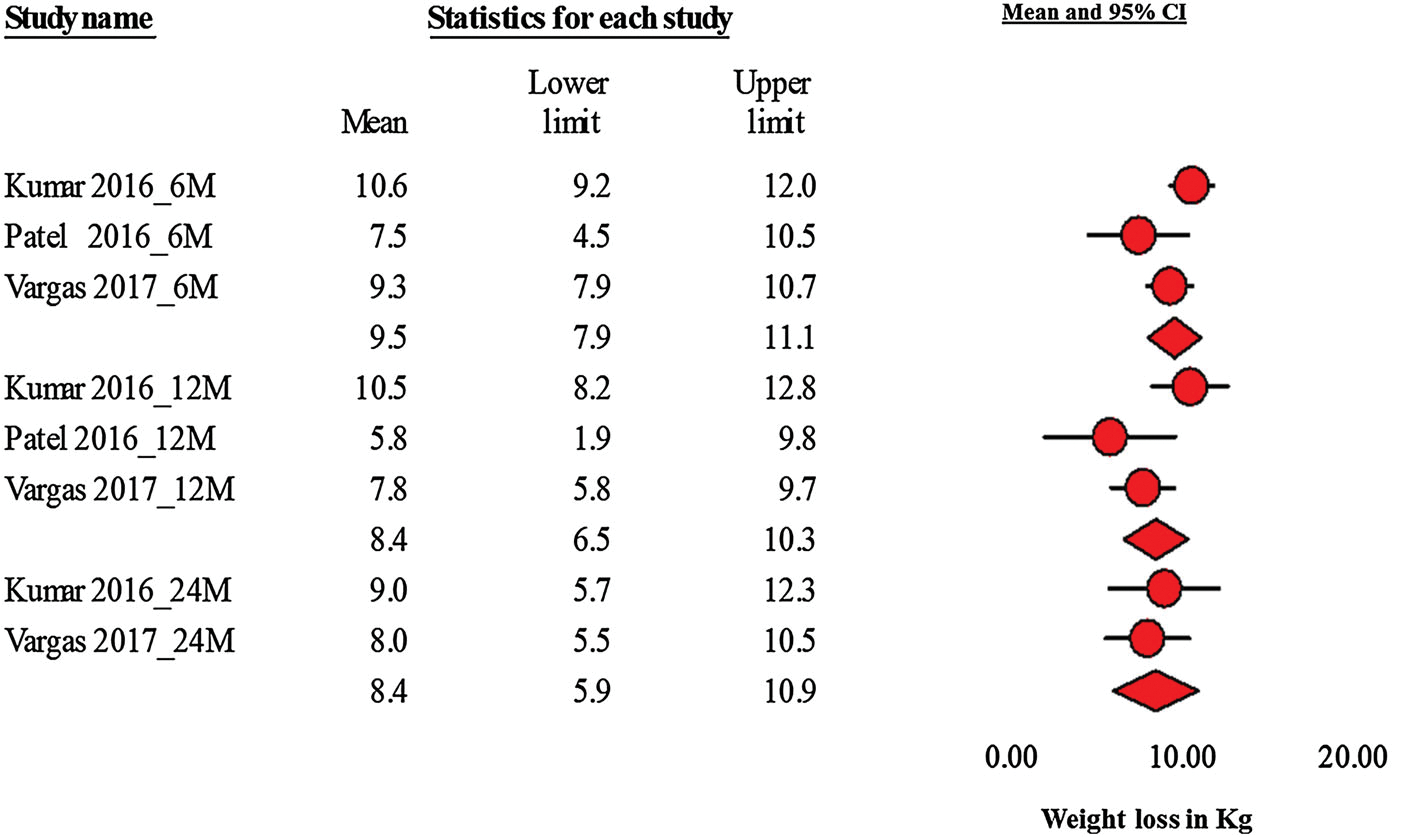
Forest plot depicting 6, 12, and 24 month absolute weight loss in Kg
Fig. 4.
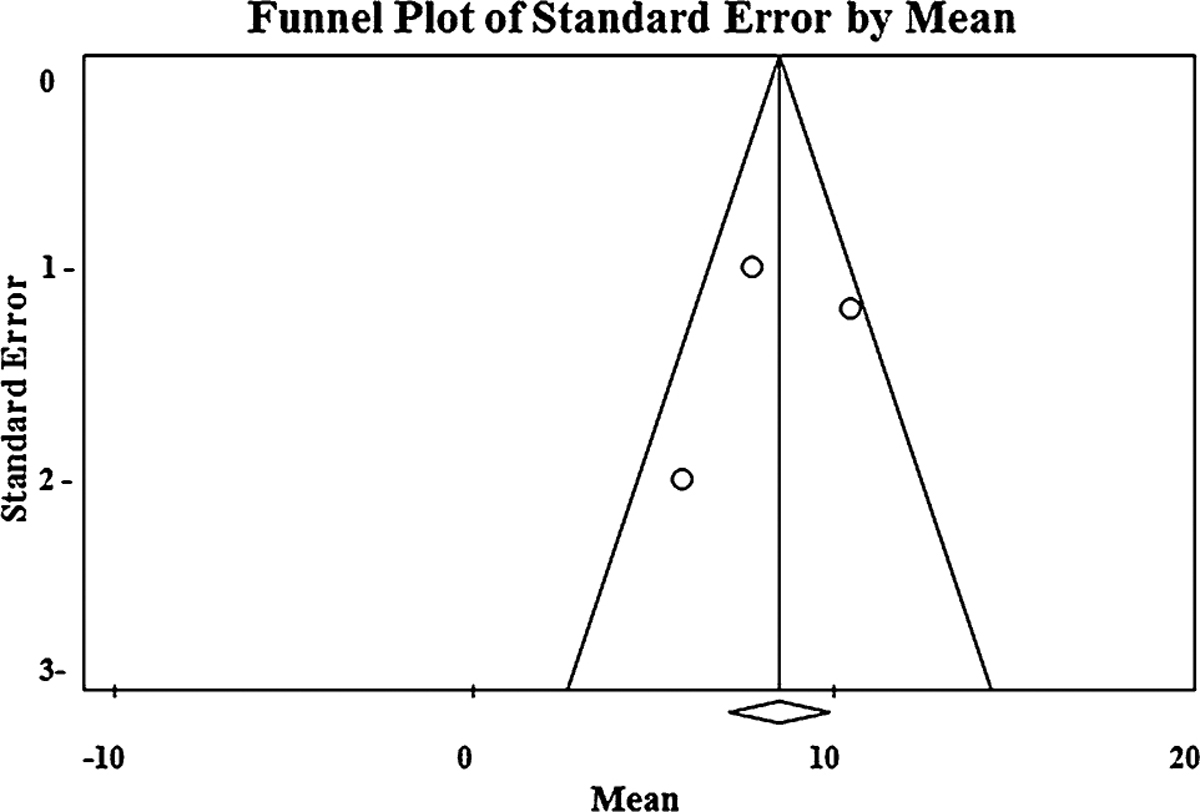
Funnel plot
Discussion
In this large prospective cohort study, we demonstrated that TORe using the endoscopic suturing device (OverStitch) is a safe and effective tool for the management of weight regain after RYGB in the setting of a dilated GJA when used in a multidisciplinary treatment setting. Furthermore, in a meta-analysis of 330 patients we validated that our results are reproducible and generalizable in different practice settings, where TORe was performed by gastroenterologists or surgical endoscopists.
According to the estimates provided by the American Society of Metabolic and Bariatric Surgery (ASMBS), the number of revision surgeries has more than doubled since 2011 and currently accounts for 13.6% of all bariatric surgeries in the US [21]. Revision procedures for a failed RYGB are technically challenging, given the potential of distorted surgical planes and anatomic changes. Additionally, these procedures are associated with higher morbidity, increased length of hospital stay and are of marginal efficacy when compared to the primary operation, with only limited literature from small cohort studies available to advise clinical decision making [15, 16, 22, 23]. Therefore, minimally invasive and effective therapeutic options were needed for the treatment of weight regain.
Compared to revision surgery for weight regain after RYGB, TORe is minimally invasive and likely cost effective option that can be performed as an outpatient procedure in under 60 min, associated with minimal risk, and potentially repeatable over the long-term in responders who derive initial benefit to maintain or enhance the weight loss. Furthermore, the literature supporting its use is robust including a randomized controlled trial showing benefit over sham even with an older generation superficial endoscopic suturing device [24, 25]. Given the above advantages and the results of this current study, TORe using the endoscopic suturing (OverStitch) device should be utilized early in the management algorithm of weight regain after RYGB in select patients with a dilated GJA in conjunction with a comprehensive lifestyle and behavioral program involving nutritionists, dieticians, and psychologists.
While the etiology of weight loss after TORe can be multifactorial and associated with the intensity of the associated lifestyle-intervention program, prior research demonstrated a correlation between the GJA diameter and eating behaviors after. [26] Reduction of the GJA has been shown to improve eating behaviors in a prospective blinded study, suggesting that restriction produced by TORe plays a major role in decreasing hunger and improving satiation and eating behaviors; thus contributing to effective weight [27]. Although restriction plays a limited role in the initial weight loss after RYGB given a primarily physiologic, not mechanical mechanism of action, it does play an important role in long-term weight maintenance [28, 29]. Indeed, both clinical and research experience have shown that overtime there is a regression in the so called “satiety response” after RYGB with increased hunger, decreased satiation, worsening eating behaviors, and tolerance of larger meal sizes [26, 30–32].
Our study has several limitations including its retrospective design, potential for selection bias given the referral practice of the participating centers, and lack of a comparator group. These are not unique to our study, but are inherent limitations to many interventional weight loss studies. We attempted to compensate for these shortcomings by analyzing reproducibility of the technique among different practice settings in a large cohort and conducting a systematic review and meta-analysis that involved contacting authors to obtain patients’ level information to provide granular data to inform clinical decision making and future research.
In conclusion, TORe is a minimally invasive weight loss intervention that, in conjunction with a robust lifestyle and behavioral intervention program, offers an effective management strategy for weight regain after RYGB in a select group of patients with dilated GJA. Future study should focus on investigating the tandem and sequential use of TORe and obesity pharmacotherapies as an effective and durable weight loss strategies in this cohort.
Footnotes
Compliance with ethical standards
Disclosures Dr. Todd Wilson has served as consultant for Bard, Olympus, and EndoEvolution. Dr. Erik B. Wilson has served as a consultant to Intuitive Surgical, Ethicon, Gore, and Apollo Endosurgery, and Olympus. Dr. Manoel Galvao Neto has served as a consultant to ALACER Biomedica, SCI Tech/CMS, MI Tech, Ethicon EndoSurgery, GI Dynamics, Apollo EndoSurgery, Fractyl Labs, GI Windows, and Alacer Biomedica. Dr. Natan Zundel is on the advisory board for Apollo Endosurgery and Olympus. Dr. Christopher J. Gostout is chief medical officer for Apollo Endosurgery. Dr. Barham K. Abu Dayyeh has served as a consultant for Apollo Endosurgery, Boston Scientific, and Metamodix. He received research support from GI dynamics, Aspire Bariatrics, and Spatz. Drs. Eric J.Vargas, Fateh Bazerbachi, Andres Acosta, Manpreet S. Mundi, Maria L. Collazo-Clavell, Shah Meera, H. S. Abu-Lebdeh, Todd A. Kellogg, Travis J. McKenzie, Michael L. Kendrick, Mark D. Topazian, Mrs. Monika Rizk and Mr. Paul A. Lorentz have no conflicts of interest or financial ties to disclose.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303(3):235–241. doi: 10.1001/jama.2009.2014 [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE et al. (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366(17):1567–1576. doi: 10.1056/NEJMoa1200225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanovski SZ, Yanovski JA (2014) Long-term drug treatment for obesity: a systematic and clinical review. JAMA 311(1):74–86. doi: 10.1001/jama.2013.281361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JW, Konz EC, Frederich RC, Wood CL (2001) Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 74(5):579–584 [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292(14):1724–1737. doi: 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ et al. (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122(3):248–256, e5. doi: 10.1016/j.amjmed.2008.09.041 [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom L (2013) Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J Intern Med 273(3):219–234. doi: 10.1111/joim.12012 [DOI] [PubMed] [Google Scholar]
- 8.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC (2008) Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg 18(6):648–651. doi: 10.1007/s11695-007-9265-1 [DOI] [PubMed] [Google Scholar]
- 9.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA et al. (2017) Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 376(7):641–651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu Dayyeh BK, Lautz DB, Thompson CC (2011) Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol 9(3):228–233. doi: 10.1016/j.cgh.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heneghan HM, Yimcharoen P, Brethauer SA, Kroh M, Chand B (2012) Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis 8(4):408–415. doi: 10.1016/j.soard.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Koball AM, Himes SM, Sim L, Clark MM, Collazo-Clavell ML, Mundi M et al. (2016) Distress tolerance and psychological comorbidity in patients seeking bariatric surgery. Obes Surg 26(7):1559–1564. doi: 10.1007/s11695-015-1926-x [DOI] [PubMed] [Google Scholar]
- 13.Clark MM, Hanna BK, Mai JL, Graszer KM, Krochta JG, McAlpine DE et al. (2007) Sexual abuse survivors and psychiatric hospitalization after bariatric surgery. Obes Surg 17(4):465–469. doi: 10.1007/s11695-007-9084-4 [DOI] [PubMed] [Google Scholar]
- 14.Coakley BA, Deveney CW, Spight DH, Thompson SK, Le D, Jobe BA et al. (2008) Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis 4(5):581–586. doi: 10.1016/j.soard.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Parikh M, Heacock L, Gagner M (2011) Laparoscopic, “gastrojejunal sleeve reduction” as a revision procedure for weight loss failure after Roux-en-Y gastric bypass. Obes Surg 21(5):650–654 [DOI] [PubMed] [Google Scholar]
- 16.Cardeal Mde A, Faria SL, Faria OP, Facundes M, Ito MK (2016) Diet-induced thermogenesis in postoperatve Roux-en-Y gastric bypass patients with weight regain. Surg Obes Relat Dis 12(5):1098–1107. doi: 10.1016/j.soard.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 17.Jirapinyo P, Slattery J, Ryan MB, Abu Dayyeh BK, Lautz DB, Thompson CC (2013) Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy 45(7):532–536. doi: 10.1055/s-0032-1326638 [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2017
- 19.Kumar N, Thompson CC (2016) Transoral outlet reduction for weight regain after gastric bypass: long-term follow-up. Gastrointest Endosc 83(4):776–779. doi: 10.1016/j.gie.2015.08.039 [DOI] [PubMed] [Google Scholar]
- 20.Patel LY, Lapin B, Brown CS, Stringer T, Gitelis ME, Linn JG et al. (2016) Outcomes following 50 consecutive endoscopic gastrojejunal revisions for weight gain following Roux-en-Y gastric bypass: a comparison of endoscopic suturing techniques for stoma reduction. Surg Endosc 2016:1–11. doi: 10.1007/s00464-016-5281-3 [DOI] [PubMed] [Google Scholar]
- 21.Estimates of Bariatric Surgery Numbers (2016) Available from https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 03 May 2017
- 22.Elnahas AI, Jackson TD, Hong D (2014) Management of Failed Laparoscopic Roux-en-Y Gastric Bypass. Bariatr Surg Pract Patient Care 9(1):36–40. doi: 10.1089/bari.2013.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gobble RM, Parikh MS, Greives MR, Ren CJ, Fielding GA (2008) Gastric banding as a salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg Endosc 22(4):1019–1022. doi: 10.1007/s00464-007-9609-x [DOI] [PubMed] [Google Scholar]
- 24.Thompson CC, Chand B, Chen YK, Demarco DC, Miller L, Schweitzer M et al. (2013) Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology 145(1):129–137, e3. doi: 10.1053/j.gastro.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Kumar N, Thompson CC (2014) Comparison of a superficial suturing device with a full-thickness suturing device for transoral outlet reduction (with videos). Gastrointest Endosc 79(6):984–989. doi: 10.1016/j.gie.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu Dayyeh BK, Jirapinyo P, Thompson CC (2017) Plasma ghrelin levels and weight regain after Roux-en-Y Gastric bypass surgery. Obes Surg 27(4):1031–1036. doi: 10.1007/s11695-016-2418-3 [DOI] [PubMed] [Google Scholar]
- 27.Jirapinyo P, Dayyeh BK, Thompson CC (2016) Gastrojejunal anastomotic reduction for weight regain in roux-en-y gastric bypass patients: physiological, behavioral, and anatomical effects of endoscopic suturing and sclerotherapy. Surg Obes Relat Dis 12(10):1810–1816. doi: 10.1016/j.soard.2016.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakravartty S, Tassinari D, Salerno A, Giorgakis E, Rubino F (2015) What is the mechanism behind weight loss maintenance with gastric bypass? Curr Obes Rep 4(2):262–268. doi: 10.1007/s13679-015-0158-7 [DOI] [PubMed] [Google Scholar]
- 29.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A et al. (2007) Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3 [DOI] [PubMed] [Google Scholar]
- 30.Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB et al. (2012) Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes 36(3):348–355 [DOI] [PubMed] [Google Scholar]
- 31.Mathes CM, Spector AC (2012) Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav 107(4):476–483. doi: 10.1016/j.physbeh.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 32.Banerjee A, Ding Y, Mikami DJ, Needleman BJ (2013) The role of dumping syndrome in weight loss after gastric bypass surgery. Surg Endosc 27(5):1573–1578. doi: 10.1007/s00464-012-2629-1 [DOI] [PubMed] [Google Scholar]


