Abstract
We investigated inflammatory and physiologic parameters in sepsis models of increasing lethality induced by cecal ligation and puncture (CLP). Mice received imipenem for antibiotic therapy, and groups were sacrificed at 2, 4, 8, 12, 16, 20, and 24 h after CLP. The severity of sepsis increased with needle puncture size (lethality with 18-gauge puncture [18G], 100%; 21G, 50%; 25G, 5%; sham treatment, 0%). While the temperature (at 12 h) and the activity and diurnal rhythm (at day 4) of the 25G-treated CLP group recovered to normal, the 21G and 18G treatment groups exhibited severe hypothermia along with decreased activities. A direct correlation was also observed between the severity of sepsis and cytokine (interleukin 1β [IL-1β], tumor necrosis factor [TNF], IL-6, and IL-10) concentrations in both the peritoneum and the plasma. There were substantially higher cytokine levels in the more severe CLP models than in the sham-treated one. Peritoneal and plasma TNF levels were always less than 40 pg/ml in all models. None of the cytokines in the septic mice peaked within the first hour, which is in contrast to the results of most endotoxin models. Chemokine (KC and macrophage inflammatory protein 2) profiles also correlated with the severity of sepsis. Except for the chemokines, levels of inflammatory mediators were always higher at the site of inflammation (peritoneum) than in the circulation. Our study demonstrated that sepsis of increasing severity induced increased cytokine levels both within the local environment (peritoneum) and systemically (plasma), which in turn correlated with morbidity and mortality.
Sepsis is a common cause of morbidity and mortality, especially following surgery or trauma (9, 14, 32). The disease ranks as the leading cause of death in intensive care units, and the incidence of sepsis has risen steadily since 1980 (14, 40). Temperature alterations, inflammation, and elevated cytokine levels are some characteristics of sepsis. Conventional therapy for sepsis involves treatment of the nidus of infection, as well as antibiotic, hemodynamic, and respiratory support (4, 8, 15). With increasing understanding of the immunopathology of sepsis, innovative strategies targeting endotoxin and inflammatory mediators have been employed and have been effective in animal models (5, 36). However, clinical trials have resulted in limited success (2, 3, 13, 31). Reasons for the clinical failures include the use of various single agents against either lipopolysaccharide or mediators of sepsis and delays between the onset of sepsis and the beginning of treatment. Most importantly, trials have been based on the wrong models, especially the endotoxemia model. Although appropriate for studying immune regulations of cytokines, this model does not adequately reproduce the complex immunology of sepsis. Cecal ligation and puncture (CLP) is another sepsis model that may be more clinically relevant and acceptable for studying the systemic response to local infection (17, 41). It is similar to the clinical situation of bowel perforation, inducing peritonitis due to mixed intestinal flora. Furthermore, this model can be manipulated to give either lethal or nonlethal sepsis. The validity of CLP has been reported in publications that showed that the blockade of tumor necrosis factor alpha (TNF-α) (20, 33) failed to prevent death, results similar to that observed in human clinical trials.
While there are numerous studies using single-size needles for CLP that have investigated different aspects of sepsis (19, 20, 28, 34, 35, 37, 38), there are presently no comprehensive studies on the kinetics of cytokine production and the accompanying pathophysiology due to different needle size in the CLP model of sepsis. Needle size can be used to manipulate CLP to give lethal and nonlethal sepsis. We investigated the kinetics of some cytokines (TNF, interleukin 1β [IL-1β], IL-10, and IL-6) and murine IL-8 analogs, macrophage inflammatory protein 2 (MIP-2) and KC, in the peritoneum (local) and plasma (systemic). In addition, we monitored the physiologic responses in lethal and nonlethal sepsis induced by CLP.
MATERIALS AND METHODS
Experimental design.
We investigated host responses to septic insults by measuring cytokines within the peritoneum (local) and the plasma (systemic). We also monitored the temperature and gross motor activities of each animal in the study. Mice were euthanized, and samples were collected 2, 4, 8, 12, 16, 20, and 24 h later. Each time point included the results of no less than two independent experiments with CLP-treated mice (25-, 21-, and 18-gauge punctures [25G, 21G, and 18G treatments, respectively]) and sham-treated mice. All surgeries were planned so that harvesting occurred at the same time each morning. Samples were analyzed for TNF, IL-6, IL-1β, and IL-10 as well as KC and MIP-2. Temperature data were averaged for a 30-min interval, analyzed on a spreadsheet, and plotted against time after surgery. Activity counts for each animal in each treatment group for a given time point were pooled to correspond to a 12-h light or dark cycle, analyzed, and plotted against time.
Animals.
BALB/c mice were obtained from Harlan-Sprague Dawley, Inc. (Indianapolis, Ind.), and maintained under standard laboratory conditions. In addition to a resting period at the institutional vivarium, mice were acclimated to the laboratory environment for at least 24 h before surgery. After surgery, the mice were housed one per cage in a temperature-controlled room with food and water provided ad libitum and were kept under a 12-h light–12-h dark diurnal cycle.
The experiments described below were performed in accordance with the National Institutes of Health guidelines, and approval was obtained from the University of Michigan Animal Care and Use Committee.
CLP. (i) Mortality study.
CLP was performed as previously described, with minor modifications (17, 30). Briefly, female BALB/c mice (19 to 23 g) were anesthetized with ketamine (87 μg/g) (Ketaset; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) and xylazine (13 μg/g) (Rompun; Bayer Corporation, Shawnee Mission, Kans.), and a 2-cm midline incision was made through the linea alba. The cecum was located, ligated with sterile 3-0 silk, and perforated twice with a 25-, 21-, or 18-gauge needle. A small amount of stool was extruded to ensure wound patency. Sham-treated mice also had surgery done along with cecal manipulations but without ligation and puncture. The cecum was then replaced in its original position within the abdomen, which was closed in two layers. Minimitter transmitters (Vitalview series 4000 E-Mitters; Minimitters Co., Sunriver, Oreg.) were implanted in all animals subcutaneously. Minimitters provide continuous monitoring of the animals' temperature and gross motor activity. Immediately after surgery, each mouse received a subcutaneous injection of 1 ml of warm normal saline (37°C) and was placed in an incubator (37°C) for 15 min. The mice were then moved to a closed room and maintained at 22°C for the remainder of the experiment. Two hours after surgery (and every 12 h up to 3 days), each mouse received a subcutaneous injection of imipenem (0.5 mg/mouse) (Primaxin; Merck & Co., Inc., West Point, Pa.), reconstituted in 1 ml of 5% dextrose for fluid resuscitation. We have previously reported that imipenem led to less mortality and morbidity in the CLP model and was superior to the use of triple antibiotics (30). Cumulative mortality was assessed for 8 days with at least six mice per CLP group. We did not collect either blood or peritoneal fluid from these mice.
(ii) Kinetics study.
Groups of mice were subjected to CLP with a 25-, 21-, or 18-gauge needle as described above. Sham-treated mice did not undergo CLP but did receive a laparotomy. Minimitters were not implanted in these mice. Mice were sacrificed at 2, 4, 8, 12, 16, 20, and 24 h, and samples were collected for later analyses.
Sample harvesting. (i) Peripheral blood.
EDTA-anticoagulated blood (20 μl) was collected at appropriate time points from the tail of each mouse, which had been lightly anesthetized with ketamine and xylazine. Blood was also collected from the retroorbital venous plexus into tubes containing 50 U of porcine-derived heparin (Elkins-Sinn, Inc., Cherry Hill, N.J.). Plasma was separated from cellular components by centrifugation at 600 × g for 5 min and stored at −20°C for later cytokine analyses.
(ii) Peritoneal lavage.
Lavages were performed as previously described (17), with minor modifications. Peritoneal washes were performed with 1 ml of ice-cold 1× Hanks balanced salt solution (without CaCl2, MgCl2, Mg2SO4, or phenol red; GIBCO, Grand Island, N.Y.) followed by a separate 10-ml Hanks balanced salt solution wash. The 1-ml wash was centrifuged (600 × g, 5 min), and the supernatant was stored at −20°C for later cytokine analyses. The cell pellet was pooled with cells from the 10-ml peritoneal wash for cell counts and differentials. To ensure that only leukocytes would be counted, erythrocytes were lysed with Zap-Oglobin II (Coulter Electronics Inc., Hialeah, Fla.), and total cell counts were carried out with a Coulter counter (model ZF; Coulter Electronics). Cells were prepared for differential counts in a final cell concentration of 5 × 105/ml in cytospin fluid (RPMI 1640, 1% fetal calf serum) and centrifuged at 700 × g for 5 min. Cytospin fluids were then stained with Diff-Quick (Baxter, Detroit, Mich.), and leukocyte types were quantified under oil immersion lens after 300 cells were tallied. The total for each cell type was calculated by dividing each cell type by 300 and multiplying by the total leukocytes obtained from the Coulter counter.
Cytokine analyses.
All samples were measured simultaneously to reduce errors due to assay variation. Cytokines from the peritoneal fluid and the plasma were measured.
IL-6 assay.
B9 cells proliferate in the presence of IL-6 and were used to quantify IL-6 concentrations (1). Samples were serially diluted across a 96-well plate, and a concentration of 5 × 104 cells/ml in Iscove's modified Dulbecco medium (GIBCO) containing 10% fetal calf serum, 5 × 10−5 M β-mercaptoethanol, 2 mM l-glutamine, 1% penicillin-streptomycin, and 25 mM HEPES (BioWhittaker) was seeded into each well. The plates were incubated in a humidified chamber (5% CO2, 37°C) for 3 days. Cell proliferation was determined by staining with MTT-tetrazolium (Sigma Chemical Co., St. Louis, Mo.). The plates were incubated for an additional 6 h, after which 150 μl of medium was aspirated from each well and cells were lysed with 0.04 N HCl-isopropyl alcohol. The plates were stored at room temperature in a darkened chamber equilibrated with HCl-isopropyl alcohol. Absorbances were quantified on a Bio-Kinetics reader (Bio-Tek Instruments, Inc.) with dual filters (550 nm with a 630-nm reference filter). Unbound IL-6 concentrations were determined from a standard curve of the human recombinant IL-6 (PeproTech, Rocky Hill, N.J.) that was employed in the same assay. This assay reliably detected samples with concentrations as low as 1 to 2 pg/ml.
TNF assay.
TNF concentrations were quantified by cytolytic activity directed against the WEHI 164 subclone 13 fibrosarcoma cell line as previously described (21, 22). Briefly, samples were serially diluted across flat-bottom 96-well microtiter plates in RPMI 1640 medium, and the WEHI 164 cell suspension was added directly on top of the diluted samples. The plates were incubated overnight in a humidified chamber (5% CO2, 37°C), and cell viability was determined with MTT-tetrazolium after an additional 4-h incubation. The concentration of unbound TNF was determined based on a standard curve generated with recombinant human TNF (Cetus Corp., Emeryville, Calif.) in the same assay. This assay was sensitive to 1 to 2 pg of TNF per ml.
ELISA.
IL-1β and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA). Plates (Immunoplate Maxisorb; Nunc, Neptune, N.J.) were coated with anti-mouse IL-10 monoclonal antibody (Pharmingen, San Diego, Calif.) or with anti-mouse monoclonal IL-1β (R&D Systems, Inc. Minneapolis, Minn.) and incubated overnight at 4°C. The plates were then washed with a wash buffer containing 0.05% Tween 20 (FisherBiotech, Fair Lawn, N.J.) in phosphate-buffered saline (PBS). Nonspecific binding sites were blocked by incubating the plates with Superblock blocking buffer in PBS (Pierce, Rockford, Ill.) for 1 h (2 h for IL-10). This and other subsequent incubations were conducted at room temperature on a shaker. After washing, samples were added, and the plates were incubated (1 h for IL-1β and 2 h for IL-10). A standard curve was prepared with either recombinant murine IL-10 or IL-1β. All standard and sample dilutions were made in dilution medium (DM; 10% Superblock in PBS supplemented with 0.1% normal rabbit plasma for nonspecific blocking and 0.05% Tween). The plates were washed, 50 μl of biotinylated rat anti-mouse IL-10 or IL-1β (diluted 1:1,000 in DM) per well was added, and the plates were incubated for 1 h. After washing, horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) was added (1:20,000 in DM) and the plates were incubated for 30 min. After a final wash, TMB (3,3′,5,5′-tetramethylbenzidine; Genzyme Diagnostics, San Carlos, Calif.) was added, the plates were incubated in the dark for 15 min, and the reaction was stopped with 1.5 N H2SO4. The plates were read by using dual wavelengths (465 and 590 nm) on the Biotek microplate reader, and cytokine concentrations were estimated by using the recombinant cytokine standard curve. The lower limits of detection were 12 pg/ml for IL-10 and 60 pg/ml for IL-1β.
Chemokines.
KC and MIP-2 were also quantitated by ELISA as described above. Briefly, rabbit polyclonal antibodies were raised against murine recombinant KC or MIP-2, purified, and used as capture antibodies. A portion of each antibody was biotinylated and used as the detection antibody. A standard curve was prepared by using recombinant KC and MIP-2. The assays were developed by using TMB. KC did not cross-react with MIP-2. The lower limits of detection for KC and MIP-2 were 100 and 25 pg/ml, respectively.
Statistical analyses.
Summary statistics are expressed as means ± standard errors of the means (SEM) in all figures and tables. Differences between all treatment groups were compared by analysis of variance. Tukey's test for pair-wise comparisons was performed when the overall F value was statistically significant (P < 0.05). Cytokine levels that were not detectable were assigned a value equal to half the lower limit of detection for that assay. Inflammatory scores were calculated by using the physiologic responses (lethality, temperature, and activity) and the circulatory inflammatory cytokines (IL-1β and IL-6) and chemokines (KC and MIP-2). Animals were assigned values (0 or 1) based on the criteria listed in Table 1. The inflammatory score was then calculated by taking the sum of each value for each cytokine and physiologic response in each treatment score.
TABLE 1.
Inflammation scoring systema
| Parameter | Characteristic for score of:
|
|
|---|---|---|
| 0 | 1 | |
| Physiologic | ||
| Lethality at day 3 | Alive | Dead |
| Temp at day 1 (°C) | >34 | ≤34 |
| Activity at day 3 (counts/5 min) | ≥20 | <20 |
| Cytokines (plasma levels at 16 h) | ||
| IL-1β (pg/ml) | ≤100 | >100 |
| IL-6 (ng/ml) | ≤55 | >55 |
| KC (ng/ml) | ≤160 | >160 |
| MIP-2 (ng/ml) | ≤7 | ≥7 |
Scores were calculated based on the physiologic responses and circulatory inflammatory cytokines and chemokines. Inflammatory scores were obtained by adding individual scores. The scores for the chemokines were added and averaged.
RESULTS
CLP lethality study.
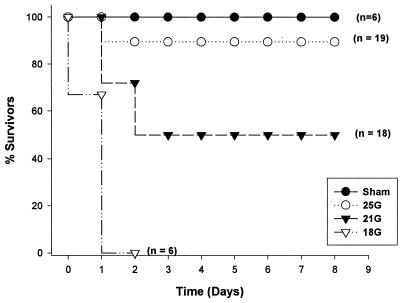
Our initial study determined the lethality associated with different sizes of needle punctures in the CLP model. As expected, increasing the size of the puncture increased mortality (Fig. 1). These results agree with previous reports (6, 17, 34) showing a clear relationship between mortality and the size of cecal puncture. The 25G-treated mice (no. of surviving mice/total no., 18/19) were considered to have undergone nonlethal sepsis, while the sepsis in the 18G-treated group was lethal (0 of 6 mice survived). The 21G-treated group showed 50% lethality, and those mice that survived showed slow recovery over the course of the study. All mice that died did so within 48 h.
FIG. 1.
CLP lethality. Mortality coincided with the severity (needle gauge) of CLP.
Physiologic responses.
We monitored physiologic reactions to determine the systemic responses to and outcomes of the different severities of insults. The physiologic response was dependent on puncture size. Increasing puncture size led to decreasing temperatures and activities. This agrees with the lethality study results shown in Fig. 1. It is important to note that the reported temperatures and activities are for those mice that did not die.
Temperature profile.
All mice developed hypothermia, but the temperature of the sham-treated mice recovered to normal within 12 h (Fig. 2A) while the temperature of the 25G-treated mice required nearly 4 days for recovery. The 21G-treated mice did not fully attain normal temperature by day 8, although the decrease was not statistically significant from that in the sham-treated group (Fig. 2B). The temperature of the 18G-treated mice continued to drop until they died. There were significant differences in the temperatures of both the 21G (to 1.5 days)- and 18G (to 1 day)-treated groups relative to that of the sham-treated mice (Tukey's test; P ≤ 0.05).
FIG. 2.
Temperature profile. The temperature of sham-treated mice (n = 6) returned to normal faster than that of the CLP-treated mice. The 25G-treated mice (n = 19) recovered by day 3, while the 21G-treated mice (n = 18) never attained the temperature of the sham-treated mice. The 18G-treated mice (n = 6) were severely hypothermic and died by 48 h. ∗, P < 0.05. Error bars indicate +SEM.
Activity profile. (i) Gross motor activity.
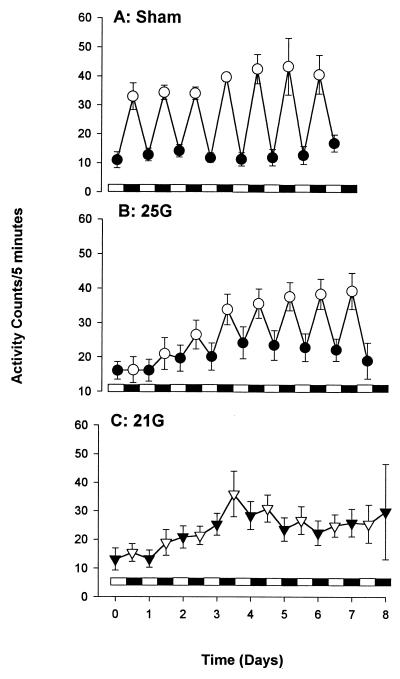
All CLP-treated mice were lethargic and exhibited piloerection, diarrhea, and labored breathing. Recovery and the time of recovery were related to needle gauge size. Sham-treated mice were initially lethargic but appeared to recover within 12 h (Fig. 3).
FIG. 3.
Gross motor activity or diurnal rhythm. (A) Recovery coincided with the severity of insult. (B) The nonlethal treated (25G-treated) mice recovered to the activity level of the sham-treated mice by day 3. (C) The 21G-treated CLP group did not fully recover to the activity level of the sham-treated mice throughout the study period. The bar along the x axis representing the dark-light cycle indicates that mice were maintained in a temperature-controlled room with a 12-h lights-on (solid bar) and 12-h lights-off (open bar) cycle. The SEM may be smaller than the symbol. Each value is the mean ± SEM for 6 to 19 mice.
(ii) Diurnal variations.
Because mice are nocturnal, with higher activity levels at night, we were able to investigate the effects of sepsis on their diurnal rhythm. Mice were maintained in a temperature-controlled room with a 12-h light (lights on)–dark (lights off) cycle. While the diurnal rhythm of the sham-treated mice was almost normal (Fig. 3A), both the 25G- and 21G-treated mice showed altered diurnal activities. However, the mice in the 25G-treated group recovered their diurnal rhythm by day 3 (Fig. 3B) while the 21G-treated mice did not fully recover (Fig. 3C) during the 8-day period. The 18G-treated group exhibited decreased activities with no diurnal rhythm before death (data not shown). There were no significant activity differences between the groups over the time course of the experiment.
Cytokine analyses.
Since cytokines may stimulate or prevent the expression of another cytokine or its receptor, we investigated the simultaneous expressions of several cytokines. Generally, our results showed that cytokine levels depended on the severity of insult. In this study, severity refers to the puncture size (needle bore size) as well as the outcome (mortality). The 21G- and 18G-treated groups exhibited higher cytokine concentrations than the sham- and 25G-treated groups. Furthermore, the concentrations at the local source of inflammation, the peritoneum, were always higher than those in the plasma. In contrast to most endotoxin models, none of the cytokines peaked within the first hour.
(i) TNF.
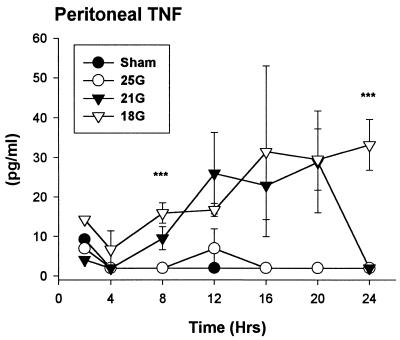
Contrary to reports that showed the presence of TNF in the circulation in the endotoxin model (20) and in the CLP model (28, 38), we did not detect biologically active TNF at any time point in the plasma of any of the CLP models that we studied (data not shown). However, we detected increasing levels of TNF at the site of inflammation, the peritoneum, in both the 21G- and 18G-treated models. Unlike the endotoxin model in which TNF peaks rapidly (within 2 h) (23), we noticed a delayed appearance of TNF in those models with higher mortality (Fig. 4). In addition, the TNF levels in these CLP models are substantially less than those reported for the endotoxin models (38).
FIG. 4.
TNF. There were higher TNF levels in the mice of the more severe treatment models. There were no detectable levels of biologically active TNF in the circulation at any time point (data not shown). ∗∗∗, P < 0.05, 18G- versus sham-treated mice (n = 6). The SEM may be smaller than the symbol.
(ii) IL-1β.
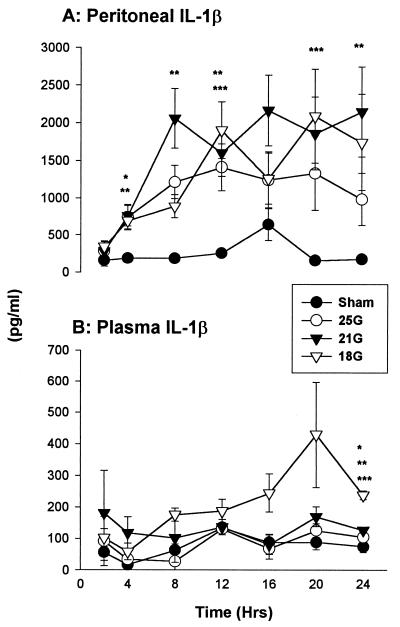
Mice that underwent CLP, regardless of puncture size, had IL-1β elevations in the peritoneum by 4 h (Fig. 5A). The sham-treated group did not show substantial elevations in the peritoneum. There were no distinct gradations between the 21G- and 18G-treated models, and the profile was similar to that observed for TNF although at significantly higher concentrations. Although levels were higher, except for those at 4 h, there were no significant differences between the 25G-treated and sham-treated models. Levels of circulating IL-1β stayed about the same in all groups except in the very severe treatment group (Fig. 5B). Unlike reports indicating undetectable IL-1β in the plasma (42), there was increasing IL-1β in the 18G-treated CLP model, peaking at 20 h (429 ± 167 pg/ml).
FIG. 5.
IL-1β. (A) All mice that underwent CLP had elevated levels of IL-1β in the peritoneum regardless of puncture size. (B) The 18G-treated CLP model showed increasing circulating levels. Each value is the mean ± SEM for 6 to 10 mice. ∗, ∗∗, and ∗∗∗, P < 0.05 for 25G, 21G, and 18G treatment groups (versus sham treatment group). The SEM may be smaller than the symbol.
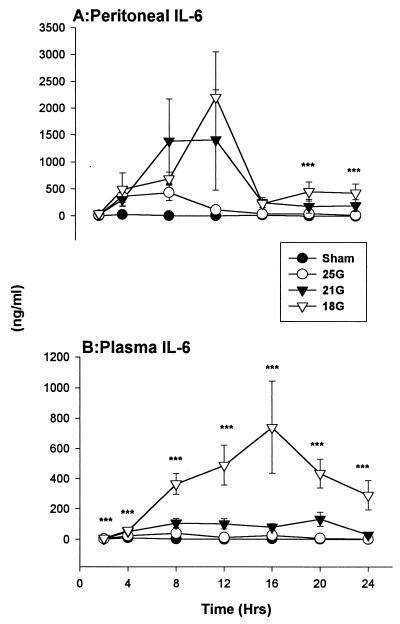
(iii) IL-6.
There were substantial quantities of IL-6 within the peritoneum 4 h after CLP (Fig. 6A). IL-6 concentrations in the 25G-treated group were near the level of the sham-treated group, while the levels in the 21G- and 18G-treated groups stayed elevated before peaking at 12 h. Levels were significantly higher at 20 and 24 h in the peritoneum of the 18G-treated mice relative to those in the sham-treated mice (Tukey's test; P < 0.05).
FIG. 6.
IL-6. There were elevated levels of IL-6 within the peritoneum (A) and in the circulation (B) (see also Table 1) in the 21G- and 18G-treated CLP models. ∗∗∗, P < 0.05 for 18G- sham-treated groups. The SEM may be smaller than the symbol. Values are expressed as means ± SEM for 6 to 10 mice.
The circulating IL-6 profile was similar to that observed with IL-1β in those groups that had higher mortalities. Like the results in the peritoneum, IL-6 concentrations within the plasma were also dependent on needle size (severity) (Fig. 6B). The gradations between the 21G, 25G, and sham treatment groups are presented in Table 2. There were significant differences in the results for the 18G treatment group (versus sham and 25G treatment groups) which were observed through all time points, and the levels of the 21G treatment group were significantly higher at 4 to 20 h (versus sham treatment group) (Tukey's test; P < 0.05).
TABLE 2.
Levels of IL-6 in plasmaa
| Time (h) | IL-6
level (ng/ml) (mean ± SEM) in indicated treatment group
|
||
|---|---|---|---|
| Sham | 25G | 21G | |
| 2 | 5 ± 3 | 15 ± 5 | 30 ± 17 |
| 4 | 27 ± 6 | 364 ± 116 | 303 ± 125b |
| 8 | 6 ± 2.5 | 435 ± 150 | 1,390 ± 781b |
| 12 | 5 ± 1 | 117 ± 50 | 1,413 ± 932b |
| 16 | 19 ± 15 | 43 ± 21 | 251 ± 93b |
| 20 | 0.32 ± 0.1 | 47 ± 12 | 183 ± 128b |
| 24 | 0.1 ± 0.07 | 19 ± 6 | 196 ± 110b |
Levels were elevated in all mice that underwent CLP. Similar to the 18G-treated group, the 21G-treated group had substantial IL-6 elevations that were significantly higher than those of the sham treatment group after 4 h.
P < 0.05 for 21G versus sham treatment groups.
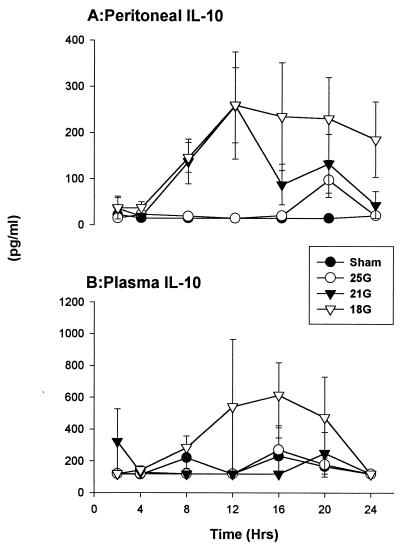
(iv) IL-10.
Elevated levels of IL-10 have been reported in the plasma of patients with sepsis (29). The 21G and 18G treatment groups had increased IL-10 levels within the peritoneum, with peaks at 12 h. Levels in the 18G treatment group were at this range during the study period, while concentrations in the 21G treatment group declined to the levels in the sham treatment group by 24 h (Fig. 7A). Circulating IL-10 concentrations of the 18G treatment group were similar to the IL-1β and IL-6 profiles described above (Fig. 7B). There were no substantial increases in IL-10 levels within the plasma of the sham, 25G, and 21G treatment groups. It is important to note that the IL-10 values are low.
FIG. 7.
IL-10. There were elevated levels of IL-10 in mice of the more severe treatment models locally and systemically. The SEM may be smaller than the symbol. Values are expressed as means ± SEM for 6 to 10 mice.
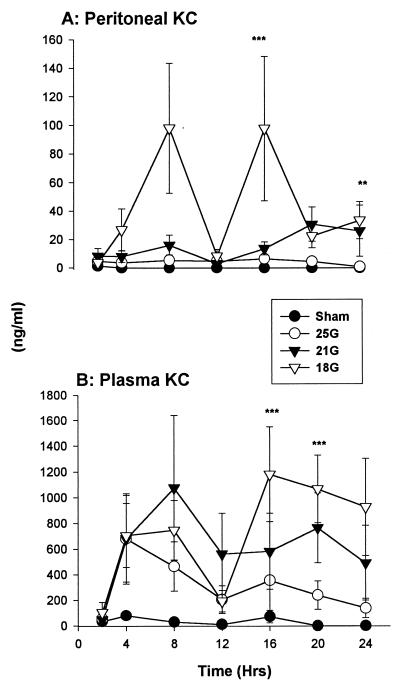
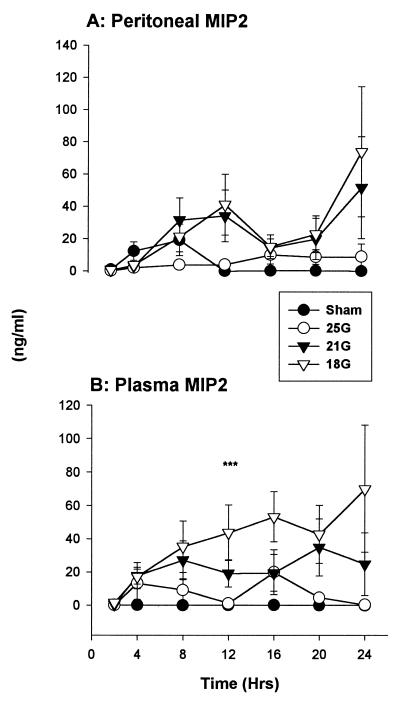
Chemokines.
As in the case of the other mediators, the levels of the murine IL-8 homologues, KC and MIP-2, coincided with needle gauge size. Larger needle sizes, with higher mortality, also coincided with higher chemokine levels. Sham-treated mice did not show significant increases in either KC or MIP-2 over the study period. Furthermore, circulating levels of KC were higher than at the site of inflammation.
(i) KC.
There were high concentrations of KC in the 18G treatment group starting at 2 h post-CLP within the peritoneum (Fig. 8A). Concentrations in all CLP-treated groups were depressed at 12 h, substantially more so in the 18G-treated mice. KC levels in plasma were elevated in all groups that had CLP (Fig. 8B). There were also depressed levels at 12 h in all CLP-treated groups. The rise in circulating KC concentration after 12 h was dependent on needle gauge size, with high levels accompanying larger needle sizes. The sham-treated mice did not exhibit increases in plasma KC. There were significant differences between the KC levels of 18G- and sham-treated groups at 16 and 20 h.
FIG. 8.
KC. (A) There were elevated KC levels within the peritoneum in the 18G-treated CLP model. (B) All mice that underwent CLP had elevations in the circulation regardless of puncture size. KC levels in plasma are substantially higher than those in the peritoneum. Values are expressed as means ± SEM for 6 to 10 mice. ∗∗, P < 0.05 for 21G versus sham treatment groups; ∗∗∗, P < 0.05 for 18G versus sham treatment groups. The SEM may be smaller than the symbol.
(ii) MIP-2.
MIP-2 concentrations were elevated within the peritoneum in the CLP-treated groups (Fig. 9A). MIP-2 levels rose substantially after 20 h in the mice that subsequently died. Both the sham- and 25G-treated groups had lesser amounts of MIP-2 locally. There were increasing concentrations of circulating MIP-2 in the 21G- and 18G-treated groups after 2 h (Fig. 9B). Circulating MIP-2 in the sham-treated mice was barely detectable (<160 pg/ml).
FIG. 9.
MIP-2. Concentrations were higher in mice in the more severe treatment models in both the peritoneum (A) and the plasma (B). Overall, MIP-2 concentrations were lower than KC levels. ∗∗∗, P < 0.05 for 18G versus sham treatment groups (n = 6); ∗∗, P < 0.05 for 21G versus sham treatment groups. The SEM may be smaller than the symbol. Values are expressed as means ± SEM for 6 to 10 mice.
Peritoneal cells.
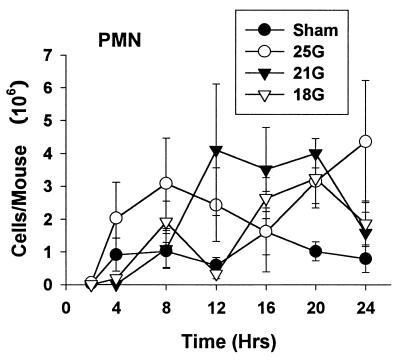
The murine chemokines KC and MIP-2 are known neutrophil chemotactic mediators. An important mechanism of host defense is neutrophil influx into the peritoneal cavity during infection. We monitored cell recruitment into the local site of inflammation, the peritoneum. There were polymorphonuclear leukocyte (PMN) influxes into the peritoneum in all groups (Fig. 10). There were no statistical differences in PMN migrations between any of the treatment groups at any time point.
FIG. 10.
Peritoneal cell counts after CLP or sham surgery. There was neutrophil influx into the local site of inflammation in all mice that underwent CLP. Each value is the mean ± SEM for 6 to 10 mice. The SEM may be smaller than the symbol.
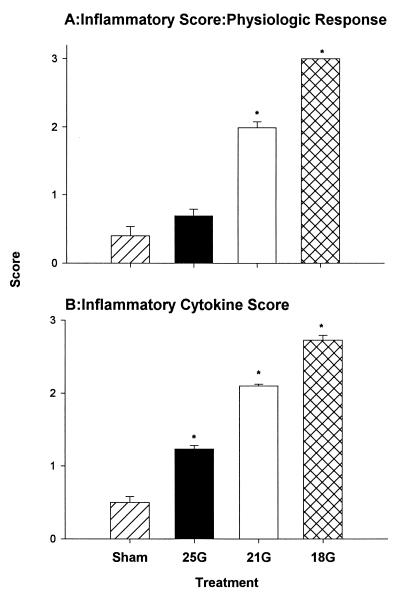
Inflammation scores.
We determined the possible relationship of proinflammatory cytokines with mortality. Figure 11 shows a clear relationship between treatment and severity scores. There were elevated levels of proinflammatory cytokines and decreased physiologic responses with increasing severity. This is in agreement with the clinical study by Casey et al. (11). The physiologic scores for those mice with higher mortality were significantly higher (Fig. 11A). The cytokine inflammatory scores were also significantly higher in mice that had been treated with CLP (Fig. 11B) (P < 0.05).
FIG. 11.
Relation between CLP treatments and inflammatory scores. There is a clear relationship between mortality and severity of sepsis. Scores were obtained from the individual physiologic responses—lethality, temperature, and activity (A) — and from the cytokines (IL-1β, TNF, IL-6) and chemokines (KC and MIP-2) (B). Each bar represents the mean + SEM. ∗, P < 0.05 versus sham treatment group.
DISCUSSION
Although the definition is still controversial in some circles, there is a general agreement that the complexity of the pathogenesis of sepsis is enormous. There is also a need to reexamine sepsis models and to continue to seek a better understanding of the basic immunopathologic alterations in sepsis. This study monitored the in vivo kinetics of cytokines and chemokines as well as physiologic changes in different levels of severity of sepsis by using the CLP model and also allowed us to monitor changes as sepsis progressed.
A pertinent goal of our study was to understand why these mice die. We observed both systemic and local alterations in these models of sepsis induced by CLP, which were directly related to needle gauge size (severity) and outcome (mortality). Cytokines are critical early mediators of sepsis, and the progression into septic shock is highly correlated with increased levels of inflammatory cytokines and chemokines. While the severe CLP model (18G treatment) group generally showed immediate increases in mediators and certain death, the sham, 25G, and, to some extent, the 21G treatment groups exhibited decreased mortality associated with delayed or decreased expression of the inflammatory mediators. TNF has been shown to afford protection during CLP-induced sepsis (18, 20). We have shown low levels of bioactive TNF at the site of infection and no detectable TNF in the plasma of the 21G and 18G treatment groups, suggesting that TNF may not be an important mediator. Elevated levels of local and systemic IL-6, IL-1β, KC, and MIP-2 in the 21G and 18G treatment groups could mean that a beneficial inflammatory response threshold had been breached with responses that are now counterproductive, reflecting important pathophysiologically disturbances and ultimately leading to higher mortality. Alternatively, responses in these animals were not adequate or appropriate, probably because the ability to tightly regulate the production of these mediators has been severely compromised due to the inadequacy of anti-inflammatory cytokines to modulate the response.
It is important to note that our study shows higher concentrations of inflammatory mediators at the local site of inflammation (peritoneum) than systemically. This pattern has been reported in other CLP studies (28) and is similar to clinical observations (26). Since it is not always possible to access the site of inflammation, circulatory cytokine levels are sometimes used to predict prognosis in septic patients. Increasing concentrations of IL-6 have been shown to correlate with the severity of sepsis (16) and clinical complications (7). Our study supports the use of plasma levels as surrogate markers. For instance, the IL-6 levels in the 18G-treated CLP model was significantly higher than those for the sham-treated group. In addition, all the 18G-treated mice died. Hence, IL-6 levels in this severely septic group may be used to predict outcomes (mortality). However, the predictive value of circulatory cytokine levels has its drawbacks. Cytokine levels in plasma tend to be lower than those at the site of inflammation, suggesting that a reduction in bioactive cytokines occurs in the circulation.
There are reports of significant IL-8 elevations in patients with multiple organ failure (24) and with adult respiratory distress syndrome (25). During sepsis, there are marked alterations in the differential count such as a significant increase in the number of neutrophils (42). Since neutrophils play a key role in the elimination of invading microorganisms, this is a physiologic and beneficial host response. Previous reports showed increased MIP-2 levels with increased severity of sepsis (39). We have shown significant increases in two murine IL-8 analogs, KC and MIP-2, particularly in the more severe septic models (21G- and 18G-treated models). However, our study showed no clear-cut relationship between levels of PMN and chemokines. We showed that all groups had neutrophils recruited into the peritoneum and that there were no differences between the numbers recruited by each group. This is similar to clinical reports in which Yamauchi et al. reported that there was no significant difference between the peak number of PMN in patients undergoing clinical major and minor surgeries (42). Since all of the sham-treated mice and 95% of the 25G-treated mice survived, we view the PMN response as adequate and sufficient. On the other hand, all of the 18G- and half of the 21G-treated mice died. It is possible that although present at very high levels, these murine IL-8 analogs are still not sufficient in these CLP models to attract an adequate number of neutrophils to the site of infection and help eliminate invading microorganisms. Also, other factors may influence neutrophil chemoattraction to the site of inflammation. We observed that a substantial number of neutrophils were engorged with bacteria. After actively engaging invading microbes, the PMN exhaust their secretory capacity as well as their internal energy supplies and/or they undergo spontaneous apoptosis. Previous studies have reported this observation in patients with severe sepsis (27). We are currently monitoring an interesting facet to this question by investigating whether these mice died from massive bacterial infection, overwhelming inflammatory response to the infection, or a combination of both.
A possible extension of this study to clinical settings is to apply the information to identify septic patients early, especially where intervention is more likely to make a difference. Furthermore, by monitoring the progression of sepsis, efforts to identify when or where to augment or suppress the effects of these mediators can be directed. In the case of treatment, a valid possibility would be a combination therapy of immunomodulating agents to improve outcome in septic patients.
While most injury scoring systems rely on anatomical injuries and/or vital signs (10, 12), we suggest that localized septic mediators may be useful as additional indicators of the severity of sepsis and tendency toward multiple organ failures.
This study provides some important insights into the complexities of sepsis with respect to inflammatory responses to infections at both the local and systemic levels. Our results shows that inflammatory mediator responses are highly correlated with the severity of sepsis, less with sham and small-gauge cecal puncture, and greater with large-gauge cecal puncture. Furthermore, the responses in the sham and nonlethal septic models are appropriate and may even be protective.
ACKNOWLEDGMENT
This work was supported by NIH grant GM44918.
REFERENCES
- 1.Aarden L A, De Groot E R, Schaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E. Cytokine modifiers: pipe dream or reality? Chest. 1998;113:224S–227S. doi: 10.1378/chest.113.3_supplement.224s. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light R B, Poole L, Allred R, Constant J, Pennington J, Porter S the NORASEPT II Study Group. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 4.Anderson I D, Fearon K C, Grant I S. Laparotomy for abdominal sepsis in the critically ill. Br J Surg. 1996;83:535–539. doi: 10.1002/bjs.1800830434. [DOI] [PubMed] [Google Scholar]
- 5.Arend W P, Welgus H G, Thompson R C, Eisenberg S P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Investig. 1990;85:1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayala A, Evans T A, Chaudry I H. Does hepatocellular injury in sepsis involve apoptosis? J Surg Res. 1998;76:165–173. doi: 10.1006/jsre.1998.5314. [DOI] [PubMed] [Google Scholar]
- 7.Baigrie R J, Lamont P M, Kwiatkowski D, Dallman M J, Morris P J. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757–760. doi: 10.1002/bjs.1800790813. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett J G. Intra-abdominal sepsis. Med Clin N Am. 1995;79:599–617. doi: 10.1016/s0025-7125(16)30059-1. [DOI] [PubMed] [Google Scholar]
- 9.Baue A E. MOF/MODS, SIRS: an update. Shock. 1996;6:S1–S5. [PubMed] [Google Scholar]
- 10.Boyd C R, Tolson M A, Copes W S. Evaluating trauma care: the TRISS method. Trauma Score and the Injury Severity Score. J Trauma. 1987;27:370–378. [PubMed] [Google Scholar]
- 11.Casey L C, Balk R A, Bone R C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Champion H R, Copes W S, Sacco W J, Lawnick M M, Keast S L, Bain L W, Jr, Flanagan M E, Frey C F. The Major Trauma Outcome Study: establishing national norms for trauma care. J Trauma. 1990;30:1356–1365. [PubMed] [Google Scholar]
- 13.Cohen J, Carlet J the International Sepsis Trial Study Group. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Cryer H G. Therapeutic approaches for clinical ischemia and reperfusion injury. Shock. 1997;8:26–32. doi: 10.1097/00024382-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Cunha B A. Antibiotic treatment of sepsis. Med Clin N Am. 1995;79:551–558. doi: 10.1016/s0025-7125(16)30056-6. [DOI] [PubMed] [Google Scholar]
- 16.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebong S J, Call D R, Bolgos G L, Newcomb D E, Granger J I, O'Reilly M, Remick D G. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12:118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Echtenacher B, Falk W, Mannel D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 19.Ertel W, Morrison M H, Wang P, Ba Z F, Ayala A, Chaudry I H. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskandari M K, Bolgos G, Miller C, Nguyen D T, DeForge L E, Remick D G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 21.Eskandari M K, Nguyen D T, Kunkel S L, Remick D G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Investig. 1990;19:69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- 22.Espevik T, Nissen Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 23.Evans G F, Snyder Y M, Butler L D, Zuckerman S H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989;29:279–290. [PubMed] [Google Scholar]
- 24.Hamano K, Gohra H, Noda H, Katoh T, Fujimura Y, Zempo N, Esato K. Increased serum interleukin-8: correlation with poor prognosis in patients with postoperative multiple organ failure. World J Surg. 1998;22:1077–1081. doi: 10.1007/s002689900520. [DOI] [PubMed] [Google Scholar]
- 25.Headley A S, Tolley E, Meduri G U. Infections and the inflammatory response in acute respiratory distress syndrome. Chest. 1997;111:1306–1321. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 26.Holzheimer R G, Schein M, Wittmann D H. Inflammatory response in peritoneal exudate and plasma of patients undergoing planned relaparotomy for severe secondary peritonitis. Arch Surg. 1995;130:1314–1320. doi: 10.1001/archsurg.1995.01430120068010. [DOI] [PubMed] [Google Scholar]
- 27.Keel M, Ungethum U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–3363. [PubMed] [Google Scholar]
- 28.Lundblad R, Sandven P, Giercksky K E. The physical nature of a large bowel perforation predicts severity of the subsequent inflammatory response. Shock. 1995;3:455–461. [PubMed] [Google Scholar]
- 29.Marchant A, Deviere J, Byl B, De Groote D, Vincent J L, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343:707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- 30.Newcomb D, Bolgos G, Green L, Remick D G. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Opal S M, Fisher C J, Jr, Dhainaut J F, Vincent J L, Brase R, Lowry S F, Sadoff J C, Slotman G J, Levy H, Balk R A, Shelly M P, Pribble J P, LaBrecque J F, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M the Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Parillo J E. The cardiovascular pathophysiology of sepsis. Annu Rev Med. 1989;40:469–485. doi: 10.1146/annurev.me.40.020189.002345. [DOI] [PubMed] [Google Scholar]
- 33.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Remick D G, Garg S J, Newcomb D E, Wollenberg G, Huie T K, Bolgos G L. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med. 1998;26:895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 35.Salkowski C A, Detore G, Franks A, Falk M C, Vogel S N. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 37.van der Poll T, Marchant A, Buurman W A, Berman L, Keogh C V, Lazarus D D, Nguyen L, Goldman M, Moldawer L L, Lowry S F. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- 38.Villa P, Sartor G, Angelini M, Sironi M, Conni M, Gnocchi P, Isetta A M, Grau G, Buurman W, van Tits L J. Pattern of cytokines and pharmacomodulation in sepsis induced by cecal ligation and puncture compared with that induced by endotoxin. Clin Diagn Lab Immunol. 1995;2:549–553. doi: 10.1128/cdli.2.5.549-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenzel R P. Anti-endotoxin monoclonal antibodies—a second look N. Engl J Med. 1992;326:1151–1153. doi: 10.1056/NEJM199204233261710. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 41.Wichterman K A, Baue A E, Chaudry I H. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi H, Kobayashi E, Yoshida T, Kiyozaki H, Hozumi Y, Kohiyama R, Suminaga Y, Sakurabayashi I, Fujimura A, Miyata M. Changes in immune-endocrine response after surgery. Cytokine. 1998;10:549–554. doi: 10.1006/cyto.1997.0322. [DOI] [PubMed] [Google Scholar]