Abstract
Nicotine has a unique profile among drugs of abuse. To the non-initiated user, nicotine has powerful aversive effects, while its relatively weak euphorigenic effects undergo rapid tolerance. Despite this, nicotine is heavily abused despite negative heath consequences and nicotine users have enormous difficulty quitting. Further, nicotine is one of the most commonly co-abused substances, in that it is often taken in combination with other drugs, in particular alcohol. One explanation of this polydrug use involving nicotine is that it has multiple appetitive and consummatory conditioning effects. For example, nicotine is a reinforcement enhancer in that it can potently increase the incentive value of other stimuli, including those surrounding drugs of abuse such as alcohol. In addition, nicotine also has a unique profile of neurobiological effects that alter regulation of alcohol intake and interoceptive status. This review discusses the psychological and biological mechanisms surrounding nicotine’s appetitive conditioning and consummatory effects, particularly its interactions with alcohol.
Keywords: Nicotine, Alcohol, Pavlovian Conditioned Approach, Reinforcement Enhancement, Incentive Salience, Operant Conditioning, Mesolimbic Dopamine, Ventral Tegmental Area, Nucleus Accumbens, Insular Cortex
INTRODUCTION
Nicotine is frequently taken in combination with other drugs, especially alcohol. Nicotine and alcohol use co-morbidity has been well established among human users, (Weinberger, Funk, & Goodwin, 2016), with tobacco usage estimates as high as 90% among alcoholics (Batel, Pessione, Maitre, & Rueff, 1995; Burling & Ziff, 1988). Intensity of use between tobacco and alcohol are positively correlated (Istvan & Matarazzo, 1984), resulting in higher levels of individual intake. Further, attempts to quit smoking are more successful in individuals who are non-alcoholic, with at success rates as low as 7% of alcoholic smokers being able to successfully terminate use (DiFranza & Guerrera, 1990). In order to better characterize the relationship between alcohol and tobacco co-use, some lines of evidence have examined intake patterns during drinking episodes. For example, ecological studies have observed that smoking increases during episodes of drinking (Harrison, Hinson, & McKee, 2009). Subjective pleasure for drinking and smoking are positively correlated and are highest during peak intoxication (Piasecki et al., 2011; Piasecki, Wood, Shiffman, Sher, & Heath, 2012), suggesting that the intake of one substance may promote the use of the other when the two are concurrently available. However, tobacco and alcohol co-use research is currently in need of both human and animal research that better model the clinical condition, as well as how the two drugs interact (Van Skike et al., 2016).
Understanding nicotine’s psychological and behavioral effects are necessary to explain the underpinnings of pathological polydrug use. First, although the direct effects of nicotine on the central nervous system certainly contribute to the maintenance of tobacco use, the psychological process underlying nicotine’s appetitive effects are equally important, both in terms of nicotine’s mediation of tobacco use and the enhancement drug use from other drug classes. For example, the sensorimotor stimuli (“cues”) associated with smoking are important for the maintenance of tobacco use because of the conditioned effects resulting from thousands of nicotine pairings with orosensory tobacco cues (Rose, Behm, & Levin, 1993; Rose, Behm, Westman, & Johnson, 2000; Rose & Levin, 1991). The motivational response to these cues that surround tobacco use are thought to be amplified by nicotine via “reinforcement enhancement” or “incentive amplification” (Bevins & Palmatier, 2004; X. Liu, Palmatier, Caggiula, Donny, & Sved, 2007; Palmatier et al., 2007), and in humans this reinforcement enhancement can extend to enhance the response other drug and non-drug rewards (Perkins & Karelitz, 2013; Perkins, Karelitz, & Boldry, 2017, 2019). As we will discuss in this chapter, in the case of alcohol, nicotine can alter the response to alcohol-paired cues and contexts, in addition to altering alcohol’s reinforcing properties directly. These conditioning effects of nicotine first result in initiation of approach behaviors towards drug and non-drug cues and elicit conditioned motivational states, which can subsequently maintain both tobacco and alcohol use.
Second, intake of one substance appears to be directly linked to the other. Experimental studies in humans have sought to establish causal links using consummatory measures of intake and concepts of reward during tobacco and alcohol use. Under experimenter-controlled conditions, alcohol administration increases both the urge to smoke and subsequent smoking behavior (Epstein, Sher, Young, & King, 2007; A. C. King & Epstein, 2005; Mitchell, de Wit, & Zacny, 1995; Rose et al., 2004), suggesting alcohol potentiates both tobacco-directed appetitive and consummatory behaviors. The reciprocal has also been demonstrated under laboratory conditions, where subjects who smoked nicotinized cigarettes report increased alcohol-induced pleasure derived relative to those who smoked de-nicotinized cigarettes (Barrett, Tichauer, Leyton, & Pihl, 2006; E. M. Kouri, McCarthy, Faust, & Lukas, 2004). Thus, alcohol and tobacco are both taken together, show positively correlated usage, and appear to increase the craving and intake of each other bidirectionally (for review see Verplaetse & McKee, 2017).
This review will discuss two major domains of nicotine’s effects on drug-taking, with special attention to its interactions with alcohol. Here, we separate these domains into being defined as affecting either the 1) appetitive (conditioning, seeking, motivational, and approach) or 2) consummatory (intake and reward) components of alcohol-directed behavior. In this regard, preclinical animal models are widely employed given their utility in characterizing precise neurobiological processes, drug-drug interactions, and long-term changes that are involved across nicotine’s various actions (Koob, Kenneth Lloyd, & Mason, 2009).
APPETITIVE EFFECTS OF NICOTINE
Although smoked tobacco has been referred to as one of the most addictive and harmful drugs (Nutt, King, Saulsbury, & Blakemore, 2007), the evidence indicating that nicotine is reinforcing in the absence of tobacco smoke or other stimuli is scant and debated, (Dar & Frenk, 2004; Henningfield & Goldberg, 1983; Henningfield, Miyasato, & Jasinski, 1983; Perkins, Grobe, Caggiula, Wilson, & Stiller, 1997) (Perkins, 2004; Perkins et al., 1997; Rose, Behm, Westman, Bates, & Salley, 2003; Rose & Corrigall, 1997). Notably, studies demonstrating intravenous nicotine self-administration in human smokers have required the presence of auditory, visual, or olfactory smoke cues coincident with nicotine infusion (Harvey et al., 2004; Rose, Behm, Westman, & Bates, 2003). As noted throughout this chapter, these external cues are critical for nicotine’s reinforcing properties.
Cues influence behavior by acquiring incentive value
Reward-associated stimuli (“cues”) strongly influence may forms of motivated behavior. In most situations, cues are inherent to the rewards they are associated with (e.g., the smell of a freshly baked brownie, the taste of alcohol). However, in modern environments, and in laboratory preparations, cues can be spatially and temporally separated from their rewards (e.g., a neon alcohol advertisement, a deck of cards at a casino, an empty cigarette box). In these examples, cues have properties that fall into two categories: they predict the availability of rewards, and they can incentivize several behavioral responses. What is the incentive value of a cue and how is it determined? Generally, a cue that has acquired incentive value promotes some form of motivated response. Specifically, in the laboratory, cues can be said to have acquired incentive value if 1) it becomes attractive and individuals will approach it (e.g., during a Pavlovian conditioned approach paradigm) 2) individual will work for it (e.g., during a conditioned reinforcement test), or 3) it energizes an ongoing instrumental action (e.g., during drug self-administration or Pavlovian to instrumental transfer Milton & Everitt, 2010; Robinson, Yager, Cogan, & Saunders, 2014). In using these measures, several laboratories have demonstrated that the incentive value of cues are experimentally dissociable from just its predictive value (Robinson & Flagel, 2009; Robinson et al., 2014) and subserved by different neurobiological mechanisms (Flagel et al., 2011; Flagel, Watson, Robinson, & Akil, 2007; Saunders & Robinson, 2012). Further, there is substantial individual variability in the attribution of incentive value to reward cues (Meyer, Lovic, et al., 2012), which may be a vulnerability trait for addiction-related traits (Meyer, Ma, & Robinson, 2012; Saunders & Robinson, 2010, 2011).
Nicotine imbues cues with incentive value via multiple mechanisms
Evidence from Pavlovian paradigms
Nicotine increases the incentive value of cues by each of these three measures, albeit via different biopsychological mechanisms. For example, our laboratory has shown that nicotine-paired cues are attractive in a Pavlovian paradigm (Fig. 1). To demonstrate this, rats were equipped with an intravenous (i.v.) catheter and were then presented with a cue (an 8-second presentation of a lever), that predicted the subsequent delivery of 0.03 mg/kg intravenous nicotine infusions (Fig. 1A). These rats learned to approach the lever cue, compared to rats that received unpaired cue/nicotine presentations (Fig. 1B). This demonstration of approach behavior shows that nicotine can imbue its associated cues with incentive value through Pavlovian mechanisms. However, paired rats did not show differences from unpaired rats when working for the cue itself via conditioned reinforcement (Fig. 1C), although a similar study demonstrated that a cue paired with nicotine did acquire this additional reinforcing value (Yager & Robinson, 2015). Thus, within strictly associative Pavlovian conditioning paradigms, nicotine-paired cues can acquire incentive value. As discussed later, the incentive value acquired through these associative processes also plays an important role in nicotine self-administration in animal models as well as smoking behavior in humans.
Figure 1: A nicotine-predictive cue elicits approach in a Pavlovian conditioning paradigm.
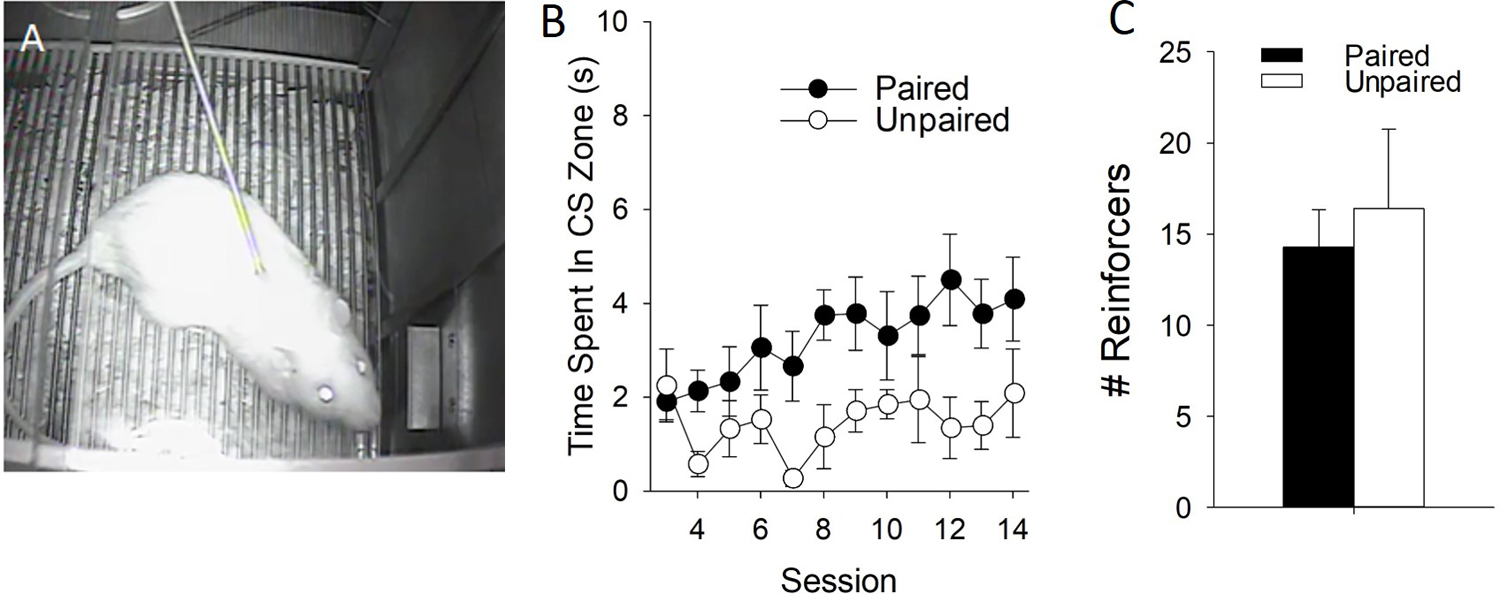
A) Rats were equipped with an intravenous catheter and trained to associate an 8-s presentation of a retractable lever with a 0.03 mg/kg infusion of nicotine over 8 trials on a VI-15m schedule for 14 days. B) Rats approach the nicotine-paired cue to a greater degree than animals presented with the lever and nicotine in an unpaired fashion. C) On a subsequent test for conditioned reinforcement, rats nose-poked for 3-s presentations of the conditioned stimulus in the absence of nicotine during a 40-min test, but there were no differences in paired and unpaired groups.
Evidence from operant paradigms
The incentive properties of cues are particularly important in the study of addiction because they strongly enhance the maintenance of drug-taking and relapse into drug addiction. For example, in a seminal study by Caggiula (Caggiula et al., 2001), rats were trained to press a lever at high rates to obtain i.v. infusions of nicotine that were paired with visual cues. When the cues were removed, the number of acquired nicotine infusions was immediately and substantially reduced, despite nicotine still being freely available. Reintroduction of the cues immediately restored responding for nicotine. Nicotine cues are therefore critical for motivating drug-taking behavior during ongoing nicotine self-administration and is another example of the cues controlling behavior via their incentive properties. Similarly, nicotine-associated cues enhance nicotine-directed behavior by increasing the motivation to obtain the drug (Chaudhri et al., 2007), slow the extinction of operant responding for nicotine when nicotine is no longer available, and reinstate nicotine seeking after extinction (Feltenstein, Ghee, & See, 2012; LeSage, Burroughs, Dufek, Keyler, & Pentel, 2004; Versaggi, King, & Meyer, 2016).
In a series of follow-up studies on Caggiula’s initial study, Palmatier et al. demonstrated that cues need not be directly associated with nicotine to produce these effects. In a particularly elegant experiment (Palmatier et al., 2006), four groups of rats were trained to respond for nicotine and/or a visual cue. One group lever-pressed for a visual stimulus (VS), one group pressed only for 0.03 mg/kg nicotine, and a third group pressed for VS-nicotine combination on a single lever. Crucially, the fourth group could concurrently press for the VS and nicotine on separate levers. The nicotine-only group responded the least, with responding lower than the VS-only group. The VS/nicotine single lever group responded at high levels, as had been shown many times. However, the separate nicotine-VS lever group responded for nicotine at similarly low levels as the nicotine group, but responded at high levels for the VS, in fact as high as the VS/nicotine single-lever group. This demonstrates a crucial finding: nicotine self-administration is primarily driven not by its own reinforcing effects, but by its ability to enhance the incentive value of other reinforcers. Subsequent studies further characterized this phenomenon, having very important implications regarding nicotine’s effects on cues 1) nicotine enhances the reinforcing value of a stimulus even when nicotine is given non-contingently at the beginning of the session (Chaudhri, Caggiula, Donny, Booth, et al., 2006; Chaudhri et al., 2007; Chaudhri, Caggiula, Donny, Palmatier, et al., 2006; X. Liu et al., 2007; Palmatier et al., 2007; Perkins et al., 2019; Versaggi et al., 2016) 2) the incentive amplification induced by nicotine depends on the initial reinforcing value of the stimulus (Palmatier et al., 2007), and 3) nicotine can enhance the incentive value of stimuli in Pavlovian and operant paradigms (Palmatier et al., 2013; Stringfield, Boettiger, & Robinson, 2018; Versaggi et al., 2016). Thus, nicotine enhances the incentive and reinforcing properties of stimuli across a variety of reward conditioning protocols, consequently imbuing those stimuli with the enhanced capacity to instigate motivated behavior or maintain responding.
In humans, nicotine-paired cues clearly instigate motivated behavior as well. Nicotine cues can induce nicotine craving (Carter & Tiffany, 1999), evoke conditioned physiological responses (Carter & Tiffany, 1999; Erblich, Bovbjerg, & Sloan, 2011; Winkler et al., 2011) maintain smoking behavior independently of nicotine intake (Donny, Houtsmuller, & Stitzer, 2007), and are necessary for the subjective positive experiences induced by nicotine (Alia-Klein et al., 2007; Rose et al., 2000). Thus, smoking, nicotine self-administration, and other smoking-associated behaviors are principally controlled by nicotine’s interactions with reward-associated cues. This incentive amplification may explain why the initiation and maintenance of smoking behavior is often highest in the presence of other rewarding situations, such as social interaction (Nguyen & Zhu, 2009). However, the isolation of the nicotine’s incentive amplifying effects have been only measured directly in a few studies, with mixed results (Addicott, Oliver, & Joseph McClernon, 2017; Barr, Pizzagalli, Culhane, Goff, & Evins, 2008; Perkins, Grottenthaler, & Wilson, 2009; Wignall & de Wit, 2011). Specifically, one study (Barr et al., 2008); found that nicotine, delivered via transdermal patch to non-smokers, biased responding toward reward stimuli, while another (Perkins et al., 2009), found that intranasal nicotine or smoking had no effect on an operant task in which non-dependent smokers responded for various non-drug rewards on an escalating fixed-ratio (FR) (FR11, FR12, etc.) schedule of responding. However, expended effort is only one measure of a reward’s incentive value, and as described above, nicotine may amplify the incentive properties of other reward (including alcohol) through multiple mechanisms (Robinson et al., 2014).
Nicotine amplifies motivation for alcohol by interacting with alcohol-associated cues.
As described above, nicotine’s conditioned effects are not limited to nicotine cues, because nicotine similarly increases responding for cues associated with other reinforcers (Caggiula et al., 2009), including alcohol (Le, Wang, Harding, Juzytsch, & Shaham, 2003b). Thus, one mechanism for the comorbidity of alcohol and nicotine use (Weinberger et al., 2016; Weinberger, Platt, Jiang, & Goodwin, 2015) is that nicotine enhances the reinforcing properties of alcohol-associated cues separately from the pharmacological interactions between these drugs. Consistent with this, rodent studies show that nicotine produces a high-approach, low-avoidance alcohol phenotype in response to alcohol associated cues. For example, nicotine enhances the approach response evoked by an alcohol-predictive cue in Pavlovian paradigms (H. Angelyn, Loney, & Meyer, in press; Loney, Angelyn, Cleary, & Meyer, 2019; Maddux & Chaudhri, 2017; Srey, Maddux, & Chaudhri, 2015). We have shown nicotine enhanced the response to a cue that predicted access to a retractable sipper bottle containing ethanol, without affecting the response to a similar, non-predictive stimulus. In other experiments, nicotine reduced the conditioned taste (but not place) aversion induced by ethanol (Loney et al., 2019; Loney, Pautassi, Kapadia, & Meyer, 2018), indicating that nicotine alters the motivational effects of alcohol-associated cues in some, but not all, Pavlovian paradigms.
Alcohol self-administration models assess the effects of motivation and intake
Alcohol self-administration involves both the preparatory responding and motivation to gain access to alcohol, as well as the subsequent consumption of alcohol following its delivery. In our laboratory, we use a procedure in which the appetitive and consummatory phases of ethanol self-administration can be dissociated [Fig. 2 and 3; see also Schier, Dilly, and Gonzales (2013); Simms, Bito-Onon, Chatterjee, and Bartlett (2010)]. First, rats are given 24-hour access to 20% ethanol or water, three times a week for four weeks. This produces escalation in ethanol intake (Fig. 2A). Then, rats make responses that are reinforced with a 30-second presentation of a bottle containing 20% ethanol (Fig. 2B). A cue-light is illuminated with the bottle presentation. Using contact lickometers, we can dissociate the appetitive (nose-poking or lever-pressing) and consummatory (licking and consumption) aspects of ethanol drinking (Fig 2C). In addition, the motivational properties of ethanol reinforcement can be assessed using a progressive ratio (PR) test, in which work requirement to access alcohol sipper becomes increasingly difficult. The PR test is used as a standard measure of ethanol reinforcement with minimal influence of post-ingestive factors. A shown in Fig. 2, the alcohol exposure provided in the home cage produces increases in both the appetitive (Fig. 2D) and consummatory measures (Fig. 2C) of alcohol-directed behavior. Using this design, it is therefore possible to specifically observe nicotine’s effects on appetitive alcohol-directed behavior, separately from its effects on regulating intake.
Figure 2: Home-cage ethanol exposure promotes operant ethanol self-administration.
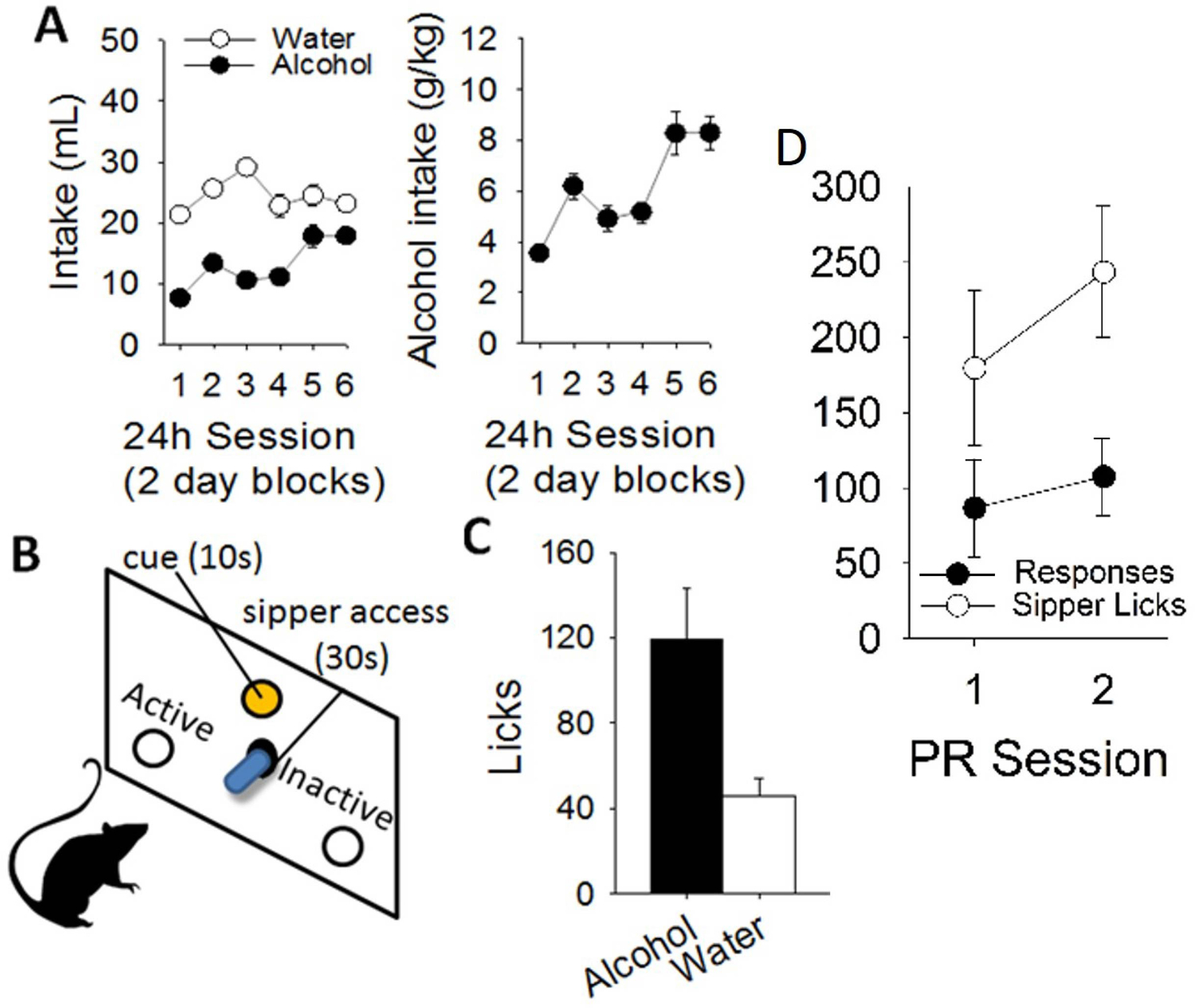
A) 20% alcohol v/v is given every other day in the home-cage for 4 weeks, resulting in escalated intake and increased preference. Then, B) rats are trained to operantly respond for alcohol by nosepoking for 30s presentations of an alcohol sipper. During self-administration, consummatory behavior is measured using contact lickometers to measure C) alcohol intake, and also D) appetitive nosepoking behavior during tests such as progressive ratio.
Figure 3: Nicotine enhanced operant responding for alcohol.
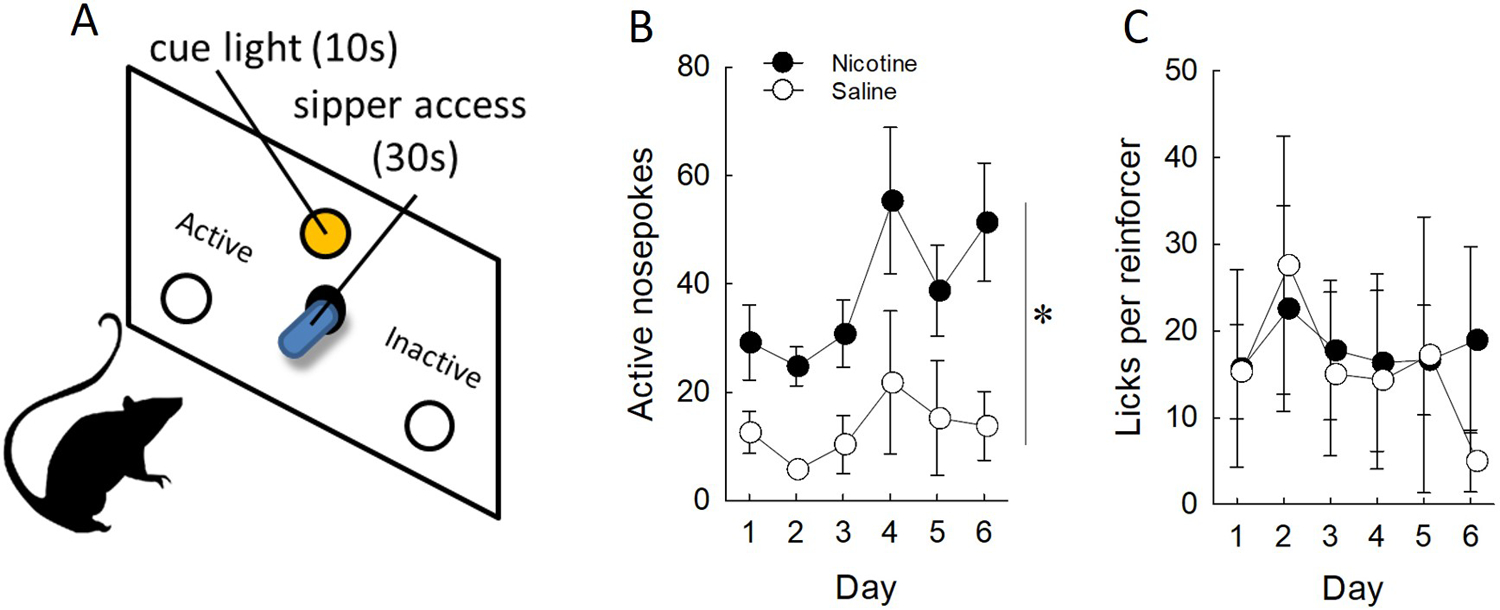
A) Rats were tested for both alcohol-directed responding and intake in daily 1-hour sessions under saline or 0.4 mg/kg injections of nicotine. B) Nicotine enhances responding for alcohol in the presence of cues relative to saline treated rats, however C) this did not result in heightened intake.
Nicotine enhances responding for alcohol in the presence of cues
Being able to distinguish between the appetitive and consummatory consequences of nicotine is important, particularly in order to dissociate the mechanisms by which nicotine modifies behavior. Factors regulating intake will be discussed in detail below, however generally speaking, nicotine reduces alcohol intake when it is given acutely, immediately prior to testing (Le, Corrigall, Harding, Juzytsch, & Li, 2000; Sharpe & Samson, 2002). In addition, chronic nicotine also increases responding for alcohol in the presence of cues (Le et al., 2003b), even when delivered continuously over a 24-hour period via osmotic pump (Clark, Lindgren, Brooks, Watson, & Little, 2001a). Using our method of alcohol self-administration to identify appetitive-specific effects of nicotine (Fig. 3A), we have found that nicotine enhances responding for presentations of an alcohol sipper (Fig. 3B) in the presence of alcohol-paired cues without affecting subsequent intake (Fig. 3C). These data fit with the general body of work suggesting nicotine can enhance responding for other reward-paired stimuli, crucially those paired with alcohol. These alcohol cues presented alongside the alcohol reinforcement can acquire incentive value, as measured by their ability to cause the reinstatement of an extinguished response (Lamparelli & Meyer, 2018). Nicotine enhances this effect and may be one reason why alcohol abstinence is hard to maintain in smokers (C. L. Smith et al., 2020).
Occasions setters and discriminative stimuli
In the experimental designs described in this chapter, cue presentation was contingent on the rats’ response. However, cues can be presented independent of the subject’s response, for example acting as “occasion-setters” to signal drug-availability or acting as a discriminative stimulus (Sd). For example, nicotine can serve as a Sd to occasion motivated responding (Randall, Cannady, & Besheer, 2016; Troisi, Dooley, & Craig; Wooters, Bevins, & Bardo, 2009). In an example using rats, we tested the effect of nicotine injections in a discriminative stimulus (Sd)-modulated alcohol self-administration and reinstatement procedure. Here, rats nose-poked for presentations of an alcohol bottle in the presence of an “alcohol-available” Sd (illumination of a houselight; Fig. 4A). We found that this Sd effectively modulated alcohol self-administration compared to a separate alcohol “non-available” DS (Sdelta), and crucially nicotine specifically enhanced appetitive responding only in the presence of the Sd during self-administration (Fig. 4B). Further, the enhancement was also observed during a reinstatement test when the alcohol bottle and its associated cues were removed (H Angelyn & Meyer, 2019). Since these cues were not response contingent, the term “reinforcement enhancement” in reference to nicotine’s effects is insufficient. Instead, because nicotine’s immediate effects on regulating appetitive behavior through cues therefore also extend to other salient stimuli that signal reward availability such as occasion setters and discriminative stimuli, nicotine is better described as acting more generally as an amplifier of the incentive value of cues Bevins and Palmatier (2004), including alcohol cues. Further this incentive amplification can apply to complex contextual stimuli, which have powerful control over not only nicotine seeking (Conklin, 2006; McClernon et al., 2016), but alcohol seeking as well (Zipori et al., 2017).
Figure 4: Nicotine enhances responding for alcohol in the presence of a discriminative stimulus signaling alcohol availability.
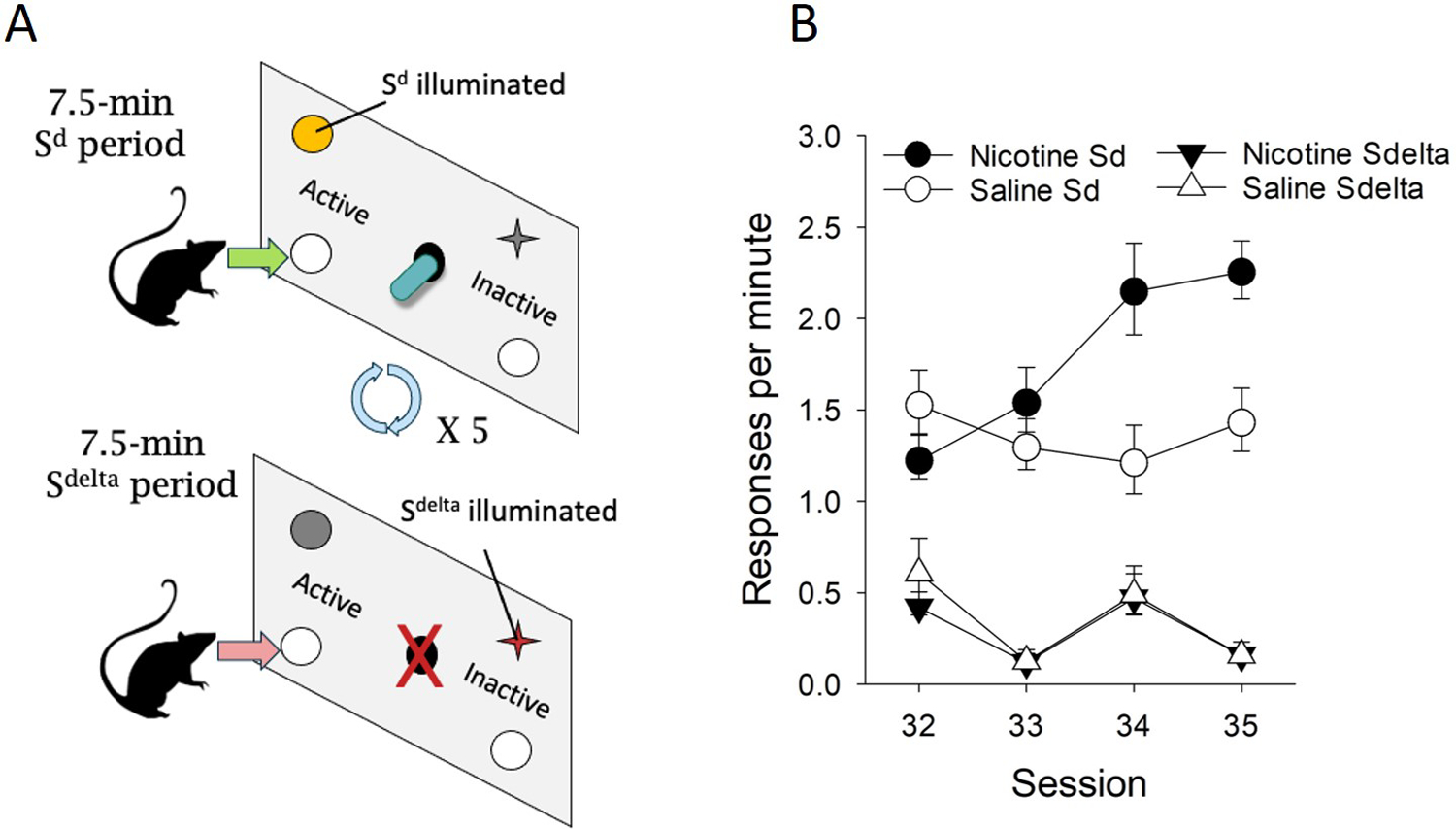
A) Rats were trained to respond for alcohol in 7.5 min “alcohol-available” (Sd) periods, where active nose-pokes resulted in presentation of an alcohol sipper solution. Each 7.5 min Sd period was followed by a 7.5 min “alcohol non-available” period signaled by a separate “Sdelta” stimulus. B) Rats specifically responded for alcohol during the Sd period, and Sd-induced responding specifically was enhanced by nicotine.
Collectively, these findings indicate that nicotine promotes appetitive alcohol responding by amplifying the incentive value of alcohol-associated cues. This is consistent with Cagiulla’s ideas regarding nicotine’s effects on reinforcement (Caggiula et al., 2009), with the modification that nicotine amplifies the incentive value of cues generally, whether they are response-contingent or not. However, one aspect of alcohol and nicotine co-use that may be unique to the combination of these drugs is the effect of nicotine on alcohol taste cues. While oral cues can instigate alcohol seeking (Knight et al., 2016), higher doses of alcohol can condition an aversion to alcohol-associated tastes (e.g., Anderson, Varlinskaya, & Spear, 2010). Nicotine interferes with this conditioned taste aversion to alcohol-associated flavors (Loney & Meyer, 2019; Loney et al., 2018) (Bienkowski, Piasecki, Koros, Stefanski, & Kostowski, 1998; Kunin, Smith, & Amit, 1999; Loney et al., 2019; Rinker et al., 2011), which is likely due to an alteration of the interoceptive properties of alcohol by nicotine (discussed more later), and not to a general effect of nicotine on learning, because nicotine does not alter a taste aversion induced by the nausea-producing drug lithium chloride. In this manner, in addition to increasing the incentive value of environmental alcohol cues, nicotine may also facilitate alcohol seeking by altering the aversive interoceptive effects of higher doses of alcohol.
CONSUMMATORY EFFECTS OF NICOTINE
Thus far we have discussed the appetitive effects of nicotine, crucially through increasing the salience of drug paired cues and instigating seeking and approach behavior and enhancing reinforcement for alcohol in the presence of cues (Le, Wang, Harding, Juzytsch, & Shaham, 2003a). During ongoing drug self-administration, in addition to the presence of discrete alcohol and nicotine cues, drug-directed behavior is influenced by lifetime experience with both substances, as well as the individual’s own ability to titrate their alcohol and nicotine intake. As discussed, nicotine’s profile of effects envelopes both appetitive and consummatory processes. including nicotine’s enhancement of responding for alcohol during self-administration and reinstatement (Le et al., 2003a), its promotion of intake in a time-dependent manner (Le et al., 2000), and its effects when given chronically at higher doses (Bito-Onon, Simms, Chatterjee, Holgate, & Bartlett, 2011). However, the relationship between intake and responding is not always straightforward. In one study using rats (Le et al., 2010), alcohol and nicotine were made available together in one group, while other groups self-administered only one drug. Alcohol self-administration occurred at the expense of nicotine, but later the extinction of responding for alcohol was delayed in rats that had nicotine concurrently available, indicating separable intake and operate response consequences of nicotine. Priming with either nicotine or alcohol during a subsequent reinstatement test increased responding for both drugs. Thus, this study showed that self-administered nicotine, although itself decreased by alcohol, increased responding for self-administered alcohol. Thus, segregation of the appetitive and consequential intake effects of nicotine is crucial to fully understand the interaction between drugs.
Several studies have used responding for alcohol and intake interchangeably as endpoints for nicotine’s effects on alcohol-directed behavior. Importantly however, the effects of nicotine are highly dependent on when it is given, for how long, and the exact behaviors being measured and when. Our studies show that although nicotine increases alcohol’s appetitive effects in standard self-administration paradigms, it either does not change or reduces alcohol intake acutely. Instead, nicotine-induced changes related to the ingestive, consummatory aspects of alcohol and are likely due to nicotine’s chronic effects, which is discussed in this section.
Nicotine’s temporal factors regulating alcohol intake
Acute effects on intake
Alcohol intake involves the reinforcing properties of alcohol during and following drinking, as well as processes involving of fluid intake and regulation (Samson, Slawecki, Sharpe, & Chappell, 1998). Naturally, given its high face validity, quantified alcohol intake is the one of the most frequently reported methods for operationalizing alcohol-directed behavior. However, the timing, dosing, and experience with nicotine may differentially alter alcohol intake, and thus with the wealth of methods to measure alcohol-directed behavior under nicotine, each measure and experimental design may target different psychological processes.
Perhaps the simplest way to model the interaction between nicotine and alcohol in rodents is to look at how consumption of one substance changes while under the influence of the other. The simplicity of this design is likely one of the reasons why this approach has historically been one of the most widely employed. For example, a number of early studies from Samson and colleagues have demonstrated that alcohol intake in rats is acutely suppressed when measured following subcutaneous injections of nicotine (Hendrickson, Zhao-Shea, Pang, Gardner, & Tapper, 2010; Nadal & Samson, 1999; Sharpe & Samson, 2002). Nicotine is anorexigenic (Jo, Talmage, & Role, 2002), which likely suppresses alcohol intake during early phases of nicotine exposure. Home-cage alcohol intake is suppressed specifically via nicotinic acetylcholine receptors (Dyr, Koros, Bienkowski, & Kostowski, 1999) including the α4 subtype (Hendrickson et al., 2010). Importantly, these studies occurred in the absence of a dedicated testing environment and alcohol-paired CSs, thus more directly reflecting the consummatory phase of alcohol intake.
Protracted effects on intake
In humans, experience and history with nicotine undoubtably contributes to the longer term behavioral and neurobiological changes, with age of smoking onset predicting alcohol use problems later (Chen et al., 2002; Grant, 1998; Jensen et al., 2003). Thus, in the laboratory, intake-specific effects of nicotine are similarly influence by an individual’s history and timing of exposure, particularly when experienced outside the testing environment. For example, alcohol-experienced rats show a suppression of intake when nicotine is given immediately prior to a relapse to alcohol intake test, but instead showed heightened intake when nicotine is was given during a withdrawal period on the days prior (Alen, Gomez, Gonzalez-Cuevas, Navarro, & Lopez-Moreno, 2009; William M. Doyon et al., 2013). Indeed, other designs have similarly demonstrated nicotine can later enhance alcohol intake when it is first given in the absence of alcohol (Hauser, Getachew, et al., 2012; Lopez-Moreno et al., 2004), and during adolescence (Kemppainen, Hyytia, & Kiianmaa, 2009). Importantly, these data suggest that longer-term intake-specific effects may be divorced from the other more immediate effects, where intake is heightened when it is temporally separated from the drinking event (Alen et al., 2009). Thus, procedures that generally employ longer nicotine exposure periods across days better facilitate alcohol intake in the home cage (Blomqvist, Ericson, Johnson, Engel, & Soderpalm, 1996; Olausson, Ericson, Lof, Engel, & Soderpalm, 2001; B. R. Smith, Horan, Gaskin, & Amit, 1999). Taken together, it is likely that the intake specific processes surrounding nicotine are a result longer term exposure periods, which eventually come to interact with the various appetitive and conditioning properties in various drug-taking settings. Indeed, nicotine delivered continuously by osmotic minipump can also promote the acquisition and maintenance of alcohol self-administration (Clark, Lindgren, Brooks, Watson, & Little, 2001b), perhaps better reflecting the chronic nature of tobacco use.
Behavioral models of co-use
A brief summary of these data suggests that, at least acutely, experimenter-delivered nicotine has suppressive effects on both alcohol intake and seeking when it is given in close temporal proximity but can enhance it when given chronically or separately from the testing session. Despite the great utility of studies that utilize non-contingent injections, controlled site-specific infusions, and other highly experimentally controlled methods, individuals can titrate their own tobacco and alcohol use under normal conditions. It stands to reason rodent studies employing dual self-administration protocols may model key components of voluntary human usage, although very few groups have tried this. Marshall and colleagues originally reported that, when nicotine and alcohol solutions were presented together, there were no changes in consumption relative to when they were presented alone (Marshall, Dadmarz, Hofford, Gottheil, & Vogel, 2003). Other attempts to model co-use using operant conditioning instead of free intake were later attempted, but either nicotine self-administration decreased in the presence of alcohol (Le et al., 2010), or the concurrent availability of both decreased the intake of both (Funk, Lo, Coen, & Le, 2016).
It is likely that the specific parameters surrounding drug-availability, timing, training, and presence of cues are important for these models. Other variations to this procedure have been deployed, but with mixed results. Orally self-administering rats demonstrate preference for an alcohol only solution over alcohol and nicotine (Hauser, Katner, et al., 2012), but show heightened intake for a cocktail of intravenous nicotine and alcohol over either by itself (Karatayev et al., 2015), likely reflecting factors surrounding relative aversion and reward due to route of administration. As discussed previously, nicotine has been shown to enhance intake when taken sufficiently in advance of alcohol, and in one instance during daily alternating access to alcohol and nicotine, nicotine enhanced alcohol intake when nicotine was taken earlier in the day (Le, Funk, Lo, & Coen, 2014), suggesting that that timing of drug-taking is important both under experimenter controlled and voluntary conditions.
Because nicotine enhances appetitive responding for alcohol in the presence of cues (see Fig. 3), to assess the immediate effect of nicotine availability on free-alcohol consumption in the absence of cues, we used a procedure in which rats were allowed to freely self-administer nicotine in the presence of an alcohol sipper (Fig. 5) that required no appetitive responding. In contrast to Le and collegues, we found that i.v. nicotine was readily self-administered (Fig. 5A), but at the expense of alcohol (Fig. 5B), crucially in rats that had an established history of home cage drinking (adapted from: (Simms et al., 2010; Simms et al., 2008)). Upon nicotine removal, responding for nicotine rapidly decreased (Fig. 5C), and alcohol consumption increased to saline levels (Fig. 5D). Recently, Chandler and colleagues have sought to try and optimize i.v. nicotine and alcohol intakes under co-use conditions, with the primary goal of evaluating pharmacological manipulations (naltrexone and varenicline) directed at reducing intake of one or both substances. Specifically, they demonstrated that alcohol consumption could escalate under nicotine, but specifically only when the fixed-ratio response requirement was sufficiently high (Chandler, Maggio, Peng, Nixon, & Bardo, 2020).
Figure 5: Alcohol and nicotine continuous access.
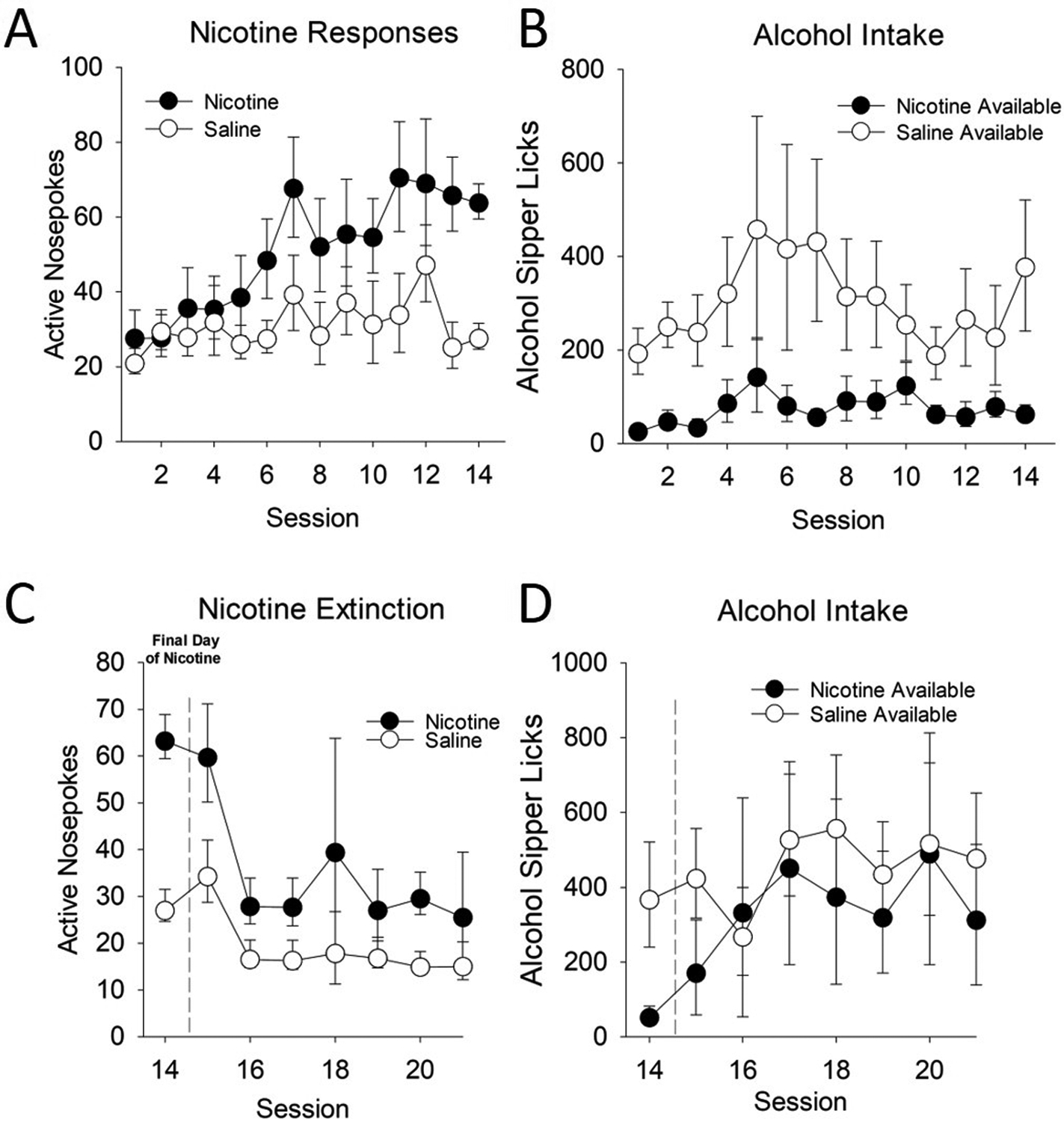
Rats were trained to lever press for nicotine or saline in the presence of a continuously available 20% alcohol sipper. Nicotine responses are shown on the left panels, and alcohol intake is shown in the right panels. A) Rats lever pressed for nicotine more than saline controls. However, B) rats with nicotine available consumed less alcohol. When nicotine was removed, C) responding on the nicotine lever decreased, and D) alcohol intake increased quickly to control levels.
These data together suggest that 1) alcohol and nicotine can both be readily self-administered, but only when 2) either the response requirement for nicotine is sufficiently high to discourage high levels of intake, or 3) nicotine-availability is sufficiently separated from alcohol to avoid the immediate alcohol suppressing effects. Further, separation of both the motivational and intake-specific effects of nicotine are especially crucial, given the differential effect of nicotine on both under various conditions.
The future utility of validating co-use models may provide important insights into the unique neuroadaptive changes surrounding motivation and regulation of intake when the subject can regulate their own consumption of both nicotine and alcohol. This may be especially useful because pharmacological treatments differ in efficacy among individuals with both tobacco and alcohol use histories, and the use of well-designed animal models of co-use may help reconcile currently existing disparities with behavioral outcomes and treatment mechanisms between sets of models, making them more generalizable (Motschman et al., 2016). Future models of co-use will thus likely be most successful when they are able to exploit the availability of cues, the long-term potentiating effects of nicotine, and dissociate both the appetitive/motivational components of drug-seeking from intake.
NEUROBIOLOGICAL UNDERPINNINGS OF NICOTINE’S REINFORCING AND INCENTIVE AMPLIFYING EFFECTS
As discussed, nicotine produces a range of appetitive and consummatory effects on alcohol. The neurobiological processes underling tobacco and alcohol co-use are complicated and involve drug actions across a range of neurotransmitter systems and brain areas. Each of these systems may engage different psychological and behavioral processes, producing an ensemble of changes working in concert to modify alcohol and nicotine directed behavior across the lifetime of the individual. It is therefore difficult to disentangle the direct effects of nicotine on alcohol-directed behavior from the synergistic effect of both taken in conjunction, although some lines of evidence have been useful in dissociating the various drug- and neuroanatomical specific effects involved in co-use.
Neurobiological substrates for nicotine and alcohol: mesolimbic dopamine
The involvement of the mesolimbic dopamine system in drug reinforcement and learning has been well documented and is probably the most widely examined neural system to date in the development of drug addiction across a variety of compounds. In addition to its involvement in the cued response to drugs, the reinforcing properties of most drugs of abuse show strong convergent evidence for the involvement in the limbic system (Everitt & Robbins, 2005, 2013; Nestler, 2005; Robinson & Berridge, 1993, 2000, 2008), and are necessary for the maintenance of drug-taking across different drug-classes such as the psychomotor stimulants, opioids, nicotine, and alcohol. It is thus reasonable to predict that the reinforcing and motivational properties of alcohol and its associated cues are directly modified by nicotine through convergent neuroadaptations in dopaminergic circuitry.
Functionally, as a key structure in the mesolimbic dopamine system, the ventral tegmental area (VTA) has been characterized for its role encoding the motivational value of rewards and their associated cues (D’Ardenne, McClure, Nystrom, & Cohen, 2008; Wolfram Schultz, 1997; W. Schultz, 1998, 2015), principally through phasic bursts of firing (Tsai et al., 2009) which can instigate reward seeking behavior (Adamantidis et al., 2011). The firing of dopamine-containing cell bodies in the VTA results in dopaminergic overflow in downstream forebrain regions such as the nucleus accumbens (NAcc) (Sombers, Beyene, Carelli, & Wightman, 2009) that contrast tonic single-spike firing (Hyland, Reynolds, Hay, Perk, & Miller, 2002). Thus, these transient increases in NAcc dopamine release become linked to reward cues and initiation of reward seeing behavior (Owesson-White, Cheer, Beyene, Carelli, & Wightman, 2008; Roitman, Stuber, Phillips, Wightman, & Carelli, 2004). These actions likely underlie the conditioned, appetitive effects of drugs such as nicotine following drug-use.
The direct, immediate actions of addictive drugs within the mesolimbic dopamine system are therefore recognized to drive abnormal motivational learning when compared to conventional reinforcers (Di Chiara, 1998; Di Chiara et al., 1999; Pierce & Kumaresan, 2006). Like many drugs of abuse, nicotine and alcohol involve actions on mesolimbic dopamine, principally by increasingly dopamine release in the NAcc through various mechanisms. Although here we discuss convergent nicotine and alcohol reinforcement mechanisms, an exhaustive review of the complex cellular and neurobiological mechanisms of alcohol and nicotine reinforcement is beyond the scope of this review. These systems have recently been reviewed in excellent detail elsewhere (e.g., for a recent review see Morel, Montgomery, & Han, 2019).
Mechanisms of nicotine reinforcement
Nicotine binds to the various subtypes of the nicotinic acetylcholine receptor (NAchR) that are neuroanatomically distributed (Gotti et al., 2009), and for example can stimulate dopamine release via VTA and NAcc actions (Ferrari, Le Novere, Picciotto, Changeux, & Zoli, 2002). In addition to being expressed at multiple nodes in the midbrain, dorsal, and ventral striatum, it is further expressed in the habenular-peduncular pathway, amygdala, hippocampus, and cortical areas (Tuesta, Fowler, & Kenny, 2011). Furthermore, nicotinic receptor subtype distribution varies anatomically as well (for a full review, see: (Fowler, Arends, & Kenny, 2008), although the α4, α7, and β2 have received a great deal of addition given their location in the VTA (Charpantier, Barneoud, Moser, Besnard, & Sgard, 1998), involvement in nicotine self-administration (Brunzell & McIntosh, 2012; Laviolette & van der Kooy, 2003), and involvement in reinforcement within in the VTA (Pons et al., 2008). Furthermore, β2-containing nicotinic receptors stimulate dopamine release from the VTA (Mameli-Engvall et al., 2006), and systemic antagonism of α4β2 reduces the conditioned reinforcing properties of nicotine (L. Liu, Zhao-Shea, McIntosh, Gardner, & Tapper, 2012; X. Liu et al., 2007). The appraisal of the β2-containing nicotinic receptor in drug related behaviors, and its proximity to the VTA-NAcc pathway have made this an attractive target for nicotine actions on reinforcement.
Mechanisms of alcohol reinforcement
Alcohol-elicited dopamine release is a key process in reinforcement (Gonzales, Job, & Doyon, 2004), and alcohol is largely been identified as reinforcing in the VTA (Gatto, McBride, Murphy, Lumeng, & Li, 1994; Rodd et al., 2004), stimulating VTA dopamine neurons directly (Brodie, Pesold, & Appel, 1999) (Appel, Liu, McElvain, & Brodie, 2003) resulting in downstream DA release in areas including the NAcc (Di Chiara & Imperato, 1985) to promote self-administration (Rassnick, Pulvirenti, & Koob, 1992). However, the VTA is a relatively large and heterogeneous structure, with separate neuroanatomical regions distinguished by various afferent targets (Swanson, 1982; Walsh & Han, 2014) and cytoarchitectural distinctions (Sanchez-Catalan, Kaufling, Georges, Veinante, & Barrot, 2014). The multiple projections of the VTA innervate the forebrain (Swanson, 1982) including areas crucial for drug reinforcement such as the NAcc. The various regions of the VTA have been simplified into dimensions based on neuroanatomical location, for example as either anterior (aVTA) or posterior (pVTA), both of which are differentially sensitive to different drugs of reinforcement (Sanchez-Catalan et al., 2014). Alcohol-stimulated dopamine release, particularly in the pVTA, innervates a variety of forebrain regions including the ventral pallidum (VP), medial prefrontal cortex (mPFC), NAcc core (Ding, Ingraham, Rodd, & McBride, 2015), and NAcc (Boileau et al., 2003; Rassnick et al., 1992; Weiss, Lorang, Bloom, & Koob, 1993) which are understood to drive the reinforcing effects of alcohol. At least in the pVTA, repeated exposure to alcohol via microinjections appears to sensitize the release of NAcc shell (NAccSh) DA (Ding, Rodd, Engleman, & McBride, 2009).
Mechanisms of nicotine-alcohol interactions
Nicotine and alcohol are thought to produce complex interactions on reinforcement through their direct actions on each other within the mesolimbic system, which have also been previously discussed in several other excellent reviews (Doyon, Thomas, Ostroumov, Dong, & Dani, 2013; Hendrickson, Guildford, & Tapper, 2013). Passive administration of nicotine and alcohol together produces and additive effect of dopamine release in the NAcc (Y. Tizabi, Bai, Copeland, & Taylor, 2007) which likely involves interactions within the VTA (Yousef Tizabi, Copeland, Louis, & Taylor, 2002). Specifically, the reinforcing value of lower doses of nicotine and alcohol together are enhanced in the pVTA (Truitt et al., 2015), as both taken at subthreshold doses together intracranially, resulting in robust brain-derived neurotrophic factor (BDNF) upregulation and drug-elicited dopamine and glutamate release in the NAccSh (Waeiss, Knight, Engleman, Hauser, & Rodd, 2020). The alcohol-enhanced firing rate of VTA neurons is further exacerbated by nicotine (Clark & Little, 2004) but is concentration-dependent. Regardless, at least locally, nicotine appears to sensitize local dopamine release in the NAccSh to the subsequent effects of alcohol (Ding et al., 2012). These studies together would support an enhancement of the reinforcing and motivational properties of combined nicotine and alcohol, although they largely model acute effects, rather than neuroadaptations following prolonged consumption and drug history. Further, these studies largely focus on localized effects under intracranial manipulations.
Protracted changes in nicotine-alcohol interactions
As discussed previously, nicotine appears to better enhance voluntary alcohol intake when it is given in exposure windows either developmentally or temporally dissociated from the period of alcohol consumption. In contrast to the previously described studies, subchronic nicotine can actually blunt alcohol-induced DA release in the NAccSh (Lopez-Moreno et al., 2008). The effect of nicotine pre-exposure can persist long after it is metabolized, attenuating the alcohol-induced release of NAcc DA and VTA firing, while producing concurrent increases in alcohol consumption (W. M. Doyon, Y. Dong, et al., 2013). In addition, although nicotine can acutely increase VTA firing, NAchRs are rapidly desensitized, producing acute tolerance to nicotine’s effects (Pidoplichko, DeBiasi, Williams, & Dani, 1997). The repeated combination of desensitization (Grady, Wageman, Patzlaff, & Marks, 2012) and NAchR upregulation (Fasoli et al., 2016; Staley et al., 2006) specifically on VTA gamma aminobutyric acid (GABA) inhibitory interneurons (Nashmi et al., 2007) may result in acceleration of DA-ergic blunting. These pre-exposures specifically in development appear to amplify inhibitory synaptic GABA mechanisms (Thomas et al., 2018) resulting in increased alcohol consumption.
Paradoxically, despite chronically induced decreases in alcohol induced DA release following nicotine, intake is amplified. Indeed, blunted DA function has thought to be a consequence of alcohol dependence (Martinez et al., 2005; Volkow et al., 1996). Interestingly, phasic firing appears to be a crucial feature surrounding the response to rewards and associated cues, as tonic stimulation of the VTA blunts alcohol intake (Bass et al., 2013). In light of this, it is likely that other mechanisms other than simply DA surges in the NAcc, such as changes in inhibitory GABA function (Chester & Cunningham, 2002) that can regulate consumption (Nie, Rewal, Gill, Ron, & Janak, 2011) may underlie persistent changes following nicotine histories. There is some evidence to suggest that the GABA-alcohol interaction may be especially important, given GABAergic inhibitory currents in the cerebellum are related to variation in alcohol consumption (Kaplan, Mohr, & Rossi, 2013; Richardson & Rossi, 2017).
Additional work separating cue and intake-specific effects under different drug histories will be important in elucidating the specific neuroadaptations following concurrent nicotine and alcohol use. It is, however, likely that the varieties of effects produced by nicotine in the mesolimbic system underlie features that alter cue conditioning and intake, and likely do so differently following prolonged experience with the drug. Further, nicotine-driven escalations in alcohol intake may involve other non-mesolimbic actions of nicotine.
Neurobiological mechanisms of nicotine amplification of the incentive value of cues
Because nicotine has weak reinforcing properties when administered alone, most studies examining nicotine reinforcement either present nicotine in combination with cues or measure the response to the cues themselves. Human studies using fMRI have indicated the appetitive activation (i.e., craving) activated by nicotine cues (typically presented on a video screen) are associated with activation of limbic brain areas described in reinforcement, including the NAcc (ventral striatum), amygdala, and areas of the cortex, including the prefrontal, cingulate, insular, and orbitofrontal cortices (Brody et al., 2002; McClernon, Hiott, Huettel, & Rose, 2005; Seungdamrong & Yasunaga, 1975; Smolka et al., 2006; Wilson, Sayette, Delgado, & Fiez, 2005). In one study, smokers were presented cues after smoking cigarettes, thus minimizing the effects of nicotine withdrawal. Not only was robust cue-induced craving observed, but robust activation of the ventral striatum, amygdala, orbitofrontal cortex, hippocampus, medial thalamus, and left insula were observed as well (Franklin et al., 2007). However, no non-nicotine sessions were included in the design, so it is unclear whether the neural cue responsivity was due to the acquired incentive properties of the cues (through Pavlovian mechanisms), or whether the brain activation was due to more generalized non-contingent incentive amplifying properties of nicotine. Another study measured the neural response to money-predictive (or neutral) cues after oral nicotine or placebo, and on some trials the outcome was unexpected (Addicott et al., 2017). Nicotine amplified the neural response to all cues in this task, including money-predictive cues, neutral cues, and those that preceded unexpected outcomes, particularly in the anterior insula/inferior frontal gyrus. This was also associated with a decrease in cue-induced activity in the dorsal striatum. Together, these data suggest a key role for the insular cortex (discussed in detail below) in nicotine’s ability to amplify the incentive value of cues.
Several studies, especially those in rodent models, indicate that nicotine self-administration is dependent on the mesolimbic dopamine system (Corrigall, Coen, & Adamson, 1994), which is likely regulated by brainstem areas that can influence the rewarding and aversive properties of nicotine (Laviolette, Alexson, & van der Kooy, 2002). However, in most of these investigations, nicotine is presented in combination with an associated cue, and thus the primary reinforcing effects of nicotine cannot be dissociated from its incentive amplifying effects. However, one study used a Pavlovian conditioned approach paradigm to demonstrate that dopamine receptors are required for the enhancement of the sign-tracking response by nicotine (Palmatier, Kellicut, Brianna Sheppard, Brown, & Robinson, 2014). This finding, in combination with others showing that sign-tracking requires dopamine neurotransmission (Danna & Elmer, 2010; Flagel et al., 2011), indicates that nicotine likely amplifies the incentive value of reward-associated stimuli via dopaminergic neurotransmission. Also, several studies have measured the role dopamine and related brain areas during cue-induced reinstatement, which largely support a role for the mesocorticolimbic circuity described above (Caggiula et al., 2001). In addition, transition to nicotine self-administration is accompanied by a reduction in the aversive properties of nicotine (and alcohol), which is associated with changes in the lateral habenula and peduncular nuclei (Baldwin, Alanis, & Salas, 2011; Fowler & Kenny, 2012; Glover, McDougle, Siegel, Jhou, & Chandler, 2016; Pang et al., 2016; Wolfman et al., 2018; Zhao-Shea et al., 2015).
At least some of the appetitive effects of nicotine are similar to other drug categories, and may be explained by actions within the mesocorticolimbic dopamine system which is important in coupling incentive salience to reward-paired cues (Berridge, 2012). In humans for example, DA-stimulating drugs such as d-amphetamine can enhance the positive response to stimuli independently of its euphorigenic effects (Wardle & de Wit, 2012). Amphetamine likely shares mechanistic overlap with nicotine, for example by enhancing the conditioned reinforcing value of CSs via its DA-promoting actions in the NAcc (Parkinson, Olmstead, Burns, Robbins, & Everitt, 1999; Taylor & Robbins, 1984, 1986; Wyvell & Berridge, 2000) and enhancing sign-tracking in the dorsolateral quadrant of the neostriatum (DLS) (DiFeliceantonio & Berridge, 2016). It is likely that these various pharmacological actions may converge simultaneously, as nicotine and amphetamine given in conjunction can further enhance VS responding (McNealy, Ramsay, Barrett, & Bevins, 2021), perhaps via their combined DA actions in the NAcc which is involved in conditioned reinforcement (Wolterink et al., 1993) and sign-tracking (Fraser & Janak, 2017). Besides the psychomotor stimulants, other drug categories including intra-NAcc agonists targeting opioid receptors can also enhance incentive salience measures such as pavlovian instrumental transfer (Peciña & Berridge, 2013) and conditioned reinforcement (Phillips, Robbins, & Everitt, 1994). Thus, it seems highly likely that there are DA-modulated actions on both reinforcement and incentive salience in the mesolimbic forebrain that can be recapitulated by different drug classes.
The neurobiological basis for the effects of nicotine on alcohol cue responsivity, however, is less well-studied and may reflect unique drug-drug interactions. The combination of these drugs can serve as a complex discriminative stimulus (Ford, Davis, McCracken, & Grant, 2013; Ford, McCracken, Davis, Ryabinin, & Grant, 2012; Randall et al., 2016; Troisi et al., 2013) that involves the NAcc and prefrontal cortex, (Chatterjee & Bartlett, 2010; Randall, McElligott, & Besheer), and alcohol cues activate similar circuitry. One study (A. King, McNamara, Angstadt, & Phan, 2010) showed that the neural response to smoking cues in the NAcc is enhanced by alcohol. The converse study has not been done, but one study found similar activation in the insular and cingulate cortices during separately presented alcohol and nicotine cues in co-users of these drugs (J. Liu, Claus, Calhoun, & Hutchison, 2014). A role for the insular cortex in nicotine’s interoceptive effects has been implicated in humans as well (Naqvi & Bechara, 2009, 2010), and in animals the insular cortex has been implicated in nicotine and alcohol self-administration (Forget, Pushparaj, & Le Foll, 2010; Jaramillo, Randall, et al., 2018; Jaramillo, Van Voorhies, Randall, & Besheer, 2018), as well as the enhancement of opioid self-administration by nicotine. Together, these data from humans and animals suggest that, whereas the reinforcing effects of nicotine, alcohol, and their associated cues are dependent on the activation of mesocorticolimbic circuitry, the incentive amplification properties of nicotine, and thereby its effects on alcohol-associated cues, are largely due to its actions within the insular cortex.
Neurobiological processes regulating intake: interoception and the insular cortex
Although initiation and regulation of alcohol intake is likely driven by concepts of reinforcement and conditioned motivation, quantity of intake is also likely to driven by post-ingestive feedback, including relative interoceptive state of intoxication, and the post-ingestive rewarding effects of alcohol. Interoception refers to perception of internal state (Craig, 2010), which can refer to both internal processes antecedent to drug-taking (Paulus & Stewart, 2014), as well perception of state when the drug is onboard. Nicotine can alter interoception, for example, by blunting the subjective effects of other drugs. In humans for example, transdermal nicotine patches reduce subjective intoxication for alcohol (McKee, O’Malley, Shi, Mase, & Krishnan-Sarin, 2008) and cocaine (Elena M. Kouri, Stull, & Lukas, 2001), and both nicotine and alcohol produce tolerance to the somatic effects of alcohol (Collins, Wilkins, Slobe, Cao, & Bullock, 1996). In rodents, experience with nicotine in periadolescence and adulthood can also reduce the subsequent aversive properties of alcohol (Rinker et al., 2011), as well as interfere with the conditioned taste aversion induced by ethanol, cocaine, and morphine in adult rats (Loney & Meyer, 2019) which is largely dependent on the interoceptive aversive properties of these drugs.
Insular Cortex
The insular cortex (IC) has received a great deal of attention for its role in interoception, and integration of limbic and sensory information (Gogolla, 2017), including properties surrounding drug conditioning (Arguello et al., 2017; Thompson, Bevins, & Murray, 2019) which likely explains its involvement in response to nicotine and alcohol cues. The interoceptive subjective effect of drugs is also important for regulation of intake and dose control, and loss of IC function can both enhance or reduce intake in rodents (Rotge et al., 2017) depending on whether the loss of function occurs prior to or following drug-experience. Nicotine and the IC have been of special interest, especially following several lines of evidence indicating damage following stroke in smokers can interrupt smoking (Naqvi, Rudrauf, Damasio, & Bechara, 2007), reduce perception of withdrawal (Abdolahi et al., 2015), and predict continued cessation of tobacco use following stroke (Gaznick, Tranel, McNutt, & Bechara, 2014; Suner-Soler et al., 2012).
The IC contains NAchRs available to nicotine, that normally receive input from the basal forebrain (Toyoda, 2019), specifically including β2-NAchRs that depress pyramidal neurons in layer V, likely via GABAergic action (Sato, Kawano, Yin, Kato, & Toyoda, 2017; Toyoda, 2018). Thus, convergent evidence for the IC in integration of interoceptive status via the nicotinic system appears likely. Crucially, both systemic nicotine and intra-IC nicotine produce rightward shifts in morphine conditioned place preference and aversion (Loney, King, & Meyer, 2021), suggesting that dose perception is blunted via the IC for both the rewarding and aversive properties of the drug under nicotine. While other studies have shown the importance of the IC specifically in drug-conditioning (Geddes, Han, Baldwin, Norgren, & Grigson, 2008; Li, Zhu, Meng, Li, & Sui, 2013), very few have demonstrated disruptions specifically as rightward dose-response shifts, such that perception of dose is less sensitive under both appetitive and aversive conditioning. This raises the interesting possibility that escalation of alcohol intake under nicotine may also be driven by interoceptive factors surrounding the perception of intoxication, in addition to changes in motivational strength involving neuroadaptive changes in mesolimbic dopamine. To date however, escalation of drug-intake under control of IC NAchRs has yet to be established but raises an interesting possibility for dysregulated intake control.
CONCLUSION
In the current review, we discuss the various nicotine effects on drug-seeking and drug-taking behavior, ranging from the antecedent “appetitive” drug-seeking and approach behaviors, to the actual consummatory intake of drug and the resulting internal consequences. For alcohol in particular, the complex characterization of appetitive and consummatory processes have come a long way since early separation of the terms (Freedland, Sharpe, Samson, & Porrino, 2001; Samson, Czachowski, Chappell, & Legg, 2003; Sharpe & Samson, 2001) to include a robust ensemble of conditioning and reinforcing effects. Here, we argue that the behavioral effects of nicotine can be conceptualized as domains that influence appetitive conditioning (cues, context, and motivation), as well as consummatory behavior (intake and interoception), summarized in Fig. 6. By increasing the incentive value of both nicotine and alcohol paired stimuli, facilitating approach behaviors and resulting in activation of conditioned motivational states, nicotine can lead to behaviors that increase the likelihood of alcohol consumption. Then, nicotine can alter the reinforcing and intake properties of alcohol directly by both interfacing with the mesolimbic dopamine system and blunting at least some of the interoceptive properties of alcohol to promote consumption. Relatively few studies have directly tried to dissociate the primary versus reinforcement enhancing effects of nicotine, which highlight the need for additional work that directly assesses modulation alcohol seeking in the presence of cues separately from the reinforcing properties of alcohol consumption directly.
Figure 6: Schematic of how nicotine’s effects on the appetitive and consummatory processes of alcohol.
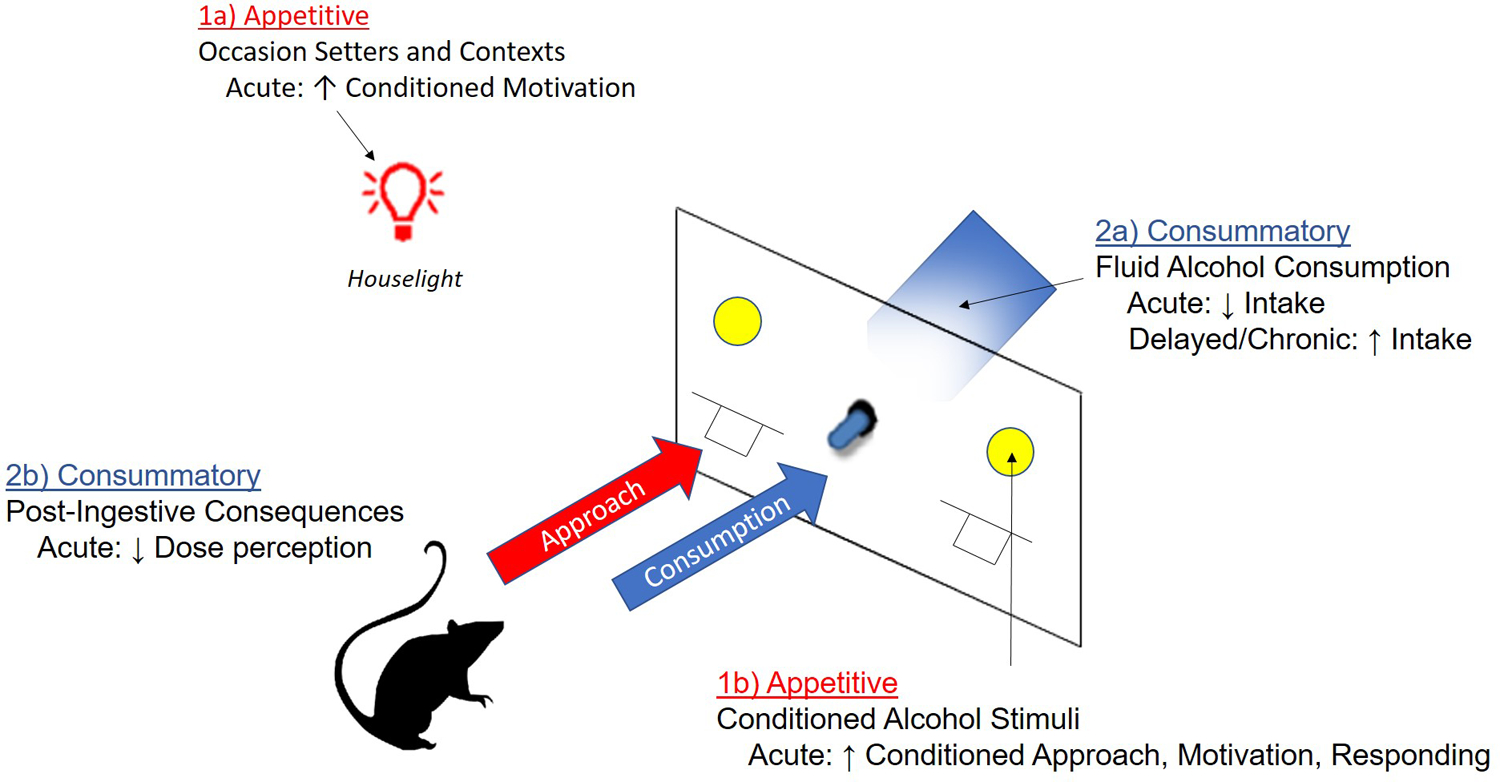
Nicotine can enhance appetitive responding and approach towards alcohol in the presence of 1a) alcohol-paired occasions setters and contexts, and 1b) discrete alcohol paired conditioned stimuli. Following approach and initiation of alcohol consumption, nicotine also can 2a) increase alcohol consumption following chronic treatment, although acute nicotine and decrease intake in some situations. Finally, 2b) nicotine is suggested to further enhance alcohol intake by blunting the post-ingestive consequences of alcohol, for example by blocking the perception of dose and intoxication.
Impact for treatment and cessation strategy
Development and validation of pharmacotherapies used for nicotine, alcohol, and codependence often involves using well validated rodent models with an understanding of the behavior concepts described in this review. Preclinical and clinical research do not always directly map onto each other. For example, although varenicline is used in treating alcohol and nicotine dependence (Bold et al., 2019; Erwin & Slaton, 2014; Hurt et al., 2018; McKee et al., 2009), preclinical data suggest that varenicline shows difficulty in reducing combined alcohol and nicotine intake (Funk et al., 2016; Maggio et al., 2018; Waeiss et al., 2019). Naltrexone has also been thought to be more effective in heavy drinkers that smoke (Fucito et al., 2012; A. King, Cao, Vanier, & Wilcox, 2009), although rodent models have had difficulty demonstrating reduced nicotine intake (Le et al., 2014; Maggio et al., 2018).
Importantly, this pattern of results show that it worth considering the behavioral model and outcome measure being used. For example, preclinical pharmacotheraputics often focus on drugs that reduce intake. Given what we know about the appetitive and motivational conditioning effects however, consideration of measures that reflect likelihood of initiating drug-use and motivation to work for drug, rather than expression free intake, may make better sense from a prevention perspective. Medications, mechanisms, and the generalization of varenicline’s effects in even in clinical data may also complicate the general pattern of findings (Motschman et al., 2016). As clinical models continue to improve the characterization of data in individuals and research groups (Gorelick & McPherson, 2015), and as animal models continue to refine observations to the various processes surrounding drug actions, well-defined behavioral measures will hopefully yield more convergent mechanistic data.
Differences in drug histories on treatment outcome
Considering the multitude of biobehavioral effects of nicotine, clinical outcomes and research may benefit by prioritizing the treatment of tobacco use disorder, an approach that has not directly been examined using preclinical modelling. Indeed, smoking is negatively related to treatment and clinical outcome for quitting drinking (Chiappetta, Garcia-Rodriguez, Jin, Secades-Villa, & Blanco, 2014; Weinberger et al., 2015). Instead, a “multimorbid” treatment approach that addresses both nicotine and other comorbid drug use may yield better outcomes (MacLean, Sofuoglu, & Rosenheck, 2018). In individuals undergoing treatment for alcohol use, smoking cessation does not appear to interfere with success maintaining alcohol abstinence (Gulliver, Kamholz, & Helstrom, 2006), which may be crucial maximizing treatment outcomes (Yokoyama et al., 2014) particularly in maximizing long-term success rates (Tsoh, Chi, Mertens, & Weisner, 2011). Approaches to encourage smoking cessation using contingency management may benefit from providing intermittent alternate reinforcement (Chudzynski, Roll, McPherson, Cameron, & Howell, 2015; Packer, Howell, McPherson, & Roll, 2012). Use of urinalysis to verify abstinence may be better predictor of substance use outcome (McPherson, Packer, Cameron, Howell, & Roll, 2014), and some recent evidence suggests that contingency management for both treatment of alcohol and tobacco use together can improve urinalysis outcomes for both (Orr et al., 2018). Preclinical models may eventually benefit from co-use models that examine behavioral outcomes following nicotine removal, or examining alternative reinforcement (Venniro et al., 2018).
Final remarks
The variety of approaches to studying drug-directed behavior under nicotine is remarkable, with the design of each study assessing different behavioral consequences of the drug. While these behavioral effects involve dissociable processes and multiple biological substrates, we suggest that it is useful to dissociate the various properties of nicotine into these various components of drug-directed behavior, specifically because it helps prevent the conflation of processes leading to, and resulting from, bouts of drug use.
Acknowledgements
We would like to acknowledge the work of Alexander Lamparelli, Hailley Angelyn, Cassandra Whalen (née Versaggi), and Laketta Jackson for collecting and analyzing the data presented in this chapter. The National Institutes of Health, and the National Institute on Alcohol Abuse and Alcoholism (AA024112) supported this work.
Abbreviations:
- aVTA
anterior ventral tegmental area
- CS
conditioned stimulus
- DLS
dorsolateral quadrant of the neostriatum
- GABA
gamma aminobutyric acid
- i.v.
intravenous
- IC
insular cortex
- DA
dopamine
- FR
fixed ratio
- mPFC
medial prefrontal cortex
- NAcc
nucleus accumbens
- NAccSh
nucleus accumbens shell
- NAchR
nicotinic acetylcholine receptor
- PR
progressive-ratio
- pVTA
posterior ventral tegmental area
- VTA
ventral tegmental area
- VP
ventral pallidum
- VS
visual stimulus
Footnotes
Conflict of Interest Statement
The authors C.P.K. and P.J.M. have no conflicts of interest to declare.
References
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, … van Wijngaarden E (2015). Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction, 110(12), 1994–2003. doi: 10.1111/add.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, … de Lecea L (2011). Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci, 31(30), 10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addicott MA, Oliver JA, & Joseph McClernon F (2017). Nicotine increases anterior insula activation to expected and unexpected outcomes among nonsmokers. Psychopharmacology (Berl), 234(7), 1145–1154. doi: 10.1007/s00213-017-4550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alen F, Gomez R, Gonzalez-Cuevas G, Navarro M, & Lopez-Moreno JA (2009). Nicotine causes opposite effects on alcohol intake: Evidence in an animal experimental model of abstinence and relapse from alcohol. Nicotine Tob Res, 11(11), 1304–1311. doi: 10.1093/ntr/ntp139 [DOI] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Tomasi D, Zhang L, Fagin-Jones S, Telang F, … Volkow ND (2007). What is in a word? No versus Yes differentially engage the lateral orbitofrontal cortex. Emotion, 7(3), 649–659. doi: 10.1037/1528-3542.7.3.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, & Spear LP (2010). Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res, 34(12), 2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelyn H, Loney GC, & Meyer PJ (in press). Nicotine enhances goal-tracking in ethanol and food Pavlovian conditioned approach paradigms. Front Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelyn H, & Meyer P (2019). NICOTINE ENHANCES REINSTATEMENT ELICITED BY A DISCRIMINATIVE STIMULUS THAT PREDICTS ALCOHOL AVAILABILITY. Paper presented at the Alcoholism-Clinical and Experimental Research. [Google Scholar]
- Appel SB, Liu Z, McElvain MA, & Brodie MS (2003). Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther, 306(2), 437–446. doi: 10.1124/jpet.103.050963 [DOI] [PubMed] [Google Scholar]
- Arguello AA, Wang R, Lyons CM, Higginbotham JA, Hodges MA, & Fuchs RA (2017). Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology (Berl), 234(16), 2431–2441. doi: 10.1007/s00213-017-4632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin PR, Alanis R, & Salas R (2011). The Role of the Habenula in Nicotine Addiction. Journal of addiction research & therapy, S1(2), 002. doi: 10.4172/2155-6105.S1-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, & Evins AE (2008). A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry, 63(11), 1061–1065. doi: 10.1016/j.biopsych.2007.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, & Pihl RO (2006). Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend, 81(2), 197–204. doi: 10.1016/j.drugalcdep.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, … Budygin EA (2013). Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci, 7, 173. doi: 10.3389/fnbeh.2013.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, & Rueff B (1995). Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction, 90(7), 977–980. doi: 10.1046/j.1360-0443.1995.90797711.x [DOI] [PubMed] [Google Scholar]
- Berridge KC (2012). From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci, 35(7), 1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, & Palmatier MI (2004). Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev, 3(3), 143–158. doi: 10.1177/1534582304272005 [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Piasecki J, Koros E, Stefanski R, & Kostowski W (1998). Studies on the role of nicotinic acetylcholine receptors in the discriminative and aversive stimulus properties of ethanol in the rat. Eur Neuropsychopharmacol, 8(2), 79–87. doi: 10.1016/s0924-977x(97)00052-7 [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, & Bartlett SE (2011). Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol, 16(3), 440–449. doi: 10.1111/j.1369-1600.2010.00309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, & Soderpalm B (1996). Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol, 314(3), 257–267. doi: 10.1016/s0014-2999(96)00583-3 [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, … Dagher A (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse, 49(4), 226–231. doi: 10.1002/syn.10226 [DOI] [PubMed] [Google Scholar]
- Bold KW, Zweben A, Fucito LM, Piepmeier ME, Muvvala S, Wu R, … O’Malley SS (2019). Longitudinal Findings from a Randomized Clinical Trial of Varenicline for Alcohol Use Disorder with Comorbid Cigarette Smoking. Alcohol Clin Exp Res, 43(5), 937–944. doi: 10.1111/acer.13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, & Appel SB (1999). Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res, 23(11), 1848–1852. doi: 10.1111/j.1530-0277.1999.tb04082.x [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, … Jarvik ME (2002). Brain metabolic changes during cigarette craving. Arch Gen Psychiatry, 59(12), 1162–1172. doi: 10.1001/archpsyc.59.12.1162 [DOI] [PubMed] [Google Scholar]
- Brunzell DH, & McIntosh JM (2012). Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burling TA, & Ziff DC (1988). Tobacco smoking: A comparison between alcohol and drug abuse inpatients. Addictive Behaviors, 13(2), 185–190. doi: 10.1016/0306-4603(88)90010-x [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, & Sved AF (2009). The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv, 55, 91–109. doi: 10.1007/978-0-387-78748-0_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, … Sved AF (2001). Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav, 70(4), 515–530. doi: 10.1016/s0091-3057(01)00676-1 [DOI] [PubMed] [Google Scholar]
- Carter BL, & Tiffany ST (1999). Meta-analysis of cue-reactivity in addiction research. Addiction, 94(3), 327–340. [PubMed] [Google Scholar]
- Chandler CM, Maggio SE, Peng H, Nixon K, & Bardo MT (2020). Effects of ethanol, naltrexone, nicotine and varenicline in an ethanol and nicotine co-use model in Sprague-Dawley rats. Drug Alcohol Depend, 212, 107988. doi: 10.1016/j.drugalcdep.2020.107988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier E, Barneoud P, Moser P, Besnard F, & Sgard F (1998). Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport, 9(13), 3097–3101. doi: 10.1097/00001756-199809140-00033 [DOI] [PubMed] [Google Scholar]
- Chatterjee S, & Bartlett SE (2010). Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS & neurological disorders drug targets, 9(1), 60–76. doi: 10.2174/187152710790966597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, … Sved AF (2006). Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl), 189(1), 27–36. doi: 10.1007/s00213-006-0522-0 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, … Sved AF (2007). Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl), 190(3), 353–362. doi: 10.1007/s00213-006-0454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, & Sved AF (2006). Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl), 184(3–4), 353–366. doi: 10.1007/s00213-005-0178-1 [DOI] [PubMed] [Google Scholar]
- Chen X, Unger JB, Palmer P, Weiner MD, Johnson CA, Wong MM, & Austin G (2002). Prior cigarette smoking initiation predicting current alcohol use. Addictive Behaviors, 27(5), 799–817. doi: 10.1016/s0306-4603(01)00211-8 [DOI] [PubMed] [Google Scholar]
- Chester JA, & Cunningham CL (2002). GABAA receptor modulation of the rewarding and aversive effects of ethanol. Alcohol, 26(3), 131–143. doi: 10.1016/s0741-8329(02)00199-4 [DOI] [PubMed] [Google Scholar]
- Chiappetta V, Garcia-Rodriguez O, Jin CJ, Secades-Villa R, & Blanco C (2014). Predictors of quit attempts and successful quit attempts among individuals with alcohol use disorders in a nationally representative sample. Drug Alcohol Depend, 141, 138–144. doi: 10.1016/j.drugalcdep.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Chudzynski J, Roll JM, McPherson S, Cameron JM, & Howell DN (2015). Reinforcement Schedule Effects on Long-Term Behavior Change. Psychological Record, 65(2), 347–353. doi: 10.1007/s40732-014-0110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, & Little HJ (2001a). Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology, 41(1), 108–117. [DOI] [PubMed] [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, & Little HJ (2001b). Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology, 41(1), 108–117. doi: 10.1016/s0028-3908(01)00037-5 [DOI] [PubMed] [Google Scholar]
- Clark A, & Little HJ (2004). Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend, 75(2), 199–206. doi: 10.1016/j.drugalcdep.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Collins AC, Wilkins LH, Slobe BS, Cao JZ, & Bullock AE (1996). Long-term ethanol and nicotine treatment elicit tolerance to ethanol. Alcohol Clin Exp Res, 20(6), 990–999. doi: 10.1111/j.1530-0277.1996.tb01936.x [DOI] [PubMed] [Google Scholar]
- Conklin CA (2006). Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol, 14(1), 12–19. doi: 10.1037/1064-1297.14.1.12 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, & Adamson KL (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain research, 653(1–2), 278–284. doi: 10.1016/0006-8993(94)90401-4 [DOI] [PubMed] [Google Scholar]
- Craig AD (2010). The sentient self. Brain Struct Funct, 214(5–6), 563–577. doi: 10.1007/s00429-010-0248-y [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, & Cohen JD (2008). BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science, 319(5867), 1264–1267. doi: 10.1126/science.1150605 [DOI] [PubMed] [Google Scholar]
- Danna CL, & Elmer GI (2010). Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacol Biochem Behav, 96(1), 40–47. doi: 10.1016/j.pbb.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar R, & Frenk H (2004). Do smokers self-administer pure nicotine? A review of the evidence. Psychopharmacology (Berl), 173(1–2), 18–26. doi: 10.1007/s00213-004-1781-2 [DOI] [PubMed] [Google Scholar]
- Di Chiara G (1998). A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol, 12(1), 54–67. doi: 10.1177/026988119801200108 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, & Imperato A (1985). Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol, 115(1), 131–132. doi: 10.1016/0014-2999(85)90598-9 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, … Carboni E (1999). Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci, 877(1 ADVANCING FRO), 461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, & Berridge KC (2016). Dorsolateral neostriatum contribution to incentive salience: opioid or dopamine stimulation makes one reward cue more motivationally attractive than another. Eur J Neurosci, 43(9), 1203–1218. doi: 10.1111/ejn.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, & Guerrera MP (1990). Alcoholism and smoking. J Stud Alcohol, 51(2), 130–135. doi: 10.15288/jsa.1990.51.130 [DOI] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, & McBride WJ (2015). The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain cortico-limbic systems. Addict Biol, 20(3), 458–468. doi: 10.1111/adb.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Katner SN, Rodd ZA, Truitt W, Hauser SR, Deehan GA Jr., … McBride WJ (2012). Repeated exposure of the posterior ventral tegmental area to nicotine increases the sensitivity of local dopamine neurons to the stimulating effects of ethanol. Alcohol, 46(3), 217–223. doi: 10.1016/j.alcohol.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, & McBride WJ (2009). Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res, 33(9), 1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, & Stitzer ML (2007). Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction, 102(2), 324–334. doi: 10.1111/j.1360-0443.2006.01670.x [DOI] [PubMed] [Google Scholar]
- Doyon William M., Dong Y, Ostroumov A, Thomas Alyse M., Zhang Tao A., & Dani John A. (2013). Nicotine Decreases Ethanol-Induced Dopamine Signaling and Increases Self-Administration via Stress Hormones. Neuron, 79(3), 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, & Dani JA (2013). Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron, 79(3), 530–540. doi: 10.1016/j.neuron.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, & Dani JA (2013). Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol, 86(8), 1181–1193. doi: 10.1016/j.bcp.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyr W, Koros E, Bienkowski P, & Kostowski W (1999). Involvement of nicotinic acetylcholine receptors in the regulation of alcohol drinking in Wistar rats. Alcohol Alcohol, 34(1), 43–47. doi: 10.1093/alcalc/34.1.43 [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, & King AC (2007). Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl), 190(3), 321–329. doi: 10.1007/s00213-006-0438-8 [DOI] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg DH, & Sloan RP (2011). Exposure to smoking cues: cardiovascular and autonomic effects. Addict Behav, 36(7), 737–742. doi: 10.1016/j.addbeh.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin BL, & Slaton RM (2014). Varenicline in the treatment of alcohol use disorders. Ann Pharmacother, 48(11), 1445–1455. doi: 10.1177/1060028014545806 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci, 8(11), 1481–1489. doi: 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2013). From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev, 37(9 Pt A), 1946–1954. doi: 10.1016/j.neubiorev.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Fasoli F, Moretti M, Zoli M, Pistillo F, Crespi A, Clementi F, … Gotti C (2016). In vivo chronic nicotine exposure differentially and reversibly affects upregulation and stoichiometry of alpha4beta2 nicotinic receptors in cortex and thalamus. Neuropharmacology, 108, 324–331. doi: 10.1016/j.neuropharm.2016.04.048 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, & See RE (2012). Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug and alcohol dependence, 121(3), 240–246. doi: 10.1016/j.drugalcdep.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, & Zoli M (2002). Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci, 15(11), 1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, … Akil H (2011). A selective role for dopamine in stimulus-reward learning. Nature, 469(7328), 53–57. doi: 10.1038/nature09588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, & Akil H (2007). Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl), 191(3), 599–607. doi: 10.1007/s00213-006-0535-8 [DOI] [PubMed] [Google Scholar]
- Ford MM, Davis NL, McCracken AD, & Grant KA (2013). Contribution of NMDA glutamate and nicotinic acetylcholine receptor mechanisms in the discrimination of ethanol-nicotine mixtures. Behav Pharmacol, 24(7), 617–622. doi: 10.1097/FBP.0b013e3283654216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, McCracken AD, Davis NL, Ryabinin AE, & Grant KA (2012). Discrimination of ethanol-nicotine drug mixtures in mice: dual interactive mechanisms of overshadowing and potentiation. Psychopharmacology (Berl), 224(4), 537–548. doi: 10.1007/s00213-012-2781-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, & Le Foll B (2010). Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry, 68(3), 265–271. doi: 10.1016/j.biopsych.2010.01.029 [DOI] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, & Kenny PJ (2008). Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol, 19(5–6), 461–484. doi: 10.1097/FBP.0b013e32830c360e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, & Kenny PJ (2012). Habenular signaling in nicotine reinforcement. Neuropsychopharmacology, 37(1), 306–307. doi: 10.1038/npp.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, … Childress AR (2007). Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology, 32(11), 2301–2309. doi: 10.1038/sj.npp.1301371 [DOI] [PubMed] [Google Scholar]
- Fraser KM, & Janak PH (2017). Long-Lasting Contribution Of Dopamine In The Nucleus Accumbens Core, But Not Dorsal Lateral Striatum, To Sign-Tracking. bioRxiv. doi: 10.1101/132977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, & Porrino LJ (2001). Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res, 25(2), 277–282. doi: 10.1111/j.1530-0277.2001.tb02209.x [DOI] [PubMed] [Google Scholar]
- Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, & O’Malley SS (2012). Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol Psychiatry, 72(10), 832–838. doi: 10.1016/j.biopsych.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Lo S, Coen K, & Le AD (2016). Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse. Behav Brain Res, 296, 157–162. doi: 10.1016/j.bbr.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, & Li TK (1994). Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol, 11(6), 557–564. doi: 10.1016/0741-8329(94)90083-3 [DOI] [PubMed] [Google Scholar]
- Gaznick N, Tranel D, McNutt A, & Bechara A (2014). Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine Tob Res, 16(4), 445–453. doi: 10.1093/ntr/ntt172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes RI, Han L, Baldwin AE, Norgren R, & Grigson PS (2008). Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. [DOI] [PMC free article] [PubMed]
- Glover EJ, McDougle MJ, Siegel GS, Jhou TC, & Chandler LJ (2016). Role for the Rostromedial Tegmental Nucleus in Signaling the Aversive Properties of Alcohol. Alcohol Clin Exp Res, 40(8), 1651–1661. doi: 10.1111/acer.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N (2017). The insular cortex. Curr Biol, 27(12), R580–R586. doi: 10.1016/j.cub.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, & Doyon WM (2004). The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther, 103(2), 121–146. doi: 10.1016/j.pharmthera.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Gorelick DA, & McPherson S (2015). Improving the analysis and modeling of substance use. Am J Drug Alcohol Abuse, 41(6), 475–478. doi: 10.3109/00952990.2015.1085264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, … Zoli M (2009). Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol, 78(7), 703–711. doi: 10.1016/j.bcp.2009.05.024 [DOI] [PubMed] [Google Scholar]
- Grady SR, Wageman CR, Patzlaff NE, & Marks MJ (2012). Low concentrations of nicotine differentially desensitize nicotinic acetylcholine receptors that include α5 or α6 subunits and that mediate synaptosomal neurotransmitter release. Neuropharmacology, 62(5–6), 1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF (1998). Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse, 10(1), 59–73. doi: 10.1016/s0899-3289(99)80141-2 [DOI] [PubMed] [Google Scholar]
- Gulliver SB, Kamholz BW, & Helstrom AW (2006). Smoking cessation and alcohol abstinence: what do the data tell us? Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 29(3), 208–212. [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Hinson RE, & McKee SA (2009). Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav, 34(5), 484–486. doi: 10.1016/j.addbeh.2008.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, & Goldberg SR (2004). Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl), 175(2), 134–142. doi: 10.1007/s00213-004-1818-6 [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, … Rodd ZA (2012). Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol Clin Exp Res, 36(1), 43–54. doi: 10.1111/j.1530-0277.2011.01579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA Jr., Ding ZM, Toalston JE, Scott BJ, … Rodd ZA (2012). Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (p) rats. Alcohol Clin Exp Res, 36(11), 1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Guildford MJ, & Tapper AR (2013). Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Frontiers in psychiatry, 4, 29. doi: 10.3389/fpsyt.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, & Tapper AR (2010). Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci, 30(30), 10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, & Goldberg SR (1983). Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav, 19(6), 989–992. doi: 10.1016/0091-3057(83)90405-7 [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, & Jasinski DR (1983). Cigarette smokers self-administer intravenous nicotine. Pharmacol Biochem Behav, 19(5), 887–890. doi: 10.1016/0091-3057(83)90099-0 [DOI] [PubMed] [Google Scholar]
- Hurt RT, Ebbert JO, Croghan IT, Schroeder DR, Hurt RD, & Hays JT (2018). Varenicline for tobacco-dependence treatment in alcohol-dependent smokers: A randomized controlled trial. Drug Alcohol Depend, 184, 12–17. doi: 10.1016/j.drugalcdep.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, & Miller R (2002). Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience, 114(2), 475–492. doi: 10.1016/s0306-4522(02)00267-1 [DOI] [PubMed] [Google Scholar]
- Istvan J, & Matarazzo JD (1984). Tobacco, alcohol, and caffeine use: A review of their interrelationships. Psychological Bulletin, 95(2), 301–326. doi: 10.1037/0033-2909.95.2.301 [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, & Besheer J (2018). Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology, 130, 42–53. doi: 10.1016/j.neuropharm.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Van Voorhies K, Randall PA, & Besheer J (2018). Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav Brain Res, 348, 74–81. doi: 10.1016/j.bbr.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MK, Sorensen TI, Andersen AT, Thorsen T, Tolstrup JS, Godtfredsen NS, & Gronbaek M (2003). A prospective study of the association between smoking and later alcohol drinking in the general population. Addiction, 98(3), 355–363. doi: 10.1046/j.1360-0443.2003.00304.x [DOI] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, & Role LW (2002). Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol, 53(4), 618–632. doi: 10.1002/neu.10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Mohr C, & Rossi DJ (2013). Opposite actions of alcohol on tonic GABA(A) receptor currents mediated by nNOS and PKC activity. Nat Neurosci, 16(12), 1783–1793. doi: 10.1038/nn.3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Lukatskaya O, Moon SH, Guo WR, Chen D, Algava D, … Leibowitz SF (2015). Nicotine and ethanol co-use in Long-Evans rats: Stimulatory effects of perinatal exposure to a fat-rich diet. Alcohol, 49(5), 479–489. doi: 10.1016/j.alcohol.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen H, Hyytia P, & Kiianmaa K (2009). Behavioral consequences of repeated nicotine during adolescence in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res, 33(2), 340–349. doi: 10.1111/j.1530-0277.2008.00838.x [DOI] [PubMed] [Google Scholar]
- King A, Cao D, Vanier C, & Wilcox T (2009). Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res, 33(6), 1044–1050. doi: 10.1111/j.1530-0277.2009.00925.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, McNamara P, Angstadt M, & Phan KL (2010). Neural substrates of alcohol-induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacology, 35(3), 692–701. doi: 10.1038/npp.2009.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, & Epstein AM (2005). Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res, 29(4), 547–552. doi: 10.1097/01.alc.0000158839.65251.fe [DOI] [PubMed] [Google Scholar]
- Knight CP, Hauser SR, Deehan GA Jr., Toalston JE, McBride WJ, & Rodd ZA (2016). Oral Conditioned Cues Can Enhance or Inhibit Ethanol (EtOH)-Seeking and EtOH-Relapse Drinking by Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res, 40(4), 906–915. doi: 10.1111/acer.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kenneth Lloyd G, & Mason BJ (2009). Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature Reviews Drug Discovery, 8(6), 500–515. doi: 10.1038/nrd2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, & Lukas SE (2004). Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend, 75(1), 55–65. doi: 10.1016/j.drugalcdep.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Kouri EM, Stull M, & Lukas SE (2001). Nicotine alters some of cocaine’s subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacology Biochemistry and Behavior, 69(1–2), 209–217. doi: 10.1016/s0091-3057(01)00529-9 [DOI] [PubMed] [Google Scholar]
- Kunin D, Smith BR, & Amit Z (1999). Nicotine and ethanol interaction on conditioned taste aversions induced by both drugs. Pharmacol Biochem Behav, 62(2), 215–221. [DOI] [PubMed] [Google Scholar]
- Lamparelli A, & Meyer P (2018). NICOTINE DIFFERENTIALLY ENHANCES ALCOHOL SELF-ADMINISTRATION IN TWO SCHEDULES OF REINFORCEMENT. Paper presented at the Alcoholism-Clinical and Experimental Research. [Google Scholar]
- Laviolette SR, Alexson TO, & van der Kooy D (2002). Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci, 22(19), 8653–8660. doi: 10.1523/jneurosci.22-19-08653.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, & van der Kooy D (2003). The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the alpha7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology (Berl), 166(3), 306–313. doi: 10.1007/s00213-002-1317-6 [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, & Li TK (2000). Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res, 24(2), 155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Lo S, & Coen K (2014). Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology (Berl), 231(20), 4019–4029. doi: 10.1007/s00213-014-3541-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Lo S, Harding S, Juzytsch W, Marinelli PW, & Funk D (2010). Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl), 208(3), 475–486. doi: 10.1007/s00213-009-1746-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, & Shaham Y (2003a). Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl), 168(1–2), 216–221. doi: 10.1007/s00213-002-1330-9 [DOI] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, & Shaham Y (2003b). Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl), 168(1–2), 216–221. doi: 10.1007/s00213-002-1330-9 [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, & Pentel PR (2004). Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav, 79(3), 507–513. doi: 10.1016/j.pbb.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Li CL, Zhu N, Meng XL, Li YH, & Sui N (2013). Effects of inactivating the agranular or granular insular cortex on the acquisition of the morphine-induced conditioned place preference and naloxone-precipitated conditioned place aversion in rats. J Psychopharmacol, 27(9), 837–844. doi: 10.1177/0269881113492028 [DOI] [PubMed] [Google Scholar]
- Liu J, Claus ED, Calhoun VD, & Hutchison KE (2014). Brain regions affected by impaired control modulate responses to alcohol and smoking cues. J Stud Alcohol Drugs, 75(5), 808–816. doi: 10.15288/jsad.2014.75.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao-Shea R, McIntosh JM, Gardner PD, & Tapper AR (2012). Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits. Mol Pharmacol, 81(4), 541–548. doi: 10.1124/mol.111.076661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, & Sved AF (2007). Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology (Berl), 194(4), 463–473. doi: 10.1007/s00213-007-0863-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney GC, Angelyn H, Cleary LM, & Meyer PJ (2019). Nicotine Produces a High-Approach, Low-Avoidance Phenotype in Response to Alcohol-Associated Cues in Male Rats. Alcohol Clin Exp Res, 43(6), 1284–1295. doi: 10.1111/acer.14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney GC, King CP, & Meyer PJ (2021). Systemic nicotine enhances opioid self-administration and modulates the formation of opioid-associated memories partly through actions within the insular cortex. Sci Rep, 11(1), 3321. doi: 10.1038/s41598-021-81955-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney GC, & Meyer PJ (2019). Nicotine pre-treatment reduces sensitivity to the interoceptive stimulus effects of commonly abused drugs as assessed with taste conditioning paradigms. Drug Alcohol Depend, 194, 341–350. doi: 10.1016/j.drugalcdep.2018.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney GC, Pautassi RM, Kapadia D, & Meyer PJ (2018). Nicotine affects ethanol-conditioned taste, but not place, aversion in a simultaneous conditioning procedure. Alcohol, 71, 47–55. doi: 10.1016/j.alcohol.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Scherma M, Rodriguez de Fonseca F, Gonzalez-Cuevas G, Fratta W, & Navarro M (2008). Changed accumbal responsiveness to alcohol in rats pre-treated with nicotine or the cannabinoid receptor agonist WIN 55,212–2. Neurosci Lett, 433(1), 1–5. doi: 10.1016/j.neulet.2007.11.074 [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Trigo-Diaz JM, Rodriguez de Fonseca F, Gonzalez Cuevas G, Gomez de Heras R, Crespo Galan I, & Navarro M (2004). Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology, 47(7), 1036–1044. doi: 10.1016/j.neuropharm.2004.08.002 [DOI] [PubMed] [Google Scholar]
- MacLean RR, Sofuoglu M, & Rosenheck R (2018). Tobacco and alcohol use disorders: Evaluating multimorbidity. Addict Behav, 78, 59–66. doi: 10.1016/j.addbeh.2017.11.006 [DOI] [PubMed] [Google Scholar]
- Maddux JN, & Chaudhri N (2017). Nicotine-induced enhancement of Pavlovian alcohol-seeking behavior in rats. Psychopharmacology (Berl), 234(4), 727–738. doi: 10.1007/s00213-016-4508-2 [DOI] [PubMed] [Google Scholar]
- Maggio SE, Saunders MA, Baxter TA, Nixon K, Prendergast MA, Zheng G, … Bardo MT (2018). Effects of the nicotinic agonist varenicline, nicotinic antagonist r-bPiDI, and DAT inhibitor (R)-modafinil on co-use of ethanol and nicotine in female P rats. Psychopharmacology (Berl), 235(5), 1439–1453. doi: 10.1007/s00213-018-4853-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, & Faure P (2006). Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron, 50(6), 911–921. doi: 10.1016/j.neuron.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Marshall CE, Dadmarz M, Hofford JM, Gottheil E, & Vogel WH (2003). Self-administration of both ethanol and nicotine in rats. Pharmacology, 67(3), 143–149. doi: 10.1159/000067801 [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, … Abi-Dargham A (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry, 58(10), 779–786. doi: 10.1016/j.biopsych.2005.04.044 [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, Addicott MA, … DeVito AM (2016). Hippocampal and Insular Response to Smoking-Related Environments: Neuroimaging Evidence for Drug-Context Effects in Nicotine Dependence. Neuropsychopharmacology, 41(3), 877–885. doi: 10.1038/npp.2015.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, & Rose JE (2005). Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology, 30(10), 1940–1947. doi: 10.1038/sj.npp.1300780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, … Balchunas E, (2009). Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry, 66(2), 185–190. doi: 10.1016/j.biopsych.2009.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, & Krishnan-Sarin S (2008). Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl), 196(2), 189–200. doi: 10.1007/s00213-007-0952-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy KR, Ramsay ME, Barrett ST, & Bevins RA (2021). Reward-enhancing effects of d-amphetamine and its interactions with nicotine were greater in female rats and persisted across schedules of reinforcement. Behavioural Pharmacology, 32(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson S, Packer RR, Cameron JM, Howell DN, & Roll JM (2014). Biochemical marker of use is a better predictor of outcomes than self-report metrics in a contingency management smoking cessation analog study. Am J Addict, 23(1), 15–20. doi: 10.1111/j.1521-0391.2013.12059.x [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, & Robinson TE (2012). Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One, 7(6), e38987. doi: 10.1371/journal.pone.0038987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, & Robinson TE (2012). A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl), 219(4), 999–1009. doi: 10.1007/s00213-011-2429-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, & Everitt BJ (2010). The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci, 31(12), 2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x [DOI] [PubMed] [Google Scholar]
- Mitchell SH, de Wit H, & Zacny JP (1995). Effects of varying ethanol dose on cigarette consumption in healthy normal volunteers. Behav Pharmacol, 6(4), 359–365. doi: 10.1097/00008877-199506000-00006 [DOI] [PubMed] [Google Scholar]
- Morel C, Montgomery S, & Han MH (2019). Nicotine and alcohol: the role of midbrain dopaminergic neurons in drug reinforcement. Eur J Neurosci, 50(3), 2180–2200. doi: 10.1111/ejn.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motschman CA, Gass JC, Wray JM, Germeroth LJ, Schlienz NJ, Munoz DA, … Tiffany ST (2016). Selection criteria limit generalizability of smoking pharmacotherapy studies differentially across clinical trials and laboratory studies: A systematic review on varenicline. Drug Alcohol Depend, 169, 180–189. doi: 10.1016/j.drugalcdep.2016.10.018 [DOI] [PubMed] [Google Scholar]
- Nadal R, & Samson HH (1999). Operant ethanol self-administration after nicotine treatment and withdrawal. Alcohol, 17(2), 139–147. doi: 10.1016/s0741-8329(98)00045-7 [DOI] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2009). The hidden island of addiction: the insula. Trends Neurosci, 32(1), 56–67. doi: 10.1016/j.tins.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct, 214(5–6), 435–450. doi: 10.1007/s00429-010-0268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, & Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531–534. doi: 10.1126/science.1135926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, … Lester HA (2007). Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci, 27(31), 8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2005). Is there a common molecular pathway for addiction? Nat Neurosci, 8(11), 1445–1449. doi: 10.1038/nn1578 [DOI] [PubMed] [Google Scholar]
- Nguyen QB, & Zhu SH (2009). Intermittent smokers who used to smoke daily: a preliminary study on smoking situations. Nicotine Tob Res, 11(2), 164–170. doi: 10.1093/ntr/ntp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, & Janak PH (2011). Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A, 108(11), 4459–4464. doi: 10.1073/pnas.1016156108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, & Blakemore C (2007). Development of a rational scale to assess the harm of drugs of potential misuse. Lancet, 369(9566), 1047–1053. doi: 10.1016/S0140-6736(07)60464-4 [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Lof E, Engel JA, & Soderpalm B (2001). Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol, 417(1–2), 117–123. doi: 10.1016/s0014-2999(01)00903-7 [DOI] [PubMed] [Google Scholar]
- Orr MF, Lederhos Smith C, Finlay M, Martin SC, Brooks O, Oluwoye OA, … McPherson SM (2018). Pilot investigation: randomized-controlled analog trial for alcohol and tobacco smoking co-addiction using contingency management. Behav Pharmacol, 29(5), 462–468. doi: 10.1097/FBP.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, & Wightman RM (2008). Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A, 105(33), 11957–11962. doi: 10.1073/pnas.0803896105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RR, Howell DN, McPherson S, & Roll JM (2012). Investigating reinforcer magnitude and reinforcer delay: a contingency management analog study. Exp Clin Psychopharmacol, 20(4), 287–292. doi: 10.1037/a0027802 [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, … Sved AF (2006). Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl), 184(3–4), 391–400. doi: 10.1007/s00213-005-0183-4 [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Kellicut MR, Brianna Sheppard A, Brown RW, & Robinson DL (2014). The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav, 126, 50–62. doi: 10.1016/j.pbb.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, & Sheppard AB (2013). The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl), 226(2), 247–259. doi: 10.1007/s00213-012-2892-9 [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, … Sved AF (2007). The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend, 89(1), 52–59. doi: 10.1016/j.drugalcdep.2006.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Liu L, Ngolab J, Zhao-Shea R, McIntosh JM, Gardner PD, & Tapper AR (2016). Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors. Neuropharmacology, 107, 294–304. doi: 10.1016/j.neuropharm.2016.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, & Everitt BJ (1999). Dissociation in Effects of Lesions of the Nucleus Accumbens Core and Shell on Appetitive Pavlovian Approach Behavior and the Potentiation of Conditioned Reinforcement and Locomotor Activity byd-Amphetamine. The Journal of Neuroscience, 19(6), 2401–2411. doi: 10.1523/jneurosci.19-06-02401.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, & Stewart JL (2014). Interoception and drug addiction. Neuropharmacology, 76 Pt B, 342–350. doi: 10.1016/j.neuropharm.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, & Berridge KC (2013). Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. European Journal of Neuroscience, 37(9), 1529–1540. doi: 10.1111/ejn.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (2004). Response to Dar and Frenk (2004), “Do smokers self-administer pure nicotine? A review of the evidence”. Psychopharmacology (Berl), 175(2), 256–258; author reply 259–261. doi: 10.1007/s00213-004-1930-7 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula A, Wilson AS, & Stiller RL (1997). Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav, 56(2), 235–241. doi: 10.1016/s0091-3057(96)00216-x [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grottenthaler A, & Wilson AS (2009). Lack of reinforcement enhancing effects of nicotine in non-dependent smokers. Psychopharmacology (Berl), 205(4), 635–645. doi: 10.1007/s00213-009-1574-8 [DOI] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (2013). Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl), 228(3), 479–486. doi: 10.1007/s00213-013-3054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Boldry MC (2017). Nicotine Acutely Enhances Reinforcement from Non-Drug Rewards in Humans. Frontiers in psychiatry, 8(65), 65. doi: 10.3389/fpsyt.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Boldry MC (2019). Reinforcement Enhancing Effects of Nicotine Via Patch and Nasal Spray. Nicotine Tob Res, 21(6), 778–783. doi: 10.1093/ntr/nty038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Robbins TW, & Everitt BJ (1994). Mesoaccumbens dopamine-opiate interactions in the control over behaviour by a conditioned reinforcer. Psychopharmacology, 114(2), 345–359. doi: 10.1007/BF02244858 [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, … Sher KJ (2011). The subjective effects of alcohol-tobacco co-use: an ecological momentary assessment investigation. J Abnorm Psychol, 120(3), 557–571. doi: 10.1037/a0023033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Wood PK, Shiffman S, Sher KJ, & Heath AC (2012). Responses to alcohol and cigarette use during ecologically assessed drinking episodes. Psychopharmacology (Berl), 223(3), 331–344. doi: 10.1007/s00213-012-2721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, & Dani JA (1997). Nicotine activates and desensitizes midbrain dopamine neurons. Nature, 390(6658), 401–404. doi: 10.1038/37120 [DOI] [PubMed] [Google Scholar]
- Pierce RC, & Kumaresan V (2006). The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev, 30(2), 215–238. doi: 10.1016/j.neubiorev.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, … Fratta W (2008). Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci, 28(47), 12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Cannady R, & Besheer J (2016). The nicotine + alcohol interoceptive drug state: contribution of the components and effects of varenicline in rats. Psychopharmacology (Berl), 233(15–16), 3061–3074. doi: 10.1007/s00213-016-4354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, McElligott ZA, & Besheer J (2020). Role of mPFC and nucleus accumbens circuitry in modulation of a nicotine plus alcohol compound drug state. Addict Biol, 25(4), e12782. doi: 10.1111/adb.12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, & Koob GF (1992). Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology, 109(1), 92–98. [DOI] [PubMed] [Google Scholar]
- Richardson BD, & Rossi DJ (2017). Recreational concentrations of alcohol enhance synaptic inhibition of cerebellar unipolar brush cells via pre- and postsynaptic mechanisms. J Neurophysiol, 118(1), 267–279. doi: 10.1152/jn.00963.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Hutchison MA, Chen SA, Thorsell A, Heilig M, & Riley AL (2011). Exposure to nicotine during periadolescence or early adulthood alters aversive and physiological effects induced by ethanol. Pharmacol Biochem Behav, 99(1), 7–16. doi: 10.1016/j.pbb.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev, 18(3), 247–291. doi: 10.1016/0165-0173(93)90013-p [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2000). The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction, 95 Suppl 2, S91–117. doi: 10.1080/09652140050111681 [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Flagel SB (2009). Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry, 65(10), 869–873. doi: 10.1016/j.biopsych.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, & Saunders BT (2014). On the motivational properties of reward cues: Individual differences. Neuropharmacology, 76, Part B(0), 450–459. doi: 10.1016/j.neuropharm.2013.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, … McBride WJ (2004). Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res, 28(8), 1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, & Carelli RM (2004). Dopamine operates as a subsecond modulator of food seeking. J Neurosci, 24(6), 1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, & Levin ED (1993). Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacol Biochem Behav, 44(4), 891–900. doi: 10.1016/0091-3057(93)90021-k [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, & Bates JE (2003). Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacol Biochem Behav, 76(2), 307–313. doi: 10.1016/j.pbb.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, & Salley A (2003). Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav, 76(2), 243–250. doi: 10.1016/j.pbb.2003.07.002 [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, & Johnson M (2000). Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav, 67(1), 71–81. doi: 10.1016/s0091-3057(00)00301-4 [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, & Lawhon D (2004). Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res, 6(1), 133–144. doi: 10.1080/14622200310001656957 [DOI] [PubMed] [Google Scholar]
- Rose JE, & Corrigall WA (1997). Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl), 130(1), 28–40. doi: 10.1007/s002130050209 [DOI] [PubMed] [Google Scholar]
- Rose JE, & Levin ED (1991). Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict, 86(5), 605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x [DOI] [PubMed] [Google Scholar]
- Rotge JY, Cocker PJ, Daniel ML, Belin-Rauscent A, Everitt BJ, & Belin D (2017). Bidirectional regulation over the development and expression of loss of control over cocaine intake by the anterior insula. Psychopharmacology (Berl), 234(9–10), 1623–1631. doi: 10.1007/s00213-017-4593-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Chappell A, & Legg B (2003). Measuring the appetitive strength of ethanol: use of an extinction trial procedure. Alcohol, 31(1–2), 77–86. doi: 10.1016/j.alcohol.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, & Chappell A (1998). Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res, 22(8), 1783–1787. doi:doi: 10.1111/j.1530-0277.1998.tb03980.x [DOI] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, & Barrot M (2014). The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience, 282, 198–216. doi: 10.1016/j.neuroscience.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Sato H, Kawano T, Yin DX, Kato T, & Toyoda H (2017). Nicotinic activity depresses synaptic potentiation in layer V pyramidal neurons of mouse insular cortex. Neuroscience, 358, 13–27. doi: 10.1016/j.neuroscience.2017.06.031 [DOI] [PubMed] [Google Scholar]
- Saunders BT, & Robinson TE (2010). A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry, 67(8), 730–736. doi: 10.1016/j.biopsych.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, & Robinson TE (2011). Individual variation in the motivational properties of cocaine. Neuropsychopharmacology, 36(8), 1668–1676. doi: 10.1038/npp.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, & Robinson TE (2012). The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci, 36(4), 2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier CJ, Dilly GA, & Gonzales RA (2013). Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the Long-Evans rat. Alcohol Clin Exp Res, 37(5), 740–747. doi: 10.1111/acer.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (1997). Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology, 7(2), 191–197. doi: 10.1016/s0959-4388(97)80007-4 [DOI] [PubMed] [Google Scholar]
- Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol, 80(1), 1–27. doi: 10.1152/jn.1998.80.1.1 [DOI] [PubMed] [Google Scholar]
- Schultz W (2015). Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev, 95(3), 853–951. doi: 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seungdamrong S, & Yasunaga S (1975). Relative severity of hemolysis in the subtypes of ABO incompatible hemolytic disease. IMJ Ill Med J, 148(5), 526–527, 530. [PubMed] [Google Scholar]
- Sharpe AL, & Samson HH (2001). Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol Clin Exp Res, 25(7), 1006–1011. doi: 10.1111/j.1530-0277.2001.tb02309.x [DOI] [PubMed] [Google Scholar]
- Sharpe AL, & Samson HH (2002). Repeated nicotine injections decrease operant ethanol self-administration. Alcohol, 28(1), 1–7. doi: 10.1016/s0741-8329(02)00238-0 [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, & Bartlett SE (2010). Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology, 35(7), 1453–1463. doi: 10.1038/npp.2010.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, & Bartlett SE (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res, 32(10), 1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, & Amit Z (1999). Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl), 142(4), 408–412. doi: 10.1007/s002130050906 [DOI] [PubMed] [Google Scholar]
- Smith CL, Jenkins G, Burduli E, Tham P, Miguel A, Roll J, & McPherson S (2020). Crossover associations of alcohol and smoking, craving and biochemically verified alcohol and nicotine use in heavy drinking smokers. Behav Pharmacol, 31(7), 702–705. doi: 10.1097/FBP.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, & Braus DF (2006). Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl), 184(3–4), 577–588. doi: 10.1007/s00213-005-0080-x [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, & Wightman RM (2009). Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci, 29(6), 1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srey CS, Maddux JM, & Chaudhri N (2015). The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Front Behav Neurosci, 9, 54. doi: 10.3389/fnbeh.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, … Baldwin RM (2006). Human tobacco smokers in early abstinence have higher levels of β2* nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience, 26(34), 8707–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfield SJ, Boettiger CA, & Robinson DL (2018). Nicotine-enhanced Pavlovian conditioned approach is resistant to omission of expected outcome. Behav Brain Res, 343, 16–20. doi: 10.1016/j.bbr.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Davalos A, … Serena J (2012). Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke, 43(1), 131–136. doi: 10.1161/STROKEAHA.111.630004 [DOI] [PubMed] [Google Scholar]
- Swanson LW (1982). The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull, 9(1–6), 321–353. doi: 10.1016/0361-9230(82)90145-9 [DOI] [PubMed] [Google Scholar]
- Taylor JR, & Robbins TW (1984). Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl), 84(3), 405–412. doi: 10.1007/BF00555222 [DOI] [PubMed] [Google Scholar]
- Taylor JR, & Robbins TW (1986). 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl), 90(3), 390–397. doi: 10.1007/bf00179197 [DOI] [PubMed] [Google Scholar]
- Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, … Dani JA (2018). Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration. Cell Rep, 23(1), 68–77. doi: 10.1016/j.celrep.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BM, Bevins RA, & Murray JE (2019). Interoceptive Stimulus Effects of Drugs of Abuse. In Neural Mechanisms of Addiction (pp. 89–101): Elsevier. [Google Scholar]
- Tizabi Y, Bai L, Copeland RL Jr., & Taylor RE (2007). Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol, 42(5), 413–416. doi: 10.1093/alcalc/agm057 [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Louis VA, & Taylor RE (2002). Effects of Combined Systemic Alcohol and Central Nicotine Administration into Ventral Tegmental Area on Dopamine Release in the Nucleus Accumbens. Alcoholism: Clinical and Experimental Research, 26(3), 394–399. doi: 10.1111/j.1530-0277.2002.tb02551.x [DOI] [PubMed] [Google Scholar]
- Toyoda H (2018). Nicotine facilitates synaptic depression in layer V pyramidal neurons of the mouse insular cortex. Neurosci Lett, 672, 78–83. doi: 10.1016/j.neulet.2018.02.046 [DOI] [PubMed] [Google Scholar]
- Toyoda H (2019). Role of nicotinic acetylcholine receptors for modulation of microcircuits in the agranular insular cortex. J Oral Biosci, 61(1), 5–11. doi: 10.1016/j.job.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Troisi JR 2nd, Dooley TF 2nd, & Craig EM (2013). The discriminative stimulus effects of a nicotine-ethanol compound in rats: Extinction with the parts differs from the whole. Behav Neurosci, 127(6), 899–912. doi: 10.1037/a0034824 [DOI] [PubMed] [Google Scholar]
- Truitt WA, Hauser SR, Deehan GA Jr., Toalston JE, Wilden JA, Bell RL, … Rodd ZA (2015). Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcohol-preferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology (Berl), 232(3), 639–649. doi: 10.1007/s00213-014-3702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, & Deisseroth K (2009). Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science, 324(5930), 1080–1084. doi: 10.1126/science.1168878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoh JY, Chi FW, Mertens JR, & Weisner CM (2011). Stopping smoking during first year of substance use treatment predicted 9-year alcohol and drug treatment outcomes. Drug Alcohol Depend, 114(2–3), 110–118. doi: 10.1016/j.drugalcdep.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, & Kenny PJ (2011). Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol, 82(8), 984–995. doi: 10.1016/j.bcp.2011.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Maggio SE, Reynolds AR, Casey EM, Bardo MT, Dwoskin LP, … Nixon K (2016). Critical needs in drug discovery for cessation of alcohol and nicotine polysubstance abuse. Prog Neuropsychopharmacol Biol Psychiatry, 65, 269–287. doi: 10.1016/j.pnpbp.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, … Shaham Y (2018). Volitional social interaction prevents drug addiction in rat models. Nat Neurosci, 21(11), 1520–1529. doi: 10.1038/s41593-018-0246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, & McKee SA (2017). An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am J Drug Alcohol Abuse, 43(2), 186–196. doi: 10.1080/00952990.2016.1189927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaggi CL, King CP, & Meyer PJ (2016). The tendency to sign-track predicts cue-induced reinstatement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology (Berl), 233(15–16), 2985–2997. doi: 10.1007/s00213-016-4341-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, … Piscani K (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res, 20(9), 1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x [DOI] [PubMed] [Google Scholar]
- Waeiss RA, Knight CP, Engleman EA, Hauser SR, & Rodd ZA (2020). Coadministration of ethanol and nicotine heightens sensitivity to ethanol reward within the nucleus accumbens (NAc) shell and increasing NAc shell BDNF is sufficient to enhance ethanol reward in naive Wistar rats. J Neurochem, 152(5), 556–569. doi: 10.1111/jnc.14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeiss RA, Knight CP, Hauser SR, Pratt LA, McBride WJ, & Rodd ZA (2019). Therapeutic challenges for concurrent ethanol and nicotine consumption: naltrexone and varenicline fail to alter simultaneous ethanol and nicotine intake by female alcohol-preferring (P) rats. Psychopharmacology (Berl), 236(6), 1887–1900. doi: 10.1007/s00213-019-5174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, & Han MH (2014). The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context. Neuroscience, 282, 101–108. doi: 10.1016/j.neuroscience.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, & de Wit H (2012). Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology (Berl), 220(1), 143–153. doi: 10.1007/s00213-011-2498-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Funk AP, & Goodwin RD (2016). A review of epidemiologic research on smoking behavior among persons with alcohol and illicit substance use disorders. Prev Med, 92, 148–159. doi: 10.1016/j.ypmed.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Platt J, Jiang B, & Goodwin RD (2015). Cigarette Smoking and Risk of Alcohol Use Relapse Among Adults in Recovery from Alcohol Use Disorders. Alcohol Clin Exp Res, 39(10), 1989–1996. doi: 10.1111/acer.12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, & Koob GF (1993). Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther, 267(1), 250–258. [PubMed] [Google Scholar]
- Wignall ND, & de Wit H (2011). Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Exp Clin Psychopharmacol, 19(3), 183–191. doi: 10.1037/a0023292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, & Fiez JA (2005). Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res, 7(4), 637–645. doi: 10.1080/14622200500185520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MH, Weyers P, Mucha RF, Stippekohl B, Stark R, & Pauli P (2011). Conditioned cues for smoking elicit preparatory responses in healthy smokers. Psychopharmacology (Berl), 213(4), 781–789. doi: 10.1007/s00213-010-2033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman SL, Gill DF, Bogdanic F, Long K, Al-Hasani R, McCall JG, … McGehee DS (2018). Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat Commun, 9(1), 2710. doi: 10.1038/s41467-018-04654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, & Everitt BJ (1993). Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology (Berl), 110(3), 355–364. doi: 10.1007/BF02251293 [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bevins RA, & Bardo MT (2009). Neuropharmacology of the interoceptive stimulus properties of nicotine. Curr Drug Abuse Rev, 2(3), 243–255. doi: 10.2174/1874473710902030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, & Berridge KC (2000). Intra-Accumbens Amphetamine Increases the Conditioned Incentive Salience of Sucrose Reward: Enhancement of Reward “Wanting” without Enhanced “Liking” or Response Reinforcement. The Journal of Neuroscience, 20(21), 8122–8130. doi: 10.1523/jneurosci.20-21-08122.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, & Robinson TE (2015). Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology (Berl), 232(17), 3149–3160. doi: 10.1007/s00213-015-3962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Mizukami T, Nakayama H, Takimura T, Sakuma H, Yoshimura A, … Higuchi S (2014). [Concurrent inpatient smoking cessation and alcohol abstinence programs for alcoholics and their outcomes]. Nihon Arukoru Yakubutsu Igakkai zasshi = Japanese journal of alcohol studies & drug dependence, 49(6), 381–390. [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, … Tapper AR (2015). Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun, 6, 6770. doi: 10.1038/ncomms7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D, Sadot-Sogrin Y, Goltseker K, Even-Chen O, Rahamim N, Shaham O, & Barak S (2017). Re-exposure to nicotine-associated context from adolescence enhances alcohol intake in adulthood. Sci Rep, 7(1), 2479. doi: 10.1038/s41598-017-02177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


