Abstract
The aperiodic exponent of the EEG power spectrum has received growing attention as a physiological marker of neurodevelopmental psychopathology, including attention-deficit/hyperactivity disorder (ADHD). However, its use as a marker of ADHD risk across development, and particularly in very young children, is limited by unknown reliability, difficulty aligning canonical band-based measures across development periods, and unclear effects of treatment in later development. Here we investigate the internal consistency of the aperiodic EEG power spectrum slope and its association with ADHD risk in both infants (n=69, 1-month-old) and adolescents (n=262, ages 11–17 years). Results confirm good to excellent internal consistency in infancy and adolescence. In infancy, a larger aperiodic exponent was associated with greater family history of ADHD. In contrast, in adolescence, ADHD diagnosis was associated with a smaller aperiodic exponent, but only in children with ADHD who had not received stimulant medication treatment. Results suggest that disruptions in cortical development associated with ADHD risk may be detectable shortly after birth via this approach. Together, findings imply a dynamic developmental shift in which the developmentally-normative flattening of the EEG power spectrum is exaggerated in ADHD, potentially reflecting imbalances in cortical excitation and inhibition that could contribute to long-lasting differences in brain connectivity.
Keywords: Neurodevelopmental Disorders, Electroencephalography, Biomarkers, Infant, Attention-deficit/hyperactivity disorder (ADHD)
Diagnosis of mental health disorders continues to rely solely on behaviorally-rated lists of symptoms rather than other types of physiological or biological markers. Attention-deficit/hyperactivity disorder (ADHD) is emblematic. Despite substantial heritability and a conceptualization of ADHD as a neurodevelopmental condition, no reliable biomarkers for ADHD yet exist. Indeed, the term biomarker is itself controversial, with debate about its appropriate definition (Lenzenweger, 2013), difficulty applying the term in the context of shifting behaviorally-based “gold standards” for diagnosis (Prata, Mechelli, & Kapur, 2014; Venkatasubramanian & Keshavan, 2016), and questions about the appropriate and ethical use of biomarkers in the fields of psychiatry and psychology (Singh & Rose, 2009). Here, we use the term biomarker to mean a physiological marker that is probabilistically associated with liability or risk for psychological disorders. Thus, while it may not yield definitive classification, a biomarker may be used to aid in clinical prediction or as part of a probabilistic algorithm for diagnosis. Because ADHD is a neurodevelopmental disorder with liability in early life, identifying biomarkers that can be used to estimate that risk and aid in clinical prediction can facilitate early prevention efforts— potentially preventing onset or minimizing long-term severity (Jaffee, 2018). During later childhood and adolescent development, physiological measures have the potential to not only aid diagnostic algorithms, but also to act as predictors of treatment response or of differential clinical courses. Thus, theoretically-motivated and objective markers for ADHD that can be measured across a wide developmental span are needed.
Electroencephalogram (EEG) measures are ideal for these purposes in that they are low cost, non-invasive and relatively easily obtained across a wide developmental range and in both clinical and non-clinical groups (Kappenman & Luck, 2016). Crucially, they are portable and field-deployable for clinical care as well. The application of EEG measures across development, and particularly in very young children, is limited by both unknown reliability and difficulty aligning canonical band-based measures across development periods (Bell & Cuevas, 2012; Saby & Marshall, 2012). Here we address that issue by investigating internal consistency of a novel EEG feature— the aperiodic exponent of the EEG power spectrum— and its association with ADHD risk in both infants and adolescents.
Establishing internal consistency is a limitation for all putative neural and physiological biomarkers for psychopathology. Similar to the way that internal consistency of self-report measures is related to the number of items on a questionnaire, the internal consistency of EEG-based markers depends, in part, on the number of data segments retained for averaging. Thus, both the length of the task (total segments) and data quality (usable segments) influence internal consistency. Internal consistency is an important psychometric property for neurophysiological biomarkers. In these cases, we expect high internal consistency because the same neurological processes should generate the signal across relatively short recording periods. If the underlying physiological signal itself is not consistent then adding more data will not help: the average of unreliable data will not be reliable (Foti, Kotov, & Hajcak, 2013). Further, the internal consistency of the signal may differ across clinical groups and developmental periods, and needs to be established separately within each population of interest (Karalunas, Bierman, & Huang-Pollock, 2016; Towers & Allen, 2009). Doing so here for the aperiodic exponent is therefore a potential a key step forward.
Periodic features of neural power spectra
The EEG signal is comprised of periodic (oscillations) and aperiodic (offset, exponent) signals (Donoghue et al., 2020b). Psychopathology research has emphasized differences in the periodic features of the power spectrum using canonical frequency bands (e.g. theta, alpha, beta, etc.). Power in several of these canonical bands have good internal consistency in both resting-state and task-based designs from middle childhood through adulthood (Salinsky, Oken, & Morehead, 1991; Tomarken, Davidson, Wheeler, & Kinney, 1992), including in clinical populations (Gold, Fachner, & Erkkilä, 2013; Lund, Sponheim, Iacono, & Clementz, 1995), but with somewhat lower reliability at the lowest and highest frequency ranges.
In ADHD, study of canonical frequency bands as biomarkers has yielded some progress, including evidence for imbalance of high versus low frequency activity (Barry, Clarke, Johnstone, & Brown, 2009; Loo et al., 2013; Newson & Thiagarajan, 2019) and low coherence in specific frequency ranges that are associated with differences in early life attention (Barry et al., 2011; Barry, Clarke, McCarthy, & Selikowitz, 2006; Barry et al., 2005; González et al., 2013; Whedon, Perry, Calkins, & Bell, 2016). Nonetheless, findings in older children and adults have been difficult to generalize to early life when the appropriate ranges for canonical frequency bands are difficult to define. In addition, internal consistency of the periodic features of the EEG signal may vary based on the specific definition of the frequency band that is used (Shackman, McMenamin, Maxwell, Greischar, & Davidson, 2010), so markers that are reliable in one developmental period may prove unreliable at other ages. Recent work in infants age 11–13 months suggests adequate internal reliability (α >.70) of some periodic EEG features (e.g., alpha band power and alpha asymmetry, Hill et al., 2020), but this work also highlights the challenges of determining the appropriate ranges for canonical bands in very young children.
Aperiodic features of neural power spectra
An alternative and complementary approach to characterizing the EEG power spectrum focuses on explicit parameterization of both periodic and aperiodic signals (Donoghue et al., 2020b). The aperiodic EEG power spectrum is characterized by an exponential decrease in power across increasing frequencies— that is, power is highest in low frequencies and gradually decreases in higher frequencies. This decrease follows approximately a 1/f distribution with the slope defined by the aperiodic exponent (i.e., χ in the 1/f^χ formulation). Aperiodic activity contributes power across frequencies. Oscillatory activity appears as “bumps” at specific frequencies where power rises above the aperiodic signal, (He, 2014; Freeman & Zhai, 2009). See Figure 1 for a visual depiction of the aperiodic and periodic activity for an exemplar resting-state EEG recording.
Figure 1.
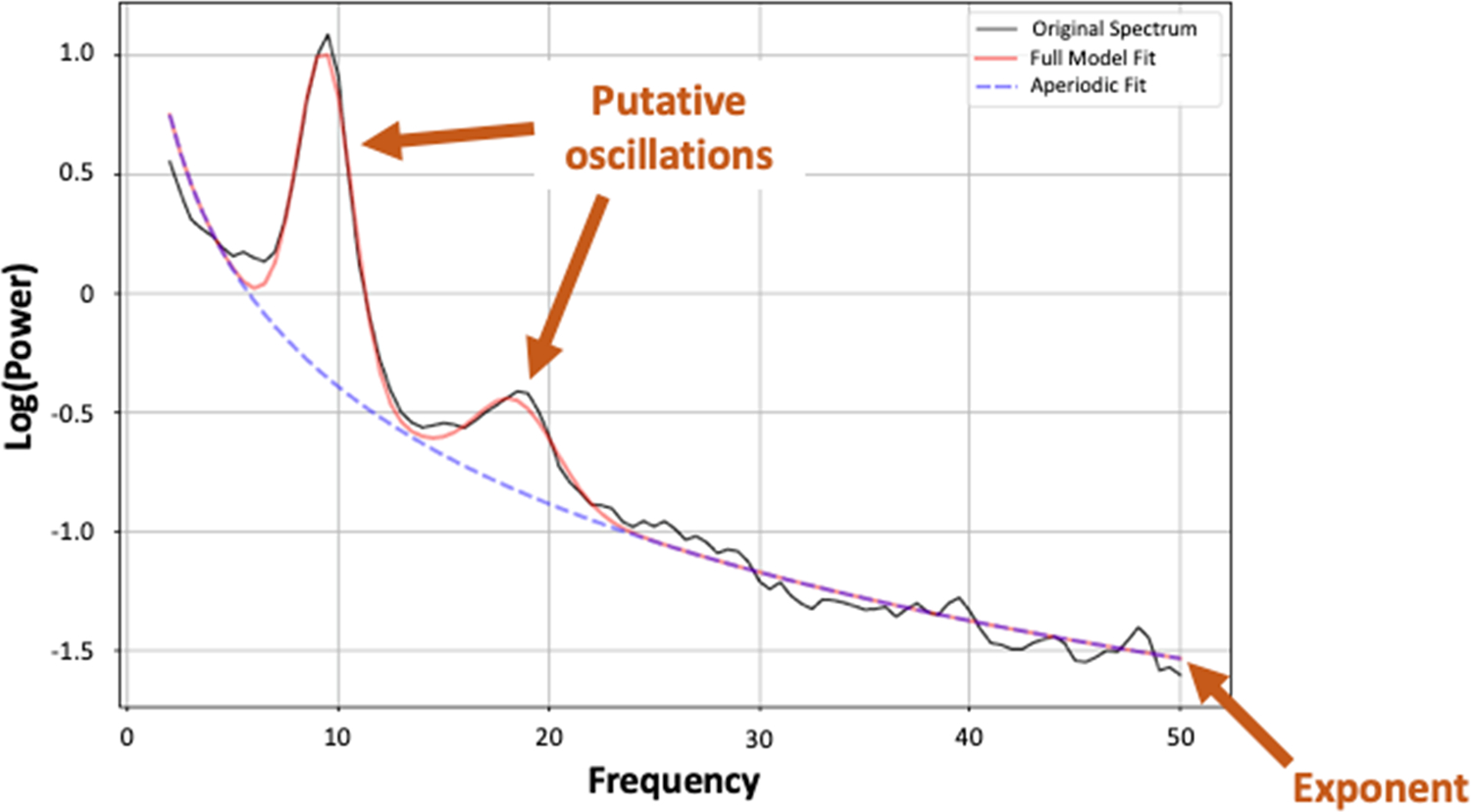
Parameterized adolescent EEG power spectrum from a single exemplar adolescent in an eyes-closed 32-channel resting state recording. In this example, two oscillatory peaks are identified with center frequencies at 9.37 Hz and 18.45 Hz. These peaks rise above the aperiodic signal (exponent = 1.63) that affects all frequencies.
Approaches that quantify both aperiodic and periodic activity have at least two potential benefits. First, standard approaches to measuring oscillatory activity risk conflating differences in aperiodic activity with differences in oscillatory power (Donoghue, Dominguez, & Voytek, 2020a; Schaworonkow & Voytek, 2021)). We do not mean to imply that spectral work is not valuable, indeed, it has and will continue to be productive in many contexts. However, ratio-based metrics such as those that have been of interest in ADHD may be particularly likely to be confounded by differences in aperiodic activity. Further, approaches that control for differences in aperiodic activity may be important for quantifying oscillatory activity in some cases (Ostlund et al., 2021b).
Secondly, and the main focus of the current study, the aperiodic exponent may be of interest in its own right. Animal and computational models suggests that this exponent reflects the ratio of excitation and inhibition in cortical circuits (Gao, Peterson, & Voytek, 2017) with a shift away from cortical inhibition reflected as a smaller exponent (flatter power spectrum). Individual differences in the aperiodic exponent have been speculatively linked to less efficient information processing due to increased “neural noise” that may underlie multiple forms of psychopathology (Voytek & Knight, 2015).
Another major benefit of an aperiodic exponent measure for studies of neurodevelopmental disorders is that is that it is readily measured at rest (so can be assessed even in extremely young infants) and that it treats the power spectrum as a continuous dimension rather than requiring a priori assumptions about specific bands of interest. Thus, it is related to differences in well-studied canonical bands but avoids some of the interpretive problems associated with them in young children. Perhaps for this reason, recent studies find that aperiodic features are better predictors of individual differences than periodic band measures (Demuru & Fraschini, 2020).
Developmental and clinical patterns in aperiodic activity
In typical development, there is a normative broadband “flattening” of EEG power spectra with age driven by changes in aperiodic activity and increases in some higher frequency bands (He et al., 2019; Schaworonkow & Voytek, 2021; Voytek & Knight, 2015). Recent work also identifies differences in the aperiodic exponent in ADHD (Robertson et al., 2019) and other neurodevelopmental psychopathology such as schizophrenia (Peterson, Rosen, Campbell, Belger, & Voytek, 2017). In ADHD, there is emerging evidence that the power spectral slope is associated with ADHD generally, and with cognitive impairments in ADHD, specifically (Ostlund, Alperin, Drew, & Karalunas, 2021a; Pertermann, Bluschke, Roessner, & Beste, 2019; Robertson et al., 2019). However, the direction of these effects has been mixed. Studies in young children have identified steeper power spectral slope (Robertson et al., 2019), while those in adolescents have found a flatter power spectral slope (Ostlund et al., 2021a) but no study has simultaneously considered both age groups. Further, while differences have been observed in children as young as 3 years old (Robertson et al., 2019), it remains unclear how early in development these disruptions emerge, and studies in even earlier developmental periods are needed (Schaworonkow & Voytek, 2021).
In addition, how group differences in the aperiodic exponent are related to treatment history is unclear. Some studies suggest that acute treatment with stimulants may normalize aperiodic activity (Pertermann et al., 2019) or that trait-like differences in aperiodic activity may only be present for stimulant-naïve children (Robertson et al., 2019). However, the few available studies have focused on early and middle childhood. Larger studies replicating results and extending into adolescence are needed, particularly because earlier treatment with stimulant medication is confounded with symptom severity.
Finally, because the approach to characterizing aperiodic features of the power spectrum is new, very little is known about the internal consistency of these features. One recent study in typically-developing adults found good test-retest reliability of the aperiodic exponent (ICCs > .80; Pathania, Schreiber, Miller, Euler, & Lohse, 2021); however, results may not generalize to clinical groups, such as ADHD, or to earlier developmental periods. In addition, internal consistency has not been assessed.
Current Study
Here we examine internal consistency of the aperiodic exponent of the EEG power spectrum in two critical developmental periods: 1) early adolescence, when ADHD symptom courses often diverge, and 2) very early infancy (1-month-olds), when early risk prediction could critically inform early prevention efforts. To inform future work, we assess internal consistency with differing amounts of available data and in clinical and non-clinical groups (ADHD versus control in the adolescents and family history of ADHD versus no family history in the infants). We then test the relationship between the aperiodic exponent and ADHD— using ADHD diagnosis in the adolescents and a dimensional measure of ADHD risk based on family history in the infants. We expected good to excellent internal consistency in the adolescents and lower but adequate internal consistency in the infants, in line with findings for canonical frequency bands (using standard criteria of > 0.9= excellent, 0.80–0.89= Good, 0.70–0.79= Adequate, and < 0.70= Poor). We also hypothesized that adolescents with ADHD would have a flatter power spectral slope (smaller exponent) than their typically-developing peers, consistent with earlier findings in a large subset of the sample here (Ostlund et al., 2021a). We sought to test whether this association differed based on lifetime treatment with stimulants which our prior study did not address. Finally, we examined whether an association between the aperiodic exponent and ADHD risk could be detected in very early development, but did not make predictions about the direction of association at this age.
Method
The current study relies on data from two different cohorts: 1) the Oregon ADHD 1000 cohort (e.g., Karalunas, Gustafsson, Fair, Musser, & Nigg, 2019; Nigg et al., 2020) and 2) the Prenatal Environment and Child Health (PEACH) study of early development (Sullivan et al., 2015).
Oregon ADHD 1000 cohort
Participants were initially recruited into the longitudinal study between the ages of 7–11 years using a community-based strategy based on public advertising and outreach. All children in the longitudinal study were invited to participate in an optional EEG testing visit at a single time point (Year 5, 6, or 8 of the larger study depending on the date of their initial enrollment). Two hundred and sixty-two (nADHD= 107) individuals between the ages of 11–17 years completed this optional EEG testing visit. A parent/legal guardian provided written informed consent, and adolescents provided written assent for the study. Ethics approval was obtained from the Institutional Review Board at Oregon Health & Science University (OHSU).
ADHD Diagnosis.
All children underwent a thorough diagnostic evaluation at baseline that included: 1) parent/guardian and teacher standardized behavior rating scales: ADHD Rating Scale (DuPaul, Power, Anastopoulos, & Reid, 1998), Conners’−3 (Conners, 2008), Strengths & Difficulties Questionnaire (Goodman, 2001); 2) a semi-structured clinical interview: Kiddie Schedule for Affective Disorders and Schizophrenia; parent only (Puig-Antich & Ryan, 1986); 3) behavior ratings of anxiety and depression: Multidimensional Anxiety Scale for Children (March, 2013) and Children’s Depression Inventory (Kovacs, 2011); and 4) IQ and academic achievement screening: Wechsler Intelligence Scale for Children, 4th Ed., Vocabulary, Block Design, and Information (Wechsler, 2002) and Wechsler Individual Achievement Test, 2nd Ed. Word Reading and Math Reasoning (Wechsler, 2003). Using all available information, baseline diagnoses were made by a clinical diagnostic team that included a board-certified child psychiatrist with over 25 years of experience and a licensed child neuropsychologist with over 10 years of experience. Blind to one another’s ratings, they formed a diagnostic opinion based on all available information. Their agreement rate was excellent (ADHD diagnosis kappa=.88). Disagreements were conferenced and consensus reached. Cases where consensus was not readily achieved were excluded from the longitudinal study.
Diagnostic assessment was repeated at all years, including the year at which EEG was recorded. In addition to the diagnostic team assessments, total symptom counts were determined by combining parent (K-SADS) and teacher (ADHD-RS) report using an “OR” algorithm (Pelham, Fabiano, & Massetti, 2005). Following the DSM, final diagnostic groups were determined as follows: Individuals with ADHD were required to have ≥ 6 hyperactive or ≥ 6 inattention symptoms, as well as parent reported impairment on the K-SADS. Individuals in the control group were required to have ≤ 3 hyperactive, ≤ 3 inattention symptoms, and ≤ 4 total symptoms with no reported impairment.
Exclusion Criteria.
Children were excluded at baseline if they were prescribed long-acting, non-stimulant psychotropic medications; had self-reported history of neurological impairment such as seizures or head injury with loss of consciousness; had a history of substance abuse; had prior diagnosis of intellectual disability, autism spectrum disorder, or psychosis; were currently experiencing a major depressive episode; or had estimated IQ < 70. Although adolescents who began treatment with non-stimulant medications after enrollment were allowed to continue in the longitudinal study, they were excluded from the EEG visit.
Stimulant Medication.
Adolescents taking stimulant medication at the time of EEG assessment were asked to do a 24- or 48-hour washout (depending on the half-life of the medication prescribed), which was verbally confirmed with the family at the start of the visit.
PEACH Cohort
Pregnant women (N=300) ages 18–40 who were less than 24 weeks gestation were recruited through OHSU, a major academic medical center located in Portland, Oregon. Patients receiving prenatal care through OHSU and its affiliated clinics were identified via electronic medical records and were contacted via email or telephone to assess interest and eligibility. When available, women’s medical records were reviewed for exclusionary conditions prior to contact, to minimize participant and staff burden. Participants were also recruited via fliers located on the OHSU campus and via social media ads targeting women living in the greater Portland metropolitan area. In recruiting, prioritization was given to patients who planned to give birth at OHSU to facilitate the collection of delivery tissue for the larger project.
All families were invited to participate in an additional, optional visit where EEG was collected when their child was 1-month-old (mean = 6.08 weeks, SD = 1.67 weeks). A total of 69 families agreed to participate in the optional EEG visit before data collection was halted at the onset of the COVID global pandemic. Because the longitudinal study is ongoing, the total N here reflects 73% agreement rate for eligible participants.
Exclusion Criteria.
All participants were screened for eligibility via telephone. Exclusionary criteria included being pregnant with multiples, known fetal anomaly or genetic condition that may influence child brain development or behavior, current substance use (illicit drugs, tobacco, alcohol, marijuana), and current use of medications that may influence inflammation (e.g., systemic corticosteroids) or that have known or suspected teratogenic effects. Women with a history of recurrent pregnancy loss in the second or third trimester and those who conceived their child using a donated oocyte were also excluded. To best serve the study’s overarching goal of examining the influence of maternal perinatal nutrition, adiposity, and metabolic state on child behavior, medical conditions that might affect inflammation or be confounded with obesity were also exclusionary criteria. This included a current diagnosis of Diabetes Type I/II, Cancer, Kidney Disease, Polycystic Ovarian Syndrome (as confirmed by hyperandrogenism or a history of medication used to treat the condition), and autoimmune diseases (e.g., Crohn’s disease).
ADHD Family History.
Parents reported on family history of ADHD (either suspected or diagnosed) in biological parents, full and half siblings, biological aunts and uncles, and grandparents. A weighted ADHD Family History variable was calculated according to the follow equation: ADHD Family History = (# first degree relatives with ADHD/ total # first degree relatives)*.5 + (number second degree relatives with ADHD/ total # second degree relatives)*.25. For the current analyses, both diagnosed and suspected cases were counted as ADHD.
EEG recording procedures
EEG was recorded with either 32 (infant n= 69 and adolescents n= 168) or 64 (adolescent n= 96) Ag-AgCl active electrodes placed based on an extended international 10–20 system using an Easycap (Easycap GmbH; configurations available at https://www.easycap.de/author/easycap/). The EEG signal was amplified with Brain Products’ ActiCHamp system and digitally recorded at 500 Hz using PyCorder v1.0.9. Impedance levels for each electrode were at or below 50kΩ during data collection. EEG was referenced online to the central midline electrode site (Cz).
Oregon ADHD 1000 EEG Recording.
Participants completed a resting baseline EEG recording as the first task in a longer laboratory protocol. EEG was continuously recorded during an eight-minute baseline task, which was divided into four two-minute blocks. Adolescents were instructed to keep their eyes open (EO) on two of the blocks during which they looked at a central fixation cross presented on a computer monitor and to keep eyes closed (EC) for the other two blocks. Blocks alternated between EC and EO conditions (EC, EO, EC, EO).
PEACH EEG Recording.
EEG data were recorded for 3 minutes. All infants were awake at the time of recording. They were held by their mothers facing away from the parent in most cases; however, when this position caused the infant to be distressed, parents were able to turn their child to face them or to a side-facing position. Parents were encouraged to remain quiet and still for the duration of recording while holding their child.
EEG data processing
EEG data were down sampled to 250 Hz and re-referenced to the average of all electrodes offline. Adolescent data were cleaned and processed using EEGLAB and ERPLAB (Brunner, Delorme, & Makeig, 2013; Lopez-Calderon & Luck, 2014) toolboxes in MATLAB. Infant data were preprocessed using identical procedures in Brain Vision Analyzer (v. 2.2.0, 2019), except where noted.
All data were filtered with an infinite impulse response bandpass filter with a half-amplitude cutoff of 0.1 Hz and 50 Hz, and a 12 dB/octave roll-off to the data. An independent components analysis was used to correct eye blink artifacts in adolescents. The data were segmented into 2-second non-overlapping epochs. Epochs were discarded from the analyses if they contained baseline drift or movement artifacts greater than 90 μV in the adolescents and 100 μV in the infants. Individual channels were interpolated if greater than 20% of epochs were flagged after artifact rejection. A Hanning window was applied and all artifact free 2-second epochs were analyzed using a fast Fourier transform (FFT). Power output (μV2) was generated in 0.5Hz bins from 2–50 Hz for adolescents and 1–30Hz for infants for each epoch.
Analytic Approach
Aperiodic exponent.
EEG power spectral features were parameterized using the specparam algorithm (version 1.0.0), an automated algorithm that disentangles periodic and aperiodic signals, although periodic activity was not considered in the current study. A detailed description of the specparam algorithm (see Donoghue et al., 2020b) and tutorials for use (Ostlund et al., 2021b) are available elsewhere.
We extracted aperiodic exponents from the 2–50 Hz frequency range for the adolescent data and 1–30 Hz frequency range for the infant data. Age-specific frequency ranges were selected to adhere to recommendations to fit the models over broad frequency ranges and to conform to prior studies. Prior research in children and adolescents has calculated the aperiodic exponent over 2–50 Hz range (Ostlund et al., 2021a; Robertson et al., 2019; Waschke et al., 2021). In infants, low frequency activity is dominant and concerns with muscle contamination at high frequencies are greater. Consistent with the only other study in very early development (Schaworonkow & Voytek, 2021), we parameterized the exponent from 1–30 Hz for infants.
For both infants and adolescents, the following spectral parameterization parameters were used: aperiodic_mode = ‘fixed’; peak_width_limits = [1, 8]; max_n_peaks = 6; default settings otherwise. Aperiodic exponent was calculated at each electrode site then all sites averaged to provide a grand average value across the scalp. Figure 2 shows scalp variation in exponent values. (Note that this approach differs from the approach used in our prior analyses in the adolescent sample; Ostlund et al., 2021, in which aperiodic slope was calculated at a single electrode site— Cz. We address these differences the Discussion.) In analyses reported here, slope estimates for adolescents did not differ based on whether recording was with 32 or 64 electrodes (EC p = .11, EO p = .73). This is perhaps not surprising. Unlike neural oscillations, the putative generator of aperiodic activity is not known to be locally defined. As such, researchers vary in the number of electrode channels considered in their analyses, ranging from a single site (Immink et al., 2021) to data averaged within a region (Cellier, Riddle, Petersen, & Hwang, 2021; Pertermann et al., 2019; Robertson et al., 2019) and across the scalp (Donoghue et al., 2020a; He et al., 2019; Molina et al., 2020). There are currently no specific recommendations regarding the number of channels to include when parameterizing aperiodic activity, and the selection of electrodes will ultimately depend on the recording context and questions of interest (e.g., in task-based studies of specific processes versus resting state recordings).
Figure 2.
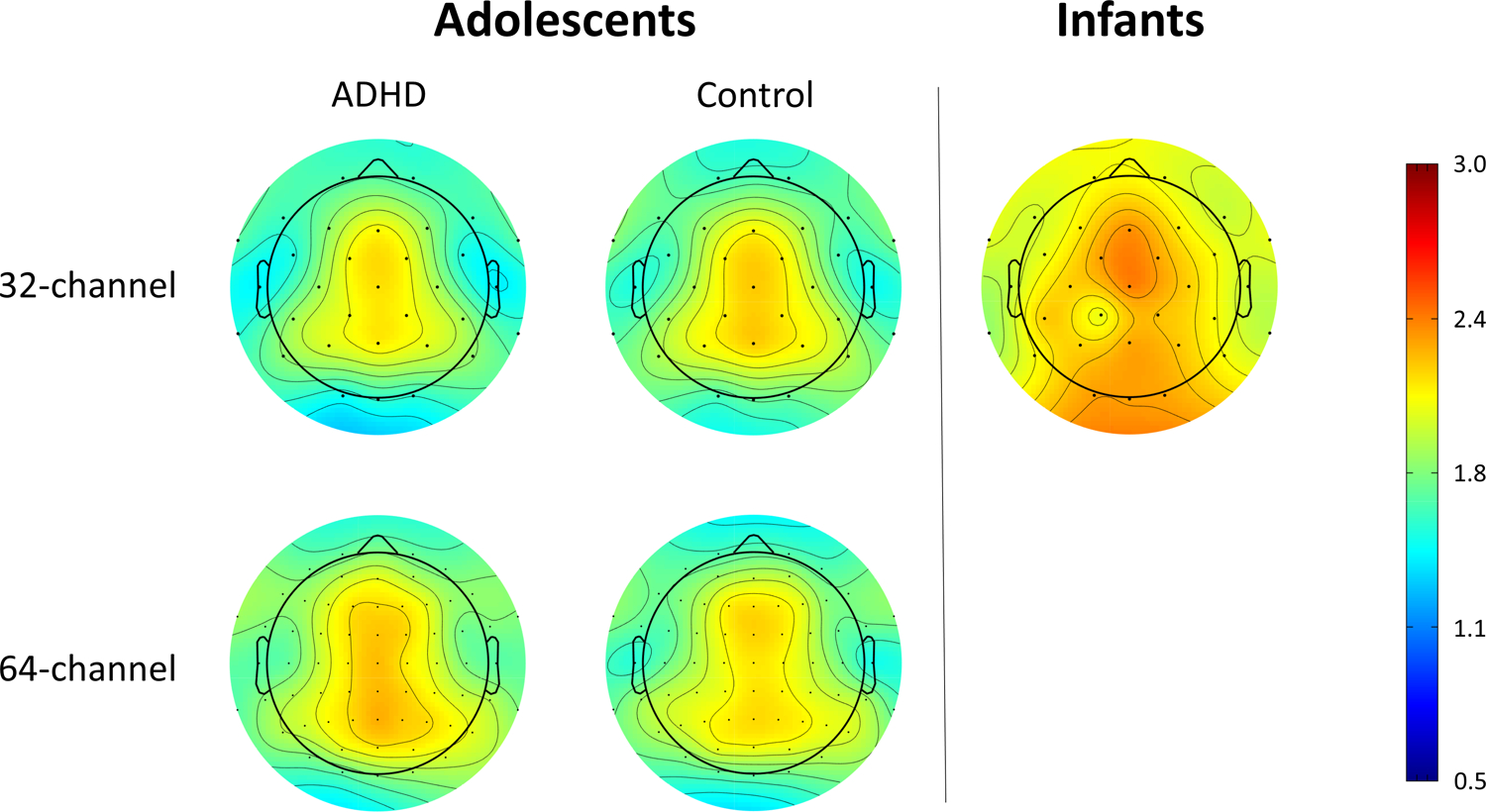
shows the variation in the aperiodic exponent across the scalp for adolescents (average of eyes-open and eyes-closed) and infants. Steeper exponents are observed in infants than in adolescents across the scalp. In general, exponents appear smaller as you move away from central midline sites. This effect appears similar across ages and diagnostic groups. Current analyses used an average of all electrodes. Additional consideration of how aperiodic exponents vary across the scalp will be important.
Internal Consistency.
We assessed reliability of the aperiodic exponent with 15 seconds to 2 minutes of available data for infants and 1–3 minutes of available data in adolescents. To assess effects of available data on internal consistency, we used a bootstrapping procedure similar to Towers & Allen (2009). First, for each participant with the minimum necessary data available, epochs were randomly selected from all available epochs. The randomly selected epochs were then split in half and the specparam algorithm described above was run on each half to obtain two estimates of the aperiodic exponent (one from each half of the data). This procedure was repeated 500 times. Scripts used to perform these analyses have been posted on OSF at: https://osf.io/hqyue/.
We next concatenated each participant’s 1st bootstrap runs into a single dataset and computed the Pearson’s correlation between the halves, then repeated this procedure 500 times (once for each bootstrap run). The resulting 500 Pearson’s r values were converted to Fisher’s z values, averaged, then converted back to Pearson’s r, and corrected via the Spearman-Brown prophecy formula. This approach yielded an average estimate of internal consistency along with an estimate of the standard deviation across runs. Estimates were derived separately for 1) eyes-open condition for adolescents with 32-channel data, 2) eyes-closed condition for adolescents with 32-channel data, 3) eyes-open condition for adolescents with 64-channel data, 4) eyes-closed condition for adolescents with 64-channel data and 5) infants. To test for differences in reliability between clinical groups, the continuous family history of ADHD variable (infants) or current ADHD diagnosis (adolescents) was included as a moderator.
Relationship to ADHD.
The relationship between either ADHD family history (infants) or current ADHD diagnosis (adolescents) was tested using standard regression approaches implemented in MPLUS v.8.5 (Muthén & Muthén, 1998–2020) with full information maximum likelihood procedures used to handle missing data and the robust maximum likelihood (MLR) estimator to handle non-normality of data. For the adolescent sample, we also tested whether lifetime history of treatment with stimulant medications as reported by parents moderated the relationship between current ADHD status and the aperiodic exponent. The exponent value for these analyses was estimated based on all available data for each person. Infant analyses were restricted to children with at least 30 seconds of available data. Sex, income, and age at EEG based on expected due date (infants) or age in months (adolescents) were used as covariates in analyses.
Results
Descriptive information for both samples is provided in Table 1. All reliability estimates are provided in Table 2 and shown in Figure 3.
Table 1.
Sample Demographics
| Typically-Developing (n = 152) | ADHD (n=107) | Infant (n=69) | |
|---|---|---|---|
| Age (years/weeks; mean (SD))a | 14.1 (1.3) | 14.0 (1.4) | 5.7 (1.6) |
| Sex (% male) | 55.9% | 72.3% | 51.5% |
| Annual Family Income (median range) | $75–100,000 | $50–75,000 | $100–199,000 |
| Median in lowest earning quartile | $50–75,000 | $25–35,000 | $50–75,000 |
| Median in highest earning quartile | $100–130,000 | $75–100,000 | $100–199,000 |
| Ethnicity (% Hispanic/Latinx) | 6.8% | 6.30% | 8.6% |
| Raceb | |||
| % American Indian/Alaskan Native | 4.2% | 0.9% | 3.0% |
| % Asian/East Indian | 5.9% | 5.4% | 12.1% |
| % Native Hawaiian/Pacific Islander | 2.5% | 1.8% | 6.1% |
| % Black/African American | 5.9% | 11.6% | 6.1% |
| % White | 98.3% | 94.6% | 86.4% |
| Current Stimulant Treatment | --- | 53.6% | --- |
| Lifetime History of Stimulant Treatment | 7.60% | 75% | --- |
| Birthweight (kg; mean (SD)) | --- | --- | 3.4 (0.5) |
| Family History Score (mean (SD)) | --- | --- | 0.06 (0.15) |
Note:
Adolescent sample age reported in years; infant sample age reported in weeks
Sex assigned at birth is report; data on gender identity were not available for the Year of EEG data collection
Percentage of participants who identified with specific NIH race categories. Because some people endorsed more than one option, numbers may add to >100%.
Table 2.
Internal consistency of Aperiodic Slope Exponents
| Condition and Amount Available Data | Mean r across bootstrap runs | SD r across bootstrap runs | N† |
|---|---|---|---|
| Eyes closed - 32 - 1 minute available data | 0.97 | 0.08 | 167 |
| Eyes closed - 32 - 2 minute available data | 0.98 | 0.09 | 159 |
| Eyes closed - 32 - 3 minute available data | 0.98 | 0.09 | 139 |
| Eyes closed - 64 - 1 minute available data | 0.97 | 0.10 | 95 |
| Eyes closed - 64 - 2 minute available data | 0.98 | 0.10 | 93 |
| Eyes closed - 64 - 3 minute available data | 0.99 | 0.12 | 79 |
| Eyes open - 32 - 1 minute available data | 0.97 | 0.08 | 166 |
| Eyes open - 32 - 1 minute available data | 0.99 | 0.07 | 162 |
| Eyes open - 32 - 1 minute available data | 0.99 | 0.08 | 143 |
| Eyes open - 64 - 1 minute available data | 0.98 | 0.09 | 95 |
| Eyes open - 64 - 2 minute available data | 0.99 | 0.09 | 92 |
| Eyes open - 64 - 3 minute available data | 0.99 | 0.09 | 82 |
| Infant resting baseline - 15 seconds available data | 0.82 | 0.18 | 42 |
| Infant resting baseline - 30 seconds available data | 0.84 | 0.22 | 30 |
| Infant resting baseline - 1 minute data available | 0.84 | 0.30 | 21 |
| Infant resting baseline - 2 minutes data available | 0.87 | 0.47 | 7 |
Note:
Number of channels varied between subjects for the adolescent sample (no children had both 32- and 64-channel recordings). Total N for each amount of available data can be obtained by adding Ns for the 32- and 64-channel conditions.
Figure 3.
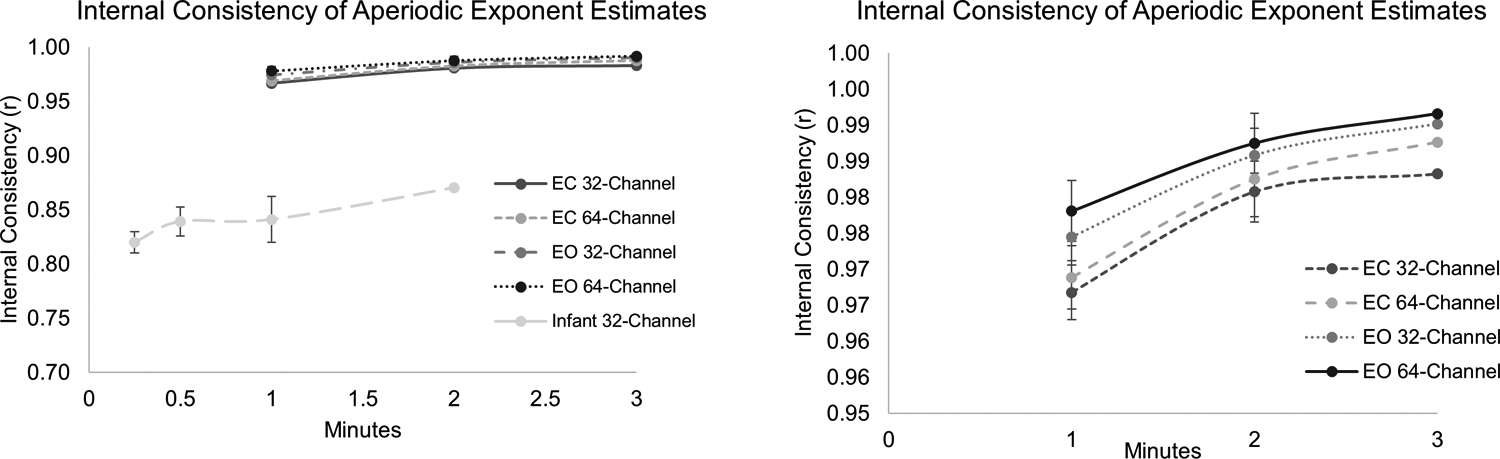
shows the internal consistency estimates (Spearman-Brown corrected spilt-half correlations) based on 500 bootstrap samples with varying amounts of available data. Panel A shows all data and Panel B shows only the range >.95 in order to visualize differences at the upper end of the reliability dimension.
Reliability of adolescent data.
Estimates of the aperiodic exponent showed excellent reliability, 0.97 or greater in all cases. Notably, all of the adolescents in our sample had at least 1 minute of available data and nearly 90% had at least 2 minutes (95% of those with 32-channel data and 89% of those with 64-channel data). Internal consistency of the aperiodic exponent was excellent in adolescents at amounts of data that are readily obtainable at this age, even in clinical groups. Reliability was not moderated by current ADHD diagnosis (β = .002, p = .394).
Reliability of infant data.
As expected, internal consistency of the infant aperiodic slope was lower than for the adolescents; however, internal consistency was still good for all amounts of available data (range 0.82–0.87). Reliability increased with 30 seconds versus 15 seconds of data (.82 versus .84). There was no additional improvement in reliability when going from 30 seconds to 1 minute of recording (both rs = 0.84) but reliability again increased when 2 minutes of data were available (r = .87). Reliability was not moderated by family history of ADHD (β = −.08, p = .176).
Relationship to ADHD Diagnosis in Adolescents.
Consistent with prior findings in a subset of this adolescent sample, adolescents with a current ADHD diagnosis had a smaller exponent (flatter power spectrum) than those without ADHD (β = −.29, p = .002). However, this relationship was moderated by history of stimulant medication treatment (β =1.3, p = .007). Children with ADHD and no history of stimulant treatment had smaller exponents than their non-ADHD peers. In contrast, children with ADHD who had been treated with stimulants did not differ from their non-ADHD peers. Figure 4 shows these effects.
Figure 4.
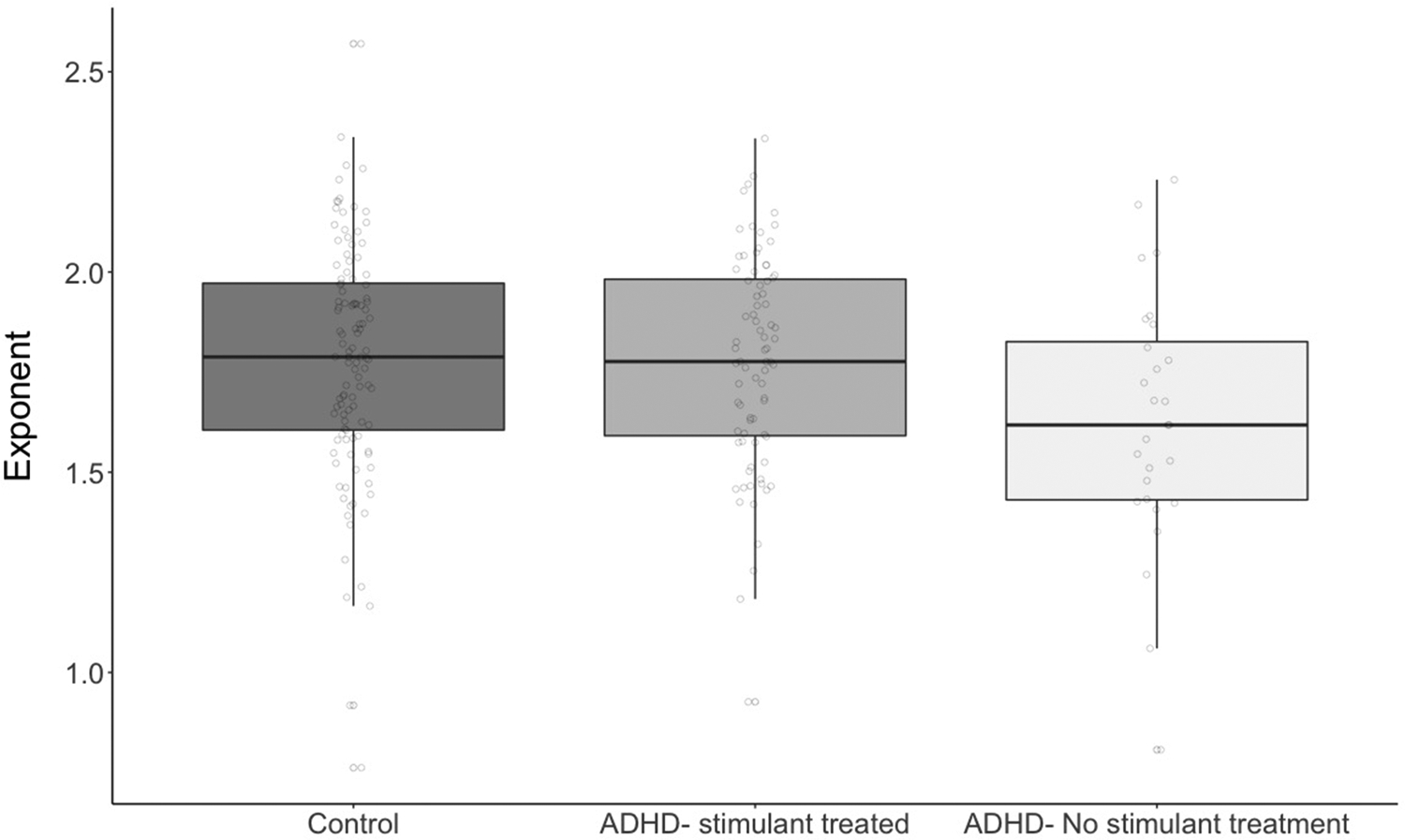
shows group differences in aperiodic exponent estimates for typically-developing children, stimulant-naïve children with ADHD, and children with ADHD and a lifetime history of treatment with stimulants. Children with ADHD who had not received stimulant treatment had smaller aperiodic exponents (flatter spectral slope) than typically-developing children; children who had been treated with stimulants did not differ from typically-developing children.
Relationship to ADHD Family History in Infants.
In the 1-month-old infants, the aperiodic exponent was significantly related to the dimensional measures of ADHD family history (β = .39, p = .010). In contrast to the adolescents, among infants, a larger aperiodic exponent (steeper power spectrum) predicted higher ADHD family history scores. Figure 5 shows the association, and Figure 6 schematically depicts the developmental trend from steeper to flatter spectral slope, which we address further in the Discussion.
Figure 5.
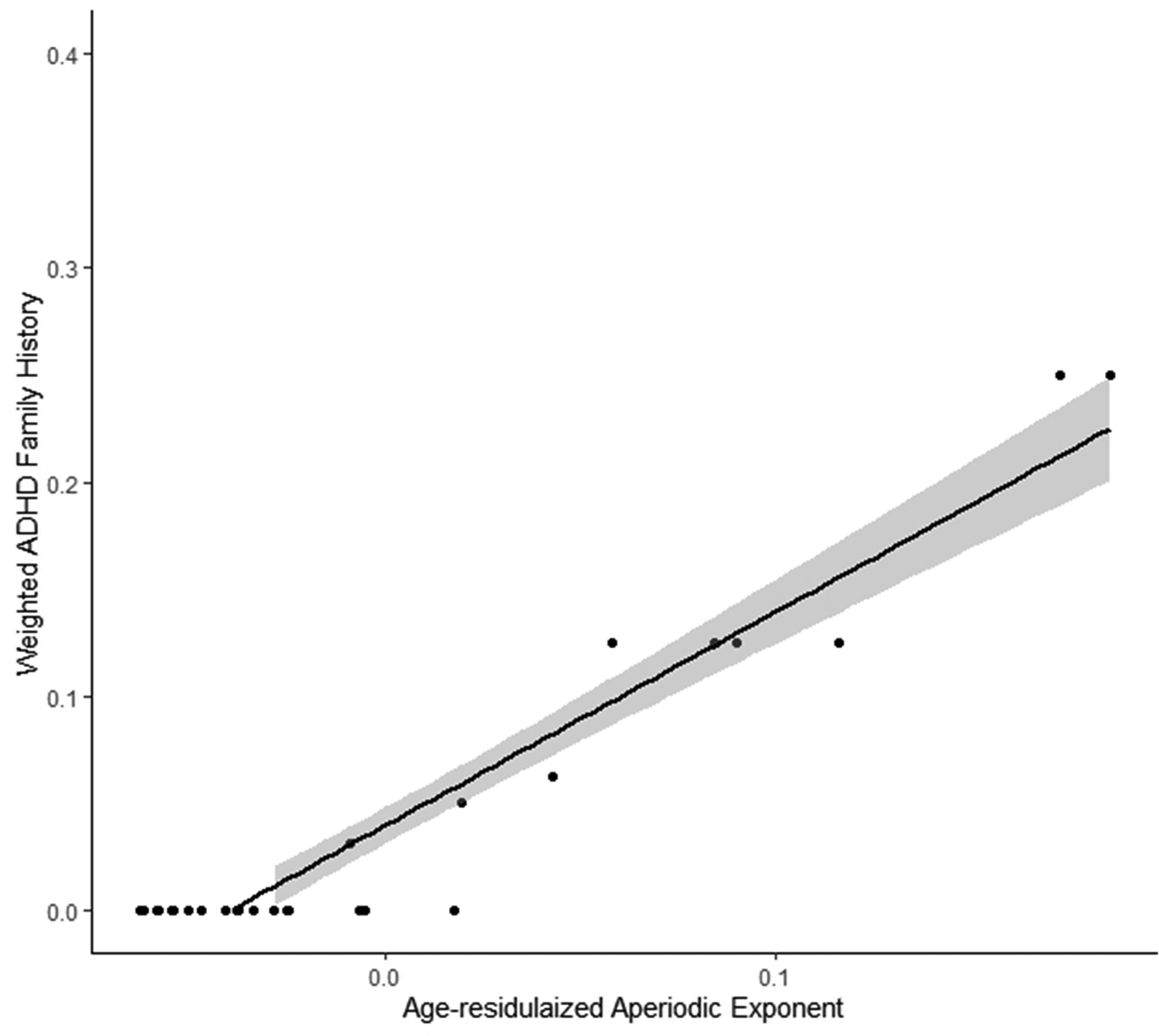
shows the linear relationship between the aperiodic exponent and a dimensional measure of ADHD family history. Greater ADHD family history scores were associated with higher aperiodic exponents (steeper spectral slope).
Figure 6.
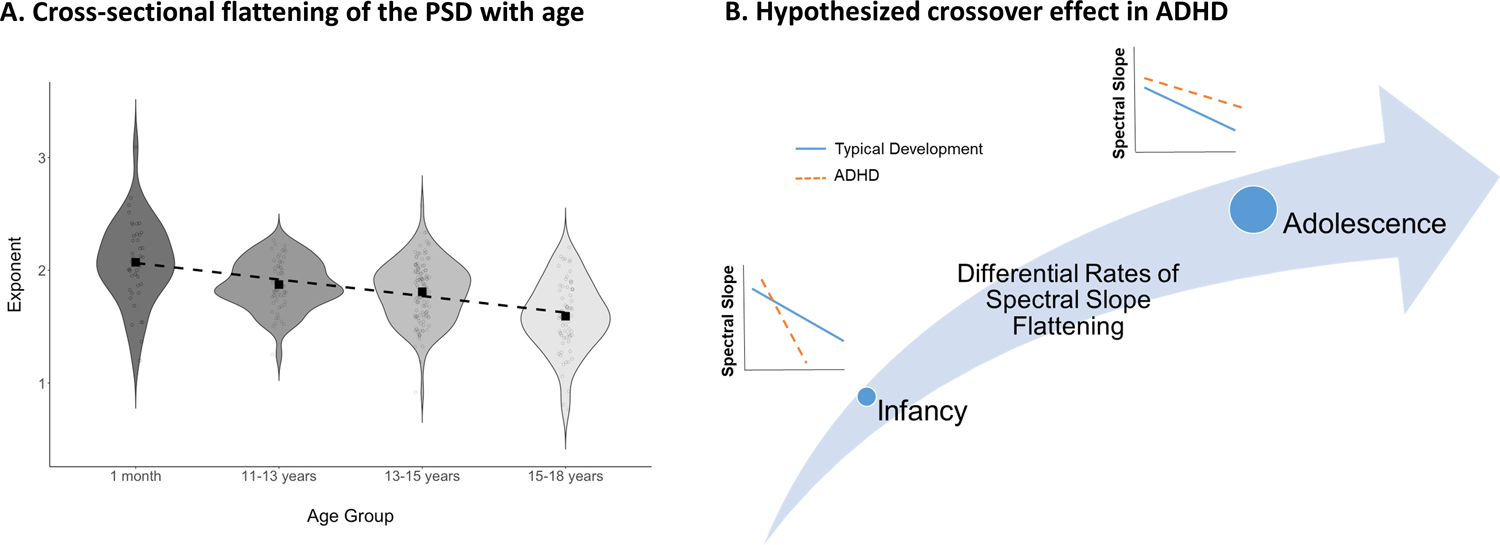
shows cross-sectional age effects in our sample (panel A) and schematically represents the developmental trend implied by the current findings (panel B). Early in development, children at high risk for ADHD demonstrate steeper spectral slopes than their low-risk peers; however, the developmentally normative flattening of the spectral slope is exaggerated in the high-risk children, such that by adolescence, the ADHD group has flatter spectral slopes than their typically-developing peers.
Discussion
EEG-recorded measures of brain activity are ideal as candidate biomarkers due to the low cost, ease of clinical translation, and applicability across a wide range of development. The aperiodic exponent of the EEG power spectrum has received growing interest as a marker of neurodevelopmental psychopathology, including ADHD (Mamiya, Arnett, & Stein, 2021; Ostlund et al., 2021a; Voytek & Knight, 2015). Here, we demonstrate for the first time that the aperiodic exponent has good to excellent internal consistency in both infancy and adolescence. We further demonstrate that the aperiodic exponent is associated with ADHD risk in children as young as 1-month-old and this association persists (yet reverses) through adolescence in untreated children with ADHD.
High internal consistency is an important psychometric property for candidate biomarkers. While periodic activity in canonical frequency bands has adequate to excellent internal consistency across a wide age range (e.g., Hill et al., 2020; Towers & Allen, 2009), this is the first study demonstrating good or better internal consistency of aperiodic features of the EEG power spectrum at any age. Internal consistency in adolescents was excellent, even with relatively short recording lengths. Unsurprisingly, internal consistency was lower in infants than in adolescents, but even in the infant group internal consistency was good with modest amounts of available data. Reliability estimates for the aperiodic exponent are similar or better than those obtained for periodic EEG power spectrum measures at the same ages (Hill et al., 2020). Internal consistency was not moderated by ADHD in either the adolescents or infants, adding to its appeal as a putative marker for neurodevelopmental risk.
Adolescents with a current ADHD diagnosis had a smaller aperiodic exponent (flatter power spectrum) than those without an ADHD diagnosis. This finding is consistent with prior work in a large subset of the sample here, which identified the same pattern (Ostlund et al., 2021a). The effect observed here is larger than that observed in the prior analyses (β = −.29 versus −.16). Only a small number of subjects were added to the dataset, so the larger effect is most likely attributable to the averaging of multiple electrode sites in the current study versus the use of a single site (Cz) in the prior publication. The topographical maps in Figure 2 suggest that the aperiodic exponent is relatively smaller (flatter PSD) as it moves away from the midline of the scalp; however, this pattern appears similar across electrode montages, age groups, and clinical groups. Thus, averaging across electrodes may have increased reliability of the final exponent measure without sacrificing important localized differences. Alternatively, it may be that the overall pattern of aperiodic activity across the scalp bears a stronger relationship to ADHD than aperiodic activity at the single Cz electrode. Future research focused on electrode selection for various types of studies will be important.
The relationship between aperiodic exponent and ADHD was specific to adolescents who had never received stimulant treatment. Results partially converge with at least one other recent study that also found differences in the aperiodic exponent specifically between stimulant naïve children with ADHD and controls (Robertson et al., 2019). Critically, in both studies children were on stimulant washout during EEG recording. Thus, results cannot be attributed to the acute effects of treatment and suggest that stimulant treatment may normalize the EEG power spectrum over the course of development, at least for some children. Such a finding would be consistent with studies showing long-term changes in brain structure and function with stimulant treatment using other brain imaging modalities, including small but provocative studies specifically showing normalization of aperiodic activity among individuals with schizophrenia (Molina et al., 2020) and ADHD (Pertermann et al., 2019) undergoing acute medication treatments.
Treatment of an excitatory/inhibitory imbalance, as reflected in the aperiodic exponent, during middle childhood may have the potential to normalize and optimize brain development in a way that mitigates neurodevelopmental risk. Critically, given the potential side effects and risk of stimulants, we do not intend to suggest stimulants as an appropriate intervention in very early development. However, other treatments may effectively lead normalization as well. For example, in a sample enriched for ADHD Sullivan and colleagues demonstrate that maternal omega-3 fatty acid levels may partially mitigate the effects of other early neurodevelopmental risk factors (Gustafsson et al., 2019). Future studies addressing similar dietary and other non-stimulant interventions, as well as developmental windows in which such effects might be most potent will be critical for informing treatment recommendations.
Although there is some convergence across studies that the aperiodic exponent of the EEG power spectrum differs between children with and without ADHD, the directions of effects have differed. Whereas here we observed a flatter power spectral slope (smaller aperiodic exponent) in adolescents with ADHD than in controls, Robertson et al. (2019) identified a steeper power spectral slope in stimulant-naïve ADHD children than in typical development at much younger ages (3–7 years-olds). Interestingly, the pattern of steeper spectral slopes identified in younger children is consistent with our own findings here that in very young infants (1-month-old) greater familial ADHD risk is also related to steeper (rather than flatter) power spectral slopes. Taken together, results suggest a dynamic disruption to patterns of cortical development. In ADHD, the normative pattern of broadband “flattening” of the EEG power spectrum (He et al., 2019; Schaworonkow & Voytek, 2021; Voytek & Knight, 2015) seems to be exaggerated. At younger ages, children with ADHD show an even steeper power spectral slope than would be expected. However, the power spectrum also flattens more rapidly across development than is typical, such that by early adolescence the power spectrum is flatter than would be expected. Figure 6 shows the cross-sectional decrease in exponent values with age in our sample (consistent with age-based flattening) and a schematic representation of the cross-over trend we describe. This cross-over pattern implies not only that the direction of effect will differ based on age, but that at some points in development (i.e., during cross-over) groups may not be distinguishable using the aperiodic exponent.
Recent evidence from animal and computational models suggests that the aperiodic exponent reflects the relative balance of cortical excitation and inhibition (Gao et al., 2017). This excitatory/inhibitory balance undergoes rapid changes starting very early in development (Zhang, Jiao, & Sun, 2011). A smaller aperiodic exponent is thought to index a relative shift towards cortical excitation, consistent with studies in older children with ADHD that show reduced GABA and increase glutamate concentrations in motor and prefrontal cortical regions, respectively (for recent review see Mamiya et al., 2021). This pattern is consistent with the flattened ADHD power spectrum observed here in adolescents. The current results suggest that early over-inhibition of cortical activity may shift to over-excitation in later development. Of course, the current results are cross-sectional, and additional longitudinal studies that span early childhood, adolescence, and into adulthood will be needed to confirm such developmental effects.
Although we focus on ADHD risk, excitatory/inhibitory imbalance and differences in the aperiodic exponent of the power spectrum have been observed in other neurodevelopmental disorders as well. Racz et al. (Racz et al., 2021) identified a trend towards shallower exponents in a small sample of adults with schizophrenia as compared to typically-developing adults, and there is interest in whether disruptions appear in autism spectrum disorder (Levin et al., 2020). Crucially, as others have highlighted (Donoghue et al., 2020b; Mamiya et al., 2021; Voytek & Knight, 2015), the excitatory/inhibitory balance is critical for optimal synaptic plasticity and neuronal developmental, generally, and for maximizing signal to noise ratio in neural circuitry, specifically. Thus, one possibility is that early differences in the aperiodic exponent reflect a general neurodevelopmental risk factor that could contribute to genetic and phenotypic correlations among these disorders. An early general factor could also interact with other features to lead to disorder-specific symptom presentations in later childhood and adolescence.
Integrating the findings here with prior work using canonical frequency bands will also be important. Theta:beta ratio has been of particular interest in ADHD because of several early studies suggesting a higher theta:beta ratio in individuals diagnosed with ADHD compared to controls (Monastra et al., 1999). The empirical support for elevated theta:beta ratio in ADHD, however, has been inconsistent. The most recent meta-analysis of this literature (Arns, Conners, & Kraemer, 2013) and studies since the meta-analysis (Arns et al., 2018; Bussalb et al., 2019) primarily find no between-group effects for theta:beta ratio in ADHD. Critically, the vast majority of studies have used samples with large age ranges that span childhood through adolescence and have not taken into account lifetime history of stimulant treatment (Arns et al., 2013), both of which we find here to be critical.
Interestingly, when age effects have been considered, there are intriguing suggestions of the developmental reversal we suggest here. For example, Loo and colleagues (2013) found no difference in theta:beta ratios for children and adolescents, but the means suggested higher theta:beta ratio for ADHD in childhood, consistent with the steeper aperiodic slope observed in our study. (Note lifetime history of stimulant use was not considered which may explain lack of significance.) In the same study, they also found that adults with ADHD had lower theta:beta ratios than controls, consistent with a flatter slope later in development. More recently, in a study including individuals ages 5–21 years-old Bussalb et al (2019) found that a subgroup of people with ADHD and high theta:beta ratio were significantly younger than those with normal or low theta:beta ratios (though representation at the extremes of their age range was too small to further characterize developmental effects). Thus, the results of the current study may align with and help clarify prior work using canonical frequency bands to study ADHD.
While the reported associations between the aperiodic EEG power spectrum in infancy are promising, they are based on a small sample that was not intentionally recruited for ADHD risk. Only a small number of infants had family history scores greater than zero. It is not unusual that large effects in small samples fail to replicate in larger samples. In addition, the infant sample had high family income (and a relatively restricted range of income). While the sample demographics overall reflected the local area, the majority of participants identified as white/Caucasian. The ADHD effects observed in the adolescents may be more robust in that they come from a larger sample; however, even in the adolescents, family income was relatively high. In the adolescents, even more participants identified as White compared to the infant sample. Both the small infant sample and the lack of income and racial representation limit the generalizability of the findings reported here.
Conclusion
The aperiodic exponent of the EEG power spectrum has received growing attention as a putative marker of neurodevelopmental psychopathology. Current findings confirm good to excellent internal consistency of this EEG feature in both very early infancy and adolescence, critical to its application as a biomarker for ADHD and other neurodevelopmental conditions. Further, results confirm a relationship between ADHD diagnosis and a flatter power spectrum in adolescence but only for children who have not received stimulant medication treatment. In contrast, a larger aperiodic exponent (indicative of a steeper power spectrum) was associated with family history of ADHD in very early infancy. Together results suggest a dynamic developmental shift in which the normative flattening of the EEG power spectrum is exaggerated in ADHD, potentially reflecting imbalances in cortical excitation and inhibition that could contribute to long-lasting differences in brain connectivity. These disruptions in cortical development may be detectable shortly after birth via this approach.
Acknowledgements:
This project was supported by R01 MH124824 (MPI: Nigg/Sullivan), R01 MH117177 (MPI: Sullivan/Nigg), R37 MH059105 (PI: Nigg), K23 MH108656 (PI: Karalunas) and K01 MH120507 (PI: Gustafsson).
Footnotes
Conflicts of Interest: Authors have no conflicts of interest to report.
Data Availability:
The data that support the findings of this study will be available in the National Institute of Mental Health Data Archive (NDA) at https://nda.nih.gov/. All data will be uploaded following publication in accordance with NIH policies. Scripts for bootstrap reliability analyses are available on OSF (https://osf.io/hqyue/).
References
- 2.2.0, B. A. V. (2019). BrainVision Analyzer (Version 2.2.0) [Software]. Gilching, Germany: Brain Products GmbH. [Google Scholar]
- Arns M, Conners CK, & Kraemer HC (2013). A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. J Atten Disord, 17(5), 374–383. doi: 10.1177/1087054712460087 [DOI] [PubMed] [Google Scholar]
- Arns M, Vollebregt MA, Palmer D, Spooner C, Gordon E, Kohn M, … Buitelaar JKJEN (2018). Electroencephalographic biomarkers as predictors of methylphenidate response in attention-deficit/hyperactivity disorder. 28(8), 881–891. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, Dupuy FE, McCarthy R, & Selikowitz M (2011). EEG coherence and symptom profiles of children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology, 122(7), 1327–1332. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, & Brown CR (2009). EEG differences in children between eyes-closed and eyes-open resting conditions. Clinical Neurophysiology, 120(10), 1806–1811. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, & Selikowitz M (2006). Age and gender effects in EEG coherence: III. Girls with attention-deficit/hyperactivity disorder. Clinical Neurophysiology, 117(2), 243–251. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Hsu C-I, … Magee CA (2005). Age and gender effects in EEG coherence: II. Boys with attention deficit/hyperactivity disorder. Clinical Neurophysiology, 116(4), 977–984. [DOI] [PubMed] [Google Scholar]
- Bell MA, & Cuevas K (2012). Using EEG to study cognitive development: Issues and practices. Journal of cognition and development, 13(3), 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Delorme A, & Makeig S (2013). Eeglab–an open source matlab toolbox for electrophysiological research. Biomedical Engineering/Biomedizinische Technik, 58(SI-1-Track-G), 000010151520134182. [DOI] [PubMed] [Google Scholar]
- Bussalb A, Collin S, Barthélemy Q, Ojeda D, Bioulac S, Blasco-Fontecilla H, … Mayaud LJCN (2019). Is there a cluster of high theta-beta ratio patients in attention deficit hyperactivity disorder?, 130(8), 1387–1396. [DOI] [PubMed] [Google Scholar]
- Cellier D, Riddle J, Petersen I, & Hwang KJDCN (2021). The development of theta and alpha neural oscillations from ages 3 to 24 years. 100969. [DOI] [PMC free article] [PubMed]
- Conners CK (2008). The Conners 3rd Edition (Conners 3). North Tonawanda, NJ: Multi-Health System. [Google Scholar]
- Demuru M, & Fraschini M (2020). EEG fingerprinting: Subject-specific signature based on the aperiodic component of power spectrum. Computers in Biology and Medicine, 120, 103748. [DOI] [PubMed] [Google Scholar]
- Donoghue T, Dominguez J, & Voytek B (2020a). Electrophysiological frequency band ratio measures conflate periodic and aperiodic neural activity. Eneuro, 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, … Knight RT (2020b). Parameterizing neural power spectra into periodic and aperiodic components. Nature Neuroscience, 23(12), 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G, Power T, Anastopoulos A, & Reid R (1998). ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation. NY, NY: Guilford Press. [Google Scholar]
- Foti D, Kotov R, & Hajcak G (2013). Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. Journal of Abnormal Psychology, 122(2), 520. [DOI] [PubMed] [Google Scholar]
- Gao R, Peterson EJ, & Voytek B (2017). Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage, 158, 70–78. [DOI] [PubMed] [Google Scholar]
- Gold C, Fachner J, & Erkkilä J (2013). Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scandinavian Journal of Psychology, 54(2), 118–126. [DOI] [PubMed] [Google Scholar]
- González JJ, Méndez LD, Mañas S, Duque MR, Pereda E, & De Vera L (2013). Performance analysis of univariate and multivariate EEG measurements in the diagnosis of ADHD. Clinical Neurophysiology, 124(6), 1139–1150. [DOI] [PubMed] [Google Scholar]
- Goodman R (2001). Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry, 40(11), 1337–1345. [DOI] [PubMed] [Google Scholar]
- Gustafsson HC, Holton KF, Anderson AN, Nousen EK, Sullivan CA, Loftis JM, … Sullivan E. L. J. F. i. n. (2019). Increased maternal prenatal adiposity, inflammation, and lower omega-3 fatty acid levels influence child negative affect. 13, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Donoghue T, Sowman PF, Seymour RA, Brock J, Crain S, … Hillebrand A (2019). Co-increasing neuronal noise and beta power in the developing brain. bioRxiv, 839258. [Google Scholar]
- Hill KE, Neo WS, Hernandez A, Hamrick LR, Kelleher BL, & Foti D (2020). Intergenerational transmission of frontal alpha asymmetry among mother–infant dyads. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink MA, Cross ZR, Chatburn A, Baumeister J, Schlesewsky M, & Bornkessel-Schlesewsky IJHMS (2021). Resting-state aperiodic neural dynamics predict individual differences in visuomotor performance and learning. 78, 102829. [DOI] [PubMed] [Google Scholar]
- Jaffee S (2018). Promises and pitfalls in the development of biomarkers that can promote early intervention in children at risk. In: Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, & Luck SJ (2016). Best practices for event-related potential research in clinical populations. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(2), 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Bierman KL, & Huang-Pollock CL (2016). Test–Retest Reliability and Measurement Invariance of Executive Function Tasks in Young Children With and Without ADHD. Journal of Attention Disorders, 1087054715627488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Gustafsson HC, Fair D, Musser ED, & Nigg JT (2019). Do we need an irritable subtype of ADHD? Replication and extension of a promising temperament profile approach to ADHD subtyping. Psychological assessment, 31(2), 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (2011). Children’s Depression Inventory (2nd ed.). North Tonawanda, NY: Multi-Health Systems Inc. [Google Scholar]
- Lenzenweger MF (2013). Thinking clearly about the endophenotype–intermediate phenotype–biomarker distinctions in developmental psychopathology research. Development and Psychopathology, 25(4pt2), 1347–1357. [DOI] [PubMed] [Google Scholar]
- Levin AR, Naples AJ, Scheffler AW, Webb SJ, Shic F, Sugar CA, … Dawson G. J. F. i. I. N. (2020). Day-to-day test-retest reliability of EEG profiles in children with Autism Spectrum Disorder and typical development. 14, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Cho A, Hale TS, McGough J, McCracken J, & Smalley SL (2013). Characterization of the Theta to Beta Ratio in ADHD Identifying Potential Sources of Heterogeneity. Journal of Attention Disorders, 17(5), 384–392. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in human neuroscience, 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TR, Sponheim SR, Iacono WG, & Clementz BA (1995). Internal consistency reliability of resting EEG power spectra in schizophrenic and normal subjects. Psychophysiology, 32(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Mamiya PC, Arnett AB, & Stein MA (2021). Precision Medicine Care in ADHD: The Case for Neural Excitation and Inhibition. Brain Sciences, 11(1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J (2013). Multidimensional Anxiety Scale for Children (2nd ed.). Toronto, Ontario, Canada: Multi-Health Systems. [Google Scholar]
- Molina JL, Voytek B, Thomas ML, Joshi YB, Bhakta SG, Talledo JA, … Light GA (2020). Memantine effects on electroencephalographic measures of putative excitatory/inhibitory balance in schizophrenia. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(6), 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastra VJ, Lubar JF, Linden M, VanDeusen P, Green G, Wing W, … Fenger TNJN (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. 13(3), 424. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2020). MPLUS User’s Guide (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Newson JJ, & Thiagarajan TC (2019). EEG frequency bands in psychiatric disorders: a review of resting state studies. Frontiers in human neuroscience, 12, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Karalunas SL, Gustafsson HC, Bhatt P, Ryabinin P, Mooney MA, … Wilmot B (2020). Evaluating chronic emotional dysregulation and irritability in relation to ADHD and depression genetic risk in children with ADHD. Journal of Child Psychology and Psychiatry, 61(2), 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Alperin BR, Drew T, & Karalunas SL (2021a). Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Developmental cognitive neuroscience, 48, 100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Donoghue T, Anaya B, Gunther K, Karalunas S, Voytek B, & Perez-Edgar K (2021b). Spectral parameterization for studying neurodevelopment: How and why, https://psyarxiv.com/btqyk/. [DOI] [PMC free article] [PubMed]
- Pathania A, Schreiber M, Miller MW, Euler MJ, & Lohse KR (2021). Exploring the reliability and sensitivity of the EEG power spectrum as a biomarker. International Journal of Psychophysiology, 160, 18–27. [DOI] [PubMed] [Google Scholar]
- Pertermann M, Bluschke A, Roessner V, & Beste C (2019). The modulation of neural noise underlies the effectiveness of methylphenidate treatment in attention-deficit/hyperactivity disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(8), 743–750. [DOI] [PubMed] [Google Scholar]
- Peterson EJ, Rosen BQ, Campbell AM, Belger A, & Voytek B (2017). 1/f neural noise is a better predictor of schizophrenia than neural oscillations. bioRxiv, 113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata D, Mechelli A, & Kapur S (2014). Clinically meaningful biomarkers for psychosis: a systematic and quantitative review. Neuroscience & Biobehavioral Reviews, 45, 134–141. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, & Ryan N (1986). Kiddie schedule for affective disorders and schizophrenia. Pittsburgh, PA: Western Psychiatric Institute. [Google Scholar]
- Racz FS, Farkas K, Stylianou O, Kaposzta Z, Czoch A, Mukli P, … Eke A (2021). Separating scale‐free and oscillatory components of neural activity in schizophrenia. Brain and Behavior, e02047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM, Furlong S, Voytek B, Donoghue T, Boettiger CA, & Sheridan MA (2019). EEG Power Spectral Slope differs by ADHD status and stimulant medication exposure in early childhood. Journal of Neurophysiology, 122(6), 2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, & Marshall PJ (2012). The utility of EEG band power analysis in the study of infancy and early childhood. Developmental Neuropsychology, 37(3), 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinsky M, Oken B, & Morehead L (1991). Test-retest reliability in EEG frequency analysis. Electroencephalography and Clinical neurophysiology, 79(5), 382–392. [DOI] [PubMed] [Google Scholar]
- Schaworonkow N, & Voytek B (2021). Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Developmental cognitive neuroscience, 47, 100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, & Davidson RJ (2010). Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage, 51(4), 1319–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, & Rose N (2009). Biomarkers in psychiatry. Nature, 460(7252), 202–207. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, & Nigg JT (2015). Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry, 56(9), 949–957. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, & Kinney L (1992). Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology, 29(5), 576–592. [DOI] [PubMed] [Google Scholar]
- Towers DN, & Allen JJ (2009). A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology, 46(1), 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, & Keshavan MS (2016). Biomarkers in psychiatry-a critique. Annals of neurosciences, 23(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, & Knight RT (2015). Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biological Psychiatry, 77(12), 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke L, Donoghue T, Fiedler L, Smith S, Garrett DD, Voytek B, & Obleser J. J. b. (2021). Modality-specific tracking of attention and sensory statistics in the human electrophysiological spectral exponent. [DOI] [PMC free article] [PubMed]
- Wechsler D (2002). Wechsler Individual Achievement Test, 2nd Ed (WIAT-II) Examiner’s Manual. San Antonio: Harcourt Brace. [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children, 4th Ed (WISC-IV) Technical and Interpretive Manual. San Antonio: Harcourt Brace. [Google Scholar]
- Whedon M, Perry NB, Calkins SD, & Bell MA (2016). Changes in frontal EEG coherence across infancy predict cognitive abilities at age 3: The mediating role of attentional control. Developmental Psychology, 52(9), 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jiao Y-Y, & Sun Q-QJN (2011). Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. 174, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study will be available in the National Institute of Mental Health Data Archive (NDA) at https://nda.nih.gov/. All data will be uploaded following publication in accordance with NIH policies. Scripts for bootstrap reliability analyses are available on OSF (https://osf.io/hqyue/).


