Abstract
Objectives
To investigate the impact of remission and lupus low disease activity state (LLDAS) on health-related quality of life (HRQoL) in systemic lupus erythematosus.
Methods
Short-Form 36 (SF-36), three-level EQ-5D (EQ-5D-3L) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue data from the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials were used. Duration in remission/LLDAS required to reach a HRQoL benefit ≥ minimal clinically important differences (MCIDs) during and post-treatment was determined using quantile regression and generalized estimating equations.
Results
Patients (n = 1684) were assessed every fourth week (15 visits). Four cumulative (β = 0.60) or four consecutive (β = 0.66) visits in remission were required to achieve a benefit ≥MCID in SF-36 physical component summary (PCS) scores, and six cumulative (β = 0.44) or five consecutive (β = 0.49) for a benefit ≥MCID in mental component summary (MCS) scores. Eight cumulative (β = 0.30 for both) or eight consecutive (β = 0.32 for both) visits in LLDAS were required for a benefit in PCS/MCS ≥MCID, respectively. For EQ-5D-3L index scores ≥MCID, six cumulative (β = 0.007) or five consecutive (β = 0.008) visits in remission were required, and eight cumulative (β = 0.005) or six consecutive (β = 0.006) visits in LLDAS. For FACIT-Fatigue scores ≥MCID, 12 cumulative (β = 0.34) or 10 consecutive (β = 0.39) visits in remission were required, and 17 cumulative (β = 0.24) or 16 consecutive (β = 0.25) visits in LLDAS.
Conclusion
Remission and LLDAS contribute to a HRQoL benefit in a time-dependent manner. Shorter time in remission than in LLDAS was required for a clinically important benefit in HRQoL, and longer time in remission for a benefit in mental compared with physical HRQoL aspects. When remission/LLDAS was sustained, the same benefit was achieved in a shorter time.
Keywords: SLE, remission, lupus low disease activity state, health-related quality of life, treat-to-target
Rheumatology key messages.
Remission and LLDAS contribute to clinically important HRQoL benefit in a time-dependent manner.
Shorter time in remission than in LLDAS was required for a clinically important HRQoL benefit.
When remission or LLDAS was sustained, the same benefit was achieved in a shorter time.
Introduction
Patients with SLE [1] experience impaired health-related quality of life (HRQoL) compared with the general population and with other chronic diseases [2, 3]. HRQoL is frequently estimated in studies of chronic diseases using different so-called patient-reported outcome measures (PROMs) [4, 5]. However, HRQoL is rarely accounted for in clinical practice in a systematic manner [6].
The importance of remission and low disease activity (LDA) in SLE was highlighted in the treat-to-target (T2T)/SLE research agenda in 2014 [7]. In 2016, the Definitions OF Remission In SLE (DORIS) international task force proposed a set of remission definitions [8] in response to the T2T/SLE task force [7, 9]. LDA is conceptualized as an alternative target when remission cannot be achieved. In 2015, experts from the Asia Pacific Lupus Collaboration introduced the Lupus Low Disease Activity State (LLDAS) [10, 11], which has been the most frequently used definition of LDA in SLE and is advocated for use in clinical trials settings [12]. Both remission and LLDAS have been associated with decelerated organ damage accrual [13–16].
Several studies have investigated the relationship between remission or LDA and HRQoL. Durable DORIS remission for five years or more was rare, but associated with better HRQoL in a study from China [17]. A Dutch study with two-year follow-up showed that patients in remission were likely to improve in physical aspects of HRQoL, but the results regarding mental aspects were inconclusive [18]. A large study from the Asia Pacific Lupus Collaboration demonstrated that patients in LLDAS experienced better physical and mental HRQoL than those not in LLDAS [10]. Both studies used Short-Form 36 (SF-36) for measuring HRQoL. Another study from Thailand showed a positive impact of LLDAS on HRQoL using the SLE-specific instrument SLEQoL [19].
In the present investigation, we aimed to study the impact of remission and LLDAS on HRQoL outcome in patients with SLE in the setting of two large phase III 52-week trials of belimumab.
Methods
Study design and population
We designed a post-hoc analysis of data from two randomized, double blind, phase III clinical trials, i.e. BLISS-52 (NCT00424476) [20] and BLISS-76 (NCT00410384) [21], which enrolled 865 and 819 patients with SLE, respectively. Inclusion criteria for both trials were age ≥18 years, SLE diagnosis according to the revised 1997 ACR criteria [22], active disease defined as a Safety of Estrogens in Lupus National Assessment SLEDAI (SELENA-SLEDAI) [23] score ≥6, and serological activity defined as ANA titre ≥1:80 and/or serum anti-double stranded (ds)DNA antibody levels ≥30 IU/ml. Organ damage was assessed using the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (SDI) [24]. Key exclusion criteria included pregnancy, severe active lupus nephritis and severe active neuropsychiatric SLE. Access to data was granted by GlaxoSmithKline (Uxbridge, UK) through the Clinical Study Data Request consortium. Baseline characteristics are shown in Table 1, and in more detail in the initial BLISS publications [20, 21].
Table 1.
Baseline characteristics of patients in BLISS-52 and BLISS-76
| BLISS-52 and BLISS-76 (n = 1684) | |
|---|---|
| Female sex | 1585 (94%) |
| Ethnicity | |
| Asian | 353 (21%) |
| Black/African-American | 146 (9%) |
| Indigenous Americana | 374 (22%) |
| White/Caucasian | 798 (47%) |
| Country/Continent | |
| Asia Pacific | 339 (20.1%) |
| Canada/USA | 436 (25.9%) |
| Europe/Israel | 393 (23.3%) |
| Latin America | 516 (30.6%) |
| Age (years) | 37.8 (11.5) |
| Mean BMI (week 0–52) | 25.8 (5.9) |
| SDI score | 0.8 (1.2); (N = 1683) |
| SLE disease duration (years) | 6.4 (6.3) |
| SLEDAI-2K score | 10.0 (3.8) |
| Clinical SLEDAI-2K score | 7.4 (3.6) |
| SELENA-SLEDAI physician’s global assessment score | 1.4 (0.5) |
| Prednisone equivalent dose (mg/day) | 10.8 (8.7) |
Data are presented as n (%) or mean (s.d.).
Alaska Native or American Indian from North, South or Central America.
SDI: Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index; SLEDAI-2K: SLEDAI 2000; SELENA-SLEDAI: Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLEDAI.
Definitions of remission and LLDAS
Supplementary Table S1, available at Rheumatology online, describes the components in the remission and LLDAS definitions, as applied in the present work. From the proposed DORIS definitions [8], we herein used the less stringent and thus most attainable remission definition, as shown in a previous report based on the same trials [25], which also constitutes the updated definition recently presented by the DORIS task force [26]. This definition required a clinical SLEDAI-2K score 0 and a SELENA-SLEDAI physician’s global assessment (PGA) score <0.5 (on a scale 0–3), while it allowed serological activity and use of low-dose glucocorticoids (prednisone or equivalent ≤5 mg daily) and immunosuppressive treatments or biological agents at standard doses.
The LLDAS definition required a SLEDAI-2K score ≤4, excluding major organ activity and fever or new activity since the previous assessment, and PGA ≤1 (on a scale 0–3), and it allowed use of prednisone (or equivalent) at a dose ≤7.5 mg/day and immunosuppressive drugs and approved biological agents at standard doses.
In addition to the analysis of remission and LLDAS, a separate analysis of patients who attained LLDAS excluding patients who fulfilled the criteria of remission at one or more visits during the 52-week study period was conducted to examine the added influence of LLDAS, hereafter termed LLDAS/no remission.
Evaluation of HRQoL and minimal clinically important differences
SLE patients’ perception of HRQoL was self-reported using generic instruments, i.e. the Medical Outcomes Study (MOS) 36-item Short Form Health Survey (SF-36) [27], the three-level European Quality of Life 5-dimension (EQ-5D-3L) health questionnaire [28], and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue (FACIT-F) scale [29]. Attainment of remission and LLDAS was calculated every fourth week and analysed in relation to HRQoL, longitudinally and at week 52, assuming that each visit reflected the condition of the patient for an average of 4 weeks. We sought to determine the cumulative (interruptions allowed) and sustained (at consecutive visits) duration in remission or LLDAS throughout the 52-week period that was needed to achieve a benefit in various aspects of HRQoL that exceeded minimal clinically important differences (MCIDs) [30].
SF-36
The SF-36 [27] consists of 36 questions, which are further grouped into eight subscales, i.e. physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), social functioning (SF), vitality (VT), role emotional (RE) and mental health (MH). Those subscales are weighted and processed into two summary scores, i.e. the physical component summary (PCS) and the mental component summary (MCS). The component summary and subscale scores range from 0 (worst possible health) to 100 (best possible health). The MCID for SF-36 PCS and MCS scores was set to 2.5, while the corresponding MCID for SF-36 subscale scores was set to 5.0, as previously commended [30].
EQ-5D
The EQ-5D-3L health questionnaire measures health status, using a Visual Analogue Scale (VAS) and a descriptive part. The EQ-VAS is scored from 0 to 100, where 0 represents the ‘worst imaginable health’ and 100 the ‘best imaginable health’. The descriptive part includes questions within five dimensions, i.e. mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Patient responses to those five questions on a three-level response scale are then summarized into a utility index score, where 1 corresponds to full health state and values below 0, which are rare, represent HRQoL experience that is considered worse than death [28]. Clinically important differences were set to 10 points for the 0–100 EQ-VAS [31] and to 0.04 for the EQ-5D utility index score [32].
FACIT-Fatigue
The FACIT-F scale was used to evaluate fatigue [29]. FACIT-F evaluates the level of fatigue and the effects of both physical and mental fatigue on daily living and functioning over the preceding seven days, and patient responses to 13 different items are transformed into a score ranging from 0 (maximal fatigue) to 52 (minimal fatigue), and it has been validated for SLE [33]. The MCID for FACIT-F scores was set to 4.0 [34].
Statistical analysis
Descriptive statistics were performed for patient characteristics. Data are presented as mean (s.d.) or median (interquartile range, IQR) for continuous variables. Numbers and percentages are reported for categorical variables. Missing values for variables used for determination of remission and LLDAS were imputed using the last observation carried forward (LOCF) method. Missing values for HRQoL were ignored in analysis.
Generalized estimating equations (GEE) were used for longitudinal analyses with an exchangeable correlation matrix, assuming a constant correlation in PCS and MCS across visits. The GEE analysis was performed on SF-36 PCS and MCS only; the information available on the SF-36 subscales, EQ-5D-3L utility index, EQ-VAS and FACIT-F was deemed insufficient for performing GEE analysis due to missing data.
For analyses of HRQoL at week 52, quantile regression to the median was used. Quantile regression confidence intervals were calculated using bootstrapped standard errors with 250 replications. Cumulative and sustained remission/LLDAS were analysed on a continuous scale yet based on data from the 52-week period, i.e. the number of cumulative/consecutive visits required to reach an MCID were extrapolated linearly from the quantile regression estimates. For example, an estimate of 0.5 means that five consecutive visits in LLDAS are required to reach a 2.5 difference in the median PCS or MCS, while an estimate of 0.1 would require 25 visits. All models were adjusted for age, sex, ethnicity, country, belimumab treatment, antimalarial treatment, immunosuppressive agents, disease duration, BMI, and organ damage (SDI score), all considered factors with confounding potentiality based on previous research [3, 35, 36] (Supplementary Table S2, available at Rheumatology online). We also tested for the interaction term between belimumab use and remission/LLDAS; as no significant interaction was noted other than in one single model (exposure: remission; outcome: SF-36 PCS), the term was excluded from the final models. P-values below 0.05 were deemed significant. All analyses were performed in R version 4.01 (Vienna, Austria).
Ethics
The study complied with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants prior to enrolment. The BLISS-52 and BLISS-76 study protocols were reviewed and approved by regional ethics review boards for all participating centres, and the study protocol for the present post-hoc analysis was reviewed and approved by the Swedish Ethical Review Authority (2019–05498).
Patient involvement
A patient research partner (Y.E.) was involved in all stages of the research process.
Results
Attainment of remission and LLDAS
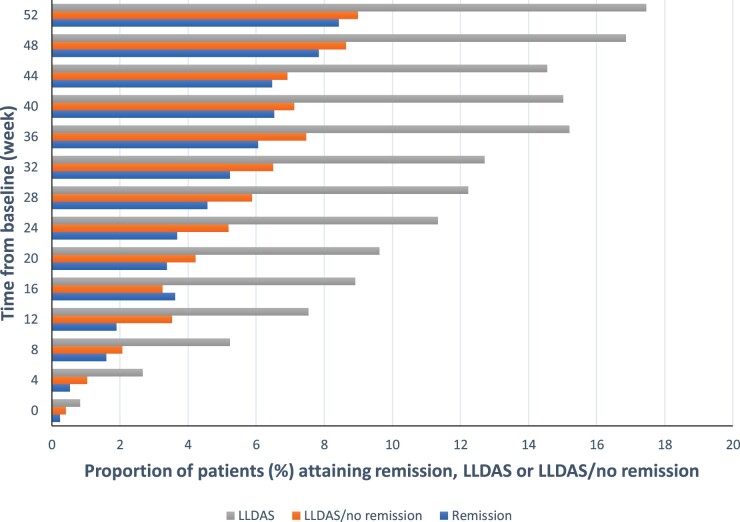
Data from 1684 patients were analysed including predominantly females (94%) with a mean age of 38 years and Caucasian ethnicity (47%). Frequencies of attainment of remission, LLDAS and LLDAS/no remission during time on treatment are presented in Fig. 1 and Supplementary Table S3, available at Rheumatology online. We next calculated the frequencies of attainment of the different components of the remission and LLDAS definitions throughout the study period (Table 2). As expected, the total number of LLDAS events exceeded the number of events of remission and was achieved at 2529/23 756 visits (10.6%) as opposed to 1012/23 756 (4.3%). At each visit, attainment of LLDAS was more common than remission and frequencies of both states increased with time on treatment.
Fig. 1.
Proportions of patients who attained remission or LLDAS
Proportions of patients who attained DORIS remission, LLDAS or LLDAS/no remission (n = 1446) at baseline and every fourth week in the BLISS-52 and BLISS-76 study population (n = 1684). DORIS: Definitions Of Remission In SLE; LLDAS: lupus low disease activity state.
Table 2.
Frequencies of different definition components
| DORIS Remission | |||||
| Out of all patients (n = 1684) | Proportion of patients fulfilling remission | Prednisone dose (mg/day) <5 | Physician’s global assessment (Scale 0–3) <0.5 | Clinicala SLEDAI-2K = 0 | |
| At week 52 (n = 1684) | 142 (8.4%) | 647 (38.4%) | 541 (32.1%) | 484 (28.7%) | |
| All visits (n = 23 756) | 1012 (4.3%) | 8118 (34.4%) | 5284 (22.4%) | 4762 (20.2%) | |
| LLDAS | |||||
| Proportion of patients fulfilling LLDAS | Prednisone dose (mg/day) ≤7.5 | Physician’s global assessment (Scale 0–3) ≤1 | SLEDAI-2K ≤4 with no activity in major organ systemsb | No new features of lupus disease activityc compared with the previous assessment | |
| At week 52 (n = 1684) | 294 (17.5%) | 818 (48.6%) | 930 (55.2%) | 726 (43.1%) | 1353 (80.3%) |
| All visits (n = 23 756) | 2529 (10.7%) | 10 399 (44.1%) | 10 523 (44.6%) | 7544 (40.0%) | 18 759 (79.6%) |
Data are presented as numbers of patients fulfilling the criterion per total number of patients in the respective category (%) in the pooled BLISS datasets. Standard maintenance doses of immunosuppressive drugs and approved biological agents were allowed and incorporated.
Serological items (anti-double stranded DNA positivity, or low C3 or C4) excluded.
SLEDAI-2K score ≤4 with no activity in major organ systems (renal, central nervous system, cardiopulmonary, vasculitis, fever).
Using SLEDAI-2K.
DORIS: Definitions Of Remission In SLE; LLDAS: lupus low disease activity state; SLEDAI-2K: SLEDAI 2000.
Associations of remission/LLDAS attainment with SF-36
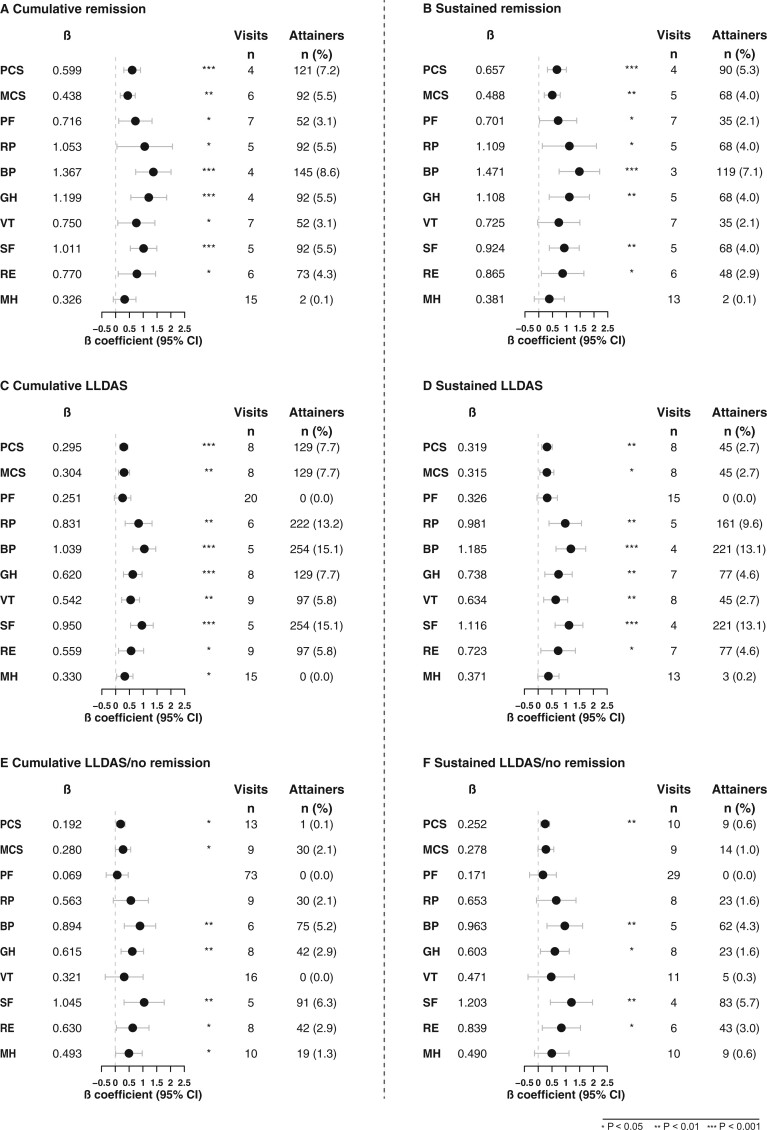
In GEE analysis, remission was associated with higher PCS (β = 2.55; SE=0.27; P < 0.001) and MCS (β = 1.75; SE = 0.33; P < 0.001) throughout the study period from baseline to week 52. The minimum cumulative number of visits in remission to achieve a clinically important benefit in PCS (≥2.5) was four, corresponding to 16 weeks (β = 0.60), while six visits (24 weeks) in remission were required for achieving a benefit in MCS ≥2.5 (β = 0.44; Fig. 2A). Bodily pain and general health were the most sensitive SF-36 subscales in yielding differences, requiring four cumulative visits in remission (β = 1.37; β = 1.20; 16 weeks) for a difference ≥MCID (≥5) at week 52. When analysing the impact of sustained remission, four consecutive visits in remission were required for achieving PCS ≥2.5 (β = 0.66), whereas five visits were required for achieving MCS ≥2.5 (β = 0.49). Again, bodily pain was the most sensitive SF-36 subscale in yielding differences, requiring a minimum of three consecutive visits in remission (β = 1.47; Fig. 2B). Supplementary Table S4, available at Rheumatology online, shows a summary of estimates, standard errors, P-values and 95% CIs.
Fig. 2.
Associations of remission, LLDAS and LLDAS/no remission with SF-36
Forest plots illustrating results from the quantile regression analyses. Number of visits refers to the number of visits in remission, LLDAS or LLDAS/no remission required to attain a clinically important benefit, based on the estimate derived from the regression model. The number of attainers refers to the actual number of patients who were in remission, LLDAS or LLDAS/no remission and attained a clinically important benefit in SF-36 within the 52-week study follow-up. Number of patients for each analysis for remission and LLDAS were: PCS (1666), MCS (1666), PF (1677), RP (1676), BP (1680), GH (1673), SF (1680), VT (1670), RE (1678) and MH (1678). Number of patients for each analysis in LLDAS/no remission was: 1446 (i.e. number of patients after exclusion of patients who attained remission at one or more visits during the 52-week study). The total number of visits was fifteen and they were scheduled every fourth week. BP: bodily pain; GH: general health; LLDAS: lupus low disease activity state; MCS: mental component summary; MH: mental health; PCS: physical component summary; PF: physical functioning; RE: role emotional; RP: role emotional; SF: social functioning; VT: vitality.
Similarly, LLDAS was associated with higher PCS (β = 2.11; SE = 0.18; P < 0.001) and higher MCS (β = 1.52; SE = 0.22; P < 0.001) compared with not being in LLDAS throughout the study period. However, the minimum cumulative number of visits in LLDAS that was required to yield a benefit in PCS and MCS (β = 0.30 for both) ≥MCID at week 52 was higher than for remission, being eight visits (corresponding to 32 weeks) for both. Bodily pain (β = 1.04) and social functioning (β = 0.95) required the lowest number of visits in LLDAS (five visits, corresponding to 20 weeks). For the subscales of physical functioning (β = 0.25) and mental health (β = 0.33), a high number of cumulative visits in LLDAS (n = 20 and n = 15, respectively) was required to yield a clinically important benefit, and no attainers were documented (Fig. 2C). When analysing the impact of sustained LLDAS, eight consecutive visits in LLDAS were required to yield PCS and MCS ≥MCID (β = 0.32 for both) at week 52. Bodily pain and social functioning required the lowest number of visits, i.e. four consecutive visits (β = 1.19 and β = 1.12, respectively). The subscales of physical functioning (β = 0.33) and mental health (β = 0.37) required a high number of visits (n = 15 and n = 13, respectively) in sustained LLDAS to yield a clinically important benefit, and no attainers were documented for physical functioning (Fig. 2D).
During follow-up, 238 patients fulfilled the criteria for remission at one or more visits. Exclusion of these patients yielded a total of 1446 patients for the LLDAS/no remission analysis. The number of cumulative visits in LLDAS/no remission that were required to yield a benefit in PCS (β = 0.19) and MCS (β = 0.28) ≥MCID at week 52 were 13 and nine visits (corresponding to 52 and 36 weeks), respectively. Social functioning (β = 1.05) and bodily pain (β = 0.89) required the lowest number of visits (five and six visits, corresponding to 20 and 24 weeks, respectively) (Fig. 2E).
For the subscales of physical functioning (β = 0.07) and vitality (β = 0.32), a high number of cumulative visits in LLDAS/no remission (n = 73 and n = 16) was required to yield a clinically important benefit, and no attainers were documented (Fig. 2E). When analysing the impact of sustained LLDAS/no remission, ten and nine consecutive visits in LLDAS/no remission were required to yield PCS and MCS ≥MCID (β = 0.25 and β = 0.28) at week 52. Social functioning (β = 1.20) and bodily pain (β = 0.96) required the lowest number of visits, i.e. four and five consecutive visits, respectively. The subscale of physical functioning (β = 0.17) required a high number of visits (n = 29) in sustained LLDAS/no remission to yield a clinically important benefit, and no attainers were documented (Fig. 2F).
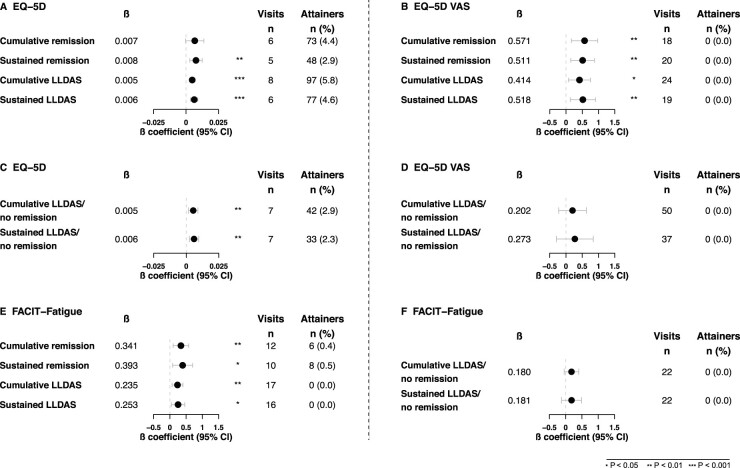
Associations of remission/LLDAS attainment with EQ-5D
A cumulative total of six visits (24 weeks) in remission was required to demonstrate EQ-5D-3L utility index scores ≥MCID at week 52 (β = 0.007). When the state of remission was sustained, remission for five consecutive visits was required (β = 0.008) (Fig. 3A). A cumulative total of 18 visits in remission (β = 0.57) was required to demonstrate EQ-VAS scale scores ≥MCID at week 52. When the state of remission was sustained, remission for 20 consecutive visits was required (β = 0.51; Fig. 3B), i.e. longer time than the 18 required cumulative vistis, with this paradoxical incongruity likely depending on statistical uncertainty. Similarly, to yield EQ-5D-3L utility index scores ≥MCID (Fig. 3A) a cumulative total of eight visits (32 weeks) in LLDAS (β = 0.005) was required, whereas if sustained, LLDAS for six visits (β = 0.006) was required. For EQ-VAS scale scores ≥MCID (Fig. 3B), a cumulative total of 24 visits in LLDAS (β = 0.41) was required, whereas if sustained, LLDAS for 19 visits (β = 0.52) was required.
Fig. 3.
Associations of remission, LLDAS and LLDAS/no remission with EQ-5D-3L, EQ-VAS and FACIT-Fatigue
Forest plots illustrating results from the quantile regression analyses. Number of visits refers to the number of visits in remission, LLDAS or LLDAS/no remission required to attain a clinically important benefit, based on the estimate derived from the regression model. The number of attainers refers to the actual number of patients who were in remission, LLDAS or LLDAS/no remission and attained a clinically important benefit in EQ-5D-3L and FACIT-Fatigue within the 52-week study follow-up. Number of patients for each analysis for remission and LLDAS were: EQ-5D (1642) and FACIT-Fatigue (1655). Number of patients for each analysis in LLDAS/no remission was: 1446 (i.e. number of patients after exclusion of patients who attained remission at one or more visits during the 52-week study). The total number of visits was 15 and they were scheduled every fourth week. LLDAS: lupus low disease activity state.
A total of seven cumulative (β = 0.005) or sustained (β = 0.006) visits (28 weeks) in LLDAS/no remission was required to yield EQ-5D-3L utility index scores ≥MCID (Fig. 3C); thus, sustainability of LLDAS/no remission did not yield any additional impact on the outcome. For EQ-VAS scale scores ≥MCID (Fig. 3D), a theoretical (extrapolated) total of 50 cumulative visits in LLDAS/no remission (β = 0.20) was required, whereas if sustained, LLDAS/no remission for 37 visits (β = 0.27) was required with zero attainers documented.
Associations of remission/LLDAS attainment with FACIT-F
A total of 12 cumulative visits in remission (β = 0.34) was required to demonstrate FACIT-F scores ≥MCID at week 52, which corresponded to 48 weeks, or 10 consecutive visits (β = 0.39; Fig. 3E). To yield FACIT-F scores at week 52 ≥MCID, a total of 17 cumulative visits (68 weeks) in LLDAS (β = 0.24) or 16 consecutive visits (β = 0.25) were required (Fig. 3E).
Finally, a total of 22 cumulative or consecutive visits in LLDAS/no remission (β = 0.18 for both; Fig. 3F) was required to demonstrate FACIT-F scores ≥MCID at week 52, corresponding to 88 weeks; thus, sustainability of LLDAS/no remission did not yield any additional impact on the outcome and zero attainers were documented.
Discussion
The treat-to-target approach has been ratified and increasingly used during the last decade, and various definitions of remission and low disease activity applied in real-life SLE cohorts have been studied in relation to various aspects of HRQoL [10, 17–19, 37–43], with positive associations with HRQoL generally reported. In this study, we applied the SLEDAI-2K-based DORIS definition of remission and LLDAS to the datasets of two large phase III clinical trials of belimumab and investigated the association between remission or LLDAS and HRQoL outcomes over one year. Along with corroboration of the known benefit of being in remission or LLDAS at a given point in time on HRQoL [10, 17–19, 37, 39, 42], the present study determined the time in remission or LLDAS needed to yield a clinically important benefit in various aspects of HRQoL. We found that, compared with LLDAS, less time in remission was needed to achieve clinically important differences in HRQoL. In addition, less time in remission or LLDAS was required to gain a clinically important benefit in HRQoL when the state was sustained. However, more patients spent longer time in LLDAS than in remission in these studies.
Several studies have investigated the relationship between remission or LDA and HRQoL, the latter assessed using various PROMs, and showed that being in remission or LDA had a positive impact on HRQoL. Most of the studies explored the impact of DORIS remission [17, 18, 38–42], and some studies investigated LLDAS [10, 19, 37]. HRQoL was most frequently assessed using the generic SF-36 questionnaire [10, 17–19, 39, 43], whereas the disease-specific instruments LupusQoL [44], SLEQoL [45] and LupusPRO [46] were used less frequently. In the present investigation, the impact of remission differed between physical and mental domains of HRQoL, wherein remission had a greater positive impact on SF-36 PCS than MCS scores at week 52, with fewer visits in remission required to yield a clinically important benefit in PCS, whereas the impact of LLDAS on SF-36 PCS and MCS outcome was similar.
We used cut-offs for MCIDs from previous literature [30–32, 34]. It is worth noting that the theoretical number of visits in remission or LLDAS that was needed to yield a benefit in EQ-VAS exceeding the MCID of 10 points (10% of the scale span) as derived from the quantile regression to the median models was greater than the total number of visits of the studies. While a 10-point difference has been suggested as the MCID for 100-point visual analogue scales in general [31], our results suggest this cut-off was rather stringent in the context of EQ-VAS in this setting. An MCID of eight points has been determined for other chronic diseases, such as chronic obstructive pulmonary disease [47], pointing to the need for derivation of an SLE-specific cut-off. This would have merit in a future investigation, especially considering the increasing adoption of EQ-5D owing to its conciseness.
In a separate analysis of LLDAS after exclusion of patients who fulfilled the criteria of remission at one or more visits, LLDAS was associated with a clinically important benefit in several aspects of HRQoL, yielding statistical significance for the SF-36 physical and mental component summaries, bodily pain, general health, social functioning, role emotional and mental health, as well as for EQ-5D index scores. As expected, the minimal required duration to achieve a clinically important benefit in HRQoL was overall longer than when patients in remission were not excluded from the analysis of LLDAS. While sustainability in LLDAS overall decreased the number of visits that were required to achieve a clinically important benefit, the statistical uncertainty increased in this analysis, presumably owing to the relatively short follow-up period. In contrast, no major impact of LLDAS/no remission on fatigue was documented. To our knowledge, this is the first analysis of the added value of LLDAS in the context of patient-reported HRQoL and fatigue after exclusion of the impact of remission, and provides additional support for LLDAS as an important and clinically relevant target in SLE management whenever remission cannot be achieved, in compliance with the T2T/SLE notion [7].
The concepts of remission and LLDAS emerged nearly a decade ago. Along with studies that have demonstrated adequate attainability and discriminant validity for the tentative DORIS remission definition (sustained remission) used herein [25] and the LLDAS definition of LDA [15, 25, 48], especially when sustained, as well as associations with deceleration of organ damage accrual [11, 13, 15], our study highlights the benefit of treat-to-target approaches in relation to HRQoL. The greater stringency of remission compared with LLDAS was reflected in the overall shorter time in remission that was required to yield a clinically important benefit in multiple HRQoL domains, although remission was attained less frequently. It is nevertheless worth noting that, compared with the amount of time in remission or LLDAS required to reduce flare rates and damage accrual as shown in some studies [49], a longer period in these states was required to achieve a clinically meaningful benefit in HRQoL which highlights a remaining urgent unmet need.
One of the limitations of the present study was its post-hoc nature; the BLISS trials were not designed to study the impact of remission or LLDAS on HRQoL. Moreover, patients with severe active lupus nephritis and severe active involvement of the central nervous system were excluded from the trials, which limits the generalizability of our findings. In fact, a clinical trial population cannot resemble real-life settings, and the results should therefore be interpreted with caution regarding applicability in daily patient care. Missing data limited us from performing GEE models for all outcomes. Furthermore, the follow-up period of the study was relatively short, and we lacked lupus-specific PROMs for the assessment of HRQoL. Although generic measures may underestimate important elements of the impact of SLE, SF-36 has the advantage of having been cross-culturally validated, which allows comparisons [50]. Moreover, positive correlations between LupusQoL and SF-36 have been reported [44, 51], as well as between LupusPRO and SF-36 or EQ-5D [46]. The SLEQoL has also been shown to have an overall moderate to strong correlation with SF-36, the strongest correlation being between the mood item in SLEQoL and the mental health subscale of the SF-36 [19].
Conclusion
Both remission and LLDAS attainment were associated with a better HRQoL outcome after therapy that exceeded MCIDs in multiple domains. However, compared with LLDAS, shorter time in remission was required to achieve a clinically meaningful benefit in HRQoL. When remission or LLDAS was sustained, shorter time in the respective state was required to yield the same clinically important benefit in HRQoL. We showed that shorter time in remission was required to yield a clinically important benefit in physical compared with mental aspects of HRQoL, but no such pattern was documented for LLDAS. Importantly, LLDAS resulted in clinically important benefit in multiple HRQoL aspects also after exclusion of patients who achieved remission during follow-up, however not in fatigue. These findings strengthen the case for adoption of DORIS remission and LLDAS as treat-to-target endpoints for SLE, and for their use in clinical trials.
Supplementary Material
Acknowledgement
The authors would like to thank GlaxoSmithKline (Uxbridge, UK) for sharing data from the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials through the Clinical Study Data Request Consortium, and all patients who participated in the trials.
S.E., Y.E., M.N. and I.P. were responsible for study conception. S.E., A.G., J.L. and I.P. were responsible for study coordination. S.E., A.G., J.L., A.B., D.G. and I.P. were responsible for data processing and statistics. S.E. and I.P. drafted the manuscript. All authors were involved in the interpretation of the results. All authors read and critically revised the manuscript for intellectual content, approved its final version prior to submission, and agree to be accountable for all aspects of the work.
Funding: This work was supported by the GlaxoSmithKline Investigator-Sponsored Studies (ISS) programme, and grants from the Swedish Rheumatism Association (R-941095), King Gustaf V’s 80-year Foundation (FAI-2020–0741), Professor Nanna Svartz Foundation (2020–00368), Ulla and Roland Gustafsson Foundation (2021–26), Region Stockholm (FoUI-955483) and Karolinska Institutet.
Disclosure statement: E.M. has received grants and/or honoraria from Astra Zeneca, Bristol Myers Squibb, Biogen, AbbVie, Eli Lilly, Janssen, Wolf, Neovacs, UCB, GlaxoSmithKline, Sanofi, EMD Serono, Novartis and Amgen. R.F.vV. has received grant/research support from AbbVie, Arthrogen, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Pfizer and UCB; consultant of AbbVie, AstraZeneca, Biotest, Bristol-Myers Squibb, Celgene, GSK, Janssen, Lilly, Medac, Merck, Novartis, Pfizer, Roche and UCB. M.N. holds an NHMRC Investigator Grant (GTN1176538) and has received research funding support and honoraria from Astra Zeneca, BMS, Boehringer Ingelheim, Eli Lilly, Janssen, GSK, Pfizer and UCB. I.P. has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis and F. Hoffmann-La Roche AG. The other authors declare that they have no conflicts of interest. The funders had no role in the design of the study, the analyses or interpretation of data, or the writing of the manuscript.
Contributor Information
Sharzad Emamikia, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Shereen Oon, Departments of Medicine and Rheumatology, The University of Melbourne at St Vincent's Hospital, Fitzroy.
Alvaro Gomez, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Julius Lindblom, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Alexander Borg, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Yvonne Enman, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Eric Morand, School of Clinical Sciences at Monash Health, Monash University Faculty of Medicine, Nursing and Health Sciences, Monash Medical Centre Clayton, Clayton, Victoria, Australia.
David Grannas, Divison of Biostatistics, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
Ronald F van Vollenhoven, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden; Department of Rheumatology, Amsterdam Rheumatology and Immunology Center, Amsterdam, The Netherlands.
Mandana Nikpour, Departments of Medicine and Rheumatology, The University of Melbourne at St Vincent's Hospital, Fitzroy.
Ioannis Parodis, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden; Department of Rheumatology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
Data availability statement
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Supplementary data
Supplementary Material, available at Rheumatology online.
References
- 1. Kaul A, Gordon C, Crow MK. et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- 2. Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–8. [PubMed] [Google Scholar]
- 3. Gomez A, Qiu V, Cederlund A. et al. Adverse health-related quality of life outcome despite adequate clinical response to treatment in systemic lupus erythematosus. Front Med 2021;8:651249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mikdashi J. Measuring and monitoring health-related quality of life responsiveness in systemic lupus erythematosus patients: current perspectives. Patient-Related Outcome Meas 2018;9:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Izadi Z, Gandrup J, Katz PP, Yazdany J.. Patient-reported outcome measures for use in clinical trials of SLE: a review. Lupus Sci Med 2018;5:e000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Annapureddy N, Devilliers H, Jolly M.. Patient-reported outcomes in lupus clinical trials with biologics. Lupus 2016;25:1111–21. [DOI] [PubMed] [Google Scholar]
- 7. van Vollenhoven RF, Mosca M, Bertsias G. et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. [DOI] [PubMed] [Google Scholar]
- 8. van Vollenhoven R, Voskuyl A, Bertsias G. et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017;76:554–61. [DOI] [PubMed] [Google Scholar]
- 9. Ríos-Garcés R, Espinosa G, van Vollenhoven R, Cervera R.. Treat-to-target in systemic lupus erythematosus: where are we? Eur J Intern Med 2020;74:29–34. [DOI] [PubMed] [Google Scholar]
- 10. Golder V, Kandane-Rathnayake R, Hoi AY. et al. Association of the lupus low disease activity state (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res Ther 2017;19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franklyn K, Lau CS, Navarra SV. et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis 2016;75:1615–21. [DOI] [PubMed] [Google Scholar]
- 12. Parodis I, Nikpour M.. How to use the Lupus Low Disease Activity State (LLDAS) in clinical trials. Ann Rheum Dis 2021;80:e119. [DOI] [PubMed] [Google Scholar]
- 13. Tsang ASMW, Bultink IE, Heslinga M, Voskuyl AE.. Both prolonged remission and Lupus Low Disease Activity State are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology 2017;56:121–8. [DOI] [PubMed] [Google Scholar]
- 14. Golder V, Tsang A.. Treatment targets in SLE: remission and low disease activity state. Rheumatology 2020;59:v19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golder V, Kandane-Rathnayake R, Huq M. et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatol 2019;1:e95–e102. [DOI] [PubMed] [Google Scholar]
- 16. Petri M, Magder LS.. Comparison of remission and lupus low disease activity state in damage prevention in a united states systemic lupus erythematosus cohort. Arthritis Rheumatol 2018;70:1790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mok CC, Ho LY, Tse SM, Chan KL.. Prevalence of remission and its effect on damage and quality of life in Chinese patients with systemic lupus erythematosus. Ann Rheum Dis 2017;76:1420–5. [DOI] [PubMed] [Google Scholar]
- 18. Tsang-A-Sjoe MWP, Bultink IEM, Heslinga M. et al. The relationship between remission and health-related quality of life in a cohort of SLE patients. Rheumatology 2019;58:628–35. [DOI] [PubMed] [Google Scholar]
- 19. Louthrenoo W, Kasitanon N, Morand E, Kandane-Rathnayake R.. Comparison of performance of specific (SLEQOL) and generic (SF36) health-related quality of life questionnaires and their associations with disease status of systemic lupus erythematosus: a longitudinal study. Arthritis Res Ther 2020;22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navarra SV, Guzmán RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 21. Furie R, Petri M, Zamani O. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 23. Petri M, Kim MY, Kalunian KC. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 24. Gladman D, Ginzler E, Goldsmith C. et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 25. Parodis I, Emamikia S, Gomez A. et al. Definitions of remission in systemic lupus erythematosus: a post-hoc analysis of two randomised clinical trials. Lancet Rheumatol 2019;1:e163–73. [DOI] [PubMed] [Google Scholar]
- 26. van Vollenhoven RF, Bertsias G, Doria A. et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med 2021;8:e000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 28. EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 29. Kosinski M, Gajria K, Fernandes AW, Cella D.. Qualitative validation of the FACIT-fatigue scale in systemic lupus erythematosus. Lupus 2013;22:422–30. [DOI] [PubMed] [Google Scholar]
- 30. Strand V, Crawford B.. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res 2005;5:317–26. [DOI] [PubMed] [Google Scholar]
- 31. Bangert E, Wakani L, Merchant M, Strand V, Touma Z.. Impact of belimumab on patient-reported outcomes in systemic lupus erythematosus: review of clinical studies. Patient-Related Outcome Meas 2019;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo N, Johnson J, Coons SJ.. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care 2010;48:365–71. [DOI] [PubMed] [Google Scholar]
- 33. Lai JS, Beaumont JL, Ogale S, Brunetta P, Cella D.. Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J Rheumatol 2011;38:672–9. [DOI] [PubMed] [Google Scholar]
- 34. Cella D, Yount S, Sorensen M. et al. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–9. [PubMed] [Google Scholar]
- 35. Gomez A, Hani Butrus F, Johansson P. et al. Impact of overweight and obesity on patient-reported health-related quality of life in systemic lupus erythematosus. Rheumatology 2021;60:1260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borg A, Gomez A, Cederlund A. et al. Contribution of abnormal BMI to adverse health-related quality of life outcomes after a 52-week therapy in patients with SLE. Rheumatology 2021;60:4205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R.. Lupus Low Disease Activity State (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab. Ann Rheum Dis 2018;77:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ugarte-Gil MF, Gamboa-Cardenas RV, Reategui-Sokolova C. et al. Better health-related quality of life in systemic lupus erythematosus predicted by low disease activity state/remission: data from the peruvian almenara lupus cohort. Arthritis Care Res 2020;72:1159–62. [DOI] [PubMed] [Google Scholar]
- 39. Margiotta DPE, Fasano S, Basta F. et al. The association between duration of remission, fatigue, depression and health-related quality of life in Italian patients with systemic lupus erythematosus. Lupus 2019;28:1705–11. [DOI] [PubMed] [Google Scholar]
- 40. Goswami RP, Chatterjee R, Ghosh P, Sircar G, Ghosh A.. Quality of life among female patients with systemic lupus erythematosus in remission. Rheumatol Int 2019;39:1351–8. [DOI] [PubMed] [Google Scholar]
- 41. Poomsalood N, Narongroeknawin P, Chaiamnuay S, Asavatanabodee P, Pakchotanon R.. Prolonged clinical remission and low disease activity statuses are associated with better quality of life in systemic lupus erythematosus. Lupus 2019;28:1189–96. [DOI] [PubMed] [Google Scholar]
- 42. Heijke R, Bjork M, Frodlund M. et al. Relationship between remission, disease activity and patient-reported outcome measures in patients with recent-onset systemic lupus erythematosus. Lupus 2020;29:625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ugarte-Gil MF, Pons-Estel GJ, Vila LM, McGwin G, Alarcón GS.. Time in remission and low disease activity state (LDAS) are associated with a better quality of life in patients with systemic lupus erythematosus: results from LUMINA (LXXIX), a multiethnic, multicentre US cohort. RMD Open 2019;5:e000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McElhone K, Abbott J, Shelmerdine J. et al. Development and validation of a disease-specific health-related quality of life measure, the LupusQoL, for adults with systemic lupus erythematosus. Arthritis Rheum-Arthritis Care Res 2007;57:972–9. [DOI] [PubMed] [Google Scholar]
- 45. Leong KP, Kong KO, Thong BY. et al. Development and preliminary validation of a systemic lupus erythematosus-specific quality-of-life instrument (SLEQOL). Rheumatology 2005;44:1267–76. [DOI] [PubMed] [Google Scholar]
- 46. Jolly M, Pickard AS, Block JA. et al. Disease-specific patient reported outcome tools for systemic lupus erythematosus. Semin Arthritis Rheum 2012;42:56–65. [DOI] [PubMed] [Google Scholar]
- 47. Zanini A, Aiello M, Adamo D. et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care 2015;60:88–95. [DOI] [PubMed] [Google Scholar]
- 48. Oon S, Huq M, Golder V. et al. Lupus Low Disease Activity State (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of belimumab in systemic lupus erythematosus. Ann Rheum Dis 2019;78:629–33. [DOI] [PubMed] [Google Scholar]
- 49. Saccon F, Zen M, Gatto M. et al. Remission in systemic lupus erythematosus: testing different definitions in a large multicentre cohort. Ann Rheum Dis 2020;79:943–50. [DOI] [PubMed] [Google Scholar]
- 50. Shi Y, Li M, Liu L. et al. Relationship between disease activity, organ damage and health-related quality of life in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev 2021;20:102691. [DOI] [PubMed] [Google Scholar]
- 51. Conti F, Perricone C, Reboldi G. et al. Validation of a disease-specific health-related quality of life measure in adult Italian patients with systemic lupus erythematosus: lupusQoL-IT. Lupus 2014;23:743–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.