Abstract
Spinal cord injury (SCI) is a devastating event that can permanently disrupt multiple modalities. Unfortunately, the combination of the inhibitory environment at a central nervous system (CNS) injury site and the diminished intrinsic capacity of adult axons for growth results in the failure for robust axonal regeneration, limiting the ability for repair. Delivering genetic material that can either positively or negatively modulate gene expression has the potential to counter the obstacles that hinder axon growth within the spinal cord after injury. A popular gene therapy method is to deliver the genetic material using viral vectors. There are considerations when deciding on a viral vector approach for a particular application, including the type of vector, as well as serotypes, and promoters. In this review, we will discuss some of the aspects to consider when utilizing a viral vector approach to as a therapy for SCI. Additionally, we will discuss some recent applications of gene therapy to target extrinsic and/or intrinsic barriers to promote axon regeneration after SCI in preclinical models. While still in early stages, this approach has potential to treat those living with SCI.
Keywords: Spinal cord injury, Axon regeneration, Gene therapy, AAV, Lentivirus, Adenovirus
Traumatic spinal cord injury (SCI) can have a catastrophic impact on an individual’s quality of life (Ahuja et al., 2017). Despite much research, there still is no clinically-available treatment that promotes repair after SCI (Yu et al., 2019; Silva et al., 2014). After an SCI, axons and cell bodies located at the lesion site are directly impacted by the trauma. Additionally, inflammation, excitotoxicity, and compromised blood-spinal cord-barrier lead to secondary damage to additional cells and axons that were initially spared after the SCI (Oyinbo, 2011). Unfortunately, axons do not readily regenerate after CNS injury. This failure is caused by factors intrinsic to the neuron, such as the decreased expression of growth associated genes in CNS neurons after development. Failure to regenerate is also caused by factors extrinsic to the neuron, such as changes in the injury environment and formation of a glial scar. Thus, much research has focused on overcoming these intrinsic and extrinsic barriers to increase the axon growth potential of neurons and foster CNS repair.
To date, there are no drug therapies that can repair the damage caused by an SCI and restore function lost due to the injury, such as voluntary muscle movement or sensation (Failli et al., 2021). Many drugs that have gone through clinical trials for SCI were shown to have minimal efficacy (Cox et al., 2015). Other drugs were shown to only treat symptoms that develop after injury, such as pain. None have been shown to regenerate axons impacted by the injury to return function (Cox et al., 2015). Drugs may also have off-target effects that lead to unwanted side effects.
One approach that appears to have much potential to increase neurons’ axon regrowth potential is gene therapy. Gene therapy would give researchers the ability to modulate protein expression in neurons damaged by SCI or non-neuronal cells within the lesion penumbra to improve axonal regeneration and functional recovery. One way to achieve this is using viral vectors, such as adeno-associated virus (AAV) and lentivirus, to deliver the transgenes. Investigators are using these vectors to modify the expression of genes or introduce novel genes to affecting extrinsic growth-inhibitory forces (such as the removal of inhibitory molecules and modulating the astrocytic response) and boosting intrinsic pro-regenerative cellular machinery (such as increasing the transcription of transcription factors and modulating components of the cytoskeleton) (Dunbar et al., 2018; Zavvarian et al., 2020). However, there are many factors to consider when applying this technology. For example, one needs to consider the size of the gene being delivered, the type and location of cells targeted for transduction, and the length of time the protein needs to be expressed (Lentz et al., 2012). Other limitations with gene therapy include high toxicity, immunogenicity, and insertional mutagenesis, which may cause other pathologies (Zavvarian et al., 2020). Further investigating, developing, and improving this technology will likely mitigate these limitations such to increase their use in the clinic.
In this review, we will provide an overview of the different types of viral vectors commonly used in SCI research studies. We will also discuss various applications of gene therapy to modulate factors extrinsic and/or intrinsic to neurons to enhance axon regeneration after injury.
1. Overview of viral vectors
1.1. Adenovirus
Unlike other viral vectors that cause permanent expression of a transgene (such as AAV and lentivirus; discussed below), adenovirus (Ad) vectors result in transient expression (Table 1). This may be considered advantageous in the context of a therapy for SCI, which is not a genetic disease requiring permanent change to a patient’s genome. It delivers a linear double-stranded DNA up to 7.5–8 kb in size that does not integrate within the host genome (Fig. 1). Once in the body, the vector binds to specific receptors such as the coxackie and adenovirus receptors (CAR) on the host cell and enters via endocytosis. After forming a pore within the endosome, it uses the motor protein dynein to retrogradely transport itself to the nucleus so the viral capsid can interact with the nuclear envelope to induce the expression of the transgene. Whereas wild-type adenovirus can use its viral genome to replicate and lyse the cell to spread and infect others, the vector used in research for gene therapy lacks the early 1 (E1) region needed for viral replication. The transient nature of the virus, along with its ability to be delivered in the neuromuscular junction and away from the anatomical and molecular complexity of the spinal cord makes it useful for research, in some cases. However, adenoviruses are strongly immunogenic, complicating its use for gene therapy (Wold and Toth, 2013; Young and Mautner, 2001; Muruve, 2004). These vectors have been shown to cause a strong innate immune response in mice models by invoking an innate response immediately after intravenous injection and lasting up to 6 h (Wold and Toth, 2013). The titer given is also important to consider, as high titers can lead to injury of organs, such as the liver, resulting in death (Wold and Toth, 2013; Young and Mautner, 2001; Muruve, 2004). Thus, adenovirus is classified in the level 2 risk group by the NIH and health risks should be considered by the researcher and proper handling [i.e., under biosafety level 2 (BSL-2) protocols] should be followed.
Table 1.
A comparison of the main types of viral vectors discussed. There are many different factors that must be considered when choosing an appropriate vector.
| Adenovirus | Lentivirus | AAV | |
|---|---|---|---|
|
| |||
| Genome type | dsDNA | ssRNA | ssDNA |
| Packaging limit | ∼7.5–8kb | ∼8–10kb | ∼4.7kb |
| Expression | Transient | Stable | Transient or stable |
| Integration | Non-integrating | Integrating | Non-Integrating |
| Cell infectivity | Dividing and nondividing cells | Slowly dividing and nondividing | Dividing and nondividing |
| Toxicity | High | Moderate to high | Low |
| Bio-safety Level | BSL-2 | BSL-2 | BSL-1 |
| Pro | High packaging capacity, transient gene expression, high protein expression | Low immune response, high packaging capacity, host genome integration allows for stable gene expression | Very low immune response, different serotypes can increase tropism |
| Con | High immune response | Host genome integration may not be desired | Low packaging capacity, low protein expression |
| References | (Lee et al., 2017; Ghosh et al., 2020) | (Parr-Brownlie et al., 2015; Lee et al., 2017; Ghosh et al., 2020) | (Wu et al., 2010; Lee et al., 2017; Ghosh et al., 2020) |
Fig. 1.
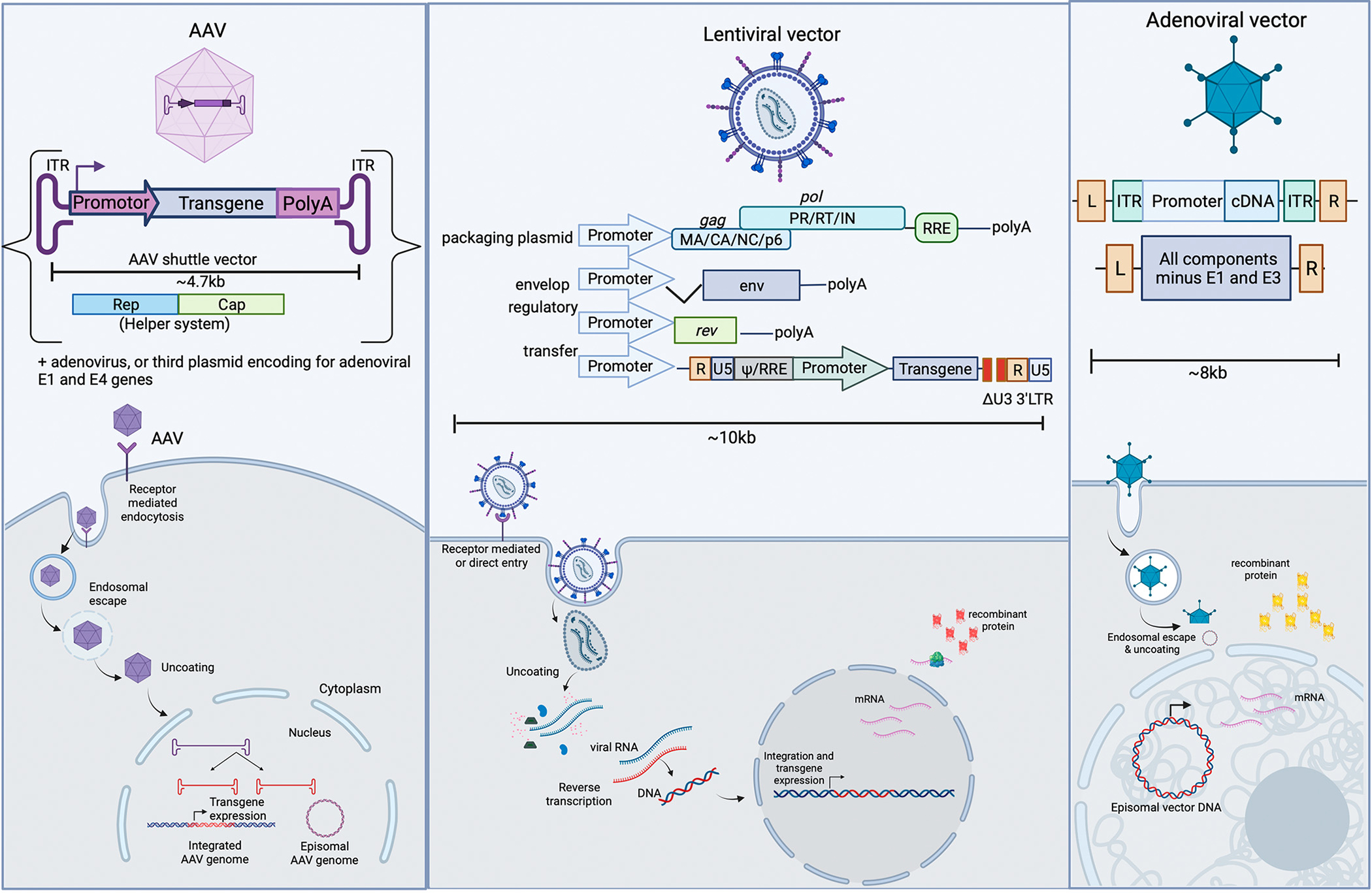
Components, route of entry, and mechanism of transgene expression of the three types of viral vectors discussed in this review—AAV, lentivirus, and adenovirus. (Created using Biorender.com)
1.2. Lentivirus
Lentiviruses are a subtype of the Retroviridae family and can be very useful when implemented in gene therapy. Unlike other subtypes of the retrovirus, the lentivirus can transduce slowly dividing or nondividing cells within the CNS (Naldini et al., 1996). They can transduce a wide range of cells, from neurons to other resident cells such as astrocytes, neural stem cells, and oligodendrocytes. This can be useful in the context of gene therapy for SCI, as virtually all types of cells are impacted by the injury. The 8–10kb packaging limit allows this vector to be used with large transgene cassettes (Fig. 1) (Parr-Brownlie et al., 2015). The capsid of the virus contains two copies of single-stranded RNA along with molecular machinery necessary for transduction—such as reverse transcriptase (Maes et al., 2019). This results in the conversion of the RNA strands into double-stranded DNA and, with the assistance of DNA integrase, can be randomly inserted into the host genome. This results in the ability of the vector to efficiently transduce cells and cause a permanent change (Choudhury et al., 2017). Because the viral machinery of the lentivirus is derived from the human immunodeficiency virus (HIV-1), there is a concern about virulence and safety. For that reason, recombinant vectors containing necessary machinery such as viral genes encoding gag (structural core protein), pol (reverse transcriptase), and env (envelope glycoprotein) are split into multiple plasmids to require more recombination events to produce a replication-competent virus. Vectors also lack the virulence factors vif, vpr, vpu, and nef, creating a safer tool for genetic manipulation, thus mitigating but not eliminating the risk of a replication competent virus (Schlimgen et al., 2016).
Insertional mutagenesis is still a risk with lentivirus integration, causing overexpression of certain genes and disturbance of others (Schlimgen et al., 2016). To deal with this, researchers have developed Non-Integrating Lentivirus Vectors (NILV) by causing point mutations on the lentivirus integrase protein found in the HIV-1 pol region (Shaw and Cornetta, 2014). Mutations in the Pol region, rather than deletion of the Pol region, are necessary because it encodes for proteins required for integration after transduction, such as reverse transcriptase and polymerase (Shaw and Cornetta, 2014). Although this non-integrating vector lessens the risk of integration, integration may still occur. Thus, even though this vector has been manipulated to mitigate health risks, lentivirus needs to be handled under BSL-2 protocols.
1.3. Adeno-associated virus
Adeno-associated virus (AAV) is a common vector of choice for gene therapy. Its low immunogenicity and risk of insertional mutagenesis combined with the ability to transduce both, dividing and non-dividing cells, make it an attractive option for researchers (Ojala et al., 2015). It is also a safer option for researchers because it can be handled under BSL-1 protocols and has been shown to be generally safe in preclinical animal models and human clinical trials. A member of the Parvoviridae family of viruses, this vector has an icosahedral capsid containing up to 4.7 kb single-stranded DNA (Fig. 1). To complete its replication cycle, it requires assistance such as cellular stress or coinfection with an adenovirus or rabies virus. When help is not available, AAV exists in a latent state by integrating itself within the genome of the host cell. Because of this, AAV vectors are produced in systems such as HEK cells, which can be transfected with other plasmids containing the various components needed for viral replication. Open reading frames (ORFs) are eliminated in recombinant AAVs, thus depriving the virus and vector the ability to replicate and increasing safety. The loss of viral genes also increases the ability to carry a larger transgene cassette, allowing use for more applications. The need to maximize the size of the transgene cassette being shuttled by the virus is important because of the 4.7 kb vector size, which is smaller than other viral vectors, such as lentivirus. It has been shown that adding transgene cassettes greater than the vector limit results in problems with packaging and only partial DNA being encapsulated (Dong et al., 2010; Lai et al., 2010). While the maximum packaging capacity in AAV is approximately 5.2kb, transduction using AAV with transgene cassettes 4.7kb or smaller in size results in highest levels of gene expression. It is believed that packaging stalls after this limit and cellular nucleases degrade the remaining portion of the gene. Thus, it may be that genes with a maximum of 4.7kb in size can reliably be packaged within an AAV vector. Beyond 4.7kb, there is an exponential rate of failure with increasing gene size (Wu et al., 2010). This is an important consideration as this limitation may make AAV unsuitable for therapies requiring expression of large genes.
Cross-packaging the AAV genome with different capsid proteins results in distinct serotypes that display differential binding to varied cell populations (Table 2) (McCown, 2011). This can affect how far the viral vector diffuses from the injection site, resulting in transduction of cells at varying distances (Haery et al., 2019). For example, the capsid for AAV2 causes a local and confined diffusion at an injection site, allowing the researcher to target specific areas of the central nervous system (CNS). Meanwhile, AAV1, -5, -9, and -rh10 can diffuse further from the administration site into the surrounding tissue, allowing for more widespread expression of the transgene (Haery et al., 2019; Sondhi et al., 2007).
Table 2.
AAV serotypes discussed in this review, along with popular routes of administration.
| AAV serotype | Cells transduced | Route of delivery | References |
|---|---|---|---|
|
| |||
| 1 | Neurons, Astrocytes | Moderate and widespread expression of neurons and glia after direct administration. | (Haery et al., 2019; Klaw et al., 2013; O’Carroll et al., 2020; Royo et al., 2008) |
| 2 | Neurons, low levels of astrocytes | More confined spread after direct injection. | (Haery et al., 2019; Klaw et al., 2013; O’Carroll et al., 2020) |
| 5 | Neurons, low levels of astrocytes, oligodendrocytes | Intraspinal injection leads to efficient retrograde transduction of the spinal cord. Intracerebroventricular (ICV) and intrathecal (IT) administration results in efficient transduction of the spinal cord and dorsal root ganglia of rats. |
(Haery et al., 2019; Petrosyan et al., 2014; Klaw et al., 2013; O’Carroll et al., 2020; Pietersz et al., 2021) |
| 6 | Neurons, Astrocytes | Transduces neurons and astrocytes after direct delivery. Has the potential to transduce microglia after modification. | (O’Carroll et al., 2020; Royo et al., 2008; Schober et al., 2016; Rosario et al., 2016) |
| 7 | Neurons, Astrocytes | Widely used in CSF delivery. | (O’Carroll et al., 2020; Royo et al., 2008) |
| 8 | Neurons, low levels of astrocytes, low levels of oligodendrocytes | Moderate and widespread expression of neurons and glia after direct administration. | (Haery et al., 2019; Klaw et al., 2013; O’Carroll et al., 2020; Royo et al., 2008; Pignataro et al., 2017) |
| 9 | Neurons, astrocytes, low levels of oligodendrocytes | IP injection of AAV9 led to the best localized transduction of cells within the spinal cord. Also used in CSF delivery. | (Haery et al., 2019; Petrosyan et al., 2014; Klaw et al., 2013; Royo et al., 2008) |
| rh10 | Neurons | Widely used in CSF delivery. | (Haery et al., 2019; Petrosyan et al., 2014; Hoshino et al., 2019) |
| PHP.B/PHP.eB | Neurons, oligodendrocytes, endothelial cells | Efficiently crosses BBB. Intrathecal administration in Rhesus Macaque leads to efficient transduction of the cortex and spinal cord. | (Reynaud-Dulaurier and Decressac, 2020; Deverman et al., 2016; Chan et al., 2017; Liguore et al., 2019; Jackson et al., 2016) |
Additionally, because of differential binding of AAV serotypes to cell types, choice of serotype can be leveraged to target multiple cell types or to limit transduction to specific cell populations. Although different AAV serotypes are known to transduce neurons, the ability to also affect cells such as glial cells, astrocytes, and oligodendrocytes is crucial in SCI research because all these cell types are impacted by the injury. In a rat SCI model, injection of AAV5, -9, and -rh10 coupled with GFP were shown to have the highest GFP expression within cells and processes at the injury site, while -9 and -rh10 also transduced cells in distant parts of the spinal cord (Petrosyan et al., 2014). Of all serotypes, -rh10 had the most versatile function as it was able to transduce neurons within the grey matter of around the injury site, as well as non-neuronal cells. This included oligodendrocytes (>10% CC1+ cells) and macrophages/microglia (>20% ED1+ cells) at or around the injury site. A separate study showed that injection of AAV5 expressing destabilized YFP (dYFP) under either the CAG or truncated-CAG promotor into the parenchyma surrounding a contusion site resulted in high transduction and transgene expression in astrocytes (Griffin et al., 2019). As research on gene therapy continues, new serotypes will be discovered or developed to manipulate gene expression more efficiently and/or specifically. For instance, a recently generated serotype AAVrh10 more effectively transduces neurons, oligodendrocytes, and astrocytes in a chronic SCI model when compared to AAV5 and -6 (Hoshino et al., 2019).
AAV serotypes with characteristics that allow for efficient retrograde targeting would have significant appeal for SCI applications. This would allow researchers to transduce neurons that project to specific regions. For example, coordinated movement requires the convergence of multiple descending tracts originating from different brainstem nuclei and the motor cortex at a given site within the spinal cord. Being able to manipulate gene expression in the various subpopulations of neurons that project to a particular region of spinal cord would allow for “casting a wide net” while concurrently conferring some specificity. Some AAV serotypes are able to travel retrogradely but require a higher dose of vector because of inefficient transduction (Haery et al., 2019). Additionally, different serotypes have different tropism for different neuron populations. We showed that injecting AAV1-, AAV2-, AAV5-, AA8-, or AAV-9 rostral to a complete SCI site resulted in differential retrograde transduction of supraspinal neuron populations. AAV5 transduced more neurons in the reticular formation via the reticulospinal tract while AAV1 transduced more neurons in the raphe nuclei via the raphespinal tract. All serotypes transduced the vestibular nuclei via the vestibulospinal tract and the red nucleus via the rubrospinal tract. Interestingly, transduction of the primary motor cortex could not be achieved by retrograde transport via the corticospinal tract even in the presence of vector particles (Klaw et al., 2013).
Recently, the AAV2-retro serotype that efficiently transduces projection neurons in a retrograde manner was engineered (Tervo et al., 2016). Injecting this viral vector into the cervical spinal cord of mice transduces cortical and brainstem neurons that project down the corticospinal, rubrospinal, and reticulospinal tracts (Wang et al., 2018a). Moreover, injecting AAV2-retro into the spinal cord after a unilateral dorsal hemisection also results in the transduction of cortical neurons, indicating that this particular viral vector can efficiency and retrogradely transduce neurons, even after injury. Thus, this new serotype is likely to be useful in the context of SCI research.
The varying characteristics of the different AAV serotypes are important to consider when deciding upon the route of administration to deliver the transgene of interest to a particular cell population. A direct delivery route, such as with intraparenchymal injections, are much more invasive but allows researchers and clinicians to better target a specific region (Hardcastle et al., 2018). One study comparing intraparenchymal injections of AAV1, -6, -8, and -9 serotypes found that AAV9 led to the best localized transduction of cells within the spinal cord (Snyder et al., 2011). A remote delivery of the vector via intramuscular (IM), intraneural (IN), intravenous (IV) or intrathecal (IT) injection allows researchers to target the injury by administering the vector at one location to transduce neurons at a completely different location that is remote to the injection site (Hardcastle et al., 2018). For example, injecting AAVs that can be transported retrogradely into muscle are taken up at the injection site by pre-synaptic axon terminals of motor neurons and carried back to the soma of within the spinal cord. Although IM and IN injections may allow for peripheral delivery of vectors, these routes are considered “segmental treatments” because only specific motor segments are targeted instead of a more diffuse delivery that transduces a large area of the spinal cord (Hardcastle et al., 2018). These methods, especially IM, needs to be more thoroughly investigated because research has shown inconsistent effectiveness, depending on the AAV serotype (Hardcastle et al., 2018). However, the advent of AAV-retro may improve this. Injecting AAV-retro into the carpi radialis muscle in the forearm transduced neurons in ipsi- and contralateral spinal cord more efficiently than similar IM injections of AAV1, -2, -5, -6, -7, -8, and -9 (Chen et al., 2020a). Moreover, IM injections of AAV-retro transduced neurons in the dorsal root ganglia (DRG) (Chen et al., 2020a). However, this vector also transduced neurons that do not project to the injected muscle, presumably via cerebrospinal fluid. Thus, IM delivery of AAV-retro may result in extensive transduction, but it still needs to be optimized.
There are other, less invasive administration routes to transduce cells within the CNS. For instance, injecting either AAV6 or AAV9 into the intrathecal space produced a broad transduction distribution within the whole spinal cord (Hardcastle et al., 2018; Snyder et al., 2011). Other means, such as systemic delivery via intravenous injection, are also less invasive but are associated with other challenges. One significant hurdle with using systemic delivery of viral vectors for CNS applications is their inefficient ability to cross the blood-brain barrier (BBB). Systemic gene therapy delivery into the CNS normally can only be achieved for a short time after birth, thus making it impossible to treat the vast majority of SCI individuals using this method (Reynaud-Dulaurier and Decressac, 2020). To circumvent this limitation, researchers created the AAV-PHP. B vector by inserting 7 amino acid sequences into the lox sites of the cap gene in the AAV9 capsid (Deverman et al., 2016). After screening for vectors that successfully crossed the BBB by using Cre mice, it was discovered that this vector variant was able to transduce neurons and astrocytes 40 times greater than AAV9 vectors and across multiple regions of the CNS. The researchers later created the AAV-PHPeB variant, which has enhanced ability to transduce cells within the CNS (Chan et al., 2017). Interestingly, the compromised blood-spinal cord barrier (BSCB) after SCI may be leveraged to enhance AAV penetration into the CNS after intravenous delivery. A recent study demonstrated delivering AAV2/9 and -2/10 under the synapsin promotor via tail-vein injection after a T8 complete crush injury in mice efficiently transduced propriospinal neurons within the thoracic spinal cord (Brommer et al., 2021). One consideration is that there appears to be a post-injury time window that determines how efficient using this method is. Tail vein injection of the vector 1 day post-injury transduced more neurons around the injury than when the same vector was injected 3 or 7 days after injury.
Retro-orbital injection is another administration route for systemic delivery of a viral vector within the CNS. Because it targets a dense capillary bed within the inside of the ocular cavity, it can be considered a type of IV injection (Chan et al., 2017). Importantly, it has been shown to be a means for vector delivery to the developing and mature CNS (Chan et al., 2017; Grames et al., 2017).
1.4. Promoter selection
Selecting the correct promoter is a crucial factor to achieve cell-specific viral transduction and efficient gene expression. While some promoters allow strong gene expression in many cell types in the CNS, others are cell type specific and limit what types of cells to express the transgene (Table 3).
Table 3.
Popular vectors, promoters, and cell types transduced discussed in this section.
| Vector(s) used | Promoter | Cell type transduced | Reference |
|---|---|---|---|
|
| |||
| AAV1, adenovirus, lentivirus | hSyn | Neurons | (Nieuwenhuis et al., 2021; Hioki et al., 2007; Kugler et al., 2003) |
| Lentivirus | E/SYN | Neurons | (Hioki et al., 2007) |
| Lentivirus | NSE | Neurons | (Hioki et al., 2007; Sonja et al., 1990) |
| Lentivirus, adenovirus, AAV2, AAV5 | CMV | Neurons, glial cells | (Hioki et al., 2007; Kugler et al., 2003; Cucchiarini et al., 2003) |
| Adenovirus | U1 | Neurons, glial cells | (Kugler et al., 2003) |
| AAV5, AAV9 | GFAP | Astrocytes | (Griffin et al., 2019; Brenner et al., 1994) |
| rAAV | MBP | Oligodendrocytes | (Chen et al., 1998; Chen et al., 1999) |
One of the most popular promoters used to target transduction of neurons is synapsin (Syn), a family of neuron specific phosphoproteins. The proximal region of this protein is important for neuron-specific gene expression and is highly conserved across species (Schoch et al., 1996). Incorporating this promoter results in highly specific transduction in neurons across different viral vector platforms, such as AAVs (Nieuwenhuis et al., 2021), lentivirus (Hioki et al., 2007), and adenovirus (Kugler et al., 2003). For instance, when compared to other popular promoters such as the short CMV early enhancer/chicken β actin (sCAG) and human cytomegalovirus (hCMV), AAV1 vectors packaged with either the mouse phosphoglycerate kinase (mPGK) or human synapsin (hSyn) promotors transduced a larger area of the sensory-motor cortex and a higher number of neurons (Nieuwenhuis et al., 2021). hSyn was the only promoter to be neuron specific as the other promoters also resulted in transgene expression in glial cells.
Although some promoters may be neuron specific, non-specific promoters such as CMV, have higher transcriptional activities. When hybrid promoters were created using a combination of neuron-specific promoters, such as Synapsin I (SYN), and CMV enhancers and packaged in a lentivirus vector (E/SYN), it had higher neuronal specificity than the CMV promoter (Hioki et al., 2007). Moreover, the expression level of the transgene (i.e., the reporter GFP) under the E/SYN promoter was higher in certain areas of the brain (e.g., the neostriatum, thalamus, and neocortex) than when using the SYN promoter. It should be noted, however, that the CMV promoter resulted in stronger GFP fluorescence in other areas, such as the neocortex and thalamus, than hybrid promoters (Hioki et al., 2007). Thus, hybrid promoters have potential in achieving strong and highly neuron-specific expression, but efficiency may be neuron-subtype specific. It is apparent that more research must be done.
The neuron-specific enolase (NSE) promoter is also thought to be neuron specific. Early research using NSE-lacZ mice showed that this promoter is highly brain-specific, as it was not detected in other organs such as the liver, kidney, and heart (Sonja et al., 1990). Expression of the lacZ transgene driven by the NSE promoter was detected throughout the brain gray matter and not in the white matter (Sonja et al., 1990). Moreover Purkinje cells, granule cells, and neurons in the cerebellar nuclei expressed the transgene, while glial cells did not. Further analysis of the spinal cord and retina also showed similar results (Sonja et al., 1990). However, findings from a more recent study complicate our understanding of NSE-promoter specificity. Adenoviral vectors with promoters Syn, CMV, U1 or NSE were injected into the striatum. The CMV or U1 promoters resulted in transgene expression mostly in non-neuronal cells. While the Syn promoter resulted in reporter expression exclusively in neurons, the NSE promoter resulted in reporter expression in both neuronal and glial cells (Kugler et al., 2003). Thus, there is some discrepancy as to how well the NSE promoter limits transduction to neurons.
To restrict transgene expression to astrocytes, one can use a promoter for glial fibrillary acidic protein (GFAP), an astrocytic intermediate-filament protein (Brenner et al., 1994). The 5’-flanking regions of the GFAP gene is sufficient to drive transgene expression in astrocytes (Besnard et al., 1991). Researchers examined transgene expression in transgenic mice with lacZ fused with a 2.2 kb fragment of the 5’-flanking region (gfa2) and found that retinal astrocytes expressed the transgene (Merienne et al., 2013). Although not all astrocytes in the brain expressed lacZ, the transgene was only expressed in astrocytes and was not present in non-astrocytic cells.
Targeted transduction of microglia remains a challenge because of a lack of specific promoters and inefficient transduction. Many studies have utilized the cytomegalovirus (CMV) promoter, although this is more a general promoter than a cell-specific promoter. Recently, researchers transduced primary microglial cultures from developing rats with AAV2 and AAV5 vectors using the macrophage specific markers CD11b, CD68, and murine F4/80 as promoters (Cucchiarini et al., 2003). However, compared to vectors using the CMV promoter, these macrophage-specific markers were not as efficient. Incubating the microglia with vectors to express the lacZ under the F4/80 promoter resulted in 25% of the cells expressing the transgene. This was a higher transduction rate of than when the primary microglia were incubated with vectors containing the promoters for CD68 (transduced ~10% of microglia) or CD68 (negligible lacZ expression). Discovering other microglia-specific promoters may identify promoters that more efficiently drive transgene expression in microglia.
To target transgene expression to oligodendrocytes, a promoter for myelin basic protein (MBP), an abundant myelin protein found in the CNS and PNS, may be used. AAV vectors using a fragment of the MBP as the promoter is able to efficiently transduce MOCH-1 oligodendrocyte cell lines (Chen et al., 1998). Additionally, injecting viral vectors with an MBP promoter into mouse brain results in much more oligodendrocyte specific transduction than the general CMV promoter (Chen et al., 1999).
1.5. Using enhancers for cell specificity
Although AAV vectors are a good choice for researchers because they do not integrate within the host’s genome, are safe for use in humans, and can transduce a variety of cells, the relatively small packaging size can be problematic. Thus, a large promoter sequence will limit the size of the transgene that can be inserted into the plasmid. One potential way around this is the use of enhancers, which are much smaller in size. If researchers can optimize the ability of enhancers to drive robust and specific transgene expression, it will allow the possibility of inserting larger sequences into the AAV vector plasmid. Recent studies have shown that it may be possible to use enhancers to target cell-type specific transgene expression after AAV transduction. For example, it was shown that using a medial entorhinal cortex enhancer (MEC13–53) leads to neuron specific expression of MEC13–53 recombinant AAVs (rAAVs) in the entorhinal cortex (Nair et al., 2020). It should be noted, however, that when the authors tried to test rAAVs with other enhancers of known specificities, only approximately half of them proved to be cell-type specific. In another study, researchers found highly specific enhancers for neuron and interneuron subtypes but also found that most enhancers screened were not cell-type specific (Mich et al., 2021). It is evident that enhancers are a promising avenue to promote strong and specific transgene expression but this technology still needs a good deal of optimization. Furthermore, enhancers for specific cell-types within the spinal cord need to be investigated more in order to utilize them as therapies for SCI.
1.6. Inflammation caused by viral vectors
Inflammation often occurs after the invasion of pathogens or from damage to tissues, and the overall inflammatory response can determine the clinical outcome and undermine the recovery process after SCI. As gene therapies predominantly use viral vectors to manipulate factors that can promote recovery, it is important to evaluate how the viral vector itself contributes to the inflammatory response in order to determine its competence as a therapeutic vehicle. Viral vectors are likely to induce immune responses as they express immunogenic epitopes recognized by the innate and adaptive arms of the immune system. AAV gene therapy is considered to be the least immunogenic, as it can transduce non-dividing cells and confer long-term stable gene expression without triggering significant inflammation and toxicity (Bessis et al., 2004). Conversely, adenoviral vectors have been shown to illicit high innate responses, such as the production of inflammatory cytokines and chemokines in the injection site (Liu and Muruve, 2003). Lentiviral vectors can elicit moderate inflammation. Additionally, the innate immune response in response to lentivirus results in its phagocytic uptake by antigen presenting cells, decreasing overall transduction efficiency (Annoni et al., 2019). Pre-existing humoral immunity can also affect transduction efficiency as capsid antigens found on the surface of adenovirus, lentivirus, and AAVs influence vector immunity, thus making factors such as administration route and dosage important considerations. Strategies to combat vector-induced immune responses would need to incorporate immunosuppression or tolerance induction, although alternative strategies to manipulate vector structure to circumvent the adaptive immune response would be ideal (Shirley et al., 2020).
It is important to consider cytotoxic effects of AAVs when used in the context of SCI research. For example, administering high doses of the AAV9 variant AAVhu68 to non-human primates appears to be toxic to DRGs (Hinderer et al., 2018). Researchers packaged the human survivor of motor neuron (SMN) protein, a protein implicated in spinal muscular atrophy (SMA), into this vector under the chicken beta-actin promoter and CMV enhancer. This viral vector was i.v. injected into three rhesus monkeys which were observed for 28 days. All three showed signs of widespread pathologies, including spinal cord damage, by 28 days. Moreover, one monkey had to be euthanized after 4 days because it became minimally responsive. Analysis showed neuronal cell body degeneration of a few DRG neurons, as well as glial cell clustering around neuronal cell bodies. Mononuclear cell infiltrates such as CD3-positive T-cells and CD-20-positive B cells were also observed to invade the cell bodies. The monkeys also exhibited axonopathy in the dorsal white matter tracts of the cervical, thoracic, and lumbar spinal cord, as well as certain peripheral nerves. When this experiment was done on piglets, similar pathologies were observed. DRG toxicity was also observed when administered through other routes such as the CSF fluid (Hordeaux et al., 2020a; Hordeaux et al., 2018a; Hordeaux et al., 2018b).
The neuronal toxicity observed was thought to be associated with high levels of transgene expression. To mitigate this, microRNA (miRNA) 183, which is expressed in DRGs, was cloned into the 3’ untranslated regions (UTRs) of the transgene for the enzyme hiDUA. This was then injected into the cisterna magna. This resulted in the transgene being expressed at reduced levels in the DRGs while also being expressed elsewhere, such as cortexes. The decrease in transgene expression also led to decreased DRG cytotoxicity (Hordeaux et al., 2020b).
Innate immunity can also cause problems when inducing cells using AAVs. For example, CpG sites are locations on a 5′–3′ strand of DNA where a cytosine nucleotide follows a guanine nucleotide. When CpG sites are unmethylated—such as that on AAVs—they can be recognized by Toll-like receptor 9 (TLR9) (Krieg, 2003; Verdera et al., 2020). This can cause an innate or adaptive immune response, thus causing inflammation and damage. To mitigate the immune response, vectors that incorporated short single-stranded oligonucleotides to target TLR9 activation were created (Chan et al., 2021). Because the oligonucleotide has a stronger binding affinity for TLR9 than the CpG sites on the vector DNA, it was thought that this would divert TLR9 away to the oligonucleotide. Interestingly, the engineered vector can evade an immune response when used in animal models, such as pigs and mice. However, when tested in non-human primates, the vector was able to delay the immune response but not evade it. Thus, this treatment has the potential to make AAVs safer for clinical applications but requires further optimization.
1.7. Timing of transgene expression
While viral vectors are an exciting way of increasing or decreasing gene expression, it is unlikely that one would want this indefinitely for a SCI application. Thus, uncontrolled transgene expression is likely not optimal. The doxycycline-regulated Tet-On or Tet-Off systems that allow for temporal control of transgene expression both in vitro and in in vivo systems show potential (Zhou et al., 2006; Burnside et al., 2018; Stieger et al., 2009). The efficacy of a two lentivirus combination treatment using an immunologically-inert, modified version of the doxycycline system (GARrtTA; reverse tetracycline-controlled transactivator coupled with a glycine-alanine repeat) showed promising results (Burnside et al., 2018). To evade T-cell mediated removal of modified cells expressing the transactivator rtTA, researchers fused the rtTA protein with the GAr domain from the Epstein-Barr Nuclear-Antigen-1 protein (EBNA-1) of the immune evasive Epstein-Barr virus (EBV; also known as human herpes virus 4) (Zaldumbide et al., 2010; Hoyng et al., 2014). This system allowed for doxycycline-inducible expression of the a transgene in the lesion penumbra following a spinal contusion in rats while also allowing the transduced cells to avoid T-cell-mediated toxicity (Burnside et al., 2018). Compared to the classical doxycycline system, the modified group caused a less prominent immune reaction after injection into the spinal cord following a cervical contusion injury. Importantly, induced transgene expression was only sustained when doxycycline was continuously administered, allowing for more precise temporal control.
2. Using viral vectors to treat SCI pathology
There are many mechanisms that contribute to the inhibition of axonal growth after an SCI. These range from a harsh extrinsic environment caused by maladaptive immune and glial responses to mature neurons intrinsically being inefficient at growth. Gene therapy can be used to tackle the various hindrances to neural repair after SCI, including removing inhibitory molecules to produce a more favorable environment, including by providing trophic support, while targeting neuro-intrinsic mechanisms to promote axon regeneration, and inducing axon remyelination.
2.1. Removal of inhibitory molecules
An inhibitory environment in the CNS after an injury is an extrinsic hindrance to potential axon regeneration. After an SCI, astrocytes within the vicinity of the injury site become activated and proliferate to help form the “glial scar”. Research has shown that the non-neuronal components in the scar — such as astrocytes, microglia, oligodendrocyte precursor cells, fibroblasts, and pericytes — are highly plastic and can play both protective and inhibitory roles in axonal regeneration (Yang et al., 2020a).
In particular, reactive astrocytes increase expression of growth inhibitory molecules, such as chondroitin sulfate proteoglycans (CSPGs). CSPGs are thought to inhibit axon growth because of their covalently attached and negatively charged chondroitin-sulphate glycosaminoglycan side chains (CS-GAGs) that bind to receptors such as transmembrane protein tyrosine phosphatase (PTPσ) and leukocyte common antigen-related phosphatase (LAR) on the axonal membrane (Fisher et al., 2011; Shen et al., 2009). Although the sugar side chains are very important in its inhibitory effects on axon growth, it is thought that the core protein also plays a role in the inhibition (Tan et al., 2005). CS-GAGs can be degraded by bacterial enzyme chondroitinase ABC (ChABC), and in vivo infusion of ChABC after SCI results in increased axonal regrowth (Burnside et al., 2018; Bradbury et al., 2002; Smith-Thomas et al., 1994).
There are limitations to using bacterial ChABC as a treatment for individuals with SCI. At the human body temperature of 37°C, ChABC quickly loses its enzymatic activity. Thus, multiple infusions of the enzyme would be required throughout the treatment window (Lee et al., 2010). However, repeated administration of ChABC to reduce the build-up of CSPGs may be too invasive as a realistic therapeutic approach for SCI. A gene therapy approach in which cells near the injury lesion are enabled to express ChABC could allow for prolonged ChABC-mediated digestion of CSPGs without the need for repeated, invasive infusions.
It is possible to modify the bacterial ChABC and package the gene into viral vectors so it can be expressed by mammalian cells in vivo (Muir et al., 2010; Zhao et al., 2011). The vector of choice for researchers trying to deliver ChABC into the CNS are lentivirus vectors because they can transduce glial cells (Hendriks et al., 2007; Baekelandt et al., 2002). Because the glial scar persists after injury, another benefit for using lentivirus to target glia associated with the scar is that it can result in long-term gene expression (Zhao et al., 2011; Baekelandt et al., 2002). For these reasons, lentivirus vectors have been extensively used by researchers to express ChABC after SCI. For example, a lentivirus vector encoding for N-glycosylation site-modified ChABC was injected into the cortex following a dorsal column crush in rats. The investigators reported fewer retraction bulbs of axons of the corticospinal tract (CST) at the injury site and increased lateral sprouting of axons from the spinal white matter into the gray matter (Zhao et al., 2011). Local injections of this lentiviral vector into the spinal cord also resulted in more axonal sprouting laterally in the spinal cord as well as along the edge of the lesion. Glial fibrillary acidic protein (GFAP) staining to visualize the astrocytic response and OX42 staining to visualize microglia and macrophages closely resembled what was observed in the control rats, providing evidence that the modified ChABC delivered by the lentivirus vector caused mostly beneficial effects and did not trigger an appreciable immune response (Zhao et al., 2011). Further studies showed that intraspinal injections of the modified ChABC rostral and caudal to the injury site after a T10/11 contusion injury in rats can cause a strong, durable, and widespread degradation of CSPGs at the injury epicenter and around the injury site (Bartus et al., 2014). Degradation of the CSPGs when the lentiviral vector is injected into an SCI epicenter 3 days after injury also resulted in smaller lesion cavities and more tissue bridges in tissues of later time points (Bartus et al., 2014). This result may be attributed to ChABC leading to an increase in phagolysosomes as well as an increased shift towards alternative, anti-inflammatory (M2) macrophages (Bartus et al., 2014). Lastly, behavioral tests showed that rats receiving the vector-derived ChABC exhibited better functional recovery as well as better sensory fiber conduction.
As mentioned above, one limitation of using gene therapy to deliver ChABC after SCI is being unable to control the expression of enzyme. As with gene therapy to express other proteins, such as GDNF and BDNF, uncontrolled expression may lead to a pathological state (Burnside et al., 2018; Georgievska et al., 2004; Fouad et al., 2013). Although there is no evidence of ChABC cytotoxicity, CSPGs are an important component in perineuronal nets that are needed for stabilizing synapses. Thus, it is likely necessary to eventually turn off ChABC expression to risk dysregulation. Excitingly, researchers recently created a lentiviral vector that incorporated a doxycycline-inducible system to drive ChABC expression, allowing for temporal control of expression of the enzyme (Burnside et al., 2018). This inducible vector was injected rostral and caudal to a C5/6 contusion injury. Animals were administered doxycycline to induce ChABC expression. Interestingly, inducing both short-term (2.5 weeks) and long-term (8 weeks) ChABC expression resulted in improvement in ladder walking, but only long-term ChABC expression resulted in continued improvement in skilled reaching and grasping. This indicates that the optimal window for ChABC delivery may be months long and illustrates the advantages of an inducible gene therapy approach, i.e., being able to control sustained transgene expression.
An alternative approach to digesting CSPGs to create a more favorable environment may be using A Disintegrin and Metalloproteinase with Thrombospondin motifs (ADAMTSs). This enzyme is a member of a family of 19 proteins that are grouped into “clades” according to their function and targets (Kelwick et al., 2015). ADAMTS-4 is a member of the aggrecanase and proteoglycanase clade, which also includes ADAMTS-1, -4, -5, -8, -9 -15, and -20, and is able to target specifically CSPGs (Kelwick et al., 2015). Repeated intrathecal injection of recombinant ADAMTS-4 following a spinal contusion in rats resulted in significant locomotor recovery (Tauchi et al., 2012). One group induced ADAMTS-4 expression specifically in astrocytes using an AAV5 vector with a truncated GFAP promotor (GfaABC1D) (Griffin et al., 2019; Griffin et al., 2020). Transduction of cultured astrocytes isolated from spinal cord to express ADAMTS-4 using this astrocyte-targeting vector did not affect astrogliosis nor the number of cells (Griffin et al., 2020). However, it did enhance degradation of TGFβ1-induced CSPGs compared to control cultures (Griffin et al., 2020). When this vector was injected into the spinal cord adjacent to a T10 contusion injury, there was a decrease of injury lesion size as well as increased CST axon sprouting throughout the grey matter, indicating that this treatment may be a viable alternative to ChABC (Griffin et al., 2020).
2.2. Modulation of astrocytic response
Reprogramming astrocytes into neurons may also prove to be a therapeutic approach to minimize the detrimental effects of scar-associated astrocytes while boosting the regeneration of neurons around the injury site. Sox2 is a transcription factor that plays a crucial role in the maintenance of neural precursor cells (NPC) (Wegner, 2011; Wegner and Stolt, 2005; Pevny and Nicolis, 2010). One group reported that using a lentiviral vector to drive Sox2 expression under the human glial fibrillary acidic protein (hGFAP) promoter, is able to reprogram cultured astrocytes from the spinal cord into multipotent neural stem cells (NSCs). The hGFAP promoter was selected because it limits transduction to astrocytes specifically and does not transduce mature oligodendrocytes nor microglia. Additionally, the investigators reported that intraspinal injection of this vector reprogrammed astrocytes in the spinal cord into neuroblasts (Su et al., 2014). When this astrocyte-specific lentiviral vector was injected into the spinal cord surrounding a T8 hemisection SCI in mouse, ~3–6% of the SOX-2+ cells expressed doublecortin (DCX; a marker for adult neurogenesis), suggesting these cells of astrocytic lineage were reprogrammed into neurons. Since a glial scar has some benefits (Sofroniew, 2015; Haan et al., 2015), reprogramming all astrocytes may lead to further damage. Thus, reprogramming only some astrocytes may be beneficial. Similarly, using AAV2/8 to transduce cells and drive expression of the Sox2 gene under the astrocyte-specific promotor gfaABC1D after T10 compression SCI in mice showed promising results (Yang et al., 2020b). The transduced cells that expressed Sox2 (and the fluorescent reporter) colocalized with immunoreactivity for ßIII-tubulin and Nissl stain, suggesting transduction of astrocytes within the scar and their reprogramming into neurons. GFAP staining used to identify reactive astrocytes showed that the astrocytic reprogramming led to a reduction in the density of the glial scar that was associated with enhanced axon regrowth in the lesion vicinity and coupled with exercise rehabilitation, functional recovery. While these are interesting, it should be noted that a strategy to stop Sox2 expression was not utilized. This may lead to abnormal cell reprogramming, Also, this treatment was only effective when combined with rehabilitation, and Sox2-induced reprogramming alone had no effects.
Overexpression of NeuroD1, a neurogenic transcription factor, has been shown to convert astrocytes to neurons in injured mouse brains and Alzheimer’s disease. A recent study demonstrated that expressing NeuroD1 leads to a highly efficient conversion (~95%) of reactive astrocytes to neurons in the dorsal horn of a mouse model (Puls et al., 2020). Using stab and contusion models at T11-T12, the researchers utilized a Cre-flox system with two AAVs vectors under the human GFAP (hGFAP) promotor, one encoding GFAP-Cre and the other a reverse form of the NeuroD1 flanked by LoxP sites. This was designed to specifically express Cre recombinase to flip the transgene and allow its expression in reactive astrocytes. They found that NeuroD1 converts astrocytes in the dorsal horn into physiologically functioning Tlx3+ glutamatergic neurons. Similarly, another study using NeuroD1-AAV based gene therapy found that ectopic expression of NeuroD1 in reactive astrocytes can regenerate a third of lost neurons in the motor cortex. Using a vasoconstrictor, a focal ischemic injury was created in the motor cortex of adult mice followed by AAV-hGFAP-NeuroD1 injection into the lesion penumbra. The investigators reported that this AAV-based gene therapy regenerated functional new neurons in this ischemic injury model, rescuing both motor and cognitive deficits (Chen et al., 2020b).
Although these results seem promising, a recent study showed that neurons induced by forced NeuroD1 expression are in fact derived from endogenous neurons and not resident astrocytes (Wang et al., 2021). They used Aldh1/1-CreERT2 mice crossed with a R26R-YFP reporter line to show that most cells expressing astrocyte markers ALDH1L1 and ALDOC were labelled with YFP. The researchers then injected an AAV5 virus to express NeuroD1 under the hGFAP promoter into the injured penumbra of the cortex following a controlled cortical impact (ICC). They found that even though most cells were NeuN+, a very small number of cells coexpressed NeuN and YFP. Thus, under injury conditions, most cells were not derived from reactive astrocytes. Using transgenic mice and lineage tracing, it was shown that injury does not induce neurons from reactive astrocytes and restricting the expression of NeuroD1 to astrocytes does not help. So, while NeuroD1 may be a promising, it needs to be studied further to confirm its efficacy and viability as a therapeutic target.
2.3. Cell adhesion molecules
Cell adhesion molecules (CAMs) are important for axonal growth because of their role in mediating the interaction of neurons with their extracellular environment. This occurs by CAMs promoting the binding of neurons to adjacent cells and proteins, causing the activation of downstream protein signaling and resulting in changes in neuronal structure and molecular activity (Kozlova et al., 2020).
The neural recognition molecule L1 (L1 or L1CAM) plays an important role in supporting axonal growth in an inhibitory environment (Castellani et al., 2002; Roonprapunt et al., 2003; Zhang et al., 2005). When AAV5-L1 was injected around an SCI lesion site in mice, a mix of neurons and glia were transduced (Chen et al., 2007). Elevated levels of L1 was accompanied by a decrease in GFAP and NG2 levels in the treated mice, indicating decreased astrogliosis and CSPG expression. Moreover, these treated mice showed an improvement in motor function (i.e., improved overground locomotion and improved plantar stepping) after 5 weeks. A more recent study shows that L1 can downregulate the CSPG phosphacan and regulate the expression of plasticity-related genes after a SCI in rats (Platek et al., 2020). Injecting AAV5 vectors using the CMV promoter to drive L1 expression into the L1/L2 spinal levels after a T10/T11 transection led to efficient transduction of neurons and GFAP+ glial cells. It was found that viral vector delivery led to higher L1 expression caudal to the site of injury and in the lumbar segments but not rostral to the injury. DiI labeling of the CST fibers showed that this caudal increase in L1 led to a decrease in retraction bulbs rostral to the injury. L1 expression led to an increase in the cyclic AMP (cAMP) synthesizing enzyme, Adcy1, which is involved in metabolism. L1 upregulation also led to an increase in myelin basic protein (MBP)—a marker for myelination—in the lumbar spinal segments. Additionally, it led to an increase in a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10). When locomotor functions were assessed using treadmill running and kinematic analysis, it was found that animals with expressing L1 had increased movement compared to control animals.
The neural cell adhesion molecule (NCAM) plays an important role in cell plasticity and migration in the nervous system (Kiss et al., 2001; Saini et al., 2016). After a spinal cord contusion injury, NCAM knockout mice display decreased locomotor recovery and increased cell apoptosis when compared to wild-type mice, suggesting NCAM plays an important role in axogenesis and functional recovery after SCI (Zhang et al., 2010). Interestingly, after a compression SCI, NCAM-expressing mice appear to have better locomotor recovery and reduced astrocytic scarring when compared to NCAM-knockout mice (Saini et al., 2016). Although these studies involved knockout mouse lines, these studies show that NCAM may be an interesting target for gene modification using viral vectors to promote repair after SCI.
2.4. Increasing neurotrophic factors
Neurotrophic factors are molecules that play an important role in cell survival, promote neuronal plasticity, and increase axonal growth (Hodgetts and Harvey, 2017). Different subgroups of neurons are varied in which neurotrophic factors they are receptive to, as they can express different combinations of neurotrophin receptors. Thus, gene therapy to modulate these molecules for different types of neurons may allow researchers to target them more efficiently to promote their ability to regenerate their axons.
Nerve growth factor (NGF) was the first neurotrophin to be discovered and is involved in neural survival and development in the PNS and neuroprotection in the CNS (Keefe et al., 2017). Studies have shown that this neurotrophin can play a role during axon regeneration, such as during axon sprouting. For example, expressing NGF at the L4-L5 dorsal root entry zone (DREZ) of rats using an adenovirus containing a pXCJL shuttle vector with a Rous sarcoma virus (RSV) long terminal repeat as the promoter and bovine growth hormone polyadenylation at the 3’ tail results in a significant increase in small-caliber cutaneous sensory fiber (CGRP+) density in lamina I, II, V, and VI of the dorsal horn (Romero et al., 2000). After 32 days of treatment, these fibers also sprouted into the dorsal horn, spinal canal, and white matter tracts of the lateral funiculus (Romero et al., 2000). Thus, NGF causes axons of small-caliber sensory fibers to sprout and penetrate deep into the gray matter.
This increased sensory axon growth may lead to pathological conditions. Using a strategy of combining expression of NGF expression and semaphorin 3A (Sema3A), which limits the growth of these nerve fibers, may be a clever means to limit the growth (Tang et al., 2007). Adenovirus vectors to express NGF and Sema3A was injected along the L4-L5 DREZ of rats following complete dorsal root crush injury. This combination treatment resulted in axon regeneration across the DREZ and into spinal cord that was more limited to laminae I and II, which is a more normal projection pattern. These regenerating sensory axons formed synaptic connections within the spinal cord. Moreover, the combination treatment appeared to decrease hindpaw withdrawal latency in a thermal nociceptive test. Thus, a comprehensive viral vector mediated gene therapy approach combining factors, such as NGF, to promote axon growth, as well as inhibitory factors, such as Sema3a, to restrict where those axons grow is one possible strategy to better target axon regrowth.
One of the best studied neurotrophins is brain-derived neurotrophic factor (BDNF). This protein plays an important role in several pro-regenerative mechanisms, such as neurogenesis, axonal sprouting, neuroprotection, and synaptic plasticity (Hodgetts and Harvey, 2017; Kovalchuk et al., 2004). The positive effect of BDNF expression on synaptic plasticity of regenerating neurons was seen using a rat model in which a graft of peripheral nerve (PNG) was transplanted to bridge a C5 and C7 spinal cord injury sites. BDNF encoding lentiviral vectors were injected into the C7 spinal segment, just distal to the caudal PNG/spinal cord interface. The axons that regenerated out of the PNG and back into spinal cord were better able to form functional synapses in the BDNF injected animals than the animals injected with the control vector (Tom et al., 2013).
Although increasing BDNF levels can enhance plasticity and regenerative growth, sustained expression can cause neuronal hyperexcitability and spasticity (Fouad et al., 2013; Weishaupt et al., 2012). Short-term expression of BDNF would allow researchers and clinicians to harness the benefits of BDNF treatment while minimizing detrimental effects. An example of this is the use of adenovirus virus to express BDNF in tiptoe-walking Yoshimura (twy/twy) mice, a model that spontaneous develops a C1-C2 spinal cord compression (Uchida et al., 2012). Injecting adenovirus-BDNF into the sternomastoid muscles of the animals retrogradely transduced the targeted spinal accessory motor neurons in the compressed spinal region and resulted in a downregulation of caspase-3, -8, -9, and p75NTR activity in neurons and oligodendrocytes and decreased apoptosis. Meanwhile, there was an increase in neurofilament (NF; marker for axonal cytoskeleton) and NG2 immunostaining, suggesting that overexpressing BDNF may also promote axonal growth and increase proliferation of NG2+ oligodendrocyte progenitor cells (Uchida et al., 2012; Polito and Reynolds, 2005).
Neurotrophin-3 is another neurotrophic factor known to help with neuron survival, growth, and differentiation of new neurons, and formation of new synapses. It has been shown that transplanting nerve bridges transduced with adenoviral vectors for NT-3 under the hCMV promoter into T12 dorsal hemisection rats results in CST fiber regrowth (Blits et al., 2000). Although axons did not grow into the nerve bridges, there was a significant increase in axons growing into the spared spinal gray matter adjacent to the injury. In a separate study, injuring the spinal cord at T8 and injecting AVV2 expressing NT-3 under the β-actin promoter into the C4-C5 region results in CST fibers growth rostral to the injury (Weishaupt et al., 2014). Using Yoshimura mice, NT-3 delivery into the sternomastoid muscles using an adenoviral vector CMV/CBA hybrid promoter results in an increase in the number of neurons in the anterior horn of the spinal region along with neurite elongation and axonal arborization (Uchida et al., 2008). Combining NT-3 with other treatments have also been shown to promote regenerative effects. After dorsal root crush in rats, delivering AAV2 encoding constitutively active Rheb (caRheb) into the spinal cord and lentivirus encoding NT-3 under the CMV promoter into the DRG led to significant growth of sensory neurons across the DREZ and into the spinal cord (Liu et al., 2016).
3. Supporting intrinsic growth mechanisms
Although the extrinsic environment plays a major role in the diminished ability for axon growth, intrinsic factors are also involved. It is known that intrinsic factors play a role in the difference between the robust regenerative capabilities of the mammalian peripheral nervous system (PNS) and the CNS (Geoffroy et al., 2017). Pathways that promote axon growth during development are downregulated with age, leading to impaired regenerative capabilities for the mature CNS. Thus, creating a favorable extrinsic environment alone is likely not optimal for efficient repair after an SCI because of the bottleneck produced by inefficient intrinsic growth mechanisms.
3.1. PTEN/mTOR
A well-studied intrinsic mechanism of axon regeneration is the modulation of the PTEN/mTORC1 pathway (mechanism and pathway reviewed by Park et al., 2010) (Park et al., 2010). PTEN (phosphatase and tensin homolog) is a negative regulator of the mammalian target of rapamycin (mTOR) pathway, with mTOR being an important factor in protein synthesis and cell growth. PI3 kinases phosphorylates phosphatidylinositol-4,5-biphosphate (PIP2) to create phosphatidylinositol-3,4,5-triphosphate (PIP3), which promotes the recruitment and activation of protein kinase B (PKB; also known as ATK) and finally mTOR (Laplante and Sabatini, 2009). PTEN converts PIP3 back into PIP2, thus reversing the action of PI3K (Laplante and Sabatini, 2009). With the deletion of PTEN, there is an accumulation of PIP3, resulting in the activation of ATK and mTOR. There is high mTOR activity during development but is suppressed in the mature CNS and even more so after CNS injury (Morgan-Warren et al., 2013). Knockout of PTEN in RGCs results in axon regeneration after optic nerve injury (Park et al., 2008). Conditional deletion of PTEN in sensorimotor cortex neurons of young mice resulted in adult CST axons being able to regenerate following a thoracic hemisection (Liu et al., 2010). A follow-up study showed that PTEN deletion in adult mice soon after a moderate cervical contusion resulted in enhanced regenerative growth and functional recovery when compared to the control group (Danilov and Steward, 2015).
Although PTEN deletion produces promising results and may be a potential therapy in the future, the studies mentioned above utilized transgenic animals. Research using viral constructs that can manipulate genes may enable transitioning this concept from the bench to a clinical setting. Along these lines, one study showed that injecting AAV2/9-shRNA-PTEN into the cortex of rats that had a dorsal hemisection that was filled with salmon fibrin in the injury site knocked down PTEN expression in cortical neurons and enhanced CST axon regeneration that was associated with improved forelimb function (Lewandowski and Steward, 2014).
Another approach is to use rAAV2-retro to express shRNA against PTEN. Since SCI affects multiple spinal pathways, rAAV-retro may allow for knockdown of PTEN expression in multiple pathways upon viral vector injection in a single location, as discussed above. Researchers packaged shRNA for PTEN in rAAV2-retro under the human U6 promoter and bilaterally injected them into C5 of rats. It was observed that PTEN expression was knocked down in Layer V neurons within sensorimotor cortex. However, the normally lower levels of PTEN within other brain regions made it difficult to verify knockdown elsewhere.
Another way to activate mTOR is with the activation of GTPase Rheb, a protein that works upstream of mTOR. Our lab found that injecting an AAV5 vector encoding for a constitutively active form of Rheb (caRheb) under the chicken β-actin promoter rostral to a T7 transection SCI site in rats that contained a PNG resulted in more axonal growth into the graft (Wu et al., 2015). Moreover, when this treatment was combined with ChABC treatment of the graft-host interface, axons from mostly propriospinal neurons were better able to grow out of the PNG and into the spinal cord. In a subsequent study, we found that transducing adult dorsal root ganglion (DRG) neurons to express caRheb enhanced the ability of the sensory axons that regenerated across a ChABC-treated dorsal root entry zone (DREZ) to form synapses on spinal neurons (Wu et al., 2016). When this study was repeated in a cervical level 2 hemisection model, it was found that Rheb expression with ChABC injection led to more axons being able to grow out of the PNG and into the intermediate spinal gray matter. Stimulation of the PNG showed more c-Fos neurons in the gray matter for the Rheb animals than control, showing the increase in functional synapses.
One factor to keep in mind is that enhanced mTOR activity in neurons results in their perpetual somal and dendritic growth (Gallent and Steward, 2018). While no obvious functional effects were observed, it is possible that this will have long-term consequences. Thus, as discussed above, it is likely that developing vectors that allow for controlled temporal regulation of mTOR activity will be optimal.
3.2. Transcription factors
There are other factors in addition to mTOR that are important for axon growth during development and that are downregulated in the mature CNS. One such target for potential therapy is Krüppel-like factor 7 (KLF7), a transcription factor involved in nervous system development as a regulator of neurogenesis and cell cycle progression. Studies have shown that silencing KLF7 in cell culture causes a downregulation of the neuronal marker microtubule-associated protein 2 (MAP2) and the NGF receptor tyrosine receptor A (TrkA), resulting in diminished neurite outgrowth, even when cells were stimulated with NGF (Caiazzo et al., 2010). Knockdown of KLF7a in combination with KLF6a in zebrafish, which are normally capable of robust retinal ganglion cell (RGC) axon regeneration, attenuated RGC axon regrowth after optic nerve injury (Veldman et al., 2007). A combination approach of using acellular nerve allografts (ANA) with AAV2 to drive KLF7 expression under the CMV promoter in mice with sciatic nerve injury resulted in an increase of myelin-forming Schwann cell marker S100 and neurofilament formation (Wang et al., 2016). This was accompanied by an increase in motor and sensory axonal regeneration through the ANA into the sciatic nerve that was accompanied with improved functional recovery. One potential limitation is that KLF7 is downregulated in mature neurons and unknown mechanisms can make it difficult to highly express KLF7 (Blackmore et al., 2012). One clever way around this is to fuse it with the VP16 transcriptional activation domain from Herpes simplex virus which recruits basal transcription factors promoting gene expression. When KLF7-VP16 was delivered via AAVs into the motor cortex after dorsal hemisection in mice, there was an increase in CST axons distal to the lesion site (Blackmore et al., 2012).
Another possible treatment is using KLF6, another member of the KLF family. In one study, a unilateral pyramidotomy was performed to injure one side of the CST. AAV8 was injected into motor cortex to transduce the cortical neurons that project only down the intact CST and were not affected by the pyramidotomy to express KLF6. Expressing KLF6 in the cortical neurons not directly impacted by the injury resulted in increased sprouting of the intact CST axons across midline in the spinal cord (Wang et al., 2018b). When the injury is performed on the side with KLF6-expressing neurons, there is an increase in CST axon growth beyond the lesion site. Using RNAseq and analyzing the promoter regions of genes upregulated by KLF6 transduction, it was found that the STAT3 recognition motif was highly enriched. Moreover, co-expression of KLF6 with constitutively active (caSTAT3) and VP16-fused STAT3 (VP16-caSTAT3) resulted in increased neurite growth when compared to control.
Regeneration associated genes have been scrutinized as a strategy to promote regeneration in the CNS. Recent studies have shown that deletion or down regulation of inhibitory factors such as PTEN, Klf4 or SOCS3, or upregulation of genes associated with PNS regeneration can induce axon growth in the CNS. STAT3 and its downstream regeneration associated targets also appear to promote axon growth. In one study, researchers fused a constitutively active variant of STAT3 (STAT3CA) with VP16 (Mehta et al., 2016). STAT3CA was able to promote neurite growth in cerebellar neurons. When these AAV constructs were used to express VP16-STAT3CA in postnatal day 3 rat cortical neurons in vitro, mRNA levels of STAT3 and downstream associated genes like ATF3 and Spr1a were increased and enhanced neurite outgrowth was promoted. Intravitreal injection of AAV-VP16-STAT3CA under a CMV promoter transduced retinal ganglion cells in an optic nerve injury model. Driving expression of STAT3CA with VP16 promoted significantly more axonal regeneration in the optic nerve than expressing STAT3CA alone, indicating that hyperactivation of STAT3 can improve its ability to promote axon growth (Mehta et al., 2016). The finding further supported the use of transcriptional enhancement of specific transcription factors seen to be elevated in the PNS to boost CNS axonal regeneration. Another study investigating the differential growth response in the PNS vs the CNS used AAV-based gene therapy to address whether and when STAT3 influences divergent growth patterns of lesioned PNS and CNS axons by genetic manipulation in DRG neurons (Bareyre et al., 2011). To understand if STAT3 plays a time-specific role during PNS and CNS axon regeneration, recombinant AAV (pAAV-MCS) to express either Cre recombinase and GFP or just Cre under the CMV promoter was injected into the L3 DRGs bilaterally in STAT3-floxed mice to delete STAT3 after a bilateral transection. They saw that deletion of STAT3 was sufficient to impair PNS axon regeneration the first two days after injury. They also noticed similar effects in the CNS and discovered that STAT3 plays a role in selectively regulating growth initiation but not subsequent axon elongation. Thus, targeting STAT3 for genetic therapy may jump start regeneration and provide the opportunity to prime axons for complimentary therapies, but more research needs to be done to test whether it can efficiently regenerate longer axons.
Multiple transcription factors, such as ATF3, c-Jun, SMAD1, and STAT3, have been implicated in regeneration. Thus, it would seem reasonable to surmise that expressing several concurrently would promote more axon regeneration that expressing them individually. Researchers wanted to investigate whether modulating these transcription factors in combination or individually, promotes regeneration of the central branch of DRG neurons after a dorsal root injury but in the absence of a peripheral nerve lesion (Fagoe et al., 2015). A pAGLWFI vector, a dual promoter AAV vector (both the CMV and CAG promoters) was injected into the L4 and L5 DRGs to express GFP control, ATF3 only, or ATF3, c-Jun and SMAD1 4 weeks prior to L4 and L5 dorsal root transection and repair or C4 dorsal column injury. The dominant active mutant of STAT3 (STAT3C) under the CAG promotor between the ITR of AAV2 in an AAV5 vector was also injected into those DRGs. In the dorsal root transection injury model, overexpressing ATF3 or the transcription factor combination had more regenerated axons at the dorsal root entry zone when compared to control groups shortly after injury (10 days post-injury) but not at later time points (20 days or 8 weeks post-injury). Moreover, there was no difference between overexpressing ATF3 alone or the combination of transcription factors, even at the early post- injury time point. Overexpressing the transcription factors did not improve sensory axon regeneration at all in the dorsal columns injury model. These data suggest that while overexpressing ATF3 can accelerate axon regeneration after a dorsal root injury, the combination of transcription factors was not synergistic and was not sufficient to result in axons across a SCI site. This suggests there may be functional redundancies between these factors. Regeneration associated genes and transcription factors that regulate their expression continue to be elucidated, so as-of-yet unknown transcription factors associated with the regeneration program and epigenetic mechanisms may also be involved.
Although transcription factors may play a vital role in axon regeneration and neuronal growth after SCI, one must be cautious of potential pitfalls. Many transcription factors display different outcomes when modulated in different cell types. For example, that partial knockout (54% transduction efficiency) of the transcription factor Sox11 using AAV2-Cre and Sox11f/f mice did not increase axon regeneration of RGCs after an optic nerve crush (ONC) (Li et al., 2018). Sox11 partial knockout also decreased the number of regenerating axons after AAV2-shRNA mediated Pten knockdown, while animal groups not receiving Sox11 partial knockout exhibited an increase in axon growth. This may be due to the cell type, as it was found that alpha retinal ganglion cells (αRGCs), which regenerate after Pten deletion, are killed by Sox11 overexpression (Norsworthy et al., 2017). Interestingly, when AAV8-Sox11 is used to overexpress Sox11 in the DRG of mice receiving a C5 dorsal hemisection, there was no observed increase in axon regeneration distal to the injury (Wang et al., 2015). However, driving Sox11 expression did reduce the initial axon retraction after injury. In cortical neurons, Sox11 upregulation after pyramidotomy resulted in increased sprouting of corticospinal tract (CST) axons near the injury site but did not enhance CST axon regeneration across the injury. Moreover, animals with Sox11 overexpression did not have improved forelimb function in the pellet retravel task and actually displayed an increase in foot slips in the horizontal ladder task. These results show that transcription factor modulation may lead to beneficial or detrimental effects, depending on the cell type. Overall and cell type- specific effects for each transcription factor should be considered.
3.3. Microtubule modulating proteins
Cytoskeleton components are very important for functions such as neuronal shape, motility, and cellular transport. Microtubules play a major role in this as it helps form the exaggerated shape of the neuron while also serving as a railroad for motor proteins to transport cellular cargo between the proximal and distal ends. They are composed of α- and β-tubulin heterodimers aligning in a head-to-tail fashion and forming a tube. Phases of tubulin assembly and disassembly, also known as dynamic instability, are very rapid but can vary depending on the region of the microtubule (Baas et al., 2016). Regions that are known to be stable go through slower exchanges in free tubulin, while labile regions go through rapid bouts of growth and shrinkage (Baas et al., 2016; Kapitein and Hoogenraad, 2015). Microtubule polarity causes the stable regions to be oriented towards the soma (the minus end), while the more labile regions (the plus end) are towards the distal end of the axon (Baas et al., 2016; Kapitein and Hoogenraad, 2015). It has been shown that microtubules are much more labile near the growth cone of axons and labile regions are much more growth permissive than stable regions (Baas et al., 2016).
Microtubule stabilizing drugs have been used to promote axon regeneration because stabilizing drugs may allow the axons to polymerize tubulin to push through inhibitory environments normal axons would not be able to penetrate through (Hellal et al., 2011; Sengottuvel and Fischer, 2011; Sengottuvel et al., 2011; Hur et al., 2012). However, this sort of growth may not be indicative of how axons would naturally grow in vivo. Instead, creating a more dynamic region near the growth cone may be an alternative approach that better mimics the normal state of the microtubule array in growing axons during development. Indeed, local infusion of a pharmacologically inhibitor of kinesin-5, which enhances microtubule dynamics within the axon, promotes axon regeneration after a complete spinal transection injury (Xu et al., 2015). More recently, we targeted fidgetin, a microtubule-severing protein which preferentially cuts and inhibits the growth of labile microtubule regions (Matamoros et al., 2019). Transduction of adult DRG neurons with an AAV5-shRNA-fidgetin to knock down expression of the protein in vivo promoted axon regeneration across the dorsal root entry zone (DREZ) – the interface of the PNS and CNS – after a dorsal root crush injury.
3.4. DREADDs
A relatively new avenue of SCI research is the use of chemogenetic technology. Engineered proteins, such as Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) may be an effective tool because of they allow for remote manipulation of neuronal and non-neuronal signal transduction in a cell-type specific manner (Fig. 2). A commonly used type of DREADD is a variety of mutated muscarinic, G-protein-coupled receptors (GPCR) that have a high affinity for a synthetic ligand, clozapine-N-oxide (CNO), and not their natural ligand, acetylcholine. DREAADs are coupled to Gi, Gq, or Gs to either activate (Gq and Gs) or silence (Gi) neurons that express the respective DREADD (Armbruster et al., 2007). CNO can be administered systemically [e.g., intraperitoneal (i.p.) or subcutaneous (s.c.) injections or in the drinking water] and is able to cross the brain-blood barrier (BBB) (Dobrzanski and Kossut, 2017). As discussed above, cells within specific regions of interest can be targeted for DREADD expression by the use of promotor-specific AAVs, transgenic animals, or a combination of both (Dobrzanski and Kossut, 2017).
Fig. 2.
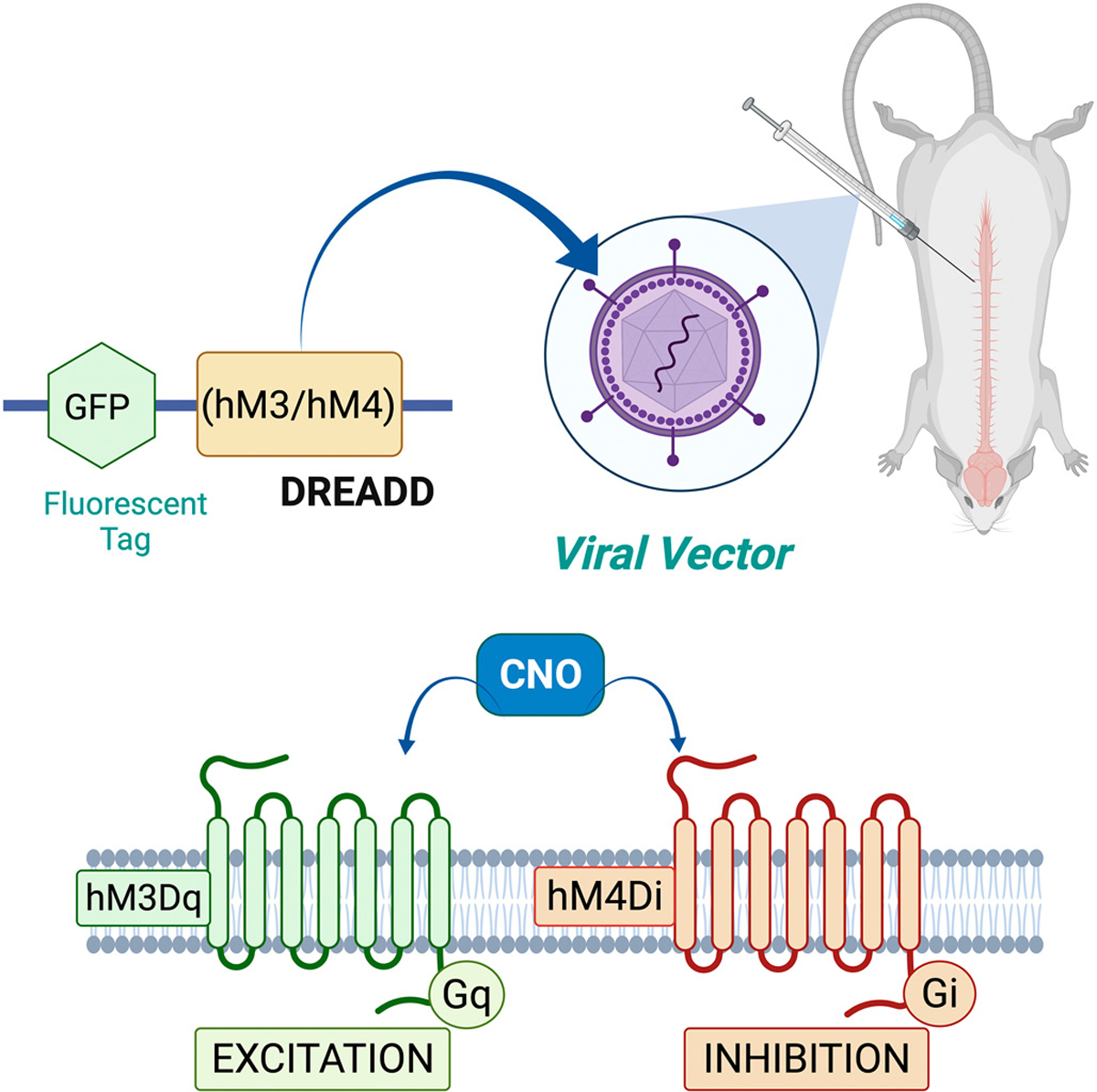
Viral vectors packaging DREADD and a fluorescent tag can be used to transduce neurons. Once the targeted neurons express either DREADD receptor, CNO can be used to activate (hM3Dq) or inactivate (hM4Di) the cell. (Created using Biorender.com)
Interestingly, repeated activation of neurons after injury with the use of electrostimulation or exercise leads to an increase in axon regeneration and functional recovery (Carmel et al., 2010; Marqueste et al., 2006; Marqueste et al., 2004). However, electric stimulation is not cell-type specific, and it is unlikely that indiscriminate neuron activation is ideal (Martin et al., 2012). With the advent of chemogenetic technology, one can relatively easily modulate neuron activity remotely in freely moving animals to elucidate if the activation or silencing of certain cells or signal transduction pathways is beneficial to axon growth and functional recovery. Allowing the animals to be freely moving also allows researchers to assess behavioral outcomes with less handling and computer manipulations, which can lessen the stress on the animals (Dobrzanski and Kossut, 2017). Below, we highlight a few studies that demonstrated that neuronal activation via virus-mediated DREADD expression can lead to more efficient repair after injury.
Recently, researchers were able to take advantage of a compromised blood-spinal cord barrier after SCI to transduce neural circuits in the spinal cord after SCI, thus elucidating a novel route of administering chemogenetic tools (Brommer et al., 2021). Tail vein administration of AAV2/9-hM3Dq under the synapsin promoter 1 h after a crush T8 SCI in mice results in efficient excitatory DREADD expression in the spinal cord. Administering CNO to these mice resulted in improved standing and stepping behaviors. This appears to be at least partly due to chemogenetic activation of Vglut2+ excitatory interneurons within the thoracic spinal cord. Moreover, chemogenetically silencing Vgat+ inhibitory neurons that express hM4Di (inhibitory) DREADDs results in improved toe lifting, hindlimb joint rotation, and height amplitude of the iliac crest. Additionally, targeting propriospinal neurons in the lumbar spinal cord for chemogenetic activation led to an increased ability to support body weight and stepping ability.
A study by this lab showed that chemogenetically activating adult DRG neurons enhanced functional axon regeneration after a dorsal root crush injury (Wu et al., 2020). AAV5-hM3Dq or -mCherry (control) under the human synapsin promoter was injected into the DRGs of rats to transduce mostly large diameter, proprioceptive DRGs. The animals were then subjected to a dorsal root crush and ChABC was injected into the DREZ once at the time of injury. CNO was given chronically (daily for 3 months). Chronic neuronal activation using systemic CNO injections increased the number of axons able to traverse the DREZ and re-enter into the grey matter of the spinal cord, where they were able to form functional synapses. This increase in axon regeneration was correlated with better performance on the proprioception dependent grid-walk test. Interestingly, neuronal activation resulted in more labile microtubules and more mTOR activation, suggesting that neuronal activation may trigger multiple mechanisms that mediate the improved axon regeneration.
3.5. DREADDs in non-neuronal cells
Since viral vectors are often used to express DREADDs, one can easily target specific types of cells within the CNS via the use of cell-specific promotors. For instance, it has been expressed in microglia via use of the CD68 promoter/enhancer (Grace et al., 2016). Since microglia has been implicated as cause for pain, including after SCI, one may be able to manipulate their activation via chemogenetics to decrease pain sensitization (Grace et al., 2016; Hains and Waxman, 2006; Hayashi et al., 2011; Hoffmann et al., 2003; Grace et al., 2018). AAV9 was injected into the spinal cord to express hM4Di (the Gi-coupled DREADD) or hM3Dq (the Gq-coupled DREADD) to transduce spinal microglia following chronic constriction injury (CCI) in rats (Grace et al., 2018). CNO administration resulted in a decrease in expression of CD11b—a marker for microglia—in hM4Di+ microglia and a reversal of CCI-induced allodynia. In contrast, CNO administration in animals with hM3Dq+ spinal microglia resulted in allodynia, even in animals without chronic constriction. These results indicate suggest that chemogenetically modulating microglia may manage injury-induced pain.
After an SCI, there is progressive oligodendrocyte death and demyelination of neurons (Sutherland and Geoffroy, 2020). One potential avenue for exploration is driving activation to promote remyelination. Learning motor skills results in strengthening of neuronal circuits and remodeling of white matter circuits within the brain (Scholz et al., 2009). This can result in additional myelin formation or remodeling of existing myelin sheaths around neurons (Zatorre et al., 2012). Researchers postulated that the increase of neuronal activity can result in an increase in myelination of those specific neurons or cause the proliferation of oligodendrocyte progenitor cells (OPCs) or oligodendrocytes—resulting in non-specific myelination of local neurons (Mitew et al., 2018). They electroporated plasmids for Gq-coupled DREADD under the CAG promoter into the neuroepithelium of embryonic day 15.5 (E15.5) mice in utero and subsequently activated them during postnatal day 19 (P19) with the administration of CNO for a week. This led to an increase in the density of OPCs, number of mitotically active OPCs, and the density of mature oligodendrocytes in the corpus callosum. Although this experiment was conducted in utero, this phenomenon appears to occur in vivo, as well. Injecting AAV1/2-hM3Dq under the CAG promoter into the primary somatosensory cortex at P5 and activating the DREADD at P19 also promoted OPC proliferation and differentiation into oligodendrocytes. Myelination was also shown to be selective, as activated, DREADD-expressing neurons had a greater chance of having myelin rings than non-transduced neurons. Although this study was not in the spinal cord, this may be an interesting strategy to explore to promote more efficient remyelination after an SCI.
3.6. Combining viral vectors with biomaterials
Viral vectors can also be combined with other treatments such as hydrogels to localize the vector much more efficiently and to target specific regions within the spinal cord. Some hydrogels, such as PF-127, are temperature sensitive by being free-flowing at ambient temperatures but gel-like when exposed to body temperatures (Wu et al., 2013). When PF-127 is combined with a lentivirus encoding shRNA for Lingo-1, a protein implicated in upregulating inhibitory signals to neurons and oligodendrocytes and ultimately preventing neurite growth. In vitro, the vector alone and vector + gel led to similar levels of OPC transduction and Lingo-1 knockdown. When used in vivo in a rat spinal cord transection model, the vector + gel group had a slightly higher transduction efficiency than the vector alone group. The results suggest that hydrogels may have the potential to increase spatial localization when delivering a virus, however, more research needs to be done to create a more efficient compound. Both treatment groups—vector alone and vector + gel—resulted in knockdown of Lingo-1 and attenuated activation of RhoA, an activator of the inhibitory signals that inhibit neurite growth, compared to the controls. The treated animals also displayed more locomotor function that correlated with increased levels of markers for axons (NF2000), dendrites (MAP-2), and synapses (synapsin I). Moreover, Lingo-1 knockdown was associated with more MBP, a myelin protein, astrocyte proliferation in the early stage of injury, and less apoptosis.
Viral vectors can also be administered as a combination therapy with polymer bridges to enhance axon regeneration. For example, combining adenoviral vectors to deliver NGF and implantation of poly(L-lactide) (PLLA) microfilaments in L4-L5 transected rats led to enhanced growth of CGRP+ nociceptive axons into the dorsal horn. This combination treatment also led to an increase in functional recovery in injured animals, as there was a decrease in paw withdrawal (Tang et al., 2004). In another interesting study, researchers used a poly(lactide-co-glycolide) (PLG) bridge to deliver lentiviral vectors encoding for IL-10 and IL-4 under the CMV promoter (Park et al., 2018). PLG is a biodegradable polymer that can be designed with channels to support axon outgrowth (Pawar et al., 2015; Tuinstra et al., 2014). This viral vector loaded bridge was then placed into a T9-T10 spinal hemisection in mice. There was increased expression of IL-10 and IL-4, as determined by ELISA, and an M2 (anti-inflammatory) polarization of macrophages. There was more regeneration of NF200+ axons throughout the bridge during the sub-acute phase of the injury (28 days). There was also an increase in MBP+ axons in the combination treatment animals compared to control. These effects were enhanced at a chronic time point (84 days). When mice were tested for locomotor function, the combination treatment resulted improved recovery when compared to animals subjected to injury alone. Thus, this technology has the potential to aid the delivery of viral vectors, but more research needs to be done to make it more efficient.
4. Future of gene therapy for SCI and conclusions
Spinal cord injury is a devastating disorder that permanently affects the quality of life for an individual. Though we still have a way to go, many strides have been taken to understand how to repair the injured spinal cord. As discussed, viral vectors to modulate gene expression can be used to target both intrinsic and extrinsic barriers to neurorepair. In the past decade, the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have already approved gene therapy treatments for spinal muscular atrophy and are developing treatments for other diseases with no known cure, such as Duchenne’s muscular dystrophy and Huntington’s disease (High and Roncarolo, 2019). Recent advancements in viral vector technology make it an exciting possibility to be a vehicle for gene therapy for SCI in the future. As more research is done to further develop viruses, including the development of novel viral vectors with strong safety profiles, additional serotypes, promoters and enhancers, we may soon have optimal methods to express transgenes of choice in specific cells within the CNS. Moreover, further improvements on viral vector design to allow for precise temporal control, ideally through the use of a systemically delivered pharmacological agent, would greatly enhance the general applicability of viral vectors to be a component of therapeutic strategies while mitigating any off-target effects. Although we have a long way to go, its potential as a new generation of therapeutics for SCI is high and exciting. This technology can revolutionize personalized medicine and improve the quality of life for those who sustained an SCI.
Acknowledgements
This work was supported by NIH R01 NS085426, NIH R01 NS106908, NIH R01 NS111761, NIH R01 NS122371 to VJT.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG, 2017. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3, 17018. 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- Annoni A, Gregori S, Naldini L, Cantore A, 2019. Modulation of immune responses in lentiviral vector-mediated gene transfer. Cell. Immunol. 342, 103802 10.1016/j.cellimm.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U. S. A. 104, 5163–5168. 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Rao AN, Matamoros AJ, Leo L, 2016. Stability properties of neuronal microtubules. Cytoskeleton (Hoboken) 73, 442–460. 10.1002/cm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekelandt V, Claeys A, Eggermont K, Lauwers E, De Strooper B, Nuttin B, Debyser Z, 2002. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum. Gene Ther. 13, 841–853. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M, 2011. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc. Natl. Acad. Sci. U. S. A. 108, 6282–6287. 10.1073/pnas.1015239108, 1015239108 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ, 2014. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. 34, 4822–4836. 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard F, Brenner M, Nakatani Y, Chao R, Purohit HJ, Freese E, 1991. Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein. J. Biol. Chem. 266, 18877–18883. 10.1016/s0021-9258(18)55145-0. [DOI] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ, Boissier MC, 2004. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 11 (Suppl. 1), S10–S17. 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL, 2012. Kruppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc. Natl. Acad. Sci. U. S. A. 109, 7517–7522. 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blits B, Dijkhuizen PA, Boer GJ, Verhaagen J, 2000. Intercostal nerve implants transduced with an adenoviral vector encoding neurotrophin-3 promote regrowth of injured rat corticospinal tract fibers and improve hindlimb function. Exp. Neurol. 164, 25–37. 10.1006/exnr.2000.7413. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB, 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640. [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A, 1994. GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 14, 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer B, He M, Zhang Z, Yang Z, Page JC, Su J, Zhang Y, Zhu J, Gouy E, Tang J, Williams P, Dai W, Wang Q, Solinsky R, Chen B, He Z, 2021. Improving hindlimb locomotor function by Non-invasive AAV-mediated manipulations of propriospinal neurons in mice with complete spinal cord injury. Nat. Commun. 12, 781. 10.1038/s41467-021-20980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside ER, De Winter F, Didangelos A, James ND, Andreica EC, Layard-Horsfall H, Muir EM, Verhaagen J, Bradbury EJ, 2018. Immune-evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain 141, 2362–2381. 10.1093/brain/awy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Colucci-D’Amato L, Esposito MT, Parisi S, Stifani S, Ramirez F, di Porzio U, 2010. Transcription factor KLF7 regulates differentiation of neuroectodermal and mesodermal cell lineages. Exp. Cell Res. 316, 2365–2376. 10.1016/j.yexcr.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH, 2010. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J. Neurosci. 30, 10918–10926. 10.1523/JNEUROSCI.1435-10.2010, 30/32/10918 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, De Angelis E, Kenwrick S, Rougon G, 2002. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 21, 6348–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V, 2017. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179. 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YK, Wang SK, Chu CJ, Copland DA, Letizia AJ, Costa Verdera H, Chiang JJ, Sethi M, Wang MK, Neidermyer WJ Jr., Chan Y, Lim ET, Graveline AR, Sanchez M, Boyd RF, Vihtelic TS, Inciong R, Slain JM, Alphonse PJ, Xue Y, Robinson-McCarthy LR, Tam JM, Jabbar MH, Sahu B, Adeniran JF, Muhuri M, Tai PWL, Xie J, Krause TB, Vernet A, Pezone M, Xiao R, Liu T, Wang W, Kaplan HJ, Gao G, Dick AD, Mingozzi F, McCall MA, Cepko CL, Church GM, 2021. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 13 10.1126/scitranslmed.abd3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCarty DM, Bruce AT, Suzuki K, Suzuki K, 1998. Gene transfer and expression in oligodendrocytes under the control of myelin basic protein transcriptional control region mediated by adeno-associated virus. Gene Ther. 5, 50–58. [DOI] [PubMed] [Google Scholar]
- Chen H, McCarty DM, Bruce AT, Suzuki K, Suzuki K, 1999. Oligodendrocyte-specific gene expression in mouse brain: use of a myelin-forming cell type–specific promoter in an adeno-associated virus. J. Neurosci. Res. 55, 504–513. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu J, Apostolova I, Skup M, Irintchev A, Kugler S, Schachner M, 2007. Adeno-associated virus-mediated L1 expression promotes functional recovery after spinal cord injury. Brain 130, 954–969. 10.1093/brain/awm049. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fan G, Li A, Yuan J, Xu T, 2020a. rAAV2-retro enables extensive and high-efficient transduction of lower motor neurons following intramuscular injection. Mol. Ther. Methods Clin. Dev. 17, 21–33. 10.1016/j.omtm.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Ma NX, Pei ZF, Wu Z, Do-Monte FH, Keefe S, Yellin E, Chen MS, Yin JC, Lee G, Minier-Toribio A, Hu Y, Bai YT, Lee K, Quirk GJ, Chen G, 2020b. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol. Ther. 28, 217–234. 10.1016/j.ymthe.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Hudry E, Maguire CA, Sena-Esteves M, Breakefield XO, Grandi P, 2017. Viral vectors for therapy of neurologic diseases. Neuropharmacology 120, 63–80. 10.1016/j.neuropharm.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Varma A, Banik N, 2015. Recent advances in the pharmacologic treatment of spinal cord injury. Metab. Brain Dis. 30, 473–482. 10.1007/s11011-014-9547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M, Ren XL, Perides G, Terwilliger EF, 2003. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene Ther. 10, 657–667. 10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- Danilov CA, Steward O, 2015. Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp. Neurol. 266, 147–160. 10.1016/j.expneurol.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V, 2016. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209. 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski G, Kossut M, 2017. Application of the DREADD technique in biomedical brain research. Pharmacol. Rep. 69, 213–221. 10.1016/j.pharep.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Dong B, Nakai H, Xiao W, 2010. Characterization of genome integrity for oversized recombinant AAV vector. Mol. Ther. 18, 87–92. 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M, 2018. Gene therapy comes of age. Science 359. 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- Fagoe ND, Attwell CL, Kouwenhoven D, Verhaagen J, Mason MR, 2015. Overexpression of ATF3 or the combination of ATF3, c-Jun, STAT3 and Smad1 promotes regeneration of the central axon branch of sensory neurons but without synergistic effects. Hum. Mol. Genet. 24, 6788–6800. 10.1093/hmg/ddv383. [DOI] [PubMed] [Google Scholar]
- Failli V, Kleitman N, Lammertse DP, Hsieh JTC, Steeves JD, Fawcett JW, Tuszynski MH, Curt A, Fehlings MG, Guest JD, Blight AR, 2021. Experimental treatments for spinal cord injury: what you should know. Top. Spinal Cord Inj. Rehabil. 27, 50–74. 10.46292/sci2702-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S, 2011. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 31, 14051–14066. 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Bennett DJ, Vavrek R, Blesch A, 2013. Long-term viral brain-derived neurotrophic factor delivery promotes spasticity in rats with a cervical spinal cord hemisection. Front. Neurol. 4, 187. 10.3389/fneur.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallent EA, Steward O, 2018. Neuronal PTEN deletion in adult cortical neurons triggers progressive growth of cell bodies, dendrites, and axons. Exp. Neurol. 303, 12–28. 10.1016/j.expneurol.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy CG, Meves JM, Zheng B, 2017. The age factor in axonal repair after spinal cord injury: a focus on neuron-intrinsic mechanisms. Neurosci. Lett. 652, 41–49. 10.1016/j.neulet.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievska B, Jakobsson J, Persson E, Ericson C, Kirik D, Lundberg C, 2004. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum. Gene Ther. 15, 934–944. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brown AM, Jenkins C, Campbell K, 2020. Viral vector systems for gene therapy: a comprehensive literature review of progress and biosafety challenges. Appl. Biosaf. 25, 7–18. 10.1177/1535676019899502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR, 2016. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U. S. A. 113, E3441–E3450. 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, Yin H, Maier SF, Watkins LR, 2018. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp. Neurol. 304, 125–131. 10.1016/j.expneurol.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grames MS, Jackson KL, Dayton RD, Stanford JA, Klein RL, 2017. Methods and tips for intravenous administration of adeno-associated virus to rats and evaluation of central nervous system transduction. J. Vis. Exp. 10.3791/55994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JM, Fackelmeier B, Fong DM, Mouravlev A, Young D, O’Carroll SJ, 2019. Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury. Gene Ther. 26, 198–210. 10.1038/s41434-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JM, Fackelmeier B, Clemett CA, Fong DM, Mouravlev A, Young D, O’Carroll SJ, 2020. Astrocyte-selective AAV-ADAMTS4 gene therapy combined with hindlimb rehabilitation promotes functional recovery after spinal cord injury. Exp. Neurol. 327, 113232 10.1016/j.expneurol.2020.113232. [DOI] [PubMed] [Google Scholar]
- Haan N, Zhu B, Wang J, Wei X, Song B, 2015. Crosstalk between macrophages and astrocytes affects proliferation, reactive phenotype and inflammatory response, suggesting a role during reactive gliosis following spinal cord injury. J. Neuroinflammation 12, 109. 10.1186/s12974-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haery L, Deverman BE, Matho KS, Cetin A, Woodard K, Cepko C, Guerin KI, Rego MA, Ersing I, Bachle SM, Kamens J, Fan M, 2019. Adeno-associated virus technologies and methods for targeted neuronal manipulation. Front. Neuroanat. 13, 93. 10.3389/fnana.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG, 2006. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 26, 4308–4317. 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle N, Boulis NM, Federici T, 2018. AAV gene delivery to the spinal cord: serotypes, methods, candidate diseases, and clinical trials. Expert. Opin. Biol. Ther. 18, 293–307. 10.1080/14712598.2018.1416089. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kawaji K, Sun L, Zhang X, Koyano K, Yokoyama T, Kohsaka S, Inoue K, Nakanishi H, 2011. Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J. Neurosci. 31, 17370–17382. 10.1523/JNEUROSCI.4152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein L. c, Strikis D, Lemmon VP, Bixby JL, Hoogenraad CC, Bradke F, 2011. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks WT, Eggers R, Verhaagen J, Boer GJ, 2007. Gene transfer to the spinal cord neural scar with lentiviral vectors: predominant transgene expression in astrocytes but not in meningeal cells. J. Neurosci. Res. 85, 3041–3052. 10.1002/jnr.21432. [DOI] [PubMed] [Google Scholar]
- High KA, Roncarolo MG, 2019. Gene therapy. N. Engl. J. Med. 381, 455–464. 10.1056/NEJMra1706910. [DOI] [PubMed] [Google Scholar]
- Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, Richman LK, Wilson JM, 2018. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29, 285–298. 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Kameda H, Nakamura H, Okunomiya T, Ohira K, Nakamura K, Kuroda M, Furuta T, Kaneko T, 2007. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 14, 872–882. 10.1038/sj.gt.3302924. [DOI] [PubMed] [Google Scholar]
- Hodgetts SI, Harvey AR, 2017. Neurotrophic factors used to treat spinal cord injury. Vitam. Horm. 104, 405–457. 10.1016/bs.vh.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kann O, Ohlemeyer C, Hanisch U-K, Kettenmann H, 2003. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (Microglia): suppression of receptor-evoked calcium signaling and control of release function. J. Neurosci. 23, 4410–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Hinderer C, Goode T, Katz N, Buza EL, Bell P, Calcedo R, Richman LK, Wilson JM, 2018a. Toxicology study of intra-cisterna magna adeno-associated virus 9 expressing human alpha-L-iduronidase in rhesus macaques. Mol. Ther. Methods Clin. Dev. 10, 79–88. 10.1016/j.omtm.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Hinderer C, Goode T, Buza EL, Bell P, Calcedo R, Richman LK, Wilson JM, 2018b. Toxicology study of intra-cisterna magna adeno-associated virus 9 expressing iduronate-2-sulfatase in rhesus macaques. Mol. Ther. Methods Clin. Dev. 10, 68–78. 10.1016/j.omtm.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Buza EL, Dyer C, Goode T, Mitchell TW, Richman L, Denton N, Hinderer C, Katz N, Schmid R, Miller R, Choudhury GR, Horiuchi M, Nambiar K, Yan H, Li M, Wilson JM, 2020a. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 31, 808–818. 10.1089/hum.2020.167. [DOI] [PubMed] [Google Scholar]
- Hordeaux J, Buza EL, Jeffrey B, Song C, Jahan T, Yuan Y, Zhu Y, Bell P, Li M, Chichester JA, Calcedo R, Wilson JM, 2020b. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci. Transl. Med. 12. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Nishide K, Nagoshi N, Shibata S, Moritoki N, Kojima K, Tsuji O, Matsumoto M, Kohyama J, Nakamura M, Okano H, 2019. The adeno-associated virus rh10 vector is an effective gene transfer system for chronic spinal cord injury. Sci. Rep. 9, 9844. 10.1038/s41598-019-46069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyng SA, Gnavi S, de Winter F, Eggers R, Ozawa T, Zaldumbide A, Hoeben RC, Malessy MJ, Verhaagen J, 2014. Developing a potentially immunologically inert tetracycline-regulatable viral vector for gene therapy in the peripheral nerve. Gene Ther. 21, 549–557. 10.1038/gt.2014.22. [DOI] [PubMed] [Google Scholar]
- Hur E, Saijilafu M, Zhou FQ, 2012. Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 35, 164–174. 10.1016/j.tins.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KL, Dayton RD, Deverman BE, Klein RL, 2016. Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAV-PHP.B. Front. Mol. Neurosci. 9, 116. 10.3389/fnmol.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Hoogenraad CC, 2015. Building the neuronal microtubule cytoskeleton. Neuron 87, 492–506. 10.1016/j.neuron.2015.05.046. [DOI] [PubMed] [Google Scholar]
- Keefe KM, Sheikh IS, Smith GM, 2017. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal Cord injury. Int. J. Mol. Sci. 18 10.3390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelwick R, Desanlis I, Wheeler GN, Edwards DR, 2015. The ADAMTS (A disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 16, 113. 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Troncoso E, Djebbara Z, Vutskits L, Muller D, 2001. The role of neural cell adhesion molecules in plasticity and repair. Brain Res. Rev. 36, 175–184. [DOI] [PubMed] [Google Scholar]
- Klaw MC, Xu C, Tom VJ, 2013. Intraspinal AAV injections immediately rostral to a thoracic spinal cord injury site efficiently transduces neurons in spinal cord and brain. Mol. Ther. Nucleic Acids 2, e108. 10.1038/mtna.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Holthoff K, Konnerth A, 2004. Neurotrophin action on a rapid timescale. Curr. Opin. Neurobiol. 14, 558–563. 10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Kozlova I, Sah S, Keable R, Leshchyns’ka I, Janitz M, Sytnyk V, 2020. Cell adhesion molecules and protein synthesis regulation in neurons. Front. Mol. Neurosci. 13, 592126 10.3389/fnmol.2020.592126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, 2003. CpG motifs: the active ingredient in bacterial extracts? Nat. Med. 9, 831–835. [DOI] [PubMed] [Google Scholar]
- Kugler S, Kilic E, Bahr M, 2003. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 10, 337–347. 10.1038/sj.gt.3301905. [DOI] [PubMed] [Google Scholar]
- Lai Y, Yue Y, Duan D, 2010. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or = 8.2 kb. Mol. Ther. 18, 75–79. 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2009. mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594. 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV, 2010. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A. 107, 3340–3345. 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC, 2017. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis 4, 43–63. 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz TB, Gray SJ, Samulski RJ, 2012. Viral vectors for gene delivery to the central nervous system. Neurobiol. Dis. 48, 179–188. 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski G, Steward O, 2014. AAVshRNA-mediated suppression of PTEN in adult rats in combination with salmon fibrin administration enables regenerative growth of corticospinal axons and enhances recovery of voluntary motor function after cervical spinal cord injury. J. Neurosci. 34, 9951–9962. 10.1523/JNEUROSCI.1996-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Struebing FL, Wang J, King R, Geisert EE, 2018. Different effect of Sox11 in retinal ganglion cells survival and axon regeneration. Front. Genet. 9, 633. 10.3389/fgene.2018.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguore WA, Domire JS, Button D, Wang Y, Dufour BD, Srinivasan S, McBride JL, 2019. AAV-PHP.B administration results in a differential pattern of CNS biodistribution in non-human primates compared with mice. Mol. Ther. 27, 2018–2037. 10.1016/j.ymthe.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Muruve DA, 2003. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10, 935–940. 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z, 2010. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 13, 1075–1081. 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kelamangalath L, Kim H, Han SB, Tang X, Zhai J, Hong JW, Lin S, Son YJ, Smith GM, 2016. NT-3 promotes proprioceptive axon regeneration when combined with activation of the mTor intrinsic growth pathway but not with reduction of myelin extrinsic inhibitors. Exp. Neurol. 283, 73–84. 10.1016/j.expneurol.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes ME, Colombo G, Schulz R, Siegert S, 2019. Targeting microglia with lentivirus and AAV: recent advances and remaining challenges. Neurosci. Lett. 707, 134310 10.1016/j.neulet.2019.134310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqueste T, Alliez J-R, Alluin O, Jammes Y, Decherchi P, 2004. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J. Appl. Physiol. 96, 1988–1995. [DOI] [PubMed] [Google Scholar]
- Marqueste T, Decherchi P, Desplanches D, Favier R, Grelot L, Jammes Y, 2006. Chronic electrostimulation after nerve repair by self-anastomosis: effects on the size, the mechanical, histochemical and biochemical muscle properties. Acta Neuropathol. 111, 589–600. 10.1007/s00401-006-0035-2. [DOI] [PubMed] [Google Scholar]
- Martin R, Sadowsky C, Obst K, Meyer B, McDonald J, 2012. Functional electrical stimulation in spinal cord injury: from theory to practice. Top. Spinal Cord Inj. Rehabil. 18, 28–33. 10.1310/sci1801-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros AJ, Tom VJ, Wu D, Rao Y, Sharp DJ, Baas PW, 2019. Knockdown of fidgetin improves regeneration of injured axons by a microtubule-based mechanism. J. Neurosci. 39, 2011–2024. 10.1523/JNEUROSCI.1888-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, 2011. Adeno-Associated Virus (AAV) vectors in the CNS. Curr. Gene Therapy 11, 181–188. [DOI] [PubMed] [Google Scholar]
- Mehta ST, Luo X, Park KK, Bixby JL, Lemmon VP, 2016. Hyperactivated Stat3 boosts axon regeneration in the CNS. Exp. Neurol. 280, 115–120. 10.1016/j.expneurol.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merienne N, Le Douce J, Faivre E, Deglon N, Bonvento G, 2013. Efficient gene delivery and selective transduction of astrocytes in the mammalian brain using viral vectors. Front. Cell. Neurosci. 7, 106. 10.3389/fncel.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mich JK, Graybuck LT, Hess EE, Mahoney JT, Kojima Y, Ding Y, Somasundaram S, Miller JA, Kalmbach BE, Radaelli C, Gore BB, Weed N, Omstead V, Bishaw Y, Shapovalova NV, Martinez RA, Fong O, Yao S, Mortrud M, Chong P, Loftus L, Bertagnolli D, Goldy J, Casper T, Dee N, Opitz-Araya X, Cetin A, Smith KA, Gwinn RP, Cobbs C, Ko AL, Ojemann JG, Keene CD, Silbergeld DL, Sunkin SM, Gradinaru V, Horwitz GD, Zeng H, Tasic B, Lein ES, Ting JT, Levi BP, 2021. Functional enhancer elements drive subclass-selective expression from mouse to primate neocortex. Cell Rep. 34, 108754 10.1016/j.celrep.2021.108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, 2018. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9, 306. 10.1038/s41467-017-02719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Warren PJ, Berry M, Ahmed Z, Scott RA, Logan A, 2013. Exploiting mTOR signaling: a novel translatable treatment strategy for traumatic optic neuropathy? Invest. Ophthalmol. Vis. Sci. 54, 6903–6916. 10.1167/iovs.13-12803. [DOI] [PubMed] [Google Scholar]
- Muir EM, Fyfe I, Gardiner S, Li L, Warren P, Fawcett JW, Keynes RJ, Rogers JH, 2010. Modification of N-glycosylation sites allows secretion of bacterial chondroitinase ABC from mammalian cells. J. Biotechnol. 145, 103–110. 10.1016/j.jbiotec.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15, 1157–1166. [DOI] [PubMed] [Google Scholar]
- Nair RR, Blankvoort S, Lagartos MJ, Kentros C, 2020. Enhancer-Driven Gene Expression (EDGE) enables the generation of viral vectors specific to neuronal subtypes. iScience 23, 100888. 10.1016/j.isci.2020.100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D, 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis B, Haenzi B, Hilton S, Carnicer-Lombarte A, Hobo B, Verhaagen J, Fawcett JW, 2021. Optimization of adeno-associated viral vector-mediated transduction of the corticospinal tract: comparison of four promoters. Gene Ther. 28, 56–74. 10.1038/s41434-020-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, Brommer B, Zhang Y, Wang C, Sanes JR, Coppola G, He Z, 2017. Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron 94. 10.1016/j.neuron.2017.05.035, 1112–1120 e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll SJ, Cook WH, Young D, 2020. AAV targeting of glial cell types in the central and peripheral nervous system and relevance to human gene therapy. Front. Mol. Neurosci. 13, 618020 10.3389/fnmol.2020.618020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala DS, Amara DP, Schaffer DV, 2015. Adeno-associated virus vectors and neurological gene therapy. Neuroscientist 21, 84–98. 10.1177/1073858414521870. [DOI] [PubMed] [Google Scholar]
- Oyinbo CA, 2011. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. 71, 281–299. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PS, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z, 2008. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z, 2010. PTEN/mTOR and axon regeneration. Exp. Neurol. 223, 45–50. S0014–4886(10)00003–8 [pii]. 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Park J, Decker JT, Margul DJ, Smith DR, Cummings BJ, Anderson AJ, Shea LD, 2018. Local immunomodulation with anti-inflammatory cytokine-encoding lentivirus enhances functional recovery after spinal cord injury. Mol. Ther. 26, 1756–1770. 10.1016/j.ymthe.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Bosch-Bouju C, Schoderboeck L, Sizemore RJ, Abraham WC, Hughes SM, 2015. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Front. Mol. Neurosci. 8, 14. 10.3389/fnmol.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar K, Cummings BJ, Thomas A, Shea LD, Levine A, Pfaff S, Anderson AJ, 2015. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: association with recovery of forelimb function. Biomaterials 65, 1–12. 10.1016/j.biomaterials.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosyan HA, Alessi V, Singh V, Hunanyan AS, Levine JM, Arvanian VL, 2014. Transduction efficiency of neurons and glial cells by AAV-1, -5, -9, -rh10 and -hu11 serotypes in rat spinal cord following contusion injury. Gene Ther. 21, 991–1000. 10.1038/gt.2014.74. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Nicolis SK, 2010. Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 42, 421–424. 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Pietersz KL, Martier RM, Baatje MS, Liefhebber JM, Brouwers CC, Pouw SM, Fokkert L, Lubelski J, Petry H, Martens GJM, van Deventer SJ, Konstantinova P, Blits B, 2021. Transduction patterns in the CNS following various routes of AAV-5-mediated gene delivery. Gene Ther. 28, 435–446. 10.1038/s41434-020-0178-0. [DOI] [PubMed] [Google Scholar]
- Pignataro D, Sucunza D, Vanrell L, Lopez-Franco E, Dopeso-Reyes IG, Vales A, Hommel M, Rico AJ, Lanciego JL, Gonzalez-Aseguinolaza G, 2017. Adeno-associated viral vectors serotype 8 for cell-specific delivery of therapeutic genes in the central nervous system. Front. Neuroanat. 11, 2. 10.3389/fnana.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek R, Grycz K, Wieckowska A, Czarkowska-Bauch J, Skup M, 2020. L1 cell adhesion molecule overexpression down regulates phosphacan and up regulates structural plasticity-related genes rostral and caudal to the complete spinal cord transection. J. Neurotrauma 37, 534–554. 10.1089/neu.2018.6103. [DOI] [PubMed] [Google Scholar]
- Polito A, Reynolds R, 2005. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J. Anat. 207, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls B, Ding Y, Zhang F, Pan M, Lei Z, Pei Z, Jiang M, Bai Y, Forsyth C, Metzger M, Rana T, Zhang L, Ding X, Keefe M, Cai A, Redilla A, Lai M, He K, Li H, Chen G, 2020. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front. Cell Dev. Biol. 8, 591883 10.3389/fcell.2020.591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud-Dulaurier R, Decressac M, 2020. PHP.B/eB vectors bring new successes to gene therapy for brain diseases. Front. Bioeng. Biotechnol. 8, 582979. 10.3389/fbioe.2020.582979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM, 2000. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 20, 4435–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roonprapunt C, Huang W, Grill R, Friedlander D, Grumet M, Chen S, Schachner M, Young W, 2003. Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J. Neurotrauma 20. [DOI] [PubMed] [Google Scholar]
- Rosario AM, Cruz PE, Ceballos-Diaz C, Strickland MR, Siemienski Z, Pardo M, Schob KL, Li A, Aslanidi GV, Srivastava A, Golde TE, Chakrabarty P, 2016. Microglia-specific targeting by novel capsid-modified AAV6 vectors. Mol. Ther. Methods Clin. Dev. 3, 16026. 10.1038/mtm.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo NC, Vandenberghe LH, Ma JY, Hauspurg A, Yu L, Maronski M, Johnston J, Dichter MA, Wilson JM, Watson DJ, 2008. Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res. 1190, 15–22. 10.1016/j.brainres.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini V, Loers G, Kaur G, Schachner M, Jakovcevski I, 2016. Impact of neural cell adhesion molecule deletion on regeneration after mouse spinal cord injury. Eur. J. Neurosci. 44, 1734–1746. 10.1111/ejn.13271. [DOI] [PubMed] [Google Scholar]
- Schlimgen R, Howard J, Wooley D, Thompson M, Baden LR, Yang OO, Christiani DC, Mostoslavsky G, Diamond DV, Duane EG, Byers K, Winters T, Gelfand JA, Fujimoto G, Hudson TW, Vyas JM, 2016. Risks associated with lentiviral vector exposures and prevention strategies. J. Occup. Environ. Med. 58, 1159–1166. 10.1097/JOM.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober AL, Gagarkin DA, Chen Y, Gao G, Jacobson L, Mongin AA, 2016. Recombinant adeno-associated virus serotype 6 (rAAV6) potently and preferentially transduces rat astrocytes in vitro and in vivo. Front. Cell. Neurosci. 10, 262. 10.3389/fncel.2016.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Cibelli G, Thiel G, 1996. Neuron-specific gene expression of synapsin I. Major role of a negative regulatory mechanism. J. Biol. Chem. 271, 3317–3323. 10.1074/jbc.271.6.3317. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H, 2009. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371. 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Fischer D, 2011. Facilitating axon regeneration in the injured CNS by microtubules stabilization. Commun. Integr. Biol. 4, 391–393. 10.4161/cib.15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D, 2011. Taxol facilitates axon regeneration in the mature CNS. J. Neurosci. 31, 2688–2699. 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Cornetta K, 2014. Design and potential of non-integrating lentiviral vectors. Biomedicines 2, 14–35. 10.3390/biomedicines2010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG, 2009. PTPs is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley JL, de Jong YP, Terhorst C, Herzog RW, 2020. Immune responses to viral gene therapy vectors. Mol. Ther. 28, 709–722. 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL, Salgado AJ, 2014. From basics to clinical: a comprehensive review on spinal cord injury. Prog. Neurobiol. 114, 25–57. 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Smith-Thomas LC, Fok-Seang J, Stevens J, Du J-S, Muir E, Geller HM, Rogers JH, Fawcett JW, 1994. An inhibitor of neurite outgrowth produced by astrocytes. J. Cell Sci. 107, 1687–1695. [DOI] [PubMed] [Google Scholar]
- Snyder BR, Gray SJ, Quach ET, Huang JW, Leung CH, Samulski RJ, Boulis NM, Federici T, 2011. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum. Gene Ther. 22, 1129–1135. 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, 2015. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16, 249–263. 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG, 2007. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 15, 481–491. 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- Sonja F-P, Danielson PE, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe GJ, 1990. Transgenic mice expressing B-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron 5, 187–197. [DOI] [PubMed] [Google Scholar]
- Stieger K, Belbellaa B, Le Guiner C, Moullier P, Rolling F, 2009. In vivo gene regulation using tetracycline-regulatable systems. Adv. Drug Deliv. Rev. 61, 527–541. 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL, 2014. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 5, 3338. 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland TC, Geoffroy CG, 2020. The influence of neuron-extrinsic factors and aging on injury progression and axonal repair in the central nervous system. Front. Cell Dev. Biol. 8, 190. 10.3389/fcell.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Zhang W, Levine JM, 2005. NG2: a component of the glial scar that inhibits axon growth. J. Anat. 207, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Cai J, Nelson KD, Peng XJ, Smith GM, 2004. Functional repair after dorsal root rhizotomy using nerve conduits and neurotrophic molecules. Eur. J. Neurosci. 20, 1211–1218. 10.1111/j.1460-9568.2004.03595.x. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Heron P, Mashburn C, Smith GM, 2007. Targeting sensory axon regeneration in adult spinal cord. J. Neurosci. 27, 6068–6078. 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi R, Imagama S, Natori T, Ohgomori T, Muramato A, Shinjo R, Matsuyama Y, Ishiguro N, Kadomatsu K, 2012. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J. Neuroinflammation 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY, 2016. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382. 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, Klaw MC, Lemay MA, Houle JD, 2013. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp. Neurol. 239, 91–100. 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Margul DJ, Goodman AG, Boehler RM, Holland SJ, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD, 2014. Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration. Tissue Eng. Part A 20, 1027–1037. 10.1089/ten.TEA.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Nakajima H, Inukai T, Takamura T, Kobayashi S, Furukawa S, Baba H, 2008. Adenovirus-mediated retrograde transfer of neurotrophin-3 gene enhances survival of anterior horn neurons of twy/twy mice with chronic mechanical compression of the spinal cord. J. Neurosci. Res. 86, 1789–1800. 10.1002/jnr.21627. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nakajima H, Hirai T, Yayama T, Chen K, Guerrero AR, Johnson WE, Baba H, 2012. The retrograde delivery of adenovirus vector carrying the gene for brain-derived neurotrophic factor protects neurons and oligodendrocytes from apoptosis in the chronically compressed spinal cord of twy/twy mice. Spine (Phila Pa 1976) 37, 2125–2135. 10.1097/BRS.0b013e3182600ef7. [DOI] [PubMed] [Google Scholar]
- Veldman MB, Bemben MA, Thompson RC, Goldman D, 2007. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev. Biol. 312, 596–612. 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Verdera HC, Kuranda K, Mingozzi F, 2020. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 28, 723–746. 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Reynolds A, Kirry A, Nienhaus C, Blackmore MG, 2015. Overexpression of sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 35, 3139–3145. 10.1523/JNEUROSCI.2832-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li WY, Sun P, Jin ZS, Liu GB, Deng LX, Guan LX, 2016. Sciatic nerve regeneration in KLF7-transfected acellular nerve allografts. Neurol. Res. 38, 242–254. 10.1080/01616412.2015.1105584. [DOI] [PubMed] [Google Scholar]
- Wang Z, Maunze B, Wang Y, Tsoulfas P, Blackmore MG, 2018a. Global connectivity and function of descending spinal input revealed by 3D microscopy and retrograde transduction. J. Neurosci. 38, 10566–10581. 10.1523/JNEUROSCI.1196-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mehra V, Simpson MT, Maunze B, Chakraborty A, Holan L, Eastwood E, Blackmore MG, Venkatesh I, 2018b. KLF6 and STAT3 co-occupy regulatory DNA and functionally synergize to promote axon growth in CNS neurons. Sci. Rep. 8, 12565. 10.1038/s41598-018-31101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Serrano C, Zhong X, Ma S, Zou Y, Zhang CL, 2021. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 184. 10.1016/j.cell.2021.09.005, 5465–5481 e5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, 2011. SOX after SOX: SOXession regulates neurogenesis. Genes Dev. 25, 2423–2428. 10.1101/gad.181487.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Stolt CC, 2005. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 28, 583–588. 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Weishaupt N, Blesch A, Fouad K, 2012. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 238, 254–264. 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Weishaupt N, Mason AL, Hurd C, May Z, Zmyslowski DC, Galleguillos D, Sipione S, Fouad K, 2014. Vector-induced NT-3 expression in rats promotes collateral growth of injured corticospinal tract axons far rostral to a spinal cord injury. Neuroscience 272, 65–75. 10.1016/j.neuroscience.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Wold WSM, Toth K, 2013. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 13, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H, Colosi P, 2010. Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86. 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HF, Cen JS, Zhong Q, Chen L, Wang J, Deng DY, Wan Y, 2013. The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials 34, 1686–1700. 10.1016/j.biomaterials.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Wu D, Klaw MC, Connors T, Kholodilov N, Burke RE, Tom VJ, 2015. Expressing constitutively active rheb in adult neurons after a complete spinal cord injury enhances axonal regeneration beyond a chondroitinase-treated glial scar. J. Neurosci. 35, 11068–11080. 10.1523/JNEUROSCI.0719-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Klaw MC, Kholodilov N, Burke RE, Detloff MR, Cote MP, Tom VJ, 2016. Expressing constitutively active rheb in adult dorsal root ganglion neurons enhances the integration of sensory axons that regenerate across a chondroitinase-treated dorsal root entry zone following dorsal root crush. Front. Mol. Neurosci. 9, 49. 10.3389/fnmol.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Jin Y, Shapiro TM, Hinduja A, Baas PW, Tom VJ, 2020. Chronic neuronal activation increases dynamic microtubules to enhance functional axon regeneration after dorsal root crush injury. Nat. Commun. 11, 6131. 10.1038/s41467-020-19914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Klaw MC, Lemay MA, Baas PW, Tom VJ, 2015. Pharmacologically inhibiting kinesin-5 activity with monastrol promotes axonal regeneration following spinal cord injury. Exp. Neurol. 263, 172–176. 10.1016/j.expneurol.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Dai Y, Chen G, Cui S, 2020a. Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front. Cell. Neurosci. 14, 78. 10.3389/fncel.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Xing L, Yu W, Cai Y, Cui S, Chen G, 2020b. Astrocytic reprogramming combined with rehabilitation strategy improves recovery from spinal cord injury. FASEB J. 34, 15504–15515. 10.1096/fj.202001657RR. [DOI] [PubMed] [Google Scholar]
- Young LS, Mautner V, 2001. The promise and potential hazards of adenovirus gene therapy. Gut 48, 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yao C, Wang Y, Mao S, Wang Y, Wu R, Feng W, Chen Y, Yang J, Xue C, Liu D, Ding F, Gu X, 2019. The landscape of gene expression and molecular regulation following spinal cord hemisection in rats. Front. Mol. Neurosci. 12, 287. 10.3389/fnmol.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldumbide A, Weening S, Cramer SJ, Rabelink MJ, Verhaagen J, Hoeben RC, 2010. A potentially immunologically inert derivative of the reverse tetracycline-controlled transactivator. Biotechnol. Lett. 32, 749–754. 10.1007/s10529-010-0218-8. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H, 2012. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536. 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavvarian MM, Toossi A, Khazaei M, Hong J, Fehlings M, 2020. Novel innovations in cell and gene therapies for spinal cord injury. F1000Res 9. 10.12688/f1000research.21989.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bo X, Schoepfer R, Holtmaat AJ, Verhaagen J, Emson PC, Lieberman AR, Anderson PN, 2005. Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc. Natl. Acad. Sci. U. S. A. 102, 14883–14888. 10.1073/pnas.0505164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xia YY, Lim HC, Tang FR, Feng ZW, 2010. NCAM-mediated locomotor recovery from spinal cord contusion injury involves neuroprotection, axon regeneration, and synaptogenesis. Neurochem. Int. 56, 919–929. 10.1016/j.neuint.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Zhao RR, Muir EM, Alves JN, Rickman H, Allan AY, Kwok JC, Roet KC, Verhaagen J, Schneider BL, Bensadoun JC, Ahmed SG, Yanez-Munoz RJ, Keynes RJ, Fawcett JW, Rogers JH, 2011. Lentiviral vectors express chondroitinase ABC in cortical projections and promote sprouting of injured corticospinal axons. J. Neurosci. Methods 201, 228–238. 10.1016/j.jneumeth.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Vink M, Klaver B, Berkhout B, Das AT, 2006. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 13, 1382–1390. 10.1038/sj.gt.3302780. [DOI] [PubMed] [Google Scholar]


