Abstract
While dietary changes are recommended to treat pediatric NAFLD, the role of specific nutrients in disease progression is unclear.
Objectives:
To 1) assess the macronutrient and micronutrient intake in adolescents with liver biopsy proven NAFLD (with and without NASH) and lean controls; 2) determine nutritional predictors of disease severity amongst these groups.
Methods:
Adolescents with biopsy proven NAFLD and lean controls completed the Harvard Food Frequency Questionnaire.
Results:
28 NAFLD and 15 lean controls were studied. NAFLD with (n=20) and without NASH (n=8) had similar total calorie, protein, fat and carbohydrate intake. Subjects with NASH had higher total sugar (122.3±48.3 vs 83.1±38.8 gm), glucose (24.3±9.3 vs 15.2±7.5 gm), sucrose (42.3±16.9 vs 28.8 ±11.7 gm) and fructose (29.4±12.5 vs 18.1±8.0 gm) intake than those with NAFLD but without NASH (p<0.05). Both NAFLD groups had similar micronutrient intake. ALT correlated with total caloric intake (ρ=0.4, p=0.04). Total carbohydrate calories correlated with a higher NAS summary score (ρ=0.38, p=0.04) and lobular inflammation (ρ=0.50, p=0.007). Percent calories from added sugar and glucose correlated with worsening NAS summary score (ρ=0.44, p=0.02; ρ=0.48, p=0.009) and lobular inflammation (ρ=0.51, p=0.006; ρ=0.53, p=0.004).) Percent calories from fructose correlated with lobular inflammation (ρ=0.56, p=0.002). Total daily calories, protein, fat, carbohydrate and micronutrient intake were similar between NAFLD and lean controls.
Conclusion:
NASH patients consume similar total calories, protein, and fat as those without NASH, but have significantly higher sugar intake. NAFLD and lean children, however, have similar macro/micronutrient intake. Histologic disease severity correlates with total carbohydrate and added sugar intake, supporting a role for simple sugar intake in NAFLD progression.
Keywords: NAFLD, fructose, vitamin E, histology, hypoxia, obstructive sleep apnea
Non-alcoholic fatty liver disease (NAFLD) affects up to 10% of all children and 38% of obese children, encompassing a spectrum of disease from isolated hepatic steatosis, non-alcoholic steatohepatitis (NASH: steatosis, hepatocyte ballooning and inflammation), and cirrhosis 1. The severity of NAFLD may be complicated by concurrent co-morbidities such as the metabolic syndrome, hyperlipidemia, type 2 diabetes mellitus, and obstructive sleep apnea 2,3. Combined lifestyle interventions targeting improvements in diet and physical activity are the primary treatment for pediatric NAFLD 4.
While general recommendations for a healthy diet exist5, it is unclear how alterations in consumption mitigate NAFLD development and progression. Given the focus on dietary modifications for pediatric NAFLD treatment, much remains to be understood about macronutrient and micronutrient intake in this population. Limited previous research has shown that adolescents with and without ultrasound diagnosed NAFLD had similar total energy, fat and protein intake, but those with NAFLD consume higher amounts of carbohydrates and sugar 6,7. Similar total caloric and macronutrient intake was also shown in a study of children with biopsy proven NAFLD, with and without NASH 8. While this study lacked specific data on glucose, fructose or sucrose consumption, differences in the consumption of sugar sweetened beverages in those with and without NASH were not seen. Fructose is a substantial component of the typical “western” diet and has been hypothesized to play a unique role in the pathophysiology of NASH. A study of Italian children with NAFLD, of whom only one third had NASH, found that those with NASH consumed more fructose per day and had higher blood uric acid concentrations than those without NASH 9. These findings suggest that the macronutrient intake in adolescents with NAFLD may be a modifiable risk factor impacting disease severity, allowing for refinement of nutritional counseling and improved weight loss in affected children.
Very little is known about the micronutrient intake of children with NAFLD. In a national survey, Taiwanese adolescents with presumed NAFLD consumed higher amounts of dietary zinc and vitamin B-2 10. Children with biopsy proven NAFLD in the NASH Clinical Research Network study had insufficient vitamin E and C intake8. Inconsistent data exist on the relationship of vitamin C, folic acid intake and adult NAFLD 11 12. Insufficient evidence is available to recommend any specific micronutrient intake or supplementation in pediatric NAFLD.
Therefore, the objectives of this study were to 1) assess the macronutrient and micronutrient intake in a well-defined cohort of adolescents with liver biopsy proven NAFLD both with and without NASH compared to lean controls and 2) determine nutritional predictors of disease severity amongst these groups.
Methods
Study participants were a subset of patients cared for in the Children’s Hospital Colorado Pediatric Liver Center between June 2009-January 2014 enrolled in a cross-sectional cohort study of pediatric NAFLD and sleep apnea who completed a food frequency questionnaire2,3. Included subjects were ages 8-18 years, with biopsy proven NAFLD. Previous studies focused on sleep apnea reported the metabolic and hepatic focused laboratory results and liver histology data from these patients 2,3,13. Lean age-matched controls (BMI<85%ile) with no evidence of hepatomegaly or liver disease (aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 40 IU/L) were also enrolled. Exclusion criteria are detailed in supplementary materials.
Demographics were collected, height/weight measured, and BMI/BMI z-scores determined. Fasting blood testing included ALT, AST, gamma-glutamyl transferase (GGT), ultra-sensitive C-reactive protein (CRP), uric acid, total cholesterol, triglyceride, high-density lipoprotein (HDL), glucose, and insulin (to calculate the homeostasis model assessment of insulin resistance [HOMA-IR]) 14. Fasting serum antioxidants (alpha tocopherol and alpha and beta carotene) were measured and normalized to total serum lipid concentrations 15. obstructive sleep apnea (OSA) has previously been associated with NAFLD disease severity2,3,13. Subjects with NAFLD underwent a standard multi-channel sleep study as part of the parent study they were enrolled in 2,3 to determine if they had OSA based on the apnea/hypopnea index (AHI) and/or hypoxia as previously defined (see supplementary material) 16,17.
NAFLD and lean control subjects completed the Harvard-Willett Food Frequency Questionnaire (FFQ), a semi-quantitative assessment of food and beverage frequency and portion sizes consumed over the past 6 months. FFQs are commonly used as the dietary assessment tool for large prospective studies, including NAFLD, given the ease of self-administration, low expense and ability to discern information on episodically consumed items18 19. The total caloric intake for each subject was reviewed to identify subjects reporting an implausibly low dietary intake, defined as < 800 kcals per day. No subjects meeting these exclusion criteria were identified.
For NAFLD subjects, liver histology was reviewed and scored by a single pediatric pathologist blinded to subject information. Biopsies with histologic NAFLD (≥ 5% of hepatocytes containing macrovesicular fat) were scored using criteria established by the NASH Clinical Research Network 20 for steatosis, lobular inflammation, and ballooning degeneration, with a NAFLD Activity Score (NAS) calculated by summing steatosis, lobular inflammation, and ballooning degeneration scores20. Subjects were classified as NASH (NAS ≥ 5) versus not NASH (NAS ≤ 4) for all statistical comparisons. Hepatic fibrosis was scored as stage 0-4 (see supplementary material)20. This study was approved by the Colorado Multiple Institutional Review Board and informed written consent was obtained from all parents/guardians, and assent from all subjects ages ≥12 years.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina) as detailed in the supplementary material.
Results
Subjects
Twenty-eight obese subjects with biopsy confirmed NAFLD were studied (mean age of 12.7 ± 1.9 years; mean BMI of 32.3 ± 5.8), 71% of whom had OSA/hypoxia. Those with definite NASH (NAS ≥ 5; 71%) had more severe obesity than those without NASH (NAS ≤ 4, 29%) but were otherwise demographically similar (Table 1). All subjects with NAFLD were also compared to 15 lean, age matched controls (mean age of 13.1 ± 1.8 years; mean BMI of 18.9 ± 2.2) (Table 1). Subjects with NAFLD had significantly (p<0.05) elevated serum aminotransferases, inflammatory markers and evidence of the metabolic syndrome compared to lean controls.
Table 1.
Demographic, laboratory and macro/micronutrient findings in NAFLD and lean control subjects.
| NAFLD (n=28) | Lean (n=15) | p value | Normal Values |
|
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 12.7 ± 1.9 | 13.1 ± 1.8 | 0.44 | -- |
| Male sex, % | 20 (71.4%) | 7 (46.7%) | 0.11 | -- |
| Ethnicity, % | ||||
| Hispanic | 24 (88.9%) | 6 (40.0%) | 0.001 | -- |
| Non-Hispanic | 3 (11.1%) | 9 (60.0%) | 0.001 | -- |
| Mean BMI | 32.3 ± 5.8 | 18.9 ± 2.2 | <0.001 | -- |
| BMI z-score | 2.3 ± 0.3 | -0.05 ± 0.8 | <0.001 | -- |
| Laboratory Testing | ||||
| ALT, IU/L | 130.9 ± 101.4 | 29.2 ± 5.2 | <0.001 | 10-45 IU/L |
| AST, IU/L | 75.4 ± 50.2 | 35.7 ± 8.2 | 0.01 | 15-40 IU/L |
| CRP, mg/dL | 2.1 ± 1.9 | 0.6 ± 0.7 | 0.01 | <0.5 mg/dL |
| Uric acid, mg/dL | 64.7 ± 22.8 | 60.0 ± 28.7 | 0.56 | 2.3-5.4 mg/dL |
| Cholesterol, mg/dL | 158.6 ± 37.9 | 133.2 ± 19.7 | 0.04 | ≤170 mg/dL |
| Triglyceride, mg/dL | 157.3 ± 83.2 | 81.42 ± 22.8 | 0.004 | ≤150 mg/dL |
| HDL, mg/dL | 39.2 ± 8.0 | 46.9 ± 6.7 | 0.006 | <35 mg/dL |
| HOMA-IR | 9.2 ± 7.1 | 2.9 ± 2.6 | 0.003 | <2.60 |
| Adiponectin, ug/mL | 7.6 ± 3.2 | 13.3 ± 5.8 | <0.001 | 3.5-14.4 ug/mL |
| Leptin, ng/mL | 29.8 ± 14.7 | 8.6 ± 8.0 | <0.001 | 3.7-11.4 ng/mL |
| Alpha-tocopherol/total lipids, mg/g | 0.015 ± 0.004 | 0.017 ± 0.002 | 0.06 | >0.8 mg/gm |
| Beta-carotene/total lipids, mg/g | 0.002 ± 0.002 | 0.004 ± 0.003 | 0.03 | -- |
| F(2)-Isoprostanes/urine creatinine | 669.2 ± 278.0 | 310.5 ± 130.0 | <0.001 | -- |
| Micro/Macronutrient | NAFLD (n=28) | Lean (n=15) | p value | |
| Total Calories, kcal | 1658.4 ± 795.1 | 1610.5 ± 760.5 | 0.85 | |
| % Protein of Total kcal | 16.7 ± 3.1 | 15.6 ± 2.0 | 0.22 | |
| % Fat of Total kcal | 28.3 ± 6.0 | 30.6 ± 4.1 | 0.19 | |
| % Carbohydrates of Total kcal | 56.9 ± 7.2 | 55.3 ± 5.1 | 0.44 | |
| % Added Sugar of Total kcal | 21.9 ± 7.6 | 21.1 ± 4.8 | 0.71 | |
| % Fructose of Total kcal | 6.6 ± 2.4 | 5.9 ± 1.9 | 0.35 | |
| % Sucrose of Total kcal | 9.8 ± 3.7 | 10.2 ± 2.3 | 0.73 | |
| % Glucose of Total kcal | 5.5 ± 2.0 | 5.0 ± 1.9 | 0.43 | |
| Total Sugar, g | 111.1 ± 48.6 | 109.7 ± 58.0 | 0.93 | |
| Sucrose, g | 38.5 ± 16.4 | 42.2 ± 24.0 | 0.55 | |
| Fructose, g | 26.2 ± 12.4 | 23.3 ± 11.4 | 0.46 | |
| Glucose, g | 21.7 ± 9.6 | 20.3 ± 12.2 | 0.67 | |
| Saturated Fat, g | 18.1 ± 9.7 | 20.6 ± 11.9 | 0.46 | |
| Monounsaturated Fat, g | 18.4 ± 10.4 | 18.5 ± 9.9 | 0.97 | |
| Polysunsaturated Fat, g | 11.3 ± 7.3 | 10.6 ± 5.1 | 0.72 | |
| Iron, μmol/L | 13.4 ± 8.7 | 19.7 ± 13.5 | 0.07 | |
| Magnesium, μmol/L | 264.3 ± 140.4 | 240.8 ± 110.5 | 0.58 | |
| Zinc, mg | 11.0 ± 6.6 | 16.5 ± 10.4 | 0.04 | |
| Vitamin A, mcg | 7620.8 ± 6113.4 | 10292.8 ± 5249.0 | 0.21 | |
| Vitamin B, mg | 6.5 ± 5.8 | 9.2 ± 5.3 | 0.15 | |
| Vitamin C, mg | 115.7 ± 51.9 | 119.6 ± 63.6 | 0.83 | |
| Vitamin D, IU | 240.6 ± 217.9 | 376.4 ± 297.9 | 0.09 | |
| Vitamin E, mg | 6.4 ± 4.9 | 11.0 ± 9.9 | 0.05 | |
| Recommended Daily Allowance | % Above Range | % Above Range | ||
| Protein | 0 | 0 | ||
| Total Fat | 11 | 13 | ||
| Saturated Fat | 54 | 80 | ||
| Added Sugar | 57 | 80 |
BMI:Body Mass Index
All values are mean ± SD unless otherwise indicated.
Dietary Intake of NAFLD subjects with and without NASH
We compared dietary intake in patient with NAFLD (n=20) and without NASH (n=8) to determine the relationships between nutrient intake and disease progression. Total daily caloric intake of subjects with NASH (1760 ± 878 kcal) and without NASH (1430 ± 490 kcals) and the proportion of calories derived from protein, fat and carbohydrates were not statistically different (p=NS) (Figure 1a). The highest proportion of total energy intake for those with (58%) and without NASH (53%) came from carbohydrates, with a modest amount of fat and protein in their diets. Subjects with NASH consumed a greater proportion of total calories (24 ± 8%) as added sugar compared to those without NASH (18 ± 3%; p=0.05). Higher added sugar consumption in those with NASH was reflected by a greater percentage of calories from fructose (7.1 ± 2.5 vs 5.2 ± 1.3, p=0.05) and glucose (6.0 ± 2.1 vs 4.2 ± 1.0, p=0.03), but not sucrose. Those with NASH also had higher absolute intake (gm/day) of sucrose, fructose and glucose (p<0.04) (Figure 1b). No differences in total saturated (18.4 ± 10.6 vs 17.6 ± 7), monounsaturated (19.0 ± 11.7 vs 16.7 ± 6.0 g) or polyunsaturated fat (12.0 ± 8.3 vs 9.8 ± 3.8 g) intake occurred between those with and without NASH. The percentage of NAFLD with and without NASH below above the Recommended Dietary Allowance (RDA; Supplementary Table 1) for protein, total and saturated fat and added sugar is shown in Table 2 21 22.
Figure 1a.
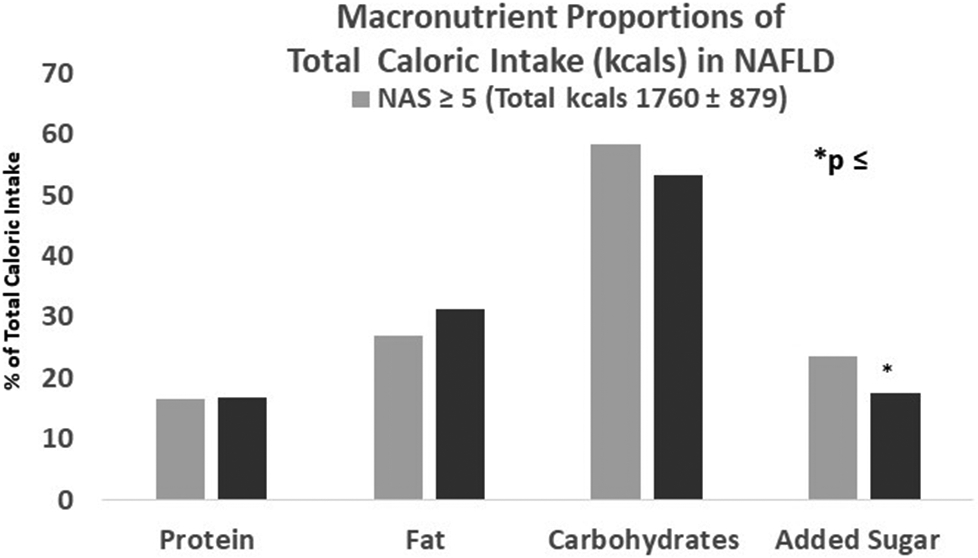
Adolescents with NAFLD, with (NAS >/= 5) and without (NAS </=4) NASH, have similar total caloric intake, as well as the proportion of calories derived from protein, fat and carbohydrates. Adolescents with NASH, however, consumed a greater proportion of their total calories as added sugar compared to those without NASH (p=0.05).
Figure 1b.
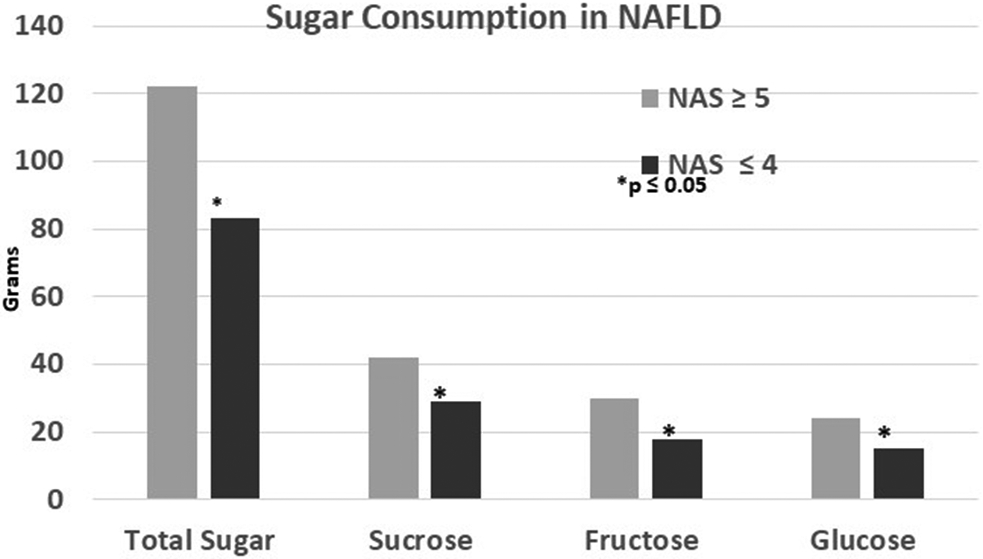
Higher total sugar consumption (p=0.05) in those with NASH was reflected by higher absolute intakes of sucrose (p=0.05), fructose (p=0.03) and glucose (p=0.02).
Table 2.
Demographic, laboratory and micronutrient findings in NAFLD patients with (NAS ≥ 5) and without NASH (NAS ≤ 4).
| NAS ≥ 5 (n=20) | NAS ≤ 4 (n=8) | p value | Normal Values for Laboratory tests |
|
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 12.9 ± 2.0 | 12.1 ± 1.7 | 0.36 | -- |
| Male sex, % | 13 (65.0%) | 7 (87.5%) | 0.37 | -- |
| Ethnicity, % | -- | |||
| Hispanic | 17 (89.5%) | 7 (87.5%) | 1.00 | -- |
| Non-Hispanic | 2 (10.5%) | 1 (12.5%) | -- | |
| Mean BMI | 33.9 ± 6.1 | 28.4 ± 2.0 | 0.02 | -- |
| BMI z-score | 2.3 ± 0.3 | 2.1 ± 0.3 | 0.04 | -- |
| Liver Histology | ||||
| NAS summary score | 5.7 ± 0.7 | 3.4 ± 0.9 | <.001 | -- |
| Liver histology | -- | |||
| Steatosis | 2.8 ± 0.56 | 1.9 ± 0.8 | 0.003 | -- |
| Lobular inflammation | 1.5 ± 0.7 | 0.5 ± 0.5 | 0.001 | -- |
| Ballooning degeneration | 1.5 ± 0.5 | 1.0 ± 0.9 | 0.11 | -- |
| Inflammation grade | 2.7 ± 0.5 | 1.6 ± 0.5 | <.001 | -- |
| Fibrosis stage | 1.8 ± 1.0 | 1.3 ± 1.2 | 0.21 | -- |
| Laboratory Testing | ||||
| ALT, IU/L | 145.6 ± 104.5 | 94.1 ± 88.7 | 0.23 | 10-45 IU/L |
| AST, IU/L | 84.2 ± 53.5 | 53.5 ± 34.2 | 0.15 | 15-40 IU/L |
| CRP, mg/dL | 2.3 ± 2.0 | 1.4 ± 1.6 | 0.25 | <0.5 mg/dL |
| Uric acid, mg/dL | 65.4 ± 24.3 | 63.05 ± 20.0 | 0.81 | 2.3-5.4 mg/dL |
| Cholesterol, mg/dL | 157.6 ± 41.4 | 160.9 ± 30.1 | 0.84 | ≤170 mg/dL |
| Triglyceride, mg/dL | 133.1 ± 53.8 | 214.9 ± 113.5 | 0.02 | ≤150 mg/dL |
| HDL, mg/dL | 40.8 ± 7.4 | 35.4 ± 8.3 | 0.10 | <35 mg/dL |
| HOMA-IR | 10.0 ± 7.8 | 7.3 ± 5.3 | 0.36 | <2.60 |
| Adiponectin, ug/mL | 7.9 ± 3.1 | 7.1 ± 3.8 | 0.58 | 3.5-14.4 ug/mL |
| Leptin, ng/mL | 32.6 ± 15.0 | 23.1 ± 12.6 | 0.13 | 3.7-11.4 ng/mL |
| Alpha-tocopherol/total lipids, mg/g | 0.014 ± 0.004 | 0.015 ± 0.002 | 0.63 | >0.8 mg/gm |
| Beta-carotene/total lipids, mg/g | 0.019 ± 0.026 | 0.015 ± 0.006 | 0.69 | -- |
| F(2)-Isoprostanes/urine creatinine | 727.0 ± 289.4 | 528.9 ± 201.1 | 0.11 | -- |
| Micro/Macronutrient | ||||
| Total Calories, kcal | 1760.4 ± 878.5 | 1403.5 ± 489.6 | 0.29 | |
| % Protein of Total kcal | 16.7 ± 3.5 | 16.8 ± 2.9 | 0.93 | |
| % Fat of Total kcal | 27.0 ± 5.8 | 31.4 ± 5.6 | 0.08 | |
| % Carbohydrates of Total kcal | 58.4 ± 7.4 | 53.2 ± 5.8 | 0.09 | |
| % Added Sugar of Total kcal | 23.6 ± 8.2 | 17.5 ± 2.8 | 0.05 | |
| % Fructose of Total kcal | 7.1 ± 2.5 | 5.2 ± 1.3 | 0.05 | |
| % Sucrose of Total kcal | 10.5 ± 4.1 | 8.2 ± 1.7 | 0.15 | |
| % Glucose of Total kcal | 6.0 ± 2.1 | 4.2 ± 1.0 | 0.03 | |
| Total Sugar, g | 122.3 ± 48.3 | 83.1 ± 38.7 | 0.05 | |
| Sucrose, g | 42.3 ± 16.7 | 28.8 ± 11.7 | 0.05 | |
| Fructose, g | 29.4 ± 12.5 | 18.1 ± 8.0 | 0.03 | |
| Glucose, g | 24.3 ± 9.3 | 15.2 ± 7.5 | 0.02 | |
| Saturated Fat, g | 18.4 ± 10.6 | 17.6 ± 7.9 | 0.85 | |
| Monounsaturated Fat, g | 19.0 ± 11.7 | 16.7 ± 6.0 | 0.59 | |
| Polysunsaturated Fat, g | 12.0 ± 8.3 | 9.8 ± 3.8 | 0.48 | |
| Iron, μmol/L | 14.5 ± 9.6 | 10.7 ± 5.8 | 0.31 | -- |
| Magnesium, μmol/L | 283.1 ± 155.9 | 217.3 ± 81.1 | 0.27 | -- |
| Zinc, mg | 11.5 ± 7.0 | 9.8 ± 5.5 | 0.54 | -- |
| Vitamin A, mcg | 8072.2 ± 6843.3 | 6341.8 ± 3453.4 | 0.56 | -- |
| Vitamin B 12, mg | 7.3 ± 6.5 | 4.7 ± 3.2 | 0.31 | -- |
| Vitamin C, mg | 124.7 ± 54.9 | 93.4 ± 37.7 | 0.15 | -- |
| Vitamin D, IU | 229.2 ± 210.9 | 269.1 ± 247.2 | 0.67 | -- |
| Vitamin E, mg | 6.7 ± 5.4 | 5.7 ± 3.5 | 0.64 | -- |
| Recommended Daily Allowance | % Above Range | % Above Range | ||
| Protein | 0 | 0 | ||
| Total Fat | 5 | 25 | ||
| Saturated Fat | 40 | 62 | ||
| Added Sugar | 70 | 62 |
BMI: Body Mass Index; NAS: NAFLD Activity Score; All values are mean ± SD unless otherwise noted.
Overall micronutrient intake was similar between those with and without NASH (Table 2). Based on the RDA, 70% of subjects with NASH had sufficient intake of folate, 50% zinc, 50% vitamin A, 85% vitamin C, 5% vitamin D and 10% vitamin E. Similarly, 63% of subjects without NASH had sufficient intake of folate, 50% zinc, 38% vitamin A, 75% vitamin C, 13% vitamin D and 13% vitamin E. While insufficient micronutrient intake was common in NAFLD, it was similar between those with and without NASH.
Relationship between dietary intake and Metabolic Syndrome components
There was no relationship between added sugar, glucose, fructose or sucrose intake and HOMA-IR in subjects with NAFLD. Serum uric acid in the NAFLD group showed moderate correlations with percent of calories from added sugar (r=0.42, p=0.03), glucose (r=0.41, p=0.3), sucrose (r=0.43, p=0.02) and fructose (r=0.44, p=0.02).
For subjects with NAFLD, higher total fat intake was associated with higher total serum cholesterol (r=0.43, p=0.02) and triglycerides (r=0.45, p=0.01), with moderate correlations. However, a smaller percentage of calories from added sugars correlated moderately and inversely with triglycerides (r=−0.46, p=0.02). There were no significant correlations noted between HDL and added sugar, glucose, sucrose or fructose, nor total fat intake amongst subjects with NAFLD.
We explored the co-varying relationship between carbohydrate and fat intake using a CoDA analysis to determine if higher caloric intake was proportional between fat and carbohydrate, or alternatively, if higher consumption of carbohydrates resulted in decreased fat intake. We found that as the relative abundance of carbohydrate intake increased, there was a small negative correlation with the intake of total fat (r=−0.33, p=0.08). Thereby, carbohydrate intake may moderate the effect of fat on serum lipid levels and vice versa.
Relationship between dietary intake and OSA/hypoxia
The total daily caloric intake and proportion of calories derived from protein, fat and carbohydrates of subjects with NAFLD with and without OSA/hypoxia were similar (p=NS). Subjects with NAFLD with and without OSA/hypoxia also had similar proportions of total calories derived from sucrose, fructose and glucose, as well as total saturated, monounsaturated and polyunsaturated fat intake. There were also no differences in micronutrient intake between subjects with NAFLD with and without OSA/hypoxia. There was a moderate correlation between worsening OSA and proportion of total calories derived from protein (ρ= 0.43, p=0.02) and iron intake (ρ= 0.047, p=0.047). There was also a modest correlation between severity of hypoxia (defined by % time saturations were <90%) and higher absolute intake of carbohydrates (ρ= 0.40, p=0.03), sucrose (ρ= 0.45, p=0.02), fructose (ρ= 0.49, p=0.008), glucose (ρ= 0.45, p=0.02) and added sugars (ρ= 0.46, p=0.01).
Associations between macro- and micronutrient intake and NAFLD severity
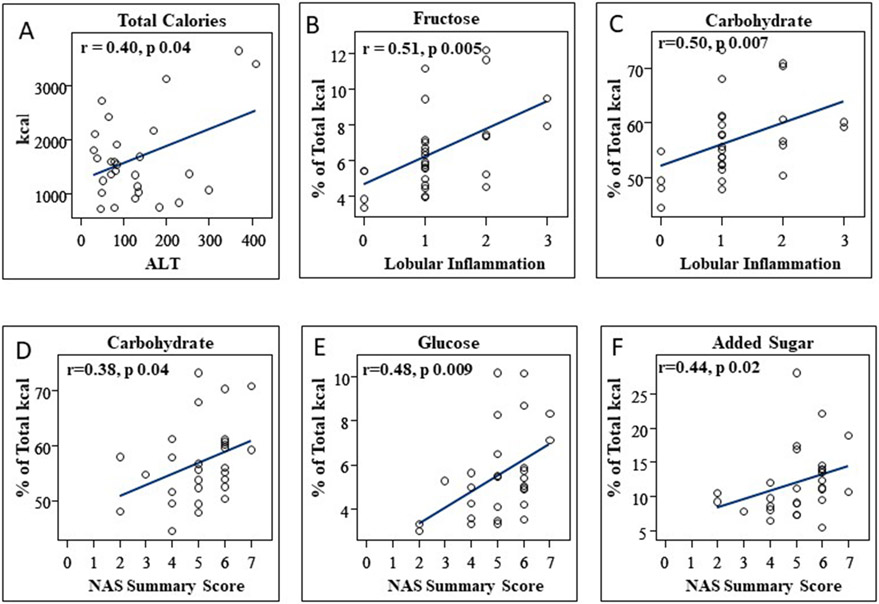
Relationships between dietary intake and NAFLD disease severity were examined (Figure 2). ALT increased concurrent with higher total caloric intake (ρ=0.4, p=0.04) and higher absolute added sugar intake (ρ=0.45, p=0.02) with moderate correlations. ALT did not, however, correlate with absolute intake of glucose, fructose or sucrose. Although total caloric intake was not associated with worsening histologic disease severity, higher absolute added sugar intake correlated with worsening histologic inflammation (ρ= 0.44, p=0.02) and higher NAS summary scores (ρ=0.40, p=0.03) with moderate correlations. Higher absolute glucose intake similarly correlated strongly with worsening histologic inflammation (ρ= 0.55, p=0.003) and moderately with higher NAS summary scores (ρ=0.39, p=0.04). An greater absolute fructose intake also moderately correlated with worsening histologic inflammation (ρ= 0.5, p=0.006) and higher NAS summary scores (ρ=0.42, p=0.03).
Figure 2.
Higher total caloric intake in adolescents with NAFLD correlates with worsening biochemical disease (A). A higher proportion of total caloric intake from fructose and carbohydrates correlates with worsening hepatic inflammation on biopsy (B, C). In addition, A higher proportion of total caloric intake from carbohydrate, glucose and added sugars correlates worsening overall histologic disease severity, as indicated by higher NAS Summary scores (D, E, F).
A higher percentage of total calories derived from carbohydrate correlated moderately with a higher NAS summary score (ρ=0.38, p=0.04) and histologic lobular inflammation (ρ=0.50, p=0.007). In addition, a higher percentage of total calories derived from added sugar also correlated moderately with worsening NAS summary score (ρ=0.44, p=0.02) and lobular inflammation (ρ=0.51, p=0.006). Similarly, an higher percent of total calories from glucose moderately correlated with worsening NAS summary score (ρ=0.48, p=0.009) and lobular inflammation (ρ=0.53, p=0.004). Finally, a greater percent of total calories from fructose correlated strongly with lobular inflammation (ρ=0.56, p=0.002) (Figure 1).
Conversely, a higher percentage of total calories derived from fat (ρ=−0.40, p=0.04) or saturated fat (ρ=−0.42, p=0.03) showed a moderate negative correlation with NAFLD lobular inflammation. We did not observe associations between total saturated, monounsaturated or polyunsaturated fat or protein intake and biochemical (ALT) or histologic disease severity, including hepatic steatosis. An increase in vitamin C intake correlated moderately with lobular inflammation (r=0.42, p=0.013), but micronutrient intake, including Vitamin E, was not otherwise associated with NAFLD biochemical (ALT) or histologic disease severity.
Dietary Intake of NAFLD compared to Lean Subjects
Total daily caloric intake, as well as proportion of calories derived from protein, fat and carbohydrates between NAFLD and lean subjects was similar (Table 1), with the greatest proportion of total energy derived from carbohydrates in both NAFLD and lean groups, with modest amounts of dietary fat and protein. There were no statistically significant differences in total saturated, monounsaturated (MUFA) or polyunsaturated (PUFA) fat nor absolute amounts or proportion of total energy intake from added sugars, fructose, sucrose or glucose between NAFLD and lean control subjects (Table 1). The percentage of NAFLD and lean subjects who were above the Recommended Dietary Allowance (RDA) for protein, total and saturated fat and added sugar is shown in Table 1 21 22.
The overall intake of micronutrients was similar between subjects with NAFLD and lean subjects, except subjects with NAFLD had lower zinc and vitamin E intake (Table 1). Based on the RDA (Supplementary Table 1), 68% of subjects with NAFLD had sufficient intake of folate, 50% zinc, 46% vitamin A, 82% vitamin C, 7% vitamin D and 11% vitamin E. In contrast, 80% of lean subjects had sufficient intake of folate, 73% zinc, 73% vitamin A, 73% vitamin C, 27% vitamin D and 33% vitamin E. These findings suggest that while many adolescents consume less than the RDA for micronutrients, those with NAFLD are disproportionately affected.
Discussion
Despite significant clinical focus on the role of dietary intake in NAFLD development and progression, data to substantiate these concerns remain sparse in adolescents with biopsy proven NAFLD. In this study, we demonstrate that while the total energy intake in adolescents with and without histologic NASH is similar, those with NASH consume a significant amount of energy from added sugars. Higher calories from added sugars are associated with worse histologic disease severity. Higher calories from carbohydrates are also associated with more severe nocturnal hypoxia in patients with NAFLD. Finally, higher total caloric intake is associated with worsening biochemical NAFLD disease severity. While simple sugar intake is implicated in NASH progression, the overall macronutrient and micronutrient intake of adolescents with NAFLD was strikingly similar to lean controls.
Subjects with NAFLD with and without histologic NASH consumed similar total calories, and proportion of calories provided by protein, fat and carbohydrates. Previous studies found similar intake of total energy, fat, or protein in obese children with and without ultrasound defined NAFLD, with disparate results regarding increased sugar intake 7 6. Children with biopsy proven NASH in the NASH CRN also had similar caloric and macronutrient intake to those without NASH8. While specific data on glucose, fructose and sucrose consumption were lacking, there were no differences in sugar sweetened beverage consumption in those with and without NASH. In contrast, we found adolescents with biopsy proven NASH consume a greater proportion of calories from fructose and sucrose and more grams of glucose, fructose and sucrose than those with NAFLD but without NASH. Furthermore, a higher percentage of calories from carbohydrates, especially added sugar and glucose, were associated with worsening histologic NASH. Patients with NASH consumed approximately 40 grams (10 teaspoons) more glucose, fructose and sucrose per day than those with NAFLD without NASH, equivalent to the sugar content of one can of regular soda. Thereby, suggesting patients with NASH decrease their soda intake is a highly practical and achievable dietary goal.
Added sugar consumption, particularly fructose, is likely important in NASH pathophysiology. Fructose is absorbed by the intestinal epithelium and transported to the liver, where it enters glycolysis and is phosphorylated to fructose-1-phosphate. Patients with NAFLD may ingest and readily absorb high fructose loads, metabolizing it quickly with resultant rapid ATP depletion 23 24. Furthermore, fructose metabolism may indirectly lead to hepatic insulin resistance and directly impede insulin signaling in the liver 25.In our cohort, we found higher fructose intake in those with the highest NAS Summary scores, reflecting the most severe histologic disease, especially inflammation. Uric acid also correlated strongly with fructose intake, suggesting its potential utility as a practical biomarker of fructose intake. A novel relationship was noted between fructose intake and sleep disordered breathing severity, which has been linked to NAFLD disease severity 2,3.
While previous NAFLD dietary studies have focused on macronutrient composition, micronutrients may also play a physiologic role. The PIVENS and TONIC treatment trials suggested some therapeutic benefit of vitamin E, attributed to an antioxidant deficiency resulting in increased lipid peroxidation and cell death26 27. Children with ultrasound or histologically diagnosed NAFLD have low Vitamin E intake 28,8. In our study, vitamin E and zinc consumption were also lower in subjects with NAFLD compared to lean controls, and much less than the RDA for folate, zinc, vitamin D and E. Furthermore, children with NAFLD may benefit from multi-vitamin supplementation to reach suggested daily intakes.
Studies delineating nutritional intake in pediatric NAFLD have largely neglected comparisons to healthy children, assuming children with NAFLD consume large numbers of calories with poor nutritional value. Unexpectedly, our study challenges this paradigm, demonstrating similar total caloric, macronutrient and micronutrient intake between biopsy proven subjects with NAFLD and age-matched lean controls. Sugar sweetened beverages and added sugars are also often blamed for the obesity epidemic and related co-morbidities, such as NAFLD. Dietary data from 2,250 adolescents in the 1999-2004 National Health and Nutrition Examination Survey found an average daily added sugar consumption of 21% of total daily caloric intake 29. In our study, both lean and subjects with NAFLD had similar sugar intake as this NHANES cohort. This suggests that differences in the metabolism of added sugars, such as fructose, may account for the development of NAFLD, rather than the absolute or relative amounts of sugars.
Potential study limitations include possible recall bias with underreporting of dietary intake by study participants. Respondents may answer FFQ questions based on current consumption instead of past 6-month average intake as directed. Additionally, NAFLD study subjects received our standard clinical care with nutritional counseling by a pediatric dietician, which may have influenced their reported dietary intake. The possibility exists that they therefore may have underreported intake or given what they considered to be socially acceptable responses to their intake. We did, however, find higher sugar consumption in adolescents with NASH, and while the questionnaire may not capture all added sugar consumption, they would be unlikely to overreport, with any misclassification strengthening the noted associations. In the future, utilization of dietary intake tools that robustly assess micronutrient intake may better characterize potential inadequacies. While the racial composition of the NAFLD and lean groups were different, the macronutrient and micronutrient intake were similar, suggesting that ethnicity did not bias our results. The NAFLD group was primarily Hispanic, reflecting the ethnic predisposition of this disease in the United States. Although Hispanic diets are not well-represented in commonly used FFQs30, ethnicity was similar in subjects with NAFLD with and without NASH, reiterating that differences in sugar intake were not due to ethnicity alone. In addition, we did not measure physical activity, a potential balancing measure of food consumption. The cross-sectional nature of our study also precludes our ability to assess a temporal association between diet and NAFLD. Finally, our relatively small sample size raises the potential for a type 2 error in detecting small differences in the dietary composition of NAFLD versus lean controls. This small sample size could also result in a Type 1 error, whereby statistical significance occurred by chance because of testing multiple associations.
Weight loss, through lifestyle modifications, remains the mainstay of treatment for pediatric NAFLD. General recommendations for children and adolescents regarding total caloric and macronutrient intake serve as the foundation for nutritional counseling for this burgeoning population. This study suggests that reduction of added sugars should be emphasized in the treatment of pediatric NAFLD, with the potential to mitigate NAFLD disease progression. The relationship between high sugar diets and OSA/hypoxia, as well as the potential impact on NAFLD severity, will require further study.
Supplementary Material
What is Known?
Combined lifestyle interventions targeting improvements in diet and physical activity are the primary treatment for pediatric NAFLD.
Fructose is a substantial component of the typical “western” diet, and it’s consumption has been associated in some studies with pediatric NASH.
What is New?
Patients with NASH consume similar total calories, protein, fat and carbohydrates as those without NASH, but have significantly higher sugar intake. Histologic disease severity correlates with total carbohydrate and added sugar intake.
NAFLD and lean children have similar macro/micronutrient intake.
These findings support the role for simple sugar intake in the progression of NAFLD.
Funding Support:
National Institutes of Health, NIDDK, K23DK085150, NCATS UL1 TR002535.
Footnotes
The authors have no conflicts of interest to disclose.
References:
- 1.Brunt EM. Nonalcoholic fatty liver disease and the ongoing role of liver biopsy evaluation. Hepatol Commun 2017;1:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundaram SS, Halbower A, Pan Z, et al. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. Journal of hepatology 2016;65:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundaram SS, Sokol RJ, Capocelli KE, et al. Obstructive sleep apnea and hypoxemia are associated with advanced liver histology in pediatric nonalcoholic fatty liver disease. The Journal of pediatrics 2014;164:699–706 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). Journal of pediatric gastroenterology and nutrition 2017;64:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidding SS, Dennison BA, Birch LL, et al. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics 2006;117:544–59. [DOI] [PubMed] [Google Scholar]
- 6.Papandreou D, Karabouta Z, Pantoleon A, Rousso I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite 2012;59:939–44. [DOI] [PubMed] [Google Scholar]
- 7.de Piano A, Prado WL, Caranti DA, et al. Metabolic and nutritional profile of obese adolescents with nonalcoholic fatty liver disease. Journal of pediatric gastroenterology and nutrition 2007;44:446–52. [DOI] [PubMed] [Google Scholar]
- 8.Vos MB, Colvin R, Belt P, et al. Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J Pediatr Gastroenterol Nutr 2012;54:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosca A, Nobili V, De Vito R, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. Journal of hepatology 2017;66:1031–6. [DOI] [PubMed] [Google Scholar]
- 10.Bai CH, Chien YW, Huang TC, et al. Increased dietary zinc and vitamin B-2 is associated with increased alanine aminotransferase in Taiwanese adolescents. Asia Pac J Clin Nutr 2017;26:78–84. [DOI] [PubMed] [Google Scholar]
- 11.Ivancovsky-Wajcman D, Fliss-Isakov N, Salomone F, et al. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig Liver Dis 2019;51:1698–705. [DOI] [PubMed] [Google Scholar]
- 12.Mahamid M, Mahroum N, Bragazzi NL, et al. Folate and B12 Levels Correlate with Histological Severity in NASH Patients. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundaram SS, Halbower AC, Klawitter J, et al. Treating Obstructive Sleep Apnea and Chronic Intermittent Hypoxia Improves the Severity of Nonalcoholic Fatty Liver Disease in Children. The Journal of pediatrics 2018;198:67–75 e1. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 15.Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr 1999;135:601–10. [DOI] [PubMed] [Google Scholar]
- 16.Halbower AC, Ishman SL, McGinley BM. Childhood obstructive sleep-disordered breathing: a clinical update and discussion of technological innovations and challenges. Chest 2007;132:2030–41. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics 2006;117:741–53. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Wang J, Li Z, et al. Consumption of Sugar-Sweetened Beverages Has a Dose-Dependent Effect on the Risk of Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Dose-Response Meta-Analysis. Int J Environ Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Fox CS, Jacques PF, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. Journal of hepatology 2015;63:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 21.Medicine Io. Dietary Reference Intakes for Energ, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein adn Amino Acids: The National Academy Press. [DOI] [PubMed] [Google Scholar]
- 22.. at https://ods.od.nih.gov/HealthInformation/Dietary_Reference_Intakes.aspx.)
- 23.Sullivan JS, Le MT, Pan Z, et al. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatric obesity 2015;10:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelmalek MF, Lazo M, Horska A, et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012;56:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Softic S, Stanhope KL, Boucher J, et al. Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci 2020;57:308–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England journal of medicine 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mager DR, Patterson C, So S, Rogenstein CD, Wykes LJ, Roberts EA. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr 2010;64:628–35. [DOI] [PubMed] [Google Scholar]
- 29.Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011;123:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullen KW, Watson K, Zakeri I. Relative reliability and validity of the Block Kids Questionnaire among youth aged 10 to 17 years. J Am Diet Assoc 2008;108:862–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.