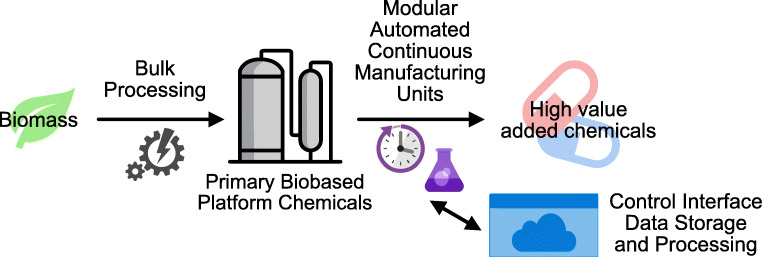
Abstract
Biomass is a renewable, almost infinite reservoir of a large diversity of highly functionalized chemicals. The conversion of biomass toward biobased platform molecules through biorefineries generally still lacks economic viability. Profitability could be enhanced through the development of new market opportunities for these biobased platform chemicals. The fine chemical industry, and more specifically the manufacturing of pharmaceuticals is one of the sectors bearing significant potential for these biobased building blocks to rapidly emerge and make a difference. There are, however, still many challenges to be dealt with before this market can thrive. Continuous flow technology and its integration for the upgrading of biobased platform molecules for the manufacturing of pharmaceuticals is foreseen as a game-changer. This perspective reflects on the main challenges relative to chemical, process, regulatory and supply chain-related burdens still to be addressed. The implementation of integrated continuous flow processes and their automation into modular units will help for tackling with these challenges.
Graphical abstract
Keywords: Biobased platforms, Flow chemistry, Active Pharmaceutical Ingredient, Automation
Introduction
To ensure optimal economic sustainability, biorefineries should be designed as an integrated production system able to produce a wide range of versatile and valuable outputs starting from raw biomass. The two main categories of outputs are energy (under the form of fuel and heat) and chemicals intended for various applications including the food industry, fragrances, materials, bulk, pharmaceuticals and fine chemicals [1]. Biorefining processes can be very complex, and, quite unexpectedly, are often associated with much less favorable environmental metrics and economics than the refining of crude oil. For compounds also found in the conventional fossil fuel supply chain, the economics of the biomass-based process must be thoroughly optimized in order to remain competitive [2].
Primary biomass-derived (or biobased) platform chemicals are compounds obtained after the first processing step of biomass [2]. Each of these compounds can be further transformed toward a number of secondary biobased building blocks [2]. Among the various chemicals that can be sourced from processing biomass, a rather limited range have attracted considerable attention with huge potential, emerging or established markets.
These biobased platforms chemicals are also classified into three categories: (a) drop-in platform chemicals, (b) smart drop-in platform chemicals and (c) dedicated platform chemicals [3]. The first category, drop-in platform chemicals, are commodity chemicals identical to an existing fossil sourced counterpart (e.g. ethylene glycol). Smart drop-in chemicals (e.g., biobased glycidol produced from glycerol 1), are commodities the biosourcing of which presents significant advantages over the petrobased supply. For example, their production process is less energy-intensive, shorter, less complex, relies on production processes involving milder conditions and lower environmental footprint chemicals or produces less harmful by-products. The last category, dedicated biobased chemicals, concerns compounds that have no direct counterpart in the petrochemical chain of value and therefore open new potential markets and products (e.g. 2,5-furanedicarboxylic acid 12) [3].
A short list of the most potent biobased platform molecules has been issued by the US Department of Energy (DoE) in 2004, emphasizing the tremendous industrial potential for 14 compounds, although this report is widely known as the “DoE Top 10” (see Fig. 1) [4]. Though the DoE list was updated in 2010 and 2016 with the addition of a few additional structures [5, 6], it is quite surprising that despite such a huge diversity and complexity, biorefineries only converge toward a very limited number of biobased platforms. While these biobased platforms are originally intended to replace petrobased building blocks in bulk and material chemistry (drop-in, smart drop-in and dedicated platforms), momentum is gained for applications targeting markets with lower volumes but higher added value, such as for the manufacturing of pharmaceuticals.
Fig. 1.
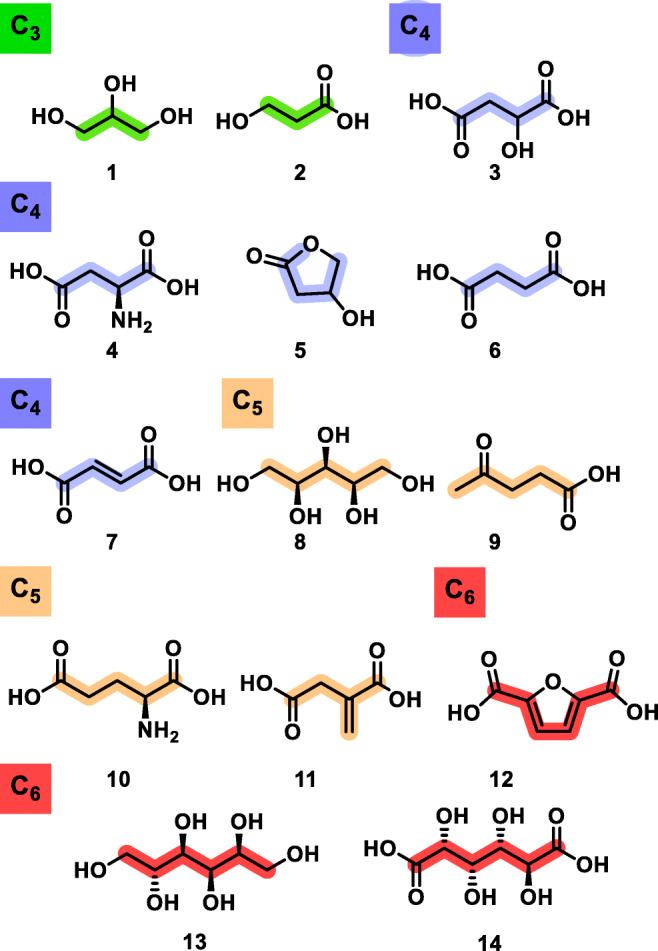
Extended list of top biobased platform molecules [4] as C3-C6 biobased building blocks. By order of appearance: (C3): glycerol 1, 3-hydroxypropionic acid 2; (C4): malic acid 3, aspartic acid 4, 3-hydroxybutyrolactone 5, succinic acid 6, fumaric acid 7; (C5): xylitol 8, levulinic acid 9, glutamic acid 10, itaconic acid 11; (C6): 2,5-furandicarboxylic acid 12, sorbitol 13, glucaric acid 14
The strength of the conventional fossil fuel approach relies mostly on an overall cost effectiveness associated with decades of process engineering. However, the chemicals obtained by fossil fuel exploitation are mainly hydrocarbon backbones with limited functionalizations. Heteroelements, such as oxygen and nitrogen, which are ubiquitous to the western pharmacopoeia, are indeed very rare in petrobased building blocks. They must be therefore inserted by selective chemical transformations prior to their end-uses.
On the other hand, biobased platforms are by contrast very rich in heteroatoms (mostly oxygen) and therefore impose a reverse scheme with the necessary removal of heteroatoms (X = O, N) to increase their inherent C/X ratio. Primary biobased building blocks are obtained through fractionation, depolymerization and defunctionnalization of the biomass. For instance, lignocellulosic biomass is mainly composed of lignin, hemicellulose and cellulose. Their hydrolysis yields a wide array of O-containing compounds [7]. Chitin obtained from crustacean and insect shells is rich in N-containing compounds. Algae and marine biomass provide a small reservoir of S-containing molecules while an abundant source of P-containing organic backbones is still missing [8].
Concerning lignin, it has now been demonstrated that the main constituents are p-coumaryl, coniferyl and sinapyl alcohols featuring phenol, alkenes and allylic alcohol scaffolds. The lignin depolymerization method, both under batch or continuous flow conditions [9], as well as lignin’s origin (hardwood, softwood or herbaceous crops) affects the proportion of these three constituents and their derivatives, opening the doors to a variety of transformations [10, 11]. Among those transformations, Antoniotti and coworkers reported in 2007 both batch and continuous flow conditions procedures allowing the selective oxidation of a broad scope of alcohols, among which coniferyl alcohol derivatives, into the corresponding aldehydes or ketones through aerobic oxidation catalyzed by heterogenous gold nanoparticles [12].
Furthermore, the synthesis of promising inhibitors to treat cardiovascular diseases was performed by Rajagopaland coworkers in 2020. This was done through the phenol moiety of coniferyl alcohol derivatives or ferulic acid, its carboxylic acid counterparts, under the form of alpha-beta unsaturated amides, hydrazines, esters and hydroxamic acids [13].
Additionally, structure-activity relationships of the three phenolic derivatives have been investigated in 2001 by Lewis and coworkers under the form of flavonolignan and flavone scaffolds with the aim of treating multidrug resistant Staphylococcus Aureus [14].
Chitin is another complex biomacromolecule featuring a repeating N-acetyl-d-glucosamine (NAG) 15 unit (see Fig. 2), and bears great interest considering its natural nitrogen content. 15 is a key intermediate to a variety of other prominent molecules such as 3-acetamido-5-acetylfuran 16, which was Sperry’s center of interest in 2018 [15]. Indeed, the authors relied on 16 to synthesize Proximicin A 17, an alkaloid known for its anticancer properties. Additionally, NAG can be transformed into chiral 3,6-anhydro-GNF 18 and 3,6-anhydro-MNF 20, two bicyclic compounds, which are key intermediates in the synthetic route proposed by Usui and colleagues in 2010 [16]. These are engaged in the synthesis of furanodictines A 19 and B 21 which both display important neuronal differentiation properties in rats.
Fig. 2.
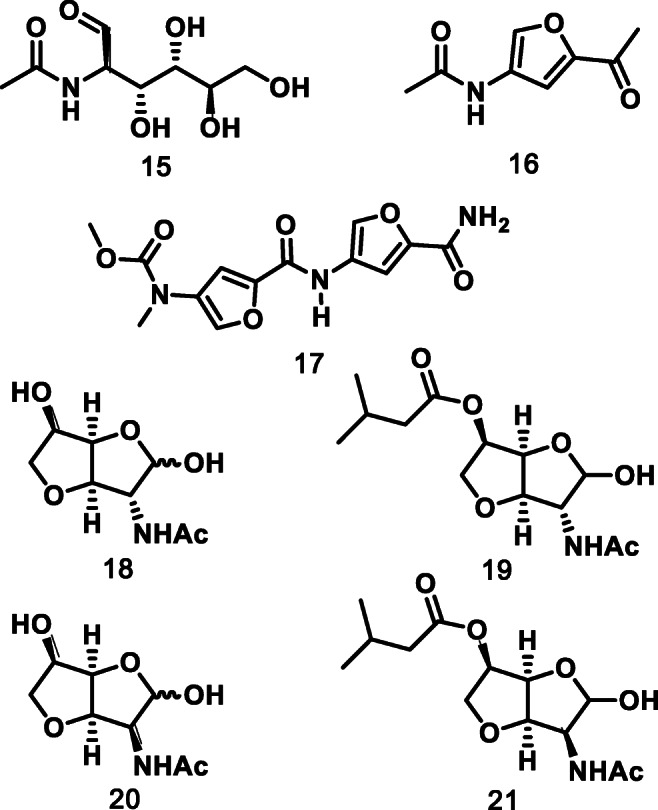
N-acetyl-d-glucosamine derivatives and some of their applications as API or API precursors [15, 16]. N-acetyl-d-glucosamine 15, 3-acetamido-5-acetylfuran 16, proximicin A 17, 3,6-anhydro-GNF 18, furanodictines A 19, 3,6-anhydro-MNF 20 and furanodictines B 21
Alongside their structural diversity, a wide range of optically active building blocks can be sourced from biobased materials. For instance, isosorbide 5-mononitrate 22 is a clinically favored substitute for isosorbide-dinitrate 23, a compound included in the World Health Organization (WHO) list of essential medicines and used as a medication against heart diseases and blood pressure issues (see Fig. 3) [17].
Fig. 3.
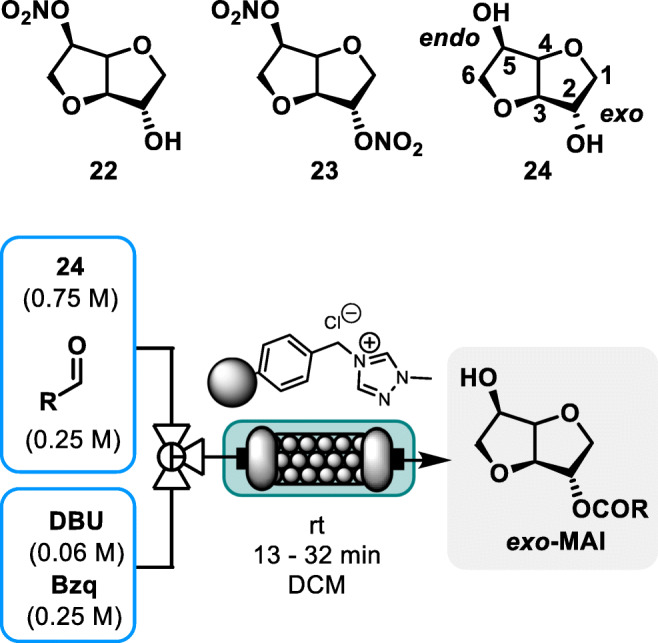
Isorbide derivatives and continuous flow synthesis of exo-MAI [18]. Bzq: 1,4-Benzoquinone
Compound 23 features an isosorbide 24 scaffold, a biobased diol building block, which is efficiently obtained by a sequence of reduction/dehydration of glucose through the intermediate formation of sorbitol. The chiral nature of isosorbide gives diverging chemical reactivity to both hydroxyl groups, although chemoselectivity remains a notable challenge. Isosorbide 5-mononitrate 22, the isomer of choice in the medical field, is obtained by nitrating the free endo-5-OH function after a selective protection through the exo-2-OH acylation. Therefore, finding a streamlined procedure towards exo-2-OH acylated products is highly relevant to the synthesis of 22. Among the most notable developments in this context, Massi and coworkers reported a tunable procedure to selectively acylate isosorbide with aldehydes through heterogeneous Nheterocyclic carbene (NHC) catalysis in batch. In a subsequent step, the authors reported the selective exo-acylation (exo-MAI) under continuous flow conditions [18]. A scope of 5 aldehydes with a limited range of molecular diversity was screened as mild acylating agents, among which, biobased furfural gave excellent results. Conversions ranging from 90 to > 95% with exo/endo ratios in the range of 4.0 to 5.3 were obtained throughout the scope. The robustness of the process was assessed with a 110 h long run, which gave consistent and steady conversion and selectivity with model substrate benzaldehyde. The authors argued their process relied on the main assets of flow chemistry to ensure strict local stoichiometry, leading to high exo/endo ratio and excellent conversion towards a key intermediate of 22.
Recycling and processing of vegetable oils are also a source of interesting chemicals. Main outputs are transesterified fatty acids (typically fatty acid methyl or ethyl esters, namely, FAME or FAEE) and glycerol 1. FAME/FAEE are primarily used in the biodiesel industry and the latter is a versatile C3 platform.
In 2019, Monbaliu and coworkers developed two separate compact and efficient fluidic modules allowing the upgrade of glycerol 1 into Active Pharmaceutical Ingredients (APIs) bearing a β-aminoalcohol scaffold [19]. This was achieved by activating neat glycerol 1 into its oxiranes counterparts (glycidol 25 and epichlorohydrin 26) through a chlorination/dechlorination sequence in flow (chlorination with 36 wt% aqueous HCl and dechlorination/epoxidation with aqueous NaOH). The product mixture was then subjected to a membrane liquid/liquid separation yielding epichlorohydrin 26 (in MTBE) and aqueous glycidol 25. MTBE was evaporated off-line and the resulting pure epichlorohydrin was fed to the second reactor module (see Fig. 4a).
Fig. 4.
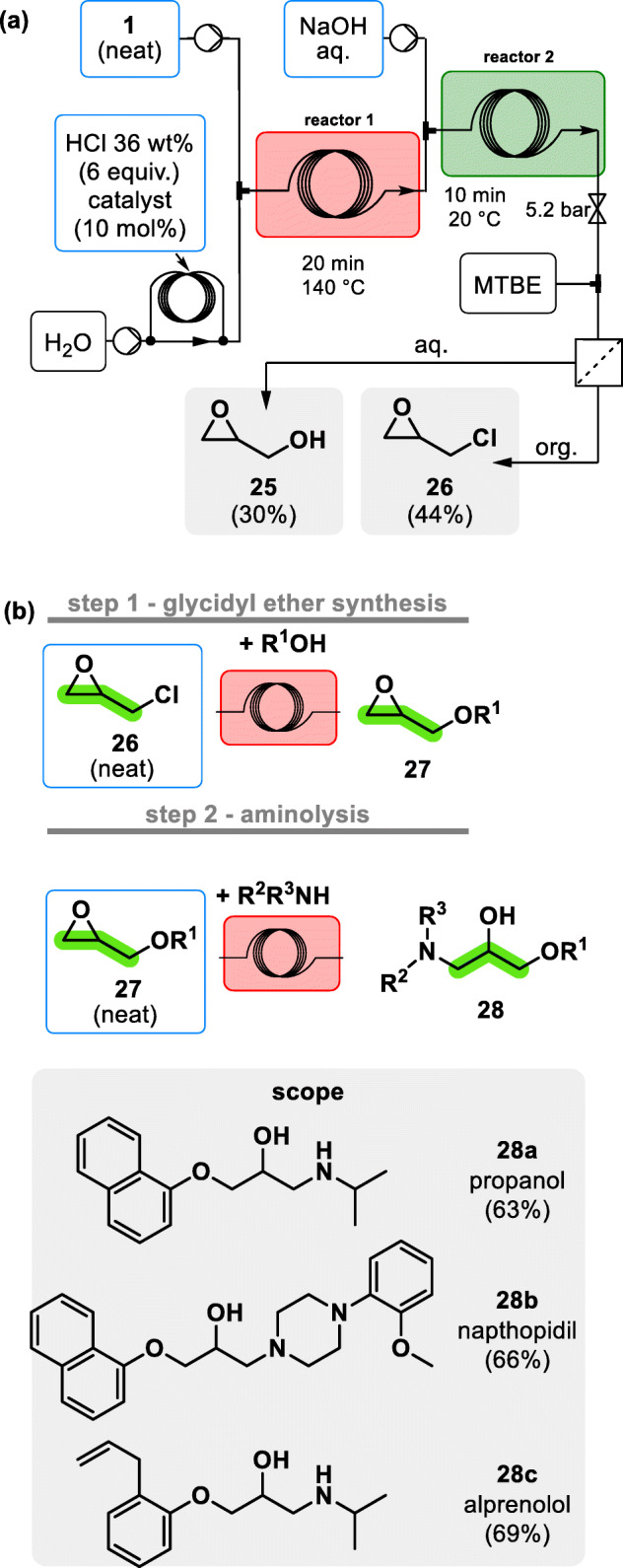
End-to-end glycerol 1 upgrading towards β-aminoalcohol APIs, including propanolol 28a, naftopidil 28b and alprenolol 28c under continuous flow conditions [19]. a Upstream activation of biobased glycerol 1 into glycidol 25 and epichlorohydrin 26. b General two-step strategy (glycidol ether synthesis – aminolysis) for converting biobased epichlorohydrin 26 toward a selected range of β-aminoalcohol APIs (28a, 28b, 28c)
The authors then used a glycidyl ether synthesis/aminolysis sequence under flow conditions to install the β-aminoalcohol scaffold from biobased epichlorohydrin (see general strategy and scope in Fig. 4b). In the first step, Williamson etherification between epichlorohydrin and a feed containing naphtol and t-BuOK was performed in 20 min at 70 °C. The coil effluent was subsequently engaged in the ring opening aminolysis of the corresponding glycidyl ether with an appropriate neat amine to yield a small selection of APIs, including propanolol 28a, naftopidil 28b and alprenolol 28c in good isolated yields.
These selected examples show that renewable platforms are of great interest as chemical precursors for accessing APIs and to further contribute in transitioning from exclusively petrobased production schemes to biobased alternatives. Though momentum is gained in the recent literature, it is worth mentioning that there is still a lack of suitable metrics to assess the incorporation of biobased materials into high valued-added scaffolds such as APIs [17]. Such metrics could potentially complement the conventional process efficiency and environmental impact metrics, such as the Process Mass Intensity, the Atom Economy and the Environmental Factor [20] alongside the green solvent selection guides [21].
This perspective emphasizes the main challenges associated with an ambitious shift of paradigm in the pharmaceutical industry with transitioning from exclusively petrobased production schemes to increasingly biobased processes and summarizes the main assets of automated continuous flow production schemes for tackling these challenges [20–23].
Challenges
Chemical and processing challenges
The main technological challenges are related to production processes (biorefining) allowing the preparation of fine chemicals from biomass. When taking the full life-cycle into account [24, 25], some biorefinery processes have a detrimental environmental impact, are very expensive or are not energy efficient [26]. The processing of the wastes and byproducts can be tedious and generates additional costs [27]. For example, the processing of crustacean shells is known to generate a huge amount of chemical waste while it provides valuable N-containing building blocks [8].There is therefore still much room for improving the overall environmental footprint and the atom economy of such processes. As a matter of fact, many of the most promising biorefinery processes are still associated with intermediate Technology Readiness Levels (TRLs) [28].
Conversion processes must also be developed with improved versatility. In an ideal case scenario, biorefining should be amenable to different substrates to minimize the impact of the seasonality of some raw material causing significant production delays and shortages due to the lack of supply when the harvesting season is finished.
To our knowledge, no study evaluates the impact of the nature of the initial biomass nor its harvest region on the impurity profile of the refined chemical platform. Differences could have consequences from a regulatory point of view as described in the subsequent section.
One of the major challenges related to chemistry and process engineering relates to the inherent features of biobased platform molecules which bear a very high oxygen content by contrast to typical petrobased building blocks [20, 29]. This inherent major difference has a profound impact on the way chemists and chemical engineers have to design new dedicated chemistries and process conditions. A conventional petrobased production scheme is indeed engineered to access molecular diversity from hydrocarbon backbones, hence relying on specific reaction conditions for incorporating heteroelements and hence access function and added-value [20]. Emerging processes feeding on biobased platforms must be tailored to lower their high O-content, hence requiring new catalysts, chemistries and process conditions [20, 28]. These new chemistries and process conditions must be compatible with variable purity profiles (see comment below on the geographical/seasonal variability of biobased platforms) and, ideally, with unrefined biobased platforms. In the current state of the art, many of these processes are still associated with depleting metal catalysts, additives, and solvents with a significant environmental impact [29]. It is therefore critical to allocate significant research efforts to rely on low environmental footprint catalysts, solvents, additives and process technologies, especially when the increasing complexity of some biobased platforms comes along with overwhelming competing reactions [29]. Consequently, the current TRL for such biobased processes is typically around TRL 4–5 [20, 28], thus still far from any implementation at commercial scale. Solving the main chemical and processing challenges associated with the upgrading of biobased platforms is expected to stimulate creativity of Chemistry and Chemical Engineering communities for the next decades.
Regulatory challenges
On a broader note than just the manufacturing of APIs, the current context within the dominant economic areas is very favorable with many governmental incentives thriving with a subsequent thrust toward biobased processes. For instance in the European Union (EU), Bioeconomy has rapidly turned into a global strategy with significant funding schemes and a stable regulatory environment [30, 31]. RoadToBio (within the H2020 program) aimed at replacing 25% of the total volume of fossil-based organic chemicals with an alternative biobased feedstock by the end of 2030. In 2021, a new program (Horizon Europe) took over to stimulate innovation, to sustain the green and digital transitions, and to strengthen EU’s leadership. Similar initiatives are echoed in the US, with directives on advancing biotechnology and biomanufacturing innovation for a sustainable, safe, and secure American Bioeconomy [32, 33].
As this perspective focuses on APIs synthesis, a brief overview of the specific regulatory challenges associated with the use of biobased materials in conjunction with continuous flow manufacturing is mandatory. Regulatory agencies are aware of the importance of continuous flow manufacturing as a breakthrough technology for the future of the pharmaceutical industry. The US Food and Drug Administration (FDA) stated that “Continuous manufacturing is an emerging technology that can enable pharmaceutical modernization and deliver potential benefits to both industry and patients” as an introduction to their Draft Guidance for Industry entitled “Quality Considerations for Continuous Manufacturing” issued in February 2019 [34]. The International Council for Harmonisation (ICH) issued a draft of the guidance Q13 on “Continuous Manufacturing of Drug Substances and Drug Products” in July 2021 [35]. It is expected the European Medicine Agency (EMA) will shortly align its objectives on FDA’s.
Altering synthetic pathway or changing raw material specifications (though still using the same chemical structure) for approved drugs or investigational new drugs are usually associated with a huge regulatory impact, especially if performed after the launch of a phase-III clinical study. As identical chemical entities can be produced from different sources of biomass, a risk assessment must be carried out for each of them. The impact on the product quality attributes must be evaluated. If the risk of change is low, in vitro equivalence demonstration is usually considered as sufficient. High change risk could require additional in vivo bioequivalence studies, which comes with significant additional costs and administrative burden [34].
The transposition of a batch process under continuous flow conditions also implies setting up a rigorous control strategy. Manufacturers must demonstrate that the quality of the finished product, even according to an equivalent combination of chemicals; obtained by continuous manufacturing is similar to the batch one. The system must be capable of real time monitoring with process analytical technologies (PAT) [36, 37] to ensure that it remains under a state of control during the whole production campaign, hence allowing for quality control [36, 37]. The software overlay should allow them to easily access, process and securely archive the acquired data at any time.
Supply chain challenges
Despite the increasing costs of fossil fuels, the biorefinery industry still lacks economic viability for many biobased platforms. This problem is identified as the main non-technical barrier to the deployment of biomass-based chemicals, especially in Europe where labor-related and energy costs are higher than anywhere else [29, 38].
In addition, there is still a need to further expand the array of secondary building blocks accessible after the transformation of the primary ones coming from the fragmentation of biomass. These high value-added products must compensate for the low incomes generated by bulk chemicals while remaining competitive towards conventional fossil fuel-sourced products [2, 39].
In order to accommodate changing input materials and to adapt to the markets demand, a change in the production paradigm is required [40]. The large-scale, delocalized and centralized units must be replaced with a network of smaller production facilities. They must be modular by design to ease the transition between different starting materials and/or finished products.
The decentralization of production facilities will reduce transport-related costs and will better accommodate the associated geographical and seasonal constraints. As some biomass sources are seasonal and perishable, their processing must be done as close to the harvesting site as possible. Besides, biomass sourced raw materials are loaded with water and minerals, which come with a deleterious impact on the transportation costs. Moreover, some mineral and organic components must be moved back to the field after the processing to avoid soil depletion [1].
To minimize the need for storage capabilities for the intermediates, raw materials should be processed as high as possible in the valorization scale. Only high value-added intermediates or finished products are worth storing or transporting to another transformation unit. These inherent features of biorefining imply the development of a robust and flexible logistical system [38]. The implementation of this disruptive production paradigm also requires modular and transportable transformation plants that are environmentally and financially efficient.
In conclusion, synergies should be created between the different actors of the supply chain through collaborative research programs for developing an integrated value chain for the different products obtained after treatment of the biomass. This newly developed supply chain brings together several aspects starting from biomass production and terminating to the market introduction and commercialization of high value-added products. This will stimulate further R&D efforts to develop efficient conversion and valorization process, and will spark the creativity for accessing new products.
Perspectives
Flow chemistry
The advantages of continuous flow production technology come with invaluable assets for the development of intensified processes, with reduced environmental impact and for the development of a decentralized production network [21, 41, 42]. However, a simple transposition of a batch process to continuous conditions does not guarantee better economics and sustainability metrics; it should always come with a careful reoptimization and redefinition of the chemistry and conditions to fully benefit from the assets of flow technology [23, 43, 44]. For instance, in 2021, Kappe and coworkers published a groundbreaking approach for the synthesis of an important Remdesivir intermediate 33, the first FDA and EMA approved drug against the late COVID-19 outburst (see Fig. 5) [45]. The high demand for this drug during the pandemic highlighted the need for faster procedures towards some intermediates. The paper describes flash chemistry under continuous flow conditions, concatenating four consecutive steps within a total residence time of 8 s with an excellent 60% yield involving a biobased ribose derivative 30.
Fig. 5.
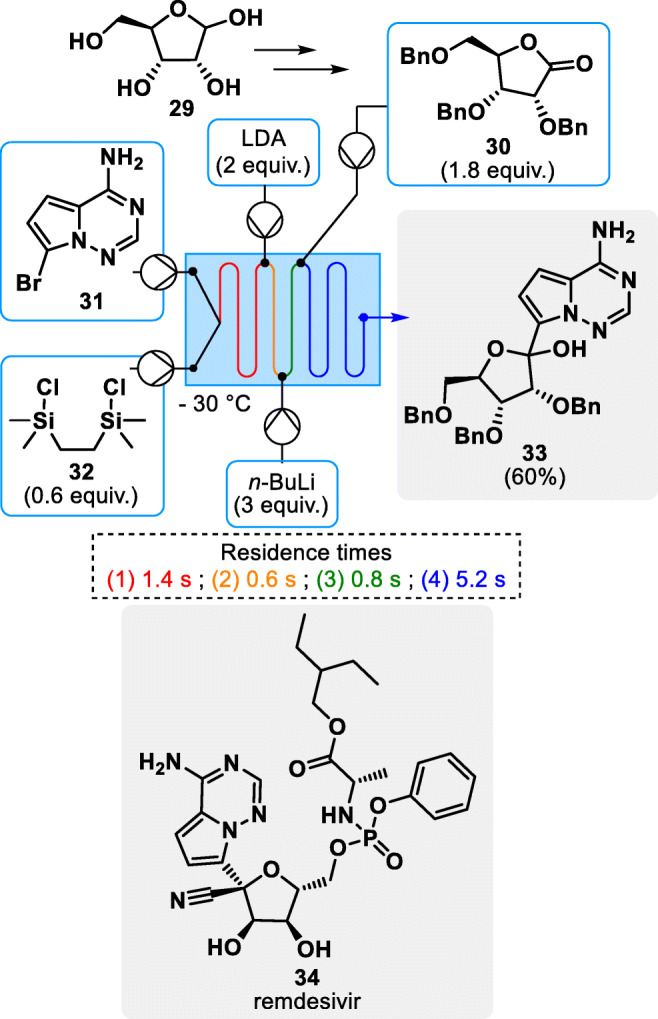
Flash synthesis of remdesevir intermediate 33 under continuous flow conditions [45]
The most remarkable feature of this highly efficient process is obviously the extremely short combined residence time which is possible thanks to the reactivity of the engaged species. However, this efficiency and speed of execution are the result of very specific and strict conditions among which, temperature, reaction time and local stoichiometry as well as an efficient mixing and heat transfer which are some of the most attractive features of microfluidic technology. The space-time yield (STY) of the process was determined to be an astonishingly high 10.4 kg∙L1∙h1 despite the very modest size of the reactor [45]. This approach is a way to reduce the production costs by optimizing the productivity of the installation while keeping a low spatial footprint.
In an industrial environment, continuous flow also provides a good answer to safety issues [46]. For example, diazo anhydrides are well-known explosives and highly carcinogenic agents. Their in situ generation under continuous flow conditions was demonstrated by Kappe and coworkers in 2015 as an elegant and efficient solution to alleviate acute hazards upon stockpiling or contact with the operator. A photochemical microfluidic reactor was developed, allowing to safely generate, handle and react this particularly hazardous substance family with non-activated arenes towards bi(hetero)aryl derivatives (see Fig. 6).
Fig. 6.
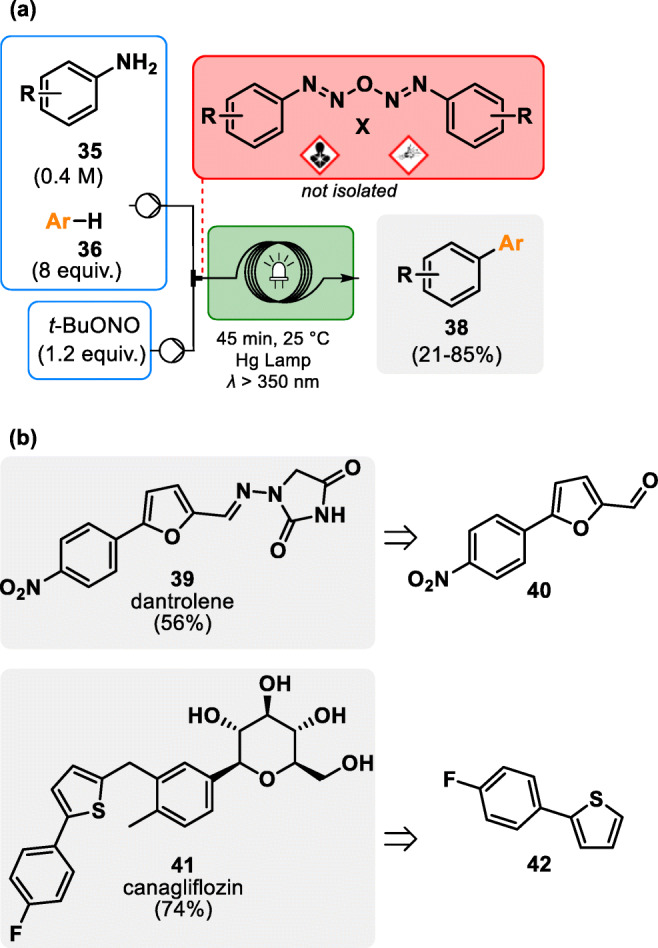
Chemical generator for diazo anhydrides under continuous flow conditions [47]. a General strategy for the photochemical synthesis of bi(hetero)aryl derivatives. b Application of diazo anhydrides for the preparation of dantrolene 39 (myorelaxant) and canagliflozin 41 (antidiabetic)
This method supports the arylation of biobased furfural 43 with 4-nitroaniline, providing a key intermediate towards dantrolene 39, an API prescribed as myorelaxant. This arylation procedure is also amenable to the coupling of thiophene and 4-fluoroaniline towards a canagliflozin 41 (antidiabetic) intermediate (see Fig. 6). The only waste materials resulting from this elegant photochemical coupling are N2, H2O and t-BuOH, while hazardous and transient diazo anhydride has been safely generated and consumed [47].
Colacino and coworkers disclosed a solvent free method for the synthesis of dantrolene and other hydrazone-based API using continuous mechanochemistry [48]. The conversion of batch mechanochemical processes to the continuous flow paradigm is another promising emerging research area. Green-minded process chemists are highly interested in ball milling as it eliminates the need for solvents [49]. This has a direct positive impact on reducing the environmental and financial footprint of the process. Rotating or mixing mills are replaced by twin screw extruders to allows the continuous mechanochemical processing of the reagents [48, 49]. Li and coworkers showed that ball milling was suitable for the upgrade of biomass-derived materials [50]. The authors demonstrated the condensation of furfural with five different ketones, which led to the desired adducts with low to excellent yields ranging from 44.0% (MIBK as ketone counterpart) to 99.8% (cyclopentanone as ketone counterpart) using CaO as catalyst under mild process conditions (40 °C) [50].
Electrochemistry has been part of the organic chemist toolkit for numerous years now. Unfortunately, it often suffers from a series of drawbacks in batch which lead to long reaction times as well as intricate conditions and side-products. The main drawbacks of electrochemistry can be in part mitigated under continuous flow conditions, as highlighted by seminal contributions from the Noël and Kappe groups, including for the preparation of APIs and relevant scaffolds [51–58]. An exemple from Noël’s group is illustrated hereafter, where the authors provided an unprecedented continuous electrochemical protocol for upgrading biobased furfural 43 simultaneously into three industrially relevant molecules: 2(5H)-furanone 44 (polymer industry and γ-butyrolactone precursor), furfuryl alcohol 45 (resins) and hydrofuroin 46 (jet-fuel precursor) (see Fig. 7) [57, 58].
Fig. 7.

Electrochemical upgrading of biobased chemicals under continuous flow conditions [57, 58]
The furfural redox reaction was performed in a divided-cell flow microreactor with a key interesting aspect: varying the applied voltage (2.4 or 2.9 V) leads to different proportions of 2(5 H)-furanone 44 (46% vs. 77%) in the cathodic cell outlet, furfuryl alcohol 45 (58% vs. 20%) and hydrofuroin 46 (29% vs. 71%) in the anodic cell outlet (see Fig. 7). In other words, a tunable reactor was devised, allowing to choose which biobased derived molecules could be synthesized in larger proportions. Unfortunately, the reaction was not amenable to a 100% selectivity between furfuryl alcohol 45 or hydrofuroin 46 [57]. In another article, the same group also demonstrated the suitability of flow electrochemistry for the preparation of suitable scaffolds for APIs with C-N cross-coupling reaction between azole derivatives and arenes. The electron-driven reaction supports a scope of both azole derivatives (10 examples) and arene substrates (11 examples) with yields ranging from 20 to 98% despite a very short 10 min residence time at room temperature in the flow reactor with the only by-product of the reaction being dihydrogen [58].
In addition to having positive effects on the efficiency of electrochemically driven reactions, microfluidic reactors offer practical solutions to similar issues encountered while performing photochemical reactions. The synergistic combination of continuous flow and photochemistry was vastly documented by the Kappe and Noël groups. For instance, in a study targeting the upgrading of 5-hydroxymethyl furfural 47, Kappe and coworkers developed a photochemical flow process for the singlet oxygen-mediated preparation of butanolide 48 (see Fig. 8a) [59]. Butenolide 48 and its derivatives hold a significant role as polyester precursors while still bearing an alkene moiety, allowing for further functionalization of either the monomer or polymer. The process takes advantage of in situ generated singlet oxygen through rose bengal photo excitation to safely and neatly oxidize 47 at low temperature. A 0.5 mol% catalyst loading sufficed to activate the slight excess of oxygen into its highly oxidizing singlet state. The efficiency of the reaction conditions was then assessed by applying them to four additional derivatives bearing ester, methyl, succinimide or acetal functions, with yields ranging from poor for the acetal-bearing substrate (< 20%) to excellent (93%). Another photochemical singlet oxygen generator was reported a few years later by Monbaliu and colleagues for the preparation of methionine sulfoxide from natural amino acid methionine [61].
Fig. 8.
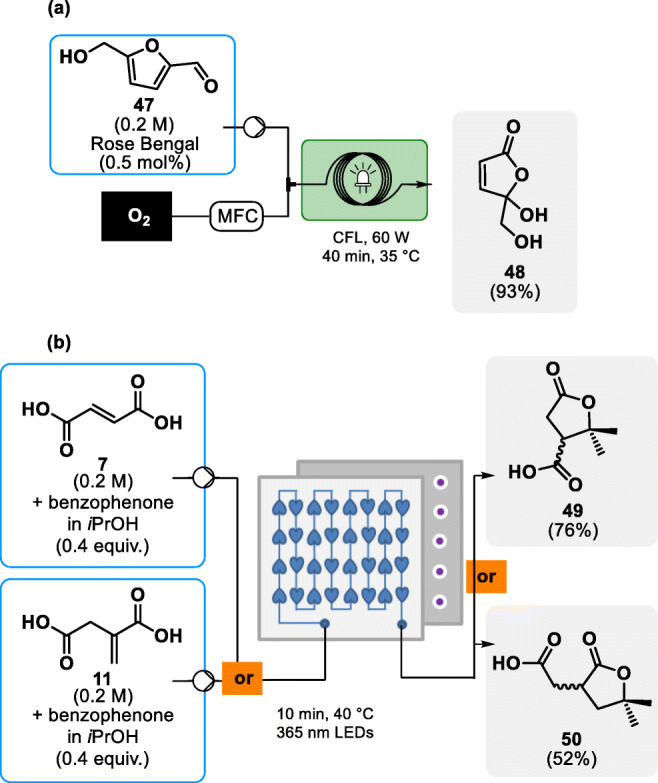
Biobased building blocks photochemical upgrading into polymer precursors [59, 60]. a Singlet oxygen generator for the preparation of butenolide 48 [46]. b Upgrading of fumaric and itaconic acids under continuous flow photochemical conditions
In 2017, Monbaliu and coworkers disclosed the photochemically-driven upgrade of plaftorms itaconic 11 and fumaric 7 acids, hence providing another illustration of the intrinsic advantages of photoflow chemistry [60]. This novel process relied on 365 nm LEDs to photochemically activate benzophenone, a radical initiator. This allows the addition of alcohol-derived radicals to the alkene substrates, forming substituted γ-butyrolactones 49 and 50, respectively (see Fig. 8b). In this process, isopropanol serves both as reagent and solvent for benzophenone and the substrates, lowering the overall footprint of the reaction. The reaction was quickly optimized by relying on in-line NMR analysis. With ideal conditions in hand, methanol and cyclohexanol were tested both for fumaric and itaconic acid in place of isopropanol. The reaction was then swiftly scaled up from a microfluidic scale to a mesofluidic Corning® Advanced-Flow™ G1 Photo Reactor resulting in an 83 g/day productivity on the model substrate (fumaric acid 7 and isopropanol). A small scope of γ-butyrolactones, including unique spiro derivatives, was documented [60].
In both electro and photochemical cases, the much larger surface to volume ratio and shorter residence time, are often associated with a significant reduction in side products. The improved yields can be ascribed to microfluidic reactor architectures; narrow channels permitting a more efficient light or current transmission to the reaction medium. Additionally, less electrolytes or photocatalysts are required and higher surface-to volume ratio ensured faster reaction rates too. Despite showing great potential for synthetic application, electrochemistry under continuous flow conditions is still a “niche” discipline, with major challenges associated to scalability yet to be addressed.
As flow processes are usually faster to upscale than their batch counterparts, early R&D development in flow are quickly transposed to commercial scales, hence coming with shorter time-to-market. With an overall lower footprint than batch reactors, flow reactors also allow the development of compact production units. These production units can be adapted to sudden change in demand, either with numbering-up or scaling-out strategies with production volume easily tunable. This property can be foreseen as paramount for deploying decentralized and/or mobile production units.
In 2016, Jensen, Jamison and coworkers reported the development of a compact, transportable and fully modular micro-fluidic setup for API manufacturing. Building upon a long history of impactful and groundbreaking technologies for the continuous flow manufacturing of APIs in low footprints, mobile setups, the MIT team pushed further the miniaturization of an end-to-end manufacturing unit combining upstream (chemical transformations, intermediates workups and extractions) with advanced downstream operations (purification and liquid formulation) [62].
This was made possible by the elaboration of individual multipurpose modules which could be connected based on the synthesized API. Coils of varying volumes, in-line separation units, pumps, gravity separation units as well as an in-line IR spectrometer and packed-bed columns constituted the functional blocks allowing the complete manufacturing of 4 different APIs. The coordination of the high number of pumps, IR and purification steps were made possible through automation. It means that a single operator can have a total control over the production of these four molecules, reducing the need for an abundant workforce. Additionally, concentrated or neat feedstocks were used to minimize solvent waste and maximize productivity [62].
Devising and relying on independent yet compatible modules opened the doors to the on-demand synthesis of several primordial pharmaceuticals. This modularity was taken advantage of to engineer a compact and portable (fridge-sized) mini-API factory, rendering this a potentially highly efficient tool for local drug delivery. In the original prototype, 4 APIs with different structures and pharmacological profiles were obtained with output ranging from 810 to 4500 doses per day. The 4 APIs included diphenhydramine hydrochloride (an antihistamine compound), lidocaine hydrochloride (an anaesthetic and antiarrhythmic compound), diazepam (a nervous system depressant) and fluoxetine (an antidepressant). Most notably, the prototype enabled a swift reconfiguration to swap from an API to another, in less than 30 min. In a further attempt to broaden the utility of such an approach, an additional series of 4 APIs was prepared in an updated prototype [63].
More recently, the same team combined advanced automation, robotics and artificial intelligence in a robotic platform dedicated to flow synthesis [64]. This innovative work is the first to propose a solution combining computer-aided synthesis planning (CASP), expert refined chemical recipe generation, automatic assembly of the fluidic setup by a robotic arm. The assembled system is then used to carry on the synthesis. The robotic arm can perform an adaptation of the setup while the synthesis is running. For example, changes of feedstock allow to rapidly access molecular diversity.
Their proof-of-concept experiment consists of the automated synthesis of fifteen APIs or pharmaceutical intermediates. The system generated synthetic routes for each compound. As all the synthesis routes are already described in the literature, the software was not allowed to use existing pathways. Then, the automated robotic arm had to construct nine different fluidic setups as some synthesis shared common apparatus and feedstocks. The recipe generation for each step was the only human intervention in the whole process as existing databases do not contain enough information to allow using a data-driven automatic generator. The main challenge relied in the translation of batch conditions into continuous flow protocols. The system carried out automatically the synthesis of the targets compounds. For example, it produced aspirin with 91% yield and a productivity of 1.72 g/h; diazepam with 75% yield and a productivity of 638 mg/h. In order to demonstrate the advantages of fluidic path rerouting by the robotic arm during a synthesis, a scope of five different ACE inhibitors based on the quinapril scaffold was prepared. The full library was produced in 68 h with productivity ranging between 342 and 459 mg/h. Another library of four celecoxib analogs was completed in 28 h with similar productivities.
Automation
A sustained effort towards automation of the production unit is mandatory to reduce the cost induced by the operation of smaller production units. Moreover, for continuous manufacturing, automated production mode should ease keeping processes under a control state and reduce transient states leading to the mandatory diversion of out of specification materials.
Other academic group are active in the field of organic synthesis automation. We already discussed of the work at MIT by Jensen, Jamison and coworkers. The group of Ley developed an automated system mixing continuous and batch reactors in the same integrated system controlled by a web-based interface. They demonstrated such concept in 2016 by synthesizing 5-methyl-4-propylthiophene-2-carboxylic acid, a precursor for the cancer drug candidate AZ82. For the needs of the synthetic pathway, they had to develop a glass reactor that can be temperature-regulated from − 70 °C to + 150 °C. The three-layer jacket enhanced the heat transfer and the thermal control of the reaction media, which are often ineffective using classical oil bath as a heat source. The web-based software allowed to control the whole system remotely, but also to receive analytical data in real time. The fully telescoped three-step synthesis gave the expected product with an overall yield of 30%. The fully manual procedure occurred with a slightly lower yield of 27% [65].
The modular software package they developed is also capable of multidimensional optimization. As a proof-of-concept, they made the five-dimensional optimization of an Appel reaction. The software made its self-optimization using the feedback provided by an IR spectrometer and mass spectrometry data. The software was able to find the optimal value of five experimental parameters after 30 experiments performed autonomously over 10 h [66].
In-line quality control devices and robust process analytical technology (PAT) are mandatory to comply with the regulatory obligations linked to the continuous manufacturing of APIs. The direct integration of PAT data into an integrated control software is sometimes problematic due to proprietary drivers or communication protocols. This year, Kappe and coworkers used a system composed of a thermoregulated microreactor linked to an inline NMR and an inline FTIR apparatus to collect real-time measurements the reaction media. Those data can then be processed with an appropriate chemometric model to optimize up to 7 reaction variables linked to a two-step process without any human intervention. The use of such automated process is not always possible at early stages of the development of pharmaceuticals as the quantity of precursor needed could be the limiting factor. For example, optimization of the considered two-step process required 14.3 g/h of starting material, meaning a total of 371.8 g for the 85 iterations performed over 26 h [67].
Cronin and coworkers unveiled their Chemputer in 2019. This platform is the combination of a modular robotic platform with an intuitive software overlay. The hardware part consists in modules dedicated to the four key steps of a chemical synthesis: reaction, workup, isolation and the subsequent purification. The modules are interconnected through a fluidic backbone consisting of pumps and valves allowing the transfer of chemicals between them. Thanks to a washing system, the system makes multisteps synthesis possible. As a proof of concept, they performed the autonomous synthesis of the antihistamine and mild sleep aid diphenydramine hydrochloride, the anticonvulsant rufinamide and sildenafil used to treat erectile dysfunction. The modules used were a reaction flask, a temperature regulated filtration module, a liquid-liquid separation module and a liquid evaporation module. This system, costing less than 10,000 USD, was able to produce the three APIs with yields comparable to the one obtained after a manual synthesis.
In order to program the synthesis unit, they developed a language allowing the translation of physical operations performed by a trained chemist to machine-readable low-level instructions through their “Chempiler”. Operations are described by the user using a Chemical Description Language (ΧDL) allowing its use by staff without any programming skills through the user-friendly “Chemical Development Environment” (ChemIDE) interface [68].
They pushed the automation one step further by developing “Synthreader”, a program based on natural language processing technology that translates, often ambiguous, literature protocol into ΧDL instructions. This program automatically splits the protocols into a list of actions, extract the relevant process and reagent information and then translate it into unambiguous ΧDL formatted sentences. The obtained ΧDL file is then compiled to be used on the user customized setup [69].
While usable in the academic environment, these systems are not suited for an industrial use. Any commercial equipment used in the industry and especially in the pharmaceutical industry must be supplied with a sufficient documentary package to allow its qualification and validation prior use. These requirements apply for the software as well. Moreover, devices should be designed to allow an efficient and easy servicing in order to minimize the downtime due to impromptus failures or planned maintenance.
Conclusion
The development of automated modular units based on integrated continuous processes is a promising technology enabler for the upgrading of biobased platform chemicals into high value-added chemicals.
From a chemical point of view, continuous flow offers a convenient framework to elaborate safe and versatile conversion processes with a moderate to low environmental footprint. Regarding regulatory aspects, PAT allows an accurate and responsive process control guaranteeing the compliance of the product with its specifications. However, the impact of the variability of biomass sourced material on impurity profile and byproducts should still be determined.
The development of modular and automated production units will fasten the shift of the actual production paradigm to smaller decentralized manufacturing sites allowing agile supply chain management. We have to keep in mind that all those developments should be made with the pharmaceutical industry documentary standard in mind.
Biographies
Geoffroy Kaisin
After a PhD in radiochemistry and organic chemistry at the Cyclotron Research Center at the University of Liège, Geoffroy joined a startup of the University, ANMI. As Head of Chemistry, he participated to the development of a patented cold kit formulation for the labelling of PSMA-11 with gallium-68. After the acquisition of ANMI by Telix Pharmaceuticals in 2018, his research focused on new methodologies for the labelling of biomolecules with radiometals. He is now R&D chemist and co-founder at SynLock where his research interests are aimed at the automation of organic synthesis.
Loïc Bovy
Loïc holds a MSc from the Université de Liège in chemical sciences (2021). He then joined the Center for Integrated Technology and Organic Synthesis (CiTOS) to conduct a PhD. His research focuses on the upgrading of biobased building blocks and their integration in active pharmaceutical ingredients by combining both batch and flow chemistry methodologies.

Yoann Joyard
Yoann graduated from INSA Rouen as PhD in organic chemistry in 2013. He focused on the development of tracers for targeting hypoxic tumors and developed new fluorination methodologies. He carried out postdoctoral research at King’s College London in the School of Biomedical Engineering and Imaging Sciences. He worked there on the development of bimodal probes for guided surgery. Yoann also worked as research engineer for two years in a company specialized in development of continuous-flow and batch process in chemistry, before joining Optimized Radiochemical Applications (ORA) in 2017 as R&D radiochemist. He is now R&D chemist and co-founder at SynLock where his research interests are aimed at the automation of organic synthesis.

Nicolas Maindron
Nicolas received his PhD from the University of Rouen, France, in 2012, in the field of the lanthanide-based luminescence probes. He then completed two postdoctoral positions. One at the University of Bourgogne, Dijon, France, where he designed multifunctional imaging probes and a second one at MNI (now Invicro) at New Haven, USA, where he started to work in the area of radiochemistry. He joined Optimized Radiochemical Applications (ORA) in 2016 as a R&D radiochemist. He is now R&D chemist and co-founder at SynLock where his research interests are aimed at the automation of organic synthesis.

Vincent Tadino
Vincent received his PhD, in Organic Chemistry at the University of Liège, Belgium, and completed a PostDoc research in Organic Chemistry at ISMRA in Caen, France. Vincent founded Optimized Radiochemical Applications (ORA) in 2006 and currently serves as its President and CTO. Prior to founding ORA, Vincent served as project chief, radiochemistry developer, and quality manager for 7 years in different companies active in PET radiopharmaceutical equipment and production, both in Europe and the USA. He holds, as inventor and/or as co-inventor, eleven patents and is the author of several abstracts/presentations in international congresses. He is now President and co-founder at SynLock where his research interests are aimed at theautomation of organic synthesis.

Jean-Christophe M. Monbaliu
is currently Associate Professor at the University of Liège (Belgium) and serves as Associate Editor of the Journal of Flow Chemistry. He is heading the Center for Integrated Technology and Organic Synthesis (CiTOS, www.citos.uliege.be), the first European Corning® Advanced-Flow™ reactor qualified lab. Research interests at CiTOS revolve around synthetic organic chemistry but are multidisciplinary in essence and aim at (a) designing cheaper and more efficient routes for the preparation of high value-added chemicals such as active pharmaceutical ingredients; (b) accelerating the transition from petrobased to biobased strategies and (c) developing efficient processes with a lower environmental impact.

Declarations
Competing interests
No funding was received to assist with the preparation of this manuscript. Yoann Joyard, Geoffroy Kaisin and Nicolas Maindron are shareholders of the Company SynLock SRL. Vincent Tadino is a shareholder and the Executive Director of the Company SynLock SRL. Loic Bovy and Jean-Christophe M. Monbaliu have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holm-Nielsen JB, Ehimen EA (2014) Biorefinery plant design, engineering and process optimisation. Advances in biorefineries: biomass and waste supply chain exploitation, pp 89–111. 10.1533/9780857097385.1.89
- 2.Brun N, Hesemann P, Esposito D. Expanding the biomass derived chemical space. Chem Sci. 2017;8:4724–4738. doi: 10.1039/c7sc00936d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carus M, Dammer L, Puente A et al (2017) Bio-based drop-in, smart drop-in and dedicated chemicals. https://roadtobio.eu/uploads/news/2017_October/RoadToBio_Drop-in_paper.pdf . Accessed on 10-29-22
- 4.Werpy T, Petersen G (2004) Top value added chemicals from biomass. Volume I - results of screening for potential candidates from sugars and synthesis gas. 10.2172/15008859
- 5.Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “top 10” revisited. Green Chem. 2010;12:539–555. doi: 10.1039/b922014c. [DOI] [Google Scholar]
- 6.Biddy MJ, Scarlata C, Kinchin C (2016) Chemicals from biomass: a market assessment of bioproducts with near-term potential. https://www.nrel.gov/docs/fy16osti/65509.pdf . Accessed on 10-29-22
- 7.Liao Y-T, Matsagar BM, Wu KC-W (2018) Metal–Organic Framework (MOF)-derived effective solid catalysts for valorization of lignocellulosic biomass. ACS Sustain Chem Eng 6:13628–13643. 10.1021/acssuschemeng.8b03683
- 8.Hülsey MJ, Yang H, Yan N (2018) Sustainable routes for the synthesis of renewable heteroatom-containing chemicals. ACS Sustain Chem Eng 6:5694–5707. 10.1021/acssuschemeng.8b00612
- 9.Abdelaziz OY, Hulteberg CP (2020) Lignin depolymerization under continuous-flow conditions: highlights of recent developments. Chemsuschem 13:4382–4384. 10.1002/cssc.202001225 [DOI] [PMC free article] [PubMed]
- 10.Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. 10.1021/cr900354u [DOI] [PubMed]
- 11.Liu X, Bouxin FP, Fan J et al (2020) Recent advances in the catalytic depolymerization of lignin towards phenolic chemicals: a review. Chemsuschem 13:4296–4317. 10.1002/cssc.202001213 [DOI] [PMC free article] [PubMed]
- 12.Giorgi PD, Elizarov N, Antoniotti S (2017) Selective oxidation of activated alcohols by supported gold nanoparticles under an atmospheric pressure of O2: batch and continuous-flow studies. ChemCatChem 9:1830–1836. 10.1002/cctc.201700179
- 13.Premkumar J, Sampath P, Sanjay R et al (2020) Synthetic guaiacol derivatives as promising myeloperoxidase inhibitors targeting atherosclerotic cardiovascular disease. ChemMedChem 15:1187–1199. 10.1002/cmdc.202000084 [DOI] [PubMed]
- 14.Guz NR, Stermitz FR, Johnson JB et al (2001) Flavonolignan and flavone inhibitors of a Staphylococcus a ureus multidrug resistance pump: structure – activity relationships. J Med Chem 44:261–268. 10.1021/jm0004190 [DOI] [PubMed]
- 15.Sadiq AD, Chen X, Yan N, Sperry J (2018) Towards the shell biorefinery: sustainable synthesis of the anticancer alkaloid proximicin A from chitin. Chemsuschem 11:532–535. 10.1002/cssc.201702356 [DOI] [PubMed]
- 16.Ogata M, Hattori T, Takeuchi R, Usui T. Novel and facile synthesis of furanodictines A and B based on transformation of 2-acetamido-2-deoxy-d-glucose into 3,6-anhydro hexofuranoses. Carbohydr Res. 2010;345:230–234. doi: 10.1016/j.carres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (2021) World Health Organization model list of essential medicines – 22nd list
- 18.Ragno D, Leonardi C, di Carmine G et al (2021) Regiodivergent isosorbide acylation by oxidative N-Heterocyclic carbene catalysis in batch and continuous flow. ACS Sustain Chem Eng 9:8295–8305. 10.1021/acssuschemeng.1c02765
- 19.Morodo R, Gérardy R, Petit G, Monbaliu JCM. Continuous flow upgrading of glycerol toward oxiranes and active pharmaceutical ingredients thereof. Green Chem. 2019;21:4422–4433. doi: 10.1039/c9gc01819k. [DOI] [Google Scholar]
- 20.Gérardy R, Debecker DP, Estager J et al (2020) Continuous flow upgrading of selected C2–C6 platform chemicals derived from biomass. Chem Rev 120:7219–7347. 10.1021/acs.chemrev.9b00846 [DOI] [PubMed]
- 21.Gérardy R, Emmanuel N, Toupy T et al (2018) Continuous flow organic chemistry: successes and pitfalls at the interface with current societal challenges. Eur J Org Chem 2018:2301–2351. 10.1002/ejoc.201800149
- 22.Gérardy R, Morodo R, Estager J et al (2019) Sustaining the transition from a petrobased to a biobased chemical industry with flow chemistry. Top Curr Chem 377:1. 10.1007/s41061-018-0222-3 [DOI] [PubMed]
- 23.Monbaliu J-CM, Legros J. Will the next generation of chemical plants be in miniaturized flow reactors? Lab Chip. 2022 doi: 10.1039/D2LC00796G. [DOI] [PubMed] [Google Scholar]
- 24.Lapkin AA, Yaseneva P (2017) Life cycle assessment of flow chemistry processes. In: Sustainable flow chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 249–276
- 25.Hessel V, Escribà-Gelonch M, Bricout J et al (2021) Quantitative sustainability assessment of flow chemistry–from simple metrics to holistic assessment. ACS Sustain Chem Eng 9:9508–9540. 10.1021/acssuschemeng.1c02501
- 26.Calvo-Serrano R, Guo M, Pozo C et al (2019) Biomass conversion into fuels, chemicals, or electricity? A network-based life cycle optimization approach applied to the European Union. ACS Sustain Chem Eng 7:10570–10582. 10.1021/acssuschemeng.9b01115
- 27.Iwaszko J, Zajemska M, Zawada A et al (2020) Vitrification of environmentally harmful by-products from biomass torrefaction process. J Clean Prod 249:119427. 10.1016/j.jclepro.2019.119427
- 28.Lindorfer J, Lettner M, Fazeni K et al (2019) Technical, economic and environmental assessment of biorefinery concepts
- 29.Muzyka C, Monbaliu J-CM (2022) Perspectives for the upgrading of bio-based vicinal diols within the developing european bioeconomy. ChemSusChem 15. 10.1002/cssc.202102391 [DOI] [PubMed]
- 30.Innovation for sustainable growth : a bioeconomy for Europe. https://op.europa.eu/en/publication-detail/-/publication/1f0d8515-8dc0-4435-ba53-9570e47dbd51. Accessed on 10-29-22
- 31.Review of the 2012 European Bioeconomy Strategy. https://ec.europa.eu/information_society/newsroom/image/document/2018-6/review_of_2012_eu_bes_2E89B85F-950B-9C84-5B426D1C24851387_49692.pdf . Accessed on 10-29-22
- 32.https://www.whitehouse.gov/briefing-room/presidential-actions/2022/09/12/executive-order-on-advancing-biotechnology-and-biomanufacturing-innovation-for-a-sustainable-safe-and-secure-american-bioeconomy/ . Accessed on 10-29-22
- 33.https://www.biopreferred.gov/BioPreferred/ . Accessed on 10-29-22
- 34.US Food & Drug Administration (2019) Draft guidance - quality considerations for continuous manufacturing
- 35.ICH Harmonised Guidelines (2021) Continuous manufacturing of drug substances and drug products Q13 - draft version
- 36.US Food & Drug Administration (2004) Guidance for industry - PAT — a framework for innovative pharmaceutical development, manufacturing, and quality assurance
- 37.Simon LL, Pataki H, Marosi G et al (2015) Assessment of recent Process Analytical Technology (PAT) trends: a multiauthor review. Org Process Res Dev 19:3–62. 10.1021/op500261y
- 38.European Commission (2021) EU Biorefinery Outlook to 2030 https://op.europa.eu/s/wTmR . Accessed on 10-29-22
- 39.Clark H, Deswarte JEI, Farmer FJ T (2009) The integration of green chemistry into future biorefineries. Biofuels Bioprod Biorefin 3:72–90. 10.1002/bbb.119
- 40.Kokossis AC, Yang A. On the use of systems technologies and a systematic approach for the synthesis and the design of future biorefineries. Comput Chem Eng. 2010;34:1397–1405. doi: 10.1016/j.compchemeng.2010.02.021. [DOI] [Google Scholar]
- 41.Hessel V (2009) Novel process windows - gate to maximizing process intensification via flow chemistry. Chem Eng Technol 32:1655–1681. 10.1002/ceat.200900474
- 42.Keil FJ. Process intensification. Rev Chem Eng. 2018;34:135–200. doi: 10.1515/revce-2017-0085. [DOI] [Google Scholar]
- 43.Newman SG, Jensen KF. The role of flow in green chemistry and engineering. Green Chem. 2013;15:1456. doi: 10.1039/c3gc40374b. [DOI] [Google Scholar]
- 44.Kassin V-EH, Gérardy R, Toupy T, et al. Expedient preparation of active pharmaceutical ingredient ketamine under sustainable continuous flow conditions. Green Chem. 2019;21:2952–2966. doi: 10.1039/C9GC00336C. [DOI] [Google Scholar]
- 45.von Keutz T, Williams JD, Kappe CO (2021) Flash chemistry approach to organometallic C-Glycosylation for the synthesis of remdesivir. Org Process Res Dev 25:1015–1021. 10.1021/acs.oprd.1c00024
- 46.Chen Y, Cattoen M, Monbaliu J-CM (2021) Mitigation of chemical hazards under continuous flow conditions. In: Flow chemistry – fundamentals. De Gruyter, pp 269–312
- 47.Cantillo D, Mateos C, Rincon JA et al (2015) Light-induced C-H Arylation of (Hetero)arenes by in situ generated diazo anhydrides. Chem---Eur J 21:12894–12898. 10.1002/chem.201502357 [DOI] [PubMed]
- 48.Crawford DE, Porcheddu A, McCalmont AS et al (2020) Solvent-free, continuous synthesis of hydrazone-based active pharmaceutical ingredients by twin-screw extrusion. ACS Sustain Chem Eng 8:12230–12238. 10.1021/acssuschemeng.0c03816
- 49.Bolt RRA, Leitch JA, Jones AC, et al. Continuous flow mechanochemistry: reactive extrusion as an enabling technology in organic synthesis. Chem Soc Rev. 2022;51:4243–4260. doi: 10.1039/D1CS00657F. [DOI] [PubMed] [Google Scholar]
- 50.Xu Q, Sheng X, Li N et al (2021) Ball-milling: a productive, economical, and widely applicable method for condensation of biomass-derived aldehydes and ketones at mild temperatures. ACS Sustain Chem Eng 9:8232–8237. 10.1021/acssuschemeng.1c02270
- 51.Noël T, Cao Y, Laudadio G (2019) The fundamentals behind the use of flow reactors in electrochemistry. Acc Chem Res 52:2858–2869. 10.1021/acs.accounts.9b00412 [DOI] [PMC free article] [PubMed]
- 52.Delparish A, Uslu A, Cao Y et al (2022) Boosting the valorization of biomass and green electrons to chemical building blocks: a study on the kinetics and mass transfer during the electrochemical conversion of HMF to FDCA in a microreactor. Chem Eng J 438:135393. 10.1016/j.cej.2022.135393
- 53.Ošeka M, Laudadio G, van Leest NP et al (2021) Electrochemical aziridination of internal alkenes with primary amines. Chem 7:255–266. 10.1016/j.chempr.2020.12.002
- 54.Cantillo D. Synthesis of active pharmaceutical ingredients using electrochemical methods: keys to improve sustainability. Chem Commun. 2022;58:619–628. doi: 10.1039/D1CC06296D. [DOI] [PubMed] [Google Scholar]
- 55.Köckinger M, Hanselmann P, Roberge DM, et al. Sustainable electrochemical decarboxylative acetoxylation of aminoacids in batch and continuous flow. Green Chem. 2021;23:2382–2390. doi: 10.1039/D1GC00201E. [DOI] [Google Scholar]
- 56.Maljuric S, Jud W, Kappe CO, Cantillo D. Translating batch electrochemistry to single-pass continuous flow conditions: an organic chemist’s guide. J Flow Chem. 2020;10:181–190. doi: 10.1007/s41981-019-00050-z. [DOI] [Google Scholar]
- 57.Cao Y, Knijff J, Delparish A et al (2021) A divergent paired electrochemical process for the conversion of furfural using a divided-cell flow microreactor. Chemsuschem 14:590–594. 10.1002/cssc.202002833 [DOI] [PMC free article] [PubMed]
- 58.Buglioni L, Beslać M, Noël T (2021) Dehydrogenative azolation of arenes in a microflow electrochemical reactor. J Org Chem 86:16195–16203. 10.1021/acs.joc.1c01409 [DOI] [PMC free article] [PubMed]
- 59.Heugebaert TSA, Stevens C, Kappe CO (2015) Singlet-oxygen oxidation of 5-Hydroxymethylfurfural in continuous flow. Chemsuschem 8:1648–1651. 10.1002/cssc.201403182 [DOI] [PubMed]
- 60.Gérardy R, Winter M, Horn CR et al (2017) Continuous-flow preparation of γ-Butyrolactone scaffolds from renewable fumaric and itaconic acids under photosensitized conditions. Org Process Res Dev 21:2012–2017. 10.1021/acs.oprd.7b00314
- 61.Emmanuel N, Mendoza C, Winter M et al (2017) Scalable photocatalytic oxidation of methionine under continuous-flow conditions. Org Process Res Dev 21:1435–1438. 10.1021/acs.oprd.7b00212
- 62.Adamo A, Beingessner RL, Behnam M, et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science (1979) 2016;352:61–67. doi: 10.1126/science.aaf1337. [DOI] [PubMed] [Google Scholar]
- 63.Zhang P, Weeranoppanant N, Thomas DA et al (2018) Advanced continuous flow platform for on-demand pharmaceutical manufacturing. Chem---Eur J 24:2776–2784. 10.1002/chem.201706004 [DOI] [PubMed]
- 64.Coley CW, Thomas DA, Lummiss JAM et al (2019) A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365:aax1566. 10.1126/science.aax1566 [DOI] [PubMed]
- 65.Fitzpatrick DE, Ley S (2016) Engineering chemistry: integrating batch and flow reactions on a single, automated reactor platform. React Chem Eng 1:629–635. 10.1039/c6re00160b
- 66.Fitzpatrick DE, Battilocchio C, Ley S (2016) A novel internet-based reaction monitoring, control and autonomous self-optimization platform for chemical synthesis. Org Process Res Dev 20:386–394. 10.1021/acs.oprd.5b00313
- 67.Sagmeister P, Ort FF, Jusner CE et al (2022) Autonomous multi-step and multi-objective optimization facilitated by real-time process analytics. Adv Sci 2105547:1–9. 10.1002/advs.202105547 [DOI] [PMC free article] [PubMed]
- 68.Steiner S, Wolf J, Glatzel S et al (2019) Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363:aav2211. 10.1126/science.aav2211 [DOI] [PubMed]
- 69.Mehr SHM, Craven M, Leonov AI et al (2020) A universal system for digitization and automatic execution of the chemical synthesis literature. Science 370:101–108. 10.1126/science.abc2986 [DOI] [PubMed]