In this final update of the living, rapid review on the role of antibodies after SARS-CoV-2 infection, the authors summarize the evidence on the durability of the antibody response and the level and duration of protection from previous infection on more current variants, including the Delta and Omicron variants.
Abstract
Background:
The durability of the antibody response after SARS-CoV-2 infection and the role of antibodies in protection against reinfection are unclear.
Purpose:
To synthesize evidence on the SARS-CoV-2 antibody response and reinfection risk with a focus on gaps identified in our prior reports.
Data Sources:
MEDLINE (Ovid), EMBASE, CINAHL, World Health Organization Research Database, and reference lists from 16 December 2021 through 8 July 2022, with surveillance through 22 August 2022.
Study Selection:
English-language, cohort studies evaluating IgG antibody duration at least 12 months after SARS-CoV-2 infection, the antibody response among immunocompromised adults, predictors of nonseroconversion, and reinfection risk.
Data Extraction:
Two investigators sequentially extracted study data and rated quality.
Data Synthesis:
Most adults had IgG antibodies after SARS-CoV-2 infection at time points greater than 12 months (low strength of evidence [SoE]). Although most immunocompromised adults develop antibodies, the overall proportion with antibodies is lower compared with immunocompetent adults (moderate SoE for organ transplant patients and low SoE for patients with cancer or HIV). Prior infection provided substantial, sustained protection against symptomatic reinfection with the Delta variant (high SoE) and reduced the risk for severe disease due to Omicron variants (moderate SoE). Prior infection was less protective against reinfection with Omicron overall (moderate SoE), but protection from earlier variants waned rapidly (low SoE).
Limitation:
Single review for abstract screening and sequential review for study selection, data abstraction, and quality assessment.
Conclusion:
Evidence for a sustained antibody response to SARS-CoV-2 infection is considerable for both Delta and Omicron variants. Prior infection protected against reinfection with both variants, but, for Omicron, protection was weaker and waned rapidly. This information may have limited clinical applicability as new variants emerge.
Primary Funding Source:
Agency for Healthcare Research and Quality. (PROSPERO: CRD42020207098)
In March 2021, we published the first version of a rapid, evolving, pragmatic review that described the antibody response in adults after an infection with SARS-CoV-2 (1, 2). In January 2022, we published a second review, meta-analysis, and data visualization (https://effectivehealthcare.ahrq.gov/products/immunity-after-covid/rapid-review) describing the risk for SARS-CoV-2 reinfection (3). Our objectives in conducting the original review were to assess the prevalence, level, and duration of the antibody response after infection; compare the risk for reinfection among those with a prior infection to persons who had never been infected; and examine the duration of protection against reinfection. We found that before the emergence of the Delta and Omicron variants, prior infection with the wild-type SARS-CoV-2 virus or the Alpha variant reduced the risk for reinfection by 80% to 97% (pooled estimate, 87% [95% CI, 84% to 90%]) compared with previously uninfected persons. Studies had a median follow-up of 8 months (range, 4 to 13 months), and protection remained above 80% for at least 7 months. There was sparse evidence on the duration of detectable antibodies beyond 6 months; whether the antibody response varied based on immunocompromised status or other factors, such as asymptomatic infection; and whether testing for SARS-CoV-2 antibodies provided clinically useful information about reinfection risk (that is, whether detectable antibodies correlated with protection).
This update examines evidence gaps identified in our previous 2 versions, with a focus on the persistence of IgG antibodies for longer than 12 months after infection, whether the antibody response varies in immunocompromised persons, and characteristics of those who do not seroconvert (key question [KQ] 1). We also evaluated available evidence regarding reinfection with Delta or Omicron variants after previous infection and the relation of antibody levels, symptoms status, and age to protection against reinfection (KQ2) as well as the duration of protection in the context of Delta and Omicron variants (KQ3).
Methods
Our protocol for this rapid, evolving, pragmatic review was developed with the American College of Physicians, registered at PROSPERO (CRD42020207098), and posted to the Agency for Healthcare Research and Quality (AHRQ) Effective Health Care website (4). We modified the scope of this update to address gaps identified in prior versions and account for the emergence of new variants and coinciding developments in SARS-CoV-2 immunity research. Methods are described in detail in our previous reports (1–3, 5), and Supplement 1 describes specific modifications for this update, including details on searches, study selection, quality assessment, data synthesis, and grading the strength of the body of evidence. Using the same search strategies as previous reports, we conducted an updated literature search for KQ1. For KQs 2 and 3, we searched the World Health Organization's COVID-19 Research Database using the search terms reinfection and Omicron. We used Google Scholar, study-specific websites, and citation lists of all newly identified articles about reinfection to find new publications from previously included cohort studies. Articles identified in searches through 22 August 2022 were eligible for inclusion in this update.
For KQs 2 and 3, we included publications that extended the results of the cohorts included in our original meta-analysis as well as newly identified retrospective or prospective cohort studies. Studies were included for KQ2 if they provided a protection estimate or data that allowed calculation of an estimate of the effect of previous infection on protection against any reinfection, symptomatic reinfection, and severe infection with the Delta or Omicron variants. Protection is calculated from the absolute risk difference (numerator) to give the proportion of reinfections prevented by previous infection (6):
(risk among previously infected − risk among not previously infected) / risk among not previously infected
We excluded studies that did not observe an uninfected, unvaccinated control cohort or did not present detailed adjusted or stratified results to characterize the effect of previous infection without vaccination. We also excluded publications if all data were collected before the emergence of the Delta variant and subsequent Delta wave. We prioritized publications (including preprints) that extended the length of follow-up of the cohorts included in our previous meta-analysis if they contributed new information about reinfection protection or duration. We also included new, published cohort studies that met our quality screening criteria. Test-negative case–control studies that did not extend the results of cohort studies in our original meta-analysis were not eligible for inclusion, but we examined their results to assess their concordance with the included studies.
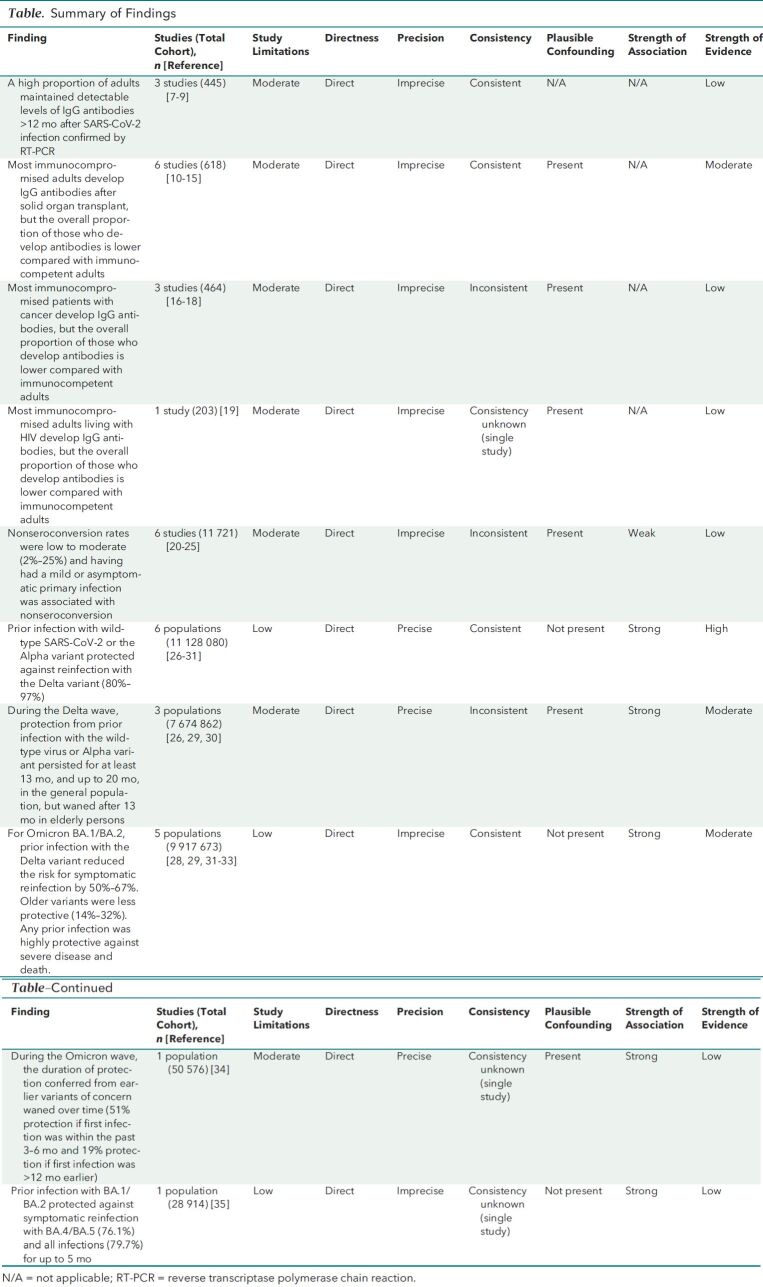
Details on study characteristics (Supplement 2), risk-of-bias assessments (Table 1 of Supplement 1), strength of evidence (SoE) (Table), and key findings (Tables 2 to 5 of Supplement 1) are provided in the supplemental materials and in the full AHRQ report (36).
Table.
Summary of Findings
Data Synthesis and Analysis
Evidence was synthesized qualitatively rather than quantitatively because of variability in study populations, outcomes, and the geographic distribution of circulating SARS-CoV-2 variants of concern. We assessed the SoE to describe our confidence in effect estimates as high, moderate, low, or insufficient. The assessment is based on our analysis of the study limitations, directness, precision, consistency, plausible confounding, and strength of association.
Role of the Funding Source
This work is based on a living, rapid review done for the AHRQ. The funding source assigned the topic and contributed to the development of the review aims and scope but was not involved in data collection, analysis, manuscript preparation, or submission.
Results
This update adds 29 observational studies to the evidence base (Appendix Figure). Our main findings are shown in the Table.
Appendix Figure. PRISMA Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram.
KQ = key question.
Durability of the Antibody Response
Immunoglobulin G Duration Greater Than 12 Months
In our first report (2), we found that IgG may remain detectable for at least 120 days, based on the study with the longest follow-up at the time (37). For this update, 3 longitudinal studies completed during the first year of the pandemic before vaccine availability met inclusion criteria; these studies had a median follow-up of at least 12 months (range, 12.7 to 14 months) (7–9).
Although a high proportion (83% to 97%) of adults had detectable IgG over the follow-up period in all 3 studies (Table 2 of Supplement 1), we have low confidence in this finding (low SoE) (Table). All studies were done early in the pandemic among adults who were mostly symptomatic during their primary infection, and we could not rule out the possibility that an asymptomatic or mild reinfection accounted for persistent antibodies. Results may not be generalizable to other settings or time periods or among adults with a mild or asymptomatic primary infection.
Immunocompromised Populations
In our original review, 3 observational studies provided insufficient evidence on the antibody response in immunocompromised populations. In this update, we identified 10 additional observational studies of the antibody response in immunocompromised patients compared with immunocompetent comparators: 3 studies in patients with cancer (16–18), 1 study in patients living with HIV (19), and 6 studies in patients who had undergone solid organ transplant (Table 3 of Supplement 1) (10–15). Immunoglobulin G antibodies were detected in most immunocompromised patients (≥65% at the first test after reverse transcriptase polymerase chain reaction diagnosis for all included studies, except for a single cohort study at just 15 days after infection, when IgG antibodies may not yet be detectable). However, IgG prevalence was consistently lower among immunocompromised patients compared with nonimmunocompromised control participants.
We are moderately confident that most adults who are immunocompromised due to solid organ transplant develop IgG antibodies after SARS-CoV-2 infection, but the overall proportion of those who develop antibodies is lower compared with immunocompetent control participants (moderate SoE) (Table). Findings were consistent and direct, although studies were small and had methodological limitations. We have low confidence that this finding is stable for patients with cancer and persons living with HIV given fewer studies overall and study methodological limitations (low SoE) (Table).
Nonseroconversion
We identified 4 prospective cohort studies (20–23) comparing characteristics of patients who did not seroconvert 6 weeks after documented SARS-CoV-2 infection with those who did seroconvert, adding to the evidence from 2 cohort studies (24, 25) identified in our first report (2) (Table 4 of Supplement 1). Across these studies, the proportion of persons who did not develop antibodies ranged from 2% to 25%. Having no or few symptoms was the most consistent factor associated with nonseroconversion. Higher minimum cycle thresholds with polymerase chain reaction testing (indicating lower viral load) were associated with nonseroconversion in 2 studies (21, 23).
Study methodological limitations give us low confidence in these findings (low SoE) (Table). We do not know to what extent the use of different immunoassays accounts for study variation. Moreover, participants could have been misclassified as not seroconverting depending on the timing of testing. Finally, the clinical significance of nonseroconversion is unclear. Persons who do not seroconvert after infection may still have a robust humoral response with repeated virus exposure because of immune memory (38).
Magnitude and Duration of Protection From Previous Infection (KQs 2 and 3)
Updates of 4 controlled, longitudinal cohort studies (26, 27, 28, 32, 34, 35, 39, 40) included in our previous meta-analysis (3) and 2 new cohort studies (29, 30, 41) contributed to estimates of protection against reinfection in the Delta and Omicron eras (Table 5 of Supplement 1). For the Delta variant, there was consistent, high-quality evidence that prior infection reduced the risk for reinfection by 80% to 97% (high SoE) (Table) (26–31). Longer follow-up for 3 of the cohorts suggested that, at least through the Delta wave, protection did not wane significantly for up to 13 months (moderate SoE) (Table) (26, 27, 29, 39). In the population-based study done in Qatar, prior infection before the emergence of the Omicron variant protected against another pre-Omicron infection by 85.5%, waning to approximately 70% by the 16th month.
Compared with earlier waves, the Omicron waves were associated with an early, marked increase in the proportion of infections that were reinfections (40–43). Subsequently, cohort studies confirmed that prior infection was less protective against reinfection with the Omicron variants (BA.1, BA.2, BA.4, and BA.5) than against reinfection with Delta and older variants (moderate SoE) (Table) (26, 28, 30, 32, 34, 39, 41).
Omicron BA.1 and BA.2
For Omicron BA.1 and BA.2, prior infection with the Delta variant reduced the risk for symptomatic infection by 50% to 67% (28, 31, 32, 39). Prior infection with older variants (for example, wild-type SARS-CoV-2 and the Alpha variant) was less protective against symptomatic infection (14% to 32%) and diminished more sharply over time. In the Qatar cohort, for example, protection against reinfection with Omicron BA.1 or BA.2 was higher among those with a recent Delta infection (approximately 60%) compared with all prior infections (39.8%) (39). In a Danish cohort study (28), protection against Omicron BA.1 or BA.2 was 43.1% if the previous infection occurred 3 to 6 months earlier and 22.2% if the previous infection had occurred at least 6 months earlier.
Omicron BA.4 and BA.5
Additional analyses in the Qatar population provided detailed information about protection against Omicron BA.4 and BA.5. Among unvaccinated persons, a previous infection with Omicron BA.1 or BA.2 reduced the risk for any infection with Omicron BA.4 or BA.5 by at least 68.7% (CI, 64.0% to 72.9%) compared with only 27.7% (CI, 19.3% to 35.2%) if the prior infection had occurred before the emergence of the Omicron variant (35). Included studies had no information about the duration of protection against Omicron BA.4 or BA.5.
Severe Disease
In unvaccinated persons, protection against severe disease with Omicron BA.1 or BA.2 was 87.8% to 90% in the Qatari cohort (35, 39) and 69.8% in the Danish cohort (28). In a multivariable analysis of a large U.K. cohort, previous infection provided moderate protection against hospitalization (55%) and very high protection against death (>80%) (30). Severe disease and death from Omicron were rare in the U.K. nursing home setting, and previous infection seemed to provide some protection (33). However, in a Cleveland Clinic cohort, protection against hospitalization was lower than in other cohorts (44.4%) (32). After adjustment for age, sex, reason for testing, and vaccination status, protection against hospitalization and intensive care unit admission was reduced to 30%. The poorer results for the Cleveland Clinic cohort may be related to a lower proportion of recent (Delta or Omicron BA.1 or BA.2) infections and a higher prevalence of major comorbidities than the population-based studies.
Role of Antibodies in Protection
Our previous report found that seroconversion was associated with substantial protection against reinfection (3), but antibody testing to predict reinfection risk provided no additional information over the more widely used reverse transcriptase polymerase chain reaction test, and the role of antibody testing in clinical practice, if any, was uncertain. Although there is still no definitive evidence to guide practice decisions about antibody testing, studies are underway to delineate reinfection risk with infection-induced antibodies compared with vaccine-induced antibodies (44, 45). The U.K. SIREN (SARS-CoV-2 Immunity and Reinfection Evaluation) study is scheduled to complete data collection in March 2023 (46).
Discussion
A central question of this review has been whether a SARS-CoV-2 antibody test obtained in everyday clinical practice provides useful information about a person's future risk for infection. In this update, we found that although the antibody response to SARS-CoV-2 infection in the Omicron era remains robust, protection against reinfection was lower.
The emergence of the Omicron variant, which evolved and spread despite high rates of vaccination and previous infection, has intensified interest in the capacity of SARS-CoV-2 variants to evade immune system protection. Recent infection with Delta or Omicron BA.1 or BA.2 seems to be protective against reinfection with Omicron for a few months but was lower than for previous variants and waned rapidly.
Although based on relatively few studies, our findings about protection against Omicron variants are likely to be robust. First, we prioritized large, well-conducted, controlled cohort studies, most of which used consistent methods throughout the entire pandemic. Second, our findings are concordant with those of test-negative case–control studies (47–50) as well as with recent cohort studies (51, 52) and preprints (53–57) identified by surveillance. In general, these studies confirm that protection against Omicron BA.1 or BA.2 from previous infection with the Delta or earlier variants was lower and waned more rapidly over time than for previous variants and that, whereas protection against BA.4 or BA.5 from BA.1 or BA.2 infection was robust for up to 4 months, this protection may wane rapidly (54). One preprint—a meta-analysis of cohort, case-negative case–control, and cross-sectional studies—confirmed that protection against death and severe infection was generally preserved (53).
The main implication of our findings about the antibody response and reinfection risk is that the presence of antibodies would be insufficient to estimate a person's degree of protection against reinfection. Although understanding population seroprevalence has important public health implications, the value of antibody testing in clinical practice remains unclear.
Supplementary Material
Footnotes
This article was published at Annals.org on 29 November 2022.
References
- 1. Mackey K, Arkhipova-Jenkins I, Armstrong C, et al. Antibody response following SARS-CoV-2 infection and implications for immunity: a rapid living review. (Prepared by the Portland VA Research Foundation under Contract No. 290-2017-00003-C). AHRQ Publication No. 21-EHC016. Agency for Healthcare Research and Quality; 2021. doi: 10.23970/AHRQEPCCOVIDIMMUNITY [DOI] [PubMed]
- 2. Arkhipova-Jenkins I , Helfand M , Armstrong C , et al. Antibody response after SARS-CoV-2 infection and implications for immunity. A rapid living review. Ann Intern Med. 2021;174:811-21. [PMID: ] doi: 10.7326/M20-7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helfand M , Fiordalisi C , Wiedrick J , et al. Risk for reinfection after SARS-CoV-2. A living, rapid review for American College of Physicians Practice Points on the role of the antibody response in conferring immunity following SARS-CoV-2 infection. Ann Intern Med. 2022;175:547-55. [PMID: ] doi: 10.7326/M21-4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agency for Healthcare Research and Quality. Immunity after COVID-19. Accessed at https://effectivehealthcare.ahrq.gov/products/immunity-after-covid/protocol on 1 November 2022.
- 5. Helfand M, Fiordalisi C, Wiedrick J, et al. Risk of reinfection from SARS-CoV-2—an update of an antibody response following SARS-CoV-2 infection and implications for immunity: a living rapid review. (Prepared by the Scientific Resource Center under Contract No. 290-2017-0003). AHRQ Publication No. 21(22)-EHC034. Agency for Healthcare Research and Quality; 2022. doi: 10.23970/AHRQEPCCOVIDIMMUNITY2 [DOI] [PubMed]
- 6. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Workforce and Career Development. Measures of risk. In: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Workforce and Career Development, eds. Principles of Epidemiology in Public Health Practice: An Introduction to Applied Epidemiology and Biostatistics. 3rd ed. U.S. Department of Health and Human Services; 2012:3-49-50. Accessed at www.cdc.gov/csels/dsepd/ss1978/ss1978.pdf on 1 November 2022.
- 7. Dehgani-Mobaraki P , Zaidi AK , Yadav N , et al. Longitudinal observation of antibody responses for 14 months after SARS-CoV-2 infection. Clin Immunol. 2021;230:108814. [PMID: ] doi: 10.1016/j.clim.2021.108814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haveri A, Ekström N, Solastie A, et al. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection. medRxiv. Preprint posted online 16 July 2021. doi: 10.1101/2021.07.13.21260426 [DOI] [PMC free article] [PubMed]
- 9. Kučinskaitė-Kodzė I , Simanavičius M , Šimaitis A , et al. Persistence of SARS-CoV-2-specific antibodies for 13 months after infection. Viruses. 2021;13:2313. [PMID: ] doi: 10.3390/v13112313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becchetti C , Broekhoven AGC , Dahlqvist G , et al. Humoral response to SARS-CoV-2 infection among liver transplant recipients. Gut. 2022;71:746-56. [PMID: ] doi: 10.1136/gutjnl-2021-326609 [DOI] [PubMed] [Google Scholar]
- 11. Caballero-Marcos A , Salcedo M , Alonso-Fernández R , et al; Spanish Society of Liver Transplantation (SETH). Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. Am J Transplant. 2021;21:2876-84. [PMID: ] doi: 10.1111/ajt.16599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Søfteland JM , Gisslén M , Liljeqvist JÅ , et al. Longevity of anti-spike and anti-nucleocapsid antibodies after COVID-19 in solid organ transplant recipients compared to immunocompetent controls. Am J Transplant. 2022;22:1245-52. [PMID: ] doi: 10.1111/ajt.16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Favà A , Donadeu L , Sabé N , et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21:2749-61. [PMID: ] doi: 10.1111/ajt.16570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Favà A , Donadeu L , Jouve T , et al. A comprehensive assessment of long-term SARS-CoV-2-specific adaptive immune memory in convalescent COVID-19 solid organ transplant recipients. Kidney Int. 2022;101:1027-38. [PMID: ] doi: 10.1016/j.kint.2021.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caballero-Marcos A , Citores MJ , Alonso-Fernández R , et al. Decreased long-term severe acute respiratory syndrome coronavirus 2-specific humoral immunity in liver transplantation recipients 12 months after coronavirus disease 2019. Liver Transpl. 2022;28:1039-50. [PMID: ] doi: 10.1002/lt.26389 [DOI] [PubMed] [Google Scholar]
- 16. Agarwal A, Baghmar S, Qureshi S, et al. Persistent antibody responses to SARS-CoV-2 infection in cancer patients: a single-center retrospective observational study. Indian J Med Paediatr Oncol. 2021;42:123-9. doi: 10.1055/s-0041-1733823 [DOI]
- 17. Marra A , Generali D , Zagami P , et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2021;32:113-9. [PMID: ] doi: 10.1016/j.annonc.2020.10.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cattaneo C , Cancelli V , Imberti L , et al. Production and persistence of specific antibodies in COVID-19 patients with hematologic malignancies: role of rituximab. Blood Cancer J. 2021;11:151. [PMID: ] doi: 10.1038/s41408-021-00546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y , Xiao Y , Wu S , et al. People living with HIV easily lose their immune response to SARS-CoV-2: result from a cohort of COVID-19 cases in Wuhan, China. BMC Infect Dis. 2021;21:1029. [PMID: ] doi: 10.1186/s12879-021-06723-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johannesen CK , Rezahosseini O , Gybel-Brask M , et al. Risk factors for being seronegative following SARS-CoV-2 infection in a large cohort of health care workers in Denmark. Microbiol Spectr. 2021;9:e0090421. [PMID: ] doi: 10.1128/Spectrum.00904-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masiá M , Telenti G , Fernández M , et al. SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response. Open Forum Infect Dis. 2021;8:ofab005. [PMID: ] doi: 10.1093/ofid/ofab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiruvengadam R , Chattopadhyay S , Mehdi F , et al; DBT India Consortium for COVID 19 Research. Longitudinal serology of SARS-CoV-2-infected individuals in India: a prospective cohort study. Am J Trop Med Hyg. 2021;105:66-72. [PMID: ] doi: 10.4269/ajtmh.21-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei J , Matthews PC , Stoesser N , et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat Commun. 2021;12:6250. [PMID: ] doi: 10.1038/s41467-021-26479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petersen LR , Sami S , Vuong N , et al. Lack of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a large cohort of previously infected persons. Clin Infect Dis. 2021;73:e3066-73. [PMID: ] doi: 10.1093/cid/ciaa1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Staines HM , Kirwan DE , Clark DJ , et al. IgG seroconversion and pathophysiology in severe acute respiratory syndrome coronavirus 2 infection. Emerg Infect Dis. 2021;27. [PMID: ] doi: 10.3201/eid2701.203074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim P , Gordon SM , Sheehan MM , et al. Duration of severe acute respiratory syndrome coronavirus 2 natural immunity and protection against the Delta variant: a retrospective cohort study. Clin Infect Dis. 2022;75:e185-90. [PMID: ] doi: 10.1093/cid/ciab999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hall V , Foulkes S , Insalata F , et al; SIREN Study Group. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-20. [PMID: ] doi: 10.1056/NEJMoa2118691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michlmayr D , Hansen CH , Gubbels SM , et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: a Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg Health Eur. 2022;20:100452. [PMID: ] doi: 10.1016/j.lanepe.2022.100452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nordström P , Ballin M , Nordström A . Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22:781-90. [PMID: ] doi: 10.1016/S1473-3099(22)00143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyberg T , Ferguson NM , Nash SG , et al; COVID-19 Genomics UK (COG-UK) consortium. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303-12. [PMID: ] doi: 10.1016/S0140-6736(22)00462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Altarawneh HN , Chemaitelly H , Hasan MR , et al. Protection against the Omicron variant from previous SARS-CoV-2 infection [Letter]. N Engl J Med. 2022;386:1288-90. [PMID: ] doi: 10.1056/NEJMc2200133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rothberg MB , Kim P , Shrestha NK , et al. Protection against the Omicron variant offered by previous severe acute respiratory syndrome coronavirus 2 infection: a retrospective cohort study. Clin Infect Dis. 2022;ciac604. [PMID: ] doi: 10.1093/cid/ciac604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krutikov M , Stirrup O , Nacer-Laidi H , et al; COVID-19 Genomics UK consortium. Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study. Lancet Healthy Longev. 2022;3:e347-55. [PMID: ] doi: 10.1016/S2666-7568(22)00093-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altarawneh HN , Chemaitelly H , Ayoub HH , et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med. 2022;387:21-34. [PMID: ] doi: 10.1056/NEJMoa2203965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altarawneh HN , Chemaitelly H , Ayoub HH , et al. Protective effect of previous SARS-CoV-2 infection against Omicron BA.4 and BA.5 subvariants. N Engl J Med. 2022;387:1620-22. [PMID: ] doi: 10.1056/NEJMc2209306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmer HK, Mackey K, Fiordalisi CV, et al. Antibody response following SARS-CoV-2 infection and implications for immunity: final update of a rapid, living review. (Prepared by the Scientific Resource Center under Contract No. 290-2017-0003). AHRQ Publication No. 22(23)-EHC037. Agency for Healthcare Research and Quality; 2022. doi: 10.23970/AHRQEPCCOVIDIMMUNITY3 [DOI] [PubMed]
- 37. Gudbjartsson DF , Norddahl GL , Melsted P , et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724-34. [PMID: ] doi: 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lustig Y , Mendelson E , Mandelboim M , et al. Existence of immunological memory response in true sero-negative individuals post COVID-19 molecular diagnosis. Clin Infect Dis. 2022. [PMID: ] doi: 10.1093/cid/ciac196 [DOI] [PubMed] [Google Scholar]
- 39. Chemaitelly H , Nagelkerke N , Ayoub HH , et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J Travel Med. 2022;taac109. [PMID: ] doi: 10.1093/jtm/taac109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 34. Accessed at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050236/technical-briefing-34-14-january-2022.pdf on 1 November 2022.
- 41. Ferguson N, Ghani A, Cori A, et al. Report 49: growth, population distribution and immune escape of Omicron in England. Imperial College London; 2021. Accessed at 10.25561/93038 on 1 November 2022. [DOI]
- 42. Washington State Department of Health. Reported COVID-19 reinfections in Washington State. Accessed at https://doh.wa.gov/sites/default/files/2022-02/421-024-ReportedReinfections.pdf on 1 November 2022.
- 43. Pulliam JRC , van Schalkwyk C , Govender N , et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376:eabn4947. [PMID: ] doi: 10.1126/science.abn4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castilla J , Lecea Ó , Martín Salas C , et al. Seroprevalence of antibodies against SARS-CoV-2 and risk of COVID-19 in Navarre, Spain, May to July 2022. Euro Surveill. 2022;27. [PMID: ] doi: 10.2807/1560-7917.ES.2022.27.33.2200619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zar HJ , MacGinty R , Workman L , et al. Natural and hybrid immunity following four COVID-19 waves: a prospective cohort study of mothers in South Africa. EClinicalMedicine. 2022;53:101655. [PMID: ] doi: 10.1016/j.eclinm.2022.101655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wallace S , Hall V , Charlett A , et al. Impact of prior SARS-CoV-2 infection and COVID-19 vaccination on the subsequent incidence of COVID-19: a multicentre prospective cohort study among UK healthcare workers - the SIREN (Sarscov2 Immunity & REinfection EvaluatioN) study protocol. BMJ Open. 2022;12:e054336. [PMID: ] doi: 10.1136/bmjopen-2021-054336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andeweg SP , de Gier B , Eggink D , et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. 2022;13:4738. [PMID: ] doi: 10.1038/s41467-022-31838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carazo S, Skowronski DM, Brisson M, et al. Protection against Omicron BA.2 reinfection conferred by primary Omicron or pre-Omicron infection with and without mRNA vaccination. medRxiv. Preprint posted online 27 June 2022. doi: 10.1101/2022.06.23.22276824 [DOI]
- 49. Cerqueira-Silva T , de Araujo Oliveira V , Paixão ES , et al. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat Commun. 2022;13:4154. [PMID: ] doi: 10.1038/s41467-022-31839-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lind ML, Robertson A, Silva J, et al. Effectiveness of primary and booster COVID-19 mRNA vaccination against infection caused by the SARS-CoV-2 Omicron variant in people with a prior SARS-CoV-2 infection. medRxiv. Preprint posted online 20 April 2022. doi: 10.1101/2022.04.19.22274056 [DOI]
- 51. Lin DY , Gu Y , Xu Y , et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. JAMA. 2022;328:1415-26. [PMID: ] doi: 10.1001/jama.2022.17876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Šmíd M, Berec L, Přibylová L, et al.. Protection by vaccines and previous infection against the Omicron variant of severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2022;226:1385-90. [PMID: ] doi: 10.1093/infdis/jiac161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of prior SARS-CoV-2 infection and hybrid immunity against Omicron infection and severe disease: a systematic review and meta-regression. medRxiv. Preprint posted online 4 October 2022. doi: 10.1101/2022.10.02.22280610 [DOI]
- 54. Malato J , Ribeiro RM , Fernandes E , et al. Rapid waning of protection induced by prior BA.1/BA.2 infection against BA.5 infection [Preprint]. med. Rxiv. 2022. [PMID: ] doi: 10.1101/2022.08.16.22278820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Malato J, Ribeiro RM, Leite PP, et al. Risk of BA.5 infection in persons exposed to prior SARS-CoV-2 variants. medRxiv. Preprint posted online 28 July 2022. doi: 10.1101/2022.07.27.22277602 [DOI] [PMC free article] [PubMed]
- 56. Nadig M, Niesen MJM, Lenehan P, et al. Individuals with recent prior SARS-CoV-2 infection are at reduced risk of Omicron infection and associated hospitalization. medRxiv. Preprint posted online 15 August 2022. doi: 10.1101/2022.08.10.22278641 [DOI]
- 57. Vicentini M, Venturelli F, Mancuso P, et al; The Reggio Emilia COVID-19 Working Group. Risk of SARS-CoV-2 reinfection by vaccination status, predominant variant, and time from previous infection: a cohort study in Italy. Lancet. Preprint posted online 9 June 2022. doi: 10.2139/ssrn.4132329 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.