Abstract
The calgranulins are a family of calcium- and zinc-binding proteins produced by neutrophils, monocytes, and other cells. Calgranulins are released during inflammatory responses and have antimicrobial activity. Recently, one of the calgranulins, human calgranulin C (CaGC), has been implicated as an important component of the host responses that limit the parasite burden during filarial nematode infections. The goal of this work was to test the hypothesis that human CaGC has biologic activity against filarial parasites. Brugia malayi microfilariae and adults were exposed in vitro to 0.75 to 100 nM recombinant human CaGC. Recombinant CaGC affected adult and larval parasites in a dose-dependent fashion. Microfilariae were more sensitive to the action of CaGC than were adult parasites. At high levels, CaGC was both macrofilariacidal and microfilariacidal. At lower levels, the percentage of parasites killed was dependent on the level of CaGC in the culture system. The larvae not killed had limited motility. The filariastatic effect of low-level CaGC was reversed when the CaGC was removed from the culture system. Immunohistochemical analysis demonstrated that human CaGC accumulated in the cells of the hypodermis-lateral chord of adult and larval parasites. The antifilarial activity of CaGC was not due to the sequestration of zinc. Thus, the cellular and molecular mechanisms that result in the production and release of CaGC in humans may play a key role in the regulation of filarial parasite numbers.
Filarial nematodes infect 140 million people worldwide, and nearly a billion people are at risk of infection (39). The mechanisms that induce and maintain the lymphatic, ocular, dermal, and renal pathology caused by infection with the filarial parasites Onchocerca volvulus, Wuchereria bancrofti, and Brugia malayi are poorly defined. In areas of endemic infection, a large majority of infected individuals maintain long-term chronic infections that persist for decades. However, there is evidence from longitudinal epidemiological and immunological surveys that certain people may develop immunity to parasite challenge (13, 38, 44). The idea that it is possible to generate a protective immune response against filarial parasites is supported by the results of animal experiments (1, 25, 48). Despite decades of investigation, the exact roles that the adaptive humoral and cellular immune responses play in pathogenesis and killing of the parasite are still not clear. In vitro, macrophages, neutrophils, and eosinophils are capable of killing larval parasites (7, 9, 23). However, there is no direct evidence that these cells play a role in killing in vivo. Defining the molecular mechanisms that mediate killing would be a significant aid in focusing efforts to develop an effective vaccine.
Although granulocytes have not been assigned a specific role in killing filarial parasites in vivo, previous studies have associated mononuclear infiltrates with adult worms (12, 31, 32). Also, proteins derived from human neutrophils and macrophages are intimately associated with adult parasites (14, 34) and are deposited adjacent to living adult worms residing in nodules (12, 14). One of these human granulocyte-derived proteins, defensin HNP1, is recognized by antibodies in patient sera and is implicated in playing a role in the hyperreactive form of onchocerciasis (Sowda) (14). Another human granulocyte-derived protein that has been found in parasite extracts is calgranulin C (CaGC) (34).
CaGC is a member of a family of calcium- and zinc-binding proteins that includes calgranulin A (CaGC; also known as MRP-8), calgranulin B (CaGB; also known as MRP-14) (3), the S100 proteins of the brain (2), calmodulin (2), and lactalbumin (36). CaGA, CaGB, and CaGC are expressed in neutrophils and monocytes (17). CaGC is also produced by keratocytes in the cornea (18, 22). CaGC makes up between 5 and 8% of the total neutrophil cytosolic protein (11, 47) and is 39% and 46% identical to CaGA and CaGB at the level of protein sequence, respectively.
Several studies have demonstrated that the CaGA and CaGB heterodimer (also known as calprotectin) has antimicrobial activity against Candida albicans (43) and a number of bacterial species (6). The antimicrobial activity of CaGC has not been tested. Given the association of calgranulins, specifically of CaGC, with filariae, we tested the hypothesis that CaGC has antifilarial activity. For these experiments, B. malayi was used as a model filarial parasite. Exposure of B. malayi in vitro demonstrated that a recombinant form of human CaGC had a dose-dependent effect on both larvae and adult parasites. At high concentrations of CaGC, the parasites were killed in as little as 5 min. At lower concentrations, CaGC was filariastatic, and this activity was reversible when the CaGC was removed from the culture system.
MATERIALS AND METHODS
Recombinant human CaGC.
The expression and purification of CaGC has been reported previously (19). Briefly, bacteria containing the pPROEXHT-CaGC expression plasmid were induced to express the recombinant CaGC with 1 mM isopropyl-β-d-thiogalactoside (IPTG). After 3 h at 37°C, the induced bacteria were lysed and the soluble form of the histidine-tagged CaGC was purified on a Ni-nitrilotriacetic acid column (Qiagen, Santa Clarita, Calif.) as specified by the manufacturer. The recombinant CaGC was specifically eluted from the column with 100 mM imidazole, dialyzed, and concentrated by ultrafiltration (UM-3 membrane; Amicon, Beverly, Mass.). The concentration of the fusion protein was determined with the Bradford protein reagent (Bio-Rad, Hercules, Calif.). The purity of the CaGC preparation, evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (28) followed by staining with silver salts, was estimated to be >95%.
CaGC immobilization and killing of adult and microfilarial B. malayi.
Microfilariae and adults of B. malayi were harvested from the peritoneal cavity of a chronically infected gerbil by peritoneal lavage (16). After the microfilariae and the adults were separated, they were washed and suspended in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Gaithersburg, Md.) containing 100 U of penicillin (Sigma, St. Louis, Mo.), 100 μg of streptomcin (Sigma) per ml, 0.25 μg of Fungizone (Life Technologies) per ml, and 1% glucose. About 50 microfilariae were transferred to the wells of a 96-well plate containing 200 μl of DMEM. Four adult parasites were place in wells of a 24-well plate containing 1.0 ml of DMEM. Recombinant CaGC was added to wells to achieve concentrations ranging from 0.75 to 100 nM. Each CaGC level was tested in triplicate, and experiments were repeated three times. Control wells contained parasites in medium only or parasites exposed to saline extracts of Escherichia coli proteins. The parasites were observed for motility at 5, 15, 30, 60, and 180 min and 18 h of culture. After 18 h, the CaGC-containing medium was removed and replaced with DMEM containing no CaGC, and the parasites were observed at 2-h intervals over the next 6 h.
Anti-CaGC.
Lewis rats were injected with 50 μg of recombinant CaGC protein emulsified in an equal volume of complete Freund's adjuvant (Difco, Detroit, Mich.). Three weeks later, the rats were challenged with 25 μg of CaGC emulsified in incomplete Freund's adjuvant. Sera were collected 2 weeks after challenge and pooled. Anti-E. coli antibodies were removed by incubating the sera with nitrocellulose membranes containing E. coli proteins.
Immunocytochemical analysis.
Adults and microfilariae were isolated from the peritoneal cavity of infected gerbils and washed. After incubation in recombinant CaGC (25 nM for 20 min at 37°C), the parasites were fixed for 18 h at 4°C in 4% paraformaldehyde. Controls included adults and microfilariae incubated under identical conditions without recombinant CaGC or microfilariae that were heat killed by exposure to 55°C for 15 min prior to exposure to the CaGC. The fixed larvae were pelleted by centrifugation, suspended in 30 μl of 1% low-melting-point agarose at 55°C, quickly transferred to a Beam capsule, and placed on ice. The worms were embedded in plastic, sectioned at 6 μm, immunostained as described previously (16), and counterstained with hematoxylin and eosin.
Zinc and CaGC activity.
Between 200 and 300 microfilariae were placed in the wells of a 24-well plate containing 100 μl of DMEM. The larvae were exposed to 50 μg of polyhistidine (18,400 kDa; Sigma) per ml; 20, 5, or 1.5 nM recombinant human CaGC; or 20, 5, or 1.5 nM His-tagged negative control protein (recombinant human endothelial-monocyte activating peptide) (28a). The mixture for each test condition was incubated with or without 10 μM ZnSO4. The microfilariae were scored at 10, 50, and 150 min of culture and reported as the percentage of organisms that remained motile.
RESULTS
Human CaGC is a macrofilaricide and a microfilaricide.
Adult female B. malayi parasites were exposed to recombinant human CaGC at levels ranging from 2.5 to 100 nM (Table 1). After 5 min of exposure, there was a dose-dependent effect on the motility of adult parasites. Although 80% of the adults exposed to 2.5, 5, and 25 nM CaGC remained motile at 5 min, the vigor and amplitude of movement were significantly reduced for all of the parasites. At 50 and 100 nM CaGC, 50 and 90% ceased their movement, respectively, and the remaining parasites moved in a sluggish fashion. This effect on worm movement was enhanced over time, so that by 120 min of exposure, only worms incubated in 2.5 or 5 nM CaGC were motile (Table 1). The vigorous movement at all time points of adult worms incubated in the presence of a saline extract of E. coli suggested that the macrofilaricidal activity of the human CaGC preparation was not due to contamination with a bacterium-derived toxin.
TABLE 1.
Effect of recombinant human CaGC on the motility of adult female B. malayi parasitesa
| Calgranulin C concn (nM) | Motility (%)a after
incubation for following time:
|
|||
|---|---|---|---|---|
| 5 | 30 | 60 | 180 | |
| 0 (E. coli) | 100 | 100 | 100 | 100 |
| 0 | 100 | 100 | 100 | 100 |
| 2.5 | 80 | 80 | 60 | 60 |
| 5.0 | 80 | 60 | 30 | 10 |
| 25.0 | 80 | 20 | 10 | 0 |
| 50.0 | 50 | 10 | 0 | 0 |
| 100.0 | 10 | 0 | 0 | 0 |
Each test condition was used in duplicate, and the experiment was repeated three times. Data presented are from a representative experiment.
The percentage of the 12 adult parasites per treatment group that remained motile.
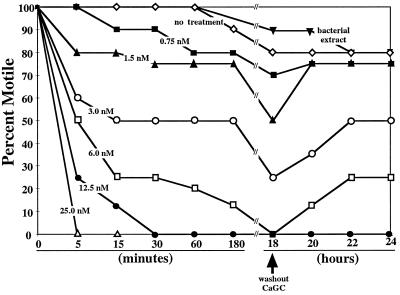
At levels as low as 0.75 nM, human CaGC inhibited the motility of B. malayi microfilariae (Fig. 1). At 5 min of exposure to levels between 1.5 and 25 nM CaGC, there was a dose-dependent inhibition of larval motility. After 5 min of exposure at 25 nM, none of the microfilariae showed any sign of movement. At 12.5 and 25 nM CaGC, the effect on microfilarial motility was almost immediate (data not shown). For 0.75, 1.5, and 3 nM, the level of inhibition increased only slightly between 5 min and 18 h of exposure. At 6 nM CaGC, the percentage of microfilariae that were motile progressively decreased over time to ∼85% inhibition at 180 min and 100% inhibition at 18 h. All of the larvae had ceased movement by 30 min of exposure at the highest levels of CaGC. As observed with the adult parasites, there was no significant inhibition of motility when the microfilariae were incubated in the presence of an E. coli extract.
FIG. 1.
Human CaGC is filariacidal and filariastatic to B. malayi microfilariae. Fifty B. malayi microfilariae were placed in culture and exposed to recombinant human CaGC at levels from 25 to 0.75 nM. In addition, parasites were incubated in medium with no human CaGC or in medium containing a crude extract of E. coli. The microfilariae were scored for motility at various times through 18 h of culture. At 18 h, the CaGC-containing medium was removed from the wells and replaced with fresh medium with no CaGC. The parasites were again scored for motility at 2-h intervals for an additional 6 h of culture. The data were expressed as the percentage of microfilariae that remained motile. Each datum point is the mean of triplicate readings for each test condition. The data are presented from a single representative experiment that was repeated three times.
For the results presented in Fig. 1, in order for a parasite to be scored as nonmotile it had to be completely motionless. However, it is important to note that the microfilariae exposed to CaGC between 3 and 12.5 nM and scored as motile did not demonstrate the rapid twisting movement of the control animals. These microfilariae took on a rod-like morphology, with movement restricted to a weak flexing of the head and tail or a slow bending at midbody (data not shown).
This altered motility phenotype suggested that it was possible that CaGC was acting as a microfilariastatic agent. To test this idea, microfilariae incubated for 18 h were washed extensively to remove the CaGC from the system and then replated in CaGC-free medium. For levels of CaGC of 6 nM and lower, a percentage of the parasites regained their motility (Fig. 1). None of the larvae incubated with 12.5 or 25 nM CaGC for 18 h regained their motility. A similar concentration-dependent ability to rescue motility was seen for adult parasites (data not shown).
Therefore, it appears that human CaGC can be both filariacidal and filariastatic, depending on the concentration of CaGC used and the time of exposure.
CaGC localizes to the hypodermis-lateral chord.
Immunocytochemical staining of adult female B. malayi parasites after a 20-min exposure to 25 nM recombinant human CaGC demonstrated that CaGC accumulated in the hypodermal-lateral chord cells (Fig. 2A and C). In contrast, control worms, incubated under identical conditions without CaGC, had no staining (Fig. 2B). There was no evidence that CaGC was associated with the surface of the parasite.
FIG. 2.
Immunolocalization of CaGC in adult female B. malayi parasites exposed to 25 nM recombinant human CaGC (A and C) or medium without CaGC (B) for 20 min. After exposure, the adult parasites were fixed, processed for plastic embedding, sectioned, incubated with a rat anti-human CaG, and counterstained with hematoxylin and eosin as outlined in Materials and Methods. Immunostaining was restricted to the hypodermis-lateral chord. Additional negative controls included incubation of tissue sections from test and control parasites with normal rat serum, an irrelevant antibody (rat anti-human serum albumin), or no antibody (conjugate control). The results from all of the negative control reactions were negative (data not shown). U, uterus.
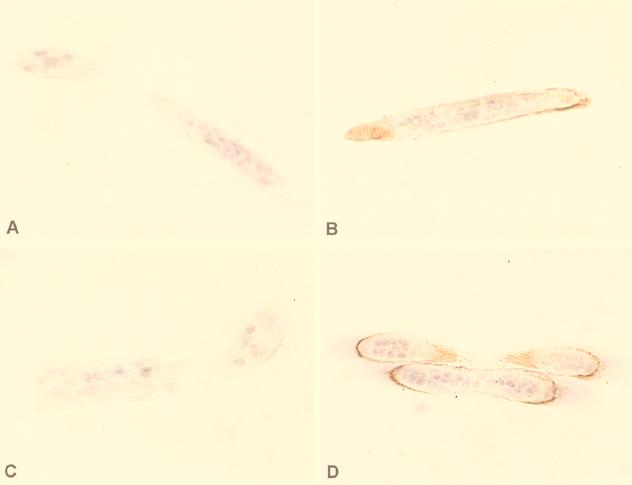
Microfilariae exposed to 25 nM CaGC for 20 min stained positive for CaGC at or near the surface (Fig. 3B and D). It was not possible to determine if CaGC localization was restricted to the hypodermis, as seen in the adults. Microfilariae killed by heat (Fig. 3C), sodium azide, or nitric oxide (data not shown) prior to exposure to CaGC had only trace amounts of CaGC uptake (Fig. 3C), suggesting that the CaGC uptake by microfilariae may be dependent on worm metabolism. There was no staining of larvae incubated under the same conditions without CaGC (Fig. 3A).
FIG. 3.
Immunolocalization of CaGC in B. malayi microfilariae exposed to 25 nM recombinant human CaGC (B and D) or medium without CaGC (A) for 20 min. After exposure, the microfilariae were fixed, processed for plastic embedding, sectioned, incubated with a rat anti-human CaG, and counterstained with hematoxylin and eosin as outlined in Materials and Methods. As an additional control, microfilariae were also heat killed prior to exposure to CaGC (C). In addition, sections of test and control parasites were reacted with normal rat serum, an irrelevant antibody (rat anti-human serum albumin), or no antibody (conjugate control). All of these controls were uniformly negative (data not shown).
Zinc does not inhibit the microfilariacidal activity of CaGC.
The calgranulins are both calcium- and zinc-binding proteins (10, 30). Several reports strongly link zinc binding to the antimicrobial activity of calprotectin (10, 30, 43). In addition, Loomans et al. (30) have demonstrated that polyhistidine, which can also bind zinc, can act as an antimicrobial agent. Because the recombinant CaGC used in these studies had a zinc-binding motif at the C terminus of the protein and carried a polyhistidine tag, it was important to determine if the filariacidal and filariastatic properties of recombinant CaGC were mediated by zinc binding.
Microfilariae were exposed to 20, 5, or 1.5 nM CaGC in the presence or absence of 10 μM zinc for up to 150 min (data not shown). Under these in vitro conditions, zinc did not change the ability of recombinant human CaGC to inhibit microfilarial motility in a dose-dependent fashion. In addition, the presence of polyhistidine or polyhistidine plus 10 μM zinc did not effect microfilarial motility (data not shown). The use of recombinant EMAP, a histidine-tagged protein with a mass and charge similar to CaGC, also did not significantly effect microfilarial motility. These results indicate that the filariacidal and filariastatic activity of CaGC is not mediated by zinc.
DISCUSSION
Filarial nematodes present a formidable challenge to the immune system. In addition to their size and constant movement, a majority of their cellular components are protected by a thick (∼2- to 4-μm) and dense acellular matrix, the cuticle, composed mainly of highly cross-linked collagens (5). The physical nature of this interface between the parasite and the host appears to restrict the options that the immune system has for the control of parasite numbers. It is thought that antibody-dependent cellular cytotoxicity leading to the generation and release of toxic molecules by granulocytes (7, 23) and the generation of nitric oxide by lymphokine-activated cells (40, 45, 46) are the main mechanisms used by the host to eliminate adult and larval parasites.
The results presented here on the nematoxic and nemastatic activity of CaGC, a protein released by neutrophils and monocytes, support the idea that granulocytes can play a role in the elimination of filarial parasites in vivo. CaGC at two levels affected filarial parasites. At high levels, CaGC killed both adult parasites and microfilariae. At lower levels, it significantly reduced the motility of the parasites. The filariastatic effect was in part reversible when the CaGC was removed. This concentration-dependent effect on filariae was similar to the activity reported for calprotectin (the CaGA-CaGB heterodimer) in its killing and inhibitory activity on yeast and bacteria (6) and mimicked the recovery of filarial larvae from the static effect of low levels of nitric oxide (45).
The mechanism through which CaGC exerts its filariacidal and filariastatic activity is not known. CaGC, like CaGA and CaGB, has two calcium-binding motifs and a single zinc-binding motif (11, 34). The positions and spacing of these cation-binding motifs are conserved within the calgranulins. CaGC binds both calcium and zinc ions (11). However, in contrast to the CaGA-CaGB heterodimer, where zinc binding appears to be key to its antimicrobial activity (10, 30, 43), the results presented here suggest that zinc is not involved in the mechanism of CaGC action of filarial parasites.
CaGC has several structural similarities to and appears to be coregulated with CaGA and CaGB in granulocytes (11). Therefore, it is possible that the mechanism of action of CaGC on filarial parasites can be extrapolated from the roles ascribed to CaGA and CaGB in the cell biology of granulocytes. Recent studies have demonstrated that CaGA and CaGB participate in calcium-dependent interactions between cytoskeletal proteins and cell membranes in neutrophils (24), monocytes (42) and epithelial cells (17) that result in changes in cell shape and motility. Indeed, the action of CaGA and CaGB on granulocyte motility led to their initial designation as migration inhibitory factor-related proteins, MRP-8 and MRP-14, respectively. The CaGA and CaGB heterodimer has also been shown to modulate kinase activity (37), inhibit cell growth (37), and induce apoptosis (49). It may be that CaGC interacts with important cytoskeletal components or regulatory proteins in the parasite and that this results in the inhibition of normal cell and tissue function. The association of CaGC with the cells of the hypodermis-lateral chord of Brugia (Fig. 2) localizes CaGC to a cellular compartment known to be important in the synthesis and maintenance of multiple systems in nematodes (4). Identifying the parasite-derived molecules that interact with or are regulated by CaGC may provide a new class of potent drug targets.
The results of the immunohistochemical staining demonstrated that after a 20-min incubation in vitro, CaGC localizes in the cells of the hypodermis-lateral chord. These findings are consistent with the results of Marti et al. (34), who described the isolation of host-derived CaGC from crude extracts of O. volvulus adults. The results are also consistent with the immunocytochemical observations of Edgeworth et al., who reported that high levels of CaGA and CaGB surround nodule-bound adult O. volvulus and that calgranulin protein was associated with the external layers of adult parasites and microfilariae (12).
The association of CaGC with a destructive corneal disease, Mooren's ulcer, raises the possibility that CaGC plays a role in filaria-induced pathology. Mooren's ulcer is an ulcerative disease of the peripheral cornea that is believed to have an autoimmune etiology (17, 19). Patients with Mooren's ulcer have autoantibodies against a cornea-associated antigen (20, 21). This antigen has been purified from corneal extracts, sequenced, and found to be identical to CaGC (20, 21, 29). The expression of CaGC in corneal keratocytes is up regulated by the proinflammatory cytokines tumor necrosis factor alpha and interleukin-1 and is believed to be one of the self antigens that mediate Mooren's ulcer formation (18). It is interesting to speculate that CaGC may play a role in mediating certain aspects of the chronic pathology associated with filarial infections. Onchocercal keratitis, or river blindness, is related to corneal invasion by microfilariae. When the microfilariae die in the cornea, they incite an inflammatory response (15, 41). The long-term consequence of this corneal exposure to parasites is the development of punctate keratitis and progressive sclerosing keratitis, which, like the pathology in the posterior chamber of the eye (8, 35), may have an autoimmune component to their etiology (8, 35). The association of CaGC with two destructive corneal diseases, Mooren's ulcer and onchocerciasis, combined with the observations that Mooren's ulcer has been associated with filarial infections in India and Nigeria (26, 27, 33), implicates CaGC as a target for autoimmune responses that lead to corneal disease.
The constant movement exhibited by filariae is likely to be one of the important mechanisms used by nematodes to avoid attack by the host immune response. The vigorous movement exhibited by filarial parasites in the mammalian host prevents cognate interactions between the parasite and immune effector cells, so that toxic molecules cannot be delivered at close range, and, for certain stages, allows the parasite to move away from toxic environments. In addition to its nematoxic activity, CaGC may contribute to killing by immobilizing parasites so that cells of the immune system can deliver a lethal blow. In this regard, the ability of cells in the cornea to produce CaGC during inflammatory responses (18) may actually be detrimental to the host during an onchocercal infection. Although Onchocerca microfilariae are freely mobile in the eye, larvae that enter the cornea are immobilized and incite a localized inflammatory response (15, 41). Ridley reported that in examining some 1,000 patients with onchocerciasis, only 1 patient had a motile worm in the cornea (41). CaGC-mediated microfilarial immobilization in the cornea may be an important component of the pathogenesis of onchocerciasis.
ACKNOWLEDGMENTS
This work was supported by grants from the National Eye Institute (NEI EY11096 to J.D.G.) and the World Health Organization (T23/181.80). Parasites were supplied under the auspices of an NIAID supply contract (AI 02642), U.S./Japan Cooperative Medical Science Program.
We thank Brian Schofield and Judy Corum for valuable assistance in carrying out the immunohistocytochemistry.
REFERENCES
- 1.Abraham D, Grieve R B, Mika Grieve M, Seibert B P. Active and passive immunization of mice against larval Dirofilaria immitis. J Parasitol. 1988;74:275–282. [PubMed] [Google Scholar]
- 2.Baudier J, Haglid K, Haiech J, Gerard D. Zinc ion binding to human brain calcium binding proteins, calmodulin and S100b protein. Biochem Biophys Res Commun. 1983;114:1138–1146. doi: 10.1016/0006-291x(83)90681-2. [DOI] [PubMed] [Google Scholar]
- 3.Berntzen H B, Fagerhol M K. L1, a major granulocyte protein; isolation of high quantities of its subunits. Scand J Clin Lab Investig. 1990;50:769–774. doi: 10.1080/00365519009091071. [DOI] [PubMed] [Google Scholar]
- 4.Bird A F, Bird J. The structure of nematodes. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1991. [Google Scholar]
- 5.Blaxter M L, Robertson W M. The cuticle. In: Perry R N, Wright D J, editors. The physiology and biochemistry of free-living and plant-parasitic nematodes. CBA International.; 1998. pp. 25–48. [Google Scholar]
- 6.Brandtzaeg P, Gabrielsen T O, Dale I, Muller F, Steinbakk M, Fagerhol M K. The leucocyte protein L1 (calprotectin): a putative nonspecific defence factor at epithelial surfaces. Adv Exp Med Biol. 1995;371A:201–206. doi: 10.1007/978-1-4615-1941-6_41. [DOI] [PubMed] [Google Scholar]
- 7.Brattig N W, Tischendorf F W, Strote G, Medina de la Garza C E. Eosinophil-larval-interaction in onchocerciasis: heterogeneity of in vitro adherence of eosinophils to infective third and fourth stage larvae and microfilariae of Onchocerca volvulus. Parasite Immunol. 1991;13:13–22. doi: 10.1111/j.1365-3024.1991.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 8.Braun G, McKechnie N M, Connor V, Gilbert C E, Engelbrecht F, Whitworth J A, Taylor D W. Immunological crossreactivity between a cloned antigen of Onchocerca volvulusand a component of the retinal pigment epithelium. J Exp Med. 1991;174:169–177. doi: 10.1084/jem.174.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterworth A E. The eosinophil and its role in immunity to helminth infection. Curr Top Microbiol Immunol. 1977;77:127–168. doi: 10.1007/978-3-642-66740-4_5. [DOI] [PubMed] [Google Scholar]
- 10.Clohessy P A, Golden B E. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol. 1995;42:551–556. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 11.Dell'Angelica E C, Schleicher C H, Santome J A. Primary structure and binding properties of calgranulin C, a novel S100-like calcium-binding protein from pig granulocytes. J Biol Chem. 1994;269:28929–28936. [PubMed] [Google Scholar]
- 12.Edgeworth J D, Abiose A, Jones B R. An immunohistochemical analysis of onchocercal nodules: evidence for an interaction between macrophage MRP8/MRP14 and adult Onchocerca volvulus. Clin Exp Immunol. 1993;92:84–92. doi: 10.1111/j.1365-2249.1993.tb05952.x. . (Erratum, 96:177, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elson L H, Calvopina M, Paredes W, Araujo E, Bradley J E, Guderian R H, Nutman T B. Immunity to onchocerciasis: putative immune persons produce a Th1-like response to Onchocerca volvulus. J Infect Dis. 1995;171:652–658. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 14.Gallin M Y, Jacobi A B, Buttner D W, Schonberger O, Marti T, Erttmann K D. Human autoantibody to defensin: disease association with hyperreactive onchocerciasis (sowda) J Exp Med. 1995;182:41–47. doi: 10.1084/jem.182.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garner A. Pathology of ocular onchocerciasis: human and experimental. Trans R Soc Trop Med Hyg. 1976;70:374–377. doi: 10.1016/0035-9203(76)90113-9. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh I, Eisinger S W, Raghavan N, Scott A L. Thioredoxin peroxidases from Brugia malayi. Mol Biochem Parasitol. 1998;91:207–220. doi: 10.1016/s0166-6851(97)00213-2. [DOI] [PubMed] [Google Scholar]
- 17.Goebeler M, Roth J, van den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J. 1995;309:419–424. doi: 10.1042/bj3090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottsch J D, Li Q, Ashraf F, O'Brien T P, Stark W J, Liu S H. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. 1999;91:34–40. doi: 10.1006/clim.1998.4681. [DOI] [PubMed] [Google Scholar]
- 19.Gottsch J D, Liu S H. Cloning and expression of human corneal calgranulin C (CO-Ag) Curr Eye Res. 1998;17:870–874. doi: 10.1076/ceyr.17.9.870.5136. [DOI] [PubMed] [Google Scholar]
- 20.Gottsch J D, Liu S H, Minkovitz J B, Goodman D F, Srinivasan M, Stark W J. Autoimmunity to a cornea-associated stromal antigen in patients with Mooren's ulcer. Investig Ophthalmol Visual Sci. 1995;36:1541–1567. [PubMed] [Google Scholar]
- 21.Gottsch J D, Liu S H, Stark W J. Mooren's ulcer and evidence of stromal graft rejection after penetrating keratoplasty. Am J Ophthalmol. 1992;113:412–417. doi: 10.1016/s0002-9394(14)76164-1. [DOI] [PubMed] [Google Scholar]
- 22.Gottsch J D, Stark W J, Liu S H. Cloning and sequence analysis of human and bovine corneal antigen (CO-Ag) cDNA: identification of host-parasite protein calgranulin C. Trans Am Ophthalmol Soc. 1997;95:111–125. [PMC free article] [PubMed] [Google Scholar]
- 23.Greene B M, Taylor H R, Aikawa M. Cellular killing of microfilariae of Onchocerca volvulus: eosinophil and neutrophil-mediated immune serum-dependent destruction. J Immunol. 1981;127:1611–1618. [PubMed] [Google Scholar]
- 24.Guignard F, Mauel J, Markert M. Identification and characterization of a novel human neutrophil protein related to the S100 family. Biochem J. 1995;309:395–401. doi: 10.1042/bj3090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horii Y, Nakanishi H, Mori A, Ueda M, Kurokawa K, Zaitsu M, Oda T, Fujita K. Induction of protective immunity to Brugia pahangiin jirds by drug-abbreviated infection. J Helminthol. 1992;66:147–154. doi: 10.1017/s0022149x00012748. [DOI] [PubMed] [Google Scholar]
- 26.Keitzman B. Mooren's ulcer in Nigeria. Am J Ophthalmol. 1968;65:679–685. doi: 10.1016/0002-9394(68)94381-x. [DOI] [PubMed] [Google Scholar]
- 27.Kuriakose E T. Mooren's ulcer etiology and treatment. Am J Ophthalmol. 1963;55:1064–1069. [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28a.Liu, S. Unpublished observations.
- 29.Liu S H, Gottsch J D. Amino acid sequence of an immunogenic corneal stromal protein. Investig Ophthalmol Visual Sci. 1996;37:944–948. [PubMed] [Google Scholar]
- 30.Loomans H J, Hahn B L, Li Q Q, Phadnis S H, Sohnle P G. Histidine-based zinc-binding sequences and the antimicrobial activity of calprotectin. J Infect Dis. 1998;177:812–814. doi: 10.1086/517816. [DOI] [PubMed] [Google Scholar]
- 31.Mackenzie C D, Oxenham S L, Gatrill A, Andrew S, Grennan D, Denham D A. Mononuclear and multinuclear macrophages in filarial infections. Immunol Lett. 1985;11:239–246. doi: 10.1016/0165-2478(85)90174-9. [DOI] [PubMed] [Google Scholar]
- 32.Mackenzie C D, Oxenham S L, Liron D A, Grennan D, Denham D A. The induction of functional mononuclear and multinuclear macrophages in murine brugian filariasis: morphological and immunological properties. Trop Med Parasitol. 1985;36:163–170. [PubMed] [Google Scholar]
- 33.Majekodunmi A A. Ecology of Mooren's ulcer in Nigeria. Doc Ophthalmol. 1980;49:211–219. doi: 10.1007/BF01886619. [DOI] [PubMed] [Google Scholar]
- 34.Marti T, Erttmann K D, Gallin M Y. Host-parasite interaction in human onchocerciasis: identification and sequence analysis of a novel human calgranulin. Biochem Biophys Res Commun. 1996;221:454–458. doi: 10.1006/bbrc.1996.0616. [DOI] [PubMed] [Google Scholar]
- 35.McKechnie N M, Braun G, Connor V, Klager S, Taylor D W, Alexander R A, Gilbert C E. Immunologic cross-reactivity in the pathogenesis of ocular onchocerciasis. Investig Ophthalmol Visual Sci. 1993;34:2888–2902. [PubMed] [Google Scholar]
- 36.Murakami K, Berliner L J. A distinct zinc binding site in the alpha-lactalbumins regulates calcium binding. Is there a physiological role for this control? Biochemistry. 1983;22:3370–3374. doi: 10.1021/bi00283a010. [DOI] [PubMed] [Google Scholar]
- 37.Murao S, Collart F, Huberman E. A protein complex expressed during terminal differentiation of monomyelocytic cells is an inhibitor of cell growth. Cell Growth Differ. 1990;1:447–454. [PubMed] [Google Scholar]
- 38.Nutman T B, Steel C, Ward D J, Zea Flores G, Ottesen E A. Immunity to onchocerciasis: recognition of larval antigens by humans putatively immune to Onchocerca volvulusinfection. J Infect Dis. 1991;163:1128–1133. doi: 10.1093/infdis/163.5.1128. [DOI] [PubMed] [Google Scholar]
- 39.Ottesen E A, Ramachandran C P. Lymphatic filariasis. Infection and disease: control strategies. Parasitol Today. 1995;11:129–131. [Google Scholar]
- 40.Rajan T V, Porte P, Yates J A, Keefer L, Shultz L D. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun. 1996;64:3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridley H. Ocular onchocerciasis, including an investigation in the Gold Coast. Br J Ophthalmol Monograph Suppl. 1945;10:58. doi: 10.1136/bjo.29.suppl.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood. 1993;82:1875–1883. [PubMed] [Google Scholar]
- 43.Santhanagopalan V, Hahn B L, Sohnle P G. Resistance of zinc-supplemented Candida albicanscells to the growth inhibitory effect of calprotectin. J Infect Dis. 1995;171:1289–1294. doi: 10.1093/infdis/171.5.1289. [DOI] [PubMed] [Google Scholar]
- 44.Steel C, Guinea A, Ottesen E A. Evidence for protective immunity to bancroftian filariasis in the Cook Islands. J Infect Dis. 1996;174:598–605. doi: 10.1093/infdis/174.3.598. [DOI] [PubMed] [Google Scholar]
- 45.Taylor M J, Cross H F, Mohammed A A, Trees A J, Bianco A E. Susceptibility of Brugia malayi and Onchocerca lienalismicrofilariae to nitric oxide and hydrogen peroxide in cell-free culture and from IFN gamma-activated macrophages. Parasitology. 1996;112:315–322. doi: 10.1017/s0031182000065835. [DOI] [PubMed] [Google Scholar]
- 46.Thomas G R, McCrossan M, Selkirk M E. Cytostatic and cytotoxic effects of activated macrophages and nitric oxide donors on Brugia malayi. Infect Immun. 1997;65:2732–2739. doi: 10.1128/iai.65.7.2732-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, deVeer M J, Gardiner E E, Devenish R J, Handley C J, Underwood J R, Robinson H C. Rabbit polymorphonuclear neutrophils form 35S-labeled S-sulfo-calgranulin C when incubated with inorganic [35S]sulfate. J Biol Chem. 1996;271:19802–19809. doi: 10.1074/jbc.271.33.19802. [DOI] [PubMed] [Google Scholar]
- 48.Yates J A, Higashi G I. Brugia malayi: vaccination of jirds with 60cobalt-attenuated infective stage larvae protects against homologous challenge. Am J Trop Med Hyg. 1985;34:1132–1137. doi: 10.4269/ajtmh.1985.34.1132. [DOI] [PubMed] [Google Scholar]
- 49.Yui S, Mikami M, Yamazaki M. Induction of apoptotic cell death in mouse lymphoma and human leukemia cell lines by a calcium-binding protein complex, calprotectin, derived from inflammatory peritoneal exudate cells. J Leukoc Biol. 1995;58:650–658. doi: 10.1002/jlb.58.6.650. [DOI] [PubMed] [Google Scholar]