Abstract
Peritonitis with Candida albicans is an important complication of bowel perforation and continuous ambulatory peritoneal dialysis. To define potential virulence factors, we investigated 50 strains of C. albicans in a murine peritonitis model. There was considerable variation in their virulence in this model when virulence was measured as release of organ-specific enzymes into the plasma of infected mice. Alanine aminotransferase (ALT) and α-amylase (AM) were used as parameters for damage of the liver and pancreas, respectively. The activities of ALT and AM in the plasma correlated with invasion into the organs measured in histologic sections and the median germ tube length induced with serum in vitro. When the activity of proteinases was inhibited in vivo with pepstatin A, there was a significant reduction of ALT and AM activities. This indicates that proteinases contributed to virulence in this model. Using strains of C. albicans with disruption of secreted aspartyl proteinase gene SAP1, SAP2, SAP3, or SAP4 through SAP6 (collectively referred to as SAP4-6), we showed that only a Δsap4-6 triple mutant induced a significantly reduced activity of ALT in comparison to the reference strain. In contrast to the Δsap1, Δsap2, and Δsap3 mutants, the ALT induced by the Δsap4-6 mutant could not be further reduced by pepstatin A treatment, which indicates that Sap4-6 may contribute to virulence in this model.
Candida albicans is a dimorphic fungus that has been the focus of considerable interest because of the increased incidence of candidiasis in immunocompromised hosts (16, 17, 34). It may cause a broad spectrum of diseases ranging from mucocutaneous infection to systemic infection with dissemination into parenchymal organs. Furthermore, C. albicans may cause peritonitis when it reaches the peritoneal cavity by iatrogenic inoculation with contaminated plastic devices and fluids during continuous ambulatory peritoneal dialysis (CAPD) (15, 26). The reported incidence of fungal peritonitis varies between 5 and 15% of all peritonitis episodes complicating CAPD (24). Peritonitis caused by C. albicans may also occur after pancreatic transplantation, in the course of severe pancreatitis or bowel perforation (2, 4).
There are several putative virulence factors of C. albicans, including the ability to form germ tubes, adherence to host cells, and secretion of proteinases (8, 14, 29). As a polymorphic organism, C. albicans can grow as a spherical yeast form or germinate and produce filaments which may appear as pseudohyphae or hyphae (22). Germ tubes have been shown to contribute to virulence in models of skin and mucosal infections and to disseminated candidiasis produced by intravenous (i.v.) infection (9, 35, 36).
Secreted aspartyl proteinases have also been demonstrated to play a role in C. albicans infections (10, 11, 18, 20, 32, 33). There are at least nine Sap isoenzymes produced by C. albicans (27, 28). The in vitro expression pattern of these isoenzymes suggests that the members of the SAP gene family may have distinct roles during Candida infection at different sites of the body (18). Saps may have auxiliary roles in promoting adhesion, as shown in studies using models of adherence of C. albicans to human mucosa (5) and cultured epidermal keratinocytes, where adherence was inhibited by the proteinase inhibitor pepstatin A (31). The relevance of the individual virulence factors depends on the infection model used (9).
The aim of our study was to investigate the contribution of germ tubes and Saps to the pathogenesis of peritonitis by C. albicans in order to describe virulence attributes which might be of importance also in humans. We used intraperitoneal (i.p.) infection of adult BALB/c mice with C. albicans. Fungal invasion from the peritoneal cavity into the parenchymal organs including the liver and pancreas was visualized by histology. The degree of tissue damage induced by strains of C. albicans was quantified via determination of activity of the hepatocyte-specific enzyme alanine aminotransferase (ALT) and the pancreatic enzyme α-amylase (AM) in the plasma. ALT is a soluble enzyme localized in the cytoplasm of hepatocytes which is released during damage of the cytoplasmic membrane. AM is localized in the secretory epithelia of glands, including the pancreas. The activities of ALT and AM detected in the blood plasma are measures of liver and pancreatic damage, respectively. ALT activity not only may be used in human infections but also is a suitable parameter for estimation of liver cell damage in mice (23). In our peritonitis model, we correlated the ALT and AM induced by clinical isolates of C. albicans with the invasion of the liver and pancreas measured by histology and the in vitro production of germ tubes. Furthermore, we investigated the role of Saps by inhibition of the proteinase activity in vivo with pepstatin A and by strains of C. albicans with disruptions of the genes SAP1, SAP2, SAP3, and SAP4 to SAP6.
MATERIALS AND METHODS
Strains.
C. albicans ATCC 10231 was obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The Δsap1, Δsap2, and Δsap3 null mutants were produced as described elsewhere (20), using the Ura− strain CAI4. The mutant with a triple disruption of the SAP4 to SAP6 genes (Δsap4-6 mutant) was produced as described elsewhere (33), using the Ura− strain CAF4-2. For all virulence tests, Δsap::hisG/Δsap::hisG::URA3::hisG (Ura+) mutants were used (20). C. albicans SC5314, the parent strain of CAF4-2 (33), was used as a reference strain for animal experiments. Fifty clinical isolates were obtained from mucosal sites of patients attending our hospital. The strains were cultured for 18 h at 30°C in Sabouraud broth containing 2% (wt/vol) glucose (Merck, Darmstadt, Germany). Under these conditions, all C. albicans strains grew in the yeast form and no germ tubes were detected microscopically (not shown). For animal experiments, the yeasts were washed three times in phosphate-buffered saline (PBS; Gibco, Eggenstein, Germany), which was composed of, per liter, 0.2 g of KCl, 0.2 g of KH2PO4, 8 g of NaCl, and 1.15 g of Na2HPO4, and resuspended in the same buffer.
Determination of germ tube length.
Strains were grown overnight in Sabouraud broth with 2% (wt/vol) glucose on a rotary shaker at 37°C. Ten microliters of the yeast suspension containing 2 × 106 cells was added to 1 ml of fetal calf serum (Gibco). Each strain was tested in triplicate. After incubation for 2 h at 37°C, the yeasts were fixed in 4% formalin (Sigma, Deisenhofen, Germany) buffered with PBS. Germ tube length was determined microscopically with an ocular micrometer. In these experiments, 100 cells per strain were examined. The median germ tube length was used for correlation with ALT and AM activities.
Mouse infection.
Eight- to twelve-week-old female BALB/c mice were purchased from Harlan (Borchen, Germany). The animals were infected i.p. with a sublethal dosage of C. albicans yeast cells (5 × 107) in 0.5 ml of PBS. In this experimental setting, both reference strains were eliminated within 10 days postinfection (not shown). For regression analysis with 50 clinical isolates, two mice per strain were inoculated. One of these mice received pepstatin A 30 min before infection, whereas the other mouse received a dimethyl sulfoxide (DMSO; Sigma) solution. For comparison of the reference strains and of the mutant strains, 10 mice were used per strain, with 5 mice pretreated with pepstatin A and 5 pretreated with DMSO. Treatment with pepstatin A was given i.p. in a dosage of 1 mg/kg of body weight (0.5 ml of isotonic saline per animal). Pepstatin A (Sigma) was dissolved in DMSO and further diluted in isotonic saline. In control experiments, animals received 1 μl of the solvent DMSO in 0.5 ml of isotonic saline before infection. Uninfected mice were used in other control experiments to determine the effect of pepstatin A and DMSO on ALT levels. Mice of all groups were killed 24 h postinfection by cervical dislocation. This time point was chosen because in preliminary experiments with the reference strains, it was the time point at which the highest levels of enzyme activities and organ invasion were detected microscopically. Blood (200 μl) was drawn by cardiac puncture and anticoagulated with 10 U of heparin. Thereafter, the organs were removed aseptically and further processed.
Tissue processing.
The organs were dissected out, and blocks of tissue were fixed in a 10% formaldehyde solution in PBS. The tissue samples were further processed by using standard methods for paraffin embedding and cutting. Two-micrometer sections were cut from the organs. For staining of C. albicans cells, the periodic acid Schiff (PAS) reaction was applied. The tissue sections were incubated in a solution of periodic acid in distilled water (1%) for 5 min, followed by washing in distilled water and further incubation with Schiff's reagent (Sigma), consisting of pararosaniline 1% (wt/vol) HCl and 4% (wt/vol) sodium bisulfite in hydrochloric acid (0.25 mol/liter). The PAS reaction was developed in tap water for 10 min. The tissue sections were counterstained with Mayer's hemalum solution (Merck) for 10 s, dehydrated, and embedded in DePeX (Serva, Heidelberg, Germany). Invasion depth of the organs was measured with an ocular micrometer, using six sections per organ. For each section, invasion was measured at 10 individual sites, and the mean of all measurements was calculated and correlated with the enzyme activities. For the reference strains, 20 sections of each of the five animals were used.
Determination of ALT and AM.
Activities of ALT (EC 2.6.1.2) were determined with the kinetic UV test recommended by the International Federation of Clinical Chemistry. This test was first described by Bergmeyer et al. (3). AM (EC 3.2.1.1) activities were measured by using maltotriose-bound 2-chloro-4-nitrophenol as the chromogenic substrate (7). The activities of both enzymes were determined with the Dade Flex cassette system (Dade Behring, Deerfield, Ill.), using a Dade Behring Dimension clinical chemistry system.
Statistics.
Linear regression analysis was used for the correlation of invasion and germ tube length with ALT and AM activities in plasma. As there was an exponential relationship between the activities of both ALT and AM with depth of invasion and germ tube length when tested with the Mathematica software (38), the ALT and AM data were logarithmically transformed before linear regression analysis was done and the coefficient of determination (r2) was determined.
McNemar's test was used for comparison of the effect of pepstatin A on the ALT and AM levels induced by the clinical isolates. Student's t test was used to determine differences between the ALT activities induced by the mutants and by the reference strains SC5314 and ATCC 10231 and also for detection of the effects of treatment with pepstatin A on the ALT activities induced by these strains. Differences were considered statistically significant at P of <0.05. All experiments were repeated at least twice. Statistical analysis was done with the Mathematica software (38).
RESULTS
Invasion and germ tube length are correlated with virulence.
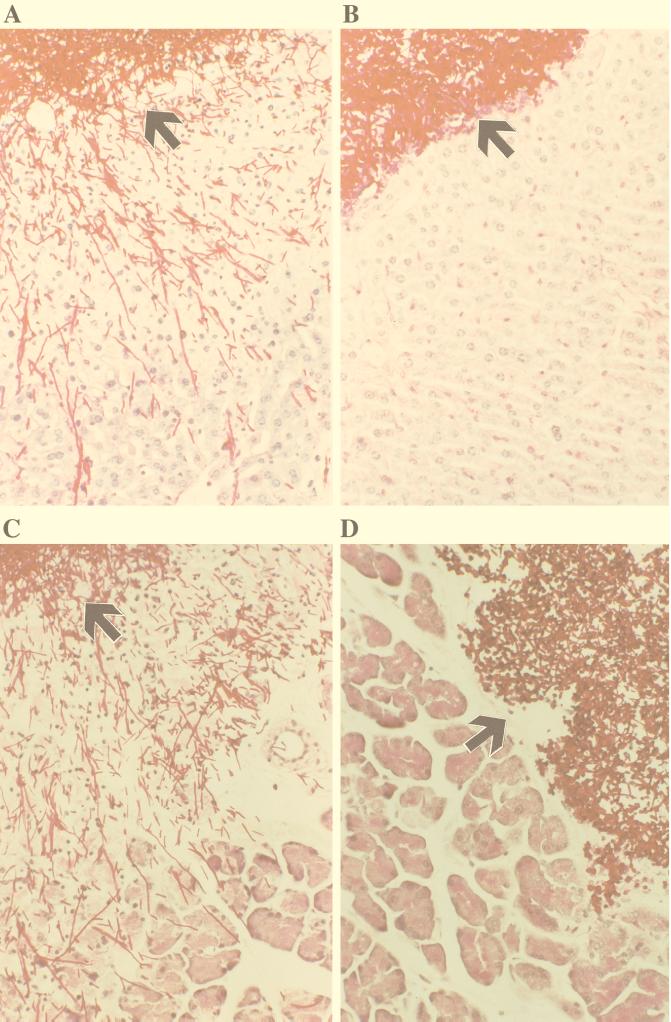
To evaluate the model of C. albicans peritonitis, we investigated the histopathological lesions induced by reference strains. There were striking differences in invasion of the parenchymal organs liver, spleen, and pancreas from the peritoneal cavity between the reference strains. As shown in Fig. 1, invasion of the liver by strain SC5314 (Fig. 1A) produced long germ tubes (12.2 ± 3.5 μm), and invasion by strain ATCC 10231 (Fig. 1B) produced short germ tubes (5.3 ± 2 μm) in vitro. There was no detectable invasion of strain ATCC 10231 into the liver from the peritoneal cavity, whereas strain SC5314, which was used as the reference strain for the Δsap mutants, invaded deeply into the liver and induced death of hepatocytes throughout the region of invasion. The sections shown are representative for all of the 20 sections cut from different sites of the organs of five animals infected at different times. Results similar to those with livers were obtained with the pancreas (Fig. 1C and D).
FIG. 1.
Histopathological analysis 24 h after i.p. infection reveals pronounced differences in virulence of reference strains of C. albicans. Deep invasion into the liver and pancreas were noted after infection with C. albicans SC5314 (A and C). This strain induced ALT and AM activities of 179 ± 32 and 6,985 ± 2,652 U/liter, respectively. In contrast, C. albicans ATCC 10231 was unable to invade the liver and pancreas (B and D). This strain induced ALT and AM activities of 45 ± 10 and 927 ± 304 U/liter, respectively. Representative photographs of 20 sections of each organ of five mice; PAS reaction and slight counterstaining with hemalum; original magnification, ×160. Arrowheads indicate the surface of the organs.
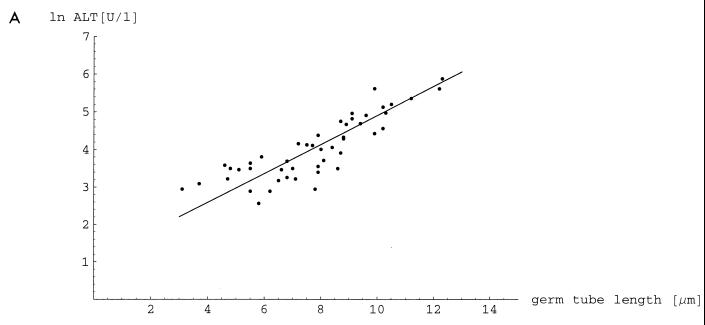
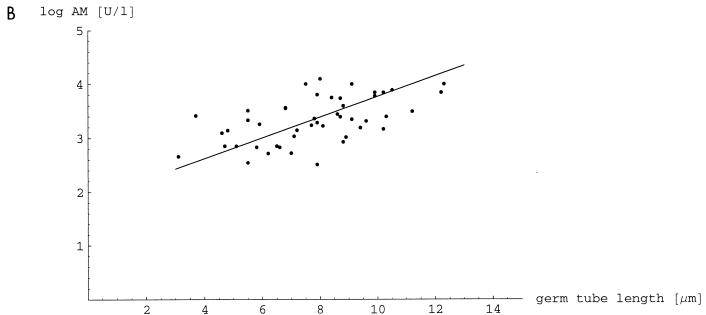
To investigate the contribution of germ tubes to tissue damage, we correlated invasion depth with the in vitro-determined length of germ tubes and both parameters with release of the ALT and AM into the blood after i.p. infection. We used 50 clinical isolates from mucosal sites. When invasion of the liver or the pancreas was correlated with liver or pancreatic damage measured by ALT or AM, there was a significant correlation (r2 = 0.515 and 0.481, respectively [data not shown]). Invasion depth of the liver and pancreas correlated with germ tube length measured in vitro (r2 = 0.408 and 0.425, respectively). Germ tube length correlated with ALT and AM, with coefficients of determination of 0.853 and 0.623, respectively (Fig. 2).
FIG. 2.
Correlation of germ tube length induced with serum in vitro and liver damage measured by ALT (A) and correlation of germ tube length and pancreatic damage measured by AM (B). Enzyme activities were measured 24 h after i.p. infection with 50 strains of C. albicans. There was a significant correlation between ALT (r2 = 0.853) and AM (r2 = 0.623) levels with the germ tube length (P < 0.001). The ALT and AM activities in sera of uninfected mice were 18.5 ± 3.7 and 143 ± 45 U/liter, respectively.
C. albicans was detected in the hearts of mice infected with all strains tested (not shown). This indicated hematogenous dissemination from the peritoneal cavity. However, using histology, we were not able to detect foci of C. albicans in the liver parenchyma distant from the invasion zone in any of the strains investigated.
Evidence for contribution of Saps to virulence.
To investigate whether proteinases of C. albicans strains contributed to virulence in vivo, we treated mice with the proteinase inhibitor pepstatin A, which did not reduce the germ tube length measured in vitro when added in the final concentration of 10 μM (not shown). Treatment with pepstatin A was not toxic by itself because the ALT levels of uninfected mice treated with pepstatin A were not higher than the ALT levels of control animals treated with DMSO (Fig. 3, control). For infected mice, we observed a reduction of invasion depth which was not statistically significant and a significant reduction of ALT and AM activities upon treatment with pepstatin A in comparison with infected mice treated with DMSO when 50 patient strains were used (P < 0.001 [data not shown]).
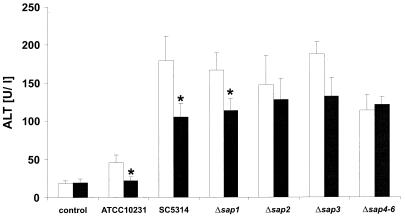
FIG. 3.
ALT activities of mice infected with sap mutants, the reference strain for animal experiments SC5314, and strain ATCC 10231 (open bars) and effect of treatment with pepstatin A (closed bars). Only in mice infected with the Δsap4-6 mutant was there a significantly (P < 0.05) reduced ALT activity in comparison to the reference strain SC5314. Treatment with pepstatin A reduced the ALT levels of all strains except the Δsap4-6 mutant; the extent of reduction was significant (P < 0.05) for strain SC5314, ATCC 10231, and the Δsap1 mutant (indicated by asterisks). Control levels of ALT were determined in uninfected mice treated with DMSO and pepstatin A (open and closed bars, respectively). Data are shown as means + standard deviations from five individual animals per group.
As the Sap isoenzymes might contribute differently to virulence of C. albicans in i.p. infection, we used isogenic Δsap null mutants. The Δsap1, Δsap2, and Δsap3 mutants had no demonstrable virulence defect in this model because there was no significant reduction in ALT activities in comparison to the reference strain SC5314 (Fig. 3). ALT activities induced by these strains were reduced by treatment of the infected mice with pepstatin A (Fig. 3). This reduction was significant for the Δsap1 mutant. In contrast, with a Δsap4-6 triple mutant, there was a significant reduction of the ALT activities (Fig. 3) in comparison to the reference strain SC5314. Furthermore, the ALT activities induced by the Δsap4-6 triple mutant could not be reduced further by pepstatin A treatment (Fig. 3). This demonstrates that Sap4-6 or individual Saps of this enzyme subfamily, which are encoded by closely related genes (19) and have therefore been disrupted collectively, might contribute to virulence in mouse peritonitis.
DISCUSSION
C. albicans exhibits a set of properties which have been shown to contribute to virulence: the ability to form germ tubes, adhesion to host cells, and production of several hydrolytic enzymes (8, 30). Most of these factors have been shown to contribute more or less to virulence in some experimental models, whereas they fail to contribute to virulence in other models (9–11, 13, 20, 21, 33).
We used a model of i.p. infection to identify virulence factors which could explain the striking differences in the ability of reference strains to invade parenchymal organs from the peritoneal cavity which can be seen in histologic sections (Fig. 1).
We investigated the correlation of the depth of invasion of the parenchymal organs liver and pancreas of human isolates of C. albicans and virulence in vivo, which was determined as liver cell damage via the ALT activities and damage of the pancreas via AM activities in the blood. ALT activity is a marker for liver cell damage which has been shown to be valuable also in mouse models (23). As there was a moderate elevation of the ALT activities of up to 30 U/liter 24 h after infection with 105 yeast cells of strain SC5314 also during systemic i.v. infection (not shown), one might argue that the i.p. model system used measured liver damage by C. albicans after colonization of the liver via the bloodstream. Although C. albicans could be detected in the hearts of infected mice, which indicated hematogenous dissemination from the peritoneal cavity, we were not able to detect foci of C. albicans in the liver parenchyma distant from the invasion zone of histologic sections 24 h after i.p. infection. This did not exclude any hematogenous dissemination into the liver, but one may conclude that there was only a minor contribution of dissemination to tissue damage and ALT release because in systemic i.v. infection with 105 CFU/animal, the fungi were readily detected in tissue sections (not shown).
Formation of long germ tubes correlated with the ALT and AM activities (Fig. 2). Germ tubes have been shown to be associated with virulence in several animal and tissue culture models of infection with C. albicans (8, 35, 36). However, a nongerminating variant of C. albicans which expressed low virulence in systemic infection was capable of causing a vaginal infection of the same duration and extent as obtained with germ tube-forming strains (9). Therefore, the contribution of germ tubes to virulence depends on the model system used. Germ tubes might improve the adherence to host cells and penetration into the host. The relationship between germination and adherence has been investigated previously. In general, germ tubes of C. albicans have been found to adhere to tissue sections (25) and isolated endothelial cells (12). This was confirmed by the inhibition of germ tube formation with new azoles, which also reduced adhesion to endothelial cells (12). Furthermore, the germ tube stage of the organism has been shown to penetrate the epithelial cell membrane (1, 30, 35). The combination of increased adherence and penetration might have contributed to the higher tissue damage in our model brought about by strains which produced long germ tubes in comparison to strains with short germ tubes. Alternatively, properties such as hydrolytic enzyme activity, associated with germ tube formation, may have been partly responsible for the observed tissue damage.
As the ALT levels are correlated not only with depth of invasion of the liver but also with germ tube formation induced by serum in vitro, one might suggest that the degree of germination of a particular strain in vitro is a predictor of virulence in mouse peritonitis. The fact that correlation of invasion depth with enzyme activities and germ tube length appeared to be weaker than the correlation of germ tube length and enzyme activities might be explained by technical problems of invasion depth measurement, which lead to higher variation coefficients in comparison with measurement of both enzyme activities and germ tube lengths. Besides germ tube length, proteinases also played a role in our model because treatment of mice with the proteinase inhibitor pepstatin A before infection significantly reduced the tissue damage of the 50 strains measured as ALT and AM levels (P < 0.001). The role of proteinases in murine infection with C. albicans has been previously established by others. For example, Fallon et al. (11) found that pepstatin A reduced mortality in intranasally challenged but not in i.v.-infected neutropenic mice. As pepstatin A was also effective in our study with i.p. infection, one may conclude that proteinases play a role in dissemination of C. albicans when barriers have to be crossed. However, a contribution of inhibition of host proteinases by pepstatin A to reduced virulence cannot be totally excluded.
From the inhibition experiments with pepstatin A, it is not possible to say which of the at least nine isoenzymes (27, 28) might contribute to virulence, because pepstatin A is likely to inhibit all Saps. Nevertheless, as there are strains with disruptions of individual SAP genes (SAP1, SAP2, and SAP3) and with a triple disruption of the SAP4 to SAP6 genes available, the contribution of these Saps in our model could be investigated. There was no demonstrable effect of the Sap1, Sap2, or Sap3 on virulence because ALT activities induced by the Δsap1, Δsap2, and Δsap3 mutants were not significantly different from the ALT levels of the reference strain SC5314 and treatment with pepstatin A was able to reduce the ALT levels induced by these strains (Fig. 3). This does not exclude the possibility of a contribution of these Saps to virulence, possibly by collective action with other Saps. Because there was a significant reduction of the ALT activities with the Δsap4-6 mutant which could not further be reduced by pepstatin A treatment (Fig. 3), we conclude that Sap4-6 might contribute to virulence either individually or collectively. Because Sap4-6 have been shown to be produced during yeast-to-hypha transition (19, 37), it is likely that they are expressed along with germ tube formation also in vivo and thereby contribute to tissue invasion and tissue damage during invasion by the germ tubes. There is also in vitro evidence for a role of Sap4-6 in mouse peritonitis because Borg-von Zepelin et al. (6) showed that Sap4-6 were expressed in isolated peritoneal macrophages and the Δsap4-6 mutant was killed more effectively after contact with macrophages than the reference strain.
The role of individual Saps has been established previously also in vivo with systemic infection models (20, 33) and vaginitis in rats (10). Using disseminated infection in guinea pigs and mice, Hube et al. (20) and Sanglard et al. (33) observed a significantly reduced but not abolished virulence of all single mutants (Δsap1, Δsap2, or Δsap3) and the Δsap4-6 mutant, which were attenuated in terms of lethality and growth in the organs in comparison to the reference strain SC5314. Therefore, none of these Saps is a single dominant virulence factor in disseminated infection. In contrast, in experimental vaginitis in rats, virulence of Δsap1, Δsap2, and Δsap3 but not Δsap4-6 mutants was attenuated in comparison to the reference strain (10). The Δsap4-6 mutant is, therefore, not attenuated in all infection models used. The fact that each of the mutants may be fully virulent in at least one of the models used argues against the possibility of unspecific effects on virulence brought about by gene disruption, although there is no proof for this assumption because complemented mutants are lacking.
In summary, we demonstrated that both invasion depth and formation in vitro of germ tubes correlate with tissue damage in a model of i.p. infection with C. albicans. Using strains with mutations of the proteinase genes, we showed that Sap4-6 but not Sap1, Sap2, and Sap3 may contribute significantly to virulence in vivo. Further studies with strains causing peritonitis in humans are needed to investigate whether germ tube formation in vitro is a predictor of virulence also in human peritonitis.
ACKNOWLEDGMENTS
This work was supported by a grant from the Faculty of Clinical Medicine, Ruprecht Karls University, Heidelberg, Germany.
We thank Martha Göller and Corina Mueller for expert technical assistance and Andreas Limmer (German Cancer Research Center, Heidelberg, Division of Molecular Immunology) for helpful discussion.
REFERENCES
- 1.Belton C M, Eversole L R. Oral hairy leukoplakia: ultrastructural features. J Oral Pathol. 1986;15:493–499. doi: 10.1111/j.1600-0714.1986.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti E, Gruessner A C, Troppmann C, Papalois B E, Sutherland D E, Dunn D L, Gruessner R W. Intra-abdominal fungal infections after pancreatic transplantation: incidence, treatment, and outcome. J Am Coll Surg. 1996;183:307–316. [PubMed] [Google Scholar]
- 3.Bergmeyer H U, Scheibe P, Wahlefeld A W. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–73. [PubMed] [Google Scholar]
- 4.Blinzler L, Fischer K, Just H M, Heuser D. Importance of mycoses in intra-abdominal infections. Langenbecks Arch Chir. 1997;382:5–8. doi: 10.1007/pl00014644. [DOI] [PubMed] [Google Scholar]
- 5.Borg M, Rüchel R. Expression of extracellular acid proteinase by proteolytic Candidaspp. during experimental infection of oral mucosa. Infect Immun. 1988;56:626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg-von Zepelin M, Beggah S, Boggian K, Sanglard D, Monod M. The expression of secreted aspartyl proteinases Sap4 to Sap6 from Candida albicansin murine macrophages. Mol Microbiol. 1998;28:543–554. doi: 10.1046/j.1365-2958.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 7.Chavez, R. G. October 1990. U.S. patent 4,963,479.
- 8.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 9.De Bernardis F, Adriani D, Lorenzini R, Pontieri E, Carruba G, Cassone A. Filamentous growth and elevated vaginopathic potential of a nongerminative variant of Candida albicansexpressing low virulence in systemic infection. Infect Immun. 1993;61:1500–1508. doi: 10.1128/iai.61.4.1500-1508.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bernardis F, Arancia S, Morelli L, Hube B, Sanglard D, Schäfer W, Cassone A. Evidence that members of the secretory aspartyl proteinases gene family, in particular SAP2, are virulence factors for Candidavaginitis. J Infect Dis. 1999;179:201–208. doi: 10.1086/314546. [DOI] [PubMed] [Google Scholar]
- 11.Fallon K, Bausch K, Noonan J, Huguenel E, Tamburini P. Role of aspartic proteases in disseminated Candida albicansinfection in mice. Infect Immun. 1997;65:551–556. doi: 10.1128/iai.65.2.551-556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fratti R A, Belanger P H, Sanati H, Ghannoum M A. The effect of a new triazole, voriconazole (UK-109,496), on the interactions of Candida albicans and Candida kruseiwith endothelial cells. J Chemother. 1998;10:7–16. doi: 10.1179/joc.1998.10.1.7. [DOI] [PubMed] [Google Scholar]
- 13.Ghannoum M A. Extracellular phospholipases as universal virulence factor in pathogenic fungi. Nippon Ishinkin Gakkai Zasshi. 1998;39:55–59. doi: 10.3314/jjmm.39.55. [DOI] [PubMed] [Google Scholar]
- 14.Ghannoum M, Abu Elteen K. Correlative relationship between proteinase production, adherence and pathogenicity of various strains of Candida albicans. J Med Vet Mycol. 1986;24:407–413. doi: 10.1080/02681218680000621. [DOI] [PubMed] [Google Scholar]
- 15.Goldie S J, Kiernan-Troidle L, Torres C, Gorban-Brennan N, Dunne D, Kliger A S, Finkelstein F O. Fungal peritonitis in a large chronic peritoneal dialysis population: a report of 55 episodes. Am J Kidney Dis. 1996;28:86–91. doi: 10.1016/s0272-6386(96)90135-3. [DOI] [PubMed] [Google Scholar]
- 16.Gyanchandani A, Khan Z K, Farooqui N, Goswami M, Ranade S A. RAPD analysis of Candida albicansstrains recovered from different immunocompromised patients (ICP) reveals an apparently non-random infectivity of the strains. Biochem Mol Biol Int. 1998;44:19–27. doi: 10.1080/15216549800201022. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg K, Meyer R D. Fungal infections in patients with AIDS and AIDS-related complex. Scand J Infect Dis. 1986;18:179–192. doi: 10.3109/00365548609032326. [DOI] [PubMed] [Google Scholar]
- 18.Hube B. Possible role of secreted proteinases in Candida albicansinfections. Rev Iberoam Micol. 1998;15:65–68. [PubMed] [Google Scholar]
- 19.Hube B, Monod M, Schofield D A, Brown A J P, Gow N A R. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 20.Hube B, Sanglard D, Odds F C, Hess D, Monod M, Schäfer W, Brown A J P, Gow N A R. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicansattenuates virulence. Infect Immun. 1997;65:3529–3538. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim A S, Mirbod F, Filler S G, Banno Y, Cole G T, Kitajima Y, Edwards J E, Nozawa Y, Ghannoum M A. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63:1993–1998. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi S D, Cutler J E. Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol. 1998;6:92–94. doi: 10.1016/s0966-842x(98)01218-9. [DOI] [PubMed] [Google Scholar]
- 23.Limmer A, Sacher T, Alferink J, Kretschmar M, Schönrich G, Nichterlein T, Arnold B, Hämmerling G J. Failure to induce organ-specific autoimmunity by breaking of tolerance: importance of the microenvironment. Eur J Immunol. 1998;28:2395–2406. doi: 10.1002/(SICI)1521-4141(199808)28:08<2395::AID-IMMU2395>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Lo W K, Chan C Y, Cheng S W, Poon J F, Chan D T, Cheng I K. A prospective randomized control study of oral nystatin prophylaxis for Candidaperitonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1996;28:549–552. doi: 10.1016/s0272-6386(96)90466-7. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Ribot J L, Vespa M N, Chaffin W L. Adherence of Candida albicansgerm tubes to murine tissues in an ex vivo assay. Can J Microbiol. 1994;40:77–81. doi: 10.1139/m94-013. [DOI] [PubMed] [Google Scholar]
- 26.Michel C, Courdavault L, Al Khayat R, Viron B, Roux P, Mignon F. Fungal peritonitis in patients on peritoneal dialysis. Am J Nephrol. 1994;14:113–120. doi: 10.1159/000168699. [DOI] [PubMed] [Google Scholar]
- 27.Monod M, Togni G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartic proteinases in Candidaspecies. Mol Microbiol. 1994;13:357–368. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 28.Monod M, Hube B, Hess D, Sanglard D. Differential regulation of SAP8 and SAP9 which encode two new members of secreted aspartic proteinase family in Candida albicans. Microbiology. 1998;144:2731–2737. doi: 10.1099/00221287-144-10-2731. [DOI] [PubMed] [Google Scholar]
- 29.Odds F C. Candida species and virulence. ASM News. 1994;60:313–318. [Google Scholar]
- 30.Odds F C. Pathogenesis of Candida infections. J Am Acad Dermatol. 1994;31:2–5. doi: 10.1016/s0190-9622(08)81257-1. [DOI] [PubMed] [Google Scholar]
- 31.Ollert M W, Söhnchen R, Korting H C, Ollert U, Bräutigam S, Bräutigam W. Mechanisms of adherence of Candida albicansto cultured human epidermal keratinocytes. Infect Immun. 1993;61:4560–4568. doi: 10.1128/iai.61.11.4560-4568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross I K, De Bernardis F, Emerson G W, Cassone A, Sullivan P A. The secreted aspartate proteinase of Candida albicans: physiology of secretion and virulence of a proteinase-deficient mutant. J Gen Microbiol. 1990;136:687–694. doi: 10.1099/00221287-136-4-687. [DOI] [PubMed] [Google Scholar]
- 33.Sanglard D, Hube B, Monod M, Odds F, Gow N A R. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicanscauses attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaberg D R, Culver D H, Gayner R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;16:72–75. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Yasue T, Ohashi M. The roles of proteinase production and germ tube formation in the invasion of Candida albicansinto newborn mouse skin. Nippon Hifuka Gakkai Zasshi. 1989;99:1227–1234. [PubMed] [Google Scholar]
- 36.Wellmer A, Bernhardt H. Adherence on buccal epithelial cells and germ tube formation in the continuous flow culture of clinical Candida albicansisolates. Mycoses. 1997;40:363–368. doi: 10.1111/j.1439-0507.1997.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 37.White T C, Agabian N. Candida albicanssecreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and activities are determined by environmental factors. J Bacteriol. 1995;177:5215–5221. doi: 10.1128/jb.177.18.5215-5221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfram S. Mathematica. 3rd ed. Munich, Germany: Addison-Wesley-Longman; 1997. [Google Scholar]