Within four weeks of the declaration of COVID-19 as a global pandemic, reports of limited access to COVID-19 diagnostic tests emerged. This—triangluated with emerging racial and ethnic disparities in cases and mortality,1 high transmission in specific settings (e.g., nursing homes, prisons, and worksites), and infrastructure challenges in rural and tribal communities—led the National Institutes of Health (NIH) to establish the Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP) initiative in April 2020. RADx-UP is one component of the NIH-wide RADx initiative.2 As with the overall NIH response to the COVID-19 pandemic, unprecedented times combined with striking disparities called for these unprecedented measures. The NIH Office of the Director committed to RADx-UP $500 million of its congressional appropriation to support science focused on COVID-19 diagnostics—which was, indeed, the largest investment in health disparities research for a single initiative.
The uniqueness of RADx-UP is its application of community-engaged research to increase access and uptake of Food and Drug Administration–authorized (or approved or cleared) COVID-19 diagnostic tests in underserved and vulnerable populations. In this context, “underserved” refers to NIH-designated populations that experience health disparities, and “vulnerable” includes groups with medical comorbidities known to increase the risk of severe COVID-19 and persons with social vulnerabilities, including environmental exposures. We were keenly aware that simply because you build does not mean they will come—especially in a time of crisis of an unknown magnitude, changing information, and elevated distrust. Thus, community-engaged approaches, including partnerships with complementary content and context expertise, were critical to identifying effective strategies to enhance access, use, and reporting of COVID-19 testing in these diverse populations.
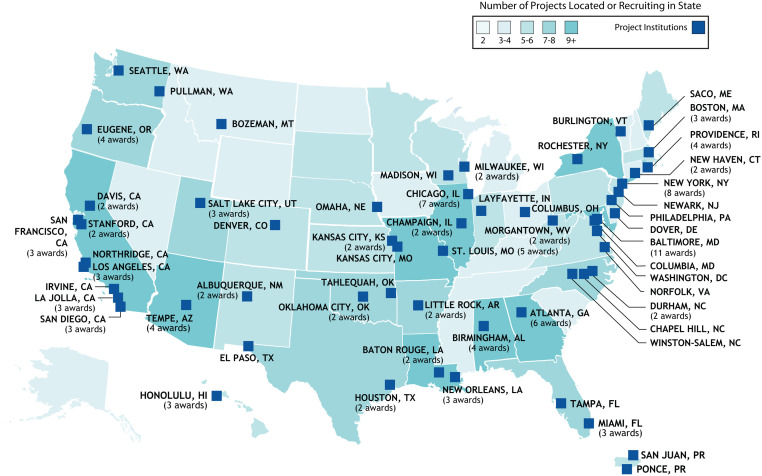
RADx-UP research teams were selected for funding based on such factors as having demonstrated track records of strong collaborations and trusted partnerships. These multidisciplinary research teams are working directly with communities to understand and reduce distrust, test interventions to increase testing access and uptake, and reduce health disparities from COVID-19. To date, the 127 RADx-UP projects are a nationwide community of practice (Figure 1) that includes partnerships with academic institutions, community-based organizations, federal qualified health centers, and historically Black colleges and universities (Table A, available as a supplement to the online version of this article at http://www.ajph.org). RADx-UP has the greatest diversity of target populations and research settings of any NIH science initiative to date (Table A).
FIGURE 1—
RADx-UP Project Map: United States
Note. RADx-UP = Rapid Acceleration of Diagnostics-Underserved Populations. The map illustrates the National Institutes of Health–funded RADx-UP projects, as well as Rapid Pilot Program projects. RADx-UP projects recruit participants in all 50 states as well as the District of Columbia, American Samoa, Guam, the Northern Mariana Islands, Puerto Rico, and the US Virgin Islands.
SCIENTIFIC GOALS
The multiphase RADx-UP framework facilitated the emergency release of funding opportunity announcements based on emerging evidence, new testing technologies, and the availability of vaccines. The targeted areas of research supported by each RADx-UP phase matched the evolving state of the pandemic and the testing-specific needs of underserved and vulnerable groups (Table B, available as a supplement to the online version of this article at https://www.ajph.org). Phase I focused on (1) building the RADx-UP infrastructure; (2) the rapid scale-up of COVID-19 testing; (3) the in-depth examination of social, ethical, and behavioral implications related to testing; and (4) project readiness for standardized data collection and sharing.
Phase II integrated new scientific and technological advances and focused on (1) interventions to reduce disparities in COVID-19 and (2) new or more intensive approaches to increase testing access and uptake given the availability of vaccines. We also initiated the RADx-UP Return to School initiative in response to the significant need for school-based testing interventions to detect and minimize the spread of COVID-19 during the return of in-person instruction.
Phase III will study strategies to expand the reach, access, and implementation of rapid testing interventions to reduce COVID-19 disparities. This phase maintains the partnership-driven approach and social, ethical, and behavioral implications research to address the challenges associated with the chronicity of the pandemic as well as the secondary impacts of testing mandates combined with other mitigation measures. In phase III, the Return to School initiative is evolving into research on minimizing educational disruptions with Safe in School research.
DATA COLLECTION AND SHARING
The richness of RADx-UP data has the potential to enhance knowledge via standardized measurement of demographics, social determinants of health, behaviors, and testing-related outcomes. In collaboration with the RADx-UP Coordination and Data Collection Center and with input from the funded projects, RADx leadership established a set of common data elements to ensure standardized data collection and reporting and to support cross-consortium data analyses in the future. Common data element collection will increase statistical power to answer research questions overall and in small populations and compare outcomes stratified by demographics, geography, and time frames. RADx-UP data will be de-identified and deposited into the RADx Data Hub, which will create a cross-initiative repository, leading to rapid and increased learning about the pandemic and its effects.
In our attempt to administer a set of common data elements, we met with challenges raised by research teams and communities. Among them were added complexity to research protocols and the limited time to engage community partners in the full scope of data collection and sharing from the start of the projects. We continue to navigate data collection and sharing among tribal nations and American Indian/Alaska Native individuals, and the new RADx Tribal Data Repository3 will serve as an independent research data repository governed under the principles and practices of tribal sovereignty.
The NIH applauds the efforts of funded research groups and, importantly, of the diverse populations of study participants to navigate these and other challenges and to ensure that collaborators trust and appreciate the importance of collecting much of the information—the same way—across the consortium. Among the key lessons learned from this community-engaged research initiative is the need for transparent and clear policies for data collection, submission, and sharing as early as possible.
DISCOVERIES AND LESSONS LEARNED
Previous RADx-UP research has produced a number of key findings regarding community-based testing efforts, promising interventions to increase testing uptake, the importance of testing implementation and school-based mitigation strategies, and COVID-19 vaccine hesitancy in occupational groups with high exposure risk. Bigelow et al.4 found a 10-fold greater positivity rate among Latino/Hispanic participants relative to White participants (31.5% vs 3.4%, respectively) in a community-based testing program. Moreover, Latino/Hispanic participants who tested positive were more likely to be younger, report Spanish as their preferred language (91.6% vs 81.7%; P < .001), and have a larger household size.
Intervention research has also demonstrated positive effects. Cioffi et al.5 found that contingency management increased COVID-19 testing among people who inject drugs, with 12.3% of unique clients tested before contingency management and 35.4% unique clients tested during contingency management. Boutzoukas et al.6 demonstrated the importance of school district–level mitigation policies, as universal masking was associated with a 72% reduction in secondary transmission compared with optional masking.
Finally, qualitative findings from social, ethical, and behavioral implications research among staff members at 50 skilled nursing facilities highlighted the important roles of social networks as sources of COVID-19 vaccine–related information, hesitancy, and misinformation among frontline occupational groups.7 This small selection of findings is consistent with the spirit of RADx-UP and underscores the importance of assessments and interventions that identify key community needs and improve outcomes among underserved and vulnerable groups across the United States.
There have also been important lessons learned that will be reinforced going forward. They include the following:
-
1.
Community engagement and gaining trust in science are essential, because of both direct and vicarious experiences among underserved groups;
-
2.
Culturally appropriate and community competent testing and vaccination strategies are important for increasing trust in COVID-19 messages from evidence-based information by contrast to the deluge of misinformation;
-
3.
Active community advisory boards and representative groups are essential for progress, as they provide key recommendations and support;
-
4.
Wraparound care and connections to resources by breaking down silos are highly valuable, as are partnerships with community clinics and clinicians everywhere; and
-
5.
Disaggregating data, where possible, can help to elucidate the impacts in smaller populations.
CONCLUSIONS
With its scope and reach, RADx–UP is unlike any previous NIH-led effort to reduce health disparities and promote health equity. Findings from all phases are expected to guide ongoing and future COVID-19 mitigation efforts in underserved and vulnerable populations, and data serve as a learning ground for research on reducing health disparities. Unless COVID-19 is eradicated, testing will remain a critical component of prevention and control efforts.
Furthermore, by sharing data across individual studies, we will have a clearer picture of how COVID-19 affects vulnerable populations and be positioned to answer key additional questions. These data will help community leaders and policymakers identify effective strategies for reducing disparities in COVID-19 testing and addressing the other health needs of their communities in the event of future pandemics.
This special issue of AJPH is part of the first wave of results from the RADx-UP consortium. Because of the impactful work of all the individuals pouring their passion, innovative scientific perspectives, and collaborative energy into this program, RADx-UP will live on in the research infrastructure, the scientific discoveries that will continue to be unearthed, and the dedication to supporting underserved and vulnerable populations. RADx-UP is an exemplar of the bold and innovative approaches that the NIH can rapidly mobilize.
ACKNOWLEDGMENTS
Funding for the Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP) initiative came from a congressional allocation (the Paycheck Protection Program and Health Care Enhancement Act, 2020, and the American Rescue Plan Act of 2021) to the National Institutes of Health (NIH) for COVID-19 testing research.
The authors would like to thank the extensive community of the NIH staff, researchers, community partners, and participants who have contributed to the RADx-UP initiative.
Note. The content of this editorial is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICTS OF INTEREST
W. M. Compton has stock holdings in General Electric, 3M, and Pfizer unrelated to this editorial. The other authors have no disclosures.
REFERENCES
- 1.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tromberg BJ, Schwetz TA, Pérez-Stable EJ, et al. Rapid scaling up of COVID-19 diagnostic testing in the United States—the NIH RADx initiative. N Engl J Med. 2020;383(11):1071–1077. doi: 10.1056/NEJMsr2022263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health. 2022. https://grants.nih.gov/grants/guide/rfa-files/RFA-OD-22-011.html
- 4.Bigelow BF, Saxton RE, Flores-Miller A, et al. Community testing and SARS-CoV-2 rates for Latinxs in Baltimore. Am J Prev Med. 2021;60(6):e281–e286. doi: 10.1016/j.amepre.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Cioffi CC, Kosty D, Capron CG, Tavalire HF, Barnes RC, Mauricio AM. Contingency management and SARS-CoV-2 testing among people who inject drugs. Public Health Rep. 2022;137(3):573–579. doi: 10.1177/00333549221074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutzoukas AE, Zimmerman KO, Inkelas M, et al. School masking policies and secondary SARS-CoV-2 transmission. Pediatrics. 2022;149(6):e2022056687. doi: 10.1542/peds.2022-05668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry SD, Johnson KS, Myles L, et al. Lessons learned from frontline skilled nursing facility staff regarding COVID-19 vaccine hesitancy. J Am Geriatr Soc. 2021;69(5):1140–1146. doi: 10.1111/jgs.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]