Abstract
At-home COVID-19 testing offers convenience and safety advantages. We evaluated at-home testing in Black and Latino communities through an intervention comparing community-based organization (CBO) and health care organization (HCO) outreach. From May through December 2021, 1100 participants were recruited, 94% through CBOs. The odds of COVID-19 test requests and completions were significantly higher in the HCO arm. The results showed disparities in test requests and completions related to age, race, language, insurance, comorbidities, and pandemic-related challenges. Despite the popularity of at-home testing, barriers exist in underresourced communities. (Am J Public Health. 2022;112(S9):S918–S922. https://doi.org/10.2105/AJPH.2022.306989)
Although free COVID-19 testing has been widely embraced in some settings, access to testing has remained challenging throughout the pandemic for many people. Limited testing sites and long lines present barriers to testing, particularly among lower-income individuals with reduced control over their schedules or limited access to transportation.1–3 Despite that, little research has evaluated strategies to enhance testing in underserved populations, and to our knowledge no previous research has examined this issue in the context of at-home testing, an increasingly popular option given its potential convenience and safety advantages.4,5
INTERVENTION AND IMPLEMENTATION
Recognizing the risks to health care workers (HCWs) during the pandemic, we developed the New Jersey Healthcare Essential Worker OutReach and Education Study-Testing Overlooked Occupations (NJ HEROES TOO) intervention as part of the National Institutes of Health Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP) initiative.6 NJ HEROES TOO engaged Black and Latino HCWs to constitute a health care organization (HCO) arm as “ambassadors” promoting at-home COVID-19 testing in their households and communities, and testing uptake in that arm was compared with uptake in a second study arm involving a traditional community-based organization (CBO) approach. Our aims were to compare the odds of at-home COVID-19 test requests and completions across study arms and examine sociodemographic factors associated with requests and completions.
The NJ HEROES TOO study was a partnership between a Rutgers University academic research team and local HCOs (n = 4) and CBOs (n = 18; Figure A, available as a supplement to the online version of this article at http://www.ajph.org). Partner organizations advertised the NJ HEROES TOO study through their preferred outreach channels, including e-mails, social media, and flyers. Hyperlinks, QR codes, and URLs provided access to the study Web site, where a screener (in English or Spanish) queried age, race and ethnicity, and NJ county residence. Respondents meeting the eligibility criteria were invited to provide informed consent and complete a study questionnaire in REDCap. Upon completion, they were e-mailed an access code to order a free COVID-19 test kit by Vault Health, a major provider of at-home COVID-19 tests.7
Once the kit was received, participants answered questions regarding COVID-19 symptoms and collected saliva samples under videoconference supervision by Vault Health staff. Participants mailed saliva samples in prepaid express envelopes to the analytic lab (with free pick-up available). Polymerase chain reaction test results were returned to participants by Vault Health clinical providers. NJ HEROES TOO staff followed up with participants who did not complete the testing process to remind them about the testing opportunity and troubleshoot challenges.
The questionnaire, developed with input from the partner organizations, included RADx-UP-required common data elements and NJ HEROES TOO–specific items focusing on demographics, lifestyle, social factors, health and health care access, pandemic-related issues, previous COVID-19 testing, and vaccine intent (see the Appendix, available as a supplement to the online version of this article at http://www.ajph.org). Parents completed abbreviated versions for participants 17 years or younger.
Logistic regression was used to assess the odds that (1) an eligible participant requested a COVID-19 test and (2) a participant who requested a test completed the testing process. Complete data were available for 40% of participants. Multiple imputation with chained equations was used to estimate a set of plausible values for the missing data. Sixty simulated data sets were generated in which each variable with missing data was regressed on covariates, including the dependent variable and variables with complete data (study arm, age, race/ethnicity, language preference, and presence of comorbidities) based on the specific distribution of the dependent variable.
Best-fit models were adjusted for covariates that were significantly related to the outcome or that improved the overall model fit, including demographic variables, the presence of chronic comorbidities, postponement of medical care during the pandemic, access to and history of COVID-19 testing, pandemic challenge score, discrimination score, and trust score (see the Appendix). Statistical analyses were performed in Stata version 17 (StataCorp LP, College Station, TX 2022).
PLACE, TIME, AND PERSONS
From May through December 2021, eligible NJ residents completed a single online questionnaire (including items on sociodemographic characteristics as well as COVID-19-related perceptions, behaviors, and challenges), after which they were able to order a free at-home saliva COVID-19 polymerase chain reaction test.
Eligibility criteria included the following: Black or Latino race/ethnicity; ability to provide informed consent or assent; ability to speak, understand, or read English or Spanish; and residence in the NJ county of Union, Passaic, Middlesex, or Essex. Participating counties were selected on the basis of high concentrations of Black and Latino residents, urbanicity, poverty rates, COVID-19 burden, proximity to participating health care sites, and extant CBO and HCO outreach infrastructure.
PURPOSE
The purpose of this intervention was to promote at-home COVID-19 testing through a nontraditional approach engaging HCWs and compare that with a traditional approach operating through CBOs. We also evaluated factors influencing engagement in the testing process across both study arms. Our focus on HCWs as ambassadors to underserved communities emerged as a result of their high level of engagement in ongoing studies of HCWs in NJ during the pandemic,8–10 the high rates of COVID-19 infection among Black and Latino hospital workers in health care support roles (e.g., hospital maintenance, housekeeping, security, food service, and facility services),9 and the high rates of COVID-19 infection and death in NJ during the pandemic.11
EVALUATION AND ADVERSE EFFECTS
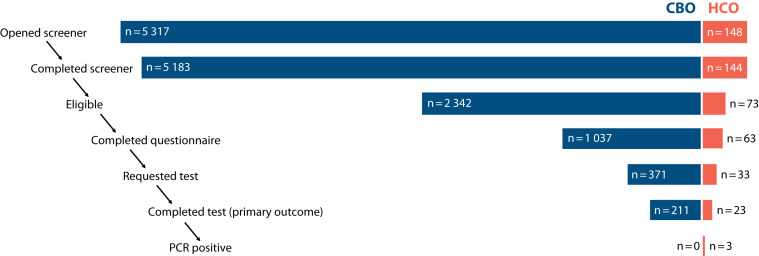
In total, 97% of individuals (5327 of 5465) who started the online screener completed it, of whom 45% (n = 2415) were eligible (ineligibility was most often due to residency outside of the participating NJ counties). Questionnaires were completed by 1100 participants, representing 46% of eligible screeners. Of these participants, 404 (37%) requested COVID-19 tests, of which 234 (58%) were completed. More participants were recruited through CBOs than HCOs at every stage in the process, including 97% (n = 5183) of screener completions, 97% (n = 2342) of informed consents, 94% (n = 1037) of questionnaire completions, 92% (n = 371) of tests requested, and 90% (n = 211) of tests completed (Figure 1; Table A, available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 1—
Recruitment and Participant Flow in the Community-Based Organization (CBO) and Health Care Organization (HCO) Arms of the NJ HEROES TOO Study: New Jersey, 2021
Note. NJ HEROES TOO = New Jersey Healthcare Essential Worker OutReach and Education Study-Testing Overlooked Occupations; PCR = polymerase chain reaction.
The median age of participants who completed a questionnaire was 29 years; 54% were female, 47% were Latino (non-Black), 36% were Black (non-Latino), and 17% were Black/Latino (Table B, available as a supplement to the online version of this article at http://www.ajph.org). In the adjusted models, the odds of test requests and completions were nonsignificantly lower among CBO versus HCO participants (Table 1).
TABLE 1—
Best-Fit Logistic Regression Models Examining Odds of COVID-19 Test Requests and Completions as Part of the NJ HEROES TOO Study (Among Participants Who Completed a Questionnaire): New Jersey, 2021
| Covariate | Requested Testa (n = 1 099), OR (95% CI) | Completed Testb (n = 403), OR (95% CI) |
| CBO (ref: HCO) | 0.58 (0.31, 1.09) | 0.45 (0.17, 1.23) |
| Age group, y (ref: 17–39) | ||
| < 17 | 1.55 (1.03, 2.32) | 0.50 (0.22, 1.15) |
| 40–59 | 1.86 (1.14, 3.00) | 1.17 (0.56, 2.43) |
| ≥ 60 | 2.03 (1.11, 3.78) | 3.36 (1.35, 8.38) |
| Race (ref: Latino, non-Black) | ||
| Black, non-Latino | 1.82 (1.28, 2.56) | 2.65 (1.47, 4.79) |
| Latino and Black | 0.64 (0.39, 1.04) | 1.76 (0.67, 4.63) |
| Survey materials in Spanish (ref: in English) | 3.02 (1.17, 7.81) | |
| Male (ref: not male) | 0.70 (0.51, 0.96) | |
| Income, $ (ref: 0–25 000) | ||
| 26 000–50 000 | 0.77 (0.48, 1.22) | 0.88 (0.42, 1.85) |
| 51 000–75 000 | 0.45 (0.27, 0.76) | 0.70 (0.26, 1.91) |
| 76 000–99 999 | 0.41 (0.2, 0.82) | 0.45 (0.15, 1.32) |
| ≥ 100 000 | 0.45 (0.22, 0.93) | 0.56 (0.18, 1.76) |
| Education (adults; ref: ≤ high school) | ||
| Some college | 1.02 (0.63, 1.63) | 1.53 (0.69, 3.37) |
| Bachelor’s degree | 0.88 (0.52, 1.48) | 2.04 (0.88, 4.71) |
| Master’s/professional degree | 1.46 (0.78, 2.77) | 1.86 (0.63, 5.52) |
| Insurance (adults; ref: private) | ||
| None | 2.25 (1.09, 4.62) | 1.42 (0.37, 5.40) |
| Public | 1.60 (1.09, 2.36) | 3.27 (1.50, 7.04) |
| Employment status (adults; ref: essential worker) | ||
| Nonessential worker | 1.31 (0.84, 2.05) | |
| Unemployed | 1.05 (0.58, 1.90) | |
| Not in labor force | 1.93 (1.14, 3.29) | |
| Body mass index (adults; ref: ≤ 25 kg/m2) | ||
| 25–≤ 30 (overweight) | 1.58 (0.99, 2.53) | |
| ≥ 30 (obese) | 2.32 (1.38, 3.94) | |
| Pandemic challenges (ref: none) | ||
| 1–4 (moderate problems) | 0.63 (0.42, 0.92) | 1.06 (0.55, 2.03) |
| 5–12 (major problems) | 0.37 (0.22, 0.61) | 0.39 (0.16, 0.99) |
| Wants to be vaccinated when available (ref: no) | 0.67 (0.44, 1.02) | 0.42 (0.22, 0.81) |
| Any chronic comorbidities (ref: no comorbidities) | 1.08 (0.79, 1.51) | 0.57 (0.33, 0.97) |
| Strongly agree/agree that it is easy to get tested for COVID-19 (ref: strongly disagree/disagree) | 1.52 (1.03, 2.27) | |
| COVID-19 testing history (ref: never tested) | ||
| Tested negative previously | 2.41 (1.62, 3.56) | |
| Tested positive previously | 1.92 (1.17, 3.13) | |
| Discrimination index score (ref: 0–9 [low]) | ||
| 10–18 | 0.48 (0.25, 0.91) | |
| 19–27 | 0.43 (0.18, 1.00) | |
| 28–45 (high) | 0.36 (0.07, 1.96) | |
| Trust index score (ref: 0–8 [low]) | ||
| 9–16 (moderate) | 2.16 (0.79, 5.87) | |
| 17–32 (high) | 1.39 (0.47, 4.14) | |
| Postponed medical care during pandemic (ref: did not postpone care) | 1.09 (0.54, 2.17) | |
Note. CBO = community-based organization; CI = confidence interval; HCO = health care organization; NJ HEROES TOO = New Jersey Healthcare Essential Worker OutReach and Education Study-Testing Overlooked Occupations; OR = odds ratio. Estimates from the best-fitting logistic regression models are displayed for each outcome. Variables were included in the best-fit model if they were significantly associated with the outcome, improved the model fit, or were selected for inclusion on the basis of model selection techniques, including elastic net regression and model fit parameters. Missing values were estimated via multiple imputation chained equations.
aThe denominator is all eligible participants who completed a questionnaire.
bThe denominator is all eligible participants who completed a questionnaire and requested a COVID-19 test through NJ HEROES TOO.
Across study arms, the odds of test requests were significantly higher for children (odds ratio [OR] = 1.55; 95% confidence interval [CI] = 1.03, 2.32), middle-aged adults (OR = 1.86; 95% CI = 1.14, 3.00), and older adults (OR = 2.03; 95% CI = 1.11, 3.78) than for younger adults (Table 1). Similarly, the odds were higher among Black participants than Latino participants (OR = 1.82; 95% CI = 1.28, 2.56).
Additional factors associated with higher odds of test requests included lower income, public (OR = 1.60; 95% CI = 1.09, 2.36) or no (OR = 2.25; 95% CI = 1.09, 4.62) insurance (vs private insurance), being outside the labor force (vs being an essential worker; OR = 1.93; 95% CI = 1.14, 3.29), and higher body mass index. Test requests were also associated with having had a prior COVID-19 test, whether with a negative (OR = 2.41; 95% CI = 1.62, 3.56) or positive (OR = 1.92; 95% CI = 1.17, 3.13) result, and self-reported ease of test access (OR = 1.52; 95% CI = 1.03, 2.27). The odds of test requests were lower among participants who experienced COVID-19-related life challenges that were either moderate (OR = 0.63; 95% CI = 0.42, 0.92) or major (OR = 0.37; 95% CI = 0.22, 0.61).
The odds of test completion were higher among adults 60 years or older (OR = 3.36; 95% CI = 1.35, 8.38; Table 1) and among Black participants (vs Latino participants; OR = 2.65; 95% CI = 1.47, 4.79). Also, participants accessing materials in Spanish (OR = 3.02; 95% CI = 1.17, 7.81) and participants with public insurance (vs private; OR = 3.27; 95% CI = 1.52, 7.04) were more likely to complete testing. Test completion rates were lower among participants reporting chronic comorbidities (OR = 0.57; 95% CI = 0.33, 0.97), those reporting major pandemic-related life challenges (OR = 0.39; 95% CI = 0.16, 0.99), and those with higher discrimination scores. Results were similar in models limited to individuals with complete case data (Table C, available as a supplement to the online version of this article at http://www.ajph.org).
Several key limitations should be noted. First, the total number of potential participants reached through each study arm cannot be quantified given the many outreach channels used by partner organizations. In addition, we observed considerable attrition at every step in the study process. This attrition, which is of central interest to our project and is relevant to understanding barriers to COVID-19 testing in vulnerable communities, also raises the possibility of bias, and thus there is the possibility that the results from our sample cannot be extrapolated to the participating counties, NJ, and the United States as a whole.
No adverse effects associated with the intervention were observed.
SUSTAINABILITY
Although the odds of completing at-home COVID-19 testing were higher among HCW study arm participants, overall engagement was much higher in the CBO arm, reinforcing the value of traditional approaches of working with community partners. At the same time, numerous barriers were identified that may limit the utility of at-home polymerase chain reaction testing in underserved communities in the absence of additional supportive measures.
PUBLIC HEALTH SIGNIFICANCE
Community-based approaches to expanding at-home COVID-19 testing among Black and Latino NJ residents were more successful than HCO-based approaches, but many sociodemographic disparities in testing uptake were observed.
ACKNOWLEDGMENTS
Research reported in this Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP) publication was supported by the National Institutes of Health under award UL1TR003017-02S2. Additional support was provided through awards U01AI122285-05S1, UL1TR003017, UL1TR003017-02S1, R01AI158911, and P30ES005022.
We thank the New Jersey Healthcare Essential Worker OutReach and Education Study-Testing Overlooked Occupations (NJ HEROES TOO) study staff, partners, and participants for their contributions to this project.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICTS OF INTEREST
Maria B. Pellerano is supported by a grant from the Johnson & Johnson Corporate Foundation and receives honorarium fees from the Patient-Centered Outcomes Research Institute and the University of Massachusetts. Daniel B. Horton reports funding for COVID-19-related research from Danisco, USA Inc. The other authors have no conflicts of interest to disclose.
HUMAN PARTICIPANT PROTECTION
The Rutgers University institutional review board approved the study activities, and all participants provided informed consent/assent.
REFERENCES
- 1.Kas-Osoka C, Moss J, Alexander L, et al. African American views of COVID-19 contact tracing and testing. Am J Infect Control. 2022;50(5):577–580. doi: 10.1016/j.ajic.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez ME, Rivera-Núñez Z, Crabtree BF, et al. Black and Latinx community perspectives on COVID-19 mitigation behaviors, testing, and vaccines. JAMA Netw Open. 2021;4(7):e2117074. doi: 10.1001/jamanetworkopen.2021.17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera-Núñez Z, Jimenez ME, Crabtree BF, et al. Experiences of black and Latinx health care workers in support roles during the COVID-19 pandemic: a qualitative study. PLoS One. 2022;17(1):e0262606. doi: 10.1371/journal.pone.0262606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall EW, Luisi N, Zlotorzynska M, et al. Willingness to use home collection methods to provide specimens for SARS-CoV-2/COVID-19 research: survey study. J Med Internet Res. 2020;22(9):e19471. doi: 10.2196/19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goggolidou P, Hodges-Mameletzis I, Purewal S, Karakoula A, Warr T. Self-testing as an invaluable tool in fighting the COVID-19 pandemic. J Prim Care Community Health. 2021;12:21501327211047782. doi: 10.1177/21501327211047782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health. RADx Underserved Populations (RADx-UP). Available at. 2022. https://radx-up.org
- 7.Vault Health. 2022. https://www.vaulthealth.com
- 8.Horton DB, Barrett ES, Roy J, et al. Determinants and dynamics of SARS-CoV-2 infection in a diverse population: 6-month evaluation of a prospective cohort study. J Infect Dis. 2021;224(8):1345–1356. doi: 10.1093/infdis/jiab411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett ES, Horton DB, Roy J, et al. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in hospital workers: results from a screening study in New Jersey, United States in spring 2020. Open Forum Infect Dis. 2020;7(12):ofaa534. doi: 10.1093/ofid/ofaa534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the US COVID-19 pandemic. BMC Infect Dis. 2020;20(1):853. doi: 10.1186/s12879-020-05587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New York Times. Tracking coronavirus in New Jersey: latest map and case count. Available. 2022. https://www.nytimes.com/interactive/2021/us/new-jersey-covid-cases.html