Abstract
The REstarting Safe Education and Testing program for children with medical complexity was implemented in May 2021 at the University of Wisconsin to evaluate the feasibility of in-home rapid antigen COVID-19 testing among neurocognitively affected children. Parents or guardians administered BinaxNOW rapid antigen self-tests twice weekly for three months and changed to symptom and exposure testing or continued surveillance. In-home testing was feasible: nearly all (92.5%) expected tests were conducted. Symptomatic testing identified seven of nine COVID-19 cases. School safety perceptions were higher among those opting for symptom testing. Clinical Trials.gov identifier: NCT04895085. (Am J Public Health. 2022;112(S9):S878–S882. https://doi.org/10.2105/AJPH.2022.306971)
Children with medical complexity (CMC)—a vulnerable population with multiple chronic conditions, functional limitations, and health services utilization1,2—have a high risk of COVID-19 morbidity and mortality.3 Regular access to in-home COVID-19 rapid antigen testing could be a valuable component of long-term pandemic management and may improve CMC’s health by facilitating earlier detection and symptom monitoring, implementation of clinical action plans (e.g., for respiratory illness), and consideration of COVID-19–directed therapies. Similarly, testing may influence CMC’s family perceptions regarding school attendance4 and has been identified as a key priority for safe return to school.5 Timely identification of a positive COVID-19 status in CMC may also benefit communities by ensuring that individuals caring for them in school and community settings take appropriate precautions.
INTERVENTION AND IMPLEMENTATION
We sought to establish the feasibility of an in-home COVID-19 surveillance and symptomatic testing program for CMC and identify associations with school safety perceptions. The testing program, REstarting Safe Education and Testing for CMC (ReSET),6 used the BinaxNOW rapid antigen system (Abbot Labs, Chicago, IL), a point-of-care lateral flow immunoassay used for the qualitative detection of SARS-CoV-2 nucleocapsid antigen from anterior nasal swabs. This test is approved for use in children aged two years and older when performed by an adult under a US Food and Drug Administration emergency use authorization. During a virtual enrollment visit with a standard checklist, study personnel trained caregivers (i.e., parents or guardians) to administer BinaxNOW rapid antigen self-tests to their CMC. ReSET staff provided families with self-test kits and mailed additional kits when families requested them.
During the first three months, we instructed all caregiver participants to conduct surveillance testing twice weekly (i.e., two tests over three days at least 24–48 hours apart, per package insert instructions). We encouraged participants to conduct additional tests when there were any concerning COVID-19 symptoms or exposures. After three months of surveillance, we asked participants to choose to either continue surveillance testing (plus as-needed testing for COVID-19 symptoms or exposures) or switch to symptom or exposure testing only (subsequently referred to as “symptom testing”). For positive BinaxNOW rapid antigen self-tests, we instructed participants to obtain polymerase chain reaction (PCR) confirmation through their community or health care providers and to follow public health isolation recommendations. We also recommended PCR confirmation for symptomatic negative BinaxNOW tests.
PLACE, TIME, AND PERSONS
Enrollment and testing began May 3, 2021, and we report data through January 31, 2022. We recruited a convenience sample of English-speaking caregivers (typically parents) of CMC aged 5 to 17 years who attended school before the pandemic. Recruitment occurred at a pediatric complex care program in the Midwest, a clinical program for CMC having three or more organ systems affected by chronic conditions, care from three or more specialists, and either five or more hospital days or ten or more specialty clinic visits in the previous year. Chronic neurologic, cardiovascular, or genetic conditions were present in 90%, 41%, and 41% of ReSET-enrolled CMC, respectively. Most CMC (73%) were assisted by enteral tubes, many (39%) received home oxygen, and 14% had tracheostomies (Table A, available as a supplement to the online version of this article at https://www.ajph.org).
PURPOSE
This study was part of the National Institutes of Health Rapid Acceleration of Diagnostics-Underserved Populations consortium, which aimed to use COVID-19 testing to support return to school for vulnerable populations. Although in-home testing may plausibly reassure families of CMC and promote safer in-person education,5,7 unknown real-world feasibility of in-home rapid antigen testing, particularly among neurocognitively affected pediatric populations, could uncover poor uptake. Yet clinicians and families depend on reliable COVID-19 testing for CMC because their baseline health can always include symptoms consistent with COVID-19 (e.g., cough, variable vital signs, oxygen needs),8 and limited communication can conceal new symptoms.9 Understanding feasibility of ReSET’s surveillance and symptom-based strategies could guide the design, implementation, and evaluation of large-scale testing programs for vulnerable child populations and inform the response of schools and public health agencies to future pandemics.
EVALUATION AND ADVERSE EFFECTS
We have no adverse effects to report.
ReSET’s in-home COVID-19 testing program resulted in 2121 BinaxNOW tests being conducted, representing 92.5% of the tests expected among the 51 CMC enrolled during the study period. The mean ±SD number of tests per child per week was 2 ±0.18 (range = 0–6). Most tests (87.1%) were conducted without symptoms. After three months, 63% chose to continue surveillance testing, and 37% chose symptom-only testing. No caregiver or child characteristics predicted the choice for surveillance or symptom testing. Test problems were rare (3.7% of tests) and included limited child cooperation (1.3%) or the child being too ill or hospitalized (0.7%).
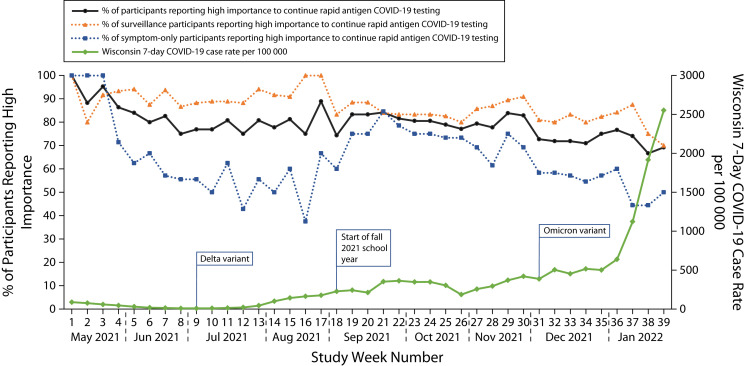
We plotted participants who reported that testing was “very” or “extremely” important to them each week (Figure 1). The proportion decreased from about 90% in early May 2021 to about 65% in January 2022. Throughout the study, participants choosing symptom testing had lower weekly ratings of importance to continue testing than did those choosing surveillance testing; however, importance ratings were similar in both groups during September 2021, when the SARS-CoV-2 delta variant cases were rising and school began. Ratings of importance to continue testing did not appreciably increase as SARS-CoV-2 omicron variant cases increased.
FIGURE 1—
Weekly Perceived Importance of Continuing In-Home COVID-19 Rapid Antigen Testing: Wisconsin, May 2021–January 2022
Among nine positive tests during the study period, seven were from symptomatic children, and eight of the nine positive tests occurred in the period after the first three months of surveillance. All positive tests had positive confirmatory PCR tests (0% false-positive rate). Although PCR confirmation for negative BinaxNOW tests was not required, no families reported positive PCRs following negative BinaxNOW tests (i.e., there were no known false-negative tests).
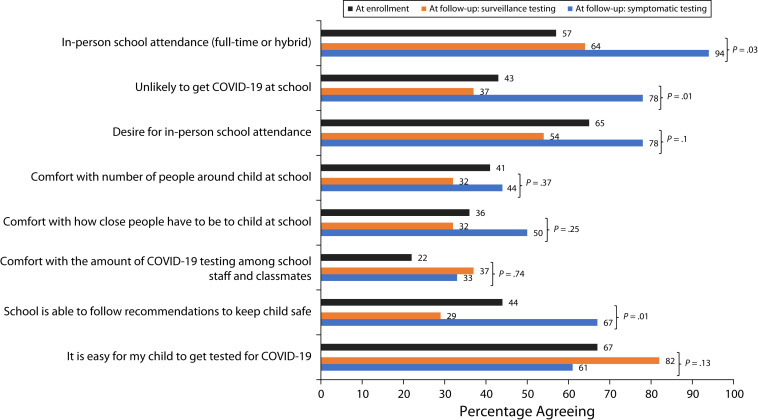
Only 57% of CMC were attending school in person at enrollment. Between enrollment and six-month follow-up, several differences existed among those choosing surveillance versus symptom testing (Figure 2). For example, CMC of caregivers selecting surveillance testing attended school in person less often than those selecting symptomatic testing (64% vs 94%, respectively; P = .03). Similarly, those selecting surveillance testing less often thought the school could follow recommendations to keep their child safe (29% surveillance vs 67% symptom; P = .01).
FIGURE 2—
Caregivers’ School Attendance Perceptions Before and After Enrollment in an In-Home COVID-19 Rapid Antigen Testing Program: Wisconsin, May 2021–January 2022
SUSTAINABILITY
In a pediatric cohort with neurologic impairment and chronic respiratory failure, the combination of high fidelity to testing frequency, test tolerability, and no attrition confirmed the feasibility of regular in-home COVID-19 testing through the ReSET program. Limitations included the single-center design and a relatively small convenience sample. False positives were rare, consistent with published BinaxNOW rapid antigen specificity greater than 99%10,11; however, confirmation in our real-world high-risk population is a valuable contribution.
Because nearly all positive tests occurred in symptomatic CMC (whether in the surveillance- or symptom-testing cohort), continuing the program with symptom testing only may be the most efficient strategy to sustainably identify cases. Quantifying false negatives is an important step: data suggest that lower sensitivity may occur when testing is conducted by non–health care professionals.10
PUBLIC HEALTH SIGNIFICANCE
The prominent role that in-home COVID-19 rapid antigen testing has in long-term pandemic mitigation was underscored by the December 2021 federal announcement that 500 million rapid tests would be freely distributed to US households.12 Although testing enthusiasm waned with time even in a high-risk population, contextual factors likely influenced enthusiasm as much as, or more than, community transmission rates. Public health professionals seeking to motivate test uptake during periods of high community transmission likely need to identify and incorporate contextual factors (e.g., new school year, virulence) in messaging to sustain enthusiasm in communities. Finally, access to in-home testing appears to have complicated relationships with school safety perceptions (e.g., perceptions were improved only among those opting for symptomatic testing). Interventions should address the concerning proportion of CMC who have not yet returned to school.
ACKNOWLEDGMENTS
This research was, in part, funded by the National Institutes of Health (NIH; agreement no. 1 OT2 HD107558-01; award number OT2 HD107558). The project was additionally supported by the Clinical and Translational Science Award program through the NIH National Center for Advancing Translational Sciences (grant UL1TR002373).
Note. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
HUMAN PARTICIPANT PROTECTION
The study was approved by the University of Wisconsin-Madison institutional review board, and participants received $250 per quarter for participation.
REFERENCES
- 1.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463–e1470. doi: 10.1542/peds.2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kompaniyets L, Agathis NT, Nelson JM, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4(6):e2111182. doi: 10.1001/jamanetworkopen.2021.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doron S, Ingalls RR, Beauchamp A, et al. Weekly SARS-CoV-2 screening of asymptomatic kindergarten to grade 12 students and staff helps inform strategies for safer in-person learning. Cell Rep Med. 2021;2(11):100452. doi: 10.1016/j.xcrm.2021.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly MM, DeMuri GP, Barton HJ, et al. Priorities for safer in-person school for children with medical complexity during COVID-19. Pediatrics. 2022;149(3):e2021054434. doi: 10.1542/peds.2021-054434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherby MR, Kalb LG, Coller RJ, et al. Supporting COVID-19 school safety for children with disabilities and medical complexity. Pediatrics. 2022;149(12 suppl 2):e2021054268H. doi: 10.1542/peds.2021-054268H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin R. Supporting the use of at-home COVID-19 testing. JAMA. 2021;326(23):2354. doi: 10.1001/jama.2021.21933. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention, COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauer J. Identifying and managing sources of pain and distress in children with neurological impairment. Pediatr Ann. 2010;39(4):198–205. doi: 10.3928/00904481-20100318-04. [DOI] [PubMed] [Google Scholar]
- 10.Frediani JK, Levy JM, Rao A, et al. Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration. Sci Rep. 2021;11(1):14604. doi: 10.1038/s41598-021-94055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020 MMWR Morb Mortal Wkly Rep. 2021703100–105.. [Erratum in: MMWR Morb Mortal Wkly Rep 10.15585/mmwr.mm7003e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettypiece S.2021. https://www.nbcnews.com/politics/white-house/biden-administration-make-500-million-home-covid-tests-available-free-n1286356