Abstract
Background
Radiotherapy and chemotherapy are used to improve survival in colorectal cancer but adverse effects can be a problem. Severe adverse effects may result in dose reduction or cessation of treatment, which have an impact on survival. Coriolus versicolor (Trametes versicolor or 'Turkey Tail') mushroom and its extracts have been used by cancer patients to help with adverse effects.
Objectives
To assess the effects of adjunctive Coriolus versicolor (Trametes versicolor) and its extracts on adverse effects and on survival during colorectal cancer treatment (chemotherapy and radiotherapy) compared with no adjunctive treatment.
Search methods
We searched databases including CENTRAL, MEDLINE, Embase, AMED and CINAHL, Chinese and Japanese databases, and trials registers to 12th April 2022 without restriction of language or publication status. We screened reference lists and attempted to contact researchers in the field to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) investigating the efficacy and safety of Coriolus versicolor and its extracts in adult participants with a confirmed diagnosis of colorectal cancer, in addition to conventional treatment. Interventions included any preparation of Coriolus versicolor (raw, decoction, capsule, tablet, tincture, extract, injection), any part of the fungus (cap, stem, mycelium or whole), in any dose or regimen. Outcomes included adverse events rates, survival, disease progression and recurrence, response rates and quality of life.
Data collection and analysis
Two review authors independently screened and selected studies, extracted outcome data, and assessed risk of bias. We evaluated the overall certainty of evidence using the GRADE approach.
Main results
We included seven parallel RCTs (1569 participants). Six studies (1516 participants) were conducted in Japan and one study (53 participants) in China. Studies included both male and female participants with colorectal cancer (five studies), colon cancer (one study) or rectal cancer (one study). Participants were diagnosed with cancer ranging from stage II to stage IV. Coriolus was used in the form of an extract in all seven studies and was generally used after curative resection, although in one study it was used preoperatively. Duration of treatment with the extract varied between four weeks and three years. Chemotherapeutic regimens in six studies consisted of an oral fluoropyrimidine which was preceded by weekly intravenous 5‐Fluorouracil (5‐FU) in one study, by mitomycin C in two studies, and which was combined with folinic acid (Leucovorin) in two studies and with radiotherapy preoperatively in one study. XELOX (oxaliplatin intravenous infusion and capecitabine) was used in the remaining study.
We found very low‐certainty evidence of little to no effect of adjunctive treatment with Coriolus (in the form of an extract, polysaccharide‐Krestin, PSK) on withdrawal from treatment due to adverse events (risk ratio (RR) 1.03, 95% confidence interval (CI) 0.45 to 2.34; 703 participants; 3 studies;). We are uncertain whether adjunctive Coriolus versicolor and its extracts compared to usual care alone resulted in a difference in adverse events including neutropenia (RR 0.41, 95% CI 0.24 to 0.71; 133 participants; 3 studies; very low certainty), oral cavity disorders such as oral dryness and mucositis (RR 0.37, 95% CI 0.13 to 1.03; 1022 participants; 5 studies; very low certainty), nausea (RR 0.73, 95% CI 0.44 to 1.22; 969 participants; 4 studies; very low certainty), diarrhoea (RR 0.77, 95% CI 0.32 to 1.86; 1022 participants; 5 studies; very low certainty), and fatigue (RR 0.76; 95% CI 0.33 to 1.78; 133 participants; 3 studies; very low certainty).
We found low‐certainty evidence of a small effect of adjunctive Coriolus on improved survival at five years compared with no adjunctive care (RR 1.08, 95% CI 1.01 to 1.15; 1094 participants; 3 studies; number needed to benefit (NNTB) = 16 (95% Cl 9 to 70). The effect at earlier time points was unclear.
Authors' conclusions
Due to the very low certainty of evidence, we were uncertain about the effect of adjunctive Coriolus (in the form of an extract PSK) on adverse events resulting from conventional chemotherapy for colorectal cancer. This includes effects on withdrawal of treatment due to adverse events and on specific adverse outcomes such as neutropenia and nausea. The uncertainty in the evidence also means that it was unclear whether any adverse events were due to the chemotherapy or to the extract itself. While there was low‐certainty evidence of a small effect on overall survival at five years, the influence of reduced adverse effects on this could not be determined. In addition, chemotherapy regimens used in assessing this outcome do not reflect current preferred practice.
Keywords: Adult, Female, Humans, Male, Agaricales, Colorectal Neoplasms, Colorectal Neoplasms/drug therapy, Colorectal Neoplasms/radiotherapy, Drug-Related Side Effects and Adverse Reactions, Nausea, Neutropenia, Polyporaceae, Randomized Controlled Trials as Topic, Trametes
Plain language summary
Coriolus versicolor mushroom in colorectal cancer
Review question
Does adding Coriolus to colorectal (bowel) cancer treatment reduce side effects and improve survival?
Background
Radiotherapy and chemotherapy are used to treat colorectal cancer but side effects can be a problem. Coriolus versicolor mushroom (also known as Trametes versicolor or 'Turkey Tail') and its extracts have been used by cancer patients to help with side effects.
Search date
We searched medical databases for trials comparing Coriolus plus chemotherapy or radiotherapy to chemotherapy or radiotherapy alone in adults (aged 18 years or greater). The evidence is current to April 2022.
Study characteristics
We included seven trials with 1569 participants who were men and women with stage 2 to 4 cancer. Six studies were carried out in Japan and one study was carried out in China. Studies measured changes in survival, frequency of side effects and changes to treatment due to side effects. One study reported on quality of life. All studies used an extract of Coriolus known as Polysaccharide‐Krestin (PSK).
Study funding sources
No study reported any information on funding.
Key results
We found that it was unclear if adding Coriolus made any difference to the number of patients whose treatment had to be stopped because of side effects.
We found that the evidence was also uncertain about whether adding Coriolus to treatment reduced side effects caused by chemotherapy or radiotherapy. We looked at evidence for a range of different side effects including effects on blood tests, and problems such as inflammation of the mouth, nausea and diarrhoea.
We found low‐certainty evidence of a small effect of adding Coriolus on survival at five years compared with no added Coriolus. Effects before five years were not clear. Cancer stages varied as did cancer treatment. Patients in some studies were being treated with combinations of cancer drugs that are not widely used in practice now and most of the studies were carried out some time ago.
Certainty of the evidence
Participants in all the studies were aware of whether they had been treated with Coriolus and this may have influenced their reporting of changes in nausea and other self‐reported problems. It should not have made any difference to laboratory tests such as blood tests. Some of the methods reported in studies were unclear and few patients were included in many of the comparisons that we examined. We found that the evidence was either low or very low certainty for all comparisons.
Summary of findings
Summary of findings 1. Adjunctive Coriolus versicolor compared to no adjunctive treatment for colorectal cancer treatment.
| Adjunctive Coriolus versicolor compared to no adjunctive treatment for colorectal cancer treatment | ||||||
| Patient or population: adults (aged 18+) with colorectal cancer Setting: hospital outpatient Intervention: Coriolus versicolor adjunctive to chemotherapy Comparison: chemotherapy without adjunctive treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no adjunctive treatment | Risk with Adjunctive Coriolus versicolor | |||||
| Survival ‐ Follow‐up: 5 years | Study population | RR 1.08 (1.01 to 1.15) | 1094 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 | May be a small improvement in survival with PSK at 5 years but not relevant to current therapy and, thus, unclear whether any advantage currently | |
| 746 per 1,000 | 806 per 1,000 (754 to 858) | |||||
| Withdrawal from treatment due to adverse events ‐ Follow‐up 6 months to 5 years | Study population | RR 1.03 (0.45 to 2.34) | 703 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | Uncertain whether there is a difference | |
| 32 per 1,000 | 32 per 1,000 (14 to 74) | |||||
| Adverse events: Oral cavity disorders (includes oral dryness and mucositis)‐ Follow‐up 6 months to 7 years | Study population | RR 0.37 (0.13 to 1.03) | 1022 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 | Uncertain whether there is a difference |
|
| 29 per 1,000 | 11 per 1,000 (4 to 30) | |||||
| Adverse events: Nausea ‐ Follow‐up 4 weeks to 7 years | Study population | RR 0.73 (0.44 to 1.22) | 969 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 | Uncertain whether there is a difference | |
| 66 per 1,000 | 48 per 1,000 (29 to 80) | |||||
| Adverse events: Diarrhoea ‐ Follow‐up 4 weeks to 7 years | Study population | RR 0.77 (0.32 to 1.86) | 1022 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 | Uncertain whether there is a difference | |
| 80 per 1,000 | 61 per 1,000 (26 to 148) | |||||
| Adverse events: Neutrophils decreased ‐ Follow‐up 4 weeks to 3 years | Study population | RR 0.41 (0.24 to 0.71) | 133 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 5 | Uncertain whether there is a difference | |
| 364 per 1,000 | 149 per 1,000 (87 to 258) | |||||
| Adverse events: Fatigue ‐ Follow‐up 4 weeks to 3 years |
Study population | RR 0.76 (0.33 to 1.78) | 133 (3 RCTs) |
⊕⊝⊝⊝
VERY LOW 4 |
Uncertain whether there is a difference |
|
| 227 per 1,000 |
173 per 1,000 (75 to 405) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for indirectness as the chemotherapy regimens include those not reflecting current preferred practice and one level for imprecision as the total number of events was less than 300.
2 Downgraded one level for indirectness as the chemotherapy regimens include those not reflecting current preferred practice, one level for risk of performance and detection bias, and two levels for imprecision as the total number of events is less than 300 and the CI includes both no effect and an important effect.
3 Downgraded one level for indirectness as the chemotherapy regimens include those not reflecting current preferred practice, one level for risk of selection, performance and detection bias, and one level for imprecision as the total number of events is less than 300.
4 Downgraded one level for indirectness as the chemotherapy regimens include those not reflecting current preferred practice, one level for risk of selection, performance and detection bias, and two levels for imprecision as the total number of events is less than 300 and the CI includes both no effect and an important effect.
5 Downgraded one level for indirectness as the chemotherapy regimens include those not reflecting current preferred practice, one level for risk of selection, performance, detection and attrition bias, and one level for imprecision as the total number of events is less than 300.
Background
Description of the condition
Approximately 19.3 million new cases of cancer were diagnosed worldwide in 2020 (Sung 2021), increasing from 18.1 million in 2018 (Bray 2018a). Colorectal cancer is the third most common cancer, with an increasing incidence in the developing world (Bray 2018b). Colorectal cancer includes colon (large bowel) cancer and cancer of the rectum (the last portion of the gastro‐intestinal tract before the anus). It may result in blood in the stools and anaemia, and gastro‐intestinal symptoms including change in bowel habit and abdominal pain (Astin 2011). The treatment of colorectal cancer may involve surgery, systemic anti‐cancer therapy (chemotherapy and targeted monoclonal antibodies designed to target specific types of cells) or radiotherapy (ESMO 2014; ASCO 2020). Both the disease and its treatment may cause symptoms such as fatigue, anorexia, or depression, which adversely affect the quality of life of patients with colorectal cancer (Gray 2011). Chemotherapy regimens incorporating oxaliplatin or irinotecan, or both, may be more effective at preventing recurrence or delaying disease progression than those based solely on 5‐fluorouracil/leucovorin, but serious adverse events and discontinuation of treatment due to toxicity are more frequent (André 2004; Pandor 2006; Schmoll 2007). Treatment‐related adverse effects, including diarrhoea, neutropenia (low levels of neutrophils), stomatitis (inflammation of the mouth), nausea and vomiting, peripheral neuropathy (nerve damage), and hand‐foot syndrome are commonly reported and toxicity may be severe in up to 57% of patients (Grothey 2018), requiring dose reduction or withdrawal of potentially life‐saving or life‐prolonging treatment. Thus, there is a need for adjunctive therapies that can support colorectal cancer patients through conventional cancer therapy by alleviating symptoms and side effects without adversely affecting overall survival.
Description of the intervention
Mushrooms have a long history of use to promote health in China and Japan and, as some mushroom preparations are orally bioavailable, they are relatively easy to administer (Lindequist 2005). Medicinal mushrooms appear to have low toxicity and immune‐modulating and health‐promoting properties have been reported based on pre‐clinical studies (Jeitler 2020). Numerous studies have also been conducted in humans and several mushroom extracts are licensed as adjunctive treatments in oncology practice in Japan (Kidd 2000; NCI 2022). There has been increasing interest in Western countries in the reported health benefits of various mushrooms (Venturella 2021), and specifically in whether there is a potential role in oncology (Jeitler 2020; Standish 2008). The four mushrooms commonly used clinically are Coriolus versicolor (C. versicolor ) (also known as Trametes versicolor, Japanese and Chinese names Kawaratake and Yun Zhi, respectively), Ganoderma lucidum (Reishi or Ling Zhi), Lentinula edodes (Shiitake or Hua Gu/Xiang Gu) and Grifola frondosa (Maitake or Hui Shu Hua) (Smith 2002; NCI 2022).
The 'Turkey Tail' fungus (Coriolus versicolor (L.) Quélor Trametes versicolor (L.) Lloyd), the focus of this review, has a colourful fruiting body with features that resemble a 'turkey tail' (Smith 2002). It grows on dead logs in many countries worldwide and is not edible. Hot‐water extracts of the whole fruiting body have been used in traditional Chinese medicine since historic times (Smith 2002). Two commercial extracts have been used clinically in the Far East: polysaccharide‐Krestin (PSK) and polysaccharopeptide (polysaccharide‐peptide or PSP) (NCI 2022). Both are orally bioavailable extracts from the cultured mycelium (the filamentous part of the mushroom that grows through the soil) of the Coriolus versicolor fungus. PSK (or Krestin®) was developed in Japan in the 1960s and is a soluble protein‐bound polysaccharide derived from the CM‐101 strain of the fungus (Wan 2013). Production of Krestin was discontinued in Japan from September 2017 (Daiichi Sankyo 2017), but similar products are still available. PSP was developed in China in the 1980s and is a polysaccharide‐peptide derived from the COV‐1 strain (Kidd 2000; Smith 2002). The molecular weights of the two preparations (about 100 kDa) and the biochemical compositions are similar, but not identical. Commercial cultivation and production aims to ensure a steady supply of the fungus, control of the polysaccharide concentration (which varies considerably in the plant depending on growth phase and storage conditions), and purity of the final product. Many products derived or extracted from Coriolus are available online, however, over‐the‐counter products may not be standardised (Memorial Sloan‐Kettering 2022).
Coriolus extracts are used as adjunctive therapy for treating cancers, either combined with herbal mixtures in Asian cultures, or combined with conventional chemotherapy/radiotherapy, and they have been reported to have an effect by boosting suppressed immune function, extending the survival rate and improving quality of life (Eliza 2012).
Recommended doses vary according to the preparation. In the Chinese Pharmacopoeia, the recommended dose of Coriolus versicolor is 9 g to 27 g used for decoction daily (Chinese Pharmacopoeia 2015 and Chinese Pharmacopoeia 2020) The daily dose of the authorised PSK product (Krestin®), according to the manufacturer, is 3 g (Daiichi‐Sankyo 2012). Doses of PSK most commonly used in clinical trials in cancer have been between 1 g and 3.6 g daily (Eliza 2012). Data available on pharmacokinetics are based mainly on studies in animal (small mammal) models. These indicate that PSK is rapidly absorbed and partly metabolised in the gastro‐intestinal tract. Peak plasma levels occur between 0.5 to 2 hours for small molecules and 4 to 24 hours for large molecules (Ikuzawa 1988). Excretion is primarily through the lungs with 70% excreted in expired air after 24 hours (Daiichi‐Sankyo 2012; Ikuzawa 1988). Radiolabelled PSK or its metabolites are also excreted in the urine and faeces with 86% excreted within 24 hours (Ikuzawa 1988). Adverse interactions between Coriolus versicolor mushroom and herbs or drugs have not been reported except for a potential interaction with cyclophosphamide (Natural Database 2022). PSP has been shown to affect phase I metabolism and hepatic cytochrome P450 in animal models, but the potential for clinically significant interactions in humans is low (Yeung 2012).
How the intervention might work
Coriolus versicolor has been used in traditional Chinese medicine as a general 'tonic' for anorexia, fatigue, and lack of strength (Chinese Pharmacopoeia 2015 and Chinese Pharmacopoeia 2020). It was recorded as an 'immune modulator' in the 2005 Chinese Pharmacopoeia (Chinese Pharmacopoeia 2005) although not in the more recent editions. The pharmacological actions of mushrooms have been studied extensively in Japan and China in animal and human studies (Rowan 2003; Sullivan 2006; Venturella 2021). These studies support the idea that they are biological response modifiers that act by stimulating the non‐specific immune system (Lindequist 2005; Ng 1998). Immune activating properties of both mycelium and its fermented substrate have been demonstrated in vitro (Benson 2019).
PSK has been shown to restore immune systems depressed by chemotherapy to normal levels in animal studies, and has been reported to improve survival in clinical studies (Sakamoto 2006). PSK is also reported to attenuate the adverse reactions induced by chemotherapy or radiotherapy, including neutropenia (Maehara 2012). Similar effects are reported for PSP: results of clinical trials in China indicated reduction in chemotherapy‐induced adverse effects, including vomiting, and restoration of chemotherapy‐induced immunosuppression when PSP was used in combination with cytotoxic agents (Chan 2006). Such findings suggest that these extracts have the potential to improve tolerance to chemotherapy and radiotherapy and to reduce adverse effects due to depressed immune function. Both products also appear to have anti‐tumour properties, which may contribute to an overall effect on survival (Eliza 2012) .
The mechanism of action is yet to be fully established. The immune‐potentiating activity is attributed to mushroom proteoglycans: these proteoglycans comprise a central linear polypeptide chain with multiple side‐branches of beta‐D‐glucans (Kidd 2000). Multiple potential actions have been reported including suppression of tumour cell growth, reversal of immune suppression, and an increase in white blood cell counts, mediated in part by scavenging of free radicals by PSK (Fisher 2002). Reviews of the biological effects of PSK suggest that it has beneficial effects on cytokines ('chemical messengers') including tumour necrosis factor‐alpha (TNFα), interferon‐gamma (IFNγ), and interleukin‐2 (IL‐2) (Standish 2008). Animal studies indicate that PSK does not affect the normally functioning immune response but can contribute to the restoration of a response depressed by tumour burden or chemotherapy. Specific mechanisms of extracts of Coriolus considered to be involved include production of antibodies and cytokines, and improved activity of natural killer cells, T cells, macrophages, and peripheral blood lymphocytes (Chang 2017).
While immune‐stimulatory actions may contribute to the apparent beneficial effects of these extracts, the mechanism of action underlying reports of reduced adverse effects when these extracts are used in combination with anticancer agents is as yet unclear. Effects on the pharmacokinetics of cytotoxic agents have been observed in some animal studies but results from human studies indicate that clinically significant effects are unlikely (Chan 2006).
Why it is important to do this review
For colorectal cancer patients in particular, radiotherapy and chemotherapy are used to improve survival, but side effects can be a problem with significant numbers of patients suffering severe effects, resulting in dose reduction or cessation of treatment, which have an impact on survival (Grothey 2018; Roeder 2020). Some of these side effects, such as fatigue and peripheral neuropathy, can persist into the survivorship period (Han 2020) and, as more cancer patients survive, the prevalence of those with persistent side effects increases. This is particularly relevant as over 50% of colorectal cancer patients in the UK currently survive more than 10 years (ONS 2019). There is a need for adjunctive therapies that can support colorectal cancer patients through conventional cancer therapy by alleviating symptoms and side effects without adversely affecting survival.
Coriolus versicolor is included in several resources aimed at cancer patients and health professionals (e.g. NCI 2022), and a number of reviews of the effects of mushrooms in cancer have been conducted previously. Within the Cochrane Database of Systematic Reviews, a review of Ganoderma lucidum (Reishi mushroom) for cancer treatment concluded that there was insufficient evidence to support its use as a first‐line treatment for cancer or long‐term cancer survival, but that it could be considered for use as an alternative adjunct to conventional treatment due to its potential to enhance tumour response and stimulate host immunity while being generally well‐tolerated (Jin 2016). Several published systematic reviews have focused on Coriolus versicolor and its extracts. One meta‐analysis published in 2012 focused on the effects of Coriolus versicolor on survival in cancer patients and concluded that there was strong evidence of a beneficial effect on survival, particularly in breast, gastric, and colorectal cancer patients (Eliza 2012). However, only research published up to 2003 was included. A second meta‐analysis focused on the efficacy of PSK for survival of patients with curatively resected colorectal cancer; PSK used as an adjuvant to conventional chemotherapy improved overall and disease‐free survival, but risk of bias and adverse events were not addressed (Sakamoto 2006). A review of safety data relating to medicinal mushrooms in cancer patients showed no evidence of cytotoxicity, mutagenicity, teratogenicity, effects on female ovulation, or reproduction at acute or chronic doses (Smith 2002). Adverse effects that have been reported include possible darkening of the fingernails and faeces (Kidd 2000). One review has assessed Coriolus efficacy and safety for a range of cancers (Zhong 2019), but no systematic review has been performed to evaluate the strength of evidence on the effects of Coriolus versicolor and its extracts on both adverse effects during cytotoxic treatment and on survival in colorectal cancer. Thus, a review focusing on evaluating the evidence on the adjuvant effects of Coriolus versicolor and its extracts in colorectal cancer was warranted.
Objectives
Primary objective
To assess the effects of adjunctive Coriolus versicolor (Trametes versicolor) and its extracts on adverse effects during cancer treatment (chemotherapy and radiotherapy) compared with no adjunctive treatment.
Secondary objectives
To assess effects due to adjunctive Coriolus versicolor (Trametes versicolor) and its extracts on survival, recurrence, and disease progression compared with no adjunctive treatment.
To evaluate the evidence in relation to the type of preparation of the mushroom (e.g. whole fresh, dried, or extract).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) investigating the efficacy and safety of Coriolus versicolor and its extracts in participants with a confirmed diagnosis of colorectal cancer, in addition to conventional treatment. We included trials regardless of language and publication status. We considered all types of RCT design for inclusion, e.g. parallel‐group and cluster‐RCTs.
We only included trials in different types of cancer if data for colorectal cancer patients were reported separately.
Types of participants
Adult patients (minimum age 18 years) diagnosed with colorectal cancer regardless of tumour stage, age, or gender. Diagnosis must have been confirmed by biopsy and tumour site and stage reported. No limitations were set in terms of location, setting, or other demographic factors.
Types of interventions
Interventions included any preparation of Coriolus versicolor (raw plant, decoction, capsule, tablet, tincture, extract, injection), any part of the mushroom (cap, stem, mycelium or whole), in any dose and regimen.
Trials were included that compared:
conventional treatment (chemotherapy with or without radiotherapy) plus Coriolus versicolor versus conventional treatment alone;
conventional treatment and a complementary therapy plus Coriolus versicolor versus the same treatment without the extract;
conventional treatment plus Coriolus versicolor versus conventional treatment plus placebo.
Types of outcome measures
We selected outcomes that directly measured effects on adverse events and those that may indicate indirect effects, such as withdrawal of treatment due to adverse events, which could ultimately affect survival and recurrence.
Primary outcomes
Overall survival at one year, three years, and five years.
Adverse event rates: the incidence of all reported adverse events or toxicities, including modification of treatment or withdrawal from the trial due to adverse events.
Secondary outcomes
Disease progression at one year (plus three years and five years, if data were available) confirmed radiologically or proven by biopsy.
Disease recurrence at one year (plus three years and five years, if data were available) confirmed radiologically or proven by biopsy.
Response rates based on recommended criteria (World Health Organization (WHO) criteria or RECIST ‐ Response Evaluation Criteria in Solid Tumors) for solid tumours (Therasse 2000).
Quality of life evaluated using any validated assessment tool.
Search methods for identification of studies
We developed the MEDLINE search strategy and search strategies for databases other than MEDLINE in close collaboration with the Cochrane Colorectal Cancer Group Information specialist. For search strategies, see Appendix 1
We imposed no language restrictions and native speakers translated studies when required.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2022 Issue 4) in the Cochrane Library (searched 12 April 2022)
MEDLINE Ovid (1946 to 12 April 2022)
Embase Ovid (1974 to 12 April 2022)
AMED Ovid (Allied and Complementary Medicine; 1985 to 12 April 2022)
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 12 April 2022)
Natural Medicines database Therapeutic Research Center: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements.aspx (2022; searched 12 April 2022)
Global Index Medicus (http://www.globalindexmedicus.net/) (searched 12 April 2022)
BIOSIS Previews (inception to 2008)*
*Access to BIOSIS Previews only included records to 2008 as no further access could be obtained by the authors through their organisations but no unique studies were found on BIOSIS Previews and all included studies were indexed on databases other than BIOSIS Previews.
Trials registers
ClinicalTrials.gov (http://clinicaltrials.gov/) (searched 12 April 2022)
WHO International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/trialsearch) (searched 12 April 2022)
Chinese databases
Chinese Science and Technology Periodical Database (CQVIP) (www.cqvip.com)(1989‐16 April 2022)
Chinese National Knowledge Infrastructure Databases (CNKI) (www.cnki.net)(1979‐16 April 2022)
Wanfang Data’s Chinese Online Journals database (http://www.wanfangdata.com/)(1985‐16 April 2022)
Chinese Biomedical Database web(CBM/Sinomed) (www.imicams.ac.cn).(1978‐16 April 2022)
Japanese databases
Ichushi Web (http://www.jamas.or.jp), web version of Igaku Chuou Zasshi (Japana centra revuo medicina) (from inception to 25 April 2022)
Searching other resources
We checked the reference lists of all retrieved studies and relevant reviews for further relevant studies. We handsearched the most recent year of the specialist International Journal of Medicinal Mushrooms to ensure that no relevant publications were missed that had not yet been indexed in the major databases (search carried out in May 2022). We attempted to contact authors and the manufacturer to check for unpublished trials (October 2020).
Data collection and analysis
Selection of studies
Two review authors independently examined all titles and abstracts retrieved by the searches of English databases (KP and LSW) and the Chinese and Japanese databases (JPL, XYJ and LT). If a record (title or abstract) could not be rejected with certainty, we obtained the full‐text article for further evaluation. We excluded duplicate records but retrieved all publications pertaining to a relevant trial. Disagreements were resolved by discussion and a third review author was available had there been any disagreements. We documented the reasons for the exclusion of studies.
Data extraction and management
Two review authors (KP and LSW) independently extracted the following information: patients (number randomised and analysed, age, sex, stage, treatment situation, setting), methods (design, observation period, analysis), interventions (type of preparation, application, dose and duration, control procedure, cancer treatments), outcomes and results (reports of adverse events, survival data, data on quality of life (QoL), number of dropouts, follow‐up). We used a pre‐defined data extraction form for recording relevant data. Differences between review authors were resolved by discussion or, if necessary, by consulting a third review author. Cancer stage(s) and chemotherapy regimen in each study were also assessed by a clinical expert (DS).
In cases where numerical data were not presented in the study reports but there was information from figures or graphs, one review author (LSW) extracted estimates of numerical data from the figures using WebPlotDigitizer (WebPlotDigitizer 2020). The extracted data were visually compared to the figures by a second review author (KP).
For the trial that was only published in Chinese, two independent translations were obtained. Data were extracted from these by two review authors (KP and LSW) and the extracted data were then checked by a review author from China (XYJ). For studies where the primary publication was in English and supplementary publications were in English and Japanese, two review authors (KP and LSW) extracted data from the primary and any further publications in English and contacted the review author in Japan (LT) to check for any additional data provided by a Japanese publication.
Assessment of risk of bias in included studies
Two review authors (KP and LSW) independently assessed the trials using the Cochrane tool for assessing risk of bias (Higgins 2011). Differences were resolved by discussion or, if necessary, by consulting another review author. Assessment of risk of bias (Appendix 2) considers the following and we assessed this as per the criteria for judging risk of bias in the risk of bias assessment tool (Table 8.5.c) in the Cochrane Handbook for Systematic Reviews of Interventions.
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcomes assessors.
Incomplete outcome data.
Selective outcome reporting. This followed the methods as per the Cochrane risk of bias assessment mentioned above. However, as a minimum we expected that trials should report adverse event data and survival or tumour response/disease progression.
Other possible sources of bias: This includes potential biases that do not correspond to the above categories. They may include study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent.
Measures of treatment effect
We extracted the number of patients in each treatment arm who experienced the outcome of interest, in order to estimate a risk ratio (RR). If sufficient data were available, we categorised these according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (NCI 2017). We planned to compare rates of adverse events at each grade of severity (NCI 2017). If data had been available, we planned to categorise adverse events as acute (during and up to six weeks after treatment) and late (after six weeks). Data for this were not available.
We present dichotomous data as RR with corresponding 95% confidence interval (CI). We present continuous data as a mean difference (MD) for common measurement units or a standardised mean difference (SMD) for differing measurement units and different scales, along with corresponding 95% CIs. We calculated effect size and 95% CI for all primary and secondary outcomes. Where possible, all data extracted are those relevant to an intention‐to‐treat analysis, in which participants are analysed in the groups to which they were assigned. We also noted the time points at which outcomes were collected and reported.
Unit of analysis issues
For individual trials, the unit of analysis is the individual patient. We had planned for the analysis of cluster‐randomised study designs, but no trials using this design were identified. See Appendix 3 for further details.
Dealing with missing data
We attempted to contact study authors for additional information on the studies. The contact author or senior author was contacted for all except one study where contact details could not be located. No responses were received. If contacting authors proved unsuccessful, we had planned to investigate the potential impact of the missing data (for further details, see Appendix 3). However, reporting of the trials was not sufficient to allow accurate assessment of the extent to which data were missing.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by a formal statistical test of the significance of the heterogeneity (I2) and, where possible, by subgroup analyses. Where heterogeneity was detected (I2 ≥ 50%), we attempted to investigate possible reasons for this and consider whether it was appropriate to report a pooled estimate. Where there was evidence of substantial heterogeneity, we attempted to investigate and report the possible reasons.
Assessment of reporting biases
We had planned to examine funnel plots to assess the potential for small‐study effects such as publication bias. We planned to check the symmetry of the funnel plot if a sufficient number of trials had been included in a meta‐analysis (more than 10 trials). As only seven trials were located, we were unable to assess publication bias.
Data synthesis
Where sufficient clinically similar studies were available, we pooled their results in meta‐analyses.
For any dichotomous outcomes, we calculated the RR for each study and then pooled these.
For continuous outcomes, we planned to pool the MDs between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we planned to pool SMDs. As a continuous outcome was only reported by one trial, pooling was not possible.
We used random‐effects models with inverse variance weighting for all primary analyses. We performed all analyses using the Cochrane statistical software, Review Manager 5.3 (RevMan 2014). One review author (LSW) oversaw all steps of data synthesis. We have summarised the results of included trials descriptively in tables.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis according to type of Coriolus versicolor preparation, dose, type of cancer (colon or rectal), stage of cancer and chemotherapy regimen. Two different commercial extracts have been used clinically in the Far East: polysaccharide‐Krestin (PSK) and polysaccharopeptide (polysaccharide‐peptide or PSP) (NCI 2022). We planned to compare the effects of these extracts and any other preparations of Coriolus that had been tested in relevant trials. The type of cancer, whether colon or rectal, has an influence on the treatment approach and the role of chemotherapy versus surgery (NICE 2020). There are also differences in recurrence rates (NICE 2020). Stage of cancer is also relevant for similar reasons. The chemotherapy regimen with which the Coriolus extract is given may affect outcomes as the nature and frequency of adverse effects vary according to the drugs involved.
Sensitivity analysis
We planned to perform sensitivity analysis excluding studies considered to be at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
Two review authors (KP and LSW) independently evaluated the certainty of evidence for each estimate of effect using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Differences were resolved by discussion or, if necessary, by consulting another review author. The certainty of evidence for the primary outcomes of survival, and adverse events was presented in 'Table 1'. Among the survival outcomes we chose five‐year survival as it was the longest prespecified survival point, and among adverse events we chose the adverse events that our clinical author (DS) identified as most concerning to patients, i.e. oral dryness and mucositis, nausea, diarrhoea, decreased neutrophils, and fatigue.
The GRADE system classifies the certainty of evidence in one of four grades.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The certainty of evidence can be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes) and imprecision (wide confidence interval, single trial). The certainty of evidence might be upgraded by one level due to a large summary effect.
We used GRADE Pro (see https://gradepro.org/cite/) to generate the summary of findings table.
Results
Description of studies
Results of the search
A total of 1355 records were retrieved across the databases and other sources. After deduplication, we screened titles and abstracts of 998 records and the full text of 101 records. Of the 53 records excluded at the full‐text stage, 10 were not original study reports and the remaining 43 studies did not match the inclusion criteria (see Excluded studies for details).
We identified 48 records corresponding to 17 studies: seven studies (reported in 38 publications) met the inclusion criteria and were included in the review, and 10 studies (10 records) are awaiting classification. Several studies were associated with multiple reference records. In all cases, the study rather than the reference was the unit of interest in the review. The flow of studies is presented in the PRISMA chart in Figure 1.
1.
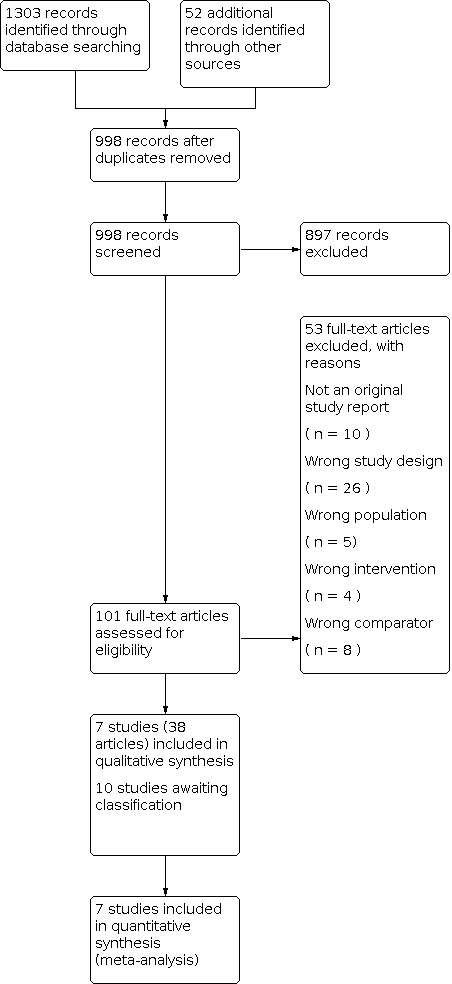
Study flow diagram.
Included studies
General characteristics
We included seven parallel RCTs (1569 participants). Six studies (1516 participants) were conducted in Japan (Ito 2004; Mitomi 1992; Ohwada 2004; Sadahiro 2010; Shichinohe 2013; Sugimoto 2012) and one study (53 participants) in China (Xu 2008). Six studies were published as full‐text papers and one was only available as a conference abstract (Shichinohe 2013). Results from the six studies from Japan were published in both English and Japanese and the study from China was published in Chinese.
Participants
Six studies included both male and female participants (one study did not report gender). Mean age of participants was between 60 and 65 years in three studies (Ito 2004; Sadahiro 2010; Sugimoto 2012); median age was 61 in one study (Xu 2008); two studies reported age range only and included participants under 40 years and up to 75 years (Mitomi 1992; Ohwada 2004) and one study did not provide details of the age of participants (Shichinohe 2013). In five studies, participants included those with colon and/or rectal cancer; in one study only those with colon cancer (Ito 2004) and, in the remaining study, only those with rectal cancer (Sadahiro 2010). Various methods were used for staging cancer including Dukes, numerical and TNM systems. All studies included patients with stage II and/or III disease, and one study had patients with stage III and IV disease (Xu 2008). In five studies, the Coriolus extract was used after curative resection, in one study it was used preoperatively (Sadahiro 2010) and, in one study involving advanced cancer patients, it was unclear if surgery had been carried out (Xu 2008). A summary of the cancer type, stage and treatment is shown in Additional Table 2.
1. Included studies: cancer type, stage and treatment regimen.
| Study | Type of cancer | Included patients (as reported) | Surgery | Radiotherapy | Post‐surgery, pre‐PSK treatment | PSK and chemotherapy | Comparison | Duration |
| Ito 2004 | Colon | Stage III (Dukes C) |
macroscopic curative resection | 5‐FU 48‐h IV infusion at a dose of 1,000 mg per m2 per 24 hr weekly for 3–4 weeks | Oral PSK 3g/day from the 29th postoperative day for 4 weeks followed by 4‐week oral 5‐FU treatment (200 mg/day) | oral 5‐FU chemotherapy (200 mg per day) at 4‐week intervals | 10 courses (10 months) | |
| Mitomi 1992 | Colorectal | Stage II and III (Any TN1,2,3 M0 T4N0M0) |
macroscopic curative resection | Mitomycin C (6mg/m2) IV on day of and day after surgery | Oral PSK 3g/day for 3 years plus oral 5‐FU (200mg/day) for 6 months | oral 5‐FU (200mg/day) for 6 months | 3 years | |
| Ohwada 2004 | Colorectal | Stage II or III | macroscopic curative resection | Mitomycin C (bolus injections of 12 and 8mg/m2) on postoperative days 1 and 2 respectively | Oral PSK (3g/day) and UFT (300 mg/day), starting 2 weeks after surgery and continuing | UFT (300 mg/day), starting 2 weeks after surgery and continuing | 2 years or until recurrence | |
| Sadahiro 2010 | Rectal | Stage II and III (cT3‐T4, Tx N+, M0) |
pre‐surgery | Preoperative CRT. External beam RT for a total dose of 20 Gy, 5 days a week for two weeks | PSK 3.0 g/day in 3 daily doses plus oral S‐1 80 mg/m2/day in 2 daily doses for 4 weeks until 3 days before surgery | oral S‐1 (Taiho Pharmaceutical Co., Japan) 80 mg/m2/day in 2 daily doses for 4 weeks until 3 days before surgery | 4 weeks | |
| Shichonohe 2013 | Colorectal | Stage III | curative resection | UFT+LV+PSK 28 days/5 weeks for 6 months, then UFT+PSK for 12 months (started within 6 months of surgery) | UFT+LV 28 days/5 weeks for 6 months, then UFT for 12 months | Up to 18 months | ||
| Sugimoto 2012 | Colorectal | Stage II and III (high‐risk stage II (T3─4N0M0) or stage III (T1─4N1─3M0)) |
curative resection | PSK (3 g/day) plus UFT (300 mg/m2 day) and LV (75 mg/day) starting 4〜8 weeks after surgery and continuing for 6 months or until recurrence (5 days‐on/2 days‐off schedule | UFT (300 mg/m2 day) and LV (75 mg/day) starting 4〜8 weeks after surgery and continuing for 6 months or until recurrence (5 days‐on/2 days‐off schedule | 6 months or until recurrence | ||
| Xu 2008 | Colorectal | Stage III and IV | Yunzhi polysaccharide (PSK) capsules 6 g/day for continuous oral administration plus XELOX regimen: oxaliplatin 130 mg/m2 intravenous infusion for 2 hours and capecitabine 2 000 mg/m2 from 1 day for a total of 14 days; 21 days is a course of treatment. | XELOX regimen: oxaliplatin 130 mg/m2 intravenous infusion for 2 hours and capecitabine 2 000 mg/m2 from 1 day for a total of 14 days; 21 days is a course of treatment. | 3‐8 cycles (median 4) |
Setting
The setting for treatment was unclear in most studies but it appeared that most participants were treated as outpatients. Treatment given orally or via an intravenous dose on the first one or two postoperative days, once weekly or once a month.
Intervention and comparators
The intervention in all seven studies was described as polysaccharide‐Krestin (PSK) used adjunctively; this was compared against no adjunctive treatment. PSK was administered orally at a dose of 3 g per day in all studies except one in which 6 g per day was given (Xu 2008). Treatment duration varied between four weeks and three years.
In one study, participants received PSK together with an oral fluoropyrimidine and radiotherapy prior to surgery (Sadahiro 2010). In the remaining six studies, PSK was used after surgery. In six studies, PSK was used alongside chemotherapy. Chemotherapeutic regimens in five studies consisted of an oral fluoropyrimidine, which was preceded by weekly intravenous 5‐fluorouracil (5‐FU) in one study (Ito 2004), and by mitomycin C in two studies (Mitomi 1992; Ohwada 2004), and which was combined with folinic acid (Leucovorin) in two studies (Shichinohe 2013; Sugimoto 2012). The XELOX regimen (oxaliplatin intravenous infusion and capecitabine) was used in the remaining study (Xu 2008).
Outcomes measured
All studies assessed adverse events but, in two studies (Ohwada 2004; Shichinohe 2013), data were not provided by group. Five studies measured overall survival as a primary outcome while the two remaining studies focused on adverse effects and completion (Sugimoto 2012) and on immune responses and adverse effects (Shichinohe 2013). Four studies investigated the impact of adverse events on modification or withdrawal of treatment (Ito 2004; Mitomi 1992; Ohwada 2004; Sugimoto 2012). Five studies reported on disease‐free survival and/or recurrence, but only one study assessed response and progression (Xu 2008). Quality of life was assessed in one study (Xu 2008). The outcomes measured by each study are presented in Table 3 and Table 4.
2. Studies that contributed to pooled effect estimates for each non‐adverse event outcome.
| Study | Overall survival | Disease‐free survival | Disease recurrence | Response/ Progression |
Treatment modification/ withdrawal |
Quality of Life |
| Ito 2004 | X | X | X | |||
| Mitomi 1992 | X | X | X | O | ||
| Ohwada 2004 | X | X | X | X | ||
| Sadahiro 2010 | ||||||
| Shichinohe 2013 | X | X | O | |||
| Sugimoto 2012 | X | X | ||||
| Xu 2008 | X | X | X |
X: Study contributed to the effect estimate for the outcome:
O: Study reported the outcome but did not contribute to the pooled effect estimate for the outcome
3. Studies that contributed to pooled effect estimates for each adverse event outcome.
| Study | AEs – blood lymphatic system | AEs ‐‐ cardiac system | AEs – GI system | AEs – general disorders | AEs ‐ investigations | AEs – metabolism/nutrition | AEs – nervous system | AEs – skin |
| Ito 2004 | X | X | X | X | X | |||
| Mitomi 1992 | X | X | X | X | X | X | ||
| Ohwada 2004 | O | O | O | O | ||||
| Sadahiro 2010 | X | X | X | X | ||||
| Shichinohe 2013 | O | O | ||||||
| Sugimoto 2012 | X | X | X | X | X | |||
| Xu 2008 | X | X | X | X | X | X | X |
X: Study contributed to the effect estimate for the outcome:
O: Study reported the outcome but did not contribute to the pooled effect estimate for the outcome
Funding sources
The source of funding was not reported in any study.
Excluded studies
Of the studies excluded at the full‐text stage, 36 were not RCTs, eight studies did not used a comparator of interest, in five studies the sample recruited did not match the population of interest, and four studies did not use the intervention of interest.
Several apparently relevant studies involved comparisons that did not meet the inclusion criteria. In two cases, this was a comparison of surgery with a combination of tegafur/uracil (UFT) and PSK (Okuno 2017; Okuno 2018). One study compared PSK against leucovorin (Miyake 2018), while another study involved appropriate test and comparator interventions but allocated patients to groups according to their 'pathological characteristics and treatment demands' (Li 2019). Two studies compared PSK against placebo after curative resection until recurrence or metastases occurred, at which point chemotherapy was introduced (Torisu 1986; Torisu 1990).
Risk of bias in included studies
A summary of the risk of bias is shown in Figure 2 and the risk of bias judgements for individual studies are shown in Figure 3. The studies contributing to each pooled effect estimate are shown in Table 3 (non‐adverse event outcomes) and Table 4 (adverse event outcomes).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three trials reported adequate randomisation methods or methods equivalent to randomisation including a modified minimisation method and permuted, stratified blocks and were judged to be at low risk of bias. The remaining four trials, which included one trial reported only as an abstract (Shichinohe 2013), provided few or no details of randomisation and the risk of bias was assessed as unclear. Three of the trials were at low risk (Ito 2004; Mitomi 1992; Ohwada 2004) and four trials were at unclear risk of bias (Sadahiro 2010; Shichinohe 2013; Sugimoto 2012; Xu 2008).
Two trials reported adequate allocation concealment through use of centralised allocation (Ito 2004; Ohwada 2004) with a low risk of bias. The remaining five trials (Mitomi 1992; Sadahiro 2010; Shichinohe 2013; Sugimoto 2012; Xu 2008) did not provide details of allocation concealment and so the risk of bias was assessed as unclear. Three of the trials contributing to meta‐analyses on overall survival (Ito 2004; Mitomi 1992; Ohwada 2004) were low risk of bias and two (Shichinohe 2013; Xu 2008) were unclear risk of bias.
No studies were assessed as at high risk of bias in this domain.
Blinding
All seven studies involved adjuvant Coriolus extract with no placebo in the control group and so were not blinded.
Blinding of participants and/or personnel
Overall survival
We judged that lack of blinding of participants and/or personnel would not likely influence the occurrence of this outcome. The five trials (Ito 2004; Mitomi 1992; Ohwada 2004; Shichinohe 2013; Xu 2008) that assessed overall survival we judged to be at low risk of bias for this outcome.
Disease recurrence
We judged that lack of blinding of participants and/or personnel would not likely influence the occurrence of this outcome. The three trials (Mitomi 1992; Ohwada 2004; Sugimoto 2012) that assessed disease recurrence we judged to be at low risk of bias for this outcome.
Disease‐free survival
We judged that lack of blinding of participants and/or personnel would not likely influence the occurrence of this outcome. The four trials (Ito 2004; Mitomi 1992; Ohwada 2004; Shichinohe 2013) that assessed disease‐free survival we judged to be at low risk of bias for this outcome.
Disease response/progression (RECIST criteria)
We judged that lack of blinding of participants and/or personnel would not likely influence the occurrence of this outcome. The one trial (Xu 2008) that assessed disease response/progression we judged to be at low risk of bias for this outcome.
Modification/withdrawal of treatment due to adverse events
We judged that lack of blinding of participants and/or personnel had an unclear influence the occurrence of this outcome. The three trials (Ito 2004; Ohwada 2004; Sugimoto 2012) that assessed modification or withdrawal of treatment we judged to be at unclear risk of bias for this outcome.
Quality of life
We judged that lack of blinding of participants and/or personnel would lead to high risk of bias for this outcome. The one trial (Xu 2008) that assessed quality of life we assessed as at high risk of bias for this outcome.
Adverse events (objective)
We judged that lack of blinding of participants and/or personnel had an unclear influence on the occurrence of these outcomes. Five trials (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008) were judged to be at unclear risk of bias for these outcomes.
Adverse events (subjective)
We judged that lack of blinding of participants and personnel would lead to high risk of bias for these outcomes. Five trials (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008) were assessed to be at high risk of bias for these outcomes.
Blinding of outcome assessment
Overall survival
We judged that lack of blinding of participants and/or personnel would not likely influence the occurrence of this outcome. The five trials (Ito 2004; Mitomi 1992; Ohwada 2004; Shichinohe 2013; Xu 2008) that assessed overall survival we judged to be at low risk of bias for this outcome.
Disease recurrence
We judged that lack of blinding of outcome assessors had an unclear influence on this outcome. The three trials (Mitomi 1992; Ohwada 2004; Sugimoto 2012) that assessed disease recurrence we judged to be at unclear risk of bias for this outcome
Disease‐free survival
We judged that lack of blinding of outcome assessors had an unclear influence on this outcome. The four trials (Ito 2004; Mitomi 1992; Ohwada 2004; Shichinohe 2013) that assessed disease‐free survival we judged to be at unclear risk of bias for this outcome.
Disease response/progression (RECIST criteria)
We judged that lack of blinding of outcome assessors had an unclear influence on this outcome. The one trial (Xu 2008) that assessed disease response/progression we judged to be at unclear risk of bias for this outcome.
Modification/withdrawal of treatment due to adverse events
We judged that lack of blinding of outcome assessors had an unclear influence the occurrence of this outcome. The three trials (Ito 2004; Ohwada 2004; Sugimoto 2012) that assessed modification or withdrawal of treatment we judged to be at unclear risk of bias for this outcome
Quality of life
We judged that lack of blinding of outcomes assessors would lead to high risk of bias for this outcome. The one trial that assessed quality of life (Xu 2008) we assessed as at high risk of bias for this outcome.
Adverse events (objective)
We judged that lack of blinding of outcomes assessors had an unclear influence on the occurrence of these outcomes. Five trials (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008) were judged to be at unclear risk of bias for these outcomes.
Adverse events (subjective)
We judged that lack of blinding of outcome assessors would lead to high risk of bias for these outcomes. Five trials (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008) were assessed to be at high risk of bias .
Incomplete outcome data
Overall survival
We judged that the risk of attrition bias due to incomplete data was low for three trials (Ito 2004; Mitomi 1992; Ohwada 2004) and unclear for two trials (Shichinohe 2013; Xu 2008) contributing data to the analysis for this outcome.
Disease recurrence
We judged that the risk of attrition bias due to incomplete data was low for two trials (Ohwada 2004; Sugimoto 2012) and unclear for one trial (Mitomi 1992) contributing data to the analysis for this outcome.
Disease‐free survival
We judged that the risk of attrition bias due to incomplete data was low for two trials (Ito 2004; Ohwada 2004) and unclear for two trials (Mitomi 1992; Shichinohe 2013) contributing data to the analysis for this outcome.
Disease response/progression (RECIST criteria)
We judged that the risk of attrition bias due to incomplete data was unclear for one trial (Xu 2008) contributing data to the analysis for this outcome.
Modification/withdrawal of treatment due to adverse events
We judged that the risk of attrition bias due to incomplete data was low for two trials (Ohwada 2004; Sugimoto 2012) and unclear for one trial (Ito 2004) contributing data to the analysis for this outcome.
Quality of life
We judged that the risk of attrition bias due to incomplete data was low for one trial (Xu 2008) contributing data to the analysis for this outcome.
Adverse events (objective)
We judged that the risk of attrition bias due to incomplete data was low for two trials (Sadahiro 2010; Sugimoto 2012) and unclear for three trials (Ito 2004; Mitomi 1992; Xu 2008) contributing data to the analysis for this outcome.
Adverse events (subjective)
We judged that the risk of attrition bias due to incomplete data was low for two trials (Sadahiro 2010; Sugimoto 2012) and unclear for three trials (Ito 2004; Mitomi 1992; Xu 2008) contributing data to the analysis for this outcome.
Selective reporting
We did not identify any accessible or published protocol or registration for six trials. We judged these trials at unclear risk of reporting bias. For one trial, we identified a trial registration with which the reported outcomes corresponded (Shichinohe 2013). We judged this trial to be at low risk of reporting bias.
One trial contributing to meta‐analyses on survival was judged low risk (Shichinohe 2013) and six trials (Ito 2004; Mitomi 1992; Ohwada 2004; Sadahiro 2010; Sugimoto 2012; Xu 2008) were at unclear risk of bias.
Other potential sources of bias
We did not identify any more general problems in study conduct such as contamination between intervention arms, or suspicion of research fraud.
Effects of interventions
See: Table 1
‐See: Table 1 for the main comparisons
We have provided a summary of the results for effects of interventions, shown in Data and analyses. Additional Table 3 and Table 4 show the studies that contributed to the pooled effect estimates for each outcome. The results of subgroup analyses comparing colon and rectal cancer are also included in Data and analyses.
Primary outcomes
Survival (Analysis 1.1). See Figure 4
1.1. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 1: Survival
4.

Forest plot of comparison: 1 Adjuvant Coriolus versicolor vs no adjunctive treament, outcome: 1.1 Survival.
Five studies assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on survival (Ito 2004; Mitomi 1992; Ohwada 2004; Shichinohe 2013; Xu 2008).
At one year, there was very low‐certainty evidence (downgraded for indirectness and imprecision) of little to no difference in survival (Analysis 1.1.1 risk ratio (RR) 1.02, 95% confidence interval (CI) 0.99 to 1.05; participants = 448; studies = 1).
At three years, there was very low‐certainty evidence (downgraded for risk of bias, inconsistency, indirectness and imprecision) of little or no difference in survival (Analysis 1.1.2 RR 1.04, 95% CI 0.94 to 1.15; participants = 958; studies = 4; I2 = 59%). Heterogeneity at this time point may be due to differences in chemotherapy regimens in the studies contributing to this analysis.
At five years, there was low‐certainty evidence (downgraded for indirectness and imprecision) of a small improvement in survival with polysaccharide‐Krestin (PSK), but not relevant to current therapy and, thus, unclear whether any advantage currently (Analysis 1.1.3 RR 1.08, 95% CI 1.01 to 1.15; participants = 1094; studies = 3; I2 = 0%). number needed to treat for an additional beneficial outcome (NNTB) 16 (95%CI 9 to 70). Absolute risk reduction 6% (95% CI 1% to 11%).
At seven years, there was low‐certainty evidence (downgraded for indirectness and imprecision) of little or no effect on survival (Analysis 1.1.4 RR 1.05, 95% CI 0.95 to 1.16; participants = 441; studies = 1).
Adverse events
Adverse events overall (Analysis 1.2)
1.2. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 2: Adverse events overall
Two studies assessed the total numbers of any adverse events due to adjunctive Coriolus versicolor versus no adjunctive treatment and of any serious adverse event (Sadahiro 2010; Sugimoto 2012).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of the effect on risk of any adverse event (Analysis 1.2.1 RR 1.27 95% CI 0.65 to 2.49; participants =80; studies = 2; I2=0%) and on the risk of any serious adverse event (Analysis 1.2.2 RR 0.95 95% CI 0.15 to 6.17; participants = 80; studies = 2; I2=0%). The 95% confidence intervals extend from reduced risk to a large increase in risk.
Blood and lymphatic system (Analysis 1.3)
1.3. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 3: Adverse events (Blood and lymphatic system)
Four studies assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of blood and lymphatic system‐related adverse events (Ito 2004; Mitomi 1992; Sadahiro 2010; Xu 2008).
There was low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of moderate benefit to no difference in risk of anaemia (Analysis 1.3.1 RR 0.78, 95% CI 0.57 to 1.07; participants = 972; studies = 4; I2 = 0%) and the effect on serious anaemia is uncertain (Analysis 1.3.2).
Cardiac system (Analysis 1.4)
1.4. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 4: Adverse events (Cardiac system)
One study assessed theeffects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of cardiac system‐related adverse events (Mitomi 1992).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of the effect on the risk of palpitations (Analysis 1.4.1 RR 0.34, 95% CI 0.01 to 8.36; participants = 448; studies = 1). The 95% confidence interval extends from a large reduction to a large increase in risk.
Gastrointestinal system (Analysis 1.5). See Figure 5
1.5. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 5: Adverse events (Gastrointestinal system)
5.

Forest plot of comparison: 1 Adjuvant Coriolus versicolor vs no adjunctive treament, outcome: 1.12 Adverse events (Gastrointestinal system).
Five studiesassessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of gastrointestinal system‐related adverse events (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision for each outcome) of the effect on the incidence of the following gastrointestinal system‐related adverse events:
oral cavity disorders (Analysis 1.5.1); loss of appetite (Analysis 1.5.3); nausea (Analysis 1.5.4); vomiting (Analysis 1.5.6); diarrhoea (Analysis 1.5.8); constipation (Analysis 1.5.10); stomach discomfort (Analysis 1.5.11); gastric ulcer (Analysis 1.5.12);
serious oral cavity disorders (Analysis 1.5.2); serious vomiting (Analysis 1.5.7); serious diarrhoea (Analysis 1.5.9);
The estimates for the effect of adjunctive Coriolus versicolor varied across specific events, however, all effects are uncertain.
General disorders (Analysis 1.6)
1.6. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 6: Adverse events (General disorders and administration site)
Five studiesassessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of general adverse events (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness (except for fatigue) and imprecision for each outcome) of the effect on the incidence of the following general adverse events:
fatigue (Analysis 1.6.1 RR 0.76, 95% CI 0.33 to 1.78; participants = 133; studies = 3; I2 = 26%). The 95% confidence interval extends from a reduction to an increase in risk;
pain (Analysis 1.6.3 RR 1.20, 95% CI 0.74 to 1.95; participants = 441; studies = 1). The 95% confidence interval extends from a reduction to an increase in risk;
malaise (Analysis 1.6.4 RR 1.03, 95% CI 0.06 to 16.32; participants = 448; studies = 1). The 95% confidence interval extends from a large reduction to a large increase in risk.
Investigations (Analysis 1.7).
1.7. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 7: Adverse events (Investigations)
Five studiesassessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of investigation‐related adverse events (Ito 2004; Mitomi 1992; Sadahiro 2010; Sugimoto 2012; Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias, inconsistency, indirectness and imprecision for each outcome) of the effect on the incidence of the following adverse event:
white blood cells (WBCs) decreased (Analysis 1.7.1 RR 0.88, 95% CI 0.44 to 1.74; participants = 972; studies = 4; I2 = 64%). Heterogeneity may be due to differences in chemotherapy regimens in the studies contributing to this analysis. The 95% confidence interval extends from a reduction to an increase in risk.
There was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of a reduction in the incidence of the following adverse events:
neutrophils decreased (Analysis 1.7.3 RR 0.41, 95% CI 0.24 to 0.71; participants = 133; studies = 3; I2 = 0%) NNTB 5 (95% CI 3 to 15). Absolute risk reduction 21% (95% CI 7% to 36%);
serious reduction in neutrophils (Analysis 1.7.4 RR 0.39, 95% CI 0.18 to 0.84; participants = 133; studies = 3; I2 = 0%). NNTB 8 (95% CI 4 to 64). Absolute risk reduction 14% (95% CI 2% to 26%).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness (except for : aspartate aminotransferase (AST)/alanine aminotransferase(ALT) and bilirubin increased) and imprecision for each outcome) of the effect on the incidence of the following adverse events:
platelets decreased (Analysis 1.7.5) ; AST increased (Analysis 1.7.7) ; ALT increased (Analysis 1.7.8) ; AST/ALT increased (Analysis 1.7.9) ; ALP increased (Analysis 1.7.11) ; bilirubin increased (Analysis 1.7.12) ; creatinine increased (Analysis 1.7.14) ; abnormal hepatic function (Analysis 1.7.16).
Metabolism and nutrition disorders (Analysis 1.8)
1.8. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 8: Adverse events (Metabolism and nutrition disorders)
One study assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of metabolism and nutrition disorders (Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision for each outcome) of the risk of low albumin levels (Analysis 1.8.1 RR 0.64, 95% CI 0.12 to 3.54; participants = 53; studies = 1). The 95% confidence interval extends from a large reduction to a large increase in risk.
Nervous system (Analysis 1.9)
1.9. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 9: Adverse events (Nervous system)
Two studies assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of nervous system‐related adverse events (Sugimoto 2012; Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness (neurotoxicity only) and imprecision for each outcome) of the effect on the incidence of nervous system‐related adverse events:
dysgeusia (Analysis 1.9.1 RR 2.00, 95% CI 0.19 to 20.67; participants = 50; studies = 1). The 95% confidence interval extends from a large reduction to a large increase in risk.
neurotoxicity (Analysis 1.9.3 RR 0.74, 95% CI 0.40 to 1.38; participants = 53; studies = 1). The 95% confidence interval extends from a reduction to an increase in risk.
Skin and subcutaneous tissue disorders (Analysis 1.10)
1.10. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 10: Adverse events (Skin and subcutaneous tissue disorders)
Four studiesassessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on incidence of investigation‐related adverse events (Ito 2004; Mitomi 1992; Sugimoto 2012; Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias and imprecision for each outcome, and for indirectness for nail discolouration, skin hyperpigmentation, skin ulceration) of the effect on the incidence of the following skin‐related adverse events:
nail discolouration (Analysis 1.10.1); skin hyperpigmentation (Analysis 1.10.2) ; skin ulceration (Analysis 1.10.4) ; eruption (Analysis 1.10.8) ; skin disorders (not defined) (Analysis 1.10.9);
serious skin hyperpigmentation (Analysis 1.10.3); serious skin ulceration (Analysis 1.10.5)
There was very low‐certainty‐evidence (downgraded for risk of bias, inconsistency, indirectness and imprecision for each outcome) of the effect on the incidence of the following skin‐related adverse events:
hand‐foot syndrome (Analysis 1.10.6 RR 1.00, 95% CI 0.11 to 9.09; participants = 103; studies = 2; I2 = 58%). The 95% confidence interval extends from a large reduction to a large increase in risk. Heterogeneity may be due to differences in chemotherapy regimens in the studies contributing to this analysis.
Modification of treatment due to adverse events (Analysis 1.11)
1.11. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 11: Modification of treatment due to adverse events
One study assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on modification of treatment due to adverse events (Sugimoto 2012).
There was very low‐certainty evidence (downgraded for risk of bias and imprecision) of the effect on modification of treatment due to adverse events (Analysis 1.11 RR 3.00, 95% CI 0.13 to 70.30; participants = 50; studies = 1). The 95% confidence interval extends from a large reduction to a large increase in risk.
Withdrawal from treatment due to adverse events (Analysis 1.12) See Figure 6
1.12. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 12: Withdrawal from treatment due to adverse events
6.

Forest plot of comparison: 1 Adjuvant Coriolus versicolor vs no adjunctive treament, outcome: 1.7 Withdrawal from treatment due to adverse events.
Three studies assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on withdrawal from treatment due to adverse events (Ito 2004; Ohwada 2004; Sugimoto 2012).
There was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of the effect on withdrawal from treatment due to adverse events (Analysis 1.12 RR 1.03, 95% CI 0.45 to 2.34; participants = 703; studies = 3; I2 = 0%). The 95% confidence interval extends from a reduction to an increase in risk.
Secondary outcomes
Disease‐free survival (Analysis 1.13)
1.13. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 13: Disease‐free survival
Four studies assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on disease‐free survival. Four studies contributed to the analyses (Ito 2004; Mitomi 1992; Ohwada 2004; Shichinohe 2013).
At one year, there was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of little or no difference in effect on disease‐free survival (Analysis 1.13.1 RR 1.05, 95% CI 0.99 to 1.13; participants = 448 studies = 1). The 95% confidence interval includes extends from a reduction to an increase in risk.
At three years, there was very low certainty evidence (downgraded for risk of bias, inconsistency, indirectness and imprecision) of little or no difference in effect on disease‐free survival (Analysis 1.13.2 RR 1.08, 95% CI 0.95 to 1.23; participants = 905; studies = 3; I2 = 58%). The 95% confidence interval extends from a reduction to an increase in risk. Heterogeneity at this time point may be due to differences in chemotherapy regimens in the studies contributing to this analysis.
At five years, there was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of no difference to a moderate benefit on disease‐free survival (Analysis 1.13.3 RR 1.12, 95% CI 1.00 to 1.24; participants = 1091; studies = 3; I2 = 41%).
At seven years, there was low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of little or no difference in disease‐free survival (Analysis 1.13.4 RR 1.04, 95% CI 0.93 to 1.17; participants = 441; studies = 1). The 95% confidence interval extends from a reduction to an increase in risk.
Disease recurrence (Analysis 1.14)
1.14. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 14: Disease recurrence
Three studies assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on disease recurrence (Mitomi 1992; Ohwada 2004; Sugimoto 2012).
At one year, there was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of the effect on disease recurrence (Analysis 1.14.1 RR 0.68, 95% CI 0.41 to 1.14; participants = 498; studies = 2; I2 = 0%). The 95% confidence interval extends from a reduction to an increase in risk.
At three years, there was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of a reduction in disease recurrence (Analysis 1.14.2 RR 0.70, 95% CI 0.52 to 0.96; participants = 448; studies = 1). NNTB 11, 95% confidence interval 6 to 76. Absolute risk reduction 10% (95% CI 1% to 18%).
At five years, there was very low‐certainty evidence (downgraded for risk of bias, indirectness and imprecision) of a reduction in disease recurrence (Analysis 1.14.3 RR 0.68, 95% CI 0.53 to 0.87; participants = 653; studies = 2; I2 = NA). NNTB 9 (95% CI 6 to 24). Absolute risk reduction 11% (95% CI 4% to 18%).
Disease response/progression (RECIST criteria) (Analysis 1.15 and Analysis 1.16)
1.15. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 15: Complete or partial response (RECIST)
1.16. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 16: Stable or progressive disease (RECIST)
One study (53 participants) assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on response rates and progression (Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias and imprecision) of the effect on complete response (Analysis 1.15.1), partial response (Analysis 1.15.2), stable disease (Analysis 1.16.1) or in progressive disease (Analysis 1.16.2 ).
Quality of life (Analysis 1.17)
1.17. Analysis.

Comparison 1: Adjuvant Coriolus versicolor vs no adjunctive treament, Outcome 17: Quality of life (continuous)
One study assessed the effects of adjunctive Coriolus versicolor versus no adjunctive treatment on quality of life (Xu 2008).
There was very low‐certainty evidence (downgraded for risk of bias and imprecision) of little to no effect on quality of life (Analysis 1.17 MD ‐0.53, 95% CI ‐1.07 to 0.01; participants = 50; studies = 1). The 95% confidence interval includes a reduction and an increase in quality of life.
Subgroup analysis
We planned to perform subgroup analysis according to type of Coriolus versicolor preparation, dose, type of cancer (colon or rectal), stage of cancer and chemotherapy regimen. We found that all trials used one extract (PSK) ,and the dose used was consistent across studies so that no subgroup analysis was necessary. While all studies included patients with stage III cancer, most studies also included patients with either stage II or stage IV cancer and results were not reported separately by stage except in one study so that subgroup analysis was not carried out. All studies except one study used a regimen based on an oral fluoropyrimidine and so a formal subgroup analysis could not be carried out (Deeks 2001).
Three studies provided data for a subgroup analysis by type of cancer (Ito 2004; Mitomi 1992; Ohwada 2004). Visual inspection suggested that the effects on survival were similar at one year (Analysis 2.1) and three years (Analysis 2.2), but formal statistical testing could not be carried out as there was only one study in each subgroup at each time point (Deeks 2001). At five years, effects upon survival appeared similar for colon cancer (Analysis 2.3 RR 1.06, 95% CI 0.99 to 1.14; participants = 690; studies = 2; I2 = 0%) and rectal cancer (Analysis 2.3; RR 1.17, 95% CI 0.98 to 1.39; participants = 282; studies = 2; I2 = 0%), and this was consistent with the test for subgroup differences (Chi² = 1.10, df = 1 (P = 0.29), I² = 9.3%). Visual inspection of data from one study (Mitomi 1992) suggested that the effects on disease recurrence appear slightly better for colon cancer than rectal cancer at all three time points (Analysis 2.4; Analysis 2.5; Analysis 2.6), however a formal subgroup test was not appropriate.
2.1. Analysis.

Comparison 2: Subgroup analyses by cancer location, Outcome 1: Survival at 1 year
2.2. Analysis.

Comparison 2: Subgroup analyses by cancer location, Outcome 2: Survival at 3 years
2.3. Analysis.

Comparison 2: Subgroup analyses by cancer location, Outcome 3: Survival at 5 years
2.4. Analysis.

Comparison 2: Subgroup analyses by cancer location, Outcome 4: Disease recurrence at 1 year
2.5. Analysis.

Comparison 2: Subgroup analyses by cancer location, Outcome 5: Disease recurrence at 3 years
2.6. Analysis.

Comparison 2: Subgroup analyses by cancer location, Outcome 6: Disease recurrence at 5 years
Sensitivity analysis
All studies were judged to be at high risk of bias for subjective outcomes (adverse events based on self‐report and/or quality of life) but unclear or low risk for all other outcomes, therefore, sensitivity analysis for high risk of bias was not carried out.
Discussion
Summary of main results
We found very low‐certainty evidence of little to no difference in withdrawal from treatment due to adverse events resulting from adjunctive treatment with Coriolus (in the form of an extract, polysaccharide‐Krestin (PSK)) (risk ratio (RR 1.03, 95% confidence interval (CI) 0.45 to 2.34; 703 participants; 3 studies). We also found the effect on modification of treatment due to adverse events to be unclear with very low certainty evidence based on one small study. Evidence of an effect on the incidence of a range of specific adverse events was also of very low certainty. The events reported included haematological‐ and gastrointestinal‐related adverse events amongst others. Thus, it is unclear if there was a difference in events including reduced white blood cell (WBC) counts, neutropenia, oral cavity disorders, nausea and diarrhoea.
We found low‐certainty evidence of a small effect of adjunctive Coriolus extract on survival at five years compared with no adjunctive extract (RR 1.08, 95% CI 1.01 to 1.15; 1094 participants; 3 studies; I2 = 0%). The effect at earlier time points was unclear with very low‐certainty evidence (three‐year survival: RR 1.04, 95% CI 0.94 to 1.15; 958 participants; 4 studies; I2 = 59%). Similar results were found for disease recurrence and disease‐free survival with very low‐certainty evidence at one, three and five years in both cases. Evidence on survival and on disease‐free survival at seven years was low certainty but indicated that there may be little or no difference with the addition of the Coriolus extract.
Only one of the seven studies located used Coriolus extract in combination with a chemotherapy regimen currently preferred in practice (capecitabine and oxaliplatin) (Xu 2008). Chemotherapeutic regimens in six studies consisted of an oral fluoropyrimidine which was preceded by weekly intravenous 5‐FU in one study and by mitomycin C in two studies, and which was combined with folinic acid (Leucovorin) in two studies while, in one study, it was combined with radiotherapy preoperatively. No evidence was found on products other than PSK or on populations outside China and Japan.
Overall completeness and applicability of evidence
Six of the studies that we found in this review were conducted in Japan and one study was carried out in China. We did not find studies that had been carried out in other countries or with other populations/ethnic groups. Participants represented both sexes, a range of ages and different stages of colorectal cancer although all studies included patients with stage III cancer. One study only included patients with rectal cancer, a second included only patients with colon cancer with the other studies including both. Only one form of Coriolus was investigated in the studies. This was PSK, an extract that was first developed as a proprietary product in Japan and which is no longer manufactured in Japan. No studies of other extracts or of the whole mushrooms were identified. While there was consistency in the dosage regimen for PSK, the duration of treatment varied from four weeks to three years. The studies had been carried out over a long period: only two of the seven studies were published in the past 10 years, the remaining five being reported more than 10 years ago. This is apparent from the varied treatment regimens used in each of the studies. In five studies, this involved oral 5‐FU or UFT and one study utilised oral S‐1. Several studies used regimens that are no longer in regular use and only one study used a regimen that is current preferred practice with the use of XELOX. Current practice was based on guidelines from China (CSCO 2020), Japan (JSCCR 2019), UK (NICE 2020) and US (NCNN 2017; NCNN 2018).
Reporting of adverse events was very variable; several studies focused on whether PSK reduced adverse events while others focused on the extent to which it caused adverse events. We found reporting of methods for eliciting adverse events to be limited or unclear. We also found varied terminology used to describe adverse events. For consistency, we categorised adverse events according to Common Terminology Criteria for Adverse Events (CTAE) headings where possible. Timing of adverse events was not clear nor was causality. Lack of causality means that it was unclear if any adverse events were due to the chemotherapy or to the Coriolus extract. One study, which was excluded as it did not involve adjunctive Coriolus, specifically assessed frequency of adverse effects due to PSK compared with placebo and found a statistically significant difference in pigmentation of nails and cough on administration compared with placebo (Torisu 1990). Other adverse effects of PSK have been reported but these are described as 'mild and tolerable' (Natural Database 2022). Several could be due to Coriolus or chemotherapy.
Quality of life, one of the secondary outcomes of interest in this review, was only assessed in one trial.
One study was published only as a conference abstract and another study as a short report so that details were limited. Several studies were reported in a series of publications and there was some inconsistency in reporting across publications. We were unable to obtain any further information on published data or any existing unpublished studies from study authors.
Quality of the evidence
We judged several studies to be at unclear risk of selection bias because randomisation was not clearly reported. None of the included studies blinded participants or providers to treatment assignment. Thus, there was a high risk of performance and detection bias for outcomes based on self‐report such as adverse events that were subjective. Reporting bias was also unclear as registration and/or protocols were not available for six of the seven studies. For most outcomes, evidence was downgraded for risk of bias, indirectness and imprecision. Where several studies reported the same outcomes, we found little heterogeneity so that only a few of the comparisons required downgrading for inconsistency. Where significant heterogeneity (I2 < 50%) was observed, this could be explained by differences in the chemotherapy regimens in the studies contributing to the analysis, although this could not be confirmed by formal statistical testing. Different disease stages may involve different treatments (surgery, chemotherapy or radiotherapy) which in themselves affect adverse events either primarily and after PSK treatment. Due to the heterogeneity of patients and treatments reported in the publications, it was not possible examine these aspects separately. Overall, none of the evidence was judged to be moderate or high certainty with most findings being very low certainty.
Potential biases in the review process
We carried out extensive searches of databases in Chinese, English and Japanese but were unable to obtain any further information on unpublished studies. This was due to many of the trials having taken place some time ago so that it was difficult to locate current contact details while the Japanese manufacturer discontinued the product used in most trials in 2017. It is possible that further unpublished data are available but, due to the small number of studies, it was not possible to examine publication bias using funnel plots. We did identity a number of clinical trials in trial registers for which we were unable to identify any publications (see Studies awaiting classification).
Several studies had been reported in a series of publications and we had to select the primary publication in each case. Where there was a discrepancy in reporting across publications, we had to make a decision on which data should be used.
We had at least two independent review authors select studies, extract data, and conduct risk of bias assessments. Where we extracted data based on translations, we consulted a review author who was fluent in the language for any points of clarification. There is, however, a slight possibility that certain aspects could be misconstrued during this process.
Agreements and disagreements with other studies or reviews
Four previous systematic reviews have addressed similar questions to our own. The first review focused on the efficacy of PSK used as an adjuvant to conventional chemotherapy in patients with curatively resected colorectal cancer (Sakamoto 2006). Similar results to those in this current review were found for PSK as an adjuvant on overall survival and disease‐free survival at five years but risk of bias and adverse events were not addressed. Two further meta‐analyses concluded that Coriolus had a beneficial effect on survival, including in colorectal cancer. In the first meta‐analysis, only research up to 2003 was included (Eliza 2012), while we included an additional five studies published subsequently. The second was a network meta‐analysis examining PSK for gastrointestinal cancer, including colorectal cancer (Ma 2017). Ma and colleagues also assessed effects on adverse effects. PSK was found to reduce the occurrence of nausea and vomiting, and leukopenia but, in contrast to the current review, the evidence was graded as moderate to high. The final review assessed Coriolus efficacy and safety for a range of cancers (Zhong 2019). Again, beneficial effects on survival were reported along with reductions in adverse effects, the most commonly encountered being similar to those in this review: nausea, vomiting, leucopenia, diarrhoea, thrombocytopenia, liver dysfunction, fatigue and anorexia. However, three of the four trials in colorectal cancer contributing to these conclusions were excluded in this current review. Overall, while there is some agreement on a possible small effect on overall survival, we rated the evidence as lower certainty. We also only found very low‐certainty evidence on whether there was any reduction in adverse events including preventing withdrawal of treatment due to adverse events.
Authors' conclusions
Implications for practice.
Due to the very low certainty of evidence, we are uncertain about the effect of Coriolus (in the form of an extract polysaccharide‐Krestin (PSK)) on adverse events resulting from conventional chemotherapy for colorectal cancer. This was true for generic outcomes such as whether there was an effect on withdrawal of treatment due to adverse events and for specific adverse outcomes such as neutropenia and nausea. The uncertainty in the evidence also means that it was unclear whether any adverse events were due to the chemotherapy or to the extract itself. While there was low‐certainty evidence of a small effect on overall survival at five years, the influence of reduced adverse effects on this could not be determined and evidence at other time points was unclear. Additionally, effect on survival was based on use of chemotherapy regimens which varied and included those that do not reflect current preferred practice. Thus, any benefits could not easily be translated into current practice.
Implications for research.
We found a lack of research on the use of Coriolus extract in combination with currently prescribed chemotherapy regimens. Any future studies to assess whether there are beneficial effects, specifically in prevention or reduction of adverse events will need to systematically and comprehensively collect and collate data on all adverse events. Researchers would also need to record timing of adverse events and carry out assessment of causality. Only PSK has been investigated in clinical trials and other products, such as Polysaccharide P, which are currently in use, require further assessment.
History
Protocol first published: Issue 2, 2016
Acknowledgements
The review authors would like to acknowledge the valuable contribution of Dr. Janine Leach in first initiating this review, recruiting the team and to the development of the protocol. Dr Leach sadly passed away in June 2016.
The review authors are grateful for the advice of the NCRI Complementary Therapies Clinical Studies Development Group in the initial stages of this work, for the support of the Cochrane Colorectal Group and thanks to Marija Barbateskovic, Information Specialist, for the development of the search strategies.
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Toby Lasserson, Cochrane Central Executive Team. Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Helen Wakeford, Cochrane Central Editorial Service. Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Executive Team. Copy Editor (copy editing and production): Heather Maxwell. Peer‐reviewers (provided comments and recommended an editorial decision): Ping Chung Leung, Director of Centre for Clinical Trials on Chinese Medicine, Chinese University of Hong Kong (clinical/content review), Jeffrey D. White, Associate Director, Office of Cancer Complementary and Alternative Medicine, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, US (clinical/content review), Nuala Livingstone, Cochrane Central Executive Team (methods review), Robin Featherstone, Cochrane Central Executive Team (search review). One additional peer reviewer provided clinical peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
Cochrane Library
#1 MeSH descriptor: [Trametes] explode all trees #2 (Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom):ti,ab,kw #3 (#1 or #2) #4 MeSH descriptor: [Colorectal Neoplasms] explode all trees #5 ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) near/3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*)):ti,ab,kw #6 (#4 or #5) #7 (#3 and #6)
MEDLINE
1. exp Trametes/ 2. (Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom).mp. 3. 1 or 2 4. exp Colorectal Neoplasms/ 5. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*)).mp. 6. 4 or 5 7. 3 and 6
Embase
1. exp trametes versicolor/ 2. (Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom).mp. 3. 1 or 2 4. exp colon cancer/ 5. exp rectum cancer/ 6. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*)).mp. 7. 4 or 5 or 6 8. 3 and 7
AMED
1. (Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or Global polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom).mp.
2. exp Intestinal Neoplasms/
3. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*)).mp.
4. 2 or 3
5. 1 and 4
BIOSIS
1. (Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom)
2. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*))
3. 1 and 2
CINAHL
1. (Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom)
2. (MH "Colorectal Neoplasms+")
3. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) N3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*))
4. 2 or 3 5. 1 and 4
Global Index Medicus
1. Coriolus versicolor or Trametes versicolor or Polyporus versicolor or Polystictus versicolor or Kawaratake or Yun Zhi or polysaccharide‐K or PSK or Krestin or polysaccharopeptide or polysaccharide‐peptide or PSP or VPS or Turkey Tail or cloud mushroom or unji mushroom
Natural Medicines database
1. Coriolus versicolor (to locate relevant monograph)
CNKI
(SU = '云芝' OR SU = '彩绒革盖菌' OR SU = '灰芝' OR SU = '瓦菌' OR SU = '彩云革盖菌' OR SU = '多色牛肝菌' OR SU = '红见手' OR SU = '千层蘑') AND (SU = '结直肠癌' OR SU = '结肠癌' OR SU = '直肠癌' OR SU = '大肠癌' OR SU = '肠癌') AND SU = '对照'
Wanfang
主题: ("云芝" + "彩绒革盖菌" + "灰芝" + "瓦菌" + "彩云革盖菌" + "多色牛肝菌" + "红见手" + "千层蘑") * 主题: ("结直肠癌" + "结肠癌" + "直肠癌" + "大肠癌" + "肠癌") * 主题: (对照)
VIP
(M=(云芝 OR 彩绒革盖菌 OR 灰芝 OR 瓦菌 OR 彩云革盖菌 OR 多色牛肝菌 OR 红见手 OR 千层蘑)) AND (M=(结直肠癌 OR 结肠癌 OR 直肠癌 OR 大肠癌 OR 肠癌)) AND R=对照
Sinomed
1. "云芝"[常用字段:智能] OR "彩绒革盖菌"[常用字段:智能] OR "灰芝"[常用字段:智能] OR "瓦菌"[常用字段:智能] OR "彩云革盖菌"[常用字段:智能] OR "多色牛肝菌"[常用字段:智能] OR "红见手"[常用字段:智能] OR "千层蘑"[常用字段:智能]
2. "结直肠癌"[常用字段:智能] OR "结肠癌"[常用字段:智能] OR "直肠癌"[常用字段:智能] OR "大肠癌"[常用字段:智能] OR "肠癌"[常用字段:智能]
3. "对照"[常用字段:智能]
4. (#3) AND (#2) AND (#1)
Ichushi Web
1. カワラタケ属/TH or カワラタケ/AL or タマチョレイタケ属/TH or タマチョレイタケ/AL or シロアミタケ属/TH or シロアミタケ/AL or クレスチン/AL or クレハ/AL or Tramete/AL or "Trametes versicolor"/AL or "Polyporus versicolor"/AL or "Polystictus versicolor"/AL or "Kawaratake"/AL or "Yun Zhi"/AL or "polysaccharide‐K"/AL or “polysaccharide‐Kureha”/AL or "PSK"/TA or "Krestin"/TH or "Krestin"/AL or "polysaccharopeptide"/AL or "polysaccharide‐peptide"/AL or "PSP"/TA or "VPS"/TA or "Turkey Tail"/AL or "cloud mushroom"/AL or "unji mushroom"/AL
2. 大腸腫瘍/TH or 結腸直腸癌/AL or 大腸癌/AL or 肛門癌/AL
3. colorectal or colon or rectum or intestine or 腸/TH or bowel/AL or 直腸/TH or rectum/AL
4. 腫瘍/TH or 腫瘍/AL or がん/AL or 癌/AL or tumor/AL or tumour/AL or cancer/AL or 肉腫/TH or 肉腫/AL or sarcoma/AL or癌腫/TH or 癌腫/AL or carcinoma/AL or 悪性/AL or malignant/AL or 腺癌/TH or 腺癌/AL or adenocarcinoma/AL or Neoplasms/AL or neoplastic/AL
5. 3 and 4
6. 2 or 5
7. 1 and 6
Clinicaltrials.gov
1. Colo‐rectal OR colorectal OR rectal OR colon (Condition)
2. Coriolus versicolor OR Trametes versicolor OR Polyporus versicolor OR Polystictus versicolor OR Kawaratake OR Yun Zhi OR polysaccharide‐K OR PSK OR Krestin OR polysaccharopeptide OR polysaccharide‐peptide OR PSP OR VPS OR Turkey Tail OR cloud mushroom (Other terms)
3. 1 and 2
ICTRP
1. Coriolus versicolor OR Trametes versicolor OR Polyporus versicolor OR Polystictus versicolor OR Kawaratake OR Yun Zhi OR polysaccharide‐K OR PSK OR Krestin OR polysaccharopeptide OR polysaccharide‐peptide OR PSP OR VPS OR Turkey Tail OR cloud mushroom
2. Colo‐rectal OR colorectal OR rectal OR colon
3. 1 and 2
Appendix 2. Assessment of risk of bias
Risk of bias in randomised trials
Extracted from the Cochrane Handbook for Systematic Reviews of Interventions (http://handbook.cochrane.org/).
Table 8.5.d: Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of 'low risk' of bias | The investigators describe a random component in the sequence generation process such as: · referring to a random number table; · using a computer random number generator; · coin tossing; · shuffling cards or envelopes; · throwing dice; · drawing of lots; · minimisation*. *Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of 'high risk' of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · sequence generated by odd or even date of birth; · sequence generated by some rule based on date (or day) of admission; · sequence generated by some rule based on hospital or clinic record number. · other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example: · allocation by judgement of the clinician; · allocation by preference of the participant; · allocation based on the results of a laboratory test or a series of tests; · allocation by availability of the intervention. |
| Criteria for the judgement of 'unclear risk' of bias | Insufficient information about the sequence generation process to permit judgement of 'low risk' or 'high risk'. |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of 'low risk' of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); · sequentially numbered drug containers of identical appearance; · sequentially numbered, opaque, sealed envelopes. |
| Criteria for the judgement of 'high risk' of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · using an open random allocation schedule (e.g. a list of random numbers); · assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); · alternation or rotation; · date of birth; · case record number; · any other explicitly unconcealed procedure. |
| Criteria for the judgement of 'unclear risk' of bias | Insufficient information to permit judgement of 'low risk' or 'high risk'. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of 'low risk' of bias | Any one of the following: · no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; · blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of 'high risk' of bias | Any one of the following: · no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; · blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for the judgement of 'unclear risk' of bias | Any one of the following: · insufficient information to permit judgement of 'low risk' or 'high risk'; · the study did not address this outcome. |
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of 'low risk' of bias | Any one of the following: · no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; · blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of 'high risk' of bias | Any one of the following: · no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; · blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for the judgement of 'unclear risk' of bias | Any one of the following: · insufficient information to permit judgement of 'low risk' or 'high risk'; · the study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data. | |
| Criteria for a judgement of 'low risk' of bias | Any one of the following: · no missing outcome data; · reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate; · for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; · missing data have been imputed using appropriate methods. |
| Criteria for the judgement of 'high risk' of bias | Any one of the following: · reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; · for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation; · potentially inappropriate application of simple imputation. |
| Criteria for the judgement of 'unclear risk' of bias | Any one of the following: · insufficient reporting of attrition/exclusions to permit judgement of 'low risk' or 'high risk' (e.g. number randomised not stated, no reasons for missing data provided); · the study did not address this outcome. |
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of 'low risk' of bias | Any of the following: · the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; · the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| Criteria for the judgement of 'high risk' of bias | Any one of the following: · not all of the study's pre‐specified primary outcomes have been reported; · one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; · one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; · the study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for the judgement of 'unclear risk' of bias | Insufficient information to permit judgement of 'low risk' or 'high risk'. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of 'low risk' of bias | The study appears to be free of other sources of bias. |
| Criteria for the judgement of 'high risk' of bias | There is at least one important risk of bias. For example, the study: · had a potential source of bias related to the specific study design used; or · has been claimed to have been fraudulent; or · had some other problem. |
| Criteria for the judgement of 'unclear risk' of bias | There may be a risk of bias, but there is either: · insufficient information to assess whether an important risk of bias exists; or · insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. Methods planned but not used in this review
Unit of analysis issues
For individual trials, the unit of analysis will be the individual patient. In the case of including cluster‐randomised study designs, the unit of analysis will be the cluster. We will extract data if the authors have adjusted for the sample size in each cluster, so that it is appropriately weighted in the following analysis. For studies in which control of clustering is not performed or reported adequately by the authors (after we have contacted them), and individual patient data are not available, we will correct for the intervention effects of cluster‐RCTs by reducing the size of each trial to its 'effective sample size', which is the number of the original sample size divided by the 'design effect'. We will calculate the design effect as 1 + (M‐1)* ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient, as described in chapter 16.3.4 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We will contact study authors to provide missing data. Where this proves unsuccessful, we will investigate the potential impact of the missing data. If trials reported the number of patients with data missing for the primary outcome, we will take a worst‐case scenario approach to data analyses, that is, taking those with missing data in the treatment group as treatment failures, while taking those with missing data in the control group as treatment successes (Gamble 2005). We will compare per protocol analysis of available data with the analysis incorporating missing data. If the effect estimate is in the same direction and there is a significant difference between groups, we will be able to make a conclusion with more confidence. If the effect estimates from the two analyses are different, then we will need to interpret the findings with more caution and make a more conservative conclusion about the treatment effect.
Data and analyses
Comparison 1. Adjuvant Coriolus versicolor vs no adjunctive treament.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Survival | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Survival at 1 year | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.99, 1.05] |
| 1.1.2 Survival at 3 years | 4 | 958 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.94, 1.15] |
| 1.1.3 Survival at 5 years | 3 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.15] |
| 1.1.4 Survival at 7 years | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 1.2 Adverse events overall | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Any adverse events | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.65, 2.49] |
| 1.2.2 Any serious adverse events | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.15, 6.17] |
| 1.3 Adverse events (Blood and lymphatic system) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Blood and lymphatic: Anaemia | 4 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.07] |
| 1.3.2 Blood and lymphatic: Serious anaemia | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 1.4 Adverse events (Cardiac system) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Cardiac: Palpitations | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.36] |
| 1.5 Adverse events (Gastrointestinal system) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Gastrointestinal: Oral cavity disorders | 5 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.03] |
| 1.5.2 Gastrointestinal: Serious oral cavity disorders | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.81] |
| 1.5.3 Gastrointestinal: Loss of appetite | 2 | 889 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.34, 1.23] |
| 1.5.4 Gastrointestinal: Nausea | 4 | 969 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.44, 1.22] |
| 1.5.5 Gastrointestinal: Serious nausea | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.5.6 Gastrointestinal: Vomiting | 2 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.06, 16.32] |
| 1.5.7 Gastrointestinal: Serious vomiting | 2 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.06, 16.32] |
| 1.5.8 Gastrointestinal: Diarrhoea | 5 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.32, 1.86] |
| 1.5.9 Gastrointestinal: Serious diarrhoea | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.05, 1.97] |
| 1.5.10 Gastrointestinal: Constipation | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.82, 2.01] |
| 1.5.11 Gastrointestinal: Stomach discomfort | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.06, 16.32] |
| 1.5.12 Gastrointestinal: Gastric ulcer | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.36] |
| 1.6 Adverse events (General disorders and administration site) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 General: Fatigue | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.33, 1.78] |
| 1.6.2 General: Serious fatigue | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.3 General: Pain | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.74, 1.95] |
| 1.6.4 General: Malaise | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.06, 16.32] |
| 1.7 Adverse events (Investigations) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Investigations: WBC decreased | 4 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.44, 1.74] |
| 1.7.2 Investigations: Serious WBC decreased | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7.3 Investigations: Neutrophils decreased | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.24, 0.71] |
| 1.7.4 Investigations: Serious neutrophils decreased | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.84] |
| 1.7.5 Investigations: Platelets decreased | 4 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.16, 2.35] |
| 1.7.6 Investigations: Serious platelets decreased | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7.7 Investigations: AST increased | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.61, 1.24] |
| 1.7.8 Investigations: ALT increased | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 1.7.9 Investigations: AST/ALT increased | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.32, 5.61] |
| 1.7.10 Investigations: Serious AST/ALT increased | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7.11 Investigations: ALP increased | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.61, 1.21] |
| 1.7.12 Investigations: Bilirubin increased | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.45, 4.90] |
| 1.7.13 Investigations: Serious bilirubin increased | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7.14 Investigations: Creatinine increased | 2 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.22, 3.00] |
| 1.7.15 Investigations: Serious creatinine increased | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7.16 Investigations: Abnormal hepatic function (not defined) | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.50, 13.10] |
| 1.8 Adverse events (Metabolism and nutrition disorders) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 Metabolism: Albumin | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.12, 3.54] |
| 1.8.2 Metabolism: Serious albumin | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Adverse events (Nervous system) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.9.1 Nervous system: Dysgeusia | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 20.67] |
| 1.9.2 Nervous system: Serious dysgeusia | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9.3 Nervous system: Neurotoxicity (not defined) | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.40, 1.38] |
| 1.10 Adverse events (Skin and subcutaneous tissue disorders) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.10.1 Skin: Nail discoloration | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.30] |
| 1.10.2 Skin: Skin hyperpigmentation | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.65] |
| 1.10.3 Skin: Serious skin hyperpigmentation | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 99.16] |
| 1.10.4 Skin: Skin ulceration | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 99.16] |
| 1.10.5 Skin: Serious skin ulceration | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.30] |
| 1.10.6 Skin: Hand‐foot syndrome | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.11, 9.09] |
| 1.10.7 Skin: Serious hand‐foot syndrome | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.10.8 Skin: Eruption | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.36] |
| 1.10.9 Skin: Skin disorders (not defined) | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.43, 5.27] |
| 1.11 Modification of treatment due to adverse events | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.30] |
| 1.12 Withdrawal from treatment due to adverse events | 3 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.45, 2.34] |
| 1.13 Disease‐free survival | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.13.1 Disease‐free survival at 1 year | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.13] |
| 1.13.2 Disease‐free survival at 3 years | 3 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.23] |
| 1.13.3 Disease‐free survival at 5 years | 3 | 1091 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.00, 1.24] |
| 1.13.4 Disease‐free survival at 7 years | 1 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.17] |
| 1.14 Disease recurrence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.14.1 Disease recurrence at 1 year | 2 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.14] |
| 1.14.2 Disease recurrence at 3 years | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.96] |
| 1.14.3 Disease recurrence at 5 years | 2 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.87] |
| 1.15 Complete or partial response (RECIST) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.15.1 Complete response | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.32, 5.19] |
| 1.15.2 Partial response | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.59, 2.36] |
| 1.16 Stable or progressive disease (RECIST) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.16.1 Stable disease | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.36, 1.99] |
| 1.16.2 Disease progression | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.26, 7.96] |
| 1.17 Quality of life (continuous) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.07, 0.01] |
Comparison 2. Subgroup analyses by cancer location.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Survival at 1 year | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.99, 1.05] |
| 2.1.1 Colon cancer | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.98, 1.05] |
| 2.1.2 Rectal cancer | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 2.2 Survival at 3 years | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.00, 1.18] |
| 2.2.1 Colon cancer | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.98, 1.20] |
| 2.2.2 Rectal cancer | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 2.3 Survival at 5 years | 3 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.01, 1.15] |
| 2.3.1 Colon cancer | 2 | 690 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.99, 1.14] |
| 2.3.2 Rectal cancer | 2 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.98, 1.39] |
| 2.4 Disease recurrence at 1 year | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.16] |
| 2.4.1 Colon cancer | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.13] |
| 2.4.2 Rectal cancer | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.68] |
| 2.5 Disease recurrence at 3 years | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.52, 0.99] |
| 2.5.1 Colon cancer | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.35, 0.96] |
| 2.5.2 Rectal cancer | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.56, 1.19] |
| 2.6 Disease recurrence at 5 years | 1 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.54, 0.95] |
| 2.6.1 Colon cancer | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 1.02] |
| 2.6.2 Rectal cancer | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ito 2004.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: Japan Study dates: enrolment February 1991 to March 1993 Recruitment: patients recruited from 93 institutions in the Chubu region of Japan Number screened / eligible: not reported Inclusion criteria: quote: "primary colon cancer with curative resection and positive macroscopic lymph node metastases (macroscopic Dukes’ C); under 75 years of age with PS 0 or 1; no serious complications; no preoperative treatment (radiation therapy, chemotherapy, immunotherapy) for cancer; no previous history of cancer; acceptable preoperative laboratory data (white blood cell count ≥3,000/mm3, platelet count ≥100,000/mm3, glutamic‐oxaloacetic transaminase and glutamic‐pyruvic transaminase ≤100 IU); preoperative records of serum carcinoembryonic antigen (CEA) level (below 5 ng/dl or not) and purified protein derivative (PPD) skin test reactions (smaller than 10 mm or not)” Exclusion criteria:no additional criteria specified Diagnostic criteria for inclusion/exclusion: quote: “primary colon cancer with curative resection and positive macroscopic lymph node metastases (macroscopic Dukes’ C)" |
|
| Participants |
Number randomised: 441; Coriolus versicolor (PSK) 220; control 221 Baseline imbalances: groups well‐matched on most variables. Quote: "The clinical characteristics of the two study groups were similar at base line (Table 1). However, there was still an imbalance in the distribution of the patients’ performance status (PS) between the two groups. Thus an adjusted analysis with two balancing factors (lymph node metastases, preoperative CEA level) and PS was adopted in the blinded review as a primary analysis." Age:Coriolus versicolor (PSK) mean 60.4 years (87% age 50 or more); Control mean 60.0 (84% age 50 years or more). Overall mean = 60.0 years Sex:Coriolus versicolor (PSK) male n =123 (55.9%); Control male n=121 (54.8%); Overall male n=244 (55%) Cancer type: colon Cancer removed or present: removed If cancer was present,was it local or metastatic: N/A. However note that there were positive lymph node metastases. What was the aim of chemo/radiotherapy (if applicable)? adjuvant. Withdrawals and exclusions:Coriolus versicolor (PSK) group withdrawals from treatment n = 25 (no hospital visits n = 11, adverse events n = 5, concomitant diseases n = 4, other n = 5); control group withdrawals from treatment n = n = 27 (no hospital visits n = 16, adverse events n = 4, concomitant diseases n = 2, others n = 5). |
|
| Interventions |
Coriolus versicolor intervention: oral protein‐bound polysaccharide K (PSK) . Dose of 3 g/day from 29 days post‐op for 4 weeks, followed by start of oral 5‐FU for 4 weeks. Ten pairs of PSK followed by 5‐FU treatments = 80 weeks of alternating treatment beginning at four weeks post‐op. Control intervention: chemotherapy alone Chemotherapy regimen: 5‐FU IV 1,000 mg/m2/24hr on postoperative days 1‐2, 8, 9, 15, 16, 22 and 23, followed by 4 weeks PSK 3 g/day alternating with 4 weeks 5‐FU oral 200 mg/day for a total of 10 cycles. Planned length of treatment: 80 weeks. Setting: not described but presumably hospital outpatient. Co‐interventions: none mentioned. |
|
| Outcomes | 1. Survival 2. Disease‐free survival 3. Treatment modification/withdrawal 4. Adverse events Planned length of follow‐up from treatment initiation: 7 years Time points: Survival and disease‐free survival reported at 5 and 7 year follow‐up. Other outcomes: cancer‐related deaths, survival from cancer‐related death, and laboratory tests, including white blood cell, neutrophil, etc., oxidative stress index using the ROMs Test to measure hydroperoxide concentration, and salivary chromogranin A. Adverse event assessment: no description of how adverse events were assessed. All adverse events are reported according to numbers of each event and to numbers of patients/year experiencing each event, not to numbers of people experiencing each adverse event as of Year 7 or any other time point. |
|
| Notes |
Study name: Study Group of Immunochemotherapy with PSK for Colon Cancer Funding source: no description of funding. Declaration of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to either the PSK group (group P, n=220) or control group (group C, n=221) according to a dynamic balancing method (modified minimization method) using three balancing factors and institution" |
| Allocation concealment (selection bias) | Low risk | Quote: "based on central‐registration method between 25 and 28 postoperative days. The randomized results processed in a computed system with the intention of concealment and adequate randomization" |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No blinding however no likely influence of unblinding upon survival. |
| Blinding of participants and personnel (performance bias) Disease free survival/recurrence/response | Low risk | No blinding however no likely effect upon disease progression or recurrence. |
| Blinding of participants and personnel (performance bias) Adverse events ‐ subjective | High risk | No blinding and may have affected experience of adverse events. |
| Blinding of participants and personnel (performance bias) Treatment modification/withdrawal | Unclear risk | No blinding and may have affected treatment decisions. |
| Blinding of participants and personnel (performance bias) Adverse events ‐ objective | Unclear risk | No blinding and effect upon adverse events unclear. |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No blinding of outcome assessors reported however low risk of bias in ascertainment of death. |
| Blinding of outcome assessment (detection bias) Disease free survival/recurrence/response | Unclear risk | No blinding described and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ subjective | High risk | No blinding described and high risk that assessment may have been affected. |
| Blinding of outcome assessment (detection bias) Treatment modification/withdrawal | Unclear risk | No blinding described and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ objective | Unclear risk | No blinding described and unclear how assessment carried out. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Appears complete outcome assessment and reporting. |
| Incomplete outcome data (attrition bias) Disease free survival/recurrence/response | Low risk | Appears complete outcome assessment and reporting. |
| Incomplete outcome data (attrition bias) Treatment modification/withdrawal | Unclear risk | Appears complete outcome assessment and reporting. |
| Incomplete outcome data (attrition bias) Adverse events ‐ objective | Unclear risk | Unclear whether complete assessment was conducted and reported. |
| Incomplete outcome data (attrition bias) Adverse events ‐ subjective | Unclear risk | Unclear whether complete assessment was conducted and reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol is mentioned but a registration or publication was not accessible. |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
Mitomi 1992.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: Japan Study dates: enrolment March 1985‐February 1987 Recruitment: patients recruited by attending physicians at 25 institutions in Kanagawa Prefecture. Number screened / eligible: 462/448 Inclusion criteria: primary cancer of the colon and rectum, any TN1, 2, 3MO or T4NOMO; macroscopically curative resection, under 75 years old, informed consent Exclusion criteria: had received cancer therapy before resection, multiple or duplicated carcinoma, severe complications, or abnormal laboratory values (WBC, PLT, protein, albumin, A/G, SGOT‐SGPT, urine protein or creatinine) Diagnostic criteria for inclusion/exclusion: primary cancer of the colon and rectum, any TN1, 2, 3MO or T4NOMO |
|
| Participants |
Number randomised: 448; 227 control; 221 intervention Baseline imbalances: groups well‐matched on most variables except for rectal tumour size (larger in PSK group). Age: PSK: under 40 n = 14; 40‐49 n = 37; 50‐59 n=67; 60‐69 n = 71; 70+ n=32. Control: under 40 n = 13; 40‐49 n = 41; 50‐59 n = 74; 60‐69 n = 63; 70+ n = 36. Overall (calculated): under 40 n = 27; 40‐49 n = 78; 50‐59 n = 141; 60‐69 n = 134; 70+ n = 68. Sex:Coriolus versicolor (PSK): 125 men/96 women; Control: 128 men/99 women; Overall: 253 men/195 women Cancer type: colorectal. PSK group colon = 124 rectum = 97; Control group colon = 125 rectum n = 102; Overall colon = 249 rectum = 199 Cancer removed or present: removed If cancer was present,was it local or metastatic?: N/A What was the aim of chemo/radiotherapy (if applicable)? adjuvant Withdrawals and exclusions: PSK group withdrawals from chemotherapy treatment n = 30 (reasons described as similar to those in control group) and withdrawals from PSK treatment n=26 (report states that few side effects were caused by PSK); control group withdrawals from treatment n =27 (side effects n = 13; postoperative complications n = 9; reasons not reported n = 5) |
|
| Interventions |
Coriolus versicolor intervention: oral protein‐bound polysaccharide K (PSK) manufactured by Kureha Chemical Industry Co. Ltd., 3 g/day, quote: "for over three years" plus chemotherapy as below Control intervention: chemotherapy alone Chemotherapy regimen: Mitomycin C (6.0 mg/m2) IV on day of and day after surgery, followed by oral 5‐FU (200 mg per day) administration quote: "for more than 6 months" Planned length of treatment: at least 6 months or until recurrence for chemotherapy and 3 years for PSK Setting: not described but presumably hospital outpatient Co‐interventions: none mentioned |
|
| Outcomes | 1. Survival 2. Disease free‐survival 3. Disease recurrence 4. Treatment modification/withdrawal 5. Adverse events Planned length of follow‐up from treatment initiation: plans unclear; follow‐up data to 5 years Time points: survival and recurrence reported at 1‐, 2‐, and 3‐year follow‐up. Adverse event assessment: no description of how adverse events were assessed. While the specific side effects for which treatment was abbreviated are reported and there is a total number of these in each group, there is no information on the timing or number of people experiencing side effects. |
|
| Notes |
Study name: The Cooperative Study Group of Surgical Adjuvant Immunochemotherapy for Cancer of Colon and Rectum (Kanagawa) Funding source: no description of funding Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "permuted blocks that were stratified according to the location of the carcinoma (colon vs. rectum) and the institution" |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No blinding however no likely influence of unblinding upon survival |
| Blinding of participants and personnel (performance bias) Disease free survival/recurrence/response | Low risk | No blinding however no likely effect upon disease progression or recurrence |
| Blinding of participants and personnel (performance bias) Adverse events ‐ subjective | High risk | No blinding and likely affected experience of adverse events |
| Blinding of participants and personnel (performance bias) Adverse events ‐ objective | Unclear risk | No blinding and may have affected experience of adverse events |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No blinding of outcome assessors reported however low risk of bias in ascertainment of death |
| Blinding of outcome assessment (detection bias) Disease free survival/recurrence/response | Unclear risk | No blinding described and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ subjective | High risk | No blinding and high risk of bias in assessment of outcome. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ objective | Unclear risk | No blinding and possible risk of bias in assessment. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Appears complete assessment and reporting. |
| Incomplete outcome data (attrition bias) Disease free survival/recurrence/response | Unclear risk | Unclear whether all data captured and reported. |
| Incomplete outcome data (attrition bias) Adverse events ‐ objective | Unclear risk | Unclear whether complete assessment was conducted and reported. |
| Incomplete outcome data (attrition bias) Adverse events ‐ subjective | Unclear risk | Unclear whether complete assessment was conducted and reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration available. |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
Ohwada 2004.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: Japan Study dates: enrolment October 1994 to March 1997 Recruitment: patients recruited at 19 affiliate hospitals of the Second Department of Surgery, Gunma University Hospital, Maebashi, Japan Number screened / eligible: not reported Inclusion criteria: age < 75 years, histologically‐confirmed colorectal cancer, stage II or III primary tumour, measurable IAP levels Exclusion criteria: previous radiotherapy, chemotherapy, or immunotherapy, or had multiple cancers, or severe complications. Also, abnormal white blood cell or platelet count; total protein, serum alanine or aspartate aminotransferase level or creatinine level. Diagnostic criteria for inclusion/exclusion: histological confirmation, stage II or III |
|
| Participants |
Number randomised: 205; Coriolus versicolor (PSK) 139 (137 analysed); control 68 Baseline imbalances: groups well‐matched on most variables. Quote: "There were no striking differences between the groups with respect to stratification factors or the histopathologic characteristics, including pathologic staging, pathologic regional lymph nodes, lymphatic invasion, venous invasion, and residual tumour excluding histopathologic grade. The histopathologic grade was higher in the PSK group than in the control group (P = 0.009)." Age:Coriolus versicolor (PSK) age 25 to 49 n = 14 (10.5%), 50 to 59 n = 33 (24.1%), 60 to 69 n = 57 (41.6%), 70 to 75 n = 33 (24.1%). Control: age 25 to 49 n = 8 (11.9%), 50 to 59 n = 17 (25.0%), 60 to 69 n = 31 (45.6%), 70 to 75 n = 12 (17.9%). Overall: age 25 to 49 n = 22 (10.7%), 50 to 59 n = 50 (24.4%), 60 to 69 n = 88 (49.2%), 70 to 75 n = 45 (22%) Sex:Coriolus versicolor (PSK) male n = 71, female n = 66; Control male n = 43, female n = 25; Overall male n = 114, female n = 91 Cancer type: colorectal. Coriolus versicolor (PSK) colon n = 88 (64.2%) rectum n = 49 (35.8%); Control colon n = 34 (50%) rectum n = 34 (50%); overall colon n = 122 rectum n = 83 Cancer removed or present: removed If cancer was present,was it local or metastatic?: N/A What was the aim of chemo/radiotherapy (if applicable)? adjuvant Withdrawals and exclusions:Coriolus versicolor (PSK) = 6 (4 refusals and 2 ineligible); Control = 1 refusal |
|
| Interventions |
Coriolus versicolor intervention: quote: "oral PSK (3.0 g day‐1) and UFT (300 mg day‐1), starting 2 weeks after surgery and continuing for 2 years or until the diagnosis of tumour recurrence." plus chemotherapy as below Control intervention: chemotherapy alone Chemotherapy regimen: quote: "'All registered patients received bolus injections of 12 and 8mgm‐2 MMC (Kyowa Hakko, Inc., Japan) on postoperative days 1 and 2, respectively. All patients received UFT (300 mg day‐1), starting 2 weeks after surgery and continuing for 2 years or until the diagnosis of tumour recurrence." Planned length of treatment: 2 years Setting: not described but presumably hospital outpatient Co‐interventions: none mentioned |
|
| Outcomes | 1. Survival 2. Disease‐free survival 3. Disease recurrence 4. Treatment modification/withdrawal 5. Adverse events Planned length of follow‐up from treatment initiation: 5 years Time points: survival and disease‐free survival reported at 3 and 5‐year follow‐up, recurrences reported at 5 years. Other outcomes: specific sites of recurrence. Adverse event assessment: follow‐up investigations (every 2 weeks for first month after discharge, then monthly for two years and then every 2‐3 months until 3 tears after surgery) included interview about drug toxicities which were recorded according to WHO criteria. |
|
| Notes |
Study name: Gunma Oncology Study Group (GOSG) Funding source: no description of funding Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment to the control or PSK‐treatment group was made by referring to a pre‐set list generated using a computer‐assisted randomisation system." |
| Allocation concealment (selection bias) | Low risk | Quote:"The subjects were randomly allocated to two groups immediately after surgery, using a centralised registration system." |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No blinding however no likely influence of unblinding upon survival |
| Blinding of participants and personnel (performance bias) Disease free survival/recurrence/response | Low risk | No blinding however no likely effect upon disease progression or recurrence |
| Blinding of participants and personnel (performance bias) Treatment modification/withdrawal | Unclear risk | No blinding and unclear whether treatment decisions were affected. |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No blinding of outcome assessors reported however low risk of bias in ascertainment of death |
| Blinding of outcome assessment (detection bias) Disease free survival/recurrence/response | Unclear risk | No blinding described and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Treatment modification/withdrawal | Unclear risk | No blinding and unclear how assessment was carried out |
| Incomplete outcome data (attrition bias) Survival | Low risk | Appears complete assessment and reporting. |
| Incomplete outcome data (attrition bias) Disease free survival/recurrence/response | Low risk | Appears complete assessment and reporting. |
| Incomplete outcome data (attrition bias) Treatment modification/withdrawal | Low risk | Appears complete assessment and reporting. |
| Selective reporting (reporting bias) | Unclear risk | Text mentions protocol but no available registration or publication. |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
Sadahiro 2010.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: Japan Study dates: not reported Recruitment: not reported Number screened/eligible: not reported Inclusion criteria: age 20‐80 years, histologically‐confirmed rectal cancer, ECOG performance status 1 or less, and adequate organ function "defined as leukocyte count ≥4,000/μL and ≤12,000/μL, neutrophil count ≥2,000/μL, platelet count ≥100,000/μl, haemoglobin ≥9 g/dL, serum creatinine ≤1.1 mg/dL, serum bilirubin ≤1.5 mg/dL, and serum aspartate aminotransferase and alanine aminotransferase ≤100 U/L." . Exclusion criteria: no additional criteria specified Diagnostic criteria for inclusion/exclusion: quote: "histologically confirmed adenocarcinoma of the middle or lower rectum (cT3‐T4, Tx N+, M0)" |
|
| Participants |
Number randomised: 30; Coriolus versicolor (PSK) 15; control 15 Baseline imbalances: groups comparable at baseline. Quote: "No differences in male/ female ratio and age were observed between the PSK and control groups (Table I). There were also no significant differences between the two groups in histological type determined from biopsies conducted before treatment, tumor diameter of resected specimens, or TNM staging of resection specimens." Age:Coriolus versicolor (PSK) group mean (SD) 60 (10) years; Control group mean (SD) 65 (6) years: overall mean (SD) 62.5 (8.5) years Sex:Coriolus versicolor (PSK) male n = 12, female n = 2 (sic); Control male n = 11, female n = 4; Overall male n = 23, female n = 6 Cancer type: rectal Cancer removed or present: removed after PSK treatment If cancer was present,was it local or metastatic?: N/A What was the aim of chemo/radiotherapy (if applicable)? neo‐adjuvant (treatment given prior to the main treatment, which was surgery) Withdrawals and exclusions: No mention of withdrawals or exclusions. |
|
| Interventions |
Coriolus versicolor intervention: quote: "PSK (Kureha Corporation, Japan) 3.0 g/day divided into 3 daily doses, for 4 weeks until 3 days before surgery" plus chemoradiotherapy as below Control intervention: chemoradiotherapy alone Chemotherapy regimen: quote: "oral S‐1 (Taiho Pharmaceutical Co., Japan) 80 mg/m2/day divided into 2 daily doses" "for 4 weeks until 3 days before surgery" Planned length of treatment: 4 weeks Setting: not described but presumably hospital outpatient Co‐interventions: preoperative CRT quote: "External beam RT for a total dose of 20 Gy was delivered in daily fractions of 2.0 Gy, 5 days a week for two weeks using a four‐field box technique.""During surgery, electron beam irradiation of 15 Gy was delivered" |
|
| Outcomes | 1. Adverse events
Planned length of follow‐up from treatment initiation: 4 weeks Time points: post‐surgery. Other outcomes: evaluation of systemic immunity in the peripheral blood, evaluation of local immunity in the resected specimen Adverse event assessment: quote:"During therapy, toxicity was assessed weekly according to the Common Toxicity Criteria of National Cancer Institute version 2.0." One serious event (Grade 3 was reported in one patient in the PSK group. Other than this, all adverse events are reported according to numbers of each event in each group (total 26 mild events in PSK group and 30 mild events in control group), not to numbers of people experiencing each event in each group. |
|
| Notes |
Study name: none Funding source: no description of funding Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) Adverse events ‐ subjective | High risk | No blinding and experience of subjective adverse events is at high risk of bias. |
| Blinding of participants and personnel (performance bias) Adverse events ‐ objective | Unclear risk | No blinding and the processes for assessment of outcomes are unclear. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ subjective | High risk | Lack of blinding puts assessment of subjective adverse events at high risk of bias. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ objective | Unclear risk | Lack of blinding puts and assessment of outcomes is unclear. |
| Incomplete outcome data (attrition bias) Adverse events ‐ objective | Low risk | Appears complete assessment and reporting of outcome data. |
| Incomplete outcome data (attrition bias) Adverse events ‐ subjective | Low risk | Appears complete assessment and reporting of outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No available protocol or registration |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
Shichinohe 2013.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: Japan Study dates: not reported Recruitment: recruited from 35 different facilities but no detail about procedures Number screened/eligible: not clear. 342 patients "registered" Inclusion criteria: patients aged 20‐80 years with primary colonic carcinoma or rectal carcinoma of histological stage III, who have undergone histological curability A or B surgeries; patients evaluated histologically as lymph node metastasis positive; performance status 0 to 2; no receipt of preoperative cancer therapy (radiotherapy, chemotherapy or immunotherapy); no diarrhoea (watery stool); no severe impairment of renal, hepatic and bone marrow functions; no serious concurrent complications (such as infection). (Data from trial registration NCT00209742) Exclusion criteria: patients graded as curability C; with stenosis not capable of oral intake; if disease stage IIIa those that are si/n(‐) and ai/n(‐); fresh haemorrhage from the gastrointestinal tract; retention of body fluid necessitating treatment; infection, intestinal palsy or intestinal occlusion; lower end of the tumour involving the peritoneal reflection; lower rectal cancer (Rb), involving the anal canal (P) or perianal skin (E); active multiple cancers; or even if the multiple cancers are metachronous, have a disease‐free period of less than 5 years (but excluding cancer in situ and skin cancer); pregnant or hope to become pregnant during the study period; poorly controlled diabetes or are treated by continuous use of insulin; a history of ischaemic heart disease; concurrent psychiatric disease or psychotic symptoms, and judged to have difficulties participating in the study; receiving continuous administration of steroids; serious drug allergy in the past. (Data from trial registration NCT00209742) Diagnostic criteria for inclusion/exclusion: quote: "histological stage III and curability A or B colorectal cancer [according to General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus, 6th edition (Japanese Classification of Colorectal Cancer, English edition), also according to pTNM classification to facilitate overseas publication]" (Data from trial registration NCT00209742) |
|
| Participants |
Number randomised: 340 "analyzed" presumably this also refers to number randomised however this is not explicit in the meeting abstract; Coriolus versicolor (PSK) with UFT+LV for 6 months and UFT for 12 months n = 171; UFT+LV for 6 months and UFT for 12 months n = 85; UFT+LV for 6 months n = 84. Baseline imbalances: quote: "At baseline, variation in QOL score was observed but histopathological parameters were not different among 3 groups." Age: NR Sex: NR Cancer type: colorectal (no further details) Cancer removed or present: removed If cancer was present,was it local or metastatic? : N/A. However, note that based on trial registration lymph node metastasis positive was an eligibility criterion. What was the aim of chemo/radiotherapy (if applicable)? adjuvant Withdrawals and exclusions: No mention of withdrawals or exclusions. |
|
| Interventions |
Coriolus versicolor intervention: oral protein‐bound polysaccharide K (PSK). Dose not reported. Given with UFT+LV "28 days/5 weeks for 6 months" then with UFT for 12 months plus chemotherapy as below Control intervention: chemotherapy as below Chemotherapy regimen: the chemotherapy regimen carried out with and without PSK was "UFT+LV 28 days/5 weeks for 6 months, then UFT for 12 months". A third group was given "UFT/LV 28 days/5 weeks for 6 months" and no UFT afterwards. Planned length of treatment: 18 months Setting: not described but presumably hospital outpatient Co‐interventions: none mentioned |
|
| Outcomes | 1. Survival 2. Disease‐free survival 3. Quality of life (instrument not mentioned, percentage with "high score" reported) 4. Adverse events Planned length of follow‐up from treatment initiation: 5 years Time points: trial registration mentions outcomes to 5 and 7 years but abstract mentions only 3‐year outcomes. Other outcomes: compliance and adverse events Adverse event assessment: methods of assessment and numbers of events or persons not mentioned, however adverse events were assessed and discussed: quote: "Adverse events ≥grade 3 included gastrointestinal symptoms and general status. There was no treatment‐related death." |
|
| Notes |
Study name: Hokkaido Gastrointestinal Cancer Study Group Funding source: no description of funding Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details as abstract only |
| Allocation concealment (selection bias) | Unclear risk | No details as abstract only |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No blinding however no likely influence of unblinding upon survival |
| Blinding of participants and personnel (performance bias) Disease free survival/recurrence/response | Low risk | No blinding however no likely effect upon disease progression or recurrence |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No blinding of outcome assessors reported however low risk of bias in ascertainment of death |
| Blinding of outcome assessment (detection bias) Disease free survival/recurrence/response | Unclear risk | No blinding described and unclear how assessment carried out. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Cannot tell from abstract publication whether complete assessment was conducted and reported. |
| Incomplete outcome data (attrition bias) Disease free survival/recurrence/response | Unclear risk | Cannot tell from abstract publication whether complete assessment was conducted and reported. |
| Selective reporting (reporting bias) | Low risk | There is a trial registration NCT00209742 and outcomes correspond. |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
Sugimoto 2012.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: Japan Study dates: enrolment May 2008‐May 2010 Recruitment: not described. Number screened / eligible: NR / NR Inclusion criteria: patients quote: "had undergone curative resection of high‐risk stage II(T3─4N0M0) or stage III(T1─4N1─3M0) adenocarcinoma of the colon and rectum" and "had an Eastern Cooperative Oncology Group performance status of 0, 1, or 2 and were less than 80 years old" Exclusion criteria: quote: "Patients were ineligible if they had synchronous or metachronous multiple cancers or severe complications." Diagnostic criteria for inclusion/exclusion: T3─4N0M0 or T1─4N1─3M0 |
|
| Participants |
Number randomised: 50; 25 Coriolus versicolor (PSK); 25 control Baseline imbalances: none quote: "With respect to the patient backgrounds, i.e. gender, age, UFT administration dose, location and other clinicopathological factors, there were no significant differences between the PSK (− ) group and the PSK (+) group (Table─1, 2." Age:Coriolus versicolor (PSK) group mean (SD) 63.8 (10.9) years; non‐PSK group mean (SD) 63.1 (8.4) years; Total not calculated. Sex:Coriolus versicolor (PSK) group male/female 13/12; non‐PSK group male/female 17/8; Total male/female 30/20 Cancer type: colorectal. PSK group colon n = 16/25 (64%) rectum n = 9/25 (36%); non‐PSK group colon n = 14/25 (56%) rectum n = 11/25 (44%); Total colon n = 30/50 (60%) rectum n = 20/50 (40%) Cancer removed or present: curative resection If cancer was present,was it local or metastatic: N/A. However note possible metastatic cancer post resection, as Stage II colorectal cancers with lymphatic/venous invasion were defined as high‐risk Stage II and eligible for inclusion. What was the aim of chemo/radiotherapy (if applicable)? adjuvant Withdrawals and exclusions: PSK group 1 dose reduction due to adverse events, 1 discontinuation due to recurrence; non‐PSK group colon 2 discontinuations due to adverse events, 1 discontinuation due to recurrence |
|
| Interventions |
Coriolus versicolor intervention: oral protein‐bound polysaccharide K (PSK) (KRESTIN®), 3 g/day, every day. The PSK was started when the UFT/LV was started. Control intervention: chemotherapy alone Chemotherapy regimen: oral UFT/LV(UFT ®;tegafur/uracil, Taiho Pharmaceuticals Co., Japan, LV;calcium leucovorin, Wako Pure Chemical Industries, Japan)dose was UFT 300mg/m2/day and LV 7 5mg/day. Dosing was started 4‐8 weeks after surgery and continued for 6 months or until the diagnosis of tumour recurrence. The UFT/LV was administered for 5 consecutive days, followed by 2 days in which the drug was not administered(5 days‐on/2 days‐off schedule.) Planned length of treatment: 6 months or until tumour recurrence Setting: not described but presumably hospital outpatient Co‐interventions: none mentioned |
|
| Outcomes | 1. Disease recurrence 2. Treatment modification/withdrawal 3. Adverse events Planned length of follow‐up from treatment initiation: 6 months or until diagnosis of tumour recurrence Time points: survival and recurrence reported at 6 months, laboratory values assessed and reported at baseline and 1, 3, and 6 month follow‐up. Other outcomes: laboratory tests (NK cells, white blood cells, neutrophils, red blood cells, platelets, AST, ALT, total bilirubin, creatine, reactive oxygen metabolites, salivary chromogranin A) Adverse event assessment: quote: "Assessment of adverse events was performed using the Common Terminology Criteria for Adverse Events ver. 4. 0(CTCAE ver. 4. 0)"(Accessed June 12, 2011, via the NIH Cancer Therapy Evaluation Program (CTEP) at http://ctep.cancer.gov). The authors do not specify how this was assessed in the patients. The authors do not specify how they categorised adverse events as serious/not serious, however based on the description at https://www.biooncology.com/clinical‐trials/safety‐endpoints.html it would appear that Grade 3 or higher would qualify as serious. |
|
| Notes |
Study name: none Funding source: no description of funding Declaration of interest: States that authors have no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random sequence is not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Disease free survival/recurrence/response | Low risk | No blinding however no likely effect upon disease progression or recurrence |
| Blinding of participants and personnel (performance bias) Adverse events ‐ subjective | High risk | No blinding and effect upon experience of adverse events is at high risk of bias |
| Blinding of participants and personnel (performance bias) Treatment modification/withdrawal | Unclear risk | No blinding and effect upon treatment decisions unclear |
| Blinding of participants and personnel (performance bias) Adverse events ‐ objective | Unclear risk | No blinding and effect upon adverse events unclear |
| Blinding of outcome assessment (detection bias) Disease free survival/recurrence/response | Unclear risk | No blinding and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ subjective | High risk | No blinding and subjective assessment of outcomes at high risk of bias. |
| Blinding of outcome assessment (detection bias) Treatment modification/withdrawal | Unclear risk | No blinding and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ objective | Unclear risk | No blinding and unclear how assessment carried out. |
| Incomplete outcome data (attrition bias) Disease free survival/recurrence/response | Low risk | Assessment and reporting of outcome appears complete. |
| Incomplete outcome data (attrition bias) Treatment modification/withdrawal | Low risk | Assessment and reporting of outcome appears complete. |
| Incomplete outcome data (attrition bias) Adverse events ‐ objective | Low risk | Assessment and reporting of outcome appears complete. |
| Incomplete outcome data (attrition bias) Adverse events ‐ subjective | Low risk | Assessment and reporting of outcome appears complete. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
Xu 2008.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Study location: China Study dates: enrolment June 2003 to June 2005. Recruitment: patients recruited at Nanfang Hospital, Guangzhou, China. Details of recruitment process not reported. Number screened / eligible: NR/NR Inclusion criteria: diagnosed as advanced or metastatic colorectal cancer; Karnofsky≥60; acceptable laboratory data including electrocardiograph, routine blood tests, liver function tests; measurable nidus by imaging examinations or physical checkup; expected survival life ≥3 months; patients have not received the chemotherapy in which the regimens include oxaliplatin. Exclusion criteria: not reported. Diagnostic criteria for inclusion/exclusion: confirmed diagnosis of advanced colorectal cancer by histopathology. |
|
| Participants |
Number randomised: 53; Coriolus versicolor (PSK) 27; control 26 Baseline imbalances: paper states that groups are comparable and the clinical status elements at baseline in Table 1 do not differ between groups. Age: PSK 64.3 ± 11.3 years; placebo 57.6 ± 10.7 years: overall median = 61 years, range 41‐71 years Sex: total male n = 31 (58%) Cancer type: colorectal (no further details) Cancer removed or present: unclear If cancer was present,was it local or metastatic?: there was mention of metastases (liver, lung, bone, abdomen, pelvis, lymph nodes, local recurrence) What was the aim of chemo/radiotherapy (if applicable)? unclear Withdrawals and exclusions: not reported. |
|
| Interventions |
Coriolus versicolor intervention: Yunzhi [PSK] polysaccharide capsules 6 g/day, continuous oral administration for over one year plus chemotherapy as below Control intervention: chemotherapy alone Chemotherapy regimen: XELOX regimen: oxaliplatin 130 mg/m2 intravenous infusion for 2 hours on the first day, capecitabine 2 000 mg/m2 taken orally with morning and evening meal from the first day to the 14th day. 21 days are defined as a cycle. All patients received 3 to 8 cycles of chemotherapy Planned length of treatment: over 1 year Setting: not described Co‐interventions: not described |
|
| Outcomes | 1. Overall survival 2. Complete remission (CR) 3. Partial remission (PR) 4. Stable disease (SD) 5. Progression (PD) 6. Quality of life (ECOG PS score, 0‐5, lower is better) 7. Adverse events Planned length of follow‐up from treatment initiation: not reported Time points: reported at 3 years with the exception of Quality of life which was quote: "evaluated after 3 cycles of chemotherapy” and the Response Evaluation Criteria in Solid Tumors (RECIST) factors (i.e., CR, PR, SD, PD), which were "evaluated after at least 3 cycles of chemotherapy”. Other outcomes: Effective rate (RR)=CR+PR, clinical benefit rate (CBR) = CR+PR+SD. Time to disease progression (TTP). Duration of overall survival. Adverse event assessment: quote: "Adverse events are evaluated according to the National Cancer Institute Common Toxicity Criteria. The study assessed nausea, vomiting, bone marrow suppression toxicity, hematotoxicity, neurotoxicity and non‐hematological toxicity." The timing of the AEs is not described, and the total number of people with any AE, or with any SAEs, is not given. Adverse events are reported according to the numbers of each events occurring in each group. In the Yunzhi group there were a total of 51 AEs and in the control group there were a total of 112 adverse events. |
|
| Notes |
Study name: none Funding source: no description of funding Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No blinding however no likely influence of unblinding upon survival |
| Blinding of participants and personnel (performance bias) Disease free survival/recurrence/response | Low risk | No blinding however no likely effect upon disease progression or recurrence |
| Blinding of participants and personnel (performance bias) Adverse events ‐ subjective | High risk | No blinding and effect upon experience of adverse events at high risk of bias |
| Blinding of participants and personnel (performance bias) Adverse events ‐ objective | Unclear risk | No blinding and effect upon adverse events unclear |
| Blinding of participants and personnel (performance bias) Quality of life | High risk | No blinding and effect upon quality of life at high risk of bias |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No blinding of outcome assessors reported however low risk of bias in ascertainment of death |
| Blinding of outcome assessment (detection bias) Disease free survival/recurrence/response | Unclear risk | No blinding and unclear how assessment carried out. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ subjective | High risk | No blinding and assessment of outcomes requiring judgment at high risk of bias. |
| Blinding of outcome assessment (detection bias) Adverse events ‐ objective | Unclear risk | No blinding and unclear how outcome assessment carried out. |
| Blinding of outcome assessment (detection bias) Quality of life | High risk | No blinding and assessment of outcomes requiring judgment at high risk of bias. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Unclear processes for assessment and whether assessment was complete. |
| Incomplete outcome data (attrition bias) Disease free survival/recurrence/response | Unclear risk | Unclear processes for assessment and whether assessment was complete. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | Outcome appears to have been assessed on all participants and presented fully. |
| Incomplete outcome data (attrition bias) Adverse events ‐ objective | Unclear risk | Unclear processes for assessment and whether assessment was complete. |
| Incomplete outcome data (attrition bias) Adverse events ‐ subjective | Unclear risk | Unclear processes for assessment and whether assessment was complete. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No potential biases were apparent that do not correspond to the above categories (including study‐specific problems such as carryover in cross‐over trials, more general problems in study conduct such as contamination between intervention arms, or that a study is claimed to be fraudulent). |
A/G: albumin/globulin; ALT: alanine aminotransferase; AST: aspartate aminotransferase;CRT: chemoradiotherapy; IU: international unit; IV: intravenous; LV: leucovorin; NR: not reported; PLT: platelet; PPD: purified protein derivative; PSK: polysaccharide Krestin; RCT: randomised controlled trial; SD: standard deviation;SGOT: serum glutamic‐oxaloacetic transaminase;SGPT: serum glutamic pyruvic transferase; UFT: tegafur/uracil; WBC: white blood cell; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agaki 2010 | Wrong study design. |
| Akao 1980 | Wrong study design. |
| Amano 1995 | Systematic review, narrative review or overview. Not an original study report. |
| Anon 1987 | Wrong study design. |
| Anon 2013 | Wrong intervention. |
| Becouarn 1996 | Systematic review, narrative review or overview. Not an original study report. |
| Boone 1994 | Systematic review, narrative review or overview. Not an original study report. |
| Hazama 2002 | Wrong study design. |
| Hiyashibe 1992 | Wrong study design. |
| Iseki 1992 | Wrong study population. |
| Ito 2008a | Wrong comparator. |
| Ito 2008b | Wrong comparator. |
| JPRN‐UMIN000001527 | Wrong comparator. |
| JPRN‐UMIN000010045 | Wrong study design. |
| Katsuno 2004 | Wrong study design. |
| Kitamura 1996 | Animal study. Wrong study population. |
| Kobayashi 1995 | Systematic review, narrative review or overview. Not an original study report. |
| Kobayshi 1994 | Wrong study design. |
| Koda 2003 | Wrong comparator. |
| Kudo 2002 | Wrong study design. |
| Li 2019 | Wrong study design. |
| Liu 2014 | Animal study. Wrong study population. |
| Maehara 2012 | Systematic review, narrative review or overview. Not an original study report. |
| Maruyama 2002 | Wrong study design. |
| Miyake 2018 | Wrong comparator. |
| Mukai 2003 | Wrong study design. |
| Nakazato 1986 | Wrong study design. |
| NCT00385970 | Wrong comparator. |
| NCT03716518 | Wrong intervention |
| Nio 1992 | Data for colorectal cancer patients not reported separately. Wrong study population. |
| Nio 1995 | Wrong study design. |
| Okuno 2017 | Wrong comparator. |
| Okuno 2018 | Wrong comparator. |
| Ooshiro 2009 | Wrong study design. |
| Saji 1997 | Systematic review, narrative review or overview. Not an original study report. |
| Sakai 2003 | Systematic review, narrative review or overview. Not an original study report. |
| Shibata 1999a | Systematic review, narrative review or overview. Not an original study report. |
| Shibata 1999b | Wrong study design. |
| Shibata 1999c | Wrong study design. |
| Shibata 2002 | Wrong study design. |
| Shibata 2011 | Wrong study design. |
| Sugiyama 1998 | Wrong study design. |
| Takashima 1988 | Wrong study design. |
| Torisu 1986 | Wrong intervention |
| Torisu 1990 | Wrong intervention |
| Torisu 1991 | Systematic review, narrative review or overview. Not an original study report. |
| Toth 2005 | Animal study. Wrong study population. |
| Tsukida 2018 | Wrong study design. |
| Watanabe 2010 | Systematic review, narrative review or overview. Not an original study report. |
| Yoshikawa 2005 | Wrong study design. |
| Yoshikawa 2006 | Wrong study design. |
| Yoshitani 2009a | Wrong study design. |
| Yoshitani 2009b | Wrong study design. |
Characteristics of studies awaiting classification [ordered by study ID]
JPRN‐UMIN000004351.
| Methods | Parallel Randomised |
| Participants | Adults with colon cancer |
| Interventions | FOLFOX4 FOLFOX6 FOLFOX/Krestin |
| Outcomes | Frequency of neutropenia Other adverse events Medication adherence QOL |
| Notes | OPEN PUBLIC RECRUITING (as of 19/10/2012) |
JPRN‐UMIN000005286.
| Methods | Parallel Randomised |
| Participants | Adults with gastric and colorectal cancer clinical stage II/III |
| Interventions | TS‐1 chemotherapy alone group TS‐1 with PSK group |
| Outcomes | Transition of PSK and induction of infiltration of cytotoxic T lymphocytes into primary tumour |
| Notes | COMPLETED (as of 09/08/2016) |
JPRN‐UMIN000005484.
| Methods | Parallel Randomised |
| Participants | Adults with colorectal cancer |
| Interventions | Group A (standard therapy group:UFT/LV 6M) Group B (trial therapy group: PSK/UFT/LV 6M). Group C (trial therapy group: PSK/UFT/LV 12M) |
| Outcomes | 3 year disease‐free survival rate Survival time Adverse event rate Completion rate |
| Notes | COMPLETED (as of 28/10/2018) |
JPRN‐UMIN000006134.
| Methods | Parallel Randomised |
| Participants | Adults with colon cancer |
| Interventions | Group A: mFOLFOX6 Group A': XELOX Group B: mFOLFOX6(or XELOX)/Krestin |
| Outcomes | The percentage of patients who complete scheduled treatment Adverse events IL‐12 and the number of NK cells in peripheral blood |
| Notes | TERMINATED (as of 16/07/2014) |
JPRN‐UMIN000006541.
| Methods | Parallel Randomised |
| Participants | Adults with colon cancer |
| Interventions | Group A (capecitabine group) Group B (capecitabine/PSK group) |
| Outcomes | Adverse events rate 3 year disease‐free survival 3 year relapse‐free survival 3 year overall survival Compliance QOL |
| Notes | Complete: follow‐up continuing (as of 02/04/2019) |
JPRN‐UMIN000007252.
| Methods | Parallel Randomised |
| Participants | Adults with colon cancer |
| Interventions | Group A(XELOX) Group B(XELOX+PSK) |
| Outcomes | Peripheral neuropathy as adverse event frequency Time to the peripheral neuropathy as adverse event Rate of treatment accomplishment Tolerated dose Disease‐free survival time Other adverse events |
| Notes | NO LONGER RECRUITING (as of 16/05/2017) |
JPRN‐UMIN000010046.
| Methods | Parallel Randomised |
| Participants | Adults with colorectal cancer |
| Interventions | 1)Curative resection 2)UFT (Stage II) or UFT/LV (Stage III) for 1 year 1)Curative resection 2)UFT (Stage II) or UFT/LV (Stage III) combined with PSK for 1 year |
| Outcomes | 3 year disease‐free survival |
| Notes | OPEN PUBLIC RECRUITING (as of 09/09/2013) |
NCT00209742.
| Methods | Parallel Randomised |
| Participants | Adults with histological stage III colorectal cancer |
| Interventions | Drug: UFT Drug: USEL/Leucovorin Drug: Krestin |
| Outcomes | 3‐year disease‐free survival rate Dose intensity (compliance) 5‐year disease‐free survival rate 3‐ and 5‐year survival rate Incidence of other adverse drug reactions QOL |
| Notes | STATUS UNKNOWN (as of 26/05/2010) |
NCT00497107.
| Methods | Parallel Randomised |
| Participants | Adults with histological stage IIIa/IIIb colorectal cancer |
| Interventions | Drug: UFT, Calcium Folinate Drug: UFT + Calcium Folinate + PSK |
| Outcomes | Disease‐free survival Overall survival Compliance Adverse events QOL Tumour markers |
| Notes | STATUS UNKNOWN (as of 31/07/2008) |
Ogawa 2021.
| Methods | Randomised Parallel |
| Participants | Patients with high‐risk stage II/III colorectal cancer |
| Interventions | 6 months of UFT/LV 6 months UFT/LV+PSK 12 months UFT/LV+PSK |
| Outcomes | 3‐year disease‐free survival |
| Notes |
NK cells: natural killer cells; PSK: Polysaccharide Krestin QoL: quality of life.
Differences between protocol and review
We amended the review title to more clearly show the focus on adverse event reduction and to include the current preferred name for Coriolus (Trametes).
We amended the objectives to clarify that Coriolus was to be used adjunctively with chemotherapy or radiotherapy (and to match the inclusion criteria).
We amended adverse effect to adverse event in describing the data extracted and analysed in view of the lack of reporting of causality in the studies.
For searching of the Japanese databases, we searched only Ichushi Web (http://www.jamas.or.jp), web version of Igaku Chuou Zasshi (Japana centra revuo medicina). The clinical trial registration site of Japan UMIN‐CTR is included in the WHO‐clinical trial registration site. The remaining two databases listed in the protocol were not found to be appropriate for searching for randomised controlled trials. Searching of the US National Cancer Institute was not carried out as the trials are included on existing trials databases nor was Current Controlled Trials searched as this website is not longer available. BIOSIS Previews could only be searched to 2008 .
We had planned to contact study authors and manufacturers for unpublished trials and any missing data. We attempted to do so, but it was difficult to locate current contact details for study authors, and we did not obtain any responses while the Japanese manufacturer had discontinued the product used in the included trials .
We changed examples in the text of other sources of risk of bias to more closely conform to the description of this category in Appendix 2.
We added that two review authors independently assessed all estimates of effect for certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Contributions of authors
Drafting of the protocol: Janine Leach, Jianping Liu, Karen Pilkington, Dawn Storey, Lida Teng.
Development of search strategies and conduct of searches: Karen Pilkington, Lida Teng, XIn Yan Jin, Jianping Liu.
Development of data extraction template: Karen Pilkington, L.Susan Wieland, Dawn Storey.
Selection and translation of trials, extraction of data: Karen Pilkington, L Susan Wieland, Lida Teng, Xin Yan Jin, Jianping Liu.
Data entry into RevMan: L Susan Wieland, Karen Pilkington.
Conduct and interpretation of the analysis: L. Susan Wieland, Karen Pilkington.
Preparation and editing of the review: Karen Pilkington, L. Susan Wieland, Lida Teng, Xin Yan Jin, Dawn Storey, Jianping Liu.
Sources of support
Internal sources
-
Innovative Research Team of Beijing University of Chinese Medicine, China
Jianping Lui's work on the review was partially supported by an internal grant (No. 2011‐CXTD‐09)
External sources
-
National Center for Complementary and Intregrative Health (NCCIH) of the US National Institutes of Health (www.nccih.nih.gov)., USA
Jianping Lui's work on the review was partially funded by grant number R24 AT001293 (NCCIH)
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations of interest
Karen Pilkington: none known
L. Susan Wieland: none known
Lida Teng: none known
Xin Yan Jin: none known
Dawn Storey: none known
Jianping Liu: none known
New
References
References to studies included in this review
Ito 2004 {published data only}
- 勝 伊藤 (Ito K). 【Tumor dormancy therapy】Impact of adjuvant postoperative immunochemotherapy for gastrointestinal cancer [【Tumor dormancy therapy】 消化器癌の術後補助免疫化学療法の意義]. G.I.Research 2004;12(5):385-92. [Google Scholar]
- 正 磯谷, 晃 山口, 博 中里, 勝 伊藤, 弘 高木, 重 佐治, 明 小池, 正 馬塲, 正 磨伊 and 靖 大橋 (Isoya K, Yamaguchi A, Nakasato H, Ito K, Takaki H, Saji A et al). Adjuvant immunochemotherapy with Krestin after curative resection of colorectal cancer, 7-year follow-up results in a randomized clinical trial (CIP Study Group) [クレスチンを併用した結腸癌治癒切除後の補助免疫化学療法 無作為化臨床試験における術後7年追跡調査結果(CIP研究会)]. In: 日本癌治療学会誌 (The Journal of Japan Society for Cancer Therapy). Vol. 36. 2001:532.
- Ito K, Nakazato H, Koike A, Takagi H, Saji S, Baba S, et al. A randomized clinical trial on adjuvant immunochemotherapy with protein bound polysaccharide K (PSK) after curative resection of colon cancer: Seven-year follow-up results. International Journal of Antimicrobial Agents 2001;17(Supplement 1):S17. [Google Scholar]
- Ito K, Nakazato H, Koike A, Takagi H, Saji S, Baba S, et al. Long-term effect of 5-fluorouracil enhanced by intermittent administration of polysaccharide K after curative resection of colon cancer. A randomized controlled trial for 7-year follow-up. International Journal of Colorectal Disease 2004;19(2):157–64. [DOI: 10.1007/s00384-003-0532-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ito K, Nakazato H, Takagi H, Saji S, Koike A, Baba S, et al. A randomized clinical trial on adjuvant immunochemotherapy with protein bound polysaccharide K (PSK) after curative resection of colon cancer: seven-year follow-up after surgery [abstract]. In: Proceedings of the American Society of Clinical Oncology. Vol. 20 (Pt 2). 2001:117b, Abstract 2220.
- Matsui T, Kojima H, Suzuki H, Hamajima H, Nakazato H, Ito K, et al. Sialyl Lewisa expression as a predictor of the prognosis of colon carcinoma patients in a prospective randomized clinical trial. Japanese Journal of Clinical Oncology 2004;34(10):588-93. [DOI] [PubMed] [Google Scholar]
- Matsui T, Omura K, Kawakami K, Morita S, Sakamoto J. Genotype of thymidylate synthase likely to affect efficacy of adjuvant 5-FU based chemotherapy in colon cancer. Oncology Reports 2006;16(5):1111-5. [PMID: PMID: 17016601] [PubMed] [Google Scholar]
- Nakazato H, Ito K, Takagi H, Saji S, Koike A, Baba S, et al. A randomized clinical trial on adjuvant immunochemotherapy with protein bound polysaccharide K (PSK) after curative resection of colon cancer [abstract]. In: Proceedings of the American Society of Clinical Oncology. Vol. 18. 1999:245a, Abstract 942.
- Sakamoto J, Hamashima H, Suzuki H, Ito K, Mai M, Saji S, et al. Sialyl-Lewisa expression predict prognosis of curatively resected colon cancer patients in an adjuvant immunochemotherapy randomized trial. In: Proceedings of the American Association for Cancer Research Annual Meeting. Vol. 42. 2001:577-8.
- Sakamoto J, Hamashima H, Suzuki H, Ito K, Mai M, Saji S, et al. Thymidylate synthase expression as a predictor of the prognosis of curatively resected colon carcinoma in patients registered in an adjuvant immunochemotherapy clinical trial. Oncology Reports 2003;10(5):1081-90. [PMID: PMID: 12883662] [PubMed] [Google Scholar]
- Takahashi Y, Mai M, Nakazato H. Preoperative CEA and PPD values as prognostic factors for immunochemotherapy using PSK and 5-FU. Anticancer Research 2005;25(2B):1377-84. [PMID: PMID: 15865094] [PubMed] [Google Scholar]
- Yamashita K, Nakazato H, Ito K, Ougolkov A, Takahashi Y, Mai M, et al. Effect of adjuvant immunochemotherapy with PSK for colon cancer patients showing oncogenic beta-catenin activation in primary tumor. Journal of Clinical Oncology 2004;22(14_Suppl):3650. [Google Scholar]
- Yamashita K, Ougolkov AV, Nakazato H, Ito K, Ohashi Y, Kitakata H, et al. Adjuvant immunochemotherapy with protein-bound polysaccharide K for colon cancer in relation to oncogenic beta-catenin activation. Diseases of the Colon and Rectum 2007;50(8):1169-81. [DOI: 10.1007/s10350-006-0842-5] [DOI] [PubMed] [Google Scholar]
- Yamashita K, Ougolkov AV, Nakazato H, Ito K, Ohashi Y, Kitakata H, et al. Erratum: Adjuvant immunochemotherapy with protein-bound polysaccharide K for colon cancer in relation to oncogenic beta-catenin activation. Diseases of the Colon & Rectum 2007;50(8):1182-7. [DOI: 10.1007/s10350-007-0291-9] [DOI] [PubMed] [Google Scholar]
Mitomi 1992 {published data only}
- 大腸癌術後免疫化学療法研究会 (Study group of postoperative immunochemotherapy for colorectal cancer). Randomized controlled study of PSK for adjuvant immunochemotherapy after curative resection of colorectal cancer, the effects of with or without blood transfusion and immunochemotherapy [大腸癌治癒切除症例に対するPSKによる補助免疫化学療法のRandomized controlled study 輸血の有無と免疫化学療法の効果]. In: 日本癌治療学会誌 (The Journal of Japan Society for Cancer Therapy). Vol. 24. 1989:2184.
- 大腸癌術後免疫化学療法研究会 (Study group of postoperative immunochemotherapy for colorectal cancer). Randomized controlled study of PSK for adjuvant immunochemotherapy after curative resection of colorectal cancer (3rd report) [大腸癌治癒切除症例に対するPSKによる補助免疫化学療法のRandomized controlled study(第3報)]. In: 日本癌治療学会誌 (Journal of Japan Society for Cancer Therapy). Vol. 26. 1991:2054.
- 大腸癌術後免疫化学療法研究会 (Study group of postoperative immunochemotherapy for colorectal cancer). Randomized controlled study of PSK for adjuvant immunochemotherapy after curative resection of colorectal cancer [大腸癌治癒切除症例に対するPSKによる補助免疫化学療法のRandomized controlled study]. In: 日本癌治療学会誌 (The Journal of Japan Society for Cancer Therapy). Vol. 25. 1990:2266.
- 隆 野登 and 他 (Noto T and Study group of postoperative immunochemotherapy for colorectal cancer). Randomized controlled study of PSK for adjuvant immunochemotherapy after curative resection of colorectal cancer, the effects of with or without blood transfusion and immunochemotherapy [大腸癌治癒切除症例に対するPSKによる補助免疫化学療法のRandomized controlled study(第4報)]. 日本癌治療学会誌 (Journal of Japan Society for Cancer Therapy) 1992;27(8):1641. [Google Scholar]
- Mitomi T, Noto TA. A randomized controlled study on adjuvant immunochemotherapy with an immunomodulator in curatively resected colorectal cancer. Journal of Cancer Research and Clinical Oncology 1990;116(SUPPL. PART 2):872. [Google Scholar]
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Nishiyama K, Amano T. [Randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. 5 Years follow-up after surgery (a final report)] [Japanese] [結腸・直腸癌の治癒切除症例に対するPSKによる補助免疫化学療法のRandomized Controlled Study 術後5年の成績(最終報告)]. Journal of Japan Society for Cancer Therapy 1993;28(1):71-83. [Google Scholar]
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Suzuki K, Nishiyama K, et al. [Randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. The Cooperative Study Group of Surgical Adjuvant Immunochemotherapy for Cancer of Colon and Rectum] [Japanese]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1989;16(6):2241-9. [PMID: PMID: 2500070] [PubMed] [Google Scholar]
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Suzuki K, Nishiyama K, et al. Randomized, controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. The Cooperative Study Group of Surgical Adjuvant Immunochemotherapy for Cancer of Colon and Rectum (Kanagawa). Diseases of the Colon and Rectum 1992;35(2):123-30. [DOI: 10.1007/BF02050666] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Suzuki K, NotoT, et al. A randomized controlled study in adjuvant immunochemotherapy with psk in curatively resected colorectal cancer. Journal of Cancer Research and Clinical Oncology 1990;116(SUPPL. PART 1):189. [Google Scholar]
- Mitomi T, Tsuchiya S, Iijima N, Noto T, Ogawa N. A randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer [abstract]. In: Proceedings of the American Society of Clinical Oncology. Vol. 11. 1992:163, Abstract 466.
Ohwada 2004 {published data only}
- 進 大和田, 俊 池谷, 忠 横森, 隆 六本木, 徹 高橋, 正 中村, 美 棚橋, 富 蒔田, 茂 岩崎, 啓 佐藤, 哲 小川, 進 川手, 邦 浜田, 泉 竹吉 and 靖 森下 (Ohwada S, Ikeya S, Yokomori S, Roppongi T, Takahashi T, Nakamura M et al). Adjuvant immunochemotherapy with UFT plus PSK improves disease-free survival in patients with Stage II or III colorectal cancer: A randomised controlled study [UFTとPSKによるアジュバント免疫化学療法は病期II及びIIIの大腸癌患者の無再発生存率を向上させる無作為化試験]. In: 日本外科学会雑誌 (Journal of Japan Surgical Society). Vol. 104. 2003:134.
- 進 川手, 進 大和田, 俊 池谷, 忠 横森, 輝 草場, 隆 六本木, 泰 高橋, 正 中村, 仁 石川, 孝 中島 and 他 (Kawate S, Owaha S, Ikeya S, Yokomori S, Kusaba A, Roppongi T, et al). UFT adjuvant immunochemotherapy with Krestin increased event-free survival rate, results of a randomized placebo-controlled study (GOSG-C study) [クレスチンとUFTを併用した術後補助免疫化学療法による無再発生存率の向上 無作為化割付試験(GOSG-C研究)結果]. In: 日本癌治療学会誌 (The Journal of Japan Society for Cancer Therapy). Vol. 36. 2001:532.
- Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, et al. Adjuvant immunochemotherapy with oral Tegafur/ Uracil plus PSK in patients with stage II or III colorectal cancer. British Journal of Cancer 2004;90(5):1003-10. [DOI: 10.1038/sj.bjc.6601619] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada S, Kawate S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, et al. Adjuvant therapy with protein-bound polysaccharide K and tegafur uracil in patients with stage II or III colorectal cancer: randomized, controlled trial. Diseases of the Colon and Rectum 2003;46(8):1060-8. [DOI: 10.1007/s10350-004-7281-y] [DOI] [PubMed] [Google Scholar]
- Ohwada S, Kawate S, Kawashima Y, Ikeya T, Yokomori T, Kusaba T, et al. A randomized controlled trial of postoperative adjuvant immunochemotherapy using protein-bound polysaccharide K (PSK) and Tegafur/Uracil for Stage II or III colorectal cancer (GOSG-C study). International Journal of Antimicrobial Agents 2001;17(Supplement 1):S114. [Google Scholar]
- Ohwada S, Morishita Y. Reply: Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer. British Journal of Cancer 2004;91:1221-1223. [DOI: 10.1038/sj.bjc.6602101] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada S, Ogawa T, Makita F, Tanahashi Y, Ohya T, Tomizawa N, et al. Beneficial effects of protein-bound polysaccharide K plus tegafur/uracil in patients with stage II or III colorectal cancer: analysis of immunological parameters. Oncology Reports 2006;15(4):861-8. [PMID: PMID: 16525672] [PubMed] [Google Scholar]
- Susumi O, Kawate S, Ogawa T, Hamada K, Makita F, Tanahashi Y, et al. Adjuvant immunochemotherapy with oral tegafur/uracil (UFT) plus PSK(protein bound polysaccharide K) in patients with stage II or III colorectal cancer: a randomized controlled study [abstract].. In: Proceedings of the American Society of Clinical Oncology. Vol. 22. 2003:262. [ISSN: 07367589]
- Tanahashi Y, Ohwada S, Nakamura S, Takeshita M, Miyamoto Y, Yokomori T, et al. A randomized controlled study on adjuvant immunochemotherapy with PSK for curatively resected stage III and IV colorectal cancer - Changes of immunological parameters [Stage III,IV大腸癌治癒切除症例に対するPSKによる補助免疫化学療法 免疫パラメーターの変動を中心に]. Journal of Japan Society for Cancer Therapy 1995;30(6):817-29. [Google Scholar]
- Totsuka O, Kawate S, Hirai K, Ogawa H, Toya H, Yoshinari D et, al. Subset analysis of preoperative lymphocyte ratio in Stage II or III colorectal cancer patients treated with oral tegafur-uracil and protein-bound polysaccharide K. Japanese Journal of Cancer and Chemotherapy 2013;40(13):2525-8. [PubMed] [Google Scholar]
Sadahiro 2010 {published data only}
- Sadahiro S, Suzuki T, Maeda Y, Tanaka A, Kamijo A, Murayama C, et al. Effects of preoperative immunochemoradiotherapy and chemoradiotherapy on immune responses in patients with rectal adenocarcinoma. Anticancer Research 2010;30(3):993-9. [PMID: ] [PubMed] [Google Scholar]
Shichinohe 2013 {published data only}
- Shichinohe T, Komatsu Y, Akazawa K, Yuki S, Fukushima H, Ohno K, et al. Randomized phase III clinical study comparing postoperative UFT/LV,UFT+LV/UFT and UFT+LV+PSK/UFT+PSK as adjuvant therapy for curatively resected stage III colorectal cancer HGCSG-CAD study. Journal of Clinical Oncology 2013;31(15 supplement):3638-3638. [DOI: 10.1200/jco.2013.31.15_suppl.3638] [EMBASE: 71098741] [DOI] [Google Scholar]
Sugimoto 2012 {published data only}
- Sugimoto K, Takahashi R, Ishiyama S, Hata M, Kamiyama H, Komiyama H, et al. Analysis of the effects of alleviating adverse events and improving completion in colorectal cancer patients with postoperative adjuvant chemotherapy with PSK. Juntendo Medical Journal 2012;58(5):422-30. [DOI: ] [Google Scholar]
Xu 2008 {published data only}
- Xu S, Song Y, Li H, Cai H. Efficacy of Yunzhi polysaccharide combined with XELOX regimen in the treatment of advanced colorectal cancer [云芝多糖联合 XELOX 方案在晚期大肠癌治疗中的疗效观察]. Acta Universitatis Medicinalis Nanjing (Natural Science) 2008;12:1616-8. [Google Scholar]
References to studies excluded from this review
Agaki 2010 {published data only}
- Akagi J, Baba H. Preoperative measurement of CD57NKT cell values as a good predictor of response to protein-bound polysaccharide (PSK). [Japanese]. Biotherapy 2010;24(2):1519. [Google Scholar]
Akao 1980 {published data only}
- Akao T, Fujimoto S, Itoh B. Studies on immunochemotherapy of the patients with colorectal cancer. [Japanese]. Japanese Journal of Cancer and Chemotherapy 1980;7(6):1063-7. [Google Scholar]
Amano 1995 {published data only}
- Amano S, Kurosu Y, Shibata M. Locoregional immunotherapy - Topics at the 13th and 14th meeting of the Japanese Research Society for Surgical Cancer Immunology. [Japanese]. Biotherapy 1995;9(7):845-51. [Google Scholar]
Anon 1987 {published data only}
- Anonymous. Treatment of postoperative urinary incontinence in rectal cancer patients with cortex moutan powder and Coriolus versicolor. Anthology of Medicine 1987;01:19. [Google Scholar]
Anon 2013 {published data only}
- Anonymous. 2nd National Congress on Medicinal Plants. Iranian Journal of Pharmaceutical Research 2013;12:43. [Google Scholar]
Becouarn 1996 {published data only}
- Becouarn Y, Seitz JF, Perkier H, Rouhier ML, Giovannini M, Brunet R. Immunotherapy of colorectal cancer. [French]. Gastroenterologie Clinique et Biologique 1996;20(1):20-32. [PubMed] [Google Scholar]
Boone 1994 {published data only}
- Boone CW, Wattenberg LW. Current strategies of cancer chemoprevention: 13th Sapporo Cancer Seminar. Cancer Research 1994;54(12):3315-8. [PubMed] [Google Scholar]
Hazama 2002 {published data only}
- Hazama S, Oka M. Analysis of type 1 and type 2 immune responses in the monocytes and neutrophils of patients with digestive cancers [Japanese]. Biotherapy 2002;16(6):541-6. [Google Scholar]
Hiyashibe 1992 {published data only}
- Hayashibe A, Kito H, Taruya E, Sakamoto K, Asada K, Tokura K et al. Immunological competence and effect of PSK in patients with gastric cancer and colon cancer: preliminary report [Japanese]. Nippon Geka Gakkai zasshi 1992;93(7):770. [PubMed] [Google Scholar]
Iseki 1992 {published data only}
- 俊 井関, 一 国友, 勝 古川 and 他. Changes in peripheral blood lymphocyte subsets before and after surgery in patients with gastric and colorectal cancer due to preoperative administration of PSK [PSK術前投与による胃・大腸癌症例における術前,術後の末梢血リンパ球サブセットの変動]. Biotherapy 1992;6(5):805-7. [Google Scholar]
Ito 2008a {published data only}
- Ito I, Mukai M, Ninomiya H, Kishima K, Tsuchiya K, Tajima T et al. Comparison between intravenous and oral postoperative adjuvant immunochemotherapy in patients with stage III colorectal cancer. Oncology Reports 2008;20(6):1521-6. [PubMed] [Google Scholar]
Ito 2008b {published data only}
- Ito I, Mukai M, Ninomiya H, Kishima K, Tsuchiya K, Tajima T, et al. Comparison between intravenous and oral postoperative adjuvant immunochemotherapy in patients with stage II colorectal cancer. Oncology Reports 2008;20(5):1189-94. [PubMed] [Google Scholar]
JPRN‐UMIN000001527 {published data only}
- JPRN-UMIN000001527. Randomized phase III trial comparing surgery-alone to UFT+PSK in Ssage II rectal cancer. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000001527%20 (first received 1st January 2009).
JPRN‐UMIN000010045 {published data only}
- JPRN-UMIN000010045. Clinical trial to research a novel biomarker of PSK responders in patients with colorectal cancer. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000010045 (first received 1st August 2007).
Katsuno 2004 {published data only}
- Katsuno H, Maeda K, Utsumi T, Hanai T, Sato H, Masumori K, et al. Variation of cytokines with administration of chemotherapeutic and immuno-therapeutic drugs for colorectal cancer. [Japanese]. Gan to kagaku ryoho 2004;Cancer & Chemotherapy 31(11):1652-4. [PubMed] [Google Scholar]
Kitamura 1996 {published data only}
- Kitamura M, Arai K, Iwasaki Y, Toi M. Antitumor effect of the angiogenesis inhibitor TNP-470 and its combination effect with 5-FU and PSK in colon 26 tumors. Oncology Reports 1996;3(1):151-3. [PubMed] [Google Scholar]
Kobayashi 1995 {published data only}
- Kobayashi H, Matsunaga K, Oguchi Y. Antimetastatic effects of PSK (Krestin), a protein-bound polysaccharide obtained from basidiomycetes: an overview. Cancer Epidemiology Biomarkers and Prevention 1995;4(3):275-81. [PubMed] [Google Scholar]
Kobayshi 1994 {published data only}
- Kobayashi Y, Kariya K, Saigenji K, Nakamura K. Oxidative stress relief for cancer-bearing hosts by the protein-bound polysaccharide of Coriolus versicolor QUEL with SOD mimicking activity. Cancer Biotherapy 1994;9(1):55-62. [DOI] [PubMed] [Google Scholar]
Koda 2003 {published data only}
- Koda K, Miyazaki M, Sarashina H, Suwa T, Saito N, Suzuki M, et al. A randomized controlled trial of postoperative adjuvant immunochemotherapy for colorectal cancer with oral medicines. International Journal of Oncology 2003;23(1):165-72. [PubMed] [Google Scholar]
Kudo 2002 {published data only}
- Kudo S, Tanaka J, Kashida H, Tamegai Y, Endo S, Yamano HO. Effectiveness of immunochemotherapy with PSK, a protein-bound polysaccharide, in colorectal cancer and changes of tumor marker. Oncology Reports 2002;9(3):635-8. [PubMed] [Google Scholar]
Li 2019 {published data only}
- Li XQ, Zhang BB, Zhang MK, Song JW. The effect of Yunzhi polysaccharide combined with XELOX regimen in the treatment of advanced colorectal cancer [[云芝多糖联合XELOX方案在晚期大肠癌治疗中的疗效观察]]. World Latest Medicine 2019;19:230-5. [Google Scholar]
Liu 2014 {published data only}
- Liu W. Mechanisms of protection against radiation-induced intestinal damage and assist in colorectal cancer radiotherapy via TLR2 agonist polysaccharide krestin. Suzhou University.
Maehara 2012 {published data only}
- Maehara Y, Tsujitani S, Saeki H, Oki E, Yoshinaga K, Emi Y, et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN()): review of development and future perspectives. Surgery Today 2012;42(1):8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maruyama 2002 {published data only}
- Maruyama S, Yagita A, Sukegawa Y, Daido A, Takeuchi S. Effect of a new immunotherapy for advanced colorectal cancer. [Japanese]. Biotherapy 2000;14(5):460-3. [Google Scholar]
Miyake 2018 {published data only}
- Miyake Y, Nishimura J, Kato T, Ikeda M, Tsujie M, Hata, T et al. Phase III trial comparing UFT + PSK to UFT + LV in stage IIB, III colorectal cancer(MCSGO-CCTG). Surgery Today 2018;48(1):66-72. [DOI] [PubMed] [Google Scholar]
Mukai 2003 {published data only}
- Mukai M, Tajima T, Nakasaki H, Sato S, Ogoshi K, Makuuchi H. Efficacy of postoperative adjuvant oral immunochemotherapy in patients with Dukes' B colorectal cancer. Annals of Cancer Research and Therapy 2003;11(1-2):201-14. [Google Scholar]
Nakazato 1986 {published data only}
- Nakazato H, Ichihashi H, Kondo T. Clinical results of a randomized controlled trial on the effect of adjuvant immunochemotherapy using Esquinon and Krestin in patients with curatively resected gastric cancer. Cooperative Study Group of Cancer Immunochemotherapy, Tokai Gastrointestinal Oncology Group. [Japanese]. Gan to kagaku Ryoho 1986;Cancer & chemotherapy 13(2):308-18. [PubMed] [Google Scholar]
NCT00385970 {published data only}
- NCT00385970. A phase III trial comparing UFT+PSK to UFT+LV in stage IIB, III colorectal cancer. https://clinicaltrials.gov/ct2/show/NCT00385970 (first received 11th October 2006).
NCT03716518 {published data only}
- NCT03716518. Herbal treatment to improve chemotherapy delivery. https://clinicaltrials.gov/ct2/show/NCT03716518 (first received 23rd October 2018).
Nio 1992 {published data only}
- Nio Y, Tsubono M, Tseng CC, Morimoto H, Kawabata K, Masai Y, et al. Immunomodulation by orally administered protein-bound polysaccharide PSK in patients with gastrointestinal cancer. Botherapy 1992;4(2):117-28. [DOI] [PubMed] [Google Scholar]
Nio 1995 {published data only}
- Nio Y, Tamura K, Masai Y, Hayashi H, Araya S, Imai S, et al. Multi-institutional study on the immunomodulatory effect of protein-bound polysaccharide, PSK on the immunity of patients after gastric or colorectal cancer surgery. [Japanese]. Journal of Japan Society for Cancer Therapy 1995;30(9):1623-34. [Google Scholar]
Okuno 2017 {published data only}
- Okuno K, Aoyama T, Oba K, Yokoyama N, Yoshida K, Kunieda K, et al. Clinical trial comparing UFT-PSK combination adjuvant therapy and surgery-alone for Stage II rectal cancer. Annals of Cancer Research and Therapy 2017;25(1):15-6. [Google Scholar]
Okuno 2018 {published data only}
- Okuno K, Aoyama T, Oba K, Yokoyama N, Matsuhashi N, Kunieda K, et al. Randomized phase III trial comparing surgery alone to UFT + PSK for stage II rectal cancer (JFMC38 trial). Cancer Chemotherapy & Pharmacology 2018;81(1):65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ooshiro 2009 {published data only}
- Ooshiro M, Kitahara T, Takagi R, Moriyama A, Urita T, Yoshida Y, et al. Ten cases of advanced gastro-intestinal cancer that required preoperative dosage of PSK. [Japanese]. Japanese Journal of Cancer and Chemotherapy 2009;36(12):1967-8. [PubMed] [Google Scholar]
Saji 1997 {published data only}
- Saji S, Sugiyama Y, Kunieda K. Pre- and/or post-operative immunochemotherapy for advanced digestive cancer. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1997;24(Suppl 1):239-49. [PubMed] [Google Scholar]
Sakai 2003 {published data only}
- Sakai T, Yamashita Y. Long-term prognosis for colorectal carcinoma treated with adjuvant immunochemotherapy. [Japanese]. Nippon rinsho Japanese journal of clinical medicine. 2003;61(Suppl 7):460-463. [PubMed] [Google Scholar]
Shibata 1999a {published data only}
- Shibata M, Kanou H, Nezu T, Takekawa M, Fukuzawa M Biotherapy. Immunotherapy for patients with highly advanced cancer. [Japanese]. Biotherapy 1999;13(7):788-93. [Google Scholar]
Shibata 1999b {published data only}
- Shibata M, Nagata Y, Kimura T, Sakamoto A, Kanou H, Nezu T, et al. Decreased immune suppressing factors after chemoimmunotherapy (5-FU, UFT+CDDP+PSK) in patients with advanced gastric and colorectal cancer. In: Proceedings of the American Association for Cancer Research Annual Meeting. Vol. 40. 1999:576.
Shibata 1999c {published data only}
- Shibata M, Takekawa M, Kanou H, Nagata Y, Kimura T, Sakamoto A, et al. Advance-matched ideal immunotherapy for gastric and colorectal cancer. [Japanese]. Biotherapy 1999;13(1):91-4. [Google Scholar]
Shibata 2002 {published data only}
- Shibata M, Abe H, Kanou H, Azuhata T, Nezu T, Ohara M, et al. In vitro and in vivo immune-modulating effects of polysaccharide-K (PSK) in patients with colorectal cancer. In: Proceedings of the American Association for Cancer Research Annual Meeting. Vol. 43. 2002:448.
Shibata 2011 {published data only}
- Shibata M, Shimura T, Nishina Y, Gonda K, Matsuo S, Abe H, et al. PSK decreased FOLFOX4-induced peripheral neuropathy and bone marrow suppression in patients with metastatic colorectal cancer. [Japanese]. Japanese Journal of Cancer and Chemotherapy 2011;38(5):797-801. [PubMed] [Google Scholar]
Sugiyama 1998 {published data only}
- Sugiyama Y, Saji S, Fukada D, Miya K, Umemoto T, Kunieda K, et al. Usefulness of administration of either lentinan or PSK in low-dose 5- FU/CDDP chemotherapy against advanced gastroenterological cancer. [Japanese]. Biotherapy 1998;12(1):127-30. [Google Scholar]
Takashima 1988 {published data only}
- Takashima S, Kinami Y, Miyazaki I. Clinical effect of postoperative adjuvant immunochemotherapy with FT-207 suppository and PSK for colo-rectal cancer patients. [Japanese]. apanese Journal of Cancer and Chemotherapy 1988;15(81):2229-36. [PubMed] [Google Scholar]
Torisu 1986 {published data only}
- Torisu M, Fujimura T, Kato M, Katano M, Takesue M, Kimura Y. Randomized trials of PSK to patients with advanced colonic cancer. In: Lapis K, Eckhardt S , editors(s). Lectures and Symposia of the 14th International Cancer Congress: Budapest August 21-27 1986. Budapest: Akadémiai Kiadó, 1987:142-142.
Torisu 1990 {published data only}
- Torisu M, Hayashi Y, Ishimitsu T, Fujimura T, Iwasaki K, Katano M, et al. Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunology, Immunotherapy: CII 1990;31(5):261-8. [DOI: 10.1007/BF01740932] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torisu 1991 {published data only}
- Torisu M, Uchiyama A, Goya T, Iwasaki K, Katano M, Yamamoto H et al. Eighteen-year experience of cancer immunotherapies--evaluation of their therapeutic benefits and future. [Japanese]. Nippon Geka Gakkai zasshi 1991;92(9):1212-1216. [PubMed] [Google Scholar]
Toth 2005 {published data only}
- Toth B, Coles M. Attempts to inhibit the development of large intestinal cancer by VPS extract of the Coriolus versicolor mushroom. In: Proceedings of the American Association for Cancer Research Annual Meeting. Vol. 46. 2005:2792005.
Tsukida 2018 {published data only}
- 茂 月田, 靖 緑川 and 清 保. A case of suspected cytomegalovirus enteritis during UFT / LV administration as adjuvant chemotherapy for colon cancer [結腸癌術後補助化学療法としてUFT/LV投与中にサイトメガロウイルス腸炎が疑われた一例]. 日本臨床外科学会雑誌 2018;79(5):1129. [Google Scholar]
Watanabe 2010 {published data only}
- Watanabe T. Ongoing colorectal cancer adjuvant trials in Japan. Current Colorectal Cancer Reports 2010;6(3):168-74. [Google Scholar]
Yoshikawa 2005 {published data only}
- Yoshikawa R, Yanagi H, Noda M, Yamamura T, Hashimoto-Tamaoki T. Polysaccharide-K (PSK) inhibits distant metastasis in colorectal cancer dependent on ECA39. In: Proceedings of the American Association for Cancer Research Annual Meeting. Vol. 46. 2005:171.
Yoshikawa 2006 {published data only}
- Yoshikawa R, Yanagi H, Shen CS, Fujiwara Y, Noda M, Yagyu T, et al. ECA39 is a novel distant metastasis-related biomarker in colorectal cancer. World Journal of Gastroenterology 2006;12(36):5884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshitani 2009a {published data only}
- Yoshitani S, Takashima S. Postoperative adjuvant immunochemotherapy using PSK in combination for colorectal cancer in the elderly [Abstract P-0195]. Annals of Oncology 2009;20(Suppl 7):772009. [Google Scholar]
Yoshitani 2009b {published data only}
- Yoshitani S, Takashima S. Efficacy of postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly patients with resected colorectal cancer. Cancer Biotherapy & Radiopharmaceuticals 2009;24(1):35-40. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
JPRN‐UMIN000004351 {published data only}
- JPRN-UMIN000004351. A study on combinational effect of Krestin to the peripheral neuropathy by the FOLFOX treatment as adjuvant chemotherapy for Stage III colon cancer [A study on combinational effect of Krestin to the peripheral neuropathy by the FOLFOX treatment as adjuvant chemotherapy for Stage III colon cancer]. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000005187 (first received 8th October 2010).
JPRN‐UMIN000005286 {published data only}
- JPRN-UMIN000005286. Preoperative immunochemotherapy with PSK for stage II/III gastric and colorectal cancer to evaluate anti-tumor effect and transition of PSK into tumor [Preoperative immunochemotherapy with PSK for stage II/III gastric and colorectal cancer to evaluate anti-tumor effect and transition of PSK into tumor]. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000006286 (first received 22nd March 2011).
JPRN‐UMIN000005484 {published data only}
- JPRN-UMIN000005484. A randomized trial of adjuvant chemotherapy with UFT / LV versus PSK / UFT / LV following curative resection for stage II / III colorectal cancer [A randomized trial of adjuvant chemotherapy with UFT / LV versus PSK / UFT / LV following curative resection for stage II / III colorectal cancer]. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000006501 (first received 22nd April 2011).
JPRN‐UMIN000006134 {published data only}
- JPRN-UMIN000006134. Pilot study of FOLFOX or XELOX Therapy Versus FOLFOX or XELOX + PSK therapy as postoperative adjuvant therapy for histological stage IIIa and IIIb colon cancer [Pilot study of FOLFOX or XELOX + PSK therapy as postoperative adjuvant therapy for stage III colon cancer]. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000007255 (first received 9th August 2011).
JPRN‐UMIN000006541 {published data only}
- JPRN-UMIN000006541. A randomized trial of postoperative adjuvant chemotherapy with capecitabine versus capecitabine/PSK following curative resection for stage III colon cancer [A randomized trial of postoperative adjuvant chemotherapy with capecitabine versus capecitabine/PSK following curative resection for stage III colon cancer]. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000006541; (first received 14th October 2011).
JPRN‐UMIN000007252 {published data only}
- JPRN-UMIN000007252. XELOX plus PSK compared with XELOX as adjuvant treatment for stage III colon cancer [XELOX plus PSK compared with XELOX as adjuvant treatment for Stage III colon cancer]. https://upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000008430 (first received 15th February 2012).
JPRN‐UMIN000010046 {published data only}
- JPRN-UMIN000010046. A randomized pilot study to search the predictive parameters of PSK responder in patients with colorectal cancer [A randomized pilot study to search the predictive parameters of PSK responder in patients with colorectal cancer]. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000011764 (first received 14th February 2013).
NCT00209742 {published data only}
- NCT00209742. Randomized phase III adjuvant study for stage III colorectal cancer [Randomized phase III clinical study comparing postoperative UFT+LV, UFT+LV/UFT and UFT+LV+PSK/UFT+PSK Therapies for Stage III colorectal cancer]. https://clinicaltrials.gov/ct2/show/NCT00209742 (first received 21st September 2005).
NCT00497107 {published data only}
- NCT00497107. Uracil and Tegafur/Leucovorin (UFT/LV) Versus UFT/LV+ Polysaccharide-K (PSK) for stage IIIa/IIIb clorectal cancer (ICOG) [Phase III randomized controlled clinical study of UFT/LV Therapy versus UFT/LV + PSK therapy as postoperative adjuvant therapy for histological stage IIIa and IIIb colorectal cancer]. https://clinicaltrials.gov/ct2/show/NCT00497107 (first received 6th July 2007).
Ogawa 2021 {published data only}
- Ogawa H, Shirabe K, Saeki H. Randomized phase II trial comparing UFT/LV to UFT/LV + PSK in high risk stage II and stage III colorectal cancer. In: Japan Society of Clinical Oncology 59th Annual Meeting (JSCO2021) http://archive.jsco.or.jp/timebox.php?session_unique_id=59-O57. Vol. 59. October 2021:O57-2.
Additional references
André 2004
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish, T et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. New England Journal of Medicine 2004;350(23):2343-51. [DOI: 10.1056/NEJMoa032709] [DOI] [PubMed] [Google Scholar]
ASCO 2020
- Chiorean EG, Nandakumar G, Fadelu T, Temin S, Alarcon-Rozas AE, Bejarano S, et al. Treatment of patients with late-stage colorectal cancer: ASCO Resource-Stratified Guideline. JCO Global Oncology 2020;6:414-38. [DOI: 10.1200/JGO.19.00367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Astin 2011
- Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. British Journal of General Practice 2011;61(586):e231-43. [DOI: 10.3399/bjgp11X572427] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benson 2019
- Benson KF, Stamets P, Davis R, Nally R, Taylor A, Slater S, et al. The mycelium of the Trametes versicolor (Turkey tail) mushroom and its fermented substrate each show potent and complementary immune activating properties in vitro. BMC Complementary and Alternative Medicine 2019;19(1):342. [DOI: 10.1186/s12906-019-2681-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bray 2018a
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer Journal for Clinicians 2018;68(6):394-424. [DOI: 10.3322/caac.21492 (Erratum: 10.3322/caac.21609)] [DOI] [PubMed] [Google Scholar]
Bray 2018b
- No authors listed. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.. CA Cancer Journal for Clinicians 2020;70(4):313. [DOI: 10.3322/caac.21609] [DOI] [PubMed] [Google Scholar]
Chan 2006
- Chan SL, Yeung JH. Effects of polysaccharide peptide (PSP) from Coriolus versicolor on the pharmacokinetics of cyclophosphamide in the rat and cytotoxicity in HepG2 cells. Food and Chemical Toxicology 2006;44(5):689-94. [DOI] [PubMed] [Google Scholar]
Chang 2017
- Chang Y, Zhang M, Jiang Y, Liu Y, Luo H, Hao C, et al. Preclinical and clinical studies of Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Discovery Medicine 2017;23(127):207-19. [PubMed] [Google Scholar]
Chinese Pharmacopoeia 2005
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press, 2005. [Google Scholar]
Chinese Pharmacopoeia 2015
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press, 2015. [Google Scholar]
Chinese Pharmacopoeia 2020
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press, 2020. [Google Scholar]
CSCO 2020
- Guidelines Working Committee of Chinese Society of Clinical Oncology (CSCO): colorectal cancer diagnosis and treatment guidelines (2020 version).. https://wenku.baidu.com/view/8eaee6da5e0e7cd184254b35eefdc8d377ee14dd.html. Accessed 16th Jan 2021.. [DOI] [PMC free article] [PubMed]
Daiichi‐Sankyo 2012
- Daiichi-Sankyo. Krestin® (PSK). Package insert 2012.
Daiichi Sankyo 2017
- Daiichi Sankyo. Discontinuation of manufacturing and sales of antineoplastic agent "Krestin ® fine granules" [抗悪性腫瘍剤「クレスチン®細粒」の製造販売の中止について]. https://www.kureha.co.jp/newsrelease/uploads/20170317.pdf 17 March 2017.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. London (UK): BMJ Publication Group, 2001. [Google Scholar]
Eliza 2012
- Eliza WL, Fai CK, Chung LP. Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: systematic review and meta-analysis. Recent Patents on Inflammation and Allergy Drug Discovery 2012;6(1):78-87. [DOI] [PubMed] [Google Scholar]
ESMO 2014
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2014;25(Supplement 3):iii1-9 Erratum in: Ann Oncol. 2015 26 Suppl 5:v174-7. [DOI: 10.1093/annonc/mdu260] [DOI] [PubMed] [Google Scholar]
Fisher 2002
- Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Research 2002;22(3):1737-54. [PubMed] [Google Scholar]
Gray 2011
- Gray NM, Hall SJ, Browne S, Macleod U, Mitchell E, Lee AJ, et al. Modifiable and fixed factors predicting quality of life in people with colorectal cancer. British Journal of Cancer 2011;104(11):1697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grothey 2018
- Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. New England Journal of Medicine 2018;378(13):1177-88. [DOI: 10.1056/NEJMoa1713709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2020
- Han CJ, Yang GS, Syrjala K. Symptom experiences in colorectal cancer survivors after cancer treatments. Cancer Nursing 2020;43(3):E132-E158. [DOI: 10.1097/NCC.0000000000000785] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at www.cochrane-handbook.org.
Ikuzawa 1988
- Ikuzawa M, Matsunaga K, Nishiyama S, Nakajima S, Kobayashi Y, Andoh T, et al. Fate and distribution of an antitumor protein-bound polysaccharide PSK (Krestin). International Journal of Immunopharmacology 1988;10(4):415-23. [DOI] [PubMed] [Google Scholar]
Jeitler 2020
- Jeitler M, Michalsen A, Frings D, Hübner M, Fischer M, Koppold-Liebscher DA, et al. Significance of medicinal mushrooms in integrative oncology: a narrative review. Frontiers in Pharmacology 2020;11:580656. [DOI: 10.3389/fphar.2020.580656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2016
- Jin X, Ruiz Beguerie J, Sze DM, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD007731. [DOI: 10.1002/14651858.CD007731.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
JSCCR 2019
- Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. International Journal of Clinical Oncology 2020;25(1):1-42. [DOI: 10.1007/s10147-019-01485-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kidd 2000
- Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Alternative Medicine Review 2000;5(1):4-27. [PubMed] [Google Scholar]
Lindequist 2005
- Lindequist U, Niedermeyer TH, Julich WD. The pharmacological potential of mushrooms. Evidence Based Complementary and Alternative Medicine 2005;2(3):285-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2017
- Ma Y, Wu X, Yu J, Zhu J, Pen X, Meng X. Can polysaccharide K improve therapeutic efficacy and safety in gastrointestinal cancer? a systematic review and network meta-analysis. Oncotarget 2017;8(51):89108-18. [DOI: 10.18632/oncotarget.19059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Memorial Sloan‐Kettering 2022
- Memorial Sloan-Kettering Cancer Center. About herbs, botanicals and other products: Coriolus versicolor. https://www.mskcc.org/cancer-care/integrative-medicine/herbs/coriolus-versicolor (accessed 16 May 2022).
Natural Database 2022
- Therapeutic Research Faculty. Natural Medicines Comprehensive Database. https://naturalmedicines.therapeuticresearch.com/ (accessed 19 April 2022).
NCI 2017
- National Cancer Institute. NCI Common Terminology Criteria for Adverse Events (CTCAE) v.5. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm 17 November 2017 (accessed 16 May 2022).
NCI 2022
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Medicinal mushrooms (PDQ®): Health Professional Version. PDQ Cancer Information Summaries [Internet]; Bethesda (MD): National Cancer Institute (US) https://www.ncbi.nlm.nih.gov/books/NBK401261/ 2022.
NCNN 2017
- Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2017;15(3):370-98. [DOI: 10.6004/jnccn.2017.0036] [DOI] [PubMed] [Google Scholar]
NCNN 2018
- Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology.. Journal of the National Comprehensive Cancer Network 2018;16(7):874-901. [DOI: 10.6004/jnccn.2018.0061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 1998
- Ng TB. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolusversicolor (Basidiomycetes: Polyporaceae). General Pharmacology 1998;30(1):1-4. [DOI] [PubMed] [Google Scholar]
NICE 2020
- National Institute for Clinical Excellence (NICE). Colorectal cancer: NICE guideline www.nice.org.uk/guidance/ng151. London: National Institute for Health and Clinical Excellence 2020.
ONS 2019
- ONS (Office for National Statistics). Cancer survival by stage at diagnosis for England. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed 2019.
Pandor 2006
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roeder 2020
- Roeder F, Meldolesi E, Gerum, S Valentini V, Rödel C. Recent advances in (chemo-)radiation therapy for rectal cancer: a comprehensive review. Radiation Oncology 2020;15:262. [DOI: 10.1186/s13014-020-01695-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowan 2003
- Rowan N, Sullivan R. Immunomodulatory activities of mushroom glucans and polysaccharide--Protein complexes in animals and humans (a review). International Journal of Medicinal Mushrooms 2003;5:95-110. [Google Scholar]
Sakamoto 2006
- Sakamoto J, Morita S, Oba K, Matsui T, Kobayashi M, Nakazato H, et al, Meta-Analysis Group of the Japanese Society for Cancer of the Colon Rectum. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunology, Immunotherapy: CII 2006;55(4):404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmoll 2007
- Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. Journal of Clinical Oncology 2007;25(1):102-9. [DOI] [PubMed] [Google Scholar]
Smith 2002
- Smith JE, Rowan NJ, Sullivan R. Medicinal Mushrooms and Cancer: Their Therapeutic Properties and Current Medical Usage with Special Emphasis on Cancer Treatments. London: Cancer Research UK, 2002. [Google Scholar]
Standish 2008
- Standish LJ, Wenner CA, Sweet ES, Bridge C, Nelson A, Martzen M, et al. Trametes versicolor mushroom immune therapy in breast cancer. Journal of the Society of Integrative Oncology 2008;6(3):122-8. [PMC free article] [PubMed] [Google Scholar]
Sullivan 2006
- Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspectives in Biology and Medicine 2006;49(2):159-70. [DOI] [PubMed] [Google Scholar]
Sung 2021
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer Journal for Clinicians 2021;71(3):209-249. [DOI: 10.3322/caac.21660] [PMID: ] [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205-16. [DOI] [PubMed] [Google Scholar]
Venturella 2021
- Venturella G, Ferraro V, Cirlincione F, Gargano ML. Medicinal mushrooms: bioactive compounds, use, and clinical trials. International Journal of Molecular Sciences 2021;22(2):634. [DOI: 10.3390/ijms22020634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2013
- Wan J MF. Chapter 27 - Polysaccaride Krestin (PSK) and Polysaccharopeptide PSP. In: Kastin AJ, editors(s). Handbook of Biologically Active Peptides. Second edition. Elsevier, 2013:180-184. [Google Scholar]
WebPlotDigitizer 2020 [Computer program]
- WebPlotDigitizer. Rohatgi, Ankit, Version 4.4. Pacifica, California: Available at https://automeris.io/, November 2020.
Yeung 2012
- Yeung JH, Or PM. Polysaccharide peptides from Coriolus versicolor competitively inhibit model cytochrome P450 enzyme probe substrates metabolism in human liver microsomes. Phytomedicine 2012;19(5):457-63. [DOI] [PubMed] [Google Scholar]
Zhong 2019
- Zhong L, Yan P, Lam WC, Yao L, Bian Z. Coriolus Versicolorand Ganoderma Lucidum related natural products as an adjunct Therapy for cancers: A systematic review and meta-analysis of randomized controlled trials. Frontiers in Pharmacology 2019;10:703. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Pilkington 2016
- Pilkington K, Leach J, Teng L, Storey D, Liu JP. Coriolus versicolor mushroom for colorectal cancer treatment. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD012053. [DOI: 10.1002/14651858.CD012053] [DOI] [PMC free article] [PubMed] [Google Scholar]


