Abstract
Rheum tanguticum (R. tanguticum) has been widely used for the treatment of inflammatory diseases in clinical. However, limited research exist on the quality evaluation of various R. tanguticum locations, which has certain drawbacks. In this study, Fourier-transform infrared spectroscopy (FTIR) and high-performance liquid chromatography (HPLC) were used to comparative study on the chemical contents of R. tanguticum, to clarify the relationship between the chemical contents and the spatial distribution of R. tanguticum. First of all, the FTIR spectra of 18 batches of R. tanguticum were examined. Following the cluster analysis, the FTIR spectra of various production locations differed. To some extent, establishing the double index analysis sequence of common and variation peaks may differentiate distinct production locations of medicinal materials. The HPLC fingerprint of R. tanguticum was constructed to further explore the link between components and their origin. PCA of common peaks of 18 batches of R. tanguticum indicated that R. tanguticum grown in Gannan and Qinghai had a tendency to separate t[2], however this trend was not noticeable. Then, OPLS-DA model was established, and the key differential components of R. tanguticum produced in Gannan and Qinghai were discovered to be R16, R37, R46, and R47 (Aloe emodin) (VIP ≥ 1 and P < 0.05). At last, Pearson’s test was used to examine the relationship between longitude, latitude, altitude, and composition. Longitude was significantly positively correlated with R28 and R30 (P < 0.05), and a very significantly positively correlated with R35, R36, R37, R46, and R47 (P < 0.01). Latitude was significantly negatively correlated with R34, R35, and R40 (P < 0.05), and extremely significantly negatively correlated with R28, R30, R36, R37, R46, and R47 (P < 0.01). Altitude was significantly positive correlation with R36 and R37 (P < 0.01). The results of our study can provide insights into R. tanguticum quality control and aid in establishing a natural medication traceability system.
Introduction
Rhubarb is a traditional Chinese medicine and the most widely used natural medication globally, with primary sources being dried roots and rhizomes. Rheum tanguticum (R. tanguticum) is one of the main sources of rhubarb. Rheum tanguticum Maxim is a Polygonaceae ex Balf plant, which is mostly grown in southern Gansu and southern Qinghai provinces [1]. The primary active constituents of rhubarb are anthraquinone, double anthrone, and tannin. Its primary pharmacological effects include inducing constipation and anti-inflammatory, diuretic, antibacterial, and antitumor properties [2–4]. Anti- inflammatory is one of the most important pharmacological effects of rhubarb. Rhubarb has been also used to treat inflammation-related diseases in clinical, such as acute pancreatitis, acute cholecystitis, appendicitis, ulcerative colitis and so on [5–9]. The administration of rhubarb extract reduced the expression of key inflammatory genes (TLR4, MCP-1, TNF-α, IL-6), macrophage-related markers (F4/80, CD68, CD11c) and oxidative markers (TLR4, NADPH oxidase). Rhubarb can eliminate inflammatory mediators from tissue and plasma and drastically reduce tumor necrosis factor (TNF), interleukin, and endotoxin levels in the serum of patients suffering from or successfully treated with severe pancreatitis by blocking the production of the inflammatory factor HMGB-1 [10]. Rhubarb aqueous extract not only inhibit the inflammatory response induced by TNF-α and the augmentation or rise of adhesion molecules in human umbilical vein endothelial cells (HUVECs), but also reduce adhesion between U937 cells and HUVECs [11]. The rhubarb extract modified host antimicrobial peptide production and gut homeostasis and was associated with profound changes in gut microbial composition [12,13]. Previous studies showed that rhubarb components, such as emodin, rhein, chrysophanol, lindleyin, and isolotus anthocyanins, have anti-inflammatory properties [11,14,15]. Emodin inhibits lipopolysaccharide (LPS)-induced apoptosis and inflammation by increasing the expression of the TUG1 (Long non-coding RNA) in cartilage-derived ATDC5 cells [16]. Emodin and Rhein exhibit potent anti-inflammatory actions in LPS-induced Raw 264.7 cells [17,18]. Chrysophanol may ameliorate LPS-induced inflammation in mice, minimize pathological harm to the liver and lung tissues, and lower blood TNF-α levels [19]. Derived from rhubarb, lindleyin and isolotus palmoside have anti-inflammatory and analgesic properties [20]. For the study of the anti-inflammatory mechanism of rhubarb, most of them focus on the study of components (such as: emodin, rhein) [21,22]. Several studies showed that the inflammatory effect of emodin was involved in the inactivation of NF-κB, an essential regulator of inflammatory processes, and PPARγ-dependent pathway was possibly involved in the NF-κB inhibitive effect of emodin [23,24]. Rhein significantly decreased IL-1β secretion via NLRP3 inflammasomes by disturbing their assembly in macrophages. Rhein also activated the Nrf2-HO1-NQO1 pathway and inhibited expression of Nox2 subunits and translocation to regulate redox balance. Moreover, rhein attenuated inflammatory responses by mediating macrophage polarization from M1 to M2 phenotype. NF-κB, AP-1, and MAPK signalling were also involved in improving inflammatory conditions by rhein [25–27]. With the development and progress of natural medication, quality control has been the most crucial factor in current research [20]. The quality of rhubarb in the market is variable because of mixed varietals, varying processing processes, and manufacturing locales [28]. Numerous studies have been conducted on the quality control of rhubarb to maximize the impact of natural medications; however, those on the variations in R. tanguticum from different production locations are limited [29–31]. This is not conducive to the clinical application of R. tanguticum from different origins.
To better manage the internal quality of natural drugs, modern analytical methods, such as spectroscopy and chromatography, have been widely utilized in quality control to compensate for the weakness of conventional morphological and anatomical identification of the source of natural pharmaceuticals. These sophisticated analytical approaches not only objectively represent internal quality, but also compensate for the lack of personal expertise. Some authors have recently described plant drug control analytical approaches, such as chromatography, spectrum, DNA barcoding, and other analytical methods [32–35]. Spectral and chromatographic approaches are mostly utilized in categorizing and identifying original plants as well as in evaluating quality and identifying the origin. These two approaches can explain some additional chemical information simultaneously, and the resulting fingerprint has become an established method for evaluating quality consistency. Fourier-transform infrared spectroscopy (FTIR) and high-performance liquid chromatography (HPLC) are two such techniques. FTIR has been widely used to determine the provenance of food and natural pharmaceuticals as well as to forecast the quantity of index components [36,37]. Many agencies, including the World Health Organization, Food and Drug Administration, and State Food and Drug Administration, have acknowledged HPLC fingerprint [38]. These two approaches, in conjunction with chemometrics, have been effectively used for the quality control and origin identification of a wide range of natural pharmaceuticals. Simple and quick measurement methods bypass the time-consuming pretreatment procedure for quality analysis and do not require a significant number of chemical reagents.
In this study, the FTIR spectra of 18 batches of R. tanguticum were examined by FTIR. Cluster analysis and the double index sequence method were used for distinguish R. tanguticum regions. The HPLC fingerprint of R. tanguticum was constructed by HPLC. And PCA, OPLS-DA was used for study the differential components of R. tanguticum in Gannan and Qinghai. At last, pearson correlation test was used for preliminarily clarified the relationship between chemical contents of R. tanguticum and longitude, latitude, altitude. The results of this study could encourage the growth of R. tanguticum business and to assist in implementing a natural medication traceability system.
Materials and methods
Herbal medicine
Yuwei Gan, Agricultural Technology Extension Researcher at the Gannan Science and Technology Development and Exchange Center, confirmed the authenticity of the R. tanguticum sample. For more information, refer to Table 1.
Table 1. Rheum tanguticum sample information.
| label | Collecting Time | Source | Longitude/°E | Latitude/°N | Altitude/m |
|---|---|---|---|---|---|
| 1 | Autumn, 2017 | Hezuo | 102.91 | 35.00 | 3136 |
| 2 | Spring, 2019 | Hezuo | 102.91 | 35.00 | 3136 |
| 3 | Autumn, 2019 | Hezuo | 102.91 | 35.00 | 3136 |
| 4 | Autumn, 2018 | Hezuo | 102.91 | 35.00 | 3136 |
| 5 | Autumn, 2018 | Hezuo | 102.91 | 35.00 | 3136 |
| 6 | Autumn, 2018 | Hezuo | 102.91 | 35.00 | 3136 |
| 7 | Autumn, 2018 | Hezuo | 102.91 | 35.00 | 3136 |
| 8 | Autumn, 2017 | Hezuo | 102.91 | 35.00 | 3136 |
| 9 | Autumn, 2019 | Xiahe, Hezuo | 102.52 | 35.20 | 3471 |
| 10 | Autumn, 2019 | Lintan, Hezuo | 103.35 | 34.69 | 2867 |
| 11 | Autumn, 2019 | Qinghai | 101.77 | 36.65 | 2261 |
| 12 | Autumn, 2019 | Qinghai | 101.77 | 36.65 | 2261 |
| 13 | Autumn, 2018 | Qinghai | 101.77 | 36.65 | 2261 |
| 14 | Autumn, 2018 | Qinghai | 101.77 | 36.65 | 2261 |
| 15 | Autumn, 2019 | Huangzhong, Qinghai | 101.57 | 36.50 | 2600 |
| 16 | Autumn, 2018 | Huangzhong, Qinghai | 101.57 | 36.50 | 2600 |
| 17 | Autumn, 2018 | Datong, Qinghai | 101.69 | 36.93 | 3451 |
| 18 | Autumn, 2017 | Guide, Qinghai | 101.44 | 36.07 | 2200 |
FTIR study
Instrument parameter setting
A Nicolet 6700 FT-IR spectrometer (Thermo Fisher, America) with a silicon carbide rod light source, DTGs detector, 4 cm-1 resolution, 30 scanning accumulation, detection in the spectral range of 4000–400 cm-1, room temperature, and the interference of H2O and CO2 was deducted.
Sample preparation
R. tanguticum was crushed and sieved through a 100 mesh sieve (Huafeng Hardware Instrument Co., Ltd., Shaoxing, China). Ultrasonic extraction of 1.00 g of powder with 50 mL methanol (AR, Lot No.: 20200825, Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China) was performed for 1 h (20 kHz, 30±5 °C). Then, suction filtration was performed and the methanol was evaporated to obtain the R. tanguticum methanol extract, which was labeled M1–M18.
Approximately 1 mg of R. tanguticum methanol-extracted powder was mixed with 100 mg of KBr (for IR, Lot No.: 20200715, Damao Chemical Reagent Factory, Tianjin, China) and compressed into a tablet. KBr was used as a blank, and infrared spectrum data for both samples were collected.
Infrared spectrum data analysis
The OMNIC 8.2 software and Origin software (version 9.0) were used for automated baseline correction, smoothing, and ordinate normalization of infrared spectrum findings.
Double index sequence analysis of common peak rate and variation peak rate
| (1) |
| (2) |
| (3) |
Note: P is the common peak rate of the two samples; N is the number of independent peaks in the two samples; Ng is the common peak number of the two samples; Pa is the variation peak rate of the sample; Na is the variation peak rate of the sample; Pb is the variation peak rate of sample B; Nb is the variation peak number of sample B, which conforms to N = Ng + Na + Nb.
The common peak rate and variation peak rate of the infrared spectrum between M1 and M18 were determined using (1)–(3), and the Rhubarb double index analysis sequence was created. The sequence has great identification ability and can accurately estimate the genetic distance between all samples in the 2+n-dimensional (n = number of samples) space [39]. Ma: Mb (P; Pa, Pb) is the double index analysis sequence of the common peak rate and variation peak rate, which implies that with Ma as the control medication, the common peak rate of Mb is P, the variation peak rate of Ma is Pa, and the variation peak rate of Mb is Pb.
HPLC study
Chromatographic conditions
The Agilent 1260 High performance liquid chromatography (Agilent Technologies, USA), with an Agilent TC-C18(2) chromatographic column (4.6×250 mm, 5 μm) was utilized. The flow rate was 1 mL·min-1, column temperature was 30 °C, detection wavelength was 280 nm, and injection volume was 5 μL. The mobile phase comprised methanol (A) and –0.1% formic acid in water (B). The gradient elution procedure was: 0–6 min, 10%–30% A; 6–13 min, 30%–33% A; 13–21 min, 33%–37% A; 21–25 min, 37%–40% A; 25–35 min, 40%–45% A; 35–45 min, 45%–48% A; 45–49 min, 48%–52% A; 49–54 min, 52%–60% A; 54–64 min, 60%–95% A; 64–74 min, 95%–10% A; 74–78 min, 10% A.
Sample preparation
Ultrasonic extraction was performed on 1.00 g rhubarb powder and 50 mL methanol for 1 h (20 kHz, 30±5 °C). The sample was filtered using 0.45 μm microporous membrane filtration. The filtered sample was marked as S1–S18.
The single standard and mixed standard solutions were prepared with methanol at a concentration of 1 mg/mL for each pharmacopoeia standard (purity ≥ 98%) of emodin (Lot No. 1205A0210), rhein (Lot No. 109C021), chrysophanol (Lot No. 523D025), aloe emodin (Lot No. 531B024), emodin methyl ether (Lot No. 718C023), catechin (Lot No. 1125H021), gallic acid (Lot No. 725G028), and sennoside A (Lot No. AF9111318) and sennoside B (Lot No. AF9111319).
Establish fingerprint
The S1–S18 chromatographic data was imported into the "Similarity evaluation system for chromatographic fingerprint of TCM (Version 2012),” for which the R. tanguticum fingerprint was created. Then, the similarity of S1–S18 was analyzed.
Analysis of chemometrics methods
The spectrum of S1–S18 chromatographic data was imported into the SIMCA software. The missing value was replaced with half of the minimum value, and the component was eliminated if the missing value reached 20%. The HPLC data matrix was statistically analyzed using PCA. The data were separated into two groups based on their origin, and OPLS-DA was performed to compare the groups.
Results and analysis
The results from the FTIR study
Similarity analysis results
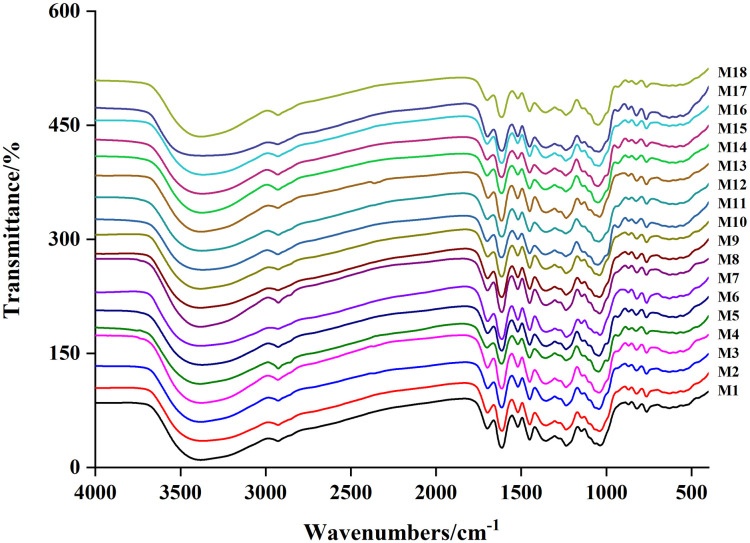
The FTIR spectra of M1–M18 are shown in Fig 1 and exhibit certain similarities. Absorption peaks were observed at 400–1800 cm-1 and 2700–3800 cm-1.
Fig 1. The FTIR spectra of M1 ~ M18.
The spectra were analyzed for similarity; the results are presented in Table 2. The similarity of the FTIR spectra ranged from 0.863 to 0.988, with a high overall similarity, demonstrating that the composition and content of R. tanguticum were relatively stable. Regarding sample similarity analysis, in M1–M10, 97.78 percent were greater than 0.9 and 96.43% of the samples in M11–M18 had similarity greater than 0.9. The similarity between M1–M10 and M11–M18 was greater than 0.9, accounting for 92.50%, demonstrating a high similarity between the M1–M10 and M11–M18 groups. The similarity between M1–M10 and M11–M18 was slightly lower.
Table 2. Similarity analysis of FTIR spectrum of M1~M18.
| Sample | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | M12 | M13 | M14 | M15 | M16 | M17 | M18 |
| M1 | 1.000 | |||||||||||||||||
| M2 | 0.929 | 1.000 | ||||||||||||||||
| M3 | 0.969 | 0.967 | 1.000 | |||||||||||||||
| M4 | 0.977 | 0.930 | 0.957 | 1.000 | ||||||||||||||
| M5 | 0.991 | 0.927 | 0.968 | 0.981 | 1.000 | |||||||||||||
| M6 | 0.919 | 0.938 | 0.966 | 0.893 | 0.915 | 1.000 | ||||||||||||
| M7 | 0.972 | 0.950 | 0.972 | 0.960 | 0.969 | 0.918 | 1.000 | |||||||||||
| M8 | 0.976 | 0.956 | 0.969 | 0.988 | 0.979 | 0.907 | 0.973 | 1.000 | ||||||||||
| M9 | 0.945 | 0.950 | 0.982 | 0.922 | 0.940 | 0.970 | 0.954 | 0.936 | 1.000 | |||||||||
| M10 | 0.951 | 0.974 | 0.989 | 0.932 | 0.945 | 0.974 | 0.963 | 0.950 | 0.985 | 1.000 | ||||||||
| M11 | 0.954 | 0.948 | 0.981 | 0.923 | 0.948 | 0.975 | 0.953 | 0.933 | 0.982 | 0.982 | 1.000 | |||||||
| M12 | 0.892 | 0.955 | 0.959 | 0.863 | 0.884 | 0.973 | 0.917 | 0.890 | 0.974 | 0.981 | 0.969 | 1.000 | ||||||
| M13 | 0.970 | 0.971 | 0.982 | 0.966 | 0.971 | 0.946 | 0.974 | 0.977 | 0.966 | 0.974 | 0.972 | 0.936 | 1.000 | |||||
| M14 | 0.967 | 0.913 | 0.944 | 0.961 | 0.960 | 0.890 | 0.951 | 0.962 | 0.924 | 0.929 | 0.930 | 0.867 | 0.964 | 1.000 | ||||
| M15 | 0.970 | 0.968 | 0.987 | 0.951 | 0.969 | 0.959 | 0.975 | 0.964 | 0.974 | 0.983 | 0.983 | 0.954 | 0.989 | 0.947 | 1.000 | |||
| M16 | 0.963 | 0.962 | 0.985 | 0.944 | 0.957 | 0.975 | 0.961 | 0.954 | 0.983 | 0.987 | 0.984 | 0.965 | 0.982 | 0.947 | 0.985 | 1.000 | ||
| M17 | 0.941 | 0.973 | 0.983 | 0.920 | 0.936 | 0.975 | 0.957 | 0.940 | 0.982 | 0.992 | 0.984 | 0.983 | 0.972 | 0.920 | 0.984 | 0.985 | 1.000 | |
| M18 | 0.927 | 0.942 | 0.965 | 0.887 | 0.915 | 0.966 | 0.933 | 0.906 | 0.969 | 0.977 | 0.973 | 0.974 | 0.940 | 0.892 | 0.964 | 0.969 | 0.982 | 1.000 |
The similarity of rhubarb methanol extract from the same origin was significant, whereas that of rhubarb from other sources was somewhat different. The rhubarb-producing locations can be differentiated using infrared chromatography.
Confirmation of common peaks and peak attribution
If the greatest difference in the wave number of a group of absorption peaks was smaller than the difference in the average wave number of its surrounding groups of peaks, the group of peaks was defined as a group of common peaks.
According to the absorption peak data reported by the OMNIC software, 17 peaks were found in the alcohol extract using standard peak identification methods. The wavenumber and common FTIR absorption peaks were listed (Table 3). Common peaks in the distinctive region were assigned as Table 4.
Table 3. Common peaks of M1~M18 FTIR absorption.
| Sample | Wave number/cm -1 | ||||||||
| M1 | 3380.70 | 2927.14 | 1697.90 | 1609.27 | 1513.93 | 1449.18 | 1354.62 | 1238.54 | — |
| M2 | 3369.65 | 2929.21 | 1697.46 | 1609.02 | 1514.56 | 1448.76 | 1355.84 | 1237.49 | 1142.48 |
| M3 | 3365.17 | 2927.10 | 1698.14 | 1609.14 | 1516.28 | 1448.93 | 1355.10 | 1238.36 | 1142.77 |
| M4 | 3372.86 | 2928.06 | 1697.77 | 1609.98 | 1513.94 | 1449.07 | 1358.24 | 1238.85 | — |
| M5 | 3373.23 | 2927.81 | 1700.74 | 1610.45 | 1514.33 | 1449.34 | 1354.00 | 1239.39 | — |
| M6 | 3377.44 | 2928.13 | 1696.30 | 1611.60 | 1518.16 | 1448.93 | 1356.21 | 1236.71 | 1143.82 |
| M7 | 3373.34 | 2925.65 | 1698.39 | 1608.92 | 1513.96 | 1448.88 | 1355.33 | 1239.76 | — |
| M8 | 3369.39 | 2928.44 | 1697.80 | 1609.48 | 1513.64 | 1449.08 | 1357.40 | 1238.86 | — |
| M9 | 3381.26 | 2926.57 | 1696.72 | 1609.89 | 1514.66 | 1448.86 | 1353.83 | 1237.44 | 1142.66 |
| M10 | 3384.23 | 2926.82 | 1697.2 | 1609.02 | 1514.14 | 1448.9 | 1355.01 | 1237.69 | 1142.93 |
| M11 | 3383.08 | 2924.68 | 1697.66 | 1610.43 | 1515.44 | 1448.74 | 1352.77 | 1238.20 | 1142.69 |
| M12 | 3380.62 | — | 1696.67 | 1609.32 | 1513.83 | 1448.65 | 1353.16 | 1235.59 | 1143.66 |
| M13 | 3372.38 | 2927.02 | 1697.51 | 1610.12 | 1514.12 | 1449.00 | 1355.88 | 1237.88 | 1141.40 |
| M14 | 3385.58 | 2925.70 | 1701.26 | 1610.35 | 1514.26 | 1449.86 | 1359.77 | 1238.85 | — |
| M15 | 3374.93 | 2927.39 | 1698.17 | 1609.31 | 1514 | 1448.81 | 1359.02 | 1238.53 | 1141.64 |
| M16 | 3377.28 | 2926.61 | 1697.03 | 1611.27 | 1512.78 | 1448.79 | 1354.26 | 1236.87 | 1142.65 |
| M17 | 3376.22 | 2926.82 | 1697.52 | 1609.02 | 1513.33 | 1448.57 | 1355.31 | 1237.06 | 1143.23 |
| M18 | 3381.82 | 2926.51 | 1697.71 | 1609.27 | 1513.68 | 1448.78 | 1356.17 | 1236.50 | 1143.94 |
| Average | 3376.62 | 2927.04 | 1697.89 | 1609.77 | 1514.39 | 1448.95 | 1355.66 | 1237.92 | 1142.82 |
| Maximum difference | 20.41 | 4.53 | 4.96 | 2.68 | 5.38 | 1.29 | 7.00 | 4.17 | 2.54 |
| Sample | Wave number/cm -1 | ||||||||
| M1 | 1048.35 | — | 869.52 | 825.15 | 766.81 | 629.04 | — | — | |
| M2 | 1044.86 | 944.03 | 869.54 | 823.00 | 767.14 | 627.93 | — | 547.63 | |
| M3 | 1044.46 | — | 869.47 | 818.49 | 766.67 | 630.74 | — | — | |
| M4 | 1048.82 | 926.47 | 870.92 | 822.44 | 767.20 | — | 594.23 | — | |
| M5 | 1048.76 | — | 870.64 | 819.03 | 767.21 | — | 598.34 | — | |
| M6 | 1038.13 | — | 868.09 | 821.76 | 767.35 | 634.63 | — | — | |
| M7 | 1045.11 | — | 869.04 | 826.91 | 766.31 | — | 590.36 | — | |
| M8 | 1049.24 | 926.40 | 868.71 | 826.88 | 767.18 | — | 593.47 | — | |
| M9 | 1037.93 | — | 871.05 | 818.49 | 766.24 | 630.89 | — | — | |
| M10 | 1038.07 | — | 869.30 | 818.37 | 766.74 | 628.60 | — | — | |
| M11 | 1038.08 | — | 869.10 | 819.10 | 766.84 | 627.45 | — | — | |
| M12 | 1035.60 | — | 870.04 | 818.61 | 766.29 | 629.53 | — | — | |
| M13 | 1044.86 | — | 868.77 | 825.29 | 766.59 | — | 596.19 | — | |
| M14 | 1045.96 | — | 867.50 | 826.28 | 765.96 | — | 584.16 | — | |
| M15 | 1041.91 | — | 869.19 | 817.62 | 766.28 | 628.76 | — | 547.37 | |
| M16 | 1040.83 | — | 868.43 | 822.38 | 766.47 | 625.63 | — | — | |
| M17 | 1038.24 | — | 869.64 | 818.32 | 766.45 | 629.04 | — | — | |
| M18 | 1035.73 | — | 869.48 | 817.30 | 765.36 | 628.65 | — | — | |
| Average | 1042.50 | 932.30 | 869.36 | 821.41 | 766.67 | 629.24 | 592.79 | 547.50 | |
| Maximum difference | 13.64 | 17.63 | 3.55 | 9.61 | 1.99 | 9.00 | 14.18 | 0.26 | |
"-" means that the sample has no this wave number.
Table 4. Vibrational modes of functional groups in common peaks (distinctive region).
| Wave number/cm-1 | Peak intensity | Vibration mode |
|---|---|---|
| 3415.27–3359.21 | wide and strong peak | polymer ν (O-H), amide ν (N-H) |
| 2932.79–2924.68 | acromion | methylene νas(C-H) |
| 1701.26–1696.30 | moderately strong peak | ν (C = O) |
| 1626.70–1608.92 | medium strong peak | amide β (N-H) |
| 1520.35–1512.78 | weak peak | amide β (N-H), aromatic hydrocarbon, and benzene ring skeleton vibration ν (C = C) |
| 1449.86–1446.70 | medium strong peak | methyl δ (C-H), methylene δ (C-H), β (O-H), aromatic hydrocarbon, and benzene ring skeleton vibration ν (C = C) |
| 1367.46–1316.93 | medium strong peak | methyl β (C-H) |
| 1243.07–1235.59 | moderately strong peak | δ (O-H), ν (C-N), and ν (C-O-C) |
| 1146.30–1141.00 | medium strong peak | ν (C-N) of lipid compounds ν (C-O-C) |
| 1053.72–1033.93 | strong peak | δ (C-O) |
R. tanguticum was mostly composed of anthraquinones, polysaccharides, and tannins. The FTIR analysis results were consistent with the composition research findings, and the methanol extract included the majority of the R. tanguticum components.
Cluster analysis
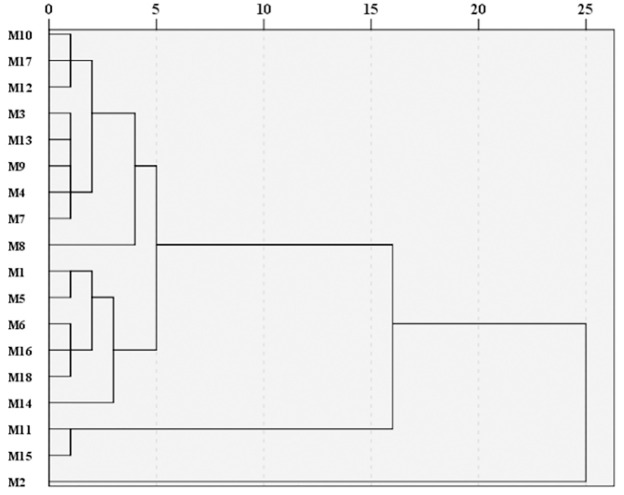
SPSS 26 software was used to perform systematic cluster analysis of M1–M18. The interval was the square Euclidean distance, and the cluster technique was the intergroup connection method. Cluster analysis was performed on 18 batches of FTIR spectra (Fig 2). When the Euclidean Distance was 10, it was separated into three groups: M10M17M12M3M13M9M4M7M8M1M5M6M16M18M14, M11M15, and M2. This might be owing to the cumulative impact of geographical location, soil, and weather. The chemical content of rhubarb varied depending on the location of its growth.
Fig 2. FTIR cluster analysis diagram of M1 ~ M18.
The results of the double index sequence analysis
Basic grouping was employed in the M1–M18 sequence. According to the common peak rate, the most comparable sample pairs and groups could be sorted into the Tables 5–8.
Table 5. The sequence with the same absorption peak (Group A).
| Control Sample | Comparison Sample | (P; Pa, Pb) | Control Sample | Comparison Sample | (P; Pa, Pb) |
|---|---|---|---|---|---|
| M3 | M6M9M10M11M16M17M18 | (1.0000; 0.0000, 0.0000) | M4 | M8 | (1.0000; 0.0000, 0.0000) |
| M5 | M7M14 | (1.0000; 0.0000, 0.0000) | M6 | M3M9M10M11M16M17M18 | (1.0000; 0.0000, 0.0000) |
| M7 | M5M14 | (1.0000; 0.0000, 0.0000) | M8 | M4 | (1.0000; 0.0000, 0.0000) |
| M9 | M3M6M10M11M16M17M18 | (1.0000; 0.0000, 0.0000) | M10 | M3M6M9M11M16M17M18 | (1.0000; 0.0000, 0.0000) |
| M11 | M3M6M9M10M16M17M18 | (1.0000; 0.0000, 0.0000) | M14 | M5M7 | (1.0000; 0.0000, 0.0000) |
| M16 | M3M6M9M10M11M17M18 | (1.0000; 0.0000, 0.0000) | M17 | M3M6M9M10M11M16M18 | (1.0000; 0.0000, 0.0000) |
| M18 | M3M6M9M10M11M16M17 | (1.0000; 0.0000, 0.0000) |
Table 8. The sequence with low common peak rate and high variation peak rate (Group D).
| Control Sample | Comparison Sample | (P; Pa, Pb) | Control Sample | Comparison Sample | (P; Pa, Pb) |
|---|---|---|---|---|---|
| M2 | M4M8 | (0.7674; 0.2308, 0.0769) | M2 | M5M7M14 | (0.7059; 0.3333, 0.0833) |
| M3 | M4M8 | (0.7500; 0.1667, 0.1667) | M4 | M2 | (0.7674; 0.0769, 0.2308) |
| M4 | M3M6M9M10M11M16M17M18 | (0.7500; 0.1667, 0.1667) | M4 | M15 | (0.7059; 0.1667, 0.1667) |
| M4 | M12 | (0.6875; 0.2727, 0.0909) | M5 | M15 | (0.7500; 0.0833, 0.2500) |
| M5 | M12 | (0.7333; 0.1818, 0.1818) | M5 | M2 | (0.7059; 0.0833, 0.3333) |
| M5 | M2 | (0.7059; 0.0833, 0.3333) | M6 | M4M8 | (0.7500; 0.1667, 0.1667) |
| M7 | M15 | (0.7500; 0.0833, 0.2500) | M7 | M12 | (0.7333; 0.1818, 0.1818) |
| M7 | M2 | (0.7059; 0.0833, 0.3333) | M8 | M2 | (0.7674; 0.0769, 0.2308) |
| M8 | M3M6M9M10M11M16M17M18 | (0.7500; 0.1667, 0.1667) | M8 | M15 | (0.7059; 0.1667, 0.2500) |
| M8 | M12 | (0.6875; 0.2727, 0.1818) | M9 | M4M8 | (0.7500; 0.1667, 0.1667) |
| M10 | M4M8 | (0.7500; 0.1667, 0.1667) | M11 | M4M8 | (0.7500; 0.1667, 0.1667) |
| M12 | M5M7 | (0.7333; 0.1818, 0.1818) | M12 | M8 | (0.6875; 0.1818, 0.2727) |
| M12 | M14 | (0.6875; 0.1818, 0.1818) | M12 | M4 | (0.6875; 0.0909, 0.2727) |
| M14 | M15 | (0.7500; 0.0833, 0.2500) | M14 | M2 | (0.7059; 0.0833, 0.3333) |
| M14 | M12 | (0.6875; 0.1818, 0.1818) | M15 | M5M7 | (0.7500; 0.2500, 0.0833) |
| M15 | M14 | (0.7500; 0.2500, 0.0833) | M15 | M8 | (0.7059; 0.2500, 0.1667) |
| M15 | M4 | (0.7059; 0.1667, 0.1667) | M16 | M4M8 | (0.7500; 0.1667, 0.1667) |
| M17 | M4M8 | (0.7500; 0.1667, 0.1667) | M18 | M4M8 | (0.7500; 0.1667, 0.1667) |
Table 6. The sequence with high common peak rate and low variation peak rate (Group B).
| Control Sample | Comparison Sample | (P; Pa, Pb) | Control Sample | Comparison Sample | (P; Pa, Pb) |
|---|---|---|---|---|---|
| M1 | M3M6M9M10M11M16M17M18 | (0.9286; 0.0000, 0.0769) | M1 | M5M7M12M14 | (0.8571; 0.0833, 0.0833) |
| M2 | M15 | (0.9375; 0.0667, 0.0000) | M2 | M1M12M13 | (0.8125; 0.2308, 0.0000) |
| M3 | M15 | (0.9333; 0.0000, 0.0714) | M3 | M1M12 | (0.9286; 0.0769, 0.0000) |
| M3 | M13 | (0.8667; 0.0769, 0.0769) | M4 | M5M7M14 | (0.9286; 0.0769, 0.0000) |
| M4 | M13 | (0.8125; 0.0769, 0.0769) | M5 | M4M8M13 | (0.9286; 0.0000, 0.0769) |
| M5 | M1 | (0.8571; 0.0833, 0.0833) | M6 | M15 | (0.9333; 0.0000, 0.0714) |
| M6 | M1M12 | (0.9286; 0.0769, 0.0000) | M6 | M13 | (0.8667; 0.0769, 0.0769) |
| M7 | M4M8M13 | (0.9286; 0.0000, 0.0769) | M7 | M1 | (0.8571; 0.0833, 0.0833) |
| M8 | M5M7M14 | (0.9286; 0.0769, 0.0000) | M8 | M13 | (0.8667; 0.0769, 0.0769) |
| M9 | M15 | (0.9333; 0.0000, 0.0714) | M9 | M1M12 | (0.9286; 0.0769, 0.0000) |
| M9 | M13 | (0.8667; 0.0769, 0.0769) | M10 | M15 | (0.9333; 0.0000, 0.0714) |
| M10 | M1M12 | (0.9286; 0.0769, 0.0000) | M10 | M13 | (0.8667; 0.0769, 0.0769) |
| M11 | M15 | (0.9333; 0.0000, 0.0714) | M11 | M1M12 | (0.9286; 0.0769, 0.0000) |
| M11 | M13 | (0.8667; 0.0769, 0.0769) | M12 | M3M6M9M10 M11M16M17M18 |
(0.9286; 0.0000, 0.0769) |
| M12 | M1 | (0.8571; 0.0833, 0.0833) | M13 | M5M7M14 | (0.9286; 0.0769, 0.0000) |
| M13 | M3M6M8M9M10 M11M16M17M18 |
(0.8667; 0.0769, 0.0769) | M13 | M4 | (0.8125; 0.0769, 0.0769) |
| M14 | M4M8M13 | (0.9286; 0.0000, 0.0769) | M14 | M1 | (0.8571; 0.0833, 0.0833) |
| M15 | M2 | (0.9375; 0.0000, 0.0667) | M15 | M3M6M9M10 M11M16M17M18 |
(0.9333; 0.0714, 0.0000) |
| M16 | M15 | (0.9333; 0.0000, 0.0714) | M16 | M1M12 | (0.9286; 0.0769, 0.0000) |
| M16 | M13 | (0.8667; 0.0769, 0.0769) | M17 | M15 | (0.9333; 0.0000, 0.0714) |
| M17 | M1M12 | (0.9286; 0.0769, 0.0000) | M17 | M13 | (0.8667; 0.0769, 0.0769) |
| M18 | M15 | (0.9333; 0.0000, 0.0714) | M18 | M1M12 | (0.9286; 0.0769, 0.0000) |
| M18 | M13 | (0.8667; 0.0769, 0.0769) |
Table 7. The sequence with high common peak rate and high variation peak rate (Group C).
| Control Sample | Comparison Sample | (P; Pa, Pb) | Control Sample | Comparison Sample | (P; Pa, Pb) |
|---|---|---|---|---|---|
| M1 | M15 | (0.8667; 0.0000, 0.1538) | M1 | M2 | (0.8125; 0.000, 0.2308) |
| M1 | M4M8M13 | (0.8000; 0.0833, 0.1667) | M2 | M3M6M9M10 M11M16M17M18 |
(0.8750; 0.1429, 0.0000) |
| M3 | M2 | (0.8750; 0.0000, 0.1429) | M3 | M5M7M14 | (0.8000; 0.1667, 0.0833) |
| M4 | M1 | (0.8000; 0.1667, 0.0833) | M5 | M3M6M9M10 M11M16M17M18 |
(0.8000; 0.0833, 0.1667) |
| M6 | M2 | (0.8750; 0.0000, 0.1429) | M6 | M5M7M14 | (0.8000; 0.1667, 0.0833) |
| M7 | M3M6M9M10M11M16M17M18 | (0.8000; 0.0833, 0.1667) | M8 | M1 | (0.8000; 0.1667, 0.0833) |
| M9 | M2 | (0.8750; 0.0000, 0.1429) | M9 | M5M7M14 | (0.8000; 0.1667, 0.0833) |
| M10 | M2 | (0.8750; 0.0000, 0.1429) | M10 | M5M7M14 | (0.8000; 0.1667, 0.0833) |
| M11 | M2 | (0.8750; 0.0000, 0.1429) | M11 | M5M7M14 | (0.8000; 0.1667, 0.0833) |
| M12 | M15 | (0.8667; 0.0000, 0.1538) | M12 | M2 | (0.8125; 0.0000, 0.2308) |
| M12 | M13 | (0.8000; 0.0833, 0.1667) | M13 | M15 | (0.8125; 0.0769, 0.1538) |
| M13 | M2 | (0.8125; 0.0000, 0.2308) | M13 | M1M12 | (0.8000; 0.1667, 0.0833) |
| M14 | M3M6M9M10M11M16M17M18 | (0.8000; 0.0833, 0.1667) | M15 | M1M12 | (0.8667; 0.1538, 0.0000) |
| M15 | M13 | (0.8125; 0.1538, 0.0769) | M16 | M2 | (0.8750; 0.0000, 0.1429) |
| M16 | M5M7M14 | (0.8000; 0.1667, 0.0833) | M17 | M2 | (0.8750; 0.0000, 0.1429) |
| M17 | M5M7M14 | (0.8000; 0.1667, 0.0833) | M18 | M2 | (0.8750; 0.0000, 0.1429) |
| M18 | M5M7M14 | (0.8000; 0.1667, 0.0833) |
The common peak rate and variation peak rate of the sequence were the same in group A, and the absorption peak corresponded completely. The sequence common peak rate was high in group B, whereas its variation peak rate was low. The sequence common peak rate and variation peak rate were higher in Group C. The common peak rate of the sequence was lower in Group D, whereas its variation peak rate was higher.
Double index analysis and sequence analysis of the methanol extract of rhubarb revealed that different results of R. tanguticum from different producing areas. However, the methanol extract can eliminate some differences caused by environmental factors. For example, Hezuo, Hezuo, Xiahe of Hezuo, Lintan of Hezuo, Qinghai, Huangzhong of Qinghai, Datong of Qinghai, and Guide of Qinghai are the corresponding producing areas of sequence M3:M6M9M10M11M16M17M18. Nonetheless, the sequence resulting from double index analysis showed that the common peak rate was 100%, with no variation peak. If this method was used, then the samples were classified into one category.
According to the results of the above analysis, environmental factors such as origin, altitude, longitude, latitude, and climatic conditions could affect the common peak rate and variation peak rate of R. tanguticum, but the specific impact could be determined. The components of R. tanguticum were determined using HPLC, and the effects of altitude, longitude, and latitude on these components were studied to further analyze the influence of the environment on the quality of R. tanguticum.
The result of HPLC study
Chromatography of pharmacopoeia standards
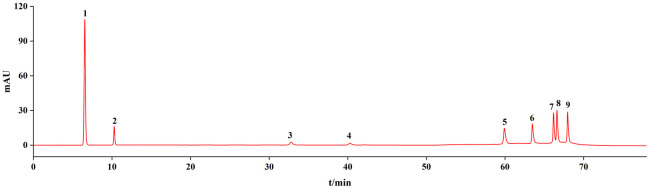
The chromatographic peaks of the mixed pharmacopoeia standards are shown in Fig 3. The chromatographic peaks of the nine components were identified using the chromatographic peak of a single pharmacopoeia standard.
Fig 3. Chromatogram of pharmacopoeia standards.
1: Gallic acid, 2: (+) -catechin, 3: Sennoside B, 4: Sennoside A, 5: Aloe emodin, 6: Rhein, 7: Emodin, 8: Chrysophanol, 9: Emodin methyl ether.
Establish rhubarb fingerprint
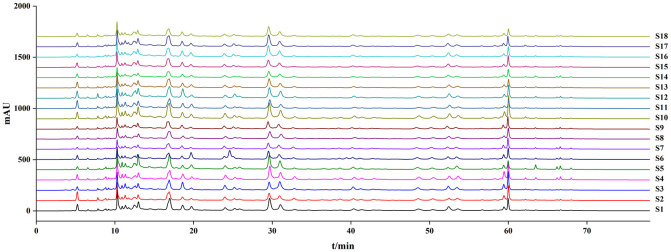
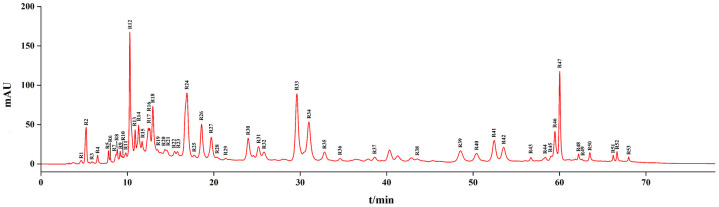
HPLC was used to acquire chromatograms of S1–S18 (Fig 4). The S1–S18 spectra were imported into the "Similarity evaluation system for chromatographic fingerprint of TCM (version 2012)" and S1 was set as the control spectrum for chromatographic peak matching to obtain the R. tanguticum common peak pattern spectrum (Fig 5). A total of 53 peaks were observed. Eight chromatographic peaks were identified after the retention time and compared with the chromatogram of the reference material.
Fig 4. The chromatograms of S1 ~ S18.
Fig 5. The chromatogram of S1 ~ S18 (common peak mode).
R4: Gallic acid, R12: (+)—catechin, R35: Sennoside B, R47: Aloe emodin, R50: Rhein, R51: Emodin, R52: Chrysophanol, R53: Emodin methyl ether.
The fingerprints of the 18 batches of Rheum tanguticum were nearly identical. The similarity of each batch to the standard fingerprint was 0.909, 0.861, 0.888, 0.895, 0.879, 0.620, 0.671, 0.899, 0.917, 0.909, 0.918, 0.914, 0.853, 0.689, 0.668, 0.779, 0.734, and 0.734, all of which are between 0.62 and 0.92.
PCA analysis results
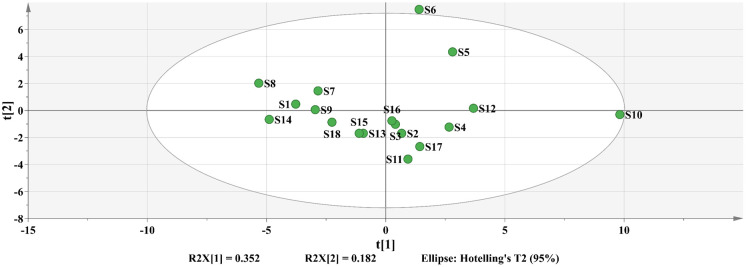
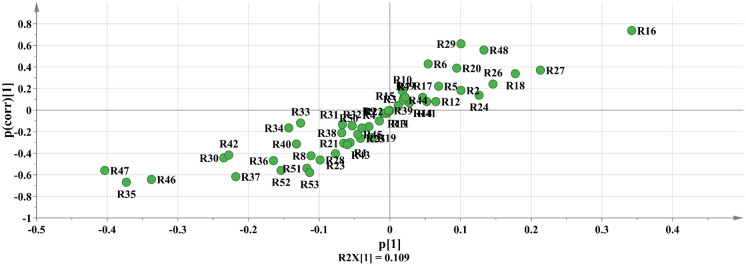
The S1-S18 common peak data were imported into the SIMCA software, the missing value was filled with half of the minimum value, and the component was eliminated if the missing value reached 20%. The PCA approach was used for statistically analyzing the HPLC data matrix (Fig 6). R. tanguticum grown in Gannan and Qinghai had a tendency to separate t[2], however this trend was not noticeable.
Fig 6. PCA analysis of S1~S18.
OPLS-DA analysis results
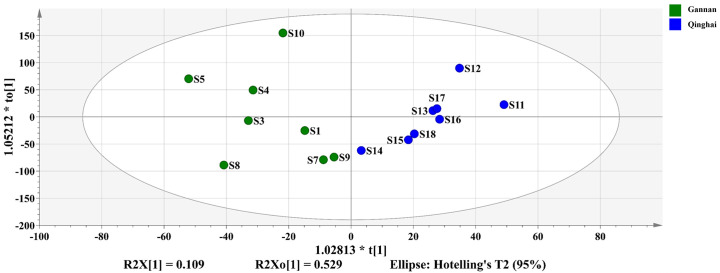
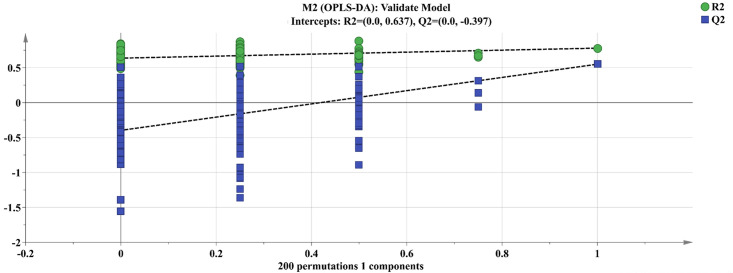
According to the PCA findings, S2 (spring harvest) and S6 (outside the 95 percent confidence interval) were removed from further investigation of the component differences of R. tanguticum produced in Gannan and Qinghai. The remaining samples were separated into two groups based on their origin, Gannan and Qinghai. OPLS-DA analysis was performed between the groups, and the OPLS-DA score diagram and S-plot diagram were generated (Figs 7–9). In the OPLS-DA study, R2Y = 0.780 and Q2 = 0.550, suggesting that the two groups were well divided and that the model could predict 55.0% of the results. Permute the OPLS-DA model 200 times (Fig 10), R2 = 0.637, Q2 = -0.397, suggesting that the model is correct.
Fig 7. OPLS-DA.
Fig 9. S-Plot.
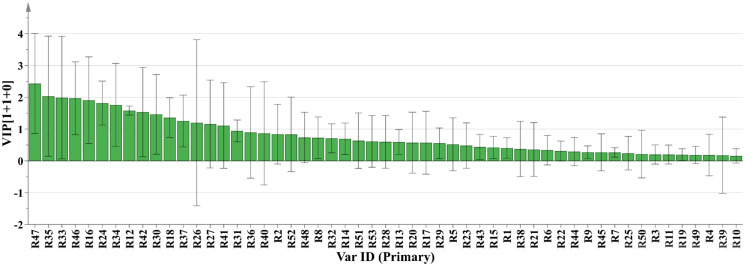
Fig 10. VIP Value.
Fig 8. Permutations.
The larger was the variable importance of projection (VIP) value of the point farther from the origin of the "s" curve in the figure, the greater was the contribution to the grouping. According to the s-plot analysis, coupled with the VIP value (Fig 9). Components with a VIP ≥ 1 were eliminated, and compounds with differences were found in conjunction with P < 0.05. According to these findings, R16, R37, R46, and R47 (Aloe emodin) are the key differentiating components of Rheum tanguticum produced in Gannan and Qinghai.
Influence of longitude, latitude and altitude on composition
Pearson’s correlation coefficient was used to examine the relationship between longitude, latitude, altitude, and composition (Table 9). Longitude was significantly positively correlated with R28 and R30 (P < 0.05), and a very significantly positively correlated with R35, R36, R37, R46, and R47 (P < 0.01). Latitude was significantly negatively correlated with R34, R35, and R40 (P < 0.05), and extremely significantly negatively correlated with R28, R30, R36, R37, R46, and R47 (P < 0.01). Altitude was significantly positive correlation with R36 and R37 (P < 0.01).
Table 9. Pearson correlation test table for the influence of longitude, latitude and altitude on composition.
| Composition | Longitude | Latitude | Altitude | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient | Significance | Correlation Coefficient | Significance | Correlation Coefficient | Significance | |
| R1 | 0.291 | 0.241 | -0.213 | 0.396 | -0.063 | 0.804 |
| R2 | 0.126 | 0.618 | 0.093 | 0.712 | -0.333 | 0.178 |
| R3 | 0.483 | 0.188 | -0.345 | 0.363 | 0.242 | 0.531 |
| R4 | 0.242 | 0.333 | -0.314 | 0.204 | -0.260 | 0.298 |
| R5 | 0.109 | 0.668 | -0.026 | 0.918 | -0.311 | 0.209 |
| R6 | -0.061 | 0.810 | 0.052 | 0.837 | -0.274 | 0.272 |
| R7 | 0.275 | 0.285 | -0.129 | 0.621 | -0.032 | 0.903 |
| R8 | 0.401 | 0.099 | -0.342 | 0.164 | 0.188 | 0.455 |
| R9 | 0.251 | 0.315 | -0.186 | 0.460 | 0.140 | 0.579 |
| R10 | -0.115 | 0.660 | 0.200 | 0.443 | -0.013 | 0.961 |
| R11 | 0.055 | 0.827 | -0.016 | 0.949 | 0.097 | 0.703 |
| R12 | 0.233 | 0.353 | -0.130 | 0.606 | -0.035 | 0.891 |
| R13 | 0.170 | 0.501 | -0.202 | 0.421 | -0.167 | 0.507 |
| R14 | 0.229 | 0.360 | -0.081 | 0.748 | 0.203 | 0.419 |
| R15 | 0.079 | 0.754 | 0.081 | 0.748 | 0.108 | 0.671 |
| R16 | -0.216 | 0.389 | 0.314 | 0.204 | -0.327 | 0.185 |
| R17 | -0.040 | 0.883 | 0.096 | 0.725 | -0.102 | 0.708 |
| R18 | -0.011 | 0.966 | 0.087 | 0.732 | -0.154 | 0.541 |
| R19 | 0.434 | 0.159 | -0.344 | 0.273 | 0.112 | 0.728 |
| R20 | 0.093 | 0.732 | -0.172 | 0.524 | 0.011 | 0.969 |
| R21 | 0.416 | 0.123 | -0.265 | 0.341 | 0.480 | 0.070 |
| R22 | 0.045 | 0.869 | 0.036 | 0.896 | -0.193 | 0.473 |
| R23 | 0.389 | 0.111 | -0.254 | 0.309 | 0.300 | 0.227 |
| R24 | 0.135 | 0.594 | -0.017 | 0.945 | -0.046 | 0.857 |
| R25 | 0.544 | 0.055 | -0.414 | 0.160 | 0.272 | 0.368 |
| R26 | -0.185 | 0.463 | 0.174 | 0.490 | -0.146 | 0.564 |
| R27 | -0.126 | 0.618 | 0.124 | 0.624 | -0.198 | 0.431 |
| R28 | 0.616* | 0.025 | -0.738** | 0.004 | 0.366 | 0.218 |
| R29 | -0.491 | 0.217 | 0.540 | 0.167 | 0.123 | 0.772 |
| R30 | 0.548* | 0.028 | -0.649** | 0.007 | 0.341 | 0.196 |
| R31 | 0.347 | 0.296 | -0.347 | 0.296 | 0.102 | 0.766 |
| R32 | 0.382 | 0.246 | -0.382 | 0.246 | 0.066 | 0.847 |
| R33 | 0.328 | 0.299 | -0.328 | 0.299 | 0.213 | 0.506 |
| R34 | 0.521 | 0.056 | -0.595* | 0.025 | 0.456 | 0.101 |
| R35 | 0.773** | 0.002 | -0.617* | 0.025 | 0.276 | 0.362 |
| R36 | 0.704** | 0.003 | -0.704** | 0.003 | 0.655** | 0.008 |
| R37 | 0.821** | 0.002 | -0.821** | 0.002 | 0.749** | 0.008 |
| R38 | 0.574 | 0.106 | -0.495 | 0.175 | 0.455 | 0.218 |
| R39 | 0.345 | 0.299 | -0.556 | 0.076 | 0.374 | 0.258 |
| R40 | 0.585 | 0.059 | -0.642* | 0.033 | 0.249 | 0.460 |
| R41 | 0.240 | 0.452 | -0.166 | 0.605 | 0.122 | 0.706 |
| R42 | 0.465 | 0.175 | -0.518 | 0.125 | 0.269 | 0.453 |
| R43 | 0.403 | 0.109 | -0.458 | 0.065 | 0.468 | 0.058 |
| R44 | 0.019 | 0.946 | -0.099 | 0.715 | 0.226 | 0.400 |
| R45 | 0.351 | 0.264 | -0.307 | 0.332 | 0.373 | 0.233 |
| R46 | 0.652** | 0.005 | -0.719** | 0.001 | 0.307 | 0.231 |
| R47 | 0.634** | 0.005 | -0.616** | 0.006 | 0.407 | 0.093 |
| R48 | -0.354 | 0.163 | 0.252 | 0.329 | -0.029 | 0.911 |
| R49 | 0.373 | 0.232 | -0.315 | 0.319 | -0.154 | 0.633 |
| R50 | 0.105 | 0.677 | -0.097 | 0.703 | 0.155 | 0.538 |
| R51 | 0.358 | 0.145 | -0.294 | 0.236 | 0.145 | 0.567 |
| R52 | 0.358 | 0.145 | -0.266 | 0.286 | 0.201 | 0.424 |
| R53 | 0.258 | 0.317 | -0.188 | 0.469 | 0.173 | 0.507 |
*, at the level of 0.05 (two tailed), the correlation is significant;
**, At the level of 0.01 (two tailed), the correlation is significant.
Discussion
The fingerprints of R. tanguticum are extensively researched; however, the distinctions between R. tanguticum from different production locations are still not well investigated. FTIR spectroscopy was used to examine the methanol extract of R. tanguticum from various production sites. According to cluster analysis, some discrepancies existed in the FTIR spectra from various manufacturing locations. The double index analysis sequence of the common peak and variation peak was further established, which can differentiate various production locations of medicinal materials to some extent. The alcohol extract may reduce the impact of several water-soluble components. To further investigate the relationship between components and production areas, the HPLC fingerprint of R. tanguticum was established, and chemical pattern discriminant analysis, such as PCA and OPLS-DA analysis, was introduced into the differential component analysis of R. tanguticum from different production areas. Differential components of R. tanguticum from Gannan and Qinghai were obtained for their distinction. The three elements of longitude, latitude, and altitude were preliminarily identified by evaluating the sample collection information, and the link between the different components and these three factors was built using multiple linear regression. Regression equations between the nine components and longitude and latitude were effectively established, and the link between longitude and latitude and components was discussed further.
The quality of R. tanguticum varied according to the growing environment. In terms of composition, R16 of R. tanguticum produced in Gannan was substantially lower than that produced in Qinghai (P < 0.05), whereas R37, R46, and R47 were significantly greater (P < 0.05). Longitude, latitude, and altitude all impacted the quality of medicinal products because they had an influence on the components. This can guide us in determining the origin of therapeutic ingredients and provides a theoretical basis for planting R. tanguticum in various production locations.
The quality of R. tanguticum depends on numerous factors, including temperature, humidity, precipitation, illumination time, longitude, latitude, altitude, slope, and soil characteristics. The longitude, latitude, and altitude evaluated in the experiment were merely a subset of the influencing elements, which have limits and cannot fully account for the impact of growth factors on the quality of R. tanguticum. Furthermore, human elements are critical for the widespread adoption of artificial farming [40]. Consequently, different growth environments can be included in the scope for investigating further development and utilization of R. tanguticum, and ecological factors that promote the accumulation of secondary metabolites can be fully considered to improve the quality of medicinal materials. In conclusion, the results of our study can provide insights in its quality control and aid in establishing a natural medication traceability system.
Conclusions
The different production locations of R. tanguticum can be distinguished using FTIR and HPLC. The double index analysis sequence of common and variation peaks may differentiate distinct production locations of medicinal materials. The composition of R. tanguticum components from various sources varied substantially by the result of OPLS-DA. The key differential components of R. tanguticum produced in Gannan and Qinghai were discovered to be R16, R37, R46, and R47 (Aloe emodin) (VIP ≥ 1 and P < 0.05). Longitude, latitude, and altitude were all found to impact R. tanguticum quality by influencing the accumulation of components. Longitude was significantly positively correlated with R28 and R30 (P < 0.05), and a very significantly positively correlated with R35, R36, R37, R46, and R47 (P < 0.01). Latitude was significantly negatively correlated with R34, R35, and R40 (P < 0.05), and extremely significantly negatively correlated with R28, R30, R36, R37, R46, and R47 (P < 0.01). Altitude was significantly positive correlation with R36 and R37 (P < 0.01). The findings of this study can be used to plan planting of R. tanguticum.
Acknowledgments
We would like to thank Yuwei Gan for his assistance in identifying R. tanguticum. Thanks to Gansu University of Chinese Medicine’s undergraduate teaching practice center for providing the instruments and locations. We would like to thank Editage (www.editage.cn) for English language editing.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the key Project of Joint Funds of the National Natural Science Foundation of China (U21A20412); the Provincial Natural Science Foundation of Gansu Province, China (21JR7RA558); Excellent doctoral student program of Gansu Province, China (22JR5RA576); the “Double First-Class” key Scientific Research Project of the Department of Education of Gansu Province, China (GSSYLXM-05); The Project of Central Government Guides the Development of Local Science and Technology, Gansu Province, China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang X, Yang R, Feng S, Hou X, Zhang Y, Li Y, et al. Genetic variation in Rheum palmatum and Rheum tanguticum (Polygonaceae), two medicinally and endemic species in China using ISSR markers. PLoS One. 2012;7(12):e51667. Epub 2013/01/05. doi: 10.1371/journal.pone.0051667 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao LL, Guo T, Xu XD, Yang JS. Rapid identification and simultaneous analysis of multiple constituents from Rheum tanguticum Maxim. ex Balf. by UPLC/Q-TOF-MS. Nat Prod Res. 2017;31(13):1529–35. Epub 2017/01/24. doi: 10.1080/14786419.2017.1280491 . [DOI] [PubMed] [Google Scholar]
- 3.Keshavarzi Z, Shakeri F, Maghool F, Jamialahmadi T, Johnston TP, Sahebkar A. A Review on the Phytochemistry, Pharmacology, and Therapeutic Effects of Rheum ribes. Adv Exp Med Biol. 2021;1328:447–61. Epub 2022/01/05. doi: 10.1007/978-3-030-73234-9_30 . [DOI] [PubMed] [Google Scholar]
- 4.Aichner D, Ganzera M. Analysis of anthraquinones in rhubarb (Rheum palmatum and Rheum officinale) by supercritical fluid chromatography. Talanta. 2015;144:1239–44. Epub 2015/10/11. doi: 10.1016/j.talanta.2015.08.011 . [DOI] [PubMed] [Google Scholar]
- 5.Cao YJ, Pu ZJ, Tang YP, Shen J, Chen YY, Kang A, et al. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin Med. 2017; 28(12):36. Epub 2017/12/28. doi: 10.1186/s13020-017-0158-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J, Li P, Zhang T. Rhubarb combined with trypsin inhibitor for severe acute pancreatitis: A systematic review and meta-analysis. Phytother Res. 2018; 32(8): 1450–1458. Epub 2018/04/19. Epub 2018 Apr 19. doi: 10.1002/ptr.6096 . [DOI] [PubMed] [Google Scholar]
- 7.Zhang WF, Li ZT, Fang JJ, Wang GB, Yu Y, Liu ZQ, et al. Expression and clinical significance of rhubarb on serum amylase and TNF-alpha of rat model of acute pancreatitis. J Biol Regul Homeost Agents. 2017; 31(3): 753–760. Epub 2017/09/06. . [PubMed] [Google Scholar]
- 8.Zhou Y, Wang L, Huang X, Li H, Xiong Y. Add-on effect of crude rhubarb to somatostatin for acute pancreatitis: A meta-analysis of randomized controlled trials. J Ethnopharmacol. 2016; 194(24): 495–505. Epub 2016/09/29. doi: 10.1016/j.jep.2016.09.053 . [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Li Y, Cao Q, Ye L, Wang J, Guo M. The Function of Natural Polysaccharides in the Treatment of Ulcerative Colitis. Front Pharmacol. 2022; 13: 927855. Epub 2022/07/04. doi: 10.3389/fphar.2022.927855 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Li P, Zhang T. Rhubarb combined with trypsin inhibitor for severe acute pancreatitis: A systematic review and meta-analysis. Phytother Res. 2018;32(8):1450–8. Epub 2018/04/20. doi: 10.1002/ptr.6096 . [DOI] [PubMed] [Google Scholar]
- 11.Moon MK, Kang DG, Lee JK, Kim JS, Lee HS. Vasodilatory and anti-inflammatory effects of the aqueous extract of rhubarb via a NO-cGMP pathway. Life Sci. 2006;78(14):1550–7. Epub 2005/11/05. doi: 10.1016/j.lfs.2005.07.028 . [DOI] [PubMed] [Google Scholar]
- 12.Neyrinck AM, Etxeberria U, Taminiau B, Daube G, Van Hul M, Everard A, et al. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol Nutr Food Res. 2017; 61(1). Epub 2016/06/01. doi: 10.1002/mnfr.201500899 . [DOI] [PubMed] [Google Scholar]
- 13.Cui HX, Zhang LS, Luo Y, Yuan K, Huang ZY, Guo Y. A Purified Anthraquinone-Glycoside Preparation From Rhubarb Ameliorates Type 2 Diabetes Mellitus by Modulating the Gut Microbiota and Reducing Inflammation. Front Microbiol. 2019; 25(10): 1423. Epub 2019/06/25. doi: 10.3389/fmicb.2019.01423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stompor-Goracy M. The Health Benefits of Emodin, a Natural Anthraquinone Derived from Rhubarb-A Summary Update. Int J Mol Sci. 2021;22(17). Epub 2021/09/11. doi: 10.3390/ijms22179522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usui TIY, Tagami T, Matsuda K, Moriyama K, Yamada K, Kuzuya H, Kohno S, Shimatsu A. <The phytochemical lindleyin isolate Source J Endocrinol SO 2002 Nov 175 2 289 96.PDF>. J Endocrinol. 2002;175(2):289–96. doi: 10.1677/joe.0.1750289 [DOI] [PubMed] [Google Scholar]
- 16.Liang Z, Ren C. Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed Pharmacother. 2018;103:897–902. Epub 2018/05/02. doi: 10.1016/j.biopha.2018.04.085 . [DOI] [PubMed] [Google Scholar]
- 17.Wen Q M J, Lau N., Zhang C., Ye P., Du S., Mei L., Weng H., Xu Q., Liu X., Chen D., Zhang F., Li C., Li H. Rhein attenuates lipopolysaccharide-primed inflammation through NF-kappaB inhibition in RAW264.7 cells: targeting the PPAR-gamma signal pathway. Can J Physiol Pharmacol. 2020;98(6):357–65. doi: 10.1139/cjpp-2019-0389 [DOI] [PubMed] [Google Scholar]
- 18.Zhu T, Zhang W, Feng SJ, Yu HP. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARgamma-dependent pathway. Int Immunopharmacol. 2016;34:16–24. Epub 2016/02/26. doi: 10.1016/j.intimp.2016.02.014 . [DOI] [PubMed] [Google Scholar]
- 19.Su S, Wu J, Gao Y, Luo Y, Yang D, Wang P. The pharmacological properties of chrysophanol, the recent advances. Biomed Pharmacother. 2020;125:110002. Epub 2020/02/18. doi: 10.1016/j.biopha.2020.110002 . [DOI] [PubMed] [Google Scholar]
- 20.Qu J, Zhang T, Liu J, Su Y, Wang H. Considerations for the Quality Control of Newly Registered Traditional Chinese Medicine in China: A Review. J AOAC Int. 2019;102(3):689–694. Epub 2018/11/02. doi: 10.5740/jaoacint.18-0301 . [DOI] [PubMed] [Google Scholar]
- 21.Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013; 341(2): 139–49. Epub 2013/08/17. doi: 10.1016/j.canlet.2013.08.023 . [DOI] [PubMed] [Google Scholar]
- 22.Ge H, Tang H, Liang Y, Wu J, Yang Q, Zeng L, et al. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017; 11: 1663–1671. Epub 2017/06/06. doi: 10.2147/DDDT.S133069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye B, Chen X, Dai S, Han J, Liang X, Lin S, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019; 25(13): 975–990. Epub 2019/03/25. doi: 10.2147/DDDT.S195412 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu T, Zhang W, Feng SJ, Yu HP. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int Immunopharmacol. 2016; 34: 16–24. Epub 2016/02/22. doi: 10.1016/j.intimp.2016.02.014 . [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Gao C, Vong CT, Tao H, Li H, Wang S, et al. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br J Pharmacol. 2022; 179(9): 1978–1997. Epub 2022/01/06. doi: 10.1111/bph.15773 . [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Wang L, Wu X, Gao K, Wang F, Cui J, et al. Rhein protects 5/6 nephrectomized rat against renal injury by reducing inflammation via NF-κB signaling. Int Urol Nephrol. 2021; 53(7): 1473–1482. Epub 2021/03/25. doi: 10.1007/s11255-020-02739-w . [DOI] [PubMed] [Google Scholar]
- 27.Zhuang S, Zhong J, Zhou Q, Zhong Y, Liu P, Liu Z. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int Immunopharmacol. 2019; 71: 321–327. Epub 2019/04/02. doi: 10.1016/j.intimp.2019.03.030 . [DOI] [PubMed] [Google Scholar]
- 28.Sun M, Wu H, He M, Jia Y, Wang L, Liu T, et al. Integrated assessment of medicinal rhubarb by combination of delayed luminescence and HPLC fingerprint with emphasized on bioactivities based quality control. Chin Med. 2020;15:72. Epub 2020/07/21. doi: 10.1186/s13020-020-00352-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y H X, Liang QH, Fan R, Qin F, Guo Y, Yan KP, Liu W, Luo JK, Li YH, Mao XL, Liu ZQ, Zhou HH. A strategy for detecting absorbed bioactive compounds for quality control in the water extract of rhubarb by ultra performance liquid chromatography with photodiode array detector. Chin J Integr Med. 2012;18(9):690–8. doi: 10.1007/s11655-012-1053-7 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Liu H, Qin L, Zhang Z, Wang Q, Zhang Q, et al. Global chemical profiling based quality evaluation approach of rhubarb using ultra performance liquid chromatography with tandem quadrupole time-of-flight mass spectrometry. J Sep Sci. 2015;38(3):511–22. Epub 2014/12/06. doi: 10.1002/jssc.201400971 . [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Li H, Jin C, Qu Y, Xiao X. Development and validation of a UPLC method for quality control of rhubarb-based medicine: fast simultaneous determination of five anthraquinone derivatives. J Pharm Biomed Anal. 2008;47(4–5):765–70. Epub 2008/04/29. doi: 10.1016/j.jpba.2008.03.011 . [DOI] [PubMed] [Google Scholar]
- 32.Klein-Junior LC, de Souza MR, Viaene J, Bresolin TMB, de Gasper AL, Henriques AT, et al. Quality Control of Herbal Medicines: From Traditional Techniques to State-of-the-art Approaches. Planta Med. 2021;87(12–13):964–88. Epub 2021/08/20. doi: 10.1055/a-1529-8339 . [DOI] [PubMed] [Google Scholar]
- 33.Song X-Y, Li Y-D, Shi Y-P, Jin L, Chen J. Quality control of traditional Chinese medicines: a review. Chinese Journal of Natural Medicines. 2013;11(6):596–607. doi: 10.1016/S1875-5364(13)60069-2 [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Zhang Y, Liu Z, Wang X, He J, Jin H. Advanced applications of mass spectrometry imaging technology in quality control and safety assessments of traditional Chinese medicines. Journal of Ethnopharmacology. 2022;284. doi: 10.1016/j.jep.2021.114760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Geng L, Liu Y, Chen M, Mu Q, Zhang X, et al. Identification of three Daphne species by DNA barcoding and HPLC fingerprint analysis. PLoS One. 2018;13(8):e0201711. Epub 2018/08/03. doi: 10.1371/journal.pone.0201711 Pass (Qinhuangdao 066200, China) which provided great financial support. The funder did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This commercial affiliation does not alter our adherence to all PLOS ONE policies on sharing data and materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javier-Astete R, Jimenez-Davalos J, Zolla G. Determination of hemicellulose, cellulose, holocellulose and lignin content using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. and Guazuma crinita Lam. PLoS One. 2021;16(10):e0256559. Epub 2021/10/28. doi: 10.1371/journal.pone.0256559 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Z, Wang Z, Shang Z, Zhao J. Classification and identification of Rhodobryum roseum Limpr. and its adulterants based on fourier-transform infrared spectroscopy (FTIR) and chemometrics. PLoS One. 2017;12(2):e0172359. Epub 2017/02/17. doi: 10.1371/journal.pone.0172359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta T, Uto T, Tanaka H. Effective methods for increasing coumestrol in soybean sprouts. PLoS One. 2021;16(11):e0260147. Epub 2021/11/19. doi: 10.1371/journal.pone.0260147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Li W, Han LF, Song XB, Li PF, Wang R, et al. Identification of Chinese Traditional Medicine Cistanches Herba from Different Places by HPLC-ESI-MS and FTIR Methods. Guang Pu Xue Yu Guang Pu Fen Xi. 2015; 35(4):1056–61. . [PubMed] [Google Scholar]
- 40.Xiong F, Nie X, Zhao X, Yang L, Zhou G. Effects of different nitrogen fertilizer levels on growth and active compounds of rhubarb from Qinghai plateau. J Sci Food Agric. 2019;99(6):2874–82. Epub 2018/11/22. doi: 10.1002/jsfa.9500 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.