Abstract
Paneth cells in crypts of the small intestine express antimicrobial peptides, including α-defensins, termed cryptdins in mice. Of the known Paneth cell α-defensins, the cryptdin 4 gene is unique, because it is inactive in the duodenum and expressed at maximal levels in the distal small bowel (D. Darmoul and A. J. Ouellette, Am. J. Physiol. 271:G68–G74, 1996). With a cryptdin 4-specific antibody, immunohistochemical staining of ileal Paneth cells was strong and specific for cytoplasmic granules, demonstrating that this microbicidal peptide is a secretory product of Paneth cells in the distal small intestine. Consistent with the pattern of cryptdin 4 mRNA distribution along the length of the gut, the cryptdin 4 peptide was not detected in duodenum. Structurally, the cryptdin 4 gene resembles other Paneth cell α-defensin genes. Its two exons, transcriptional start site, intron, splice sites, and 3′ flanking sequences are characteristic of the highly conserved mouse α-defensin genes. However, in the region upstream of the transcriptional initiation site, the cryptdin 4 gene contains a repeated 130-bp element that is unique to this α-defensin gene. Every independent cryptdin 4 genomic clone examined carries the repeated element, which contains putative recognition sequences for TF-IID-EIIA, cMyc-RS-1, and IgHC.2/CuE1.1; the repeat proximal to the start of transcription replaces DNA at the corresponding position in other mouse α-defensin genes. We speculate that this unique duplicated element may have a cis-acting regulatory role in the positional specificity of cryptdin 4 gene expression.
Located in the crypts of Lieberkühn, Paneth cells synthesize and release proteinaceous granules into the lumen of the small bowel. These secretory granules are rich in antimicrobial peptides and proteins, supporting the hypothesis that these cells contribute to innate immunity of the intestinal mucosa (20). Paneth cell α-defensins, also termed cryptdins, are gene-encoded antibiotic peptides expressed by this epithelial lineage, and mouse and human Paneth cells contain high levels of these peptides and their corresponding mRNAs (15, 16, 18). Six cryptdin peptide variants have been purified from mouse small intestine (7, 26), and five of those isoforms, cryptdins 1 to 3, 5, and 6, are coded by separate genes clustered in the proximal region of chromosome 8 (18). Transcripts of cryptdin genes are approximately 1 kb in length and coded by two-exon genes in which the first exon codes for the preprosegment of the precursor and the second exon codes for the mature α-defensin peptide (14). Human Paneth cells express two α-defensin genes, HD-5 and HD-6 (2, 15, 16), but the mouse α-defensin gene family is larger, coding for at least 19 different cryptdin isoforms (18).
At the level of both the peptide and the gene, cryptdin 4 has several features that distinguish it from the known Paneth cell α-defensins. For example, the peptide has the highest antimicrobial activity of the known mouse enteric α-defensins in in vitro assays (18), and the cryptdin 4 gene has a unique pattern of expression along the longitudinal axis of the small intestine (5). In contrast to cryptdins 1 and 5, whose mRNAs occur at approximately equivalent levels along the length of the mouse small bowel, cryptdin 4 mRNA levels increase along the proximal-to-distal axis to reach maximal concentration in the distal ileum. To test for potential structural differences correlating with differential cryptdin 4 gene activity, DNA sequences up-stream of mouse Paneth cell α-defensin gene transcription initiation sites have been analyzed and compared.
Here, we demonstrate that the cryptdin 4 peptide is a Paneth cell granule constituent, and thus is secreted into the lumen, and that peptide distribution corresponds to the increasing cryptdin 4 mRNA concentration along the length of the gut. Also we report on the structure of the 129/SVJ mouse cryptdin 4 gene and show that its two exons are identical in sequence to cryptdin 4 cDNAs from intestinal crypts of C3H/HeJ and outbred Swiss mice. The region just upstream of the cryptdin 4 gene transcriptional initiation site contains a 130-bp repeated element that does not occur in other known mouse α-defensin genes.
(This work was presented in preliminary form at the Annual Meeting of the American Gastroenterological Association, Washington, D.C., May 1997.)
MATERIALS AND METHODS
Solid-phase synthesis of cryptdin 4.
The cryptdin 4 peptide chain was assembled on a Millipore 9050 automated continuous flow peptide synthesizer by sodium 9-fluorenylmethoxycarbonyl methodology (3). Amino acids were activated in situ with N,N-diisopropylcarboxydiimide–N,N-diisopropylethylamine–1-hydroxybenzotriazole chemistry (27), and the coupling yield was monitored on line with quinoline yellow (29). The synthetic peptide was released from the polyethylene glycol-polystyrene resin support by treatment with trifluoroacetic acid-water-phenol-triisopropylsilane (88:5:5:2, reagent B [27]) for 6 h at 20°C and filtered. The peptide solution was adjusted with acetic acid (HOAc) to a final concentration of 30%, extracted three times with equal volumes of dichloromethane, diluted fivefold with H2O, and lyophilized. The lyophilized peptide was dissolved in 10% HOAc and lyophilized again prior to biochemical analysis.
Approximately 100 mg of crude synthetic peptide was linearized by reduction for 8 h at 60°C with a solution containing 100 mM dithiothreitol in 6 M guanidine HCl, 200 mM Tris-Cl, and 2 mM EDTA (pH 8.2) and acidified to 30% HOAc. The reduced, acidified synthetic peptide was dialyzed stepwise against 4 liters each of the following aqueous solutions: 30% HOAc for 24 h; 15% HOAc for 24 h; and 1% HOAc for 24 h. The peptide was refolded in 1% sodium acetate (pH 8.0) containing glutathione (2 mol of oxidized glutathione and 1 mol of reduced glutathione per 20 cysteine-SH molar equivalents). The folded peptide was purified to homogeneity by successive reverse-phase high-pressure liquid chromatography (RP-HPLC) with Vydac C-18 columns (diameters, 10 and 22 mm; length, 250 mm) developed with linear gradients of water-acetonitrile containing 0.1% trifluoroacetic acid or 0.13% heptafluorobutyric acid. Peak resolution was optimized for each round of purification by varying the water-to-acetonitrile gradient from 1 to 0.25% per min.
Preparation of anti-cryptdin 4 antibody.
A peptide-specific polyclonal rabbit antibody to the synthetic, folded cryptdin 4 was prepared. A sample (4 mg) of cryptdin 4 conjugated to ovalbumin was used to immunize two New Zealand White rabbits (Zymed Laboratories, Inc., San Francisco, Calif.). Serum samples were collected for 8 to 12 weeks, until the anti-cryptdin 4 titer, determined by enzyme-linked immunosorbent assay, reached 1:10,000 for each rabbit. Immunoglobulin G (IgG) was isolated from antiserum by DEAE Econo-Pac chromatography (Bio-Rad, Richmond, Calif.) as described by the manufacturer. Peptide specificity of the antibody was determined by assessing immunoreactivity against cryptdins 1 to 6 immobilized on polyvinylidene difluoride and nitrocellulose membranes, with the antibody demonstrating reactivity only with cryptdin 4.
Immunohistochemical detection of cryptdin 4.
Paraffin sections of formalin-fixed mouse small bowel were deparaffinized with xylenes, treated for 30 min with 0.3% H2O2, and washed extensively with water and then with phosphate-buffered saline (PBS). Slides were incubated three times for 5 min each in the microwave oven with antigen unmasking solution (Vector Laboratories, Inc., Burlingame, Calif.) and then cooled in unmasking solution (Vector Laboratories, Inc.) for 30 min at room temperature. After being rinsed with PBS, the sections were blocked by incubation with normal goat serum for 30 min and with Avidin D blocking solution for 15 min, rinsed briefly with PBS, and then incubated with biotin blocking solution (Vector Laboratories, Inc.) for 15 min. Slides were incubated with a 1:50 dilution of rabbit anti-cryptdin 4 IgG, a 1:10 dilution of anti-cryptdin 4 IgG in 1× PBS preabsorbed at 4°C overnight with 80 μg of synthetic, folded cryptdin 4, or with IgG prepared from serum of rabbits prior to immunization. After 30 min, slides were washed three times with PBS, incubated for 30 min with a 1:200 dilution of biotinylated goat anti-rabbit IgG, and washed as before. After 60 min of incubation with Vectastain ABC peroxidase reagent (Vector Laboratories, Inc.), slides were washed and flooded for 3 min with diaminobenzidine as a peroxidase substrate, washed, counterstained with hematoxylin, cleaned, and mounted.
Cryptdin 4 gene isolation.
Bacteriophages containing cryptdin 4 gene sequences were identified in a 129/SVJ mouse genomic library in λ DASH II (Stratagene Cloning Systems, La Jolla, Calif.). Phage plates (1.1 × 105 PFU/dish) were screened in duplicate by hybridization of filter lifts with oligonucleotide cryp4 (5′-CGGCG GGGGC AGCAG TA-3′), the reverse complement of nucleotides 259 to 275 in cryptdin 4 cDNA, labeled with [γ-32P]ATP by using polynucleotide kinase (Promega Corporation, Madison, Wis.). Conditions for hybridization and washing of filters have been described previously (5). Subsequently, a mixed probe for putative cryptdin gene promoter sequences was prepared by amplification of mouse genomic DNA (30 cycles of 94°C for 40 s, 60°C for 40 s, and 72°C for 40 s) with oligonucleotide Defcrm150 (5′-GCTGC TCCTC AGTAT TAGTC TCTT-3′) paired with sense strand primer CIA7 (5′-TAAAT (A/G)(C/T)TCA AGCAG ATGG-3′). Defcrm150 primes on the antisense strand of all known cryptdin cDNAs near the midpoint of exon 1, and the CIA7 priming site is conserved in several mouse Paneth cell α-defensin genes between residues −216 and −234 relative to the transcription start site (13a). This 384-bp PCR product was used to screen Southern blots of genomic clones to identify isolates containing sequences upstream of the transcription start site of this gene.
DNA sequencing and analysis.
Denatured plasmid DNAs from subcloned fragments of genomic clones were sequenced by dideoxy nucleotide termination (23) with modified Sequenase II (United States Biochemical Corp., Cleveland, Ohio). Computations for similarity searches of DNA sequences in nonredundant nucleic acid and protein sequence databases were performed at the National Center for Biotechnology Information by using the BLAST network service (1, 12). DNA sequences were analyzed by using programs in the University of Wisconsin Genetics Computer Group suite (6) as well as routines in the MacVector version 4.5.3 program (Eastman Kodak Company, Rochester, N.Y.).
Nucleotide sequence accession numbers.
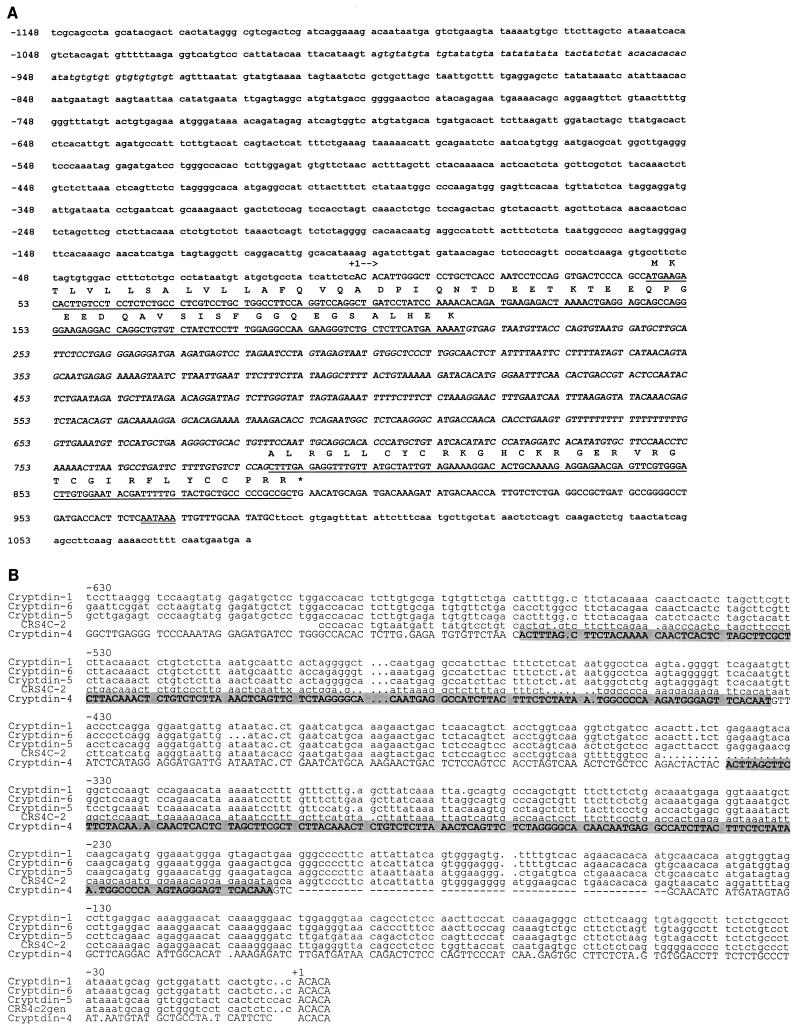
The GenBank accession number for the cryptdin 4 gene sequence (Fig. 4A) is AF178040. Those for the 5′ UTRs of cyrptdin 1, cryptdin 5, cryptdin 6, and CRS4C-2 (Fig. 4B) are AF178043, AF178042, AF178041, and AF178044, respectively.
FIG. 4.
Nucleotide sequence and structure of the mouse cryptdin 4 gene. (A) Overlapping fragments of cryptdin 4 genomic clones were sequenced as described above (see Materials and Methods). The transcribed sequence is depicted in uppercase characters; the sequence coding for the deduced cryptdin 4 precursor is underlined; the intron, determined by sequence alignment with cryptdin 4 cDNA (18), is shown in uppercase italics; and untranscribed sequences in the 5′ and 3′ flanking regions of the gene are shown in lowercase. The deduced amino acid sequence of the cryptdin 4 precursor is shown in uppercase, single-letter notation above the coding regions, with an asterisk denoting the termination codon. The deduced polyadenylation start site is shown as double underlined characters, beginning 21 nucleotides from the transcription termination site. Nucleotide residue positions are numbered with the first nucleotide of the transcription start site assigned residue position +1. (B) Alignment of 5′ upstream sequences from mouse α-defensin family genes. Sequences corresponding to nucleotide positions −630 to +5 in four mouse cryptdin genes (14) and in the related CRS4C-2 gene (13) were aligned with the UWGCG program Pileup (6) by using a gap weight of 5.00 and a gap length weight of 0.30. Dot characters (.) in lines of sequence were introduced by the program to maximize the alignment score. The cryptdin 4 nucleotide sequence is shown in uppercase type with a tandemly repeated element denoted as shaded text. Residue positions are numbered in relation to the transcriptional start site, designated to be position +1.
RESULTS AND DISCUSSION
Analysis of synthetic cryptdin 4.
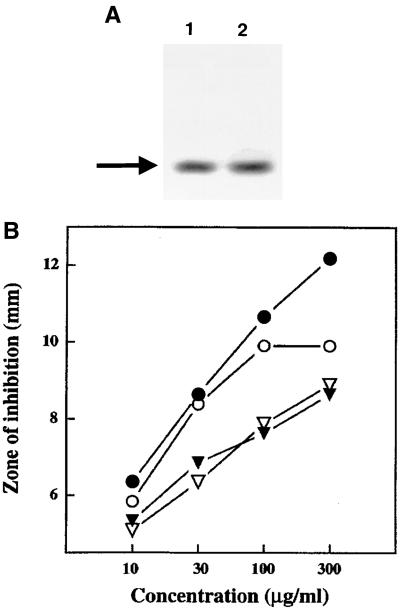
The cryptdin 4 peptide (GLLCY CRKGH CKRGE RVRGT CGIRF LYCCP RR), isolated previously from full-length, full-thickness mouse small intestine (26), is the most potent of the known mouse Paneth cell α-defensins (18). Because the overall recovery of cryptdin 4 from intestinal extracts is low relative to that of cryptdins 1, 2, 5, and 6, which are expressed along the entire length of the small bowel, the peptide was synthesized to obtain sufficient quantities of peptide for biochemical analysis and antibody production (see Materials and Methods). The overall peptide yield was estimated at 4.1% with a recovery of 35 mg of RP-HPLC-purified, folded cryptdin 4 recovered from 860 mg of crude peptide. The purified synthetic cryptdin 4 peptide was characterized by mass spectroscopy, amino acid analysis, and analytical RP-HPLC and shown to be identical to the natural cryptdin 4 reference peptide in all respects (data not shown). In acid-urea polyacrylamide gel electrophoresis (AU-PAGE), a system that provides high resolution of the protonated forms of defensins and small basic peptides (21, 25), the synthetic and natural cryptdin 4 peptides had identical mobilities (Fig. 1A). As may be predicted from their identical biochemical properties, synthetic and natural cryptdin 4 were functionally equivalent in assays of antimicrobial activity against Escherichia coli ML35 and Staphylococcus aureus (Fig. 1B). Therefore, this synthetic cryptdin 4 peptide was used for preparation of antiserum to analyze the tissue distribution of cryptdin 4 peptide in Paneth cells along the length of the mouse small intestine.
FIG. 1.
Characterization of synthetic cryptdin 4. Panel A, AU-PAGE of natural (lane 1) and synthetic (lane 2) cryptdin 4. Samples (1 μg) of peptide dissolved in 2% acetic acid were electrophoresed for 6 h at 200 V in 12.5% polyacrylamide gels as described previously (25) with migration toward the cathode proceeding from top to bottom. The gel was stained with Coomassie blue as described previously (24). Panel B, antimicrobial activities of natural and synthetic cryptdin 4. The combined bactericidal and bacteriostatic activities of the peptides were evaluated by a radial diffusion assay (see Materials and Methods [18]). The similarity of zones of bacterial growth inhibition obtained with the corresponding natural or synthetic peptides shows that they are functionally equivalent. Symbols: ○, E. coli exposed to natural cryptdin 4; ●, E. coli exposed to synthetic cryptdin 4; ▿, S. aureus exposed to natural cryptdin 4; ▾, S. aureus exposed to synthetic cryptdin 4.
Localization of cryptdin 4 in mouse small intestine.
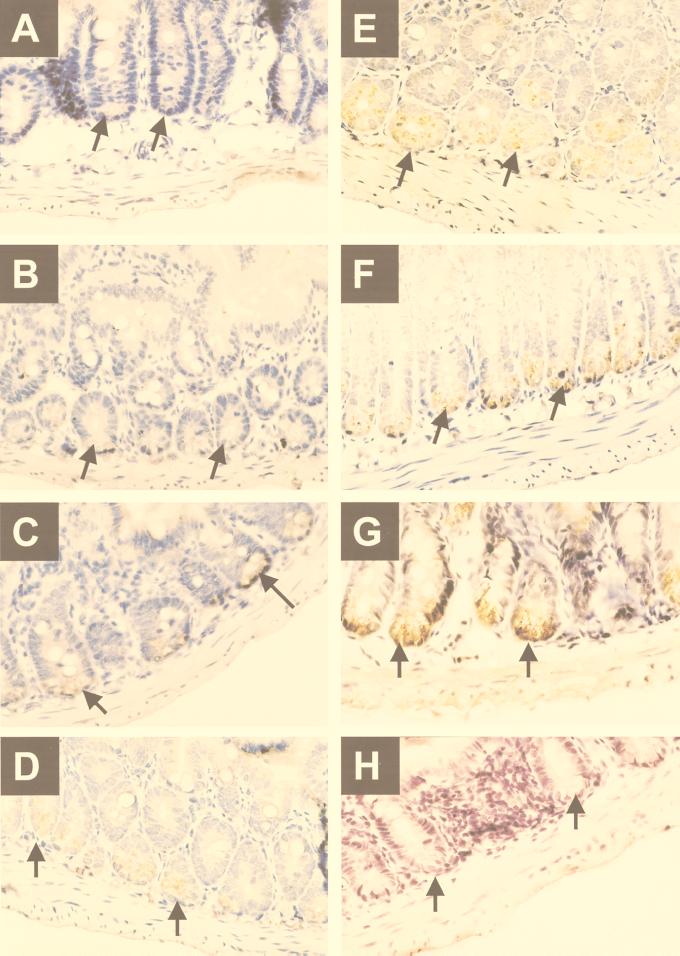
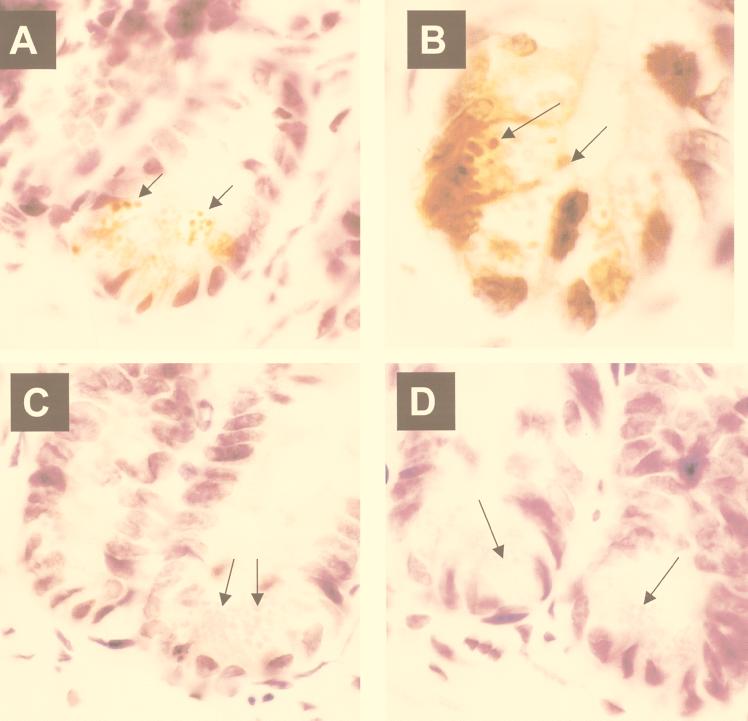
In the small bowel, cryptdin 4 gene expression is restricted to Paneth cells, and the highest levels of peptide occur in the distal regions of the organ. Immunoperoxidase staining of full-thickness sections of mouse small intestine with anti-cryptdin 4 IgG failed to identify the peptide in the duodenum (Fig. 2A), showed intermediate levels along the jejunum (Fig. 2B to D), and demonstrated maximal levels in terminal ileum (Fig. 2G). In contrast to these results, Paneth cells in duodenum are strongly immunoreactive with the anti-cryptdin 1 antibody (26). Cryptdin 4 is present in Paneth cells at the base of every ileal crypt (Fig. 2E to G). When sections of ileum stained with the antibody were viewed at higher magnification, the cryptdin 4 antigen was found to be concentrated within Paneth cell secretory granules (Fig. 3A and B). Thus, as with the known enteric α-defensins, cryptdin 4 is a constituent of secretory granules and is therefore released into the crypt lumen.
FIG. 2.
Immunohistochemical localization of cryptdin 4 peptide in Paneth cells of distal mouse small intestine. Sections of adult mouse duodenum (A), jejunum (B through D), and ileum (E through G) were analyzed for the presence of the cryptdin 4 peptide, by using purified IgG from a polyclonal rabbit cryptdin 4-specific antibody (see Materials and Methods). In each panel, arrows indicate immunoperoxidase localization in Paneth cells of the crypts. Magnification, ×40. IgG prepared from preimmune serum is negative (H). Note that duodenal crypts are negative, jejunal crypts are modestly positive, and ileal crypts are strongly immunoreactive.
FIG. 3.
Cryptdin 4 in Paneth cell secretory granules. As described for Fig. 2, sections of adult mouse ileum were incubated with IgG prepared from polyclonal rabbit cryptdin 4-specific antiserum (A and B), with preimmune serum (C), or with immune IgG preadsorbed with 80 μg of synthetic cryptdin 4 (D). Magnification, ×100. In panels A and B, the arrows indicate secretory granules in Paneth cells that are strongly immunoreactive; in panels C and D, arrows indicate eosinophilic granules that are immunoperoxidase negative after exposure to preimmune and preadsorbed IgG controls, respectively.
The anti-cryptdin 4 antibody used in these experiments is specific for the cryptdin 4 isoform, and it does not cross-react with cryptdins 1 to 3, or cryptdin 5 or 6 (data not shown). No immunoperoxidase activity is visible in slides treated with preimmune IgG or IgG preabsorbed with antigen as a negative control (Fig. 3C and D). Leukocytes in the lamina propria of the villi also were negative, showing that all the peptide in the small bowel accumulates in the epithelial compartment. A rabbit polyclonal antibody to synthetic cryptdin 1 displays comparable specificity (26). That antibody recognizes the mouse cryptdins 1 to 3 and 6, four cryptdin 1-like isoforms, but it does not detect cryptdin 4 or 5, nor does it stain rat or human Paneth cells. The immunolocalization results (Fig. 2 and 3) correlate well with the previous amplification and cloning of cryptdin 4 cDNA from isolated intestinal crypts (5, 18) and are consistent with the strong positive signals obtained by in situ hybridization of Paneth cells with α-defensin probes (9, 15, 17) and with the detection of other enteric α-defensins in mouse and human cells (22, 26).
Cryptdin 4 gene structure.
The structural features of the cryptdin 4 gene are conserved relative to those of known mouse and human Paneth cell α-defensin genes (Fig. 4 [14–16]). Bacteriophage clones in a 129/SVJ mouse genomic library were identified by hybridization with the cryp4 oligonucleotide (4, 5), and several isolates contained a positive, 2-kb EcoRI fragment that contained the complete coding sequence for the cryptdin 4 precursor (see Materials and Methods). DNA sequencing of those clones showed that the cryptdin 4 gene primary transcript is 987 nucleotides long and contains two exons (Fig. 4A). Exon 1 contains a 45-bp 5′ untranslated region and sequence coding for the preprosegment of the precursor; exon 2 codes for the mature cryptdin 4 peptide and contains approximately 100 bp of 3′ untranslated sequence (Fig. 4A). The two cryptdin 4 exons of the 129/SVJ mouse are identical to cryptdin 4 cDNA from C3H/HeJ and outbred Swiss mice (18). The sequences upstream of transcriptional start sites of mouse cryptdin genes are conserved (Fig. 4B). A multiple sequence alignment produced by the program Pileup (6) illustrates the extensive sequence similarity between the 5′ upstream regions of the cryptdin 1, 4, 5, and 6 genes and also with the defensin-related mouse CRS4C-2 (13) gene (Fig. 4B). Also, cryptdin 4-coding genomic sequences from 129/SVJ (Fig. 4) and C3H/HeJ, BALB/cJ, and outbred Swiss mice are identical (data not shown), as are cryptdin 4 cDNAs from outbred Swiss and several inbred strains of mice (20a).
Detection of new cryptdin 4 isoform.
The mouse cryptdin 1-like α-defensin subfamily has undergone gene duplication events to generate many peptide isoforms in Mus musculus (14, 18). However, evidence of cryptdin 4 isoforms has not been found in outbred Swiss or in inbred strains of mice. Studies of cryptdin 4-coding sequences amplified from genomic DNA of wild-derived strains revealed that the Mus czech II/Ei genome codes for the following deduced cryptdin 4 peptide variant: DLTCYCRKGHCKRGGRVRGTCGIRFLYCCPRR (replaced amino acids are indicated by italics), compared to GLLCYCRKGHCKRGERVRGTCGIRFLYCCPRR, the primary structure of cryptdin 4 isolated from M. musculus domesticus. To our knowledge, the M. czech II/Ei cryptdin 4 variant, with D1G, T3L, and G15E amino acid replacements, represents the first evidence of evolutionary diversity of this particular α-defensin gene in mice.
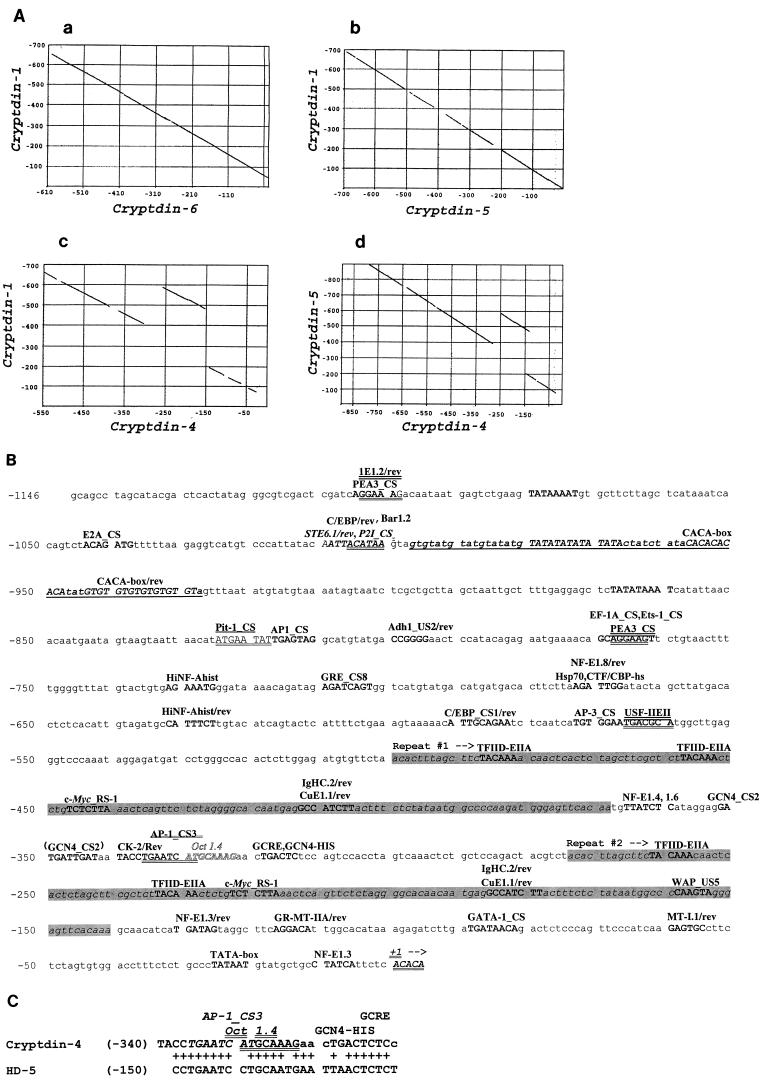
Tandemly repeated element specific to the cryptdin 4 gene.
Dot matrix alignments of sequences corresponding to 600 bp 5′ of the transcriptional start sites of the cryptdin 1 gene and the cryptdin 5 and 6 genes produce continuous diagonal lines, indicative of their 95% nucleotide sequence identities (Fig. 5Aa and Ab). However, a similar comparison of the cryptdin 1 and cryptdin 5 genes with the cryptdin 4 gene sequence showed that the 5′ flanking DNA of the cryptdin 4 gene contains a distinctive, tandemly repeated element (Fig. 5Ac and 5d). The alignment of the cryptdin 1 and cryptdin 4 upstream regions becomes discontinuous near nucleotide position −300 (Fig. 5Ac), indicative of a direct repeat in the region upstream of the cryptdin 4 gene transcriptional start site. We infer that the second repeat and the 70 bp adjacent to its 3′ end, i.e., nucleotides −270 to −70, have replaced DNA present in other mouse Paneth cell α-defensin genes, because the corresponding regions (nucleotides −270 to −70) of the cryptdin 1, 5, and 6 genes lack similarity with the cryptdin 4 repeated element (data not shown). The tandem repeats, located from nucleotides −495 to −365 and −270 to −140 (Fig. 5B) are nearly identical in sequence, except that the element distal to the initiation site has a 3-bp gap (data not shown). The role of this duplicated element and the doubling of certain putative transcription factor binding sites in the regulation of cryptdin 4 gene activity remains to be determined, but to our knowledge, no known α-defensin genes contain such a structural feature.
FIG. 5.
A repeated sequence element upstream of the cryptdin 4 gene transcriptional start site. (A) DNA sequences in the region from positions +1 to −700 upstream of the cryptdin genes shown were compared by the Pustell dot matrix alignment routine in the MacVector suite (see Materials and Methods). In contrast to comparisons of cryptdins 1 and 6 (a) as well as of cryptdins 1 and 5 (b), which were colinear and nearly identical, alignment comparisons involving the cryptdin 4 gene revealed a discontinuity in the sequence alignment that is indicative of a tandem repeat (c and d). (B) Potential transcription factor recognition sites (10, 11, 28) in the region upstream of the cryptdin 4 transcriptional start site are indicated in uppercase type and identified above lines of nucleotide sequence. The duplicated element described for panel A, from nucleotides −494 to −367 and −269 to −140, is indicated by shaded text. (C) The only region of similarity between the 1.2 kb of nucleotide sequence 5′ of the mouse cryptdin 4 and human HD-5 Paneth cell α-defensin gene transcription start sites is shown. Nucleotide sequences from the 5′ untranscribed regions of cryptdin 4 and HD-5 genes were aligned using the program Bestfit. Pluses between lines of DNA sequence denote identical nucleotide positions. Conserved potential DNA recognition sequences for AP-1 (italics), Oct 1.4 (double underlining), and GCRE/GCN4-HIS (single underlining) transcription factors are indicated.
Sequences upstream of the 129/SVJ mouse cryptdin 4 gene were compared with those of mouse and human Paneth cell α-defensin genes to identify putative transcriptional regulatory sites near the transcription initiation site. Because the 2.0-kb EcoRI cryptdin 4 gene DNA fragment contained only 230 bp of 5′ upstream sequence, clones with additional DNA upstream of the transcriptional start site were isolated with a hybridization probe consisting of nucleotides −225 to +150 of the cryptdin 4 gene. Subcloned restriction fragments were sequenced, revealing the presence of several consensus transcription factor binding sites within 1.15 kb of the transcriptional start site, including TFIID-EIIA, Oct 1.4, AP-1, GATA-1, NF-E1, PEA3, GRE, and C/EBP sites of potential regulatory importance (Fig. 5B). Also, between the nucleotides at positions −1000 and −930 relative to the transcriptional start site, the gene contains a continuous tract of alternating purines and pyrimidines, a motif known to modulate transcription by introducing Z-DNA structures (Fig. 5B). Comparisons of the regions consisting of 1.15 kb immediately upstream of the mouse cryptdin 4 and human HD-5 α-defensin genes by Bestfit (6) and by Pustell dot matrix alignment analyses (see Materials and Methods) showed that the genes lack overall similarity, but between nucleotides −340 and −310, they share putative AP-1, Oct-1.4, and GCRE DNA binding sites (Fig. 5C). The involvement of these apparent recognition sequences in regulating transcription of the cryptdin 4 gene or of any Paneth cell α-defensin gene is not known, because cell lines that express constructs of reporter transgenes under the control of putative cryptdin gene promoters are not available. Interestingly, a mouse line transgenic for an HD-5 minigene construct expresses the human gene appropriately in small intestinal crypts (14a), providing preliminary evidence that human and mouse Paneth cell α-defensin gene promoters may contain conserved cis-acting elements. At present, however, the lack of systems for functional analysis of Paneth cell-specific gene promoters precludes definition of the role of those putative recognition sequences.
The repeated upstream element was found in every cryptdin 4 genomic clone isolated. To confirm that the cryptdin 4 gene containing the tandemly repeated element was representative of the functional gene, eight cryptdin 4 genomic clones, judged to be independent isolates on the basis of their distinctive restriction patterns, were tested for the presence of the repeated element. The region comprising the repeated elements was amplified with oligonucleotides Cr4ut630, corresponding to residue positions −585 to −561 on the sense strand (5′-GAATC TCAAT CATGT GGAAT GACG-3′) paired with Cr4ut110, corresponding to nucleotides −125 to −110 on the antisense strand (5′-ATGTC CTGAA GCCTA CTATC ATGAT-3′), by using 35 cycles at 94°C for 40 s, 55°C for 40 s, and 72°C for 40 s. One genomic clone, phage D (ϕD), contained only 223 bp upstream of the transcriptional start site and provided a negative control, since it lacks the upstream priming site. DNA corresponding to the region containing the repeat was amplified, and samples of each reaction were electrophoresed to distinguish between PCR fragments indicative of two (473 bp) or one (345 bp) copy of the repeated element (data not shown). The duplicated element was detected in every clone, except for the ϕD negative control, and restriction analyses confirmed that the 473-bp PCR fragment contained two repeats and therefore that all cryptdin 4 genomic isolates contained the tandemly repeated DNA (data not shown).
The cryptdin 4 gene shares the general features of the two-exon Paneth cell α-defensin genes, and comparisons of mouse cryptdin gene sequences upstream of their highly conserved transcriptional start site (13, 14) revealed extensive similarities but also the presence of a repeated element unique to the cryptdin 4 gene (Fig. 5 and data not shown). The molecular mechanism(s) regulating the distinctive, proximal-to-distal pattern of cryptdin 4 gene expression (5) remains unexplained by these nucleotide sequence comparisons. In addition, seven pedigrees of mice transgenic for 1.15 kb of cryptdin 4 gene 5′ upstream DNA with a luciferase promoter failed to express the transgene in the small intestine (4a). Perhaps a silencer element(s) outside of the region analyzed is involved in inactivating the gene in the proximal intestine. Since the cryptdin 4 gene intron has pyrimidine-rich islands and an oligo(T)18 tract not found in introns of other cryptdin genes (data not shown), we cannot exclude the possibility that the intron may have regulatory importance. Consensus transcription factor recognition sequences (10, 11, 28) that are associated with tissue-specific gene expression in other systems (8) were found both within the intron and upstream of the cryptdin 4 gene transcriptional start site.
The recovery of cryptdin 4 from extracts of full-length, full-thickness mouse small bowel is low relative to that for cryptdins 1, 2, 5, and 6 (19), and therefore it was necessary to exclude the possibility that the peptide might be released along a constitutive basolateral route rather than trafficking along the exocytic pathway for secretion. Experiments showing that the peptide is an abundant constituent of ileal Paneth cell secretory granules (Fig. 2 and 3) both demonstrated that cryptdin 4 is secreted apically and confirmed the increasing proximal-to-distal distribution of the peptide as predicted by genetic evidence (5). Two additional lines of evidence support the conclusion that cryptdin 4 is secreted into the crypt lumen. First, cryptdin 4 and a modified (des-Gly)-cryptdin 4 variant have been isolated from rinses of the mouse small intestinal lumen (19). Second, by using the cryptdin 4 antibody (Fig. 2 and 3) for enzyme-linked immunosorbent assay and Western blot analysis, cryptdin 4 has been detected in secretions collected from isolated crypts stimulated to degranulate with carbamyl choline (1a). Collectively, these findings implicate this position-specific, enteric α-defensin in mucosal innate immunity.
ACKNOWLEDGMENTS
This work was supported by NIH grants DK44632 and DK33506 (A.J.O.) and by NIH grant AI 22931 and Biosource Technologies, Inc. (M.E.S.).
We thank Robert S. Akeson, Dana Frederick, Robin Huffman, Joseph S. Piraino, and Hong Yang for excellent technical assistance.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1991;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 1a.Ayabe, T., D. P. Satchell, M. E. Selsted, C. L. Wilson, and A. J. Ouellette. Unpublished results.
- 2.Bevins C L. Antimicrobial peptides as agents of mucosal immunity. In: Marsh J, Goode J A, editors. Antimicrobial peptides. Chichester, England: John Wiley & Sons; 1994. pp. 250–269. [DOI] [PubMed] [Google Scholar]
- 3.Choi H, Aldrich J V. Comparison of methods for the Fmoc solid-phase synthesis and cleavage of a peptide containing both tryptophan and arginine. Int J Pept Protein Res. 1993;42:42–58. doi: 10.1111/j.1399-3011.1993.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 4.Darmoul D, Brown D, Selsted M E, Ouellette A J. Cryptdin gene expression in developing mouse small intestine. Am J Physiol. 1997;272:G197–G206. doi: 10.1152/ajpgi.1997.272.1.G197. [DOI] [PubMed] [Google Scholar]
- 4a.Darmoul, D., D. M. Frederick, and A. J. Ouellette. Unpublished results.
- 5.Darmoul D, Ouellette A J. Positional specificity of defensin gene expression reveals Paneth cell heterogeneity in mouse small intestine. Am J Physiol. 1996;271:G68–G74. doi: 10.1152/ajpgi.1996.271.1.G68. [DOI] [PubMed] [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1985;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer P B, Harwig S S, Lehrer R I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992;60:3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faisst S, Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992;20:3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz T, Liu L, Valore E V, Oren A. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood. 1993;82:641–650. [PubMed] [Google Scholar]
- 10.Ghosh D. TFD: the transcription factors database. Nucleic Acids Res. 1992;20(Suppl.):2091–2093. doi: 10.1093/nar/20.suppl.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh D. Status of the transcription factors database (TFD) Nucleic Acids Res. 1993;21:3117–3118. doi: 10.1093/nar/21.13.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 13.Huttner K M, Ouellette A J. A family of defensin-like genes codes for diverse cysteine-rich peptides in mouse Paneth cells. Genomics. 1994;24:99–109. doi: 10.1006/geno.1994.1586. [DOI] [PubMed] [Google Scholar]
- 13a.Huttner, K. M., and A. J. Ouellette. Unpublished results.
- 14.Huttner K M, Selsted M E, Ouellette A J. Structure and diversity of the murine cryptdin gene family. Genomics. 1994;19:448–453. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 14a.Huttner, K. M., N. Salzman, and C. L. Bevins. Unpublished results.
- 15.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 16.Jones D E, Bevins C L. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315:187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 17.Ouellette A J, Greco R M, James M, Frederick D, Naftilan J, Fallon J T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989;108:1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellette A J, Hsieh M M, Nosek M T, Cano-Gauci D F, Huttner K M, Buick R N, Selsted M E. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouellette A J, Hsieh M M, Selsted M E. Structure and function of cryptdins isolated from mouse small intestinal lumen. Gastroenterology. 1996;110(Suppl.):A985. [Google Scholar]
- 20.Ouellette A J, Selsted M E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 20a.Ouellette, A. J. Unpublished observations.
- 21.Panyim S, Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969;130:337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- 22.Porter E M, Liu L, Oren A, Anton P A, Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65:2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selsted M E. Investigational approaches for studying the structures and biological functions of myeloid antimicrobial peptides. Genet Eng. 1993;15:131–147. doi: 10.1007/978-1-4899-1666-2_6. [DOI] [PubMed] [Google Scholar]
- 25.Selsted M E, Becker H W., III Eosin Y: a reversible stain for detecting electrophoretically resolved protein. Anal Biochem. 1986;155:270–274. doi: 10.1016/0003-2697(86)90436-7. [DOI] [PubMed] [Google Scholar]
- 26.Selsted M E, Miller S I, Henschen A H, Ouellette A J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Abel R J, Tang Y Q, Rao V S V, Dobbs C H, Tran D, Barany G, Selsted M E. Synthesis and characterization of indolicidin, a tryptophan-rich antimicrobial peptide from bovine neutrophils. Int J Pept Protein Res. 1995;45:401–409. doi: 10.1111/j.1399-3011.1995.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 28.Wingender E, Dietze P, Karas H, Kneuppel R. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res. 1996;24:238–241. doi: 10.1093/nar/24.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young S C, White P D, Davies J W, Owen E E I A, Salisbury S A, Tremeer E J. Counterion distribution monitoring—a novel method for acylation monitoring in solid-phase peptide synthesis. Biochem Soc Trans. 1990;18:1311–1312. doi: 10.1042/bst0181311. [DOI] [PubMed] [Google Scholar]