Abstract
Background
The cardiovascular system is strongly dependent on the gravitational environment. Gravitational changes cause mechanical fluid shifts and, in turn, autonomic effectors influence systemic circulation and cardiac control. We implemented a tilt paradigm to (1) investigate the acute hemodynamic response across a range of directions of the gravitational vector, and (2) to generate specific dose‐response relationships of this gravitational dependency.
Methods and Results
Twelve male subjects were tilted from 45° head‐up tilt to 45° head‐down tilt in 15° increments, in both supine and prone postures. We measured the steady‐state hemodynamic response in a range of variables including heart rate, stroke volume, cardiac output, oxygen consumption, total peripheral resistance, blood pressure, and autonomic indices derived from heart rate variability analysis. There is a strong gravitational dependence in almost all variables considered, with the exception of oxygen consumption, whereas systolic blood pressure remained controlled to within ≈3% across the tilt range. Hemodynamic responses are primarily driven by differential loading on the baroreflex receptors, combined with differences in venous return to the heart. Thorax compression in the prone position leads to reduced venous return and increased sympathetic nervous activity, raising heart rate, and systemic vascular resistance while lowering cardiac output and stroke volume.
Conclusions
Gravitational dose‐response curves generated from these data provide a comprehensive baseline from which to assess the efficacy of potential spaceflight countermeasures. Results also assist clinical management of terrestrial surgery in prone posture or head‐down tilt positions.
Keywords: altered gravity, dose‐response, heart rate variability, hemodynamics, spaceflight countermeasures, tilt
Subject Categories: Autonomic Nervous System, Hemodynamics, Physiology
Nonstandard Abbreviations and Acronyms
- BRS
baroreflex sensitivity
- CO
cardiac output
- DBP
diastolic blood pressure
- GLMM
generalized linear mixed‐effects model
- HDT
head‐down tilt
- HF
high frequency
- HFNorm
normalized high frequency
- HR
heart rate
- HRV
heart rate variability
- HRVTi
heart rate variability triangular index
- HUT
head‐up tilt
- LF
low frequency
- LFNorm
normalized low frequency
- LMM
linear mixed‐effects model
- RMSSD
root mean square of successive differences of NN intervals
- RPP
rate pressure product
- SBP
systolic blood pressure
- SDNN
standard deviation of NN intervals
- SV
stroke volume
- TPR
total peripheral resistance
- VO2
oxygen consumption
Clinical Perspective
What Is New?
In this study, we develop acute gravitational dose‐response curves for multiple hemodynamic parameters and autonomic indices, quantifying changes in the systemic circulation as a result of changes in the gravitational vector through a tilt paradigm in the range 45° head‐up tilt to 45° head‐down tilt (supine and prone).
We find that there is a strong gravitational dependence in almost all variables considered, with the exception of oxygen consumption, and that the effect of posture (supine versus prone) is significant in multiple parameters, with thorax compression in the prone position leading to impaired baroreflex function and associated hemodynamic effects.
What Are the Clinical Implications?
These gravitational dose‐response curves act as a comprehensive baseline that maps the acute effects of changes in the gravitational vector on the cardiovascular system, informing the development of spaceflight countermeasures. The curves also provide a reference for clinical management of surgeries in unusual postures such as Trendelenburg positioning or the use of prone spinal frames.
The human cardiovascular system is strongly dependent on the gravitational environment. Changes in the gravitational vector influence the systemic circulation through mechanical and autonomic effects. Mechanically, any alteration in gravity causes redistribution of fluid volumes and dynamic pressures because of changing hydrostatic pressure gradients. For example, in weightless conditions, the total loss of hydrostatic gradients induces a cephalad fluid shift. Among other acute effects, this fluid shift promotes venous return, increasing stroke volume (SV) via the Frank‐Starling mechanism. 1 Other changes include a fall in interstitial fluid pressure, 2 reduced systemic vascular resistance, 3 decreased heart rate (HR), 4 slightly decreased systolic blood pressure (SBP) and diastolic blood pressure (DBP), 3 , 5 and decreased central venous pressure. 6 Simultaneously, these acute dynamic pressure and volume changes influence autonomic receptors, including arterial baroreceptors in the carotid sinus and aortic arch, and cardiopulmonary receptors in the atriocaval junctions, atrial and ventricular walls, and pulmonary vasculature. 7 On an acute timescale, autonomic response because of stimulation of the arterial baroreflex and cardiopulmonary reflex receptors leads to changes in sympathetic and vagal activity, further altering the homeostatic set points of the hemodynamic system. Chronically, longer durations in a reduced gravity environment can lead to overall cardiovascular deconditioning, atrophy of cardiac muscles, reduction in circulating fluid volume, and impaired autonomic response. 8 Together, these changes induce an elevated risk of syncope when subjected to orthostatic stress on return to a gravitational environment. 9
Upcoming long‐duration exploration missions to the Moon and Mars will require significant time periods of weeks, months, or even years in altered gravity conditions. Thus, there is a need to develop novel countermeasures to counteract cardiovascular degradation, ensuring astronauts are healthy and fully operationally capable on return to a gravitational environment. This need is further enhanced by the arrival of commercial spaceflight and space tourism, where the broader medical profile of spaceflight participants may include older demographics and potentially individuals with preexisting cardiac pathologies. 10
Tilt studies are an important analog for the understanding of cardiovascular changes in altered gravity conditions. By changing the angle of tilt, we adjust the effective gravitational stress in the rostrocaudal Gz direction. Six‐degree head‐down tilt (HDT) has been used as an analog for microgravity conditions in multiple studies, including studies of the acute effects of tilt 11 , 12 , 13 , 14 , 15 , 16 , 17 and long‐duration HDT bed rest. 18 , 19 , 20 , 21 , 22 , 23 , 24 To date, most studies of head‐up tilt (HUT) or HDT have focused on 1 or a few physiological parameters and considered limited tilt angles. These include studies related to systemic, 25 , 26 , 27 , 28 , 29 cerebral, 12 , 30 ocular hemodynamics, 13 and autonomic function. 30 , 31 Studies that cover both HUT and HDT (for example Lieshout et al 29 ) are generally limited to 1 or 2 tilt angles in each tilt condition. A limited number of studies have also considered differences between supine and prone postures during tilt. 15 , 32 Further insight into postural differences (ie, supine versus prone posture) may lead to a deeper understanding of the importance of tissue weight on gravitational hemodynamics by isolating changes purely because of the reversal of the gravitational vector in the Gx axis. 33 Such changes have been demonstrated to influence regional hemodynamics. 34 , 35 , 36 Understanding the physiological response to altered gravity through a tilt paradigm can also be directly beneficial to the development of countermeasures. 37 , 38 For example, artificial gravity is a posited countermeasure for combating cardiovascular deconditioning. Although artificial gravity generated through short radius centrifugation creates a gravity gradient (as opposed to a constant gravity field), quantifying the baseline response to gravity through tilt can provide insight into what level of centrifugation should be targeted to provide a given physiological response. 39 , 40 , 41 , 42
The aim of this study was to construct dose‐response curves to quantify the behavior of the cardiovascular system across a large range of HUT and HDT. These curves will encompass a wide range of hemodynamic and autonomic measures, providing a holistic picture of cardiovascular circulation and control. Although there have been many studies using tilt in specific angles (for example 6° HDT as a microgravity analog), it is not currently possible to estimate the physiological response to any particular gravitational dose. Thus, in this study, we measure the acute response to altered gravity across a wide range of tilt angles (45° HUT to 45° HDT, supine and prone). Furthermore, we use the experimental data to build hemodynamic and autonomic gravitational dose‐response curves, thus indicating the predicted response in a representative nonpathological population. We hypothesize that many of the relationships between tilt angle and a given cardiovascular or autonomic parameter can be explained by linear models. Together, these results lead to a greater understanding of the gravitational influence on the cardiovascular system, aiding in the future development of spaceflight countermeasures as well as clinical applications.
METHODS
The data sets analyzed for this study are publicly available, and a repository can be found on GitHub: https://github.com/rswhittle/cv‐dose‐response. Code availability is not applicable.
Subjects and Study Approval
Twelve healthy, recreationally active male subjects aged between 23 and 33 years were recruited from the Texas A&M University System to participate in the study. From an initial pool of volunteers, the age range of selected subjects was limited as much as possible to avoid confounding factors related to changes in the cardiovascular system with age. Sample size and the number of tilt angles required was determined based on a power curve analysis of pilot data. Subject characteristics (mean±SD), including blood pressure at screening, are shown in Table 1. Before participating in the study, subjects completed a questionnaire designed to identify any exclusion criteria, including current use of any cardiac, blood pressure, muscle relaxant, anticoagulant, or stimulant medications, thyroid disease, chronic cardiovascular pathologies, extreme obesity, and history of hypertension. One subject was unable to complete 1 single condition (45° HDT, supine position) because of discomfort; however, he was returned to a HUT position and experienced no lasting symptoms. The remainder of his data are included in the results. All other subjects completed the full protocol and experienced no adverse effects. Each subject received written and verbal explanations of the study protocols and gave written informed consent to participate in the experiment. All procedures performed in the study were in accordance with the 1964 Helsinki Declaration and its later amendments. The study protocol was approved by the Texas A&M Human Research Protection Program with institutional review board number IRB2020‐0724F.
Table 1.
Characteristics of the 12 Recreationally Active Male Subjects Who Participated in the Study
| Characteristic | Value |
|---|---|
| N | 12 |
| Race | 8 White, 1 Black, 3 Asian |
| Age, y | 26.8±2.9 |
| Height, cm | 179.0±8.3 |
| Weight, kg | 84.7±18.7 |
| BMI, kg/m2 | 26.3±4.9 |
| SBP, mm Hg | 129.5±14.5 |
| DBP, mm Hg | 82.3±6.5 |
Characteristics were recorded during the baseline session before testing sessions. Data are reported as mean±SD where appropriate. BMI indicates body mass index; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Experimental Design and Testing Protocol
We implemented a counterbalanced, within‐subjects, experimental design such that every subject experienced every tilt condition and posture. Subjects were tilted from 45° HUT to 45° HDT in 2 separate postures: supine (face up) and prone (face down). The procedure was identical for each posture. Experimental sessions took place on 3 separate days within a 2‐week period. In the first session, subjects gave informed consent, and baseline measurements were collected in a seated posture. In the additional 2 experimental sessions, subjects were tested once in supine position and once in prone position (order counterbalanced). To control for potential circadian effects, all sessions were scheduled in the morning at approximately the same time. In addition, subjects were asked to refrain from drinking caffeine and exercising before each test session.
In a single experimental session (supine or prone), subjects were placed on a tilt table (World Triathlon, Tampa Bay, FL) initially at 45° HUT. Continuous measurements of blood pressure and electrocardiography were recorded throughout the test. Subjects initially remained at rest for a period of 6 minutes to allow any hemodynamic transients to settle. After the rest period, an inert gas rebreathing device was used to collect discrete measurements of cardiac parameters. Following this, several further measurements were collected from the subjects, including ocular tonometry, ultrasonography, and noninvasive measurement of internal jugular venous pressure. These further measurements will be described in a separate publication. The total procedure at a single tilt angle lasted for ≈12 minutes. Subjects were then tilted downward 15°, and the entire process repeated, starting with the 6‐minute resting period. The total protocol included 7 tilt angles: 45° HUT, 30° HUT, 15° HUT, 0° (horizontal), 15° HDT, 30° HDT, and 45° HDT. The procedure for the seated baseline conducted on the first experimental session was identical to the procedure for a single tilt angle.
Dependent Variables
Dependent variables include 8 hemodynamic metrics and 7 autonomic indices. The hemodynamic measurements considered were: (1) HR (beats per minute), (2) SV (milliliters), (3) cardiac output (CO; liters per minute), (4) total peripheral resistance (TPR; millimeters of mercury per milliliter per second), (5) SBP (millimeters of mercury), (6) DBP (millimeters of mercury), (7) rate pressure product (RPP; millimeters of mercury per minute) used as a metric for myocardial stress and energy consumption, 43 and (8) oxygen consumption (VO2; liters per minute).
Autonomic analysis was performed from measurements of HR variability (HRV) and baroreflex sensitivity (BRS). HRV analysis can be performed over short duration timescales, often of the order of 5 minutes, although shorter analyses have been used successfully to analyze autonomic changes in parabolic flight over a single parabola. 44 In particular, 2 key classes of HRV indices exist 45 : time‐domain measures and frequency‐domain measures. Time‐domain measures are metrics related to the variation in the intrabeat interval between normal sinus beats (the NN interval). Frequency‐domain metrics consider the distribution of intrabeat interval variation in the power spectral density of various frequency bands. Three time‐domain and 3 frequency‐domain indices were considered. The 3 time‐domain indices were: (1) the standard deviation of the NN intervals (SDNN), (2) the root mean square of successive differences of the NN interval (RMSSD), and (3) HR variability triangular index (HRVTi). As a time‐dependent measure of autonomic function, BRS was also included in this set of metrics. SDNN and HRVTi represent estimates of total HRV incorporating sympathetic and parasympathetic activity. 45 RMSSD represents short‐term variability and thus is closely correlated with vagal‐mediated cardiac control. 46 Finally, baroreflex efferents translate into HRV via the cardiac sinoatrial node, providing blood pressure buffering and cardioprotection. 47 Thus, BRS represents a metric of total autonomic control over the cardiovascular system via the arterial baroreflex, with implications in the regulation of systemic fluid pressures. 48 , 49 The 3 frequency‐domain indices were: (1) spectral power density in the low‐frequency (LF) (0.04–0.15 Hz) band, (2) spectral power density in the high‐frequency (HF) (0.15–0.4 Hz) band, and (3) the ratio between LF and HF power spectral densities (LF/HF). Following the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, 45 LF and HF are shown in both absolute units (milliseconds squared) and normalized units (LFNorm and HFNorm), which represent relative contributions of each power component in proportion to the total power minus the very LF (0.0033–0.04 Hz) component. LF is used as a marker of sympathetic activity (particularly when expressed in normalized units), HF is closely correlated with vagal activity, whereas LF/HF represents sympathovagal balance. 45
Instrumentation and Data Collection
Hemodynamic measurements were collected using 2 instruments, an Innocor inert gas rebreathing device (Cosmed: The Metabolic Company, Rome, Italy) and a Finapres NOVA (Finapres Medical Systems, Enschede, the Netherlands). Full calibration was performed on devices daily, and ambient data calibrations were also performed before each subject’s test (mean±SD: temperature 20.5±2.0 °C, relative humidity 53.9±11.0%, pressure 767.2±4.8 mm Hg). Innocor rebreathes were performed at every tilt angle. The inert gas rebreathing method was used to obtain noninvasive measures of pulmonary blood flow by analyzing the changing concentrations of a soluble gas (NO, 5%) and an insoluble gas (sulfur hexafluoride, 1%) in an oxygen‐enriched air mixture over 5 to 6 breaths. The mixture is rebreathed using a bag for ≈30 seconds. During the rebreathe, subjects inspired and expired at a rate of 20 breaths per minute, following this rhythm with a metronome (respiration at all other times was at a normal relaxed respiration rate). After each rebreathe, the gas concentration traces were visually inspected by a trained operator to ensure correct function of the device. The Innocor device was used to measure HR, SV, CO, and VO2. Further details on the inert gas rebreathing methodology can be found in Whittle et al. 16
Finapres data (finger arterial pulse contour waveform and 5‐lead ECG) were collected continuously throughout the protocol. Pressure was corrected to heart level with a hydrostatic height sensor placed laterally on the midcoronal plane at the fifth intercostal space. At each tilt angle, the Finapres pressure waveform was calibrated with a discrete blood pressure measurement using a brachial sphygmomanometer. Finapres data were used to measure SBP, DBP, and RPP. Furthermore, TPR was calculated by Equation 1:
| (1) |
using the mean arterial pressure from the Finapres, and the CO from the Innocor. Autonomic indices were derived from the Finapres ECG trace and beat‐to‐beat RR interval. Calculations were performed automatically by the Finapres software. Three of the 4 time‐domain measures (SDNN, RMSSD, and HRVTi) and all of the frequency‐domain measures were continuously calculated using a 300‐second sliding window. The BRS measure used a 10‐second sliding window to compute baroreflex sensitivity as the transfer gain of the cross‐spectra between beat‐to‐beat SBP and RR interval, resampled to 1 Hz. 50 After visual inspection of the traces, measurements from the Finapres (hemodynamic and autonomic) were averaged using a 95% trimmed mean during the entire sixth minute at each tilt angle to give a single value for each subject condition. This ensured that there was no temporal overlap with the forced respiration rate imposed during the Innocor measurements, which could have influenced HRV.
Statistical Analysis
Data from the hemodynamic measurements were distributed approximately normally at each tilt Angle and Position (supine or prone) combination, assessed using Shapiro‐Wilk tests. Two‐factorial linear mixed‐effects models (LMMs) were used to assess the effects of Angle and Position on the hemodynamic measurements within subjects. Subjects were included as random factors with Angle and Position (supine or prone) as fixed factors. LMMs were fit using restricted maximum likelihood. Diagnostic plots for all models were examined visually to confirm normality of residuals, and homoscedasticity was assessed using the Levine test. Data related to the autonomic response were severely right‐skewed, with multiple violations of normality. Because all autonomic indices used are bounded by , and following a methodology used in multiple studies, 51 , 52 , 53 , 54 data were fit to generalized linear mixed‐effects models (GLMMs) with a γ distribution and a log link (ie, expected value of the dependent variable µ is given by log[µ]=η, where is the linear predictor) using the same fixed (ie, Angle and Position) and random (ie, subjects) factors as the LMMs. GLMMs were fit to maximum likelihood estimated via adaptive Gauss‐Hermite quadrature. 55 Diagnostic plots of all GLMMs were examined visually, and fit assessed using tests for dispersion, outliers, and distribution (Kolmogorov‐Smirnof). LFNorm and HFNorm did not present the same heteroscedasticity, so were fit with LMMs as per the hemodynamic parameters. Significant effects of angle, position, or their interaction were followed by post hoc contrasts between the LMM/GLMM estimated marginal means and the seated baseline condition, which was used as the control condition. Significance was adjusted using the Dunnett many‐to‐one comparison test (). 56 When only the factor Angle was significant, contrasts were performed disregarding the factor Position (ie, supine and prone values were pooled). Furthermore, when the factor Position was significant, a post hoc contrast between supine and prone was performed excluding the seated baseline. If this contrast was significant, then further contrasts were performed between supine and prone at each tilt angle on the EMMs using the Benjamini‐Hochberg correction for false discovery rates (). 57
Gravitational dose‐response curves between 45° HUT and 45° HDT were constructed for each dependent variable by refitting the models (LMM and GLMM) without the seated baseline, using tilt angle as a quantitative continuous variable. Model fit was assessed as above. The following linear predictor (Equation 2) was used to generate dose‐response curves for each dependent variable measured:
| (2) |
where, for each dependent variable, the linear predictor for subject () in Position (, supine and prone) is described by the tilt Angle (from +45°, HUT to –45°, HDT), the fixed effects (where represents the intercept), the random intercept (associated with each subject and the within‐subjects design), and the residual error . Given that the gravitational vector is aligned with the global vertical plane (as opposed to the subject's axis), tilt angle was transformed using a sinusoid function, 58 as can be seen in Equation 2. Dose‐response curves are shown as mean and 95% confidence band. If the main effect of the factor Position was not significant, supine and prone data were pooled (ie, the dose‐response curve is modeled using only the factor Angle). If the main effect of Angle was not significant, tilt angle data were pooled (ie, the dose‐response curve is just modeled using the factor Position). None of the interaction effects were statistically significant, and therefore, they were not included in the model.
All statistical analyses were completed using R version 4.1.0 59 with LMMs and GLMMs fit using the lme4 55 and glmmTMB 60 packages. Diagnostics were assessed using the lmerTest 61 and DHARMa 62 packages. Adjusted means and contrasts were calculated using the emmeans package. 63 Significance level was set at (2‐sided).
RESULTS
Hemodynamic Response
Figure 1 shows the evolution of hemodynamic parameters (mean±SE) as a function of tilt angle (including the seated baseline). Table 2 reports the results of the LMM analyses. There were no significant interaction effects between Angle and Position in any of the models. All hemodynamic parameters except for VO2 showed a significant effect of Angle, and all hemodynamic parameters except for SBP showed a significant effect of Position. A follow‐up contrast between supine and prone positions (excluding the seated baseline) also showed no difference in DBP. HR decreased with increasing HDT (P<0.001), and HR in the prone position was significantly higher than in the supine position at most of the tilt angles. On average, HR in the prone position was 5.5±2.1 bpm (95% CI, 1.6–9.3 bpm) higher than HR in the supine position. The SV and CO increased significantly with increasing HDT (P<0.001 and P<0.001, respectively). On average, SV in the supine position was 8.8±1.5 mL (95% CI, 5.8–11.8 mL; P<0.001) higher than SV in the prone position, with significant differences in a pairwise comparison between supine and prone at 15° HUT, 0°, 15° HDT, 30° HDT, and 45° HDT. Differences between CO in the supine and prone positions were found marginally significant (P=0.048), with CO in the supine position being 0.2±0.1 L/min (95% CI, 0.0–0.4 L/min; P=0.048) higher than CO in the prone position. However, no significant differences were found in an adjusted pairwise comparisons at each tilt angle. TPR decreased significantly with increasing HDT (P<0.001). There was no main effect of Position on SBP (P=0.251). However, SBP showed a gentle (and significant) decrease with increasing HDT (P=0.005). SBP decreased from 128.6±3.3 mm Hg (95% CI, 123.2–133.9 mm Hg) at 45° HUT to 124.4±2.7 mm Hg (95% CI, 119.0–129.8 mm Hg) at 45° HDT. SBP was only significantly different from the seated baseline at 30° HDT. DBP also decreased significantly with increasing HDT (P<0.001). Although there was a main effect for Position in DBP (P<0.002), significant differences were not found between supine and prone positions (P=0.172) (ie, the main effect in Position is most likely driven by differences from the seated baseline). DBP was significantly different from the seated baseline at 0°, 15° HDT, 30° HDT, and 45° HDT. On average, the RPP in the prone position was 480±141 mm Hg/min (95% CI, 200–759 mm Hg/min; P<0.001) higher than in the supine position. In addition, RPP also decreased with increasing HDT (P<0.001), showing significant differences between the seated baseline and 45° HUT (prone), 30° HDT (supine and prone), and 45° HDT (supine and prone). Finally, there was no main effect of Angle on VO2 (P=0.244). However, VO2 was significantly higher in the prone position than in the supine position, with an average increase of 0.04±0.01 L/min (95% CI, 0.02–0.06 L/min; P<0.001) between the 2 conditions.
Figure 1. Hemodynamic variables as a function of tilt angle in supine (Sup) (―, •) and prone (Pr) (‐ ‐ ‐, ○) positions, collected on 12 male subjects.
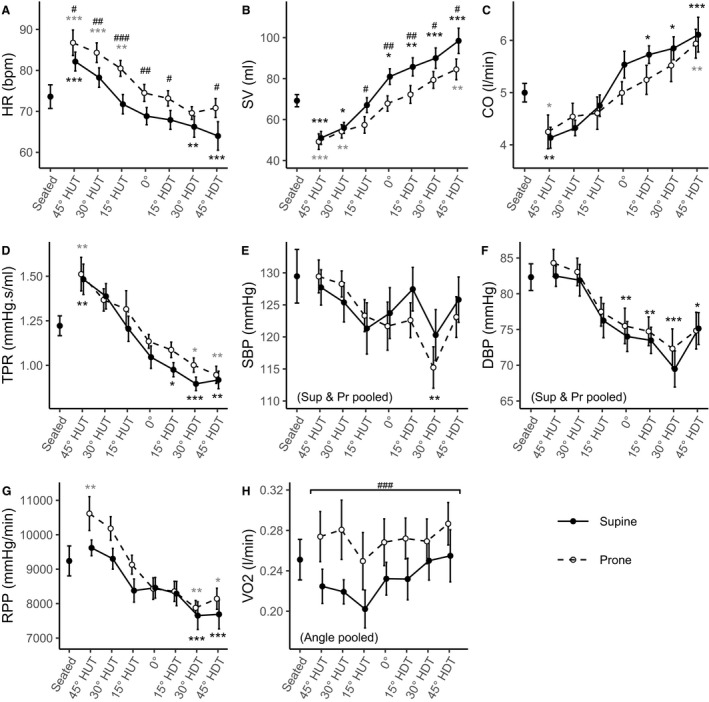
Measurements were taken at a seated baseline, 45° head‐up tilt (HUT), 30° HUT, 15° HUT, 0°, 15° head‐down tilt (HDT), 30° HDT, and 45° HDT. Data are presented as mean±SE at each tilt angle. Asterisks (*; black, supine; grey, prone) indicate statistically significant differences between a specific tilt condition and the seated baseline condition. When the statistical analysis indicated no significant differences between the supine and prone positions, these conditions were pooled (see systolic blood pressure [SBP] and diastolic blood pressure [DBP]; in these cases, black asterisks represent both positions together). Pound signs (#) represent statistically significant differences between prone and supine postures at a given angle. A, Heart rate (HR). B, Stroke volume (SV). C, Cardiac output (CO). D, Total peripheral resistance (TPR). E, Systolic blood pressure (SBP). F, Diastolic blood pressure (DBP). G, Rate pressure product (RPP). H, Oxygen consumption (VO2). ***P<0.001. **P<0.01. *P<0.05. ### P<0.001. ## P<0.01. # P<0.05.
Table 2.
Statistical Results of the Linear Mixed Model and Generalized Linear Mixed Model Analysis
| Variable | P value | |||
|---|---|---|---|---|
| Angle | Position* | Angle × position | Supine vs prone † | |
| Hemodynamic measurements | ||||
| HR | <0.001 ‡ | <0.001 ‡ | 0.609 | <0.001 ‡ |
| SV | <0.001 ‡ | <0.001 ‡ | 0.157 | <0.001 ‡ |
| CO | <0.001 ‡ | <0.001 ‡ | 0.266 | 0.048 § |
| TPR | <0.001 ‡ | <0.001 ‡ | 0.834 | 0.024 § |
| SBP | 0.005 ‖ | 0.251 | 0.645 | … |
| DBP | <0.001 ‡ | 0.003 ‖ | 0.997 | 0.172 |
| RPP | <0.001 ‡ | <0.001 ‡ | 0.308 | <0.001 ‡ |
| VO2 | 0.244 | <0.001 ‡ | 0.915 | <0.001 ‡ |
| Time‐domain autonomic indices | ||||
| SDNN | <0.001 ‡ | 0.214 | 0.656 | … |
| RMSSD | <0.001 ‡ | 0.002 ‖ | 0.789 | <0.001 ‡ |
| HRVTi | <0.001 ‡ | 0.710 | 0.555 | … |
| BRS | <0.001 ‡ | <0.001 ‡ | 0.386 | 0.066 |
| Frequency‐domain autonomic indices | ||||
| LF | <0.001 ‡ | 0.106 | 0.776 | … |
| HF | <0.001 ‡ | 0.250 | 0.515 | … |
| LFNorm | <0.001 ‡ | 0.745 | 0.615 | … |
| HFNorm | <0.001 ‡ | 0.746 | 0.615 | … |
| LF/HF | <0.001 ‡ | 0.300 | 0.084 | … |
Fixed factors included Angle, Position, and their interaction. Subjects were included as random factors. See text for model details. BRS, baroreflex sensitivity; CO, cardiac output; DBP, diastolic blood pressure; HF, high frequency; HFNorm, normalized high frequency; HR, heart rate; HRVTi, heart rate variability triangular index; LF, low frequency; LFNorm, normalized low frequency; RMSSD, , root mean square of successive differences of NN intervals; RPP, rate pressure product; SBP, systolic blood pressure; SDNN, standard deviation of NN intervals; SV, stroke volume, TPR, total peripheral resistance; and VO2, oxygen consumption.
Main effect of Position includes seated baseline.
Post hoc contrast to determine whether there is a true difference between supine and prone positions (ie, does not include seated baseline).
P<0.001.
P<0.05.
P<0.01.
Autonomic Response
Figure 2 shows the evolution of time‐domain autonomic indices (mean±SE) as a function of tilt angle (including the seated baseline). Table 2 reports the results of the GLMM analyses. There were no statistically significant interaction effects between Angle and Position in any of the indices. All 4 indices showed statistically significant main effects of Angle (SDNN: P<0.001; RMSSD: P<0.001; HRVTi: P<0.001; BRS: P<0.001), where all parameters increased with increased angles of HDT. The statistical analysis did not reveal a significant effect of Position in SDNN (P=0.214) or HRVTi (P=0.710). Results for BRS did not reveal statistically significant differences between the prone and supine position (P=0.066). Thus, supine and prone results were pooled for SDNN, HRVTi, and BRS. The SDNN index increased from 36.4±3.7 ms (95% CI, 30.0–44.8 ms) at 45° HUT to 58.0±5.9 ms (95% CI, 47.4–70.8 ms) at 45° HDT and was significantly different from the seated baseline at 45° HUT and 30° HUT. The HRVTi index increased from 9.0±0.8 (95% CI, 7.5–10.7) at 45° HUT to 12.3±1.1 (95% CI, 10.3–14.8) at 45° HDT and was significantly different from the seated baseline at 45° HUT and 30° HUT. The BRS index increased from 6.6±0.7 ms/mm Hg (95% CI, 5.3–8.3 ms/mm Hg) at 45° HUT to 17.1±2.0 ms/mm Hg (95% CI, 13.6–21.5 ms/mm Hg) at 45° HDT and was significantly different from the seated baseline at 45° HUT, 30° HDT, and 45° HDT. Finally, RMSSD was, on average, 1.16±0.05 (95% CI, 1.06–1.25; P<0.001) times (statistically significantly) higher in supine than in prone (ratio and tests on log scale for GLMMs), although adjusted pairwise comparisons did not show significant differences at any tilt angle. The RMSSD index increased in the supine position from 17.6±2.7 ms (95% CI, 13.0–23.8 ms) at 45° HUT to 40.5±6.4 ms (95% CI, 29.7–55.0 ms) at 45° HDT, and in prone position from 16.4±2.6 ms (95% CI, 12.1–22.3 ms) at 45° HUT to 33.8±5.2 ms (95% CI, 25.0–45.8 ms) at 45° HDT. RMSSD was significantly different from the seated baseline at 45° HUT, 30° HUT, and 15° HUT (in both the supine and prone positions), and at 0° (only in prone position).
Figure 2. Time‐domain autonomic indices as a function of tilt angle in supine (Sup) (―, •) and prone (Pr) (‐ ‐ ‐, ○) positions, collected on 12 male subjects.
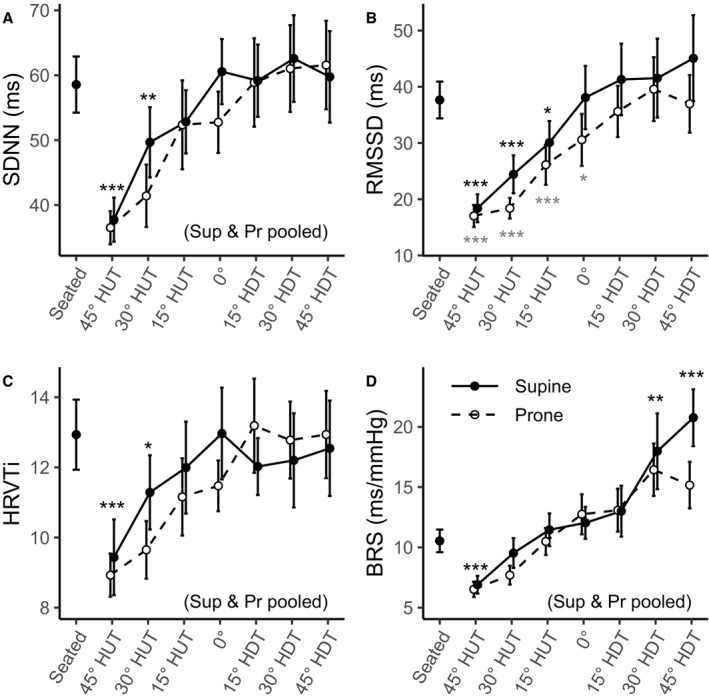
Measurements were taken at a seated baseline, 45° head‐up tilt (HUT), 30° HUT, 15° HUT, 0°, 15° head‐down tilt (HDT), 30° HDT, and 45° HDT. Data are presented as mean±SE at each tilt angle. Asterisks (*; black, supine; grey, prone) indicate statistically significant differences between a specific tilt condition and the seated baseline condition. When the statistical analysis indicated no significant differences between the supine and prone positions, these conditions were pooled (where noted, black asterisks represent both positions together). A, Standard deviation of NN intervals (normalized RR intervals) (SDNN). B, Root mean square of successive differences of NN intervals (RMSSD). C, Heart rate variability triangular index (HRVTi). D, Baroreceptor sensitivity (BRS). ***P<0.001. **P<0.01. *P<0.05.
Figure 3 shows the evolution of the frequency‐domain autonomic indices (mean±SE) as a function of tilt angle (including the seated baseline). Table 2 reports the results of the LMM and GLMM analyses. There were no statistically significant interaction effects between Angle and Position or statistically significant main effect of Position in any of the indices. Thus, supine and prone results were pooled for all frequency‐domain variables considered. All indices showed statistically significant main effects of Angle (LF: P<0.001; LFNorm: P<0.001; HF: P<0.001; HFNorm: P<0.001; LF/HF: P<0.001). LF increased from 516±77 ms2 (95% CI, 385–691 ms2) at 45° HUT to 888±134 ms2 (95% CI, 661–1192 ms2) at 45° HDT, with statistically significant differences from the seated baseline at 45° HUT and 30° HUT. Similarly, HF increased from 98±24 ms2 (95% CI, 60–160 ms2) at 45° HUT to 407±102 ms2 (95% CI, 249–665 ms2) at 45° HDT, with statistically significant differences from the seated baseline at 45° HUT, 30° HUT, 15° HUT, and 0°. However, when expressed in normalized units, LFNorm (ie, the proportion of total power minus very LF power) decreased from 83.8±2.9% (95% CI, 78.0%–89.6%) at 45° HUT to 68.2±2.9% (95% CI, 62.3%–74.0%) at 45° HDT. Accordingly, HFNorm increased from 16.2±2.9% (95% CI, 10.4%–22.0%) at 45° HUT to 31.8±2.9% (95% CI, 26.0%–37.7%) at 45° HDT. LFNorm and HFNorm differed from the seated baseline at 45° HUT and 30° HUT. Thus, LF/HF decreased from 5.7±0.9 (95% CI, 4.2–7.8) at 45° HUT to 2.5±0.4 (95% CI, 1.8–3.4) at 45° HDT, differing from the seated baseline at 45° HUT.
Figure 3. Frequency‐domain autonomic indices as a function of tilt angle in supine (Sup) (―, •) and prone (Pr) (‐ ‐ ‐, ○) positions, collected on 12 male subjects.
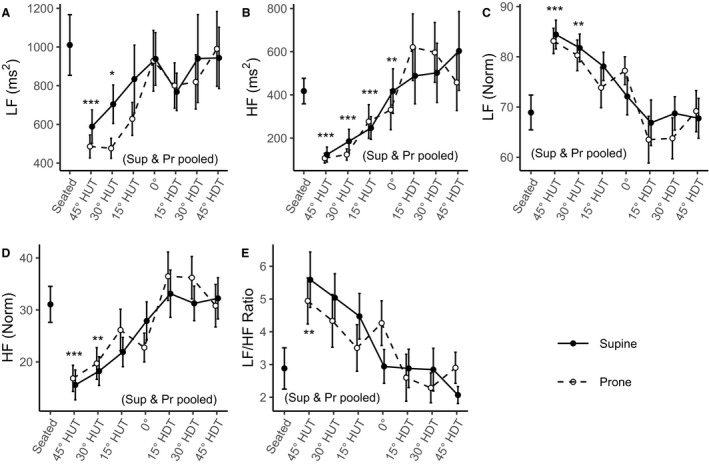
Measurements were taken at a seated baseline, 45° head‐up tilt (HUT), 30° HUT, 15° HUT, 0°, 15° head‐down tilt (HDT), 30° HDT, and 45° HDT. Data are presented as mean±SE at each tilt angle. Asterisks (*; black, supine; grey, prone) indicate statistically significant differences between a specific tilt condition and the seated baseline condition. When the statistical analysis indicated no significant differences between the supine and prone positions, these conditions were pooled (where noted, black asterisks represent both positions together). A, Power density in the low‐frequency range (0.04–0.15 Hz) (LF). B, Power density in the high‐frequency range (0.15–0.4 Hz) (HF). C, Normalized low frequency (LFNorm). D, Normalized high frequency (HFNorm). E, Ratio of low to high power densities (LF/HF ratio). ***P<0.001. **P<0.01. *P<0.05.
Dose‐Response Curves
Figures 4 and 5 show the estimated dose‐response curves for all of the hemodynamic and autonomic parameters considered within the range of 45° HUT to 45° HDT. Curves are shown as mean and 95% CI. Because there was no difference between supine and prone for SBP, DBP, SDNN, HRVTi, BRS, LF, LFNorm, HF, HFNorm, and LF/HF, supine and prone position results were pooled, and these dose‐response curves are combined into a single estimate. The dose‐response curves corresponding to VO2 are modeled as constant functions for supine and prone positions, because the statistical analysis did not show a significant effect of Angle. Model details are shown in Table S1.
Figure 4. Estimated gravitational dose‐response curves for hemodynamic parameters in the range 45° head‐up tilt (HUT) to 45° head‐down tilt (HDT).
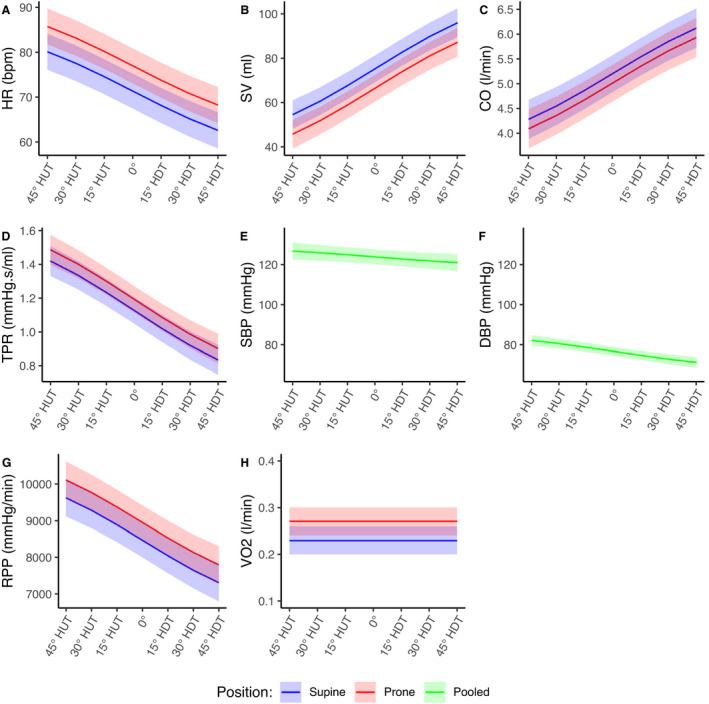
Curves were fit via linear mixed‐effects models as described in the main text. Curves are presented as mean±95% CI. Blue, supine; red, prone; green, supine and prone pooled. A, Heart rate (HR). B, Stroke volume (SV). C, Cardiac output (CO). D, Total peripheral resistance (TPR). E, Systolic blood pressure (SBP). F, Diastolic blood pressure DBP). G, Rate pressure product (RPP). H, Oxygen consumption (VO2).
Figure 5. Estimated gravitational dose‐response curves for autonomic parameters in the range 45° head‐up tilt (HUT) to 45° head‐down tilt (HDT).
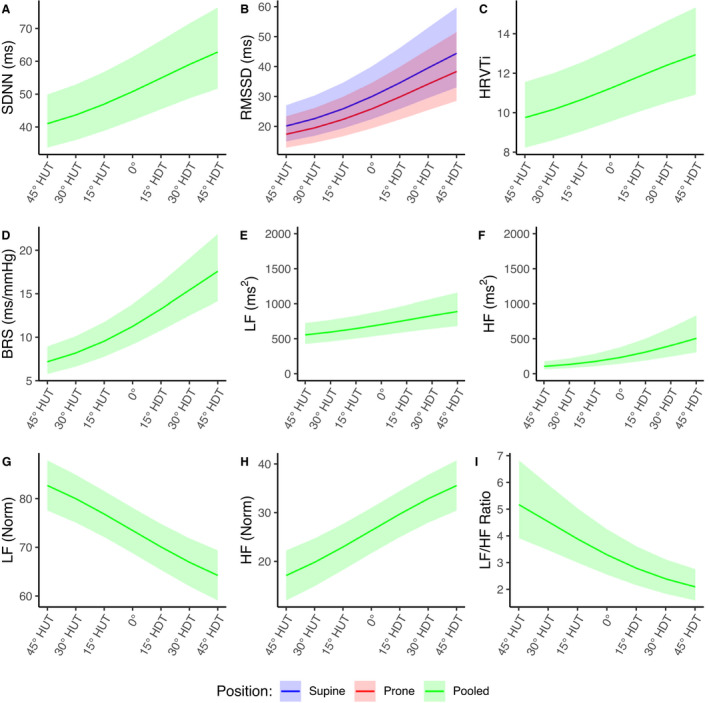
Curves were fit via linear mixed‐effects models (LFNorm and HFNorm) and generalized linear mixed‐effects models (remaining parameters) as described in the main text. Curves are presented as mean±95% CI. Blue, supine; red, prone; green, supine and prone pooled. A, Standard deviation of NN intervals (normalized RR intervals) (SDNN). B, Root mean square of successive differences of NN intervals (RMSSD). C, Heart rate variability triangular index (HRVTi). D, Baroreceptor sensitivity (BRS). E, Power density in the low frequency range (0.04–0.15 Hz) (LF). F, Power density in the high frequency range (0.15–0.4 Hz) (HF). G, Normalized low frequency (LFNorm). H, Normalized high frequency (HFNorm). I, Ratio of low to high power densities (LF/HF ratio).
DISCUSSION
This study investigated the acute gravitational dependence of cardiovascular hemodynamics and autonomic control in a tilt paradigm. To our knowledge, this study represents the most comprehensive analysis of hemodynamic and autonomic responses over the widest range of tilt angles to date, while also considering supine and prone differences. Our main findings show that: (1) Almost all hemodynamic parameters and autonomic indices present a strong gravitational dependence. (2) The effect of body position (supine or prone) is important for HR, SV, CO, TPR, and VO2, but not for blood pressure or autonomic regulation. (3) In the range between 45° HUT and 45° HDT, linear models can effectively describe the relationship between tilt angle and hemodynamic/autonomic response. Some studies have previously considered the hemodynamic, 27 , 28 autonomic, 64 or endocrine 58 response to acute graded HUT. Other studies have considered the hemodynamic 25 , 26 or autonomic 26 , 65 response to graded HDT. However, studies that investigated both HUT and HDT are scarce. Lieshout et al 29 considered hemodynamic response in 9 subjects at 20° HUT, horizontal 0°, and 20° HDT (all supine). Furthermore, we could find no studies that considered supine and prone hemodynamic differences in HUT or HDT. Additionally, only the László et al 58 study on autonomic response to graded HUT attempts to fit dose‐response curves to the experimental data. Thus, the present study gives unique insight into the complete gravitational and postural cardiovascular response over both HUT and HDT, in the supine and prone positions, with applications in spaceflight and terrestrial surgery.
Multiple studies have shown either a decrease in CO with increased angles of HUT from the supine posture, or an increase in CO with increased angles of HDT. 11 , 27 , 30 , 66 With respect to the horizontal supine 0° posture, our 21.9% (95% CI, 12.8%–31.0%) decrease in CO to 30° HUT matches closely the 19% decrease found by Tuckman et al. 67 They did not measure CO at 15°, but our decrease of 14.2% (95% CI, 5.1%–23.3%) at 15° HUT is between their reported changes at 10° HUT (5% decrease) and 20° HUT (17% decrease). Similarly, Bundgaard‐Nielsen et al 68 found a decrease in CO by 0.7 L/min (95% CI, 0.2–1.2 L/min) from 0° supine to 45° HUT, which is within our 95% confidence limits of a 1.4 L/min (95% CI, 0.6–2.2 L/min) decrease, although they did not see an increase in CO during any angles of HDT (15°, 45°, 70°, or 90° HDT). Stroke volume is principally controlled by the Frank‐Starling mechanism. During HUT, a reduction in central blood volume and reduced venous return because of pooling in the abdominal and lower extremity vasculature leads to decreased cardiac filling and left ventricular end‐diastolic pressure, reduced myocyte stretch, and hence, reduced contraction force and lower SV. Conversely, we expect the opposite behavior in graded HDT; the cephalad fluid shift leads to increased central blood volume, increased left ventricular end‐diastolic pressure and thus increased SV. 69 The reduction in HR with HDT (and increase with HUT), along with the reduction in TPR, are primarily driven by autonomic activity. 70 , 71 In HDT, increased pressure on the arterial baroreflex stimulates vagal activity while simultaneously withdrawing sympathetic nervous stimulation, promoting a bradycardic response together with vasorelaxation. 69 The converse is true in HUT; HR and TPR increase with HUT, driven by vagal withdrawal and sympathetic stimulation, which promote tachycardia and vasoconstriction. This explanation is supported by our findings on HRV. However, the fact that CO still increases in HDT indicates that the increase in SV is proportionally greater than the reduction in HR.
Our results also indicate a reduction of SBP with increasing HDT, but this is only a small change. Between 45° HUT and 45° HDT, SBP decreases by 4.2±2.7 mm Hg (95% CI, −3.1 to 11.5 mm Hg), which is equivalent to 3.3% of the seated baseline SBP. On the other hand, DBP presents a larger decrease over the same interval. Between 45° HUT and 45° HDT, DBP decreases by 8.6±1.9 mm Hg (95% CI, 3.5–13.7 mm Hg) or 10.4% of the seated baseline value. In the absence of syncope (which we did not observe in any of our subjects), this is to be expected, because maintenance of arterial pressure is the primary function of cardiovascular control. 69 In effect, the rest of the hemodynamic and autonomic changes we observe in tilt are affected to maintain arterial pressure. Our results fall between Mukai and Hayano, 72 who observed no changes in either SBP or DBP in graded HUT, and Zaidi et al, 27 who found an increase of 11.9% and 20.3% in SBP and DBP, respectively, from horizontal supine to 45° HUT. The data also align with Mosqueda‐Garcia et al, 73 who found little change in SBP, a small increase in DBP, and an increase of around 14 bpm in HR when subjects were tilted from 0° supine to 45° HUT compared with our increase of 13.3±1.9 bpm (95% CI, 7.6–19.1 bpm) under the same conditions. Although SBP is essentially controlled in tilt, we hypothesize that the apparent reduction in DBP with HDT is an artifact of bradycardia combined with vasorelaxation in the terminal resistance arterioles, increasing both the diastolic time interval and the rate of pressure drop during diastole. 74 Because blood pressure is largely maintained, bradycardia in HDT also leads to a reduction in RPP, indicating a reduction in myocardial oxygen consumption with HDT. Although, on appearance, this may provide evidence for a reduced risk of cardiovascular events during reduced gravity conditions, 75 long‐term cardiovascular deconditioning likely outweighs any acute benefits. 76
VO2 was the only hemodynamic parameter that did not show a strong response to tilt. Studies on cardiopulmonary response to graded tilt are scarce. Diaz‐Artiles et al 14 found no difference in VO2 consumption at rest across a range of tilt angles from 90° HUT to 6° HDT. Furthermore, our results are concordant with Prisk and his colleagues, who noted no significant change to VO2 between standing and supine on Earth, or in microgravity, in a study of 8 subjects on space shuttle missions Spacelab Life Sciences‐1 (SLS‐1) and SLS‐2. 77 Based on these findings, we preliminarily conclude that VO2, and more broadly pulmonary function, is more dependent on the gravitational vector in the direction (which also supports our experimental results indicating VO2 differences between prone and supine positions) than in the direction, most likely as a result of the weight of the thoracic cavity. 78
HRV indices provide additional insight into autonomic responses to changes in gravitational loads. Our results indicate that, in general, HRV increases with increasing angles of HDT (shown by the increase in HRVTi; Figure 2C). The increase in SDNN (Figure 2A) points to a combined increase in sympathetic and vagal activity. 45 Results indicate, based on changes in RMSSD (Figure 2B) and HF (Figure 3B and 3D), that vagal activity increases with HDT. Conversely, although the total power spectral density in the LF band increases with HDT (Figure 3A), we noticed that, in normalized units (Figure 3C), LF power decreases with increasing HDT. The Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology 45 recommends using LF in absolute units as an index of total sympathovagal activity, whereas LFNorm is more indicative as marker for sympathetic activity only. Taking all the indices together, results indicate an increase in vagal activity and sympathetic withdrawal with HDT. Once again, comparison with previous literature is precluded by the limited number of studies considering HRV in graded tilt. Our results are congruent with Sharma et al, 79 who considered 10° and 70° HUT, finding an increase in sympathetic activity (increased LFNorm and LF/HF ratio) and vagal withdrawal (decreased HFNorm and RMSSD) combined with an overall decrease in autonomic activity (decreased SDNN), compared with the 0° supine position. Similarly, Malhotra et al 80 found a decrease in sympathetic activity in 30° HDT (decrease in LFNorm) compared with the 0° supine position, whereas both Mosqueda‐Garcia et al 73 and Saito et al 81 found an increase in sympathetic activity in HUT. We also noted an increase in BRS (Figure 2D) from 6.6±0.8 ms/mm Hg (95% CI, 5.3–8.3 ms/mm Hg) at 45° HUT to 17.1±2.0 ms/mm Hg (95% CI, 13.6–21.5 ms/mm Hg) at 45° HDT. This is congruent with Schroeder et al, 30 who noted that HUT suppressed baroreflex sensitivity. Our values in the seated position (baseline) and horizontal 0° closely match those given in a review by Rovere et al. 49 Although our study did not differentiate between the relative sensitivity of the 2 divisions of the autonomic system, O'Leary et al 82 suggest that during HUT, the sympathetic arm is the dominant mediating cardiovascular control.
Differences in supine versus prone positioning on cardiac function also match what has been previously reported in the literature. Dharmavaram et al 83 compared HR, SV, and CO between 0° supine and a variety of prone positioning systems designed for spinal surgery. Although they do not use a control group (ie, simple 0° prone position with no positioning device), their data using the Jackson spinal table and the longitudinal bolster are the most insightful (horizontal position, body anatomically straight). They reported a nonsignificant decrease of 0.5±0.6 L/min in cardiac output from supine to prone on the Jackson table, which closely matches our 0.54±0.25 L/min (95% CI, −0.15 to 1.23 L/min; P=0.202) decrease from supine to prone at 0°. Furthermore, they reported a decrease of 7.2±4.7 mL in SV on the Jackson table, and 14.8±6.6 mL using the longitudinal bolster. These values are in close agreement with our 13.1±4.0 mL (95% CI, 2.2–24.1 mL; P=0.005) decrease from supine to prone at 0°. It must be noted that Dharmavaram et al 83 also found a decrease in HR of 6±3 bpm from supine to prone (Jackson table) compared with the increase that we found at 0° (5.7±1.9 bpm; 95% CI, 0.5–10.9 bpm; P=0.008). We hypothesize that this decrease could potentially be attributable to differences in their methodology; patients were in an anesthetized state, with supine measurements performed first and prone measurements performed sometime later. Fentanyl, vecuronium, and thiopental used by Dharmavaram et al 83 in the anesthetization process have varying temporal effects on HR and autonomic function. 84 , 85 , 86 Further studies found no change in blood pressure between the prone and supine positions, but increased TPR and reduced SV and CO in the prone position compared with the supine position. 87 , 88 , 89 , 90 Studies by Sudheer et al, 89 Schaefer et al, 90 Yap et al, 91 and Pump et al 88 also found increased HR in the prone position. These data are all in agreement with our findings. Taken together, the hemodynamic differences between prone and supine are likely explained by compression of the thorax and inferior vena cava, reducing venous return. 90 , 92 This is combined with attenuated pulsation of the arteries while in the prone position, further inhibiting baroreflex function, leading to increased sympathetic nervous system activity and hence an increase in HR and vascular resistance. 88 Blood pressure is maintained between prone and supine postures via the concomitant reflexive peripheral vasoconstriction. 92 We further suggest that the increase in VO2 seen in the prone position may be a result of this thorax compression. Studies have shown that limited thorax compression can increase ventilatory efficiency by reducing expiratory cost, 93 and the additional pressure on the musculature of the thorax may lead to a dyspnea sensation in subjects, reflexively inducing hyperventilation and thus increasing VO2. 94 Pump et al 88 further suggested that these findings identify a limitation of the 6° HDT microgravity analog in that cardiac function is regulated by the gravitational vector in the direction as well as the direction. Our results also support this conclusion. Furthermore, we found RMSSD to be significantly higher in the supine position. Viewing RMSSD as an index of vagal activity, this supports the hypothesis of vagal withdrawal because of baroreflex inhibition when prone, leading to a higher HR compared with the supine position.
Our study only considers angles from 45° HUT to 45° HDT; over this interval the tilt angle and its respective sine differ by <10%. Therefore, we construct dose‐response curves using the sine of the tilt angle rather than the angle itself to capture the underlying mechanisms as accurately as possible. This follows the methodology of multiple other studies including Critchley et al 95 (mean arterial pressure, HR, and SV), Khurana et al 96 (HR, SBP, and DBP), and Smith et al 97 (SV, CO, and TPR). In line with these studies, if we were to expand our range of measurement from 90° HUT to 90° HDT, we would hypothesize to see the linear trend continue in the sine of the angle between ±1. Thus, this would set maximal and minimal responses when angle is represented on a linear scale. These dose‐response curves form a comprehensive baseline for the range of hemodynamic parameters and autonomic indices across a range of tilt angles representing a change in the direction of the gravitational vector.
The development of dose‐response curves for cardiovascular and autonomic function is an important stage in the development of countermeasures for cardiovascular degradation during spaceflight. In particular, cardiac deconditioning is currently limited by a mixture of aerobic and resistive exercise on the International Space Station, 98 , 99 , 100 but mass and volume constraints necessitate novel concepts to protect astronaut health on future missions to the Moon and Mars. 101 Furthermore, spaceflight associated neuro‐ocular syndrome, the name given to the collective set of degenerative ocular changes that occur in long‐duration spaceflight, is widely hypothesized to be associated with the cephalad fluid shift caused by removal of hydrostatic gradients. 15 , 102 , 103 Potential countermeasures, such as lower body negative pressure 104 , 105 , 106 , 107 or short radius centrifugation, 39 , 41 , 42 , 108 may provide relief from these degrading effects. However, to accurately measure their effectiveness, it is necessary to have a comprehensive baseline to support countermeasure development.
Tilt studies also have important application outside of human spaceflight. Multiple surgeries, including lower abdominal surgery, central venous catheter placement, and minimally invasive glaucoma surgery, are often performed in the Trendelenburg position (ie, 15°–30° HDT) to facilitate access to pelvic organs and/or improve surgeon positioning. However, there is controversy over the efficacy of this positioning and its potential adverse complications. 109 , 110 , 111 Similarly, the use of a Wilson frame (or equivalent) in back surgery places the patient in a prone position where the head is hydrostatically lower than the heart. 83 , 112 A greater understanding of the hemodynamic and operative response to such conditions may improve perioperative management and clinical decision making. 113 Finally, the recent COVID‐19 pandemic has brought to public attention the practice of proning patients with SARS‐CoV‐2 to improve respiratory function. 114 These dose‐response curves provide a reference from nonpathological subjects that can be used for clinical management purposes to assess expected hemodynamic and autonomic variation. This has applications in surgical cases such as Trendelenburg positioning or surgeries using spinal frames, along with tests of orthostatic response. 113
Limitations
We acknowledge several limitations of our study. First, our study population consisted of only male subjects, which limits variability in our results. However, Arzeno et al 115 noted sex differences in baroreflexive control of blood pressure. On the other hand, Patel et al 116 found no significant effect of sex on autonomic indices in a protocol involving HUT, HDT, and lower body negative pressure. Future work should examine the impact of sex on the metrics considered as well as their underlying physiological mechanisms. Second, our study only measured acute responses. Much literature has reported on the long‐term effects of altered gravity environments, and our investigation did not consider those hemodynamic changes with a longer time course, for example cardiovascular degradation, 4 , 76 reduction in total blood volume because of endocrine response, or long‐term autonomic changes. 7 , 31 , 117 However, we believe that understanding and mapping the physiological mechanisms behind the acute response to altered gravity still provides insight into the expected response to countermeasures. Furthermore, there are also situations in spaceflight where optimal operational performance is critical immediately after a gravity transition (for example, immediately after entering orbit or landing), when the acute response is dominant.
The entire study was conducted using noninvasive methods. Future work should consider the addition of more invasive measurements to improve accuracy and provide additional dose‐response relationships. In particular, a direct measurement of cardiac output would provide the most accurate dose‐response relationship, because there are observable differences between the results of different methodologies. 16 , 118 Furthermore, for the autonomic measures, samples of blood plasma catecholamines, and other neurohormones, along with intracellular magnesium levels, 119 , 120 could provide further insights into cardiovascular control. Finally, invasive measurement of central venous pressure would provide informative data on cardiac loading conditions and thoracic blood volume. 121 , 122
CONCLUSIONS
We implemented a tilt paradigm to investigate the acute changes in multiple hemodynamic parameters and autonomic indices across a range of 45° HUT to 45° HDT in both supine and prone positions. Our data revealed a strong gravitational dependence of almost all metrics considered, explained by cephalad fluid shift in HDT combined with alterations of baroreflex function. Based on the experimental data collected, we constructed gravitational dose‐response curves for all variables across the tilt ranges considered. Furthermore, we confirmed statistically significant differences between supine and prone positions in HR, SV, CO, TPR, and VO2, but not in blood pressure. This difference is likely attributable to thorax compression inhibiting baroreflex function while prone, leading to an increase in sympathetic activity. These findings lead to a greater understanding of acute cardiovascular hemodynamics in altered gravity, whereas the gravitational dose‐response curves provide a unique and comprehensive baseline to support spaceflight countermeasure development, as well as other Earth applications, such as terrestrial surgery in prone or HDT positions.
Sources of Funding
This work was supported by the National Aeronautics and Space Administration Human Research Program, grant 80NSSC20K1521.
Disclosures
None.
Supporting information
Table S1
Acknowledgments
Author contributions: R. S. Whittle and Dr Diaz‐Artiles contributed to the study conception and design. Material preparation, data collection, and analyses were performed by R. S. Whittle, N. Keller, E. A. Hall, H. S. Vellore, L. M. Stapleton, Dr Findlay, and Dr Diaz‐Artiles. The first draft of the article was written by R. S. Whittle, and all authors commented on previous versions of the article. All authors read and approved the final article.
For Sources of Funding and Disclosures, see page 15.
References
- 1. Whittle RS, Diaz‐Artiles A. Modeling individual differences in cardiovascular response to gravitational stress using a sensitivity analysis. J Appl Physiol. 2021;130:1983–2001. doi: 10.1152/japplphysiol.00727.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hargens AR, Richardson S. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respir Physiol Neurobiol. 2009;169:S30–S33. doi: 10.1016/j.resp.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 3. Norsk P, Asmar A, Damgaard M, Christensen NJ. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J Physiol. 2015;593:573–584. doi: 10.1113/jphysiol.2014.284869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verheyden B, Liu J, Beckers F, Aubert AE. Adaptation of heart rate and blood pressure to short and long duration space missions. Respir Physiol Neurobiol. 2009;169:S13–S16. doi: 10.1016/j.resp.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 5. Karemaker JM, Berecki‐Gisolf J. 24‐h blood pressure in Space: the dark side of being an astronaut. Respir Physiol Neurobiol. 2009;169:S55–S58. doi: 10.1016/j.resp.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 6. Buckey JC, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW, Meyer DM, Blomqvist CG. Central venous pressure in space. J Appl Physiol. 1996;81:19–25. doi: 10.1152/jappl.1996.81.1.19 [DOI] [PubMed] [Google Scholar]
- 7. Eckberg DL, Halliwill JR, Beightol LA, Brown TE, Taylor JA, Goble R. Human vagal baroreflex mechanisms in space. J Physiol. 2010;588:1129–1138. doi: 10.1113/jphysiol.2009.186650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Limper U, Moestl S, Tank J, Prisk GK, Heusser K, Hoffmann F, Goßmann A, Migeotte P‐F, Gauger P, Beck LEJ, et al. A 20‐year evolution of cardiac performance in microgravity in a male astronaut. Clin Auton Res. 2020;31:139–141. doi: 10.1007/s10286-019-00657-1 [DOI] [PubMed] [Google Scholar]
- 9. Iwase S, Nishimura N, Tanaka K, Mano T. Effects of microgravity on human physiology. Beyond LEO—Hum Heal Issues Deep Sp Explor [Working Title]. 2020. doi: 10.5772/INTECHOPEN.90700 [DOI]
- 10. Hodkinson PD, Anderton RA, Posselt BN, Fong KJ. An overview of space medicine. Br J Anaesth. 2017;119:i143–i153. doi: 10.1093/bja/aex336 [DOI] [PubMed] [Google Scholar]
- 11. Shiraishi M, Schou M, Gybel M, Christensen N, Norsk P. Comparison of acute cardiovascular responses to water immersion and head‐down tilt in humans. J Appl Physiol. 2002;92:264–268. doi: 10.1152/jappl.2002.92.1.264 [DOI] [PubMed] [Google Scholar]
- 12. Marshall‐Goebel K, Ambarki K, Eklund A, Malm J, Mulder E, Gerlach D, Bershad E, Rittweger J. Analogs of microgravity: space research without leaving the planet: effects of short‐term exposure to head‐down tilt on cerebral hemodynamics: a prospective evaluation of a spaceflight analog using phase‐contrast MRI. J Appl Physiol. 2016;120:1466. doi: 10.1152/japplphysiol.00841.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marshall‐Goebel K, Mulder E, Bershad E, Laing C, Eklund A, Malm J, Stern C, Rittweger J. Intracranial and intraocular pressure during various degrees of head‐down tilt. Aerosp Med Hum Perform. 2017;88:10–16. doi: 10.3357/AMHP.4653.2017 [DOI] [PubMed] [Google Scholar]
- 14. Diaz‐Artiles A, Navarro Tichell P, Perez F. Cardiopulmonary responses to sub‐maximal ergometer exercise in a hypo‐gravity analog using head‐down tilt and head‐up tilt. Front Physiol. 2019;10:720. doi: 10.3389/fphys.2019.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen LG, Whittle RS, Lee JH, Sieker J, Carlson J, Finke C, Shelton CM, Petersen JCG, Diaz‐Artiles A. Gravitational effects on intraocular pressure and ocular perfusion pressure. J Appl Physiol. 2022;132:24–35. doi: 10.1152/japplphysiol.00546.2021 [DOI] [PubMed] [Google Scholar]
- 16. Whittle RS, Stapleton LM, Petersen LG, Diaz‐Artiles A. Indirect measurement of absolute cardiac output during exercise in simulated altered gravity is highly dependent on the method. J Clin Monit Comput. 2021. doi: 10.1007/s10877-021-00769-y [DOI] [PubMed] [Google Scholar]
- 17. Shankhwar V, Singh D, Deepak KK, Shankhwar V, Singh D, Deepak KK. Immediate changes in cardiac autonomic tone and stroke volume during microgravity simulation using head‐down tilt. Indian J Physiol Pharmacol. 2021;65:86–93. doi: 10.25259/IJPP_2_2021 [DOI] [Google Scholar]
- 18. Prisk GK, Fine JM, Elliott AR, West JB. Effect of 6 degrees head‐down tilt on cardiopulmonary function: comparison with microgravity. Aviat Space Environ Med. 2002;73:8–16. [PubMed] [Google Scholar]
- 19. Amirova L, Navasiolava N, Rukavishvikov I, Gauquelin‐Koch G, Gharib C, Kozlovskaya I, Custaud M‐A, Tomilovskaya E. Cardiovascular system under simulated weightlessness: head‐down bed rest vs. dry immersion. Front Physiol. 2020;11:395. doi: 10.3389/fphys.2020.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meck JV, Dreyer SA, Warren LE. Long‐duration head‐down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med. 2009;80:A01–A08. doi: 10.3357/ASEM.BR01.2009 [DOI] [PubMed] [Google Scholar]
- 21. Marshall‐Goebel K, Mulder E, Donoviel D, Strangman G, Suarez JI, Rao CV, Frings‐Meuthen P, Limper U, Rittweger J, Bershad EM. An international collaboration studying the physiological and anatomical cerebral effects of carbon dioxide during head‐down tilt bed rest: the SPACECOT study. J Appl Physiol. 2017;122:1398–1405. doi: 10.1152/japplphysiol.00885.2016 [DOI] [PubMed] [Google Scholar]
- 22. Basner M, Stahn AC, Nasrini J, Dinges DF, Moore TM, Gur RC, Mühl C, Macias BR, Laurie SS. Effects of head‐down tilt bed rest plus elevated CO2 on cognitive performance. J Appl Physiol. 2021;130:1235–1246. doi: 10.1152/japplphysiol.00865.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laurie SS, Christian K, Kysar J, Lee SMC, Lovering AT, Macias BR, Moestl S, Sies W, Mulder E, Young M, et al. Unchanged cerebrovascular CO2 reactivity and hypercapnic ventilatory response during strict head‐down tilt bed rest in a mild hypercapnic environment. J Physiol. 2020;598:2491–2505. doi: 10.1113/JP279383 [DOI] [PubMed] [Google Scholar]
- 24. Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effects of head‐down‐tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol. 1997;83:2139–2145. doi: 10.1152/jappl.1997.83.6.2139 [DOI] [PubMed] [Google Scholar]
- 25. Vijayalakshmi P, Madanmohan. Acute effect of 30 degrees, 60 degrees and 80 degrees head‐down tilt on blood pressure in young healthy human subjects. Indian J Physiol Pharmacol. 2006;50:28–32. [PubMed] [Google Scholar]
- 26. Nagaya K, Wada F, Nakamitsu S, Sagawa S, Shiraki K. Responses of the circulatory system and muscle sympathetic nerve activity to head‐down tilt in humans. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1289–R1294. doi: 10.1152/ajpregu.1995.268.5.R1289 [DOI] [PubMed] [Google Scholar]
- 27. Zaidi A, Benitez D, Gaydecki PA, Vohra A, Fitzpatrick AP. Haemodynamic effects of increasing angle of head up tilt. Heart. 2000;83:181–184. doi: 10.1136/heart.83.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hainsworth R, Al‐Shamma YMH. Cardiovascular responses to upright tilting in healthy subjects. Clin Sci. 1988;74:17–22. doi: 10.1042/cs0740017 [DOI] [PubMed] [Google Scholar]
- 29. Van LJJ, Harms MPM, Pott F, Jenstrup M, Secher NH. Stroke volume of the heart and thoracic fluid content during head‐up and head‐down tilt in humans. Acta Anaesthesiol Scand. 2005;49:1287–1292. doi: 10.1111/j.1399-6576.2005.00841.x [DOI] [PubMed] [Google Scholar]
- 30. Schroeder C, Tank J, Boschmann M, Diedrich A, Sharma AM, Biaggioni I, Luft FC, Jordan J. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105:347–353. doi: 10.1161/hc0302.102597 [DOI] [PubMed] [Google Scholar]
- 31. Convertino VA, Doerr DF, Eckberg DL, Fritsch JM, Vernikos‐Danellis J. Head‐down bed rest impairs vagal baroreflex responses and provokes orthostatic hypotension. J Appl Physiol. 1990;68:1458–1464. doi: 10.1152/jappl.1990.68.4.1458 [DOI] [PubMed] [Google Scholar]
- 32. Anderson AP, Swan JG, Phillips SD, Knaus DA, Kattamis NT, Toutain‐Kidd CM, Zegans ME, Fellows AM, Buckey JC. Acute effects of changes to the gravitational vector on the eye. J Appl Physiol. 2016;120:939–946. doi: 10.1152/japplphysiol.00730.2015 [DOI] [PubMed] [Google Scholar]
- 33. Buckey JC, Phillips SD, Anderson AP, Chepko AB, Archambault‐Leger V, Gui J, Fellows AM. Microgravity‐induced ocular changes are related to body weight. Am J Physiol Regul Integr Comp Physiol. 2018;315:R496–R499. doi: 10.1152/ajpregu.00086.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tugrul M, Camci E, Pembeci K, Al‐Darsani A, Telci L. Relationship between peripheral and central venous pressures in different patient positions, catheter sizes, and insertion sites. J Cardiothorac Vasc Anesth. 2004;18:446–450. doi: 10.1053/j.jvca.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 35. Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kästner S, Godau J, Schüler M, Tryba M, Gehling M. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21:186–191. doi: 10.1007/s12028-014-0004-x [DOI] [PubMed] [Google Scholar]
- 36. Nekludov M, Bellander BM, Mure M. Oxygenation and cerebral perfusion pressure improved in the prone position. Acta Anaesthesiol Scand. 2006;50:932–936. doi: 10.1111/j.1399-6576.2006.01099.x [DOI] [PubMed] [Google Scholar]
- 37. Diaz‐Artiles A. Exercise under artificial gravity—experimental and computational approaches. PhD thesis. 2015.
- 38. Whittle RS, Diaz‐Artiles A. Metabolic modeling in altered gravity. IEEE Aerosp Conf. 2020;2020:1–17. doi: 10.1109/AERO47225.2020.9172582 [DOI] [Google Scholar]
- 39. Diaz A, Trigg C, Young LR. Combining ergometer exercise and artificial gravity in a compact‐radius centrifuge. Acta Astronaut. 2015;113:80–88. doi: 10.1016/j.actaastro.2015.03.034 [DOI] [Google Scholar]
- 40. Diaz Artiles A, Heldt T, Young LR. Effects of artificial gravity on the cardiovascular system: computational approach. Acta Astronaut. 2016;126:395–410. doi: 10.1016/j.actaastro.2016.05.005 [DOI] [Google Scholar]
- 41. Diaz‐Artiles A, Heldt T, Young LR. Short‐term cardiovascular response to short‐radius centrifugation with and without ergometer exercise. Front Physiol. 2018;9:1492. doi: 10.3389/fphys.2018.01492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diaz‐Artiles A, Heldt T, Young LR. Computational model of cardiovascular response to centrifugation and lower‐body cycling exercise. J Appl Physiol. 2019;127:1453–1468. doi: 10.1152/japplphysiol.00314.2019 [DOI] [PubMed] [Google Scholar]
- 43. Ansari M, Javadi H, Pourbehi M, Mogharrabi M, Rayzan M, Semnani S, Jallalat S, Amini A, Abbaszadeh M, Barekat M, et al. The association of rate pressure product (RPP) and myocardial perfusion imaging (MPI) findings: a preliminary study. Perfusion. 2012;27:207–213. doi: 10.1177/0267659112436631 [DOI] [PubMed] [Google Scholar]
- 44. Widjaja D, Vandeput S, Van Huffel S, Aubert AE. Cardiovascular autonomic adaptation in lunar and martian gravity during parabolic flight. Eur J Appl Physiol. 2015;115:1205–1218. doi: 10.1007/s00421-015-3118-8 [DOI] [PubMed] [Google Scholar]
- 45. TFESCNASPE . Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology: heart rate variability—standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 46. Berntson GG, Lozano DL, Chen Y‐J. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology. 2005;42:246–252. doi: 10.1111/j.1469-8986.2005.00277.x [DOI] [PubMed] [Google Scholar]
- 47. Swenne CA. Baroreflex sensitivity: mechanisms and measurement. Neth Heart J. 2013;21:58. doi: 10.1007/s12471-012-0346-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eckberg DL, Sleight P. Human baroreflexes in health and disease. Clarendon Press; 1992:1–572. [Google Scholar]
- 49. La RMT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Westerhof B, Gisolf J, Stok W, Wesseling K, Karemaker J. Time‐domain cross‐correlation baroreflex sensitivity: performance on the EUROBAVAR data set. J Hypertens. 2004;22:1371–1380. doi: 10.1097/01.hjh.0000125439.28861.ed [DOI] [PubMed] [Google Scholar]
- 51. Fernández E, Neto ES, Abry P, Macchiavelli R, Balzarini M, Cuzin B, Baude C, Frutoso J, Gharib C. Assessing erectile neurogenic dysfunction from heart rate variability through a Generalized Linear Mixed Model framework. Comput Methods Programs Biomed. 2010;99:49–56. doi: 10.1016/j.cmpb.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 52. Oh DY, Park SM, Choi SW. Daytime neurophysiological hyperarousal in chronic insomnia: a study of qEEG. J Clin Med. 2020;9:3425. doi: 10.3390/jcm9113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park SM, Jung HY. Respiratory sinus arrhythmia biofeedback alters heart rate variability and default mode network connectivity in major depressive disorder: a preliminary study. Int J Psychophysiol. 2020;158:225–237. doi: 10.1016/j.ijpsycho.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 54. Barbeau DY, Krueger C, Huene M, Copenhaver N, Bennett J, Weaver M, Weiss MD. Heart rate variability and inflammatory markers in neonates with hypoxic‐ischemic encephalopathy. Physiol Rep. 2019;7:e14110. doi: 10.14814/PHY2.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed‐effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 56. Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. doi: 10.1080/01621459.1955.10501294 [DOI] [Google Scholar]
- 57. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 58. László Z, Rössler A, Hinghofer‐Szalkay HG. Cardiovascular and hormonal changes with different angles of head‐up tilt in men. Physiol Res. 2001;50:71–82. [PubMed] [Google Scholar]
- 59. R Core Team . R: A Language and Environment for Statistical Computing. 2021. Available at: https://www.r‐project.org/. Accessed July 1, 2021. [Google Scholar]
- 60. Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R J. 2017;9:378–400. doi: 10.32614/RJ-2017-066 [DOI] [Google Scholar]
- 61. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26. [Google Scholar]
- 62. Hartig F. DHARMa: residual diagnostics for hierarchical (multi‐level/mixed) regression models. 2021. Available at: https://cran.r‐project.org/package=DHARMa. Accessed July 1, 2021.
- 63. Lenth RV. emmeans: estimated marginal means, aka least‐squares means. 2021. Available at: https://cran.r‐project.org/package=emmeans. Accessed July 1, 2021.
- 64. Porta A, Tobaldini E, Guzzetti S, Furlan R, Montano N, Gnecchi‐Ruscone T. Assessment of cardiac autonomic modulation during graded head‐up tilt by symbolic analysis of heart rate variability. Am J Physiol Heart Circ Physiol. 2007;293:702–708. doi: 10.1152/ajpheart.00006.2007 [DOI] [PubMed] [Google Scholar]
- 65. Yamazaki F, Matsumura N, Nagata J, Ando A, Imura T. Spontaneous arterial baroreflex control of the heart rate during head‐down tilt in heat‐stressed humans. Eur J Appl Physiol. 2001;85:208–213. doi: 10.1007/s004210100482 [DOI] [PubMed] [Google Scholar]
- 66. London G, Levenson J, Safar M, Simon A, Guerin A, Payen D. Hemodynamic effects of head‐down tilt in normal subjects and sustained hypertensive patients. Am J Physiol. 1983;245:H194–H202. doi: 10.1152/AJPHEART.1983.245.2.H194 [DOI] [PubMed] [Google Scholar]
- 67. Tuckman J, Shillingford J. Effect of different degrees of tilt on cardiac output, heart rate, and blood pressure in normal man. Br Heart J. 1966;28:32. doi: 10.1136/hrt.28.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bundgaard‐Nielsen M, Sørensen H, Dalsgaard M, Rasmussen P, Secher NH. Relationship between stroke volume, cardiac output and filling of the heart during tilt. Acta Anaesthesiol Scand. 2009;53:1324–1328. doi: 10.1111/j.1399-6576.2009.02062.x [DOI] [PubMed] [Google Scholar]
- 69. Rowell LB. Human Cardiovascular Control. Oxford University Press; 1993. [Google Scholar]
- 70. Olufsen MS, Alston AV, Tran HT, Ottesen JT, Novak V. Modeling heart rate regulation—part I: sit‐to‐stand versus head‐up tilt. Cardiovasc Eng. 2007;8:73–87. doi: 10.1007/s10558-007-9050-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hall JE. Guyton and Hall Textbook of Medical Physiology. 13th ed. Elsevier; 2016. [Google Scholar]
- 72. Mukai S, Hayano J. Heart rate and blood pressure variabilities during graded head‐up tilt. J Appl Physiol. 1995;78:212–216. doi: 10.1152/jappl.1995.78.1.212 [DOI] [PubMed] [Google Scholar]
- 73. Mosqueda‐Garcia R, Furlan R, Fernandez‐Violante R, Desai T, Snell M, Jarai Z, Ananthram V, Robertson RM, Robertson D. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;99:2736–2744. doi: 10.1172/JCI119463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Printz MP, Jaworski RL. Hypertension; overview. In: Encyclopedia of Endocrine Diseases. Reference module in biomedical sciences. Vol. 3, 2nd ed. Amsterdam, Netherlands: Elsevier; 2018:369–380. doi: 10.1016/B978-0-12-801238-3.03801-0 [DOI] [Google Scholar]
- 75. Silvani A. Sleep disorders, nocturnal blood pressure, and cardiovascular risk: a translational perspective. Auton Neurosci. 2019;218:31–42. doi: 10.1016/j.autneu.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 76. Gallo C, Ridolfi L, Scarsoglio S. Cardiovascular deconditioning during long‐term spaceflight through multiscale modeling. NPJ Microgravity. 2020;6:1–14. doi: 10.1038/s41526-020-00117-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Prisk GK, Elliott AR, Guy HJ, Kosonen JM, West JB. Pulmonary gas exchange and its determinants during sustained microgravity on Spacelabs SLS‐1 and SLS‐2. J Appl Physiol. 1995;79:1290–1298. doi: 10.1152/jappl.1995.79.4.1290 [DOI] [PubMed] [Google Scholar]
- 78. Prisk GK, Paiva M, West JB. Gravity and the Lung: Lessons From Microgravity. Marcel Dekker, Inc.; 2001. [Google Scholar]
- 79. Sharma P, Paudel B, Singh P, Linmbu P. Heart rate variability: response to graded head up tilt in healthy men. Kathmandu Univ Med J. 2009;7:252–257. doi: 10.3126/kumj.v7i3.2733 [DOI] [PubMed] [Google Scholar]
- 80. Malhotra V, Thakare AE, Hulke SM, Wakode SL, Parashar R, Ravi N. Effect of head down tilt on heart rate variability. J Family Med Prim Care. 2021;10:439. doi: 10.4103/jfmpc.jfmpc_1642_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Saito M, Foldager N, Mano T, Iwase S, Sugiyama Y, Oshima M. Sympathetic control of hemodynamics during moderate head‐up tilt in human subjects. Environ Med. 1997;41:151–155. [PubMed] [Google Scholar]
- 82. O’Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head‐up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol. 2003;88:769–774. doi: 10.1113/eph8802632 [DOI] [PubMed] [Google Scholar]
- 83. Dharmavaram S, Jellish WS, Nockels RP, Shea J, Mehmood R, Ghanayem A, Kleinman B, Jacobs W. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: an echocardiographic study. Spine (Phila Pa 1976). 2006;31:1388–1393. doi: 10.1097/01.brs.0000218485.96713.44 [DOI] [PubMed] [Google Scholar]
- 84. Vettorello M, Colombo R, De Grandis C, Costantini E, Raimondi F. Effect of fentanyl on heart rate variability during spontaneous and paced breathing in healthy volunteers. Acta Anaesthesiol Scand. 2008;52:1064–1070. doi: 10.1111/j.1399-6576.2008.01713.x [DOI] [PubMed] [Google Scholar]
- 85. Cozanitis D, Pouttu J, Rosenberg P. Bradycardia associated with the use of vecuronium. A comparative study with pancuronium with and without glycopyrronium. Anaesthesia. 1987;42:192–194. doi: 10.1111/j.1365-2044.1987.tb02998.x [DOI] [PubMed] [Google Scholar]
- 86. Sharma S, Nair P, Murgai A, Selvaraj R. Transient bradycardia induced by thiopentone sodium: a unique challenge in the management of refractory status epilepticus. BMJ Case Rep. 2013;2013:bcr2013200484. doi: 10.1136/BCR-2013-200484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ma M, Noori S, Maarek J, Holschneider D, Rubinstein E, Seri I. Prone positioning decreases cardiac output and increases systemic vascular resistance in neonates. J Perinatol. 2015;35:424–427. doi: 10.1038/jp.2014.230 [DOI] [PubMed] [Google Scholar]
- 88. Pump B, Talleruphuus U, Christensen NJ, Warberg J, Norsk P. Effects of supine, prone, and lateral positions on cardiovascular and renal variables in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R174–R180. doi: 10.1152/ajpregu.00619.2001 [DOI] [PubMed] [Google Scholar]
- 89. Sudheer PS, Logan SW, Ateleanu B, Hall JE. Haemodynamic effects of the prone position: a comparison of propofol total intravenous and inhalation anaesthesia. Anaesthesia. 2006;61:138–141. doi: 10.1111/j.1365-2044.2005.04464.x [DOI] [PubMed] [Google Scholar]
- 90. Schaefer WM, Lipke CSA, Kühl HP, Koch K‐C, Kaiser H‐J, Reinartz P, Nowak B, Buell U. Prone versus supine patient positioning during gated 99m Tc‐sestamibi SPECT: effect on left ventricular volumes, ejection fraction, and heart rate. J Nucl Med. 2004;45:2016–2020. [PubMed] [Google Scholar]
- 91. Yap K, Campbell P, Cherk M, McGrath C, Kalff V. Effect of prone versus supine positioning on left ventricular ejection fraction (LVEF) and heart rate using ECG gated Tl‐201 myocardial perfusion scans and gated cardiac blood pool scans. J Med Imaging Radiat Oncol. 2012;56:525–531. doi: 10.1111/j.1754-9485.2012.02438.x [DOI] [PubMed] [Google Scholar]
- 92. Relton J, Conn A. Anaesthesia for the surgical correction of scoliosis by the Harrington method in children. Can Anaesth Soc J. 1963;10:603–615. doi: 10.1007/BF03002093 [DOI] [PubMed] [Google Scholar]
- 93. Malehorn K, Hiniker J, Mackey T, Heumann K, Murray S, Pettitt R. Kinesio Tape® applied to the thorax augments ventilatory efficiency during heavy exercise. Int J Exerc Sci. 2013;6:157–163. [Google Scholar]
- 94. West JB, Luks AM. Respiratory Physiology: The Essentials. 10th ed. Wolters Kluwer; 2016. [Google Scholar]
- 95. Critchley LAH, Conway F, Anderson PJ, Tomlinson B, Critchley JAJH. Non‐invasive continuous arterial pressure, heart rate and stroke volume measurements during graded head‐up tilt in normal man. Clin Auton Res. 1997;7:97–101. doi: 10.1007/BF02267754 [DOI] [PubMed] [Google Scholar]
- 96. Khurana RK, Nicholas Ba EM, Khurana RK. Head‐up tilt table test: how far and how long? Clin Auton Res. 1996;6:335–341. doi: 10.1007/BF02556304 [DOI] [PubMed] [Google Scholar]
- 97. Smith JJ, Hughes CV, Ptacin MJ, Barney JA, Tristani FE, Ebert TJ. The effect of age on hemodynamic response to graded postural stress in normal men. J Gerontol. 1987;42:406–411. doi: 10.1093/geronj/42.4.406 [DOI] [PubMed] [Google Scholar]
- 98. Diaz A, Heldt T, Young LR. Cardiovascular responses to artificial gravity combined with exercise. IEEE Aerosp Conf Proc. 2015;2015‐June:1–11. doi: 10.1109/AERO.2015.7118969 [DOI] [Google Scholar]
- 99. Keller N, McHenry N, Duncan C, Johnston A, Whittle RS, Koock E, Bhattacharya SS, De La Torre G, Ploutz‐Snyder L, Sheffield‐Moore M, et al. Augmenting exercise protocols with interactive virtual reality environments. IEEE Aerosp Conf Proc. 2021;2021‐March:1–13. doi: 10.1109/AERO50100.2021.9438234 [DOI] [Google Scholar]
- 100. Loerch LH. Exercise countermeasures on ISS: summary and future directions. Aerosp Med Hum Perform. 2015;86:A92–A94. doi: 10.3357/AMHP.EC12.2015 [DOI] [PubMed] [Google Scholar]
- 101. Laws JM, Caplan N, Bruce C, McGrogan C, Lindsay K, Wild B, Debuse D, Wotring V, Winnard A. Systematic review of the technical and physiological constraints of the Orion Multi‐Purpose Crew Vehicle that affect the capability of astronauts to exercise effectively during spaceflight. Acta Astronaut. 2020;170:665–677. doi: 10.1016/j.actaastro.2020.02.038 [DOI] [Google Scholar]
- 102. Lee AG, Mader TH, Gibson CR, Brunstetter TJ, Tarver WJ. Space flight‐associated neuro‐ocular syndrome (SANS). Eye. 2018;32:1164–1167. doi: 10.1038/s41433-018-0070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mader T, Gibson C, Miller N, Subramanian P, Patel N, Lee A. An overview of spaceflight‐associated neuro‐ocular syndrome (SANS). Neurol India. 2019;67:206. doi: 10.4103/0028-3886.259126 [DOI] [PubMed] [Google Scholar]
- 104. Greenwald SH, Macias BR, Lee SMC, Marshall‐Goebel K, Ebert DJ, Liu JHK, Ploutz‐Snyder RJ, Alferova IV, Dulchavsky SA, Hargens AR, et al. Intraocular pressure and choroidal thickness respond differently to lower body negative pressure during spaceflight. J Appl Physiol. 2021;131:613–620. doi: 10.1152/japplphysiol.01040.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Marshall‐Goebel K, Terlević R, Gerlach DA, Kuehn S, Mulder E, Rittweger J. Lower body negative pressure reduces optic nerve sheath diameter during head‐down tilt. J Appl Physiol. 2017;123:1139–1144. doi: 10.1152/japplphysiol.00256.2017 [DOI] [PubMed] [Google Scholar]
- 106. Petersen L, Hargens A, Bird E, Ashari N, Saalfeld J, Petersen J. Mobile lower body negative pressure suit as an integrative countermeasure for spaceflight. Aerosp Med Hum Perform. 2019;90:993–999. doi: 10.3357/AMHP.5408.2019 [DOI] [PubMed] [Google Scholar]
- 107. Petersen LG, Lawley JS, Lilja‐Cyron A, Petersen JCG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, et al. Lower body negative pressure to safely reduce intracranial pressure. J Physiol. 2019;597:237–248. doi: 10.1113/JP276557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Diaz‐Artiles A, Heldt T, Young LR. Effects of artificial gravity on the cardiovascular system: computational approach. Acta Astronaut. 2016;126:395–410. doi: 10.1016/j.actaastro.2016.05.005 [DOI] [Google Scholar]
- 109. Kim SY, Park SJ. The effect of the lithotomy‐trendelenburg position on respiratory and hemodynamic changes during general anesthesia. Korean J Anesthesiol. 2002;42:722. doi: 10.4097/kjae.2002.42.6.722 [DOI] [Google Scholar]
- 110. Reuter DA, Felbinger TW, Schmidt C, Moerstedt K, Kilger E, Lamm P, Goetz AE. Trendelenburg positioning after cardiac surgery: effects on intrathoracic blood volume index and cardiac performance. Eur J Anaesthesiol. 2003;20:17–20. doi: 10.1097/00003643-200301000-00003 [DOI] [PubMed] [Google Scholar]
- 111. Arvizo C, Mehta ST, Yunker A. Adverse events related to Trendelenburg position during laparoscopic surgery: recommendations and review of the literature. Curr Opin Obstet Gynecol. 2018;30:272–278. doi: 10.1097/GCO.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 112. DePasse JM, Palumbo MA, Haque M, Eberson CP, Daniels AH. Complications associated with prone positioning in elective spinal surgery. World J Orthop. 2015;6:351. doi: 10.5312/wjo.v6.i3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rozet I, Vavilala MS. Risks and benefits of patient positioning during neurosurgical care. Anesthesiol Clin. 2007;25:631–653. doi: 10.1016/j.anclin.2007.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Asar S, Acicbe O, Sabaz MS, Tontu F, Canan E, Cukurova Z, Cakar N. Comparison of respiratory and hemodynamic parameters of COVID‐19 and non‐COVID‐19 ARDS patients. Indian J Crit Care Med. 2021;25:704. doi: 10.5005/jp-journals-10071-23856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Arzeno NM, Stenger MB, Lee SMC, Ploutz‐Snyder R, Platts SH. Sex differences in blood pressure control during 6° head‐down tilt bed rest. J Appl Physiol. 2013;304:1114–1123. doi: 10.1152/ajpheart.00391.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Patel K, Rössler A, Lackner HK, Trozic I, Laing C, Lorr D, Green DA, Hinghofer‐Szalkay H, Goswami N. Effect of postural changes on cardiovascular parameters across gender. Medicine (Baltimore). 2016;95:e4149. doi: 10.1097/MD.0000000000004149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Eckberg DL, Diedrich A, Cooke WH, Biaggioni I, Buckey JC, Pawelczyk JA, Ertl AC, Cox JF, Kuusela TA, Tahvanainen KUO, et al. Respiratory modulation of human autonomic function: long‐term neuroplasticity in space. J Physiol. 2016;594:5629–5646. doi: 10.1113/JP271656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Geerts BF, Aarts LP, Jansen JR. Methods in pharmacology: measurement of cardiac output. Br J Clin Pharmacol. 2011;71:316–330. doi: 10.1111/j.1365-2125.2010.03798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Takase B, Akima T, Satomura K, Ohsuzu F, Mastui T, Ishihara M, Kurita A. Effects of chronic sleep deprivation on autonomic activity by examining heart rate variability, plasma catecholamine, and intracellular magnesium levels. Biomed Pharmacother. 2004;58:S35–S39. doi: 10.1016/S0753-3322(04)80007-6 [DOI] [PubMed] [Google Scholar]
- 120. Benditt DG, van Dijk JG, Krishnappa D, Adkisson WO, Sakaguchi S. Neurohormones in the pathophysiology of vasovagal syncope in adults. Front Cardiovasc Med. 2020;7:76. doi: 10.3389/fcvm.2020.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Su L, Pan P, Li D, Zhang Q, Zhou X, Long Y, Wang X, Liu D. Central venous pressure (CVP) reduction associated with higher cardiac output (CO) favors good prognosis of circulatory shock: a single‐center, retrospective cohort study. Front Med. 2019;6:216. doi: 10.3389/fmed.2019.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gaffney FA, Nixon JV, Karlsson ES, Campbell W, Dowdey ABC, Blomqvist CG. Cardiovascular deconditioning produced by 20 hours of bedrest with head‐down tilt (‐5°) in middle‐aged healthy men. Am J Cardiol. 1985;56:634–638. doi: 10.1016/0002-9149(85)91025-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


