Abstract
Background
Until recently, a large right ventricle outflow tract interfered with the feasibility of standard transcatheter pulmonary valve replacement (PVR). We are describing our experience using a hybrid approach for PVR using a left anterior thoracotomy approach to allow for plication of the main pulmonary artery followed by a transcatheter PVR using a Sapien S3 valve.
Methods and Results
This is a single‐center, retrospective review of patients who were evaluated to be appropriate for a hybrid PVR approach. The patients' demographics, procedure details, and follow‐up data were collected. Between May 2018 and April 2021, a total of 11 patients presented for hybrid transcatheter PVR. The median age and weight were 24 years (interquartile range, 19–43 years) and 81.8 kg (interquartile range, 69–91 kg), respectively. Nine out of 11 patients received a transcatheter PVR after main pulmonary artery plication. There were no procedurally related deaths. One major complication was encountered in which the valve was malpositioned requiring successful surgical PVR. Minor complications included acute kidney injury (n=1) and a broken rib (n=1). The median length of stay was 4 days (interquartile range, 2–4 days), with median follow‐up of 7 months (interquartile range, 3–18 months). A well‐functioning pulmonary valve was observed in all patients at the last follow‐up.
Conclusions
A hybrid approach using left anterior thoracotomy with pulmonary artery plication followed by transcatheter Sapien S3 PVR provides a less‐invasive option for patients with an enlarged right ventricular outflow tract. Preliminary results demonstrated this to be a safe option with good short‐term outcomes.
Keywords: congenital heart disease, hybrid, Sapien valve, tetralogy of Fallot, transcatheter
Subject Categories: Congenital Heart Disease, Valvular Heart Disease, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- MPA
main pulmonary artery
- PVR
pulmonary valve replacement
Clinical Perspective.
What Is New?
This is a single‐center experience showing consistent outcome using left anterior thoracotomy with main pulmonary artery plication as a hybrid approach for pulmonary valve replacement.
What Are the Clinical Implications?
Our approach provides a less invasive alternative option for patients with a large right ventricular outflow tract who are not candidates for a classic transcatheter approach.
Hybrid repairs in patients with congenital heart disease have emerged as methods that combine cardiac surgery and cardiac catheterization to provide less‐invasive options and better outcomes. 1 , 2 Although it has been >2 decades since the first transcatheter pulmonary valve replacement (PVR), this technology has benefited only one‐quarter of patients who require PVR. There are 2 common causes. The first is the risk of coronary and/or aortic compression by the transcatheter valve. The second and most frequent impediment is the lack commercially available valves large enough to be implanted via a transcatheter approach in patients with enlarged right ventricle outflow tracts (RVOTs). 3 , 4 There is a sizable number of patients with larger RVOTs who have historically required surgical valves for significant pulmonary insufficiency. These patients mostly fall into 2 groups, those who underwent tetralogy of Fallot repairs that included a transannular patch and patients born with congenital pulmonary valve stenosis after balloon valvuloplasty. Both anomalies can result in significant pulmonary insufficiency. Lately, these patients are being increasingly served by the development of advanced transcatheter valves and stents including the recently approved Harmony valve (Medtronic, Minneapolis, MN). 5 Another innovation that was also recently approved by the US Food and Drug Administration is the combined transcatheter placement of the Alterra stent along with the Sapien valve (Edwards Lifesciences, Irvine, CA). 6 Another approach includes the off‐label placement of isolated transcatheter valves with or without a stent. This is typically performed with the Sapien valve if a landing zone exists. 7 Many hybrid methods have also been developed to allow for the use of commercially available transcatheter devices in complex anatomies by either surgical alternation of the existing anatomy (typically off‐pump surgical plication or main pulmonary artery [MPA] banding) or surgically providing direct access to the heart through the right ventricle. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 We are presenting in this article our institution's experience using the hybrid approach for PVR in patients with a large RVOT. The method we adapted included a left anterior thoracotomy with pulmonary artery banding followed by transcatheter PVR using a Sapien S3 valve. In this case series, we demonstrated our systematic approach of patient selection, preparation, procedure details, and short‐term outcomes.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is a single‐center, retrospective review of our experience with surgical and catheter‐based hybrid PVR (Figure 1) between May 2018 and April 2021. Institutional review board approval was obtained according to the Journal of the American Heart Association (JAHA) guidelines to authors. No informed consent was required. All patients with significant pulmonary insufficiency (severe pulmonary insufficiency on echocardiogram and/or cardiac magnetic resonance imaging) who presented for treatment were offered for this hybrid approach, which incorporated a left anterior thoracotomy and MPA plication to facilitate a transcatheter PVR (Table). The data collected included demographics, medical and surgical history, details about prior diagnostic studies and multidisciplinary conference decisions, procedure details, recovery period, and details on the follow‐up visits. The procedural details include the surgical and catheter‐based interventional data such as total procedure duration, fluoroscopy time, radiation dose, contrast volume, hemodynamics, and angiograms. Procedure duration was defined as the time the patients entered the hybrid catheterization laboratory suite until the departure time. Length of stay was defined as the time from admission to discharge. Procedural safety was evaluated by reviewing any postprocedure adverse events.
Figure 1. Hybrid PVR cohort.
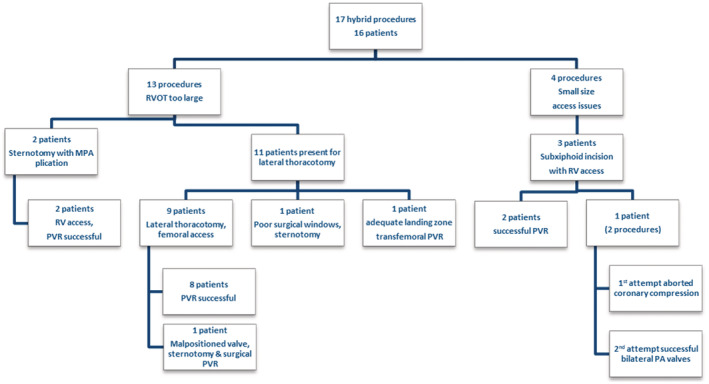
MPA indicates main pulmonary artery; PA, pulmonary arteries; PVR, pulmonary valve replacement; RV, right ventricle; and RVOT, right ventricular outflow tract.
Table 1.
Hybrid Main Pulmonary Artery Plication+Transcatheter Pulmonary Valve Replacement Cohort
| Patients | Diagnosis | Surgeries | No. of attempted plications | Balloon waist,mm | Prestent | Valve placed | Chest tube, d | Length of stay/ICU, d | PRBC/inotropes | Complications (%) | Other associated procedures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TOF | TAP | 2 | 26 | None | Sapien S3 29 mm | 1 | 4/1 | No/none | None | … |
| 2 | CPS | BTS, TAP | 2 | 26.9 | None | Sapien S3 29 mm | 2 | 5/2 | No/yes | Valve malposition, converted to surgy via sternotomy (11.1%) | … |
| 3 | TOF | Valvotomy | 5 | 27 | None | Sapien S3 29 mm | 2 | 2/1 | No/none | None | … |
| 4 | CPS | Valvotomy | 1 | 23.6 | None | Sapien S3 29 mm | 1 | 4/1 | No/none | None | ASD device closure |
| 5 | PA/IVS | BTS, TAP, ASD closure | 1 | 24.3 | None | Sapien S3 29 mm | 2 | 4/2 | No/yes | None | … |
| 6 | CPS | None | 2 | 22.9 | None | Sapien S3 29 mm | 1 | 1/1 | No/none | None | … |
| 7 | CPS | Valvotomy | 1 | 25 | None | Sapien S3 29 mm | 2 | 6/4 | No/yes | AKI during recovery (11.1%), broken rib (11.1%) | … |
| 8 | TOF | Brock, VSD closure | 2 | 25.1 | 10 Zig CP stent on 30 mm BIB | Sapien S3 29 mm | 1 | 2/1 | No/yes | None | ICD generator replacement |
| 9 | CPS | None | 1 | 25.6 | None | Sapien S3 29 mm | 2 | 3/1 | No/none | None | … |
AKI indicates acute kidney injury; ASD, atrial septal defect; BTS, Blalock‐Taussig shunt; BIB, balloon‐in‐balloon catheter; CPS, congenital pulmonary valve stenosis; ICD, implantable cardioverter‐defibrillator; ICU, intensive care unit; PA/IVS, pulmonary atresia with intact ventricular septum; PRBC, packed red blood cell transfusion; TAP, transannular patch; TOF, tetralogy of Fallot; and VSD, ventricular septal defect; and 10 zig CP stent, 10 zig configuration Cheatham‐Platinum covered stent.
Procedure
Evaluation of each patient involved a multidisciplinary team approach reviewing patients' medical records and all the relevant imaging (computed tomography angiography and/or magnetic resonance imaging) studies to determine a patient’s candidacy for the hybrid approach. Candidacy for our hybrid approach included meeting criteria for PVR and having a large RVOT that is not amenable to regular transcatheter PVR using balloon expandable devices. On the day of the procedure, catheterization, surgical, and cardiopulmonary bypass personnel were present in the catheterization laboratory. All potentially necessary cardiopulmonary bypass equipment was also present on standby. A general anesthetic was administered, and invasive lines were placed in accordance with our protocols for a patient potentially having a redo sternotomy and necessitating cardiopulmonary bypass. All of the patients were intubated using a double‐lumen endotracheal tube to selectively ventilate the right lung and enhance visualization of the structures in the left chest. The groin, chest, and abdomen were prepped in case a full sternotomy was required if the hybrid procedure could not be accomplished. The interventional cardiology team typically placed a 7‐Fr sheath in the right femoral vein and 5‐Fr sheaths in both the left femoral vein and artery. A right heart catheterization was performed followed by pulmonary artery angiography (Figure 2A and 2B). If the pulmonary artery measurement was <27 to 28 mm, balloon sizing was performed using a 0.035‐inch Lunderquest wire (Cook Medical, Bloomington, IN). If the balloon measurement was >28 mm, we proceeded to surgical plication. We positioned a radio‐opaque clip at the mid axillary line at the level of the nipple, and then using the lateral fluoroscopy camera measured a distance from the marker to the area that we intended to plicate (Figure 2B). This measurement was used to help the surgeons choose the appropriate intercostal space incision site. The surgical team created a 5‐ to 7‐cm left anterior thoracotomy incision, typically at the second intercoastal space (Figure 3). The pericardium was longitudinally opened anterior to the left phrenic nerve. In patients with previous cardiac surgical history, the left appendage may have been adhered to the left posterior aspect of the MPA; if so, this was carefully separated from the MPA. The MPA was then plicated.
Figure 2. Serial angiograms showing pre‐ and postsurgical plication.
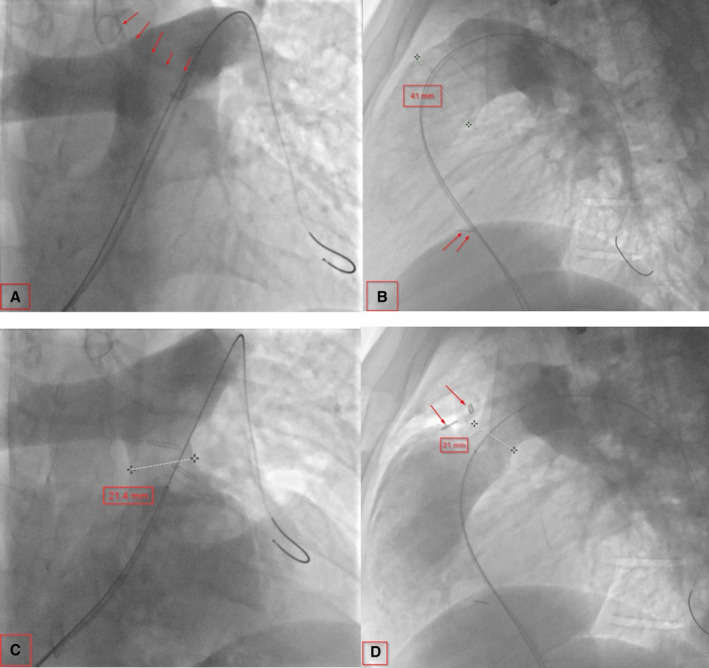
A, Baseline AP angiogram showing a large main pulmonary artery (MPA). The arrow indicates the double‐lumen endotracheal tube. B, Baseline lateral angiogram showing a large MPA. The arrow indicates an outside clip at the level of the nipple. C, After MPA plication on AP. D, After MPA plication on lateral; the arrows indicate surgical clips at the site of plication. AP indicates anterioposterior view.
Figure 3. Schematic figure of the surgical plication technique.
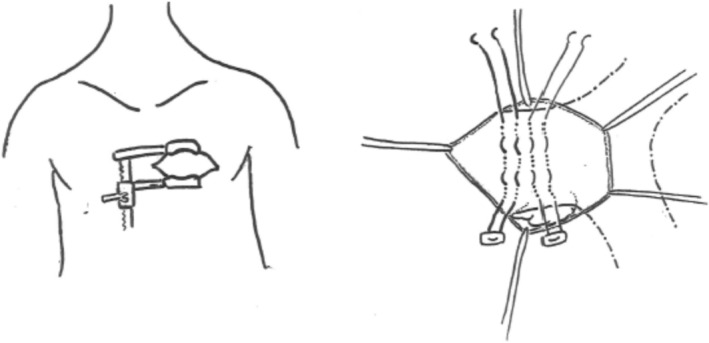
In patients with a preserved native pulmonary root where a transannular patch was not performed, pulmonary valve sinuses were identifiable, which gave us a landmark for the plication site. However, in patients with a previous history of transannular patch repair, a dissection of the right ventricle infundibulum inferiorly to identify the border between the right ventricle muscle and the posterior native MPA was necessary. MPA plication was performed using 3‐0 Ethibond (Ethicon, Somerville, NJ) horizontal mattress pledgeted sutures placed longitudinally to achieve a 2‐cm length landing zone. It was felt that plication of the MPA for a 2‐cm length would provide a safety advantage over a shorter, discrete area (Figure 2C and 2D). In addition, the surgical team placed a radio‐opaque marker at the suture site that was visible on fluoroscopy at the time of valve placement (Figure 2D). The catheterization team then performed a simultaneous balloon sizing of the plicated pulmonary artery and an aortic root angiogram to rule out coronary compression (Figure 4A and 4B). If the plicated pulmonary artery diameter continued to be too large on angiogram and/or balloon sizing, the surgical team would adjust the caliber of the plicated area. If the landing zone was deemed appropriate (typically 24–27 mm), the transvenous transcatheter PVR was completed, followed by either intracardiac echocardiogram or pulmonary and right ventricle angiograms (Figure 4C and 4D) to check for placement and perivalvular leak.
Figure 4. Transcatheter pulmonary valve replacement (PVR).
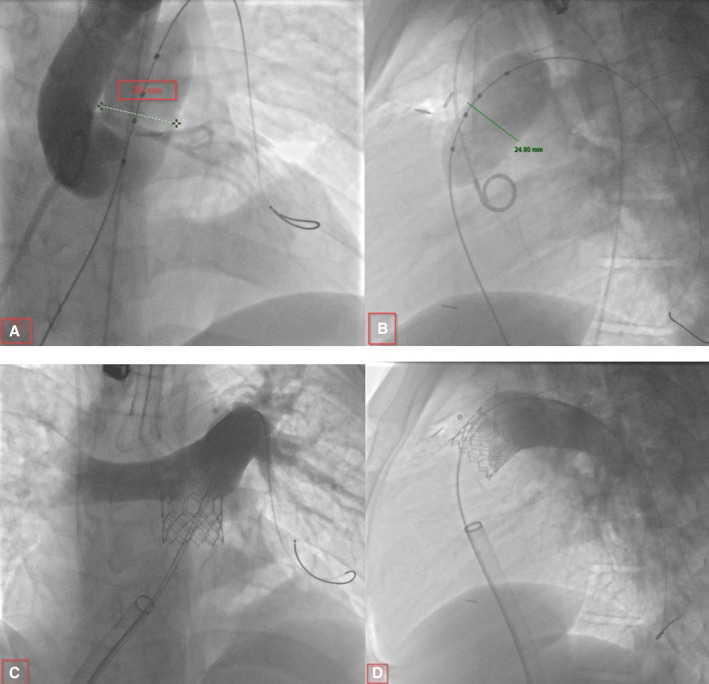
A and B, Balloon sizing with simultaneous aortic root angiogram for coronary compression testing. C and D, Main pulmonary artery angiogram showing a competent valve after transcatheter PVR.
The incision site was then closed, a chest tube was placed, and a nerve block was performed by the surgeon to help control postoperative pain. Typically, femoral vein hemostasis was achieved using a figure‐of‐8 suture. The patients who were appropriate candidates for extubation at the end of the procedure had their stomach thoroughly suctioned and were then extubated while still anesthetized but spontaneously breathing. This was done to avoid coughing. A portable chest x‐ray was obtained the same night of the procedure and follow‐up echocardiogram the next day. Ordinarily, the chest tube was removed the following day at the surgeon's discretion. The timing of discharge was based on assessments of the patient's condition made by both the interventional and surgical teams. All patients were discharged on 81 mg of aspirin. Follow‐up appointments were typically scheduled with cardiothoracic surgery and cardiology 4 weeks after hospital discharge.
RESULTS
A total of 11 patients were offered a hybrid PVR using left anterior thoracotomy with MPA plication (Figure 1). Upon balloon sizing, it was determined that 1 patient did not require MPA plication and underwent a conventional transcatheter PVR using a 29‐mm Sapien S3 valve. During surgical dissection, another patient was found to have anatomy that was not amenable to exposure of the MPA via left anterior thoracotomy and so was converted to surgical PVR via sternotomy. The remaining 9 patients who underwent left anterior thoracotomy, MPA plication, and transcatheter PVR using a 29‐mm Sapien S3 valve are the focus of this study.
The median age of the 11 patients at the time of the procedure was 24 years (interquartile range [IQR], 19–43 years) and the median weight was 81.8 kg (IQR, 69–91 kg). The final 9‐patient cohort included 3 born with tetralogy of Fallot, 5 with congenital pulmonary valve stenosis, and 1 with congenital pulmonary atresia with intact ventricular septum. Three patients had a previous transannular patch as part of their initial repair, 4 underwent a surgical valvotomy, and 2 patients only had a balloon valvuloplasty (Table) for palliation of their congenital lesion. Four patients had successful plication sizing on the first attempt, 4 other patients needed 2 times, and 1 patient required 5 attempts. The median waist observed with balloon sizing after acceptable MPA plication was 25.1 mm (IQR, 24.3–26 mm). Only 1 patient underwent stenting before placement of a valve (5010 Palmaz stent). All patients received a 29‐mm Sapien S3 valve. Two patients underwent additional procedures during the hybrid PVR, transcatheter atrial septal defect closure in 1, and implantable cardioverter‐defibrillator generator change in another patient.
The median contrast given during the procedure was 3.3 mL/kg (IQR, 2.4–4.2 mL/kg), fluoroscopy time was 50.5 minutes (IQR, 13.7–54.2 minutes), radiation exposure was 5235.6 cGy/cm2 (IQR, 1970.2–9667.25 cGy/cm2), and procedure length was 359 minutes (IQR, 216–450 minutes). None of the patients required a blood transfusion during these procedures. A total of 4 patients were extubated at the procedure’s end (as described previously), and the rest were extubated within 24 hours.
A major complication occurred in only 1 patient (11.1%) whose deployed valve became malpositioned and then failed transcatheter repositioning attempts. This patient underwent a successful surgical removal of the Sapien valve and then operative implantation of a bioprosthetic 27‐mm porcine Medtronic bioprosthetic valve. Additionally, 1 patient (11.1%) developed transient acute kidney injury, and another patient (11.1%) suffered a fractured rib at the thoracotomy site. Four patients (44.4%) required temporary use of inotropes during the procedure, 2 of these patients with a median age 58 years and comorbidities and another younger patient (20 years old) who was thought to have transient pulmonary hypertension during selective right lung ventilation for surgical plication. The fourth patient was the one who required conversion to a surgical PVR after the deployed valve became malpositioned (Table).
The median length of stay was 4 days (IQR, 2–4 days), with the longest length of 6 days being related to acute kidney injury management. The second longest stay was 5 days in the patient who required surgical placement via sternotomy and removal of the malpositioned valve. The median follow up was 7 months (IQR, 3–18 months). Transthoracic echocardiogram findings of follow‐up demonstrated adequate valve function in all of the patients. The median peak pressure gradient through these valves was 17 mm Hg (IQR, 16–17 mm Hg), and the mean pressure gradient was 9 mm Hg (IQR, 8–10 mm Hg). None of the patients had more than mild pulmonary insufficiency.
DISCUSSION
We believe that for patients with significant pulmonary insufficiency with dilated native RVOTs, a transcatheter PVR using MPA plication via a left anterior thoracotomy provided a safe alternative treatment to an entirely surgical valve replacement. Our protocol demonstrated consistent outcomes as the result of thorough understanding of the patients’ anatomy. To date, this is the largest case series of hybrid PVR using MPA plication via lateral thoracotomy and transcatheter PVR using the Sapien S3 valve.
Historically, hybrid PVR was performed using different surgical approaches for pulmonary artery size reduction. These approaches included full sternotomy for MPA banding with or without RV access, subxyphoid incision with RV access, and more recently, lateral thoracotomy for MPA plication. 10 , 11 , 13 , 14 , 15 , 17 , 18 , 19 , 20 The method studied in this article avoids cardiopulmonary bypass and sternotomy. By avoiding cardiopulmonary bypass, the potential sequences from systemic inflammatory process, including end‐organs injury, can be eliminated. In addition, the potential need for blood transfusion using this method is less in the absence of a complication. It also offers the potential for shorter hospital stays and faster return to full activity, because less restriction is needed compared with sternotomy. 21 , 22 Although subjective, patients consistently voiced a high satisfaction, which was the case especially with patients who had previously undergone an open cardiac procedure.
The surgical field access for MPA plication via left anterior thoracotomy can be challenging in patients who have undergone prior cardiac surgeries. Significant calcification can be present in patients who have had prior transannular patch repair. These calcifications can interfere with reliable suture placement leading to an increased risk of postoperative bleeding. The hybrid approach was abandoned, and instead an open surgical valve placement was performed in 1 of our patients because of poor exposure and patch calcifications. An additional challenge is that because the plication sutures are placed along the anterior lateral aspect of the MPA, it does not create an easily recognizable landing zone for the valve. A complete main pulmonary artery band would potentially provide a more reliable landing zone. This would require extensive surgical dissection to provide circumferential exposure of the MPA, which is not easily achieved from the mini anterior thoracotomy incision we presently use. Although surgical plication has been our approach, complete MPA banding has been achieved by Serfas et al in their 2‐case series through left lateral thoracotomy. 14 We believe that the fluoroscopic balloon sizing evaluation we perform has helped us overcome this issue. A complete encirclement of the MPA with a band was only used at our institution when full sternotomies were performed, and these cases are not included in this study cohort.
As a result of these challenges, we have adjusted our procedural criteria and we now avoid offering this option to patients who have undergone a previous transannular patch and who have had multiple surgeries. We believe the ideal candidates are those who have previously undergone pulmonary balloon valvuloplasty for congenital pulmonary stenosis and surgical repairs that did not incorporate patch material as part of an MPA. We continue to consider candidates for this hybrid approach who did not fulfill our modified criteria, but the alternative, a completely surgical approach, is deemed to bear a high morbidity and/or mortality risk for valve replacement.
Another challenge involved with this approach was how to determine the optimal amount of surgical plication necessary to achieve the appropriate vessel caliber for PVR. Our typical goal is to place a 29‐mm Sapien S3 valve, and therefore, our targeted waist upon balloon sizing is 24 to 27 mm. Frequently, there was a need to serially adjust plication sutures multiple times to achieve a tighter waist before proceeding with PVR. There was only 1 case where there was a need to loosen the sutures to accommodate a larger valve. It is our observation that the need for multiple adjustments decreased over time, reflecting the accumulation of surgical experience. We have also found that ensuring an adequate length of vessel wall plication helps support a secure implantation. This is especially important when using the 29‐mm Sapien S3 valve, because it is relatively short and measures 22.5 mm in height. This might have prevented the movement of one of the valves to a more distal position than intended, as noticed during a postimplantation angiogram interrogation. Transcatheter repositioning of the valve was attempted, but conversion to a fully surgical correction with sternotomy and cardiopulmonary bypass was required. This hybrid method also allows for the application of sutures through the vessel wall to further secure the valve and/or stent. We used this reinforcing method once to secure a stent in a patient with a large RVOT and branch pulmonary arteries before proceeding with PVR.
In the recent era, multiple devices designed for deployment on native RVOT tissue have been approved by the Food and Drug Administration or are under clinical trial. The Harmony valve, which was recently approved by the Food and Drug Administration, is a self‐expanding porcine pericardium valve sutured within a nitinol frame and sewn onto a polyester fabric tube. It comes in 2 sizes, transcatheter pulmonary valve 22 mm and transcatheter pulmonary valve 25 mm. 5 , 23 The Alterra stent, which is self‐expandable and has a nitinol frame with a polyethylene terephthalate fabric covering was also recently approved by the Food and Drug Administration. The design of the stent is to provide a reduction and reconfiguration of the RVOT to provide a stable landing zone for the 29‐mm Sapien S3 valve. 6 , 24 , 25 The Venus P valve made by Venus Medtech (Shanghai, China) is also a self‐expanding valve with multiple sizes. 26
In the early feasibility study of transcatheter PVR of the Harmony valve, only 24% of the patients screened were enrolled into the study, and 8% underwent implant. 27 Combining early feasibility and the pivotal studies, 71 out of 340 (21%) enrolled patients underwent cardiac catheterization for valve placement. 5 , 27 Similarly, 52% of the screened patient were enrolled in the Alterra study. 6 More recent data, although not published, suggested a passing rate of 9% for the 22‐mm Harmony valve and up to 75% for the 25‐mm Harmony valve as per Dr Cheatham’s presentation at the Society for Cardiovascular Angiography and Interventions in 2020.
With the developing technology of transcatheter PVR for native RVOT, the expectation would be a decline in the need for such a hybrid approach. Nonetheless, these devices do not cover all anatomy, and for certain cases, the hybrid approach will still be a valid option.
Limitations
This is a small, retrospective patient cohort at a single institution. Because of the small sample size, we were unable to perform subgroup analysis such as comparing the length of stay and outcomes in patients who underwent sternotomy/subxiphoid access versus left anterior thoracotomy. We were also unable to compare this group with classic surgical valve placement with sternotomy including the length of stay and analgesics use.
CONCLUSIONS
A hybrid approach via left anterior thoracotomy and MPA plication serves as a less‐invasive alternative for patients who are not candidates for a traditional transcatheter approach. Based on this small cohort, a hybrid PVR approach appears to be technically feasible and safe as demonstrated by the good short‐term outcomes. Further studies are required to refine the procedure and to verify our findings.
Sources of Funding
None.
Disclosures
None.
This article was sent to John S. Ikonomidis, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Kalfa D, Torres AJ. Indications and results for hybrid interventions in patients with congenital heart disease. Arch Cardiovasc Dis. 2020;113:96–103. doi: 10.1016/j.acvd.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 2. Holoshitz N, Kenny D, Hijazi ZM. Hybrid interventional procedures in congenital heart disease. Methodist Debakey Cardiovasc J. 2014;10:93–98. doi: 10.14797/mdcj-10-2-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right‐ventricle to pulmonary‐artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0 [DOI] [PubMed] [Google Scholar]
- 4. McElhinney DB, Hennesen JT. The Melody(R) valve and Ensemble(R) delivery system for transcatheter pulmonary valve replacement. Ann N Y Acad Sci. 2013;1291:77–85. doi: 10.1111/nyas.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benson LN, Gillespie MJ, Bergersen L, Cheatham SL, Hor KN, Horlick EM, Weng S, McHenry BT, Osten MD, Powell AJ, et al. Three‐year outcomes from the harmony native outflow tract early feasibility study. Circ Cardiovasc Interv. 2020;13:e008320. doi: 10.1161/CIRCINTERVENTIONS.119.008320 [DOI] [PubMed] [Google Scholar]
- 6. Shahanavaz S, Balzer D, Babaliaros V, Kim D, Dimas V, Veeram Reddy SR, Leipsic J, Blanke P, Shirali G, Parthiban A, et al. Alterra adaptive prestent and SAPIEN 3 THV for congenital pulmonic valve dysfunction: an early feasibility study. JACC Cardiovasc Interv. 2020;13:2510–2524. doi: 10.1016/j.jcin.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 7. Shahanavaz S, Zahn EM, Levi DS, Aboulhousn JA, Hascoet S, Qureshi AM, Porras D, Morgan GJ, Bauser Heaton H, Martin MH, et al. Transcatheter pulmonary valve replacement with the sapien prosthesis. J Am Coll Cardiol. 2020;76:2847–2858. doi: 10.1016/j.jacc.2020.10.041 [DOI] [PubMed] [Google Scholar]
- 8. Abu‐Anza O, Carr K, Aldoss O. Combined hybrid pulmonary valve placement and atrial septal defect closure: case report and literature review. Cardiol Young. 2020;30:737–739. doi: 10.1017/S1047951120000724 [DOI] [PubMed] [Google Scholar]
- 9. Berman DP, Burke R, Zahn EM. Use of a novel hybrid approach to salvage an attempted transcatheter pulmonary valve implant. Pediatr Cardiol. 2012;33:839–842. doi: 10.1007/s00246-012-0224-9 [DOI] [PubMed] [Google Scholar]
- 10. Breatnach CR, McGuinness J, Ng LY, Franklin O, Redmond M, Nolke L, McMahon C, Oslizlok P, Walsh K, Kenny D. Procedural technique for hybrid pulmonary valve replacement in infants and small children. Eur J Cardiothorac Surg. 2021;59:823–830. doi: 10.1093/ejcts/ezaa410 [DOI] [PubMed] [Google Scholar]
- 11. Guzeltas A, Tanidir IC, Gokalp S, Haydin S. Hybrid transcatheter pulmonary valve implantation: the first case series from Turkey. Anatol J Cardiol. 2018;20:190–191. doi: 10.14744/AnatolJCardiol.2018.06981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McElhinney DB. Hybrid transcatheter pulmonary valve replacement: moving into the mainstream? Catheter Cardiovasc Interv. 2016;88:811–813. doi: 10.1002/ccd.26816 [DOI] [PubMed] [Google Scholar]
- 13. Porras D, Gurvitz M, Marshall AC, Emani SM. Hybrid approach for off‐pump pulmonary valve replacement in patients with a dilated right ventricular outflow tract. Ann Thorac Surg. 2015;100:e99–e101. doi: 10.1016/j.athoracsur.2015.02.124 [DOI] [PubMed] [Google Scholar]
- 14. Serfas JD, Turek J, Haney J, Krasuski RA, Fleming GA. Hybrid transcatheter pulmonary valve replacement with a SAPIEN S3 valve after pulmonary artery banding via left lateral thoracotomy. Catheter Cardiovasc Interv. 2020;95:E78–E83. doi: 10.1002/ccd.28591 [DOI] [PubMed] [Google Scholar]
- 15. Sosnowski C, Matella T, Fogg L, Ilbawi M, Nagaraj H, Kavinsky C, Wolf AR, Diab K, Caputo M, Kenny D. Hybrid pulmonary artery plication followed by transcatheter pulmonary valve replacement: comparison with surgical PVR. Catheter Cardiovasc Interv. 2016;88:804–810. doi: 10.1002/ccd.26620 [DOI] [PubMed] [Google Scholar]
- 16. Thalmann R, Merkel EM, Akra B, Bombien R, Kozlik‐Feldmann RG, Schmitz C. Evaluation of hybrid surgical access approaches for pulmonary valve implantation in an acute swine model. Comp Med. 2019;69:299–307. doi: 10.30802/AALAS-CM-18-000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suleiman T, Kavinsky CJ, Skerritt C, Kenny D, Ilbawi MN, Caputo M. Recent development in pulmonary valve replacement after tetralogy of fallot repair: the emergence of hybrid approaches. Front Surg. 2015;2:22. doi: 10.3389/fsurg.2015.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips AB, Nevin P, Shah A, Olshove V, Garg R, Zahn EM. Development of a novel hybrid strategy for transcatheter pulmonary valve placement in patients following transannular patch repair of tetralogy of fallot. Catheter Cardiovasc Interv. 2016;87:403–410. doi: 10.1002/ccd.26315 [DOI] [PubMed] [Google Scholar]
- 19. Dittrich S, Gloeckler M, Arnold R, Sarai K, Siepe M, Beyersdorf F, Schlensak C. Hybrid pulmonary valve implantation: injection of a self‐expanding tissue valve through the main pulmonary artery. Ann Thorac Surg. 2008;85:632–634. doi: 10.1016/j.athoracsur.2007.08.010 [DOI] [PubMed] [Google Scholar]
- 20. Travelli FC, Herrington CS, Ing FF. A novel hybrid technique for transcatheter pulmonary valve implantation within a dilated native right ventricular outflow tract. J Thorac Cardiovasc Surg. 2014;148:e145–e146. doi: 10.1016/j.jtcvs.2014.04.046 [DOI] [PubMed] [Google Scholar]
- 21. Olds A, Saadat S, Azzolini A, Dombrovskiy V, Odroniec K, Lemaire A, Ghaly A, Lee LY. Improved operative and recovery times with mini‐thoracotomy aortic valve replacement. J Cardiothorac Surg. 2019;14:91. doi: 10.1186/s13019-019-0912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al Otaibi A, Gupta S, Belley‐Cote EP, Alsagheir A, Spence J, Parry D, Whitlock RP. Mini‐thoracotomy vs. conventional sternotomy mitral valve surgery: a systematic review and meta‐analysis. J Cardiovasc Surg (Torino). 2017;58:489–496. doi: 10.23736/S0021-9509.16.09603-8 [DOI] [PubMed] [Google Scholar]
- 23. Gillespie MJ, Bergersen L, Benson LN, Weng S, Cheatham JP. 5‐Year outcomes from the harmony native outflow tract early feasibility study. JACC Cardiovasc Interv. 2021;14:816–817. doi: 10.1016/j.jcin.2021.01.046 [DOI] [PubMed] [Google Scholar]
- 24. Eicken A, Ewert P. Size matters‐new percutaneous catheter treatment for large dysfunctional right ventricular outflow tracts: alterra plus sapien. JACC Cardiovasc Interv. 2020;13:2525–2527. doi: 10.1016/j.jcin.2020.06.043 [DOI] [PubMed] [Google Scholar]
- 25. Zahn EM, Chang JC, Armer D, Garg R. First human implant of the Alterra Adaptive Prestent(TM): a new self‐expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018;91:1125–1129. doi: 10.1002/ccd.27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgan G, Prachasilchai P, Promphan W, Rosenthal E, Sivakumar K, Kappanayil M, Sakidjan I, Walsh KP, Kenny D, Thomson J, et al. Medium‐term results of percutaneous pulmonary valve implantation using the Venus P‐valve: international experience. EuroIntervention. 2019;14:1363–1370. doi: 10.4244/EIJ-D-18-00299 [DOI] [PubMed] [Google Scholar]
- 27. Gillespie MJ, Benson LN, Bergersen L, Bacha EA, Cheatham SL, Crean AM, Eicken A, Ewert P, Geva T, Hellenbrand WE, et al. Patient selection process for the harmony transcatheter pulmonary valve early feasibility study. Am J Cardiol. 2017;120:1387–1392. doi: 10.1016/j.amjcard.2017.07.034 [DOI] [PubMed] [Google Scholar]


