Abstract
Background
Pulmonary and cardiac functions decline with age, but the associations of pulmonary dysfunction with cardiac function and heart failure (HF) risk in late life is not known. We aimed to determine the associations of percent predicted forced vital capacity (ppFVC) and the ratio of forced expired volume in 1 second (FEV1) to forced vital capacity (FVC; FEV1/FVC) with cardiac function and incident HF with preserved or reduced ejection fraction in late life.
Methods and Results
Among 3854 HF‐free participants in the ARIC (Atherosclerosis Risk in Communities) cohort study who underwent echocardiography and spirometry at the fifth study visit (2011–2013), associations of FEV1/FVC and ppFVC with echocardiographic measures, cardiac biomarkers, and risk of HF, HF with preserved ejection fraction, and HF with reduced ejection fraction were assessed. Multivariable linear and Cox regression models adjusted for demographics, body mass index, coronary disease, atrial fibrillation, hypertension, and diabetes. Mean age was 75±5 years, 40% were men, 19% were Black, and 61% were ever smokers. Mean FEV1/FVC was 72±8%, and ppFVC was 98±17%. In adjusted analyses, lower FEV1/FVC and ppFVC were associated with higher NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; both P<0.001) and pulmonary artery pressure (P<0.004). Lower ppFVC was also associated with higher left ventricular mass, left ventricular filling pressure, and high‐sensitivity C‐reactive protein (all P<0.01). Lower FEV1/FVC was associated with a trend toward higher risk of incident HF with preserved ejection fraction (hazard ratio [HR] per 10‐point decrease, 1.31; 95% CI, 0.98–1.74; P=0.07) and HF with reduced ejection fraction (HR per 10‐point decrease, 1.24; 95% CI, 0.91–1.70; P=0.18), but these associations did not reach statistical significance. Lower ppFVC was associated with incident HF with preserved ejection fraction (HR per 10‐unit decrease, 1.21; 95% CI, 1.04–1.41; P=0.013) but not with HF with reduced ejection fraction (HR per 10‐unit decrease, 0.90; 95% CI, 0.76–1.07; P=0.24).
Conclusions
Subclinical reductions in FEV1/FVC and ppFVC differentially associate with cardiac function and HF risk in late life.
Keywords: cardiopulmonary, elderly, heart dysfunction, lung function, respiratory disease
Subject Categories: Heart Failure, Aging, Epidemiology, Echocardiography, Pulmonary Biology
Nonstandard Acronyms and Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- CARDIA
Coronary Artery Risk Development in Young Adults
- FEV1/FVC
forced expired volume in 1 second and Forced vital capacity ratio
- Health ABC
Health, Aging, and Body Composition
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- JHS
Jackson Heart Study
- MESA
Multi‐Ethnic Study of Atherosclerosis
- PASP
estimated pulmonary arterial systolic pressure
- PIROUETTE
Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction
- ppFVC
percent predicted forced vital capacity
Clinical Perspective
What Is New?
In a community‐based cohort free of heart failure (HF) or pulmonary disease, both obstructive and restrictive spirometric patterns associate with higher pulmonary pressure, higher NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and higher risk of incident HF.
A restrictive spirometric pattern is further associated with greater left ventricular mass and filling pressure, and with a higher incidence of HF with preserved ejection fraction beyond traditional risk factors.
What Are the Clinical Implications?
Subclinical lung dysfunction in late life, assessed using low cost and widely available spirometry, may help to identify older adults at increased risk of HF beyond traditional risk factors.
Restrictive spirometric pattern in particular is associated with diastolic dysfunction and heighted risk of incident HF with preserved ejection fraction, the most prevalent HF type in late life.
Prospective studies are necessary to determine whether pulmonary dysfunction represents a modifiable risk factor for HF and whether interventions targeting pulmonary dysfunction decrease risk of HF, and HF with preserved ejection fraction in particular, in older adults.
Subclinical impairments in pulmonary function detected by spirometry are associated with alterations in cardiac structure and function and cardiovascular events in early adulthood and midlife. 1 , 2 Among persons in early and midlife, an obstructive spirometric pattern reflected in a reduction in the forced expired volume in 1 second/forced vital capacity ratio (FEV1/FVC) has been associated with left ventricular (LV) underfilling and lower cardiac output, 3 even in the absence of a clinical diagnosis of chronic obstructive pulmonary disease or asthma. In contrast, reduced vital capacity, as seen in restrictive phenotypes, has been associated with increased LV mass and higher cardiac output. 2 Reductions in FVC have been independently associated with cardiovascular events 4 and HF hospitalizations in particular, 5 and these associations appear more robust than those with obstructive spirometric patterns. 6 In addition, the rate of subclinical decline in both FEV1/FVC and FVC is associated with heightened risk of cardiovascular events, such that the risk of incident HF in particular is higher in rapid decliners. 1 , 5
The prevalence of HF increases exponentially with age, disproportionately burdening the elderly, among whom HF with preserved ejection fraction (HFpEF) accounts for up to 80% of prevalent HF. 7 Age‐related alterations in both cardiac and pulmonary structure and function are well described. 8 , 9 However, the extent to which subclinical lung‐heart interactions previously described in early life and midlife extend into late life is unclear. 10 Furthermore, pulmonary dysfunction—and reduced FEV1/FVC in particular—has previously been associated with risk of incident HFpEF compared with HF with reduced ejection fraction (HFrEF). 11 However, few data exist as to whether reduced FVC and FEV1/FVC differentially associate with incident HFpEF in late life.
We hypothesized that worse FEV1/FVC and FVC differently associate with cardiac structure and function and consequently with incident HF phenotypes in late life. We leveraged the comprehensive phenotyping of elderly participants in the ARIC (Atherosclerosis Risk in Communities) study to (1) define the extent to which FEV1/FVC and FVC assessed in late life, and their change from midlife to late life, associate with cardiac structure and function in late life; and (2) determine their associations with incident HFpEF and HFrEF in late life.
Methods
Detailed policies for requesting ARIC study data can be found at https://sites.cscc.unc.edu/aric/pubs‐policies‐and‐forms‐pg. ARIC study data can also be obtained from the National Heart, Lung, and Blood Institute BioLINCC repository (https://biolincc.nhlbi.nih.gov/studies/aric/).
Study Population
ARIC is an ongoing cohort that enrolled 15 792 participants from 4 US communities between 1987 and 1989. 12 This analysis included 3854 HF‐free participants who underwent echocardiography and spirometry at the fifth study visit (2011–2013; age ≥65 years) (Figure S1). For analyses using FEV1/FVC as the primary exposure (obstructive ventilatory pattern), we excluded participants with an FVC below the lower limits of normal from the National Health and Nutrition Examination Survey III equation 13 , 14 to avoid mixed deficits, resulting in 3476 participants. Conversely, for analyses with percent predicted FVC (ppFVC) as the primary exposure (restrictive pattern), we excluded participants with FEV1/FVC below the National Health and Nutrition Examination Survey III lower limit of normal on the basis of age, sex, race, and height, leaving 3325 participants in this analysis. The ARIC study was approved by institutional review boards from each site, and all participants provided written informed consent.
Clinical Characteristics
Methods for ascertaining participant characteristics and laboratory measures in ARIC have previously been described 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 and are detailed in Data S1. All laboratory measures were performed at visit 5.
Assessment of Lung Function
Lung function was assessed using spirometry at ARIC visits 1 (1987–1989), 2 (1990–1992), and 5 (2011–2013) following standard protocols and American Thoracic Society quality criteria. 13 Employed equipment and methods are detailed in Data S1. Predicted values for all 3 visits derived from National Health and Nutrition Examination Survey III equations. 14 The primary exposures were FEV1/FVC and ppFVC assessed at visit 5. Secondary analyses further assessed longitudinal changes in these variables by calculating the differences between the visit 5 and the highest of visit 1 and 2 values.
Assessment of Cardiac Structure and Function
Echocardiography at visit 5 has been described in detail. 23 All studies were acquired using uniform imaging equipment and acquisition protocol. 24 , 25 Images were analyzed in a dedicated echocardiography reading center, blinded to clinical information, in accordance with the American Society of Echocardiography recommendations. 26 , 27
Incident HF After Visit 5
Incident HF after visit 5 was based on active surveillance of hospitalizations and by participant self‐report. Hospitalizations with International Classification of Diseases (ICD) codes associated with HF undergo chart abstraction and adjudication by centrally trained and certified physicians as previously described. 21 , 22 LV ejection fraction (LVEF) at the time of hospitalization was abstracted. If LVEF at time of hospitalization was unavailable, then the most recent LVEF available within 6 months of the index hospitalization was used if no intercurrent myocardial infarction was present. Death was ascertained through the National Death Index. Participants were followed up through December 31, 2018.
Statistical Analysis
Participant characteristics at visit 5 were described according to sex‐specific quartiles of FEV1/FVC and ppFVC, with tests for trend adjusted for demographics (age, sex, and race). FEV1/FVC is expressed as a percentage, in which the ratio was multiplied by 100. For associations with echocardiographic outcomes, additional models further adjusted for body mass index, current or prior smoking, hypertension, diabetes, atrial fibrillation, and log‐transformed NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and high‐sensitivity C‐reactive protein. The continuous associations between FEV1/FVC and ppFVC with echocardiographic measurements were assessed using restricted cubic splines for possible nonlinear associations. Similar analyses were performed using spirometry change as the exposure.
For associations with incident HF, Cox proportional hazards regression models adjusted for demographics, and then additionally for obesity, coronary disease, atrial fibrillation, diabetes, hypertension, and NT‐proBNP. We quantified the magnitude to which each covariate attenuated the association of ppFVC with incident HF by comparing the ppFVC model coefficient in models with or without each covariate. All models adjusted for demographics and 95% confidence intervals were derived from 2000‐bootstrap samples. Nonlinear associations were investigated using restricted cubic splines with the number of knots selected to minimize the model Akaike information criterion (3 to 7 knots tested). The proportional hazards assumption was tested for all models using Schoenfeld residuals.
Statistical analysis was performed using STATA 14.2 (StataCorp LP, College Station, TX).
Results
Population Characteristics
Among the 3854 participants included, age was 75.0±5.0 years, 60% were women, 19% were Black, and 61% were ever smokers (Table S1). Participants excluded because of missing or poor‐quality spirometry or echocardiography or to prevalent HF were older, more frequently Black, with a higher body mass index (BMI), higher prevalence of cardiovascular and metabolic diseases, and higher levels of high‐sensitivity C‐reactive protein and NT‐proBNP (Table S1). Sex and smoking status were similar between those included and excluded.
Obstructive Ventilatory Pattern
Among the 3476 participants without restrictive deficits, the average FEV1/FVC was 72.5±8.3, FVC was 3.1±0.9 L, and ppFVC was 100±15%. Of participants in quartile 1 of FEV1/FVC, 75% had FEV1/FVC >60%. Lower FEV1/FVC was associated with older age and non‐Black race. Accounting for age, sex, and race, lower FEV1/FVC was also associated with smoking and atrial fibrillation but less diabetes and lower BMI (Table 1). In adjusted models, lower FEV1/FVC associated with higher NT‐proBNP but was not independently associated with high‐sensitivity C‐reactive protein or measures of cardiac structure or function (Table 2). When modeled continuously, lower FEV1/FVC was also associated with higher estimated pulmonary arterial systolic pressure (PASP) in fully adjusted models (ß coefficient, −0.04; 95% CI, −0.07 to −0.01; P=0.004; Figure 1; Table S2). At a median follow‐up of 5.6 (25th–75th percentile 5.1–6.1) years, 335 participants died and 160 developed HF (78 HFpEF, 64 HFrEF, 18 unknown LVEF). Lower FEV1/FVC was associated with heightened risk for incident HF overall (Table 3 and Figure S2), and all‐cause mortality (Table 3; Figure 2). Similar results were observed in sensitivity analysis excluding participants with moderate or greater valvular heart disease (n=64; Table S3).
Table 1.
Characteristics of the Study Population According to Sex‐Specific Quartiles of FEV1/FVC ratio (n=3476) and ppFVC (n=3325) at ARIC Baseline Visit 5
| Participants, n | FEV1/FVC | ppFVC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend* | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend* | |
| 870 | 869 | 869 | 868 | 832 | 831 | 831 | 831 | |||
| Female, range, % | 28.21–69.84 | 69.86–74.69 | 74.70–78.90 | 78.91–96.26 | 34.40–87.26 | 87.31–97.66 | 97.68–109.64 | 109.65–173.90 | ||
| Male, range, % | 25.19–66.24 | 66.27–71.84 | 71.89–76.74 | 76.75–91.65 | 43.23–85.23 | 85.24–95.61 | 95.67–106.34 | 106.35–154.83 | ||
| Demographics | ||||||||||
| Age, y | 76.2±5.3 | 75.6±4.8 | 74.7±5.0 | 74.2±4.7 | <0.001 | 74.2±4.6 | 74.3±4.6 | 75.0±4.8 | 76.5±5.4 | <0.001 |
| Male | 338 (40) | 338 (40) | 338 (40) | 337 (40) | … | 325 (39) | 325 (39) | 325 (39) | 325 (39) | … |
| Black | 112 (13) | 132 (15) | 176 (20) | 272 (31) | <0.001 | 159 (19) | 137 (17) | 169 (20) | 209 (25) | <0.001 |
| Center | <0.001 | <0.001 | ||||||||
| Forsyth | 215 (25) | 196 (23) | 160 (18) | 168 (19) | 155 (19) | 179 (21) | 168 (20) | 180 (22) | ||
| Jackson | 97 (11) | 122 (14) | 163 (19) | 253 (29) | 150 (18) | 128 (15) | 154 (18) | 189 (23) | ||
| Minneapolis | 297 (34) | 297 (34) | 268 (31) | 228 (26) | 256 (31) | 258 (31) | 278 (33) | 249 (30) | ||
| Washington | 261 (30) | 254 (29) | 278 (32) | 219 (25) | 271 (33) | 266 (32) | 231 (28) | 213 (26) | ||
| Medical history | ||||||||||
| Hypertension | 682 (78) | 691 (79) | 691 (79) | 697 (80) | 0.81 | 712 (86) | 670 (81) | 649 (78.1) | 642 (77.3) | <0.001 |
| Diabetes | 233 (27) | 276 (32) | 312 (36) | 310 (36) | <0.001 | 363 (44) | 289 (35) | 259 (31.2) | 234 (28.2) | <0.001 |
| Smoking status | ||||||||||
| Current | 72 (8) | 41 (5) | 43 (5) | 25 (3) | <0.001 | 54 (7) | 43 (5) | 36 (4) | 24 (3) | 0.002 |
| Ever | 622 (71) | 498 (57) | 484 (56) | 471 (54) | <0.001 | 505 (61) | 486 (58) | 466 (56) | 453 (54) | 0.02 |
| Atrial fibrillation | 52 (6) | 44 (5) | 32 (4) | 22 (3) | 0.01 | 37 (4) | 35 (4) | 32 (4) | 36 (4) | 0.34 |
| Chronic kidney disease | 242 (28) | 208 (24) | 204 (24) | 191 (22) | 0.31 | 194 (23) | 197 (24) | 215 (26) | 194 (23) | 0.05 |
| Coronary artery disease | 90 (11) | 78 (9) | 70 (8) | 83 (10) | 0.49 | 81 (10) | 80 (10) | 80 (10) | 71 (9) | 0.06 |
| Myocardial infarction | 67 (8) | 59 (7) | 44 (5) | 64 (8) | 0.91 | 64 (8) | 53 (7) | 49 (6) | 55 (7) | 0.19 |
| Physical examination | ||||||||||
| Height, cm | 165±9 | 166±10 | 165±9 | 165±9 | <0.001 | 166±9 | 166±9 | 166±9 | 164±9 | <0.001 |
| BMI, kg/m2 | 26.8±5.0 | 27.8±4.8 | 29.0±5.4 | 29.4±5.2 | <0.001 | 30.6±6.1 | 29.0±5.2 | 28.1±4.9 | 27.2±4.5 | <0.001 |
| BMI >30 kg/m2 | 196 (23) | 258 (30) | 310 (36) | 334 (39) | <0.001 | 408 (49) | 301 (36) | 264 (32) | 186 (22) | <0.001 |
| Heart rate, bpm | 62±10 | 61±10 | 62±10 | 62±10 | 0.46 | 63±10 | 62±10 | 61±10 | 61±10 | <0.001 |
| Systolic BP, mm Hg | 129±17 | 130±18 | 130±17 | 130±17 | 0.17 | 131±18 | 129±16 | 130±17 | 130±17 | 0.004 |
| Diastolic BP, mm Hg | 66±10 | 66±10 | 67±10 | 68±10 | 0.06 | 68±10 | 67±10 | 67±10 | 67±10 | 0.22 |
| Laboratory tests | ||||||||||
| Hemoglobin, g/dL | 13.4±1.4 | 13.4±1.3 | 13.5±1.7 | 13.3±1.5 | 0.67 | 13.3±1.4 | 13.4±1.4 | 13.4±1.8 | 13.3±1.3 | 0.01 |
| eGFR, mL/min, 1.73 m2 | 69.3±16.9 | 71.0±15.4 | 71.8±15.9 | 72.9±16.6 | 0.16 | 71.9±17.1 | 71.8±16.2 | 70.6±16.6 | 71.1±16.1 | 0.16 |
| Spirometry | ||||||||||
| FEV1, L | 1.9±0.6 | 2.2±0.6 | 2.3±0.6 | 2.4±0.6 | <0.001 | 1.8±0.5 | 2.2±0.5 | 2.3±0.6 | 2.5±0.7 | <0.001 |
| FVC, L | 3.0±0.8 | 3.1±0.9 | 3.1±0.8 | 2.9±0.8 | <0.001 | 3.0±0.8 | 3.1±0.9 | 3.0±0.8 | 2.9±0.8 | <0.001 |
| FEV1/FVC, % | 61.8±7.4 | 71.1±2.1 | 75.8±1.8 | 81.3±2.9 | … | 74.8±5.2 | 74.6±4.9 | 74.6±5.1 | 73.6±5.3 | <0.001 |
| ppFVC, % | 101±17 | 102±15 | 100±14 | 99±15 | <0.001 | 77.0±8.1 | 92.0±3.2 | 102.3±3.6 | 119.9±11.2 | … |
Values are expressed as mean±SD or n(%). Chronic kidney disease was considered if Chronic Kidney Disease Epidemiology Collaboration glomerular filtration rate is <60 mL/min per 1.73 m2. ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; FEV1/FVC indicates forced expired volume in 1 second and forced vital capacity ratio; and ppFVC, percent predicted forced vital capacity ratio.
P value for trend adjusted for age sex and race.
Table 2.
Biomarkers and Echocardiography Variables of the Study Population According to Sex‐Specific FEV1/FVC Ratio Quartiles at ARIC Baseline Visit 5 (n=3476)
| FEV1/FVC | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
Model1 P‐trend |
Mode 2 P‐trend |
|---|---|---|---|---|---|---|
| Biomarkers | ||||||
| High‐sensitivity CRP, mg/L | 1.7 (0.9–3.7) | 1.7([0.8–3.4) | 2.0 (1.0–4.3) | 1.9 (0.9–4.0) | 0.29* | … |
| NT‐proBNP, pg/mL | 150 (77–272) | 127 (70–239) | 107 (56–200) | 88 (49–168) | <0.001* | <0.001* |
| Structure | ||||||
| Mean wall thickness, cm | 0.96±0.14 | 0.97±0.14 | 0.98±0.13 | 0.97±0.12 | 0.14 | … |
| Relative wall thickness | 0.42±0.07 | 0.42±0.07 | 0.43±0.07 | 0.42±0.07 | 0.67 | … |
| LV mass index, g/m2 | 77±19 | 79±19 | 77±18 | 76±16 | 0.55 | … |
| LV mass, g | 140±42 | 145±3 | 145±41 | 142±36 | 0.45 | … |
| LVEDV index, mL/m2 | 43±10 | 44±10 | 43±10 | 43±10 | 0.14 | … |
| Systolic function | ||||||
| LV ejection fraction, % | 65.9±5.9 | 65.9±6.0 | 66.0±58 | 66.1±5.7 | 0.09 | … |
| Longitudinal strain, % | −18.1±2.4 | −18.2±2.4 | −18.1±2.3 | −18.3±2.3 | 0.15 | … |
| Stroke volume index, mL/m2 | 50±15 | 49±13 | 47±13 | 48±16 | 0.98 | … |
| Diastolic function | ||||||
| E wave, cm/sec | 67±19 | 66±18 | 66±17 | 66±17 | 0.10 | … |
| A wave, cm/sec | 79±19 | 78±19 | 80±18 | 80±18 | 0.05 | … |
| E/A ratio | 0.87±0.28 | 0.87±0.27 | 0.84±0.26 | 0.85±0.27 | 0.004 | 0.56 |
| Lateral e′, cm/s | 7.2±2.1 | 7.1±2.0 | 7.1±2.0 | 7.2±2.0 | 0.04 | 0.47 |
| E/e′ lateral | 10.1±3.9 | 9.9±3.6 | 9.9±3.7 | 9.7±3.3 | 0.84 | … |
| LA volume index, mL/m2 | 25.5±9.9 | 26.1±7.9 | 25.0±8.0 | 25.1±7.4 | 0.38 | … |
| Right ventricle and pulmonary pressure | ||||||
| Estimated PASP, mm Hg | 28.2±5.5 | 27.5±5.1 | 27.5±5.0 | 27.3±5.2 | 0.09 | … |
| RV fractional area change | 0.53±0.08 | 0.53±0.08 | 0.52±0.07 | 0.52±0.07 | 0.26 | … |
Values are expressed as mean±SD or median (25th–75th percentile). Model 1: age, sex, race. Model 2: age, sex, race, current or prior smoking, hypertension, diabetes, atrial fibrillation, body mass index, log high‐sensitivity CRP, log NT‐proBNP. Model 2 analyses were performed only when P<0.05 in model 1. ARIC indicates Atherosclerosis Risk in Communities; CRP, C‐reactive protein; FEV1/FVC, forced expired volume in 1 second and forced vital capacity ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; LA, left atrial; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; PASP, pulmonary artery systolic pressure; and RV, right ventricular.
P value for the log‐transformed CRP and NT‐proBNP trend.
Figure 1. Continuous association of FEV1/FVC (blue) and percent predicted FVC (light red) with LV mass, E/e′ ratio, and PASP at visit 5 using restricted cubic splines.

Models were adjusted for age, sex, and race and primary exposure variables (FEV1/FVC and percent predicted FVC) using restricted cubic splines with 3 knots. *P<0.05 in models further adjusted for body mass index, prevalent coronary artery disease, prevalent atrial fibrillation, hypertension, diabetes, log(NT‐proBNP) and the other spirometric measure (FEV1/FVC or ppFVC). FEV1/FVC indicates forced expired volume in 1 second and forced vital capacity ratio; LV, left ventricular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PASP, estimated pulmonary arterial systolic pressure; and ppFVC, percent predicted forced vital capacity ratio.
Table 3.
Association of Spirometric Function at the Fifth ARIC Visit With Incident HFpEF and HFrEF (Median Follow‐Up Time, 5.6 y), and Overall Mortality (Median Follow‐Up Time, 5.7 y)
| Outcome | Events | Model 1* | Model 2* | ||
|---|---|---|---|---|---|
|
HR (95% CI) per 10‐point decrease |
P value |
HR (95% CI) per 10‐point decrease |
P value | ||
| FEV1/FVC (n=3476) | |||||
| HFpEF | 78 | 1.24 (0.97–1.60) | 0.09 | 1.31 (0.98–1.74) | 0.07 |
| HFrEF | 64 | 1.28 (0.98–1.68) | 0.07 | 1.24 (0.91–1.70) | 0.18 |
| Heart failure | 160 | 1.27 (1.07–1.51) | 0.006 | 1.28 (1.06–1.57) | 0.012 |
| Mortality | 335 | 1.37 (1.23–1.54) | <0.001 | 1.29 (1.14–1.46) | <0.001 |
| Percent predicted FVC (n=3325) | |||||
| HFpEF | 78 | 1.32 (1.15–1.51) | <0.001 | 1.21 (1.04–1.41) | 0.013 |
| HFrEF | 58 | 1.00 (0.86–1.16) | 0.96 | 0.90 (0.76–1.07) | 0.24 |
| Heart failure | 157 | 1.20 (1.09–1.32) | <0.001 | 1.09 (0.98–1.21) | 0.11 |
| Mortality | 310 | 1.14 (1.07–1.22) | <0.001 | 1.12 (1.04–1.21) | 0.002 |
Model 1: age, sex, and race. Model 2: age, sex, race, body mass index, prevalent coronary artery disease, ever smoking, hypertension, diabetes, log(NT‐proBNP), and stratified by prevalent atrial fibrillation, all at baseline visit 5. ARIC indicates Atherosclerosis Risk in Communities; FEV1/FVC, forced expired volume in 1 second and forced vital capacity ratio; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 2. Continuous associations of FEV1/FVC (blue) and percent predicted FVC (light red) at visit 5 with subsequent incidence of HF overall, HFpEF, and HFrEF.
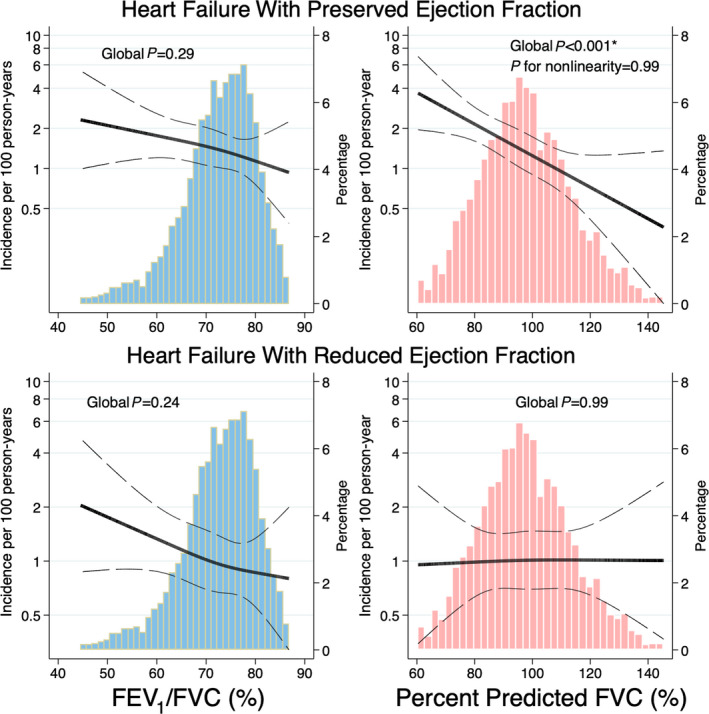
Models were adjusted for age, sex, race, and primary exposure variables (FEV1/FVC and percent predicted FVC) using restricted cubic splines with 3 knots. *P<0.05 in models further adjusted for body mass index, prevalent coronary artery disease, prevalent atrial fibrillation, hypertension, diabetes, log(NT‐proBNP), and the other spirometric measure (FEV1/FVC or ppFVC). FEV1/FVC indicates forced expired volume in 1 second and forced vital capacity ratio); HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and ppFVC, percent predicted Forced vital capacity ratio).
The mean absolute decline in FEV1/FVC from visit 1 or 2 to visit 5 was −4.6±5.5. Change in FEV1/FVC was not associated with late‐life cardiac structure or function beyond the visit 5 FEV1/FVC in the multivariable models (Table S4). Decline in FEV1/FVC from midlife to late life was not significantly associated with risk of incident HF or all‐cause mortality in late life beyond the visit 5 FEV1/FVC value (Table S5).
Restrictive Ventilatory Pattern
Among the 3325 participants without obstructive deficits, the average FEV1/FVC was 75.0±4.9, FVC was 3.0±0.9 L, and ppFVC was 98±17% (Table 1). Of participants in quartile 1 of ppFVC, 75% had a ppFVC >73%. Lower ppFVC was associated with younger age and non‐Black race. Accounting for age, sex, and race, lower ppFVC was associated with smoking, hypertension, diabetes, and higher BMI (Table 1). In models adjusted for demographics, lower ppFVC was associated with higher high‐sensitivity C‐reactive protein and NT‐proBNP, greater LV wall thickness and mass, lower LV end‐diastolic volume index, worse longitudinal strain, lower stroke volume index, larger left atrial volume, and higher E/e′ and PASP (Table 4). After further adjustment for clinical comorbidities and biomarkers, the associations with LV wall thickness and mass, LV end‐diastolic volume index, longitudinal strain, E/e′ and PASP persisted (Table 4 and Figure 1). Similar findings were observed when ppFVC was modeled continuously (Table S6). Similar findings were observed in analyses using quartiles of FVC adjusted for sex, age, height, and race, instead of ppFVC (Table S7).
Table 4.
Biomarkers and Echocardiography Variables of the Study Population According to Sex‐Specific Percent Predicted FVC Quartiles at ARIC Baseline Visit 5 (n=3325)
| ppFVC | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
Model1 P trend |
Mode 2 P trend |
|---|---|---|---|---|---|---|
| Biomarkers | ||||||
| High‐sensitivity CRP, mg/L | 2.5 (1.2–5.3) | 2.0 (1.0–4.1) | 1.7 (0.8–3.5) | 1.4 (0.7–3.1) | <0.001* | <0.001* , † |
| NT‐proBNP, pg/mL | 126 (64–241) | 111 (55–218) | 113 (59–213) | 111 (63–208) | <0.001* | <0.001* |
| Structure | ||||||
| Mean wall thickness, cm | 1.00±0.14 | 0.98±0.12 | 0.97±0.13 | 0.96±0.12 | <0.001 | 0.005 |
| Relative wall thickness | 0.43±0.08 | 0.42±0.07 | 0.43±0.07 | 0.42±0.07 | 0.01 | 0.55 |
| LV mass index, g/m2 | 80.1±18.8 | 77.4±18.6 | 76.9±18.1 | 76.0±16.7 | <0.001 | 0.31 |
| LV mass, g | 155±44 | 146±42 | 143±41 | 137±37 | <0.001 | 0.002 |
| LVEDV index, mL/m2 | 42±9 | 43±10 | 43±10 | 44±10 | <0.001 | <0.001 |
| Systolic function | ||||||
| LV ejection fraction, % | 65.7±6.1 | 65.8±5.8 | 66.1±6.1 | 66.0±5.4 | 0.06 | … |
| Longitudinal strain, % | −17.9±2.6 | −18.2±2.4 | −18.4±2.3 | −18.2±2.3 | <0.001 | 0.03 |
| Stroke volume index, mL/m2 | 48±14 | 47±13 | 49±16 | 49±15 | 0.013 | 0.31 |
| Diastolic function | ||||||
| E wave, cm/sec | 69±19 | 67±17 | 66±17 | 64±16 | <0.001 | <0.001 |
| A wave, cm/sec | 81±20 | 80±18 | 79±18 | 78±18 | <0.001 | 0.006 |
| E/A ratio | 0.87±0.30 | 0.86±0.26 | 0.86±0.26 | 0.84±0.26 | 0.53 | … |
| Lateral e′, cm/s | 7.1±2.0 | 7.1±2.0 | 7.1±2.0 | 7.2±2.1 | 0.001 | 0.04 |
| E/e′ lateral | 10.5±3.9 | 10.0±3.6 | 9.8±3.4 | 9.5±3.3 | <0.001 | <0.001 |
| LA volume index, mL/m2 | 25.9±9.1 | 25.3±8.5 | 25.2±7.2 | 25.5±7.8 | 0.005 | 0.79 |
| Right ventricle and pulmonary pressure | ||||||
| Estimated PASP, mm Hg | 28.7±6.1 | 27.7±5.3 | 27.2±4.8 | 27.2±4.7 | <0.001 | 0.005 |
| RV fractional area change | 0.52±0.08 | 0.52±0.07 | 0.52±0.08 | 0.53±0.07 | 0.08 | … |
Values are expressed as mean±SD or median (25th–75th percentile). Model 1: age, sex, and race. Model 2: age, sex, race, current or prior smoking, body mass index, hypertension, diabetes, log Hs‐CRP, log NT‐proBNP. Model 2 analyses were performed only when P<0.05 in model 1. ARIC indicates Atherosclerosis Risk in Communities; CRP, C‐reactive protein; FEV1/FVC, forced expired volume in 1 second and forced vital capacity ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; LA, left atrial; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; PASP, pulmonary artery systolic pressure; and RV, right ventricular.
P‐value for the log‐transformed CRP and NT‐proBNP trend.
Log NT‐proBNP was excluded for model 2.
At a median follow‐up of 5.6 (25th–75th percentile 5.1–6.1) years, 157(5.1%) participants developed HF and 310 (9.3%) died. Of incident HF cases, 78 were HFpEF, 58 were HFrEF, and 21 occurred with unknown LVEF. Accounting for age, sex, and race, lower ppFVC was associated with heightened risk of HFpEF, and all‐cause mortality, but not of incident HFrEF (Table 3, Figure 2). In fully adjusted models, associations with incident HFpEF and all‐cause mortality persisted. NT‐proBNP and BMI accounted for the greatest attenuation of the association of ppFVC with incident HF (Table S8). Similar findings were observed with absolute FVC as the exposure (Table S9).
The mean absolute ppFVC decline from visit 1 or 2 to visit 5 was –3.1±13.3, ranging from a mean of −18.6±7.1 (quartile 1) to +13.8±9.9 (quartile 4) (Table S10). Accounting for demographics and the visit 5 ppFVC value, greater ppFVC decline from midlife to late life was associated with greater late‐life mass, left atrial volume, E/A ratio, and PASP. Associations were also observed with lower LVEF and higher right ventricular fractional area change. In fully adjusted models, associations with LV mass, left atrial volume, E/A ratio, and right ventricular fractional area change persisted, but the mid‐to‐late‐life decline in ppFVC was not significantly associated with incident HF or death beyond the visit 5 value (Table S6).
Discussion
While previous studies have demonstration the association of spirometric deficits with risk of HF in mid‐ and early‐late life, and with clinical outcomes in prevalent HF, their prognostic relevance—and that of reduced FVC in particular—for incident HF and HF phenotype (HFpEF, HFrEF) have not been well established in late life. In this analysis of a large number of well‐characterized community‐based people in late life, we report the following novel findings: (1) lower ppFVC, and greater decline in ppFVC from midlife to late life, are robustly associated with higher LV mass, higher LV filling pressure, and higher PASP in late life, accounting for common comorbidities; (2) lower ppFVC is associated with heightened risk of incident HFpEF beyond BMI and other cardiovascular comorbidities; and (3) lower FEV1/FVC is independently associated with higher PASP but not with HF phenotypes (Figure 3). Together, these findings demonstrate that important subclinical associations between pulmonary and cardiovascular dysfunctions persist into late life, and highlight cardiovascular associations with restrictive spirometric patterns that have been relatively understudied.
Figure 3. Differential associations of obstructive and restrictive ventilatory patterns cardiovascular structure and function and incident HF in late life.

Lower FEV1/FVC was associated with higher PASP and with incident overall HF. In contrast, lower ppFVC was associated with higher LV mass, higher LV filling pressure, and higher PASP, and with incident HFpEF. FEV1/FVC indicates forced expired volume in 1 second and forced vital capacity ratio; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; PASP, estimated pulmonary arterial systolic pressure; and ppFVC, percent predicted forced vital capacity ratio.
Prevalent lung disease associates with heightened risk of cardiovascular diseases, particularly among the elderly. 28 Community‐based longitudinal cohorts of people in early life and midlife have demonstrated that lung disease of lesser severity, or even subclinical alterations in lung function, associate with subclinical impairments in cardiac structure and function and with prognosis. Among 2816 participants in MESA (Multi‐Ethnic Study of Atherosclerosis; mean age, 61±10 years), greater airway obstruction based on percentage of emphysema from chest computed tomography or lower FEV1/FVC was associated with lower LV end‐diastolic volume, stroke volume, and cardiac output. 3 Furthermore, in a younger sample of 3000 participants at study year 25 of the CARDIA (Coronary Artery Risk Development in Young Adults) study (mean age, 50±4 years), greater decline in FEV1/FVC from early adulthood to middle age was associated with smaller left atrial size and lower cardiac output. 2 However, less is known if such relationship persists in late life, when multiple accumulated cardiovascular comorbidities may influence cardiac structure and function. In our analysis of people 75.0±5.0 years of age, worse FEV1/FVC was associated with higher PASP. Unlike CARDIA, change in FEV1/FVC from midlife to late life was not independently associated with cardiac structure and function. It is possible that age‐related changes and cumulative burden of multiple cardiovascular comorbidities exert more robust effects on cardiac structure and function relative to pulmonary function in elderly compared with younger cohorts.
While established chronic obstructive pulmonary disease is associated with cardiovascular disease and HF, subclinical reductions in FEV1/FVC also appear predictive of incident HF, 1 , 28 but the relationship with HF phenotype is less clear. In a cross‐sectional analysis of the Gutenberg Health Study (mean age, 55±11 years), lower FEV1/FVC was associated with higher odds of both HFpEF and HFrEF in adjusted analyses. 29 In contrast, in the Framingham Heart Study (mean age, 76±5 years), lower FEV1/FVC was predictive of incident HFpEF—but not HFrEF—in adjusted analyses. We observed associations of lower FEV1/FVC with incident HF and all‐cause mortality, but not with HFpEF and HFrEF individually.
Fewer data are available regarding the association of restrictive ventilatory patterns with cardiac structure and function, although existing data suggest associations even in the absence of overt cardiovascular disease. Restrictive physiology may result from alterations in the lung parenchyma, pleura, chest wall, or neuromuscular apparatus, 30 especially in the elderly, among whom kyphoscoliosis and sarcopenia are frequent. In the Jackson Heart Study (median age, 55 years), a restrictive spirometry was associated with higher E/A ratio and PASP, but not LV mass index. 31 Similarly, in the Gutenberg Health study, lower FVC was also associated higher E/A ratio and E/e′. 29 In the CARDIA study, greater longitudinal decline in FVC from early adulthood to midlife was associated with higher LV mass but lower E/A ratio in midlife. 2 Few data exist regarding these associations in late life. In our study, participants were ≈20 to 25 years older than those in the JHS (Jackson Heart Study) and CARDIA. Lower ppFVC was associated with greater LV mass, worse diastolic indices, and higher PASP independent of common cardiovascular comorbidities including BMI. Furthermore, greater longitudinal decline in ppFVC from midlife to late life predicted higher E/A ratio, left atrial volume, and LV mass independent of the late‐life ppFVC value. Importantly, greater LV mass, worse diastolic function, and higher PASP all characterize HFpEF, which is particularly prevalent in late life.
Reduced FVC has consistently been associated with mortality, 32 and since the initial observation in the Framingham Heart Study, 33 associations with incident HF are well described. 5 , 31 , 34 However, whether reductions in FVC differentially predict incident HFpEF versus HFrEF is unclear. In our study, lower ppFVC was associated with heightened risk of incident HFpEF, but not HFrEF, independent of BMI, common cardiovascular comorbidities, and NT‐proBNP. Our findings are supported by previous observations in 2125 participants from the Health ABC (Health, Aging, and Body Composition) study, among whom each 10% decrease in ppFVC was associated with incident HFpEF (LVEF>40%) independent of BMI. 35 Of note, in that study, lower ppFVC was also associated with incident HFrEF, and associations were not adjusted for NT‐proBNP.
The mechanisms underlying the association of a restrictive spirometry pattern with alterations in cardiac structure and function and risk of incident HFpEF are unclear. It is possible that reduced ppFVC may reflect subtle/early interstitial pulmonary edema. The association of lower ppFVC with higher NT‐proBNP levels maybe consistent with this. However, if this were the primary mechanism linking lower ppFVC to incident HFpEF, we would expect clinical manifestation of HF to occur relatively soon after pulmonary assessment. We therefore believe that the median time to incident HF of 5.6 years argues against this as a primary mechanism. Obesity is a common cause of restrictive spirometry and an important contributor to the HFpEF syndrome. 36 However, the associations of lower ppFVC with LV structure, diastolic indices, and PASP, and with incident HFpEF, persisted in models adjusted for BMI and other cardiovascular comorbidities. Notably, in the National Health and Nutrition Examination Survey, the prevalence of ventilatory restriction was higher in participants who were underweight than in participants who were obese, and despite increases in the prevalence of obesity over time, the prevalence of a restrictive spirometry pattern remained relatively stable. These data emphasize that a restrictive spirometry pattern should not be viewed as solely a manifestation or epiphenomenon of obesity. 32
However, it is likely that close associations of FVC with cardiovascular risk factors contribute to our findings. Lower FVC, but not FEV1/FVC, is consistently associated with incident hypertension 37 and diabetes. 38 While age‐related changes of the lung have classically been characterized by airspace enlargement with alveolar dilatation and reduced static elastic recoil resulting in an “emphysema‐type” pattern, 39 , 40 , 41 recent advances in CT‐based chest imaging increasingly recognize interstitial fibrosis in asymptomatic community dwelling individuals and patients with even mild chronic obstructive pulmonary disease. 42 , 43 These fibrotic areas associate with decreased lung compliance and increased resistance in late life in animal models, 44 increase in prevalence and progression with advancing age in humans, 43 , 45 and predict mortality in general population samples. 43 , 45 While the underlying drivers of this age‐related fibrosis in the lungs are unclear, they may overlap with the factors promoting well‐recognized age‐related changes in the heart, including increases in LV mass, higher filling pressure, and higher pulmonary pressure. 46 , 47 , 48 , 49 , 50 , 51 Indeed, fibrosis also appears to be important in HFpEF in clinical and preclinical studies, and may represent one common pathophysiological mechanism underlying both lung and heart dysfunction. 52 , 53 Recently, data from the PIROUETTE (Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction) trial 54 demonstrate that the antifibrotic agent pirfenidone, effective in restrictive lung disease caused by pulmonary fibrosis, reduces myocardial fibrosis compared with placebo in HFpEF, supporting potential shared cardiopulmonary inflammation‐fibrosis underlying HFpEF in at least a subset of patients. 55 It is possible that chronic systemic inflammation related to cardiometabolic risk factors and cardiovascular disease acts as a shared underlying driver for pulmonary and cardiac fibrosis and associated dysfunction. 32 Markers of systemic inflammation predict greater decline in FEV1 and FVC, 56 and associate with both cardiac and extracardiac comorbidities. 36
Beyond shared risk factors driving inflammation and pulmonary and cardiac fibrosis in parallel, recent data from the UK Biobank using Mendelian randomization suggest a potential causal association between lower FVC and coronary artery disease. 57 It is possible that causal associations also exist with subclinical coronary disease and microvascular dysfunction, leading to the myocardial ischemia, fibrosis, and remodeling that underlie HF development. Future studies with phenotyping of coronary morphology and microvascular function will be necessary to explore this hypothesis.
Several limitations should be noted. First, the observational nature precludes determinations of causality, and residual/unmeasured confounders likely exist for the observed associations. Only a subset of ARIC participants alive at the time of the study visit chose to attend, and only a subset of those attending had the necessary data for analysis, potentially introducing selection bias. Indeed, among visit 5 attendees, participants included in this analysis tended to be healthier (Table S1), which may have resulted in an attenuation of the observed associations. Furthermore, given the time difference between ARIC visits 1 to 2 and 5, survival bias may have limited our ability to detect associations between spirometry changes and study outcomes. Spirometry was performed without bronchodilators, so we were unable to detect reversible obstruction and may have overestimated the prevalence of restrictive patterns. Total lung capacity was unavailable, and restrictive ventilatory pattern was based solely on FVC as reported in other community‐based epidemiologic cohorts like ARIC. Diffusion capacity for CO2 and ventilatory strength measurements were unavailable to further investigate reduced FVCs. An isolated ppFVC value cannot distinguish nonspecific ventilatory patterns (low FVC but normal total lung capacity), which potentially lowers specificity for true restrictive patterns. 58 Data on conditions such as sarcoidosis, autoimmune diseases, prior chest radiation, and amyloidosis, which may underlie both interstitial lung disease and HF, were not available in ARIC, nor were data on supplemental oxygen use. Chest computed tomography imaging to assess for interstitial lung disease was not available at ARIC visit 5. Longitudinal echocardiographic data from visit 1 or 2 and visit 5 were not available to assess the association of changes in spirometric measures with changes in cardiac function. Our analysis could have been underpowered to detect an association of reduced FEV1/FVC with the incidence HFpEF or HFrEF.
Conclusions
In this large community‐based cohort of persons in late life, lower ppFVC was independently associated with greater LV mass, filling pressures, and PASP, and with incident HFpEF but not HFrEF. Lower FEV1/FVC was associated with higher PASP and with HF overall, which did not appear differential by incident HF phenotype. These findings highlight the importance of pulmonary dysfunction with cardiac dysfunction interactions and the differential associations of obstructive and restrictive spirometric deficits with HF risk and particularly HFpEF in late life.
Sources of Funding
The ARIC study was funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HSN268201700004I). The work for this article was also supported by National Heart, Lung, and Blood Institute grants R01HL135008, R01HL143224, R01HL150342, R01HL148218 (Dr Shah), and K24HL152008 (Dr Shah) and a Watkins Discovery Award from the Brigham and Women’s Heart and Vascular Center (Dr Shah). The role of funding organizations was to support the collection and management of data. They had no role in the design and conduct of the study; interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
Dr Claggett reports receiving consulting fees from Amgen, Boehringer Ingelheim, Corvia, Myokardia, and Novartis. Dr Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, and Theracos; and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, and Tremeau. Dr Skali reports receiving ownership interest from OptimizeRx. Dr Shah reports receiving research support from Novartis through Brigham and Women’s Hospital and consulting fees from Philips Ultrasound. The remaining authors report no relevant disclosures.
Supporting information
Data S1
Tables S1–S10
Figures S1–S2
Acknowledgments
Drs Ramalho and Shah had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the staff and participants of the ARIC study for their important contributions.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Silvestre OM, Nadruz W Jr, Querejeta Roca G, Claggett B, Solomon SD, Mirabelli MC, London SJ, Loehr LR, Shah AM. Declining lung function and cardiovascular risk: the ARIC study. J Am Coll Cardiol. 2018;72:1109–1122. doi: 10.1016/j.jacc.2018.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cuttica MJ, Colangelo LA, Shah SJ, Lima J, Kishi S, Arynchyn A, Jacobs DR, Thyagarajan B, Liu K, Lloyd‐Jones D, et al. Loss of lung health from young adulthood and cardiac phenotypes in middle age. Am J Resp Crit Care Med. 2015;192:76–85. doi: 10.1164/rccm.201501-0116OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JAC, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wannamethee SG, Shaper AG, Papacosta O, Lennon L, Welsh P, Whincup PH. Lung function and airway obstruction: associations with circulating markers of cardiac function and incident heart failure in older men‐the British Regional Heart Study. Thorax. 2016;71:526–534. doi: 10.1136/thoraxjnl-2014-206724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuttica MJ, Colangelo LA, Dransfield MT, Bhatt SP, Rana JS, Jacobs DR, Thyagarajan B, Sidney S, Lewis CE, Liu K, et al. Lung function in young adults and risk of cardiovascular events over 29 years: the CARDIA study. J Am Heart Assoc. 2018;7:e010672. doi: 10.1161/JAHA.118.010672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle‐aged cohort. Thorax. 2008;63:599–605. doi: 10.1136/thx.2007.088112 [DOI] [PubMed] [Google Scholar]
- 7. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients>or=65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. [DOI] [PubMed] [Google Scholar]
- 8. Fleg JL, Strait J. Age‐associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17:545–554. doi: 10.1007/s10741-011-9270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67:264–275. doi: 10.1093/gerona/glr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramalho SHR, Shah AM. Lung function and cardiovascular disease: a link. Trends Cardiovasc Med. 2021;31:93–98. doi: 10.1016/j.tcm.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam CSP, Lyass A, Kraigher‐Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 14. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Resp Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 15. Folsom AR, Yamagishi K, Hozawa A, Chambless LE.; Atherosclerosis Risk in Communities Study I . Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, Loehr LR, McNeill AM, Poole C, Soliman EZ, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129:971–980. doi: 10.1161/CIRCULATIONAHA.113.004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, et al. Comparison of risk prediction using the CKD‐EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA; The ARIC Investigators . Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 19. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- 20. Michos ED, Selvin E, Misialek JR, McEvoy JW, Ndumele CE, Folsom AR, Ballantyne CM, Lutsey PL. 25‐hydroxyvitamin D levels and markers of subclinical myocardial damage and wall stress: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2016;5:e003575. doi: 10.1161/JAHA.116.003575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061 [DOI] [PubMed] [Google Scholar]
- 23. Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community‐dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah AM, Claggett B, Kitzman D, Biering‐Sørensen T, Jensen JS, Cheng S, Matsushita K, Konety S, Folsom AR, Mosley TH, et al. Contemporary assessment of left ventricular diastolic function in older adults: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135:426–439. doi: 10.1161/CIRCULATIONAHA.116.024825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135:224–240. doi: 10.1161/CIRCULATIONAHA.116.023361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 27. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 28. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 29. Baum C, Ojeda FM, Wild PS, Rzayeva N, Zeller T, Sinning CR, Pfeiffer N, Beutel M, Blettner M, Lackner KJ, et al. Subclinical impairment of lung function is related to mild cardiac dysfunction and manifest heart failure in the general population. Int J Cardiol. 2016;218:298–304. doi: 10.1016/j.ijcard.2016.05.034 [DOI] [PubMed] [Google Scholar]
- 30. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow‐up. Int J Tuberc Lung Dis. 2005;9:613–621. [PubMed] [Google Scholar]
- 31. Jankowich M, Elston B, Liu Q, Abbasi S, Wu WC, Blackshear C, Godfrey M, Choudhary G. Restrictive spirometry pattern, cardiac structure and function, and incident heart failure in African Americans. The Jackson Heart Study. Ann Am Thorac Soc. 2018;15:1186–1196. doi: 10.1513/AnnalsATS.201803-184OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Godfrey MS, Jankowich MD. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 2016;149:238–251. doi: 10.1378/chest.15-1045 [DOI] [PubMed] [Google Scholar]
- 33. Kannel WB, Seidman JM, Fercho W, Castelli WP. Vital capacity and congestive heart failure. The Framingham Study. Circulation. 1974;49:1160–1166. doi: 10.1161/01.CIR.49.6.1160 [DOI] [PubMed] [Google Scholar]
- 34. Engstrom G, Melander O, Hedblad B. Population‐based study of lung function and incidence of heart failure hospitalisations. Thorax. 2010;65:633–638. doi: 10.1136/thx.2010.135392 [DOI] [PubMed] [Google Scholar]
- 35. Georgiopoulou VV, Kalogeropoulos AP, Psaty BM, Rodondi N, Bauer DC, Butler AB, Koster A, Smith AL, Harris TB, Newman AB, et al. Lung function and risk for heart failure among older adults: the Health ABC Study. Am J Med. 2011;124:334–341. doi: 10.1016/j.amjmed.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018;39:2780–2792. doi: 10.1093/eurheartj/ehy301 [DOI] [PubMed] [Google Scholar]
- 37. Jacobs DR Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van den Borst B, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in diabetes: a metaanalysis. Chest. 2010;138:393–406. doi: 10.1378/chest.09-2622 [DOI] [PubMed] [Google Scholar]
- 39. Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol. 1968;25:664–671. doi: 10.1152/jappl.1968.25.6.664 [DOI] [PubMed] [Google Scholar]
- 40. Butler C III, Kleinerman J. Capillary density: alveolar diameter, a morphometric approach to ventilation and perfusion. Am Rev Respir Dis. 1970;102:886–894. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez‐Roisin R, Burgos F, Roca J, Barberà JA, Marrades RM, Wagner PD. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1183/09031936.99.14614549 [DOI] [PubMed] [Google Scholar]
- 42. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RSJ, Lynch DA, Brehm JM, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, et al. Association between interstitial lung abnormalities and all‐cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elliott JE, Mantilla CB, Pabelick CM, Roden AC, Sieck GC. Aging‐related changes in respiratory system mechanics and morphometry in mice. Am J Physiol Lung Cell Mol Physiol. 2016;311:L167–L176. doi: 10.1152/ajplung.00232.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, et al. Development and progression of interstitial lung abnormalities in the Framingham heart study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short‐ and long‐term change in the Framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age‐related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuznetsova T, Herbots L, López B, Jin YU, Richart T, Thijs L, González A, Herregods M‐C, Fagard RH, Díez J, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627 [DOI] [PubMed] [Google Scholar]
- 50. Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3:614–622. doi: 10.1161/CIRCIMAGING.109.926022 [DOI] [PubMed] [Google Scholar]
- 51. Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Oliva MJ, et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging. 2015;16:1031–1041. doi: 10.1093/ehjci/jev083 [DOI] [PubMed] [Google Scholar]
- 52. Zile MR, Baicu CF, S. Ikonomidis J, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol. 2017;2:995–1006. doi: 10.1001/jamacardio.2017.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lewis GA, Schelbert EB, Naish JH, Bedson E, Dodd S, Eccleson H, Clayton D, Jimenez BD, McDonagh T, Williams SG, et al. Pirfenidone in heart failure with preserved ejection fraction‐rationale and design of the PIROUETTE Trial. Cardiovasc Drugs Ther. 2019;33:461–470. doi: 10.1007/s10557-019-06876-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lewis GA, Dodd S, Clayton D, Bedson E, Eccleson H, Schelbert EB, Naish JH, Jimenez BD, Williams SG, Cunnington C, et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat Med. 2021;27:1477–1482. doi: 10.1038/s41591-021-01452-0 [DOI] [PubMed] [Google Scholar]
- 56. Kalhan R, Tran BT, Colangelo LA, Rosenberg SR, Liu K, Thyagarajan B, Jacobs DR Jr, Smith LJ. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5:e11431. doi: 10.1371/journal.pone.0011431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Higbee DH, Granell R, Sanderson E, Davey Smith G, Dodd JW. Lung function and cardiovascular disease: a two‐sample Mendelian randomisation study. Eur Respir J. 2021;58. doi: 10.1183/13993003.03196-2020 [DOI] [PubMed] [Google Scholar]
- 58. Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test: longitudinal follow‐up and outcomes. Chest. 2011;139:878–886. doi: 10.1378/chest.10-0804 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S10
Figures S1–S2


