Abstract
Background
Recent studies have identified an increased risk of dementia in patients with atrial fibrillation (AF). However, both AF and dementia usually manifest late in life. Few studies have investigated this association in adults with early‐onset dementia. The aim of this study was to investigate the relationship between AF and early‐onset dementia.
Methods and Results
We searched the PubMed/MEDLINE, Embase, and Scopus databases through April 15, 2022, for studies reporting on the association between AF and dementia in adults aged <70 years, without language restrictions. Two reviewers independently performed the study selection, assessed the risk of bias, and extracted the study data. We performed a meta‐analysis of early‐onset dementia risk according to occurrence of AF using a random‐effects model. We retrieved and screened 1006 potentially eligible studies. We examined the full text of 33 studies and selected the 6 studies that met our inclusion criteria. The pooled analysis of their results showed an increased risk of developing dementia in individuals with AF, with a summary relative risk of 1.50 (95% CI, 1.00–2.26) in patients aged <70 years, and 1.06 (95% CI, 0.55–2.06) in those aged <65 years.
Conclusions
In this systematic review and meta‐analysis, AF was a risk factor for dementia in adults aged <70 years, with an indication of a slight and statistically imprecise excess risk already at ages <65 years. Further research is needed to assess which characteristics of the arrhythmia and which mechanisms play a role in this relationship.
Keywords: Alzheimer dementia, atrial arrhythmia, atrial fibrillation, dementia, frontotemporal dementia
Subject Categories: Risk Factors, Primary Prevention, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- EOD
early‐onset dementia
Clinical Perspective
What Is New?
This article provides the first systematic review and meta‐analysis that details the association between atrial fibrillation and the risk of dementia in adults aged <70 years.
The data identify an increased risk of dementia in adults with atrial fibrillation, especially in those aged 60 to 69 years, although the risk is not as pronounced as that which has been identified in adults aged ≥70 years.
What Are the Clinical Implications?
A timely diagnosis of atrial fibrillation and appropriate therapy may be warranted, even in adults who are aged <70 years.
Atrial fibrillation (AF) is the most common clinically important cardiac arrhythmia worldwide. It is characterized by a rapid and irregular excitation of the atria, which occurs when a chaotic pattern of electrical activity replaces the normal sinus mechanism. AF is associated with increased mortality and morbidities, including a 5‐fold increase in the risk for stroke, and it is a major cause of health care costs. 1 , 2 The prevalence of AF in the general population of Western countries tends to be between 1% and 2%. Age is the most important risk factor for AF, with a sharp increase in prevalence after the age of 65 years. Because of increased survival and progressive aging, models predict almost 18 million adults in Europe and 12 million adults in the United States will have dementia by 2050 to 2060. 3 , 4 , 5 Age and sex are major predictors of incident AF, but other important risk factors include hypertension, valvular heart disease, systolic dysfunction, obesity, and alcohol consumption. 1 , 6
The term early‐onset dementia (EOD) indicates dementia with symptom onset at a younger than usual age, usually arbitrarily set at 65 years but less frequently at 70 years or other age cut points. With a prevalence of ≈67 to 98 per 100 000, Alzheimer disease is the most frequent form of dementia, followed by frontotemporal dementia. 7 , 8 EOD attributable to Alzheimer disease accounts for about 4% to 6% of all Alzheimer disease, with an annual incidence rate of ≈6 per 100 000 and a prevalence rate of about 24 per 100 000 people. 7 , 8 Alcohol consumption, cigarette smoking, traumatic brain injuries, cardiovascular and metabolic diseases, genetic susceptibility, neurotoxic agent exposure, like heavy metals and selenium, pesticides, and solvents are some of the factors involved in an increased risk of EOD, but the exact mechanisms of its pathophysiology are still uncertain. 9 , 10 , 11 , 12
In a 1997 report from the Netherlands, an association was noted between AF and dementia risk, 13 and since then the association has been identified in many studies. 14 , 15 , 16 , 17 , 18 , 19 , 20 However, this association has been established for the overall risk of dementia and of dementia in elderly people, but limited evidence exists for the association between AF and EOD, and no systematic review and meta‐analysis has been performed on this potential relationship. 21 , 22 , 23
We performed a systematic review and meta‐analysis on the association between AF and EOD, using an upper age cut point for symptom onset of 70 years given the low number of studies available on this topic.
METHODS
In performing this systematic review and meta‐analysis, we followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis 2020 statement. 24 The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis checklist is reported in Data S1. We declare that this study review did not directly involve human subjects; thus, no informed consent was required. Finally, we declare that all supporting data are available within the article and its online supplementary files.
Study Identification
We conducted a systematic search in the PubMed/MEDLINE, Scopus, and Embase databases with no time restrictions up to April 15, 2022, using the following keywords: “atrial fibrillation,” “heart atrium arrhythmia,” “dementia,” “amentia,” “Alzheimer's dementia,” and “frontotemporal dementia.” Two authors (M.E.G. and T.F.) independently evaluated the retrieved studies to check eligibility for inclusion in our analysis. In case of disagreement, a third author (M.V.) was consulted to resolve conflicts. We made no preliminarily restrictions on language, on study design, and on diagnosis of AF and dementia. Our search was restricted to original research studies that had investigated the association between AF and dementia in adults aged <70 years, and excluded reviews, letters, commentaries, studies without a group control not affected by AF, studies with an outcome other than dementia or with an exposure other than AF, and studies that did not provide data stratified by age if the study population encompassed also individuals aged >70 years. Table S1 reports additional details of the search.
Risk of Bias
We assessed the quality of the selected studies using the Risk of Bias for Nonrandomized Studies of Exposure tool. 25 Table S2 reports the criteria for risk of bias evaluation. In this assessment, we considered 7 domains as having low, moderate, or high risk of bias: (1) bias attributable to confounding; (2) bias in selecting participants; (3) bias in exposure classification; (4) bias attributable to departures from intended exposures; (5) bias attributable to missing data; (6) bias in outcome measurement; and (7) bias in selection of reported results. The overall risk of bias was considered high or moderate if at least one domain was judged at high or moderate risk; otherwise, it was classified as having a low risk of bias.
Data Extraction and Statistical Analysis
We extracted the following data from the included studies: (1) first author name, (2) publication year, (3) location, (4) exposure assessment, (5) outcome, (6) overall population and number of cases, (7) mean age, (8) age cutoff, (9) length of follow‐up, (10) risk ratio (RR) estimates with 95% CIs, and (11) adjustment factors. When >1 statistical model was available, we extracted the most adjusted estimates. When 95% CIs were not reported, we used P values to calculate them, according to established methods. 26
We performed a meta‐analysis of EOD risk according to AF occurrence using a random‐effects model. We assessed the heterogeneity of the included studies using the I2 statistic, considering the 25%, 50%, and 75% cut points to classify the set of studies as having low, moderate, and high inconsistency. 27 Because of the high heterogeneity, we used the random‐effects model, as proposed by Paule and Mandel, for meta‐analysis of dichotomous data. 28 We also explored the heterogeneity within stratified analyses whenever possible by cut point of EOD diagnosis, censoring for vascular dementia, and certainty of EOD diagnosis. Finally, we assessed the occurrence of small‐study effects using visual inspection of funnel plots along with computation of the Egger regression asymmetry test for publication bias. We used “meta” routine of Stata 17.0 software (StataCorp, College Station, TX; 2021) for all statistical analysis.
RESULTS
The process of study selection is shown in Figure 1. Following duplicate removal, our preliminary literature search identified 1006 potentially eligible studies. After screening the title and abstract, we excluded 973 studies. We then retrieved the full text of the remaining 33 studies. Of these studies, 27 were excluded because they did not meet the inclusion criteria (15 did not provide data stratified by age, 2 investigated a population aged >70 years, and 10 analyzed outcomes or exposures other than AF and EOD). Six studies were therefore left for analysis, all characterized by a cohort design, published between 2015 and 2021. The Table describes the main characteristics of these studies. 21 , 22 , 29 , 30 , 31 , 32 Overall, they encompassed ≈1.6 million participants with a mean age at recruitment ranging from 42 to 85 years, with a dementia outcome in 3647 adults in the studies that studied those aged <70 years. Duration of follow‐up ranged from 1 month to 8 years. The study outcome was incidence of dementia in participants with no history of AF and in those who had a previous history of AF or an AF diagnosis at baseline. Three of these studies reported both the incidence of overall dementia at all ages and of EOD. 29 , 30 , 31 Diagnosis of AF was mainly identified by hospital discharge or admission records, or following such a diagnosis, confirmed on at least 2 occasions in an outpatient clinic (see Table S3 for detailed information). The studies differ in their assessment of dementia. Dementia diagnosis was identified through medical records, prescriptions of disease‐specific medications (eg, donepezil and rivastigmine), clinical neuroimaging, hospital discharge records, diagnosis confirmation at least twice in outpatient clinic, and use of neuropsychological/cognitive tests. Concerning the 2 latter approaches to diagnosis, the Mini‐Mental State Examination and the Geriatric Mental State Schedule were used. One study 29 confirmed the diagnosis of Alzheimer dementia through the combination of clinical diagnosis and documented use of Alzheimer disease medication, and that of vascular dementia through clinical neuroimaging (ie, positron emission tomography/single‐photon emission computed tomography) (Table S3).
Figure 1. Flowchart of the literature search and study identification.
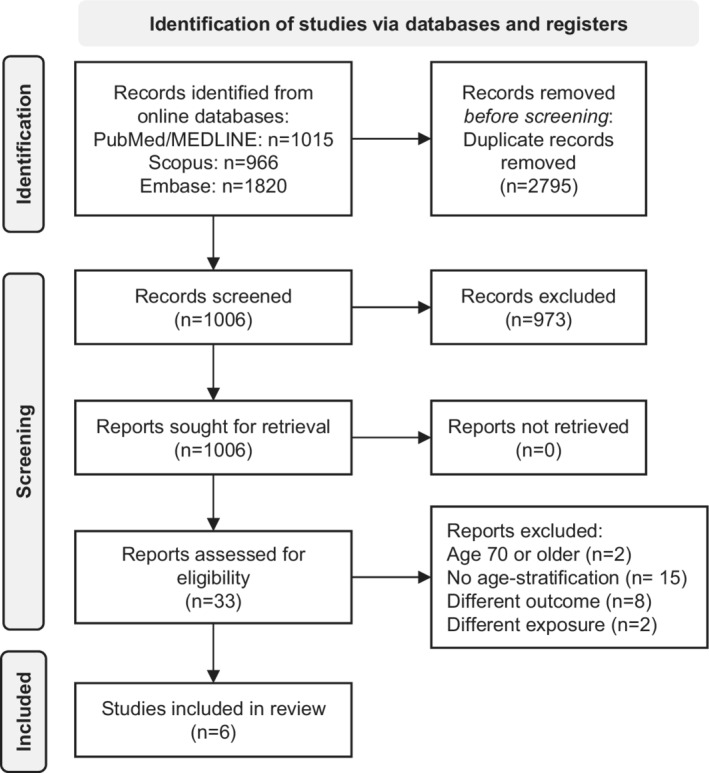
Table Table.
Characteristics of Included Studies
| Reference | Country | Period | Design | Total population (men/women) | Cases (men/women) | Outcome | Early‐onset dementia cutoff, y | Age, mean±SD, y |
|---|---|---|---|---|---|---|---|---|
| Bunch et al 201021 | United States | Not reported | Cohort | 37 025 (10 161 incident AF) | 1535 cases of dementia (all ages); dementia for those aged <70 y not reported | Incidence of dementia | <70 |
60.6±17.9 (57.8±18.9 AF free; 68.1±12.2 AF) |
| Chen et al 202129 | Taiwan | Jan 2001–Dec 2013 | Case‐cohort AF and AF free | 200 130 (100 065/100 065) | 1897 (919/978) | Incidence of dementia (overall and for AD and VaD) | <65 | 72.5±12.5 |
| de Bruijn et al 201522 | The Netherlands | Sept 2014–Apr 2015 | Cohort | 6194 | 932 cases of dementia (all ages); dementia for those aged <70 y not reported | Incidence of dementia | <67 | 68.3 |
| Kim et al 201930 | South Korea | 2005–2012 | Cohort | 262 611 (10 435 incident AF) | 638 | Incidence of dementia | <70 | 70.7±5.4 (AF); 71.7±5.7 (AF free) |
| Kim et al 202031 | South Korea | 2005–2010 | Cohort | 428 262 (232 513/195 749) | 1112 (955 censoring for stroke) | Incidence of AF and dementia | <65 |
61.7±9.9 (AF) 55.5±9.1 (AF free) |
| Liao et al 201532 | Taiwan | Jan 1996–Dec 2011 | Case‐cohort AF and AF free | 665 330 (372 000/293 330) | 56 901 cases of dementia (all ages); dementia for those aged <70 y not reported | Incidence of dementia | <65 | 70.3±13.0 (AF and AF free) |
AD indicates Alzheimer dementia; AF, atrial fibrillation; and VaD, vascular dementia.
Assessment of AF as a risk factor for dementia was performed for those with different forms of dementia (ie, Alzheimer dementia, vascular dementia, senile dementia, or nonspecific dementia). 21 , 22 , 29 , 30 , 31 , 32 Results still showed an association of AF with incidence of dementia in study participants without a history of stroke, compared with the entire study population. Almost all of the studies exhibited a moderate risk of bias (Table S4), mainly because of a lack of adjustment for educational attainment (confounding domain). One study, however, was characterized by a low risk of bias. 22
The meta‐analysis of the results of these studies showed an increased EOD risk associated with presence of AF (summary RR, 1.50 [95% CI, 1.00–2.26]; Figure 2). 21 , 22 , 29 , 30 , 31 , 32 When we restricted the analysis using stroke‐censored results or excluding vascular dementia, we found a similarly increased AF‐related risk of EOD (summary RR, 1.38 [95% CI, 0.91–2.11]; Figure 3). 21 , 22 , 29 , 30 , 31 , 32 The only study with a low overall risk of bias reported an RR of EOD (defined as symptom onset at age <67 years) in individuals with a diagnosis of AF (RR, 1.81 [95% CI, 1.11–2.95]). 22
Figure 2. Forest plot for risk of early‐onset (aged <70 years) dementia in association to atrial fibrillation in all studies.
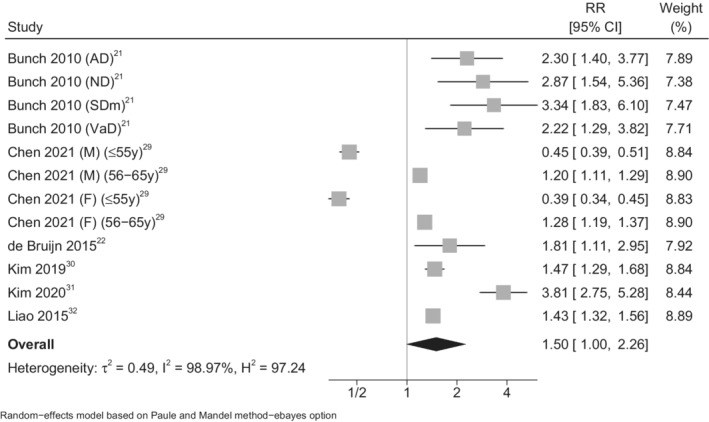
AD indicates Alzheimer dementia; F, female; M, male; ND, nonspecified dementia; RR, risk ratio; SDm, senile dementia; and VaD, vascular dementia.
Figure 3. Forest plot for the risk of early‐onset (aged <70 years) dementia in association with atrial fibrillation in studies using stroke‐censored data or excluding vascular dementia (VaD).
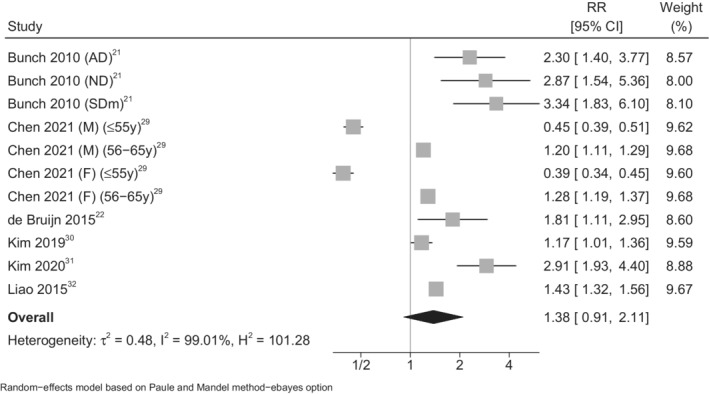
AD indicates Alzheimer dementia; F, female; M, male; ND, nonspecified dementia; RR, risk ratio; and SDm, senile dementia.
In age‐stratified analyses, we identified larger RRs with increasing age ranges (age group <65 years: summary RR, 1.06 [95% CI, 0.54–2.06 29 , 31 , 32 ]; age group <67 years: summary RR, 1.81 [95% CI, 1.11–2.95 22 ]; age group <70 years: summary RR, 2.13 [95% CI, 1.58–2.87 21 , 30 ]) (Figure 4). However, one of the included studies that assessed the risk of dementia in younger adults affected by AF (≤55 years) yielded markedly different results in this specific age group (≤55 years group: RR, 0.45 [95% CI, 0.39–0.51] in men and RR, 0.39 [95% CI, 0.34–0.45] in women; 56–65 years group: RR, 1.20 [95% CI, 1.11–1.29] in men and RR, 1.28 [95% CI, 1.19–1.37] in women). 29 This latter study was also characterized by a wide range of follow‐up (1 month–8 years), with the duration in those with the least follow‐up being too short to allow a reliable assessment of the association between AF and incident dementia. A sensitivity analysis in which this study was excluded still identified an increased risk of dementia (summary RR, 2.16 [95% CI, 1.63–2.88]; Figure S1). 21 , 22 , 30 , 31 , 32 A sensitivity analysis confined to those in whom there was certainty of an EOD diagnosis (dementia onset before 65 years) also identified an increased risk of dementia (summary RR, 1.14 [95% CI, 0.64–2.05]), although the estimate was imprecise (Figure S2). 21 , 22 , 29 , 30 , 31 , 32
Figure 4. Forest plot for risk of early‐onset dementia associated with atrial fibrillation, according to age categories of the populations studied.
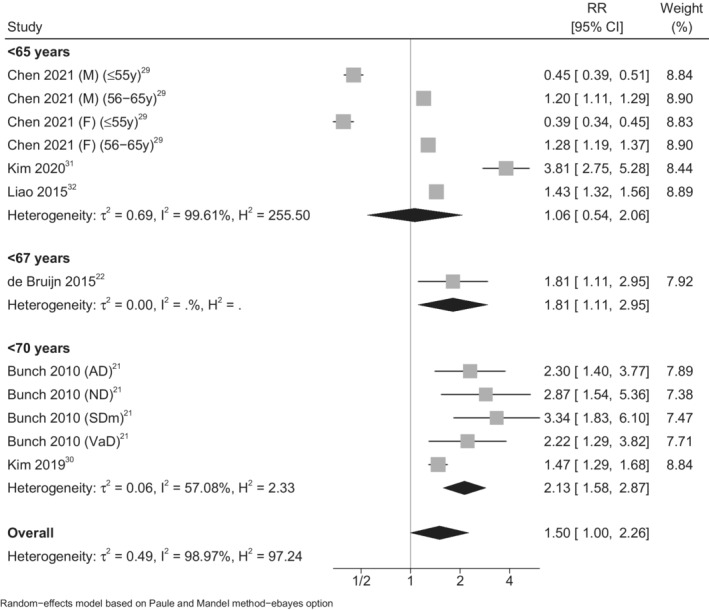
AD indicates Alzheimer dementia; F, female; M, male; ND, nonspecified dementia; RR, risk ratio; SDm, senile dementia; and VaD, vascular dementia.
Funnel plot analysis for small‐study bias showed an asymmetric distribution in line with results using the Egger test, yielding an indication of presence of small‐study effect (4.21 [95% CI, 1.18–7.25]; Figure 5). 21 , 22 , 29 , 30 , 31 , 32
Figure 5. Funnel plot for publication bias and small‐study effects.
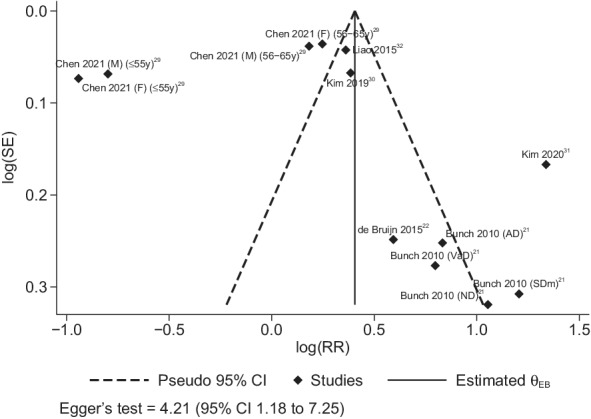
AD indicates Alzheimer dementia; F, female; M, male; ND, nonspecified dementia; RR, risk ratio; SDm, senile dementia; and VaD, vascular dementia.
DISCUSSION
To the best of our knowledge, this systematic review and meta‐analysis is the first to assess the relationship between AF and dementia in studies of adults aged <70 years. It provides evidence that adults with AF experience an increase in the risk of developing dementia before the age of 65 to 70 years. Our findings are consistent with the overall association between AF and dementia risk. 17 , 18 , 19 The biological mechanisms underpinning an excess EOD risk in adults with AF are unclear. Cerebral hypoperfusion and altered cerebral blood flow inducing brain atrophy could play a role, as could a decrease in cardiac output in patients with AF, secondarily to the loss of atrial systole and atrioventricular synchrony. 33 , 34 , 35 , 36 A low or high ventricular rate response, leading to an important reduction or marked variability in cardiac output, could play a role in the development of dementia in individuals affected by AF. 37 , 38 Another potential mechanism could be vascular inflammation, leading to damage of the blood‐brain barrier and adversely affecting the brain. 39 Both the hemodynamic alterations and the vascular inflammation could favor amyloid beta‐protein accumulation and the phosphorylation of tau protein in patients with Alzheimer dementia. 39 , 40 , 41 In addition, blood stasis in the atrium may lead to development of thrombi and consequently increase the risk of strokes and silent cerebral infarct. 42 Last, genetic factors, such as the AF‐related gene PITX2, whose expression results in both structural and electrical remodeling of the atrium, may be associated with an increased risk of ischemic cerebral events and resulting dementia. 43 , 44 , 45 , 46
A causal link between AF and dementia in all age groups is also supported by findings of studies that have investigated the effect of AF therapy in dementia prevention. In particular, an association between oral anticoagulants and a reduced risk of dementia and cognitive impairment in patients with AF has been suggested. 47 , 48 , 49 , 50 In addition, the capacity of rhythm‐control therapy by means of catheter ablation to reduce the risk of dementia has recently been suggested, further supporting a causal link between AF and dementia onset. 51 , 52 In young adults (aged ≤55 years), AF does not seem to correlate positively with dementia, as a decreased risk was reported. 29 This negative association could be attributable to the low AF and dementia incidence before 55 years, 53 or by better adherence to anticoagulant therapy with a relatively low number of comorbid conditions in younger compared with older adults. 54 , 55 As a consequence, we observed a stronger association between AF and dementia after removing the study with young participants from our analysis (summary RR, 2.16 [95% CI, 1.63–2.88]).
Our systematic review and meta‐analysis had several limitations. Because the number of studies available for analysis was limited, we could not conduct subgroup analyses that stratified for geographical area. Interestingly, the quality of the included studies, although generally belonging to the intermediate category (ie, showing a moderate risk of bias), did not appear to influence the results of our meta‐analysis. In fact, our summary estimate for the RR of developing dementia was similar to the RR reported in the only study with a low risk of bias (RR, 1.81 [95% CI, 1.11–2.95]). 22
Our results were generally affected by high heterogeneity. For this reason, we used a random‐effects model that can be considered more robust when implementing meta‐analysis of dichotomous data. 28 We also explored possible sources of heterogeneity in several stratified and sensitivity analyses. We noted a somewhat diminished heterogeneity in the stratified analysis by age of study participants, as well as in our sensitivity analysis in which the study with younger participants was excluded. 29 The remaining heterogeneity may have resulted from various reasons, including the different methods used for dementia assessment (ie, drug prescription, medical records, Mini‐Mental State Examination, or Geriatric Mental State Schedule) and the different covariates that were considered in the multivariable analyses, although we systematically used the most adjusted estimates in our meta‐analysis. Unfortunately, the limited number of studies hampered the implementation of stratified analyses for some of the previously mentioned sources of heterogeneity. In addition, our findings could have been partially affected by the moderate to substantial small‐study effect, with some potential for publication bias that suggests caution in evaluating our results. We were also unable to assess whether AF type (paroxysmal, persistent, or permanent) and duration may contribute differently to the risk of developing dementia, because none of the available studies reported such details, and the role of comorbidities could not be investigated in detail. Last, the lack of information on concurrent treatments, and specifically anticoagulant therapy following the occurrence of AF, did not allow us to assess the effects of the specific pharmacological therapy for AF on EOD risk.
Sources of Funding
This study was supported by the “Dipartimenti di Eccellenza 2018–2022” grant to the Department of Biomedical, Metabolic, and Neural Sciences of the University of Modena and Reggio Emilia from the Italian Ministry of Education, University, and Research.
Disclosures
G.B. received small speaker's fees from Boston and Daiichi‐Sankyo outside of the submitted work.
Supporting information
Data S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025653
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Zimetbaum P. Atrial fibrillation. Ann Intern Med. 2017;166:ITC33–ITC48. doi: 10.7326/AITC201703070 [DOI] [PubMed] [Google Scholar]
- 2. Zoni Berisso M, Landolina M, Ermini G, Parretti D, Zingarini GL, Degli Esposti L, Cricelli C, Boriani G. The cost of atrial fibrillation in Italy: a five‐year analysis of healthcare expenditure in the general population. From the Italian Survey of Atrial Fibrillation Management (ISAF) study. Eur Rev Med Pharmacol Sci. 2017;21:175–183. [PubMed] [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anumonwo JM, Kalifa J. Risk factors and genetics of atrial fibrillation. Heart Fail Clin. 2016;12:157–166. doi: 10.1016/j.hfc.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 7. Chiari A, Vinceti G, Adani G, Tondelli M, Galli C, Fiondella L, Costa M, Molinari MA, Filippini T, Zamboni G et al. Epidemiology of early onset dementia and its clinical presentations in the province of Modena, Italy. Alzheimers Dement. 2020; 17 :81–88. doi: 10.1002/alz.12177 [DOI] [PubMed] [Google Scholar]
- 8. Mendez MF. Early‐onset Alzheimer disease. Neurol Clin. 2017;35:263–281. doi: 10.1016/j.ncl.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adani G, Filippini T, Garuti C, Malavolti M, Vinceti G, Zamboni G, Tondelli M, Galli C, Costa M, Vinceti M, et al. Environmental risk factors for early‐onset Alzheimer's dementia and frontotemporal dementia: a case‐control study in northern Italy. Int J Environ Res Public Health. 2020;17:7941. doi: 10.3390/ijerph17217941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filippini T, Adani G, Malavolti M, Garuti C, Cilloni S, Vinceti G, Zamboni G, Tondelli M, Galli C, Costa M, et al. Dietary habits and risk of early‐onset dementia in an Italian case‐control study. Nutrients. 2020;12:3682. doi: 10.3390/nu12123682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park SH, Lee SR, Choi EK, Lee H, Chung J, Choi J, Han M, Ahn HJ, Kwon S, Lee SW, et al. Low risk of dementia in patients with newly diagnosed atrial fibrillation and a clustering of healthy lifestyle behaviors: a Nationwide population‐based cohort study. J Am Heart Assoc. 2022;11:e023739. doi: 10.1161/JAHA.121.023739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vinceti M, Chiari A, Eichmuller M, Rothman KJ, Filippini T, Malagoli C, Weuve J, Tondelli M, Zamboni G, Nichelli PF, et al. A selenium species in cerebrospinal fluid predicts conversion to Alzheimer's dementia in persons with mild cognitive impairment. Alzheimers Res Ther. 2017;9:100. doi: 10.1186/s13195-017-0323-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population‐based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316 [DOI] [PubMed] [Google Scholar]
- 14. Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta‐analysis. Ann Intern Med. 2013;158:338–346. doi: 10.7326/0003-4819-158-5-201303050-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myserlis PG, Malli A, Kalaitzoglou DK, Kalaitzidis G, Miligkos M, Kokkinidis DG, Kalogeropoulos AP. Atrial fibrillation and cognitive function in patients with heart failure: a systematic review and meta‐analysis. Heart Fail Rev. 2017;22:1–11. doi: 10.1007/s10741-016-9587-y [DOI] [PubMed] [Google Scholar]
- 16. Papanastasiou CA, Theochari CA, Zareifopoulos N, Arfaras‐Melainis A, Giannakoulas G, Karamitsos TD, Palaiodimos L, Ntaios G, Avgerinos KI, Kapogiannis D, et al. Atrial fibrillation is associated with cognitive impairment, all‐cause dementia, vascular dementia, and Alzheimer's disease: a systematic review and meta‐analysis. J Gen Intern Med. 2021;36:3122–3135. doi: 10.1007/s11606-021-06954-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proietti R, AlTurki A, Vio R, Licchelli L, Rivezzi F, Marafi M, Russo V, Potpara TS, Kalman JM, de Villers‐Sidani E, et al. The association between atrial fibrillation and Alzheimer's disease: fact or fallacy? A systematic review and meta‐analysis. J Cardiovasc Med (Hagerstown). 2020;21:106–112. doi: 10.2459/JCM.0000000000000917 [DOI] [PubMed] [Google Scholar]
- 18. Saglietto A, Matta M, Gaita F, Jacobs V, Bunch TJ, Anselmino M. Stroke‐independent contribution of atrial fibrillation to dementia: a meta‐analysis. Open Heart. 2019;6:e000984. doi: 10.1136/openhrt-2018-000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Horton R, Burkhardt JD, Lakkireddy D, Reddy YM, et al. Atrial fibrillation and the risk of incident dementia: a meta‐analysis. Heart Rhythm. 2012;9:1761–1768. doi: 10.1016/j.hrthm.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 20. Wang HT, Chen YL, Lin YS, Chen HC, Chong SZ, Hsueh S, Chung CM, Chen MC. Differential risk of dementia between patients with atrial flutter and atrial fibrillation: a national cohort study. Front Cardiovasc Med. 2021;8:787866. doi: 10.3389/fcvm.2021.787866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm. 2010;7:433–437. doi: 10.1016/j.hrthm.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 22. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288–1294. doi: 10.1001/jamaneurol.2015.2161 [DOI] [PubMed] [Google Scholar]
- 23. Koh YH, Lew LZW, Franke KB, Elliott AD, Lau DH, Thiyagarajah A, Linz D, Arstall M, Tully PJ, Baune BT, et al. Predictive role of atrial fibrillation in cognitive decline: a systematic review and meta‐analysis of 2.8 million individuals. Europace. 2022; euac003. doi: 10.1093/europace/euac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan RL, Thayer KA, Santesso N, Holloway AC, Blain R, Eftim SE, Goldstone AE, Ross P, Ansari M, Akl EA, et al. A risk of bias instrument for non‐randomized studies of exposures: a users' guide to its application in the context of GRADE. Environ Int. 2019;122:168–184. doi: 10.1016/j.envint.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090. doi: 10.1136/bmj.d2090 [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen YL, Chen J, Wang HT, Chang YT, Chong SZ, Hsueh S, Chung CM, Lin YS. Sex difference in the risk of dementia in patients with atrial fibrillation. Diagnostics (Basel). 2021;11: 760. doi: 10.3390/diagnostics11050760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH, Pak HN, Lee MY, Lee MH, Lip GYH, et al. Risk of dementia in stroke‐free patients diagnosed with atrial fibrillation: data from a population‐based cohort. Eur Heart J. 2019;40:2313–2323. doi: 10.1093/eurheartj/ehz386 [DOI] [PubMed] [Google Scholar]
- 31. Kim D, Yang PS, Lip GYH, Joung B. Atrial fibrillation increases the risk of early‐onset dementia in the general population: data from a population‐based cohort. J Clin Med. 2020;9:3665. doi: 10.3390/jcm9113665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liao JN, Chao TF, Liu CJ, Wang KL, Chen SJ, Tuan TC, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Risk and prediction of dementia in patients with atrial fibrillation—a nationwide population‐based cohort study. Int J Cardiol. 2015;199:25–30. doi: 10.1016/j.ijcard.2015.06.170 [DOI] [PubMed] [Google Scholar]
- 33. Aldrugh S, Sardana M, Henninger N, Saczynski JS, McManus DD. Atrial fibrillation, cognition and dementia: a review. J Cardiovasc Electrophysiol. 2017;28:958–965. doi: 10.1111/jce.13261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chinta V, Askandar S, Nanda A, Sharma A, Abader P, Kabra R, Khouzam RN. Atrial fibrillation and deterioration in cognitive function. Curr Probl Cardiol. 2019;44:100386. doi: 10.1016/j.cpcardiol.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 35. Ihara M, Washida K. Linking atrial fibrillation with Alzheimer's disease: epidemiological, pathological, and mechanistic evidence. J Alzheimers Dis. 2018;62:61–72. doi: 10.3233/JAD-170970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, Launer LJ, Gudnason V. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44:1020–1025. doi: 10.1161/STROKEAHA.12.679381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cacciatore F, Testa G, Langellotto A, Galizia G, Della‐Morte D, Gargiulo G, Bevilacqua A, Del Genio MT, Canonico V, Rengo F, et al. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: a 10‐year study. Dement Geriatr Cogn Disord. 2012;34:143–148. doi: 10.1159/000342195 [DOI] [PubMed] [Google Scholar]
- 38. Saglietto A, Scarsoglio S, Ridolfi L, Gaita F, Anselmino M. Higher ventricular rate during atrial fibrillation relates to increased cerebral hypoperfusions and hypertensive events. Sci Rep. 2019;9:3779. doi: 10.1038/s41598-019-40445-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25‐year follow‐up of the Honolulu‐Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265 [DOI] [PubMed] [Google Scholar]
- 40. Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bell RD, Zlokovic BV. Neurovascular mechanisms and blood‐brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rivard L, Friberg L, Conen D, Healey JS, Berge T, Boriani G, Brandes A, Calkins H, Camm AJ, Yee Chen L, et al. Atrial fibrillation and dementia: a report from the AF‐SCREEN International Collaboration. Circulation. 2022;145:392–409. doi: 10.1161/circulationaha.121.055018 [DOI] [PubMed] [Google Scholar]
- 43. Bevan S, Traylor M, Adib‐Samii P, Malik R, Paul NL, Jackson C, Farrall M, Rothwell PM, Sudlow C, Dichgans M, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760 [DOI] [PubMed] [Google Scholar]
- 44. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007 [DOI] [PubMed] [Google Scholar]
- 45. Rivard L, Khairy P. Mechanisms, clinical significance, and prevention of cognitive impairment in patients with atrial fibrillation. Can J Cardiol. 2017;33:1556–1564. doi: 10.1016/j.cjca.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 46. Rollo J, Knight S, May HT, Anderson JL, Muhlestein JB, Bunch TJ, Carlquist J. Incidence of dementia in relation to genetic variants at PITX2, ZFHX3, and ApoE epsilon4 in atrial fibrillation patients. Pacing Clin Electrophysiol. 2015;38:171–177. doi: 10.1111/pace.12537 [DOI] [PubMed] [Google Scholar]
- 47. Chopard R, Piazza G, Gale SA, Campia U, Albertsen IE, Kim J, Goldhaber SZ. Dementia and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Am J Med. 2018;131:1408–1417. doi: 10.1016/j.amjmed.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 48. Lee SR, Choi EK, Park SH, Jung JH, Han KD, Oh S, Lip GYH. Comparing warfarin and 4 direct oral anticoagulants for the risk of dementia in patients with atrial fibrillation. Stroke. 2021;52:3459–3468. doi: 10.1161/STROKEAHA.120.033338 [DOI] [PubMed] [Google Scholar]
- 49. Mongkhon P, Naser AY, Fanning L, Tse G, Lau WCY, Wong ICK, Kongkaew C. Oral anticoagulants and risk of dementia: a systematic review and meta‐analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev. 2019;96:1–9. doi: 10.1016/j.neubiorev.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 50. Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39:453–460. doi: 10.1093/eurheartj/ehx579 [DOI] [PubMed] [Google Scholar]
- 51. Hsieh YC, Chen YY, Chien KL, Chung FP, Lo LW, Chang SL, Chao TF, Hu YF, Lin CY, Tuan TC, et al. Catheter ablation of atrial fibrillation reduces the risk of dementia and hospitalization during a very long‐term follow‐up. Int J Cardiol. 2020;304:75–81. doi: 10.1016/j.ijcard.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 52. Kantharia BK. Impact of catheter ablation of atrial fibrillation on reduction of the risks of dementia and hospitalization. Int J Cardiol. 2020;304:47–49. doi: 10.1016/j.ijcard.2020.01.030 [DOI] [PubMed] [Google Scholar]
- 53. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng LC, Lunetta KL, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;361:k1453. doi: 10.1136/bmj.k1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cadogan SL, Powell E, Wing K, Wong AY, Smeeth L, Warren‐Gash C. Anticoagulant prescribing for atrial fibrillation and risk of incident dementia. Heart. 2021;107:1898–1904. doi: 10.1136/heartjnl-2021-319672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. An J, Bider Z, Luong TQ, Cheetham TC, Lang DT, Fischer H, Reynolds K. Long‐term medication adherence trajectories to direct oral anticoagulants and clinical outcomes in patients with atrial fibrillation. J Am Heart Assoc. 2021;10:e021601. doi: 10.1161/JAHA.121.021601 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


