Abstract
Background
Diltiazem, a moderate cytochrome P450 3A4 isozyme/P‐glycoprotein inhibitor, may potentiate the bleeding risk of direct oral anticoagulants (DOACs) through pharmacokinetic interactions. We evaluated the association between concomitant use of diltiazem with DOACs and bleeding among patients with atrial fibrillation, across varying degrees of kidney function.
Methods and Results
We identified 4544 patients with atrial fibrillation who were initiated on rivaroxaban (n=1583), apixaban (n=2373), or dabigatran (n=588), between 2010 and 2019 in Geisinger Health, with a mean age of 72 years and an estimated glomerular filtration rate of 70 mL/min per 1.73 m2. At the time of DOAC initiation, 15% patients were taking diltiazem and an additional 5% were initiated on diltiazem during follow‐up. Among DOAC users, using diltiazem concurrently (versus DOAC alone) was associated with an increased risk of any bleeding‐related hospitalization (unadjusted risk difference, 2.4; 95% CI, 0.6–4.2 events per 100 person‐years; adjusted hazard ratio, 1.56, 95% CI, 1.15–2.12), as well as major bleeding (unadjusted risk difference, 1.4 [95% CI, 0.1–2.6 events per 100 person‐years]; adjusted hazard ratio, 1.84 [95% CI, 1.18–2.85]). Increased risk of any/major bleeding with diltiazem was observed in both patients with and without CKD (estimated glomerular filtration rate <60 mL/min per 1.73 m2) (P for interaction=0.524 and 0.629, respectively). Among 13 179 warfarin users (the negative control), concomitant diltiazem use was not associated with bleeding.
Conclusions
Concomitant use of diltiazem with DOACs was associated with a higher bleeding risk in patients with atrial fibrillation, consistently in both subgroups of chronic kidney disease and non–chronic kidney disease. For DOAC users, concomitant diltiazem should be prescribed only when the benefit outweighs the risk, with close monitoring for signs of bleeding.
Keywords: atrial fibrillation, chronic kidney disease, diltiazem, direct oral anticoagulants, drug–drug interactions, hemorrhage
Subject Categories: Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- CHA2DS2‐VASc
congestive heart failure, hypertension, age ≥75, diabetes, prior stroke/transient ischemic attack, vascular disease, age 65 to 74 years, and sex category
- CYP
cytochrome P450
- CYP3A4
cytochrome P450 3A4 isozyme
- DOAC
direct oral anticoagulant
- FDA
Food and Drug Administration
- HAS‐BLED
hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (not included in the score calculation), elderly >65 years, drugs/alcohol concomitantly
- IQI
interquartile interval
- P‐gp
P‐glycoprotein
- RD
risk difference
- ROCKET AF
Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation
Clinical Perspective.
What Is New?
This real‐world study found a 56% higher risk of bleeding‐related hospitalization associated with concomitant use of diltiazem, a moderate cytochrome P450 3A4 isozyme/P‐glycoprotein inhibitor, with direct oral anticoagulants (rivaroxaban, apixaban, or dabigatran), among 4544 patients with atrial fibrillation, consistently in both subgroups of patients with and without chronic kidney disease.
These findings support the current Food and Drug Administration label for rivaroxaban, which recommends avoidance of concurrent use of diltiazem with rivaroxaban, and also provides important safety information for apixaban users, given that the Food and Drug Administration guidance for concurrent use of diltiazem with apixaban is lacking.
What Are the Clinical Implications?
Diltiazem should be prescribed only in patients treated with direct oral anticoagulants when the benefit outweighs the risk, with close monitoring for signs of bleeding.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its prevalence is noted as 15% to 20% in people with chronic kidney disease (CKD). 1 , 2 It causes significant morbidity and mortality, including a 5‐fold increased risk of ischemic stroke. 3 , 4 Direct oral anticoagulants (DOACs) have been approved to reduce the risk of ischemic stroke for more than a decade, and are now recommended over warfarin in patients with AF by clinical guidelines. 5 , 6 , 7 However, safety concerns are still present. For example, P‐glycoprotein (P‐gp) and cytochrome P450 (CYP) inhibitors such as CYP 3A4 isozyme (CYP3A4) may increase plasma DOAC concentrations and increase the risk of bleeding, 8 , 9 particularly among patients with CKD. The US Food and Drug Administration’s (FDA’s) prescribing information recommends avoidance of DOACs with strong CYP3A4/P‐gp inhibitors, but provides little guidance with moderate to weak CYP3A4/P‐gp inhibitors. 10 , 11 , 12 As improving safer use of anticoagulants is prioritized by the National Action Plan for Adverse Drug Event Prevention, 13 it is of substantial importance to better understand impacts of interactions between DOACs and moderate to weak CYP3A4/P‐gp inhibitors in real‐world scenarios.
Diltiazem, a moderate CYP3A4/P‐gp inhibitor, is commonly used for heart rate control among patients with AF and increases plasma concentration of apixaban and rivaroxaban by 31% and 40%, respectively, via pharmacokinetic interactions when coadministrated. 14 , 15 , 16 , 17 Based on the post hoc analyses of ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), 18 the FDA labeling for rivaroxaban recommends avoidance of concomitant moderate CYP3A4/P‐gp inhibitors, including diltiazem, in patients with creatinine clearance (CrCl) between 15 mL/min and <80 mL/min. 10 However, current FDA labeling has no guidance on the use of apixaban or dabigatran when diltiazem is coadministrated because of limited trial data on evaluating diltiazem‐apixaban/dabigatran interactions. Real‐world evidence is also sparse, with one case report of bleeding with concurrent use of diltiazem and apixaban 19 and three cohort studies with controversial results. 20 , 21 , 22
Patients with CKD are at higher risk of bleeding compared with those without CKD. 23 , 24 , 25 , 26 , 27 Patients with CKD are also vulnerable to adverse events caused by drug–drug interactions, 28 , 29 , 30 as they are often older, with multiple comorbidities, 31 , 32 and at increased risk of polypharmacy. 33 Nevertheless, current understanding of drug–drug interactions with DOACs mostly comes from animal models, case reports, and limited pharmacokinetic studies conducted in younger and healthier populations. 34 , 35 Previous real‐world studies are limited by the lack of kidney function assessment or exclusion of patients with CKD. 20 , 21 Therefore, the clinical effects of concomitant diltiazem use with DOACs remain unclear, particularly in this high‐risk population with CKD.
The aim of this study was to evaluate whether concomitant use of diltiazem was associated with the risk of bleeding among DOAC users for AF and whether the associations differed by CKD status using real‐world data.
METHODS
Data Source
Geisinger is a large health care system that serves >3 million citizens in central and northeastern Pennsylvania, with <1% outmigration per year. We used deidentified electronic health record data from inpatient and outpatient encounters, sociodemographic characteristics, outpatient prescription records, and laboratory results. The study protocol was reviewed and approved by the institutional review boards of Geisinger Medical Center and Johns Hopkins University. The requirement of informed consent was waived. Per data use agreements, we are not allowed to share the health care data of patients in the Geisinger Health System. However, all reasonable requests for verification analyses will be fulfilled.
Study Population
Eligible patients were adults aged >18 years with a diagnosis of AF who were initiated on rivaroxaban, apixaban, or dabigatran between January 1, 2010, and February 1, 2019. We defined the index date as the date of the first prescription of DOACs, and required at least 1 year of prior engagement with the Geisinger system and at least one outpatient serum creatinine measurement within the year before the index date for inclusion. We excluded patients undergoing dialysis or with a history of kidney transplant, and those with diagnoses of venous thromboembolism. In a sensitivity analysis, we further excluded patients diagnosed with left ventricular thrombus based on International Classification of Diseases (ICD) diagnosis codes (“I51.3” and “429.79”).
Study Design
The exposure of interest was the concomitant use of diltiazem with DOACs (versus DOACs alone) and was treated as time‐varying based on the outpatient prescription records. The start and end dates of medications were extracted and used to determine the time‐varying diltiazem exposure status. Patients were considered exposed during the days when they had both prescriptions for diltiazem and DOACs, and unexposed during the days when they were using DOACs only. We allowed patients to have a grace period up to 7 days after the last day with diltiazem prescription and still be considered exposed, to make sure that patients had no diltiazem at hand when they were considered unexposed. In a sensitivity analysis, patients were only considered exposed when they had prescriptions for both diltiazem and DOACs on the same day with no grace period.
The outcome of bleeding was defined as hospitalization (emergency department visit or inpatient stay) with an issued primary diagnosis of any bleeding regardless of sites based on published, validated ICD versions 9 and 10 codes (Table S1). 36 , 37 Patients were followed until the first bleeding occurrence, or censored at the following event times: DOAC discontinuation, last encounter with Geisinger, kidney failure (dialysis or kidney transplantation), death, or end of study period on February 28, 2019, whichever came first. Discontinuation of DOACs was defined as a gap in use ≥90 days, and patients were taking rivaroxaban, apixaban, or dabigatran since initiation (index date) over the follow‐up.
To take into account the severity of bleeding, we conducted a secondary analysis on major bleeding, defined as hospitalization with a primary diagnosis code of intracranial or gastrointestinal hemorrhage, or other bleeding episodes (eg, retroperitoneal, intraocular, or intra‐articular hemorrhage; see a full list of validated diagnosis codes in Table S1). 36 , 37
Covariates
Covariates at DOAC initiation included demographics (ie, age, sex, and race); drinking history; smoking status; body mass index; CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75, diabetes, prior stroke/transient ischemic attack, vascular disease, age 65 to 74 years, and sex category) score 38 ; HAS‐BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio [not included in the score calculation], elderly >65 years, drugs/alcohol concomitantly) score 39 ; Charlson Comorbidity Index; number of hospitalizations within 1‐year pre‐index period; initiation year of DOACs; and history of comorbidities (ie, diabetes, hypertension, heart failure, stroke, coronary heart disease, peripheral vascular disease, past bleeding, liver diseases, chronic pulmonary disease, rheumatic disease, and cancer). A higher CHA2DS2‐VASc score (maximum of 9) indicates a higher risk of stroke; likewise, the HAS‐BLED score (maximum of 8) predicts 1‐year risk for major bleeding. The Charlson Comorbidity Index is a composite score for 17 comorbidity categories. All diagnosis codes are presented in Table S1. 40 , 41
We used serum creatinine measurement to calculate estimated glomerular filtration rate (eGFR) with the 2021 Chronic Kidney Disease Epidemiology Collaboration creatinine‐based equation. 42 The eGFR, use of antiplatelets (aspirin alone, adenosine diphosphate P2Y12 receptor inhibitors alone, and dual antiplatelet therapy), and NSAIDs were updated over the follow‐up and thus adjusted as time‐varying covariates. We also adjusted for time‐varying use of other CYP3A4/P‐gp inhibitors (ie, azole‐antimycotics, amiodarone, clarithromycin, dronedarone, erythromycin, pibrentasvir, ritonavir, quinidine, and verapamil) and CYP3A4/P‐gp inducers (ie, apalutamide, carbamazepine, fosphenytoin, phenobarbital/primidone, phenytoin, rifampin, and St John's wort), since they may potentially interact with DOACs and affect the bleeding risk.
Statistical Analysis
Demographic and clinical characteristics, overall and by baseline diltiazem use, were reported as proportion with count for categorical variables and mean with SD or median with interquartile interval (IQI) for continuous variables. Missing data on drinking (0.3%), smoking (2%), and body mass index (15%) were imputed using chained equations, and 25 imputed data sets were created. 43 , 44 , 45 For each imputed data set, we used Cox proportional hazards regression model with time‐varying exposure of diltiazem to estimate hazard ratio (HR) and 95% CI for risk of bleeding. Then, we pooled the HRs using Rubin's rule to estimate the effect size. 46 We assessed whether the associations differed by CKD status (defined as eGFR <60 mL/min per 1.73 m2 according to the Kidney Disease: Improving Global Outcomes guideline), 47 using the same analytic approach with an interaction term between diltiazem use and indicator for CKD status. Last, as an exploratory analysis, we examined DOAC dosage using creatinine clearance calculated by Cockcroft‐Gault equation 48 to determine overdose or underdose for rivaroxaban and dabigatran according to the FDA labels (Table S2). 10 , 11 , 12 Serum creatinine, body weight, and age were used to determine overdose or underdose for apixaban.
Results with 2‐sided P values <0.05 were considered statistically significant. All analysis was performed using SAS software version 9.4 (SAS Institute Inc), and figures were constructed using R version 3.6.0 (R Foundation for Statistical Computing).
Propensity Score–Matched Analysis
As a sensitivity analysis, we fit logistic regression to estimate the propensity score of receiving diltiazem (versus not receiving diltiazem) at baseline (ie, DOAC initiation) including all of the aforementioned baseline covariates in each imputed data set. Then, we used 1‐to‐3 nearest neighbor matching without replacement to create a matched cohort. In each matched cohort, we estimated the HR (95% CI) of bleeding using Cox proportional hazards regression model and pooled the estimates using Rubin's rule. 46
Analysis With Negative Controls
We used a series of negative controls to address residual/unmeasured confounding. First, we used warfarin users as the negative control study population since there is no known interaction between warfarin and diltiazem. 49 In other words, we repeated the same analysis with time‐varying exposure of diltiazem among warfarin initiators within the same study period. Second, we selected metoprolol, another commonly used medication recommended for heart rate control for AF but does not interact with DOACs, 50 , 51 as the negative control exposure for diltiazem. We conducted a separate analysis using time‐varying exposure to metoprolol among DOAC initiators. Last, we chose urinary tract infection as a negative control outcome because it is not a known side effect for either diltiazem or anticoagulants. We applied the same statistical techniques evaluating the association of time‐varying exposure to diltiazem with the outcome of urinary tract infection among DOAC initiators.
RESULTS
Patient Characteristics
A total of 4544 patients with AF who initiated rivaroxaban (n=1583), apixaban (n=2373), or dabigatran (n=588), were identified between January 2010 and February 2019 (Figure S1). The duration of median follow‐up was 182 days from DOAC initiation (IQI, 33–540 days). The patients were 45% women, with a mean age of 72 years (SD, 12 years) and an average eGFR of 70 mL/min per 1.73 m2 (SD, 21 mL/min per 1.73 m2) (Table 1). The average CHA2DS2‐VASc score was 3.8 (SD, 2.0), and more than half of the patients had a history of stroke, coronary heart disease, or peripheral vascular disease. The average HAS‐BLED score was 3.2 (SD, 1.5), and more than half had a bleeding history. At the time of DOAC initiation, 15% were taking diltiazem, and an additional 5% (203 of 3862) initiated diltiazem during the follow‐up afterwards (median of 58 days; IQI, 13–241 days). Diltiazem users were younger and more likely to be women and healthier (higher eGFR, lower CHA2DS2‐VASc score, lower HAS‐BLED score, and fewer comorbidities). Overall, few patients were concurrently using other CYP3A4/P‐gp inducers or inhibitors, of which amiodarone was most frequently prescribed at baseline and more frequently prescribed among diltiazem nonusers than users (5.9% versus 3.1%). Patient characteristics of each individual DOAC user are presented in Table S3 through S5.
Table 1.
Baseline Characteristics of Initiators of Any DOAC, Overall and by Baseline Diltiazem Use
| Baseline characteristics | Overall (N=4544) | Diltiazem nonusers (n=3862) | Diltiazem users (n=682) |
|---|---|---|---|
| Age, mean (SD), y | 72 (12) | 72 (12) | 70 (12) |
| Age <65 y | 1243 (27.4) | 1040 (26.9) | 203 (29.8) |
| Age 65–74 y | 1383 (30.4) | 1173 (30.4) | 210 (30.8) |
| Age 75–84 y | 1334 (29.4) | 1140 (29.5) | 194 (28.4) |
| Age ≥85 y | 584 (12.9) | 509 (13.2) | 75 (11.0) |
| Women | 2021 (44.5) | 1674 (43.3) | 347 (50.9) |
| White race | 4444 (97.8) | 3783 (98.0) | 661 (96.9) |
| Body mass index, mean (SD), kg/m2 * | 31.8 (7.9) | 31.8 (7.8) | 32.1 (8.8) |
| Underweight (<18.5) | 68 (1.5) | 62 (1.5) | 6 (1.3) |
| Normal (18.5–24.9) | 751 (16.4) | 672 (16.3) | 79 (17.2) |
| Overweight (25.0–29.9) | 1292 (28.3) | 1170 (28.4) | 122 (26.6) |
| Obese (≥30) | 2462 (53.8) | 2210 (53.7) | 252 (54.9) |
| Drinking history* | 1940 (42.4) | 1750 (42.5) | 190 (41.4) |
| Smoking status* | |||
| Never smoker | 1955 (42.7) | 1761 (42.8) | 194 (42.3) |
| Former smoker | 2183 (47.7) | 1966 (47.8) | 217 (47.3) |
| Current smoker | 435 (9.5) | 387 (9.4) | 48 (10.5) |
| eGFR, mean (SD), mL/min per 1.73 m2 | 70 (21) | 69 (21) | 71 (21) |
| ≥60 | 3021 (66.5) | 2540 (65.8) | 481 (70.5) |
| 30–59 | 1363 (30.0) | 1182 (30.6) | 181 (26.5) |
| <30 | 160 (3.5) | 140 (3.6) | 20 (2.9) |
| CHA2DS2‐VASc score, mean (SD) | 3.8 (2.0) | 3.8 (1.9) | 3.6 (2.0) |
| 0 | 158 (3.5) | 129 (3.3) | 29 (4.3) |
| 1–3 | 1931 (42.5) | 1620 (41.9) | 311 (45.6) |
| 4+ | 2455 (54.0) | 2113 (54.7) | 342 (50.1) |
| HAS‐BLED score, mean (SD) | 3.2 (1.5) | 3.2 (1.5) | 3.0 (1.5) |
| 0 | 135 (3.0) | 107 (2.8) | 28 (4.1) |
| 1 to 3 | 2542 (55.9) | 2152 (55.7) | 390 (57.2) |
| 4+ | 1867 (41.1) | 1603 (41.5) | 264 (38.7) |
| Charlson Comorbidity Index, mean (SD) | 2.1 (2.0) | 2.2 (2.0) | 2.0 (1.9) |
| 0 | 1035 (22.8) | 850 (22.0) | 185 (27.1) |
| 1 | 997 (21.9) | 854 (22.1) | 143 (21.0) |
| 2+ | 2512 (55.3) | 2158 (55.9) | 354 (51.9) |
| No. of hospitalizations, median (IQI) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Diabetes | 1441 (31.7) | 1248 (32.3) | 193 (28.3) |
| Hypertension | 3720 (81.9) | 3165 (82.0) | 555 (81.4) |
| Cardiovascular disease | 2574 (56.6) | 2247 (58.2) | 327 (47.9) |
| Stroke | 822 (18.1) | 719 (18.6) | 103 (15.1) |
| Coronary heart disease | 1826 (40.2) | 1613 (41.8) | 213 (31.2) |
| Peripheral vascular disease | 779 (17.1) | 694 (18.0) | 85 (12.5) |
| Bleeding history | 13 (0.3) | 12 (0.3) | 1 (0.1) |
| Liver disease | 203 (4.5) | 174 (4.5) | 29 (4.3) |
| Chronic pulmonary disease | 451 (9.9) | 369 (9.6) | 82 (12.0) |
| Rheumatic disease | 242 (5.3) | 206 (5.3) | 36 (5.3) |
| Cancer | 804 (17.7) | 668 (17.3) | 136 (19.9) |
| NSAIDs | 1289 (28.4) | 1114 (28.8) | 175 (25.7) |
| Aspirin alone | 2191 (48.2) | 1878 (48.6) | 313 (45.9) |
| Adenosine diphosphate P2Y12 receptor inhibitors alone | 120 (2.6) | 109 (2.8) | 11 (1.6) |
| Dual antiplatelet therapy | 134 (3.0) | 126 (3.2) | 8 (1.2) |
| Aspirin plus clopidogrel | 125 (2.8) | 117 (3.0) | 8 (1.2) |
| Aspirin plus ticagrelor | 5 (0.1) | 5 (0.1) | 0 (0) |
| Aspirin plus prasugrel | 4 (0.1) | 4 (0.1) | 0 (0) |
| P‐gp inducers | 37 (0.8) | 28 (0.7) | 9 (1.3) |
| Phenobarbital/Primidone | 25 (0.6) | 21 (0.5) | 4 (0.6) |
| Phenytoin | 8 (0.18) | 6 (0.16) | 2 (0.29) |
| Carbamazepine | 6 (0.13) | 4 (0.10) | 2 (0.29) |
| Rifampin | 1 (0.02) | 0 (0) | 1 (0.15) |
| P‐gp inhibitor | 343 (7.6) | 311 (8.1) | 32 (4.7) |
| Amiodarone | 247 (5.4) | 226 (5.9) | 21 (3.1) |
| Verapamil | 46 (1.0) | 42 (1.1) | 4 (0.59) |
| Dronedarone | 24 (0.53) | 22 (0.57) | 2 (0.29) |
| Erythromycin | 21 (0.46) | 20 (0.52) | 1 (0.15) |
| Clarithromycin | 5 (0.11) | 2 (0.05) | 3 (0.44) |
| Azole‐antimycotics (itraconazole/ketoconazole/fluconazole) | 5 (0.11) | 4 (0.10) | 1 (0.15) |
CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75, diabetes, prior stroke/transient ischemic attack, vascular disease, age 65 to 74 years, and sex category; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HAS‐BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (not included in the score calculation), elderly >65 years, drugs/alcohol concomitantly; IQI, interquartile interval; and P‐gp, P‐glycoprotein.
Data on these variables were from a randomly selected imputed data set. Values are expressed as number (percentage) unless otherwise indicated.
Risk of Bleeding Associated With Concurrent Use of Diltiazem Among DOAC Users
Overall, 56 any bleeding‐related hospitalizations occurred during 852 person‐years with diltiazem coprescribed with DOACs and 200 events during 4766 person‐years with DOACs only. Among DOAC users, concomitant use of diltiazem was associated with a higher risk of any bleeding (unadjusted risk difference [RD], 2.4 [95% CI, 0.6–4.2] events per 100 person‐years; adjusted HR, 1.56 [95% CI, 1.15–2.12]) (Table 2). For rivaroxaban users, the unadjusted incidence rate of any bleeding associated with diltiazem exposure was twice the rate without diltiazem exposure (8.7 versus 4.3 events per 100 person‐years; unadjusted RD, 4.4 [95% CI, 1.0–7.8] events per 100 person‐years), and the bleeding risk remained significant after controlling for potential confounders (adjusted HR, 1.90; 95% CI, 1.20–3.01). Likewise, among apixaban users, the unadjusted incidence rate of any bleeding associated with diltiazem exposure was higher (7.2 versus 4.6 events per 100 person‐years) and the bleeding risk remained significant after adjustment (adjusted HR, 1.75; 95% CI, 1.05–2.93). For dabigatran users, there were only 7 events associated with diltiazem exposure, without significant association.
Table 2.
Risk of Any Bleeding‐Related Hospitalization Associated With Diltiazem Among DOAC Users
| Exposure | No. of events | Person‐y | Unadjusted incidence rate, events per 100 person‐y | Unadjusted RD (95% CI), events per 100 person‐y | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Any DOAC users | |||||
| Diltiazem unexposed | 200 | 4765.6 | 4.2 | Reference | Reference |
| Diltiazem exposed | 56 | 851.7 | 6.6 | 2.4 (0.6 to 4.2)* | 1.56 (1.15 to 2.12)* |
| Rivaroxaban users | |||||
| Diltiazem unexposed | 75 | 1763.5 | 4.3 | Reference | Reference |
| Diltiazem exposed | 27 | 311.1 | 8.7 | 4.4 (1.0 to 7.8)* | 1.90 (1.20 to 3.01)* |
| Apixaban users | |||||
| Diltiazem unexposed | 83 | 1818.5 | 4.6 | Reference | Reference |
| Diltiazem exposed | 20 | 276.8 | 7.2 | 2.7 (−0.6 to 6.0) | 1.75 (1.05 to 2.93)* |
| Dabigatran users | |||||
| Diltiazem unexposed | 30 | 861.2 | 3.5 | Reference | Reference |
| Diltiazem exposed | 7 | 200.9 | 3.5 | <0.001 (−2.9 to 2.9) | 0.62 (0.22 to 1.74) |
DOAC indicates direct oral anticoagulant; HR, hazard ratio; and RD, risk difference.
Two‐sided P value <0.05.
More than half of the bleeding‐related hospitalizations were attributable to major bleeding. Among DOAC users, there was an increase in major bleeding risk comparing using diltiazem with DOACs with using DOACs alone (unadjusted RD, 1.4 [95% CI, 0.1–2.6 events per 100 person‐years]; adjusted HR, 1.84 [95% CI, 1.18–2.85]) (Table 3). For both rivaroxaban and apixaban users, concomitant use of diltiazem was associated with a higher risk of major bleeding (unadjusted RD, 2.4 [95% CI, 0.03–4.7] events per 100 person‐years; adjusted HR, 2.08 [95% CI, 1.04–4.15] for rivaroxaban users; unadjusted RD, 2.6 [95% CI, 0.06–5.0] events per 100 person‐years; adjusted HR, 3.05 [95% CI, 1.48–6.30] for apixaban users).
Table 3.
Risk of Major Bleeding Associated With Diltiazem Among DOAC Users
| Exposure | No. of events | Person‐y | Unadjusted incidence rate, events per 100 person‐y | Unadjusted RD (95% CI), events per 100 person‐y | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Any DOAC users | |||||
| Diltiazem unexposed | 89 | 4770.9 | 1.9 | Reference | Reference |
| Diltiazem exposed | 28 | 864.1 | 3.2 | 1.4 (0.1 to 2.6)* | 1.84 (1.18 to 2.85)* |
| Rivaroxaban users | |||||
| Diltiazem unexposed | 31 | 1757.3 | 1.8 | Reference | Reference |
| Diltiazem exposed | 13 | 315.7 | 4.1 | 2.4 (0.03 to 4.7)* | 2.08 (1.04 to 4.15)* |
| Apixaban users | |||||
| Diltiazem unexposed | 31 | 1813.1 | 1.7 | Reference | Reference |
| Diltiazem exposed | 12 | 281.9 | 4.3 | 2.6 (0.06 to 5.0)* | 3.05 (1.48 to 6.30)* |
| Dabigatran users | |||||
| Diltiazem unexposed | 19 | 880.2 | 2.2 | Reference | Reference |
| Diltiazem exposed | 2 | 201.2 | 1.0 | −1.2 (−2.9 to 0.5) | 0.23 (0.03 to 1.96) |
DOAC indicates direct oral anticoagulant; HR, hazard ratio; and RD, risk difference.
Two‐sided P value <0.05.
The results were consistent in both patients with CKD (eGFR <60 mL/min per 1.73 m2) and without CKD (eGFR ≥60 mL/min per 1.73 m2) (all P for interaction >0.05) (Figures 1 and 2). The results were materially unchanged in the sensitivity analysis with no grace period of diltiazem use (Table S6, S7 and Figure S2 and S3), as well as in the sensitivity analysis excluding patients with left ventricular thrombus (Table S8 and Figure S4). In the propensity score–matched analysis (682 diltiazem users and 2046 diltiazem nonusers), diltiazem use at baseline with DOACs was associated with higher risk of bleeding (HR, 1.48; 95% CI, 1.04–2.10), similarly in both CKD and non‐CKD (interaction P=0.245) (Figure S5).
Figure 1. Risk of any bleeding‐related hospitalization associated with diltiazem among direct oral anticoagulant (DOAC) users.
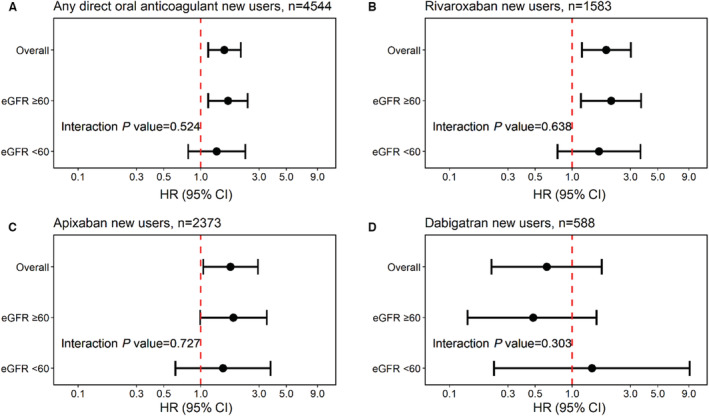
A, Hazard ratio (HRs) and 95% CIs for any bleeding estimated using Cox proportional hazards regression models with time‐varying exposure to diltiazem among DOAC users, overall and stratified by chronic kidney disease status (estimated glomerular filtration rate [eGFR] <60 vs ≥60 mL/min per 1.73 m2). For individual DOAC agents, the same analysis was repeated to estimate overall and stratum‐specific HRs and 95% CIs in rivaroxaban users (B), in apixaban users (C), and in dabigatran users (D).
Figure 2. Risk of major bleeding associated with diltiazem among direct oral anticoagulant (DOAC) users.

A, Hazard ratios (HRs) and 95% CIs for major bleeding estimated using Cox proportional hazards regression models with time‐varying exposure to diltiazem among DOAC users, overall and stratified by chronic kidney disease status (estimated glomerular filtration rate [eGFR] <60 vs ≥60 mL/min per 1.73 m2). For individual DOAC agents, the same analysis was repeated to estimate overall and stratum‐specific HRs and 95% CIs in rivaroxaban users (B), and in apixaban users (C), while an interaction model was not possible because of zero major bleeding events in dabigatran users with eGFR <60 mL/min per 1.73 m2 (D).
DOAC Dosing Patterns by CKD
At the time of DOAC initiation, 86% of patients were administrated the appropriate dosage (rivaroxaban 82%, apixaban 87%, and dabigatran 90%). The adherence to the FDA dosing recommendation was lower among patients with CKD compared with those without CKD (any DOAC 76% versus 91%; rivaroxaban 71% versus 87%; apixaban 78% versus 92%; and dabigatran 81% versus 93%). Overall, underdosing of DOACs was more common than overdosing (12% versus 2%), and underdosing was more common among patients with CKD than those without CKD (18% versus 9%).
Risk of All Bleeding in the Analysis With Negative Controls
Using warfarin users as a negative control study population (n=13 179), the risk of all bleeding was similar regardless of diltiazem exposure among warfarin users (adjusted HR, 0.98; 95% CI, 0.84–1.15) (Table 4). Using metoprolol as a negative control exposure, concomitant use of metoprolol was not associated with bleeding among DOAC users (adjusted HR, 1.21; 95% CI, 0.92–1.60), as well as among individual agent users (Table 5). Using urinary tract infection as a negative control outcome, there were no associations between concomitant use of diltiazem and urinary tract infection among DOAC users (adjusted HR, 1.04; 95% CI, 0.86–1.26), as well as among users of the individual agent (Table S9).
Table 4.
Risk of Any Bleeding‐Related Hospitalization Associated With Diltiazem Among Warfarin Users (Negative Control Population, n=13 179), Overall and by eGFR
| Exposure | No. of events | Person‐y | Unadjusted incidence rate, events per 100 person‐y | Unadjusted RD (95% CI), events per 100 person‐y | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Overall | |||||
| Diltiazem unexposed | 1280 | 37 418.7 | 3.4 | Reference | Reference |
| Diltiazem exposed | 177 | 4711.1 | 3.8 | 0.3 (−0.3 to 0.9) | 0.98 (0.84 to 1.15) |
| eGFR ≥60 mL/min per 1.73 m2 | |||||
| Diltiazem unexposed | 750 | 22 588.9 | 3.3 | Reference | Reference |
| Diltiazem exposed | 111 | 3058.8 | 3.6 | 0.3 (−0.4 to 1.0) | 0.98 (0.80 to 1.20) |
| eGFR <60 mL/min per 1.73 m2 | |||||
| Diltiazem unexposed | 530 | 14 093.5 | 3.8 | Reference | Reference |
| Diltiazem exposed | 66 | 1581.2 | 4.2 | 0.4 (−0.6 to 1.5) | 0.98 (0.76 to 1.27) |
eGFR indicates estimated glomerular filtration rate; HR, hazard ratio; and RD, risk difference.
Table 5.
Risk of Any Bleeding‐Related Hospitalization Associated With Metoprolol (Negative Control Exposure) Among DOAC Users
| Exposure | No. of events | Person‐y | Unadjusted incidence rate, events per 100 person‐y | Unadjusted RD (95% CI), events per 100 person‐y | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Any DOAC users | |||||
| Metoprolol unexposed | 84 | 2135.5 | 3.9 | Reference | Reference |
| Metoprolol exposed | 153 | 3082.2 | 5.0 | 1.0 (−0.1 to 2.2) | 1.21 (0.92 to 1.60) |
| Rivaroxaban users | |||||
| Metoprolol unexposed | 33 | 784.4 | 4.2 | Reference | Reference |
| Metoprolol exposed | 61 | 1148.1 | 5.3 | 1.1 (−0.9 to 3.1) | 1.39 (0.89 to 2.17) |
| Apixaban users | |||||
| Metoprolol unexposed | 31 | 755.3 | 4.1 | Reference | Reference |
| Metoprolol exposed | 65 | 1235.4 | 5.3 | 1.2 (−0.8 to 3.1) | 1.32 (0.84 to 2.07) |
| Dabigatran users | |||||
| Metoprolol unexposed | 16 | 460.6 | 3.5 | Reference | Reference |
| Metoprolol exposed | 18 | 503.0 | 3.6 | 0.1 (−2.3 to 2.5) | 0.64 (0.24 to 1.77) |
DOAC indicates direct oral anticoagulant; HR, hazard ratio; and RD, risk difference.
DISCUSSION
In this study using real‐world data from a large US health system, we demonstrated that patients with AF who used diltiazem concurrently with DOACs had a 56% higher risk of any bleeding‐related hospitalization than those who used DOACs only, and the strength of such an association was similar between those with and without CKD. The risk of major bleeding was nearly 2‐fold among any DOAC users coadministrated with diltiazem, with doubled risk in rivaroxaban users and tripled risk in apixaban users. These findings were specific to the concomitant use of diltiazem with DOACs because we did not find an increased bleeding risk related to combinations of diltiazem with warfarin (negative control study population) or metoprolol with DOACs (negative control exposure). The results persisted in the series of sensitivity analysis including propensity score–matched analysis.
Although it is well established that CYP3A4 and/or P‐gp inhibitors can increase DOAC bioavailability and inhibit ≥1 clearance pathway simultaneously, 52 real‐world evidence is limited and controversial. An early case‐cohort study of 286 patients from a single center found no difference in bleeding risk comparing patients taking rivaroxaban with and without diltiazem. 22 However, the results were likely biased because of inclusion of prevalent rivaroxaban users in the diltiazem‐exposed group only (ie, less susceptible to bleeding). A recent retrospective cohort study at the same institution used an incident user design and propensity score matching, and found an increased risk of bleeding with combined P‐gp and moderate CYP3A4 inhibitors including diltiazem (HR, 1.80; 95% CI, 1.19–2.73). 53 Chang et al 20 did not find a difference in the risk of bleeding with diltiazem use in a large cohort of 91 330 patients with AF who used DOACs in Taiwan. However, Chang et al’s study results were likely biased because of the incomplete control of confounders such as kidney function and concurrent medication use, and the inclusion of medications that may not interact with DOACs (eg, digoxin and atorvastatin). 54 , 55 , 56 In our study, we adjusted for eGFR and concurrent medications such as NSAIDs, antiplatelets, and other CYP3A4/P‐gp agents in a time‐varying manner. On the other hand, Pham et al 57 found increased bleeding risk in patients using dabigatran with diltiazem compared with those with amlodipine (HR, 1.52; 95% CI, 1.05–2.20) or with metoprolol (HR, 1.43; 95% CI, 1.02–2.00). 21 However, this study excluded patients with CKD, and the study population of patients without CKD was younger (≈60% aged <65 years) and healthier than the general AF population (mean age, 70 to 74 years), which limited the ability to generalize the results to real‐world DOAC users. In the present study, we had limited power in dabigatran users (≈12% of the study population with 37 bleeding events). Of note, while we used a validated algorithm to ascertain bleeding outcome, heterogeneity in definitions of bleeding outcome among the studies (eg, inclusion of minor bleeding) may have partly contributed to inconsistencies in the studies.
Our findings align with the current FDA labeling for rivaroxaban that recommends avoidance of concomitant use with diltiazem. 10 This study also adds to the existing body of evidence around the impacts of interaction of diltiazem with apixaban. 11 Inconsistent findings on dabigatran in our study and previous studies may be largely attributable to the temporal trends in utilization. Dabigatran was the first DOAC introduced as an alternative to warfarin and available on the market in 2010, followed by rivaroxaban and apixaban in 2011 and 2012, respectively. There has been a substantial decrease in dabigatran use, particularly among patients with low eGFR in recent years, 58 and we observed more patients with AF initiated with apixaban (52%) or rivaroxaban (35%) than dabigatran (12%) in the present study. The decrease in dabigatran use may suggest that patients are switching to or initiating new DOACs, particularly apixaban, for better safety. In other words, patients with a low eGFR or high bleeding risk could have preferred apixaban or rivaroxaban over dabigatran, which may explain the few dabigatran users and few bleeding events in our study. On the other hand, this also suggests an underestimation of bleeding risk among apixaban users in previous studies that excluded patients with CKD. Therefore, our findings with apixaban may fill in the knowledge gap of the clinical impacts of interaction with diltiazem as little guidance is provided in current FDA labeling for apixaban.
Although reduced kidney function may increase plasma DOAC concentrations with varying degrees 17 , 59 and is an independent risk factor for bleeding, 10 , 11 , 12 , 23 , 55 we found a similar increase in bleeding risk associated with concomitant use of diltiazem with DOACs by CKD status. These findings were contrary to our hypothesis that patients with CKD may be more vulnerable to bleeding events associated with concomitant use of diltiazem with DOACs. One potential explanation may be that patients with CKD are more frequently underdosed than recommended compared with those without CKD. Consistent with previous observations, 58 18% and 9% of our patients with CKD and without CKD received a reduced dose of DOAC, respectively, which may explain the lack of interaction.
This population‐based study was designed for examining the risk of bleeding with concomitant use of diltiazem in DOAC users. The strengths include a heterogeneous study population with both CKD and non‐CKD, and the adjustment for laboratory measurement of kidney function. Our study also has some limitations. Despite multivariable adjustment including time‐varying eGFR and medication use to minimize confounding, there might be incomplete control for changes in factors such as liver function during follow‐up or unmeasured factors that might contribute to bleeding risk (eg, socioeconomic status). However, we used a series of negative controls in our analyses to address this concern. The majority of our study population was of White race and from a single health care system, which may limit generalizability of our findings. There were few bleeding events in the subgroups by individual anticoagulant (eg, dabigatran) and by CKD status such as eGFR <60 mL/min per 1.73 m2. Furthermore, we could not examine whether risk differs by severity of CKD because of the small sample size of patients with advanced CKD. Medication use was determined by prescription, which may not reflect true use. The dosages of DOACs and diltiazem were not considered in the regression analysis, which could alter DOAC exposure and affect bleeding risk. Last, the follow‐up duration was relatively short. Further large‐scale studies with longer follow‐up are warranted to confirm our findings.
In our study of real‐world data, concomitant use of diltiazem with rivaroxaban or apixaban was associated with a higher risk of bleeding in patients with AF, with similar risk between those with and those without CKD. These findings support current FDA labeling for rivaroxaban, which recommends avoidance of concurrent use of diltiazem with rivaroxaban. This study also provides important safety information for apixaban users, given that the FDA's guidance for concurrent use of diltiazem with apixaban is lacking. Alternative drugs to diltiazem may be a safer choice to reduce the risk of bleeding for patients taking rivaroxaban or apixaban.
Sources of Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [grant numbers R01 DK115534 and K24 HL155861 (PI: Dr. Grams), and K01 DK121825 (PI: Dr. Shin)]. The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, and preparation or final approval of the manuscript before publication.
Disclosures
J. Shin reports receiving research funding from Merck and the National Institutes of Health. L. Inker reports having consultancy agreements with Diamtrix; reports receiving research funding to the institution for research and contracts with the National Institutes of Health, National Kidney Foundation, Omeros, Reata Pharmaceuticals; reports having consulting agreements to her institution with Omeros and Tricida Inc.; reports having an advisory or leadership role with the Alport Syndrome Foundation; and reports having other interests or relationships as a member of the American Society of Nephrology, the National Kidney Disease Education Program, and the National Kidney Foundation. The remaining authors have nothing to disclose.
Supporting information
Tables S1–S9
Figures S1–S5
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025723
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Kulkarni N, Gukathasan N, Sartori S, Baber U. Chronic kidney disease and atrial fibrillation: a contemporary overview. J Atr Fibrillation. 2012;5:448. doi: 10.4022/jafib.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magnocavallo M, Bellasi A, Mariani MV, Fusaro M, Ravera M, Paoletti E, Di Iorio B, Barbera V, Della Rocca DG, Palumbo R, et al. Thromboembolic and bleeding risk in atrial fibrillation patients with chronic kidney disease: role of anticoagulation therapy. J Clin Med. 2021;10:83. doi: 10.3390/jcm10010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575 [DOI] [PubMed] [Google Scholar]
- 4. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida J‐M, Capodanno D, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 6. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 7. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, Rowell N, et al. European heart rhythm association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23:1612–1676. doi: 10.1093/europace/euab065 [DOI] [PubMed] [Google Scholar]
- 8. Foerster KI, Hermann S, Mikus G, Haefeli WE. Drug–drug interactions with direct oral anticoagulants. Clin Pharmacokinet. 2020;59:967–980. doi: 10.1007/s40262-020-00879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiggins BS, Dixon DL, Neyens RR, Page RL, Gluckman TJ. Select drug‐drug interactions with direct oral anticoagulants. J Am Coll Cardiol. 2020;75:1341–1350. doi: 10.1016/j.jacc.2019.12.068 [DOI] [PubMed] [Google Scholar]
- 10. Xarelto (rivaroxaban) [package insert] Janssen ; 2011. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022406s036,202439s036lbl.pdf. Accessed January 5, 2021.
- 11. Eliquis (apixaban) [package insert] Bristol‐Myers Squibb ; 2012. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf. Accessed January 5, 2021.
- 12. Pradaxa (dabigatran) [package insert] Boehringer Ingelheim ; 2012. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022512s028lbl.pdf. Accessed January 5, 2021.
- 13. National Action Plan for Adverse Drug Event Prevention. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2014. Available at: https://health.gov/sites/default/files/2019‐09/ADE‐Action‐Plan‐508c.pdf. Accessed February 19, 2021. [Google Scholar]
- 14. Shu‐Feng Z. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310–322. doi: 10.2174/138920008784220664 [DOI] [PubMed] [Google Scholar]
- 15. Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA, Zhang D, Yu Z, Dias C, Shenker A, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79:838–846. doi: 10.1111/bcp.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim M, Son H, Noh K, Kim E, Shin BS, Kang W. Effects of verapamil and diltiazem on the pharmacokinetics and pharmacodynamics of rivaroxaban. Pharmaceutics. 2019;11:133. doi: 10.3390/pharmaceutics11030133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, Goldsmith D. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol. 2019;74:2204–2215. doi: 10.1016/j.jacc.2019.08.1031 [DOI] [PubMed] [Google Scholar]
- 18. Washam JB, Hellkamp AS, Lokhnygina Y, Piccini JP, Berkowitz SD, Nessel CC, Becker RC, Breithardt G, Fox KAA, Halperin JL, et al. Efficacy and safety of rivaroxaban versus warfarin in patients taking nondihydropyridine calcium channel blockers for atrial fibrillation (from the ROCKET AF trial). Am J Cardiol. 2017;120:588–594. doi: 10.1016/j.amjcard.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 19. Aktas H, Inci S, Dogan P, Izgu I. Spontaneous rectus sheath hematoma in a patient treated with apixaban. Intractable Rare Dis Res. 2016;5:47–49. doi: 10.5582/irdr.2015.01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, See LC, Kuo CF. Association between use of non‐vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318:1250–1259. doi: 10.1001/jama.2017.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pham P, Schmidt S, Lesko L, Lip GYH, Brown JD. Association of oral anticoagulants and verapamil or diltiazem with adverse bleeding events in patients with nonvalvular atrial fibrillation and normal kidney function. JAMA Netw Open. 2020;3:e203593. doi: 10.1001/jamanetworkopen.2020.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bartlett JW, Renner E, Mouland E, Barnes GD, Kuo L, Ha NB. Clinical safety outcomes in patients with nonvalvular atrial fibrillation on rivaroxaban and diltiazem. Ann Pharmacother. 2019;53:21–27. doi: 10.1177/1060028018795140 [DOI] [PubMed] [Google Scholar]
- 23. Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open‐label, parallel‐group, single‐centre study. Clin Pharmacokinet. 2010;49:259–268. doi: 10.2165/11318170-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 24. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez‐Sendon J, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. doi: 10.1093/eurheartj/ehs274 [DOI] [PubMed] [Google Scholar]
- 25. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation. Circulation. 2014;129:961–970. doi: 10.1161/CIRCULATIONAHA.113.003628 [DOI] [PubMed] [Google Scholar]
- 26. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E. Impact of renal function on outcomes with edoxaban in the ENGAGE AF‐TIMI 48 trial. Circulation. 2016;134:24–36. doi: 10.1161/CIRCULATIONAHA.116.022361 [DOI] [PubMed] [Google Scholar]
- 27. Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, Berkowitz SD, Breithardt G, Fox KAA, Mahaffey KW, et al. On‐treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin. Circulation. 2016;134:37–47. doi: 10.1161/CIRCULATIONAHA.116.021890 [DOI] [PubMed] [Google Scholar]
- 28. Sharifi H, Hasanloei MAV, Mahmoudi J. Polypharmacy‐induced drug‐drug interactions; threats to patient safety. Drug Res (Stuttg). 2014;64:633–637. doi: 10.1055/s-0033-1363965 [DOI] [PubMed] [Google Scholar]
- 29. Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug—drug and drug—food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7:55–61. doi: 10.15420/aer.2017.50.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansen KL, Chertow GM, Foley RN, Gilbertson DT, Herzog CA, Ishani A, Israni AK, Ku E, Tamura MK, Li S, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2021;77:A7–A8. doi: 10.1053/j.ajkd.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80 [DOI] [PubMed] [Google Scholar]
- 32. Vallianou NG, Mitesh S, Gkogkou A, Geladari E. Chronic kidney disease and cardiovascular disease: is there any relationship? Curr Cardiol Rev. 2019;15:55–63. doi: 10.2174/1573403X14666180711124825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roux‐Marson C, Baranski JB, Fafin C, Exterman G, Vigneau C, Couchoud C, Moranne O; Investigators PSPA . Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr. 2020;20:87. doi: 10.1186/s12877-020-1485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez S, Lenoir C, Samer C, Rollason V. Drug interactions with apixaban: a systematic review of the literature and an analysis of VigiBase, the World Health Organization database of spontaneous safety reports. Pharmacol Res Perspect. 2020;8:e00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandez S, Lenoir C, Samer CF, Rollason V. Drug‐drug interactions leading to adverse drug reactions with rivaroxaban: a systematic review of the literature and analysis of VigiBase. J Pers Med. 2021;11:250. doi: 10.3390/jpm11040250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 37. Jun M, James MT, Manns BJ, Quinn RR, Ravani P, Tonelli M, Perkovic V, Winkelmayer WC, Ma Z, Hemmelgarn BR. Alberta kidney disease network. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. BMJ. 2015;350:h246. doi: 10.1136/bmj.h246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 39. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 40. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 41. Webster‐Clark M, Huang T‐Y, Hou L, Toh S. Translating claims‐based CHA2DS2‐VaSc and HAS‐BLED to ICD‐10‐CM: impacts of mapping strategies. Pharmacoepidemiol Drug Saf. 2020;29:409–418. doi: 10.1002/pds.4973 [DOI] [PubMed] [Google Scholar]
- 42. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New creatinine‐ and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The MI Procedure. In: SAS/STAT® 14.1 User's Guide. SAS Institute Inc.; 2015. Available at: https://support.sas.com/documentation/onlinedoc/stat/141/mi.pdf. [Google Scholar]
- 44. Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–213. doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- 45. Pedersen AB, Mikkelsen EM, Cronin‐Fenton D, Kristensen NR, Pham TM, Pedersen L, Petersen I. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–166. doi: 10.2147/CLEP.S129785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. doi: 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 47. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 48. Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 49. Stoysich AM, Lucas BD, Mohiuddin SM, Hilleman DE. Further elucidation of pharmacokinetic interaction between diltiazem and warfarin. Int J Clin Pharmacol Ther. 1996;34:56–60. [PubMed] [Google Scholar]
- 50. Li A, Li MK, Crowther M, Vazquez SR. Drug‐drug interactions with direct oral anticoagulants associated with adverse events in the real world: a systematic review. Thromb Res. 2020;194:240–245. doi: 10.1016/j.thromres.2020.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Konieczny K, Dorian P. Clinically important drug–drug interactions between antiarrhythmic drugs and anticoagulants. J Innov Card Rhythm Manag. 2019;0:3552–3559. doi: 10.19102/icrm.2019.100304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa®) after co‐medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hanigan S, Das J, Pogue K, Barnes GD, Dorsch MP. The real world use of combined P‐glycoprotein and moderate CYP3A4 inhibitors with rivaroxaban or apixaban increases bleeding. J Thromb Thrombolysis. 2020;49:636–643. doi: 10.1007/s11239-020-02037-3 [DOI] [PubMed] [Google Scholar]
- 54. Li Y, Dong S, Soria‐Saucedo R. Drug interactions with non–vitamin K oral anticoagulants. JAMA. 2018;319:827. doi: 10.1001/jama.2017.20830 [DOI] [PubMed] [Google Scholar]
- 55. Vazquez SR, Allen A. Drug interactions with non–vitamin K oral anticoagulants. JAMA. 2018;319:829. doi: 10.1001/jama.2017.20842 [DOI] [PubMed] [Google Scholar]
- 56. Sennesael A‐L, Henrard S, Spinewine A. Drug interactions with non–vitamin K oral anticoagulants. JAMA. 2018;319:829. doi: 10.1001/jama.2017.20846 [DOI] [PubMed] [Google Scholar]
- 57. Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, Ezekowitz MD, Fonarow GC, Gersh BJ, Goldhaber S, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD‐AF, ORBIT‐AF I, and ORBIT‐AF II registries. Am Heart J. 2017;194:132–140. doi: 10.1016/j.ahj.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 58. Shin J‐I, Secora A, Alexander GC, Inker LA, Coresh J, Chang AR, Grams ME. Risks and benefits of direct oral anticoagulants across the spectrum of GFR among incident and prevalent patients with atrial fibrillation. Clin J Am Soc Nephrol. 2018;13:1144–1152. doi: 10.2215/CJN.13811217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, et al. The 2018 European heart rhythm association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figures S1–S5


