Abstract
Background
Plasma biomarkers may aid in the detection of anthracycline‐related cardiomyopathy (ACMP). However, the currently available biomarkers have limited diagnostic value in long‐term childhood cancer survivors. This study sought to identify diagnostic plasma biomarkers for ACMP in childhood cancer survivors.
Methods and Results
We measured 275 plasma proteins in 28 ACMP cases with left ventricular ejection fraction <45%, 29 anthracycline‐treated controls with left ventricular ejection fraction ≥53% matched on sex, time after cancer, and anthracycline dose, and 29 patients with genetically determined dilated cardiomyopathy with left ventricular ejection fraction <45%. Multivariable linear regression was used to identify differentially expressed proteins. Elastic net model, including clinical characteristics, was used to assess discrimination of proteins diagnostic for ACMP. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and the inflammatory markers CCL19 (C‐C motif chemokine ligands 19) and CCL20, PSPD (pulmonary surfactant protein‐D), and PTN (pleiotrophin) were significantly upregulated in ACMP compared with controls. An elastic net model selected 45 proteins, including NT‐proBNP, CCL19, CCL20 and PSPD, but not PTN, that discriminated ACMP cases from controls with an area under the receiver operating characteristic curve (AUC) of 0.78. This model was not superior to a model including NT‐proBNP and clinical characteristics (AUC=0.75; P=0.766). However, when excluding 8 ACMP cases with heart failure, the full model was superior to that including only NT‐proBNP and clinical characteristics (AUC=0.75 versus AUC=0.50; P=0.022). The same 45 proteins also showed good discrimination between dilated cardiomyopathy and controls (AUC=0.89), underscoring their association with cardiomyopathy.
Conclusions
We identified 3 specific inflammatory proteins as candidate plasma biomarkers for ACMP in long‐term childhood cancer survivors and demonstrated protein overlap with dilated cardiomyopathy.
Keywords: anthracycline‐related cardiomyopathy, biomarkers, cancer therapy–related cardiac dysfunction, cardio‐oncology, chemokine ligands, childhood cancer survivors
Subject Categories: Cardio-Oncology, Cardiotoxicity, Cardiomyopathy
Nonstandard Abbreviations and Acronyms
- ACMP
anthracycline‐related cardiomyopathy
- CCL19
C‐C‐motif chemokine ligand 19
- CCL20
C‐C‐motif chemokine ligand 20
- CCS
childhood cancer survivor
- DCM
dilated cardiomyopathy
- PSPD
pulmonary surfactant protein D
Clinical Perspective.
What Is New?
Candidate inflammatory biomarkers for diagnosing anthracycline cardiomyopathy in childhood cancer survivors were identified.
These biomarkers were also dysregulated in patients with genetically determined dilated cardiomyopathy.
What Are the Clinical Implications?
As findings were exploratory, the diagnostic value of the identified plasma biomarkers should be confirmed in a larger cohort.
Childhood cancer survivors (CCSs) treated with anthracyclines, mitoxantrone, and/or chest‐directed radiotherapy are at high risk for heart failure, with 11.6% developing heart failure within 40 years from cancer diagnosis. 1 Because of the high risk of heart failure and the potential benefits of early detection and treatment of cardiac dysfunction, life‐long echocardiographic surveillance is currently recommended. 2
Blood biomarkers with a high sensitivity and sufficient specificity could be useful as a time‐efficient and cost‐effective triage test, where survivors with a normal biomarker level can safely be deferred from further workup with an echocardiogram. 3 Blood biomarkers could also be used in addition to an echocardiogram to improve its diagnostic accuracy or for prognostic reasons. Up until now, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and troponins have been studied but lack sufficient sensitivity to detect asymptomatic left ventricular (LV) dysfunction in long‐term CCSs and are therefore not recommended for surveillance purposes. 2 , 4 Few studies have used plasma proteomics to identify additional biomarkers that might improve detection of anthracycline‐related cardiomyopathy (ACMP), some of which were in patients with pediatric cancer in the short‐term phase 5 and others that assessed the value of natriuretic peptides, cardiac troponin T, soluble suppression of tumorigenicity‐2, and galectin‐3 carnitine, in long‐term CCSs. 6 , 7 However, most of the studies using larger‐scale proteomic analyses have been conducted in adult patients with cancer during or shortly after anthracycline treatment. 8 , 9 , 10
In this discovery case‐control study in the DCCSS LATER 2 CARD (Dutch Childhood Cancer Survivor Study, LATER cohort, part 2, cardiology), we sought to identify candidate plasma proteins that would be able to discriminate ACMP cases from anthracycline‐treated controls with normal LV function, using a large biomarker panel consisting of markers for ventricular wall stress, oxidative stress, inflammation, cellular adhesion, apoptosis, and extracellular matrix remodeling. To further support the hypothesis that the selected markers are associated with cardiomyopathy and not with a systemic effect of anthracyclines in those sensitive to them, we compared plasma levels of the proteins that we identified in ACMP with plasma levels in patients with genetically determined dilated cardiomyopathy (DCM).
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design and Study Participants
We conducted a cross‐sectional case–control study as part of the DCCSS LATER 2 CARD. The design of this cohort study has been published. 11 In short, DCCSS LATER 2 CARD is a multicenter study in 5‐year CCSs diagnosed with a malignancy before the age of 18 years and between January 1, 1963, and December 31, 2001, who were treated with (potentially) cardiotoxic cancer treatments. Participants visited the outpatient clinic between February 2016 and February 2020 for questionnaires, physical examination, blood sampling, electrocardiography, and echocardiography. For primary analysis of this biomarker case‐control study, we included CCSs treated with anthracyclines or mitoxantrone, with or without concomitant chest‐directed radiotherapy. CCSs with congenital heart disease were excluded. The first 30 ACMP cases (defined as a LV ejection fraction [LVEF] <45%) included in the DCCSS LATER 2 CARD were selected and matched with 30 anthracycline‐treated controls without ACMP (defined as LVEF ≥53% without grade ≥2 diastolic dysfunction or valvular disease) (Figure 1). We chose to include these first 30 ACMP cases (1) because the inclusion for the DCCSS LATER 2 CARD was still ongoing at time of this case‐control study and (2) to ensure a random selection of ACMP cases. Controls were propensity score matched to ACMP cases, where the propensity score was estimated with logistic regression of case status on the covariates sex, time since cancer diagnosis, and cumulative anthracycline/mitoxantrone dose (calculated as doxorubicin equivalents). 12 For secondary analysis, we included patients with DCM with LVEF <45% and a pathogenic titin truncating variant or a lamin A/C mutation from the Amsterdam University Medical Center and the UNRAVEL database at the University Medical Center Utrecht in the Netherlands. 13 These patients with DCM were included to test whether the plasma proteins selected to be discriminative for ACMP would also be discriminative for DCM.
Figure 1. Study design of the LATER CARD biomarker case‐control study.
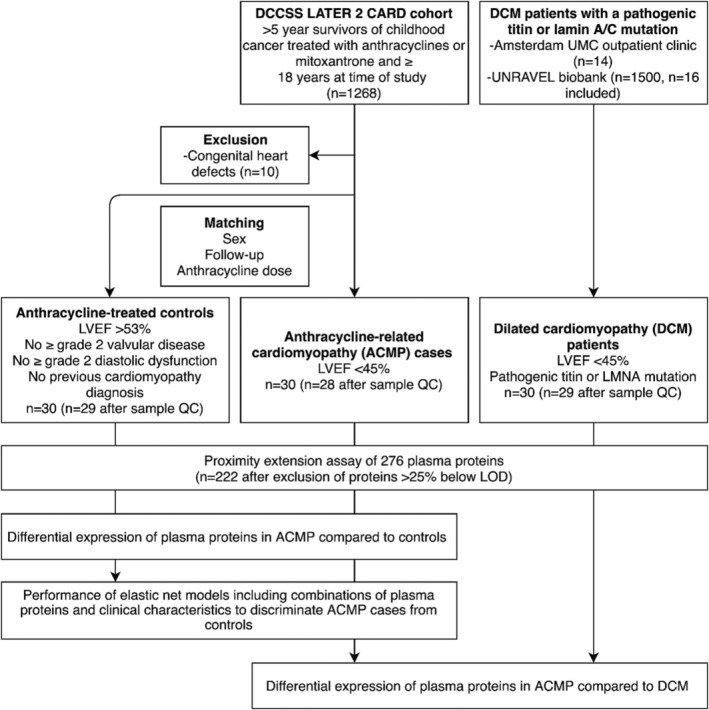
ACMP indicates anthracycline‐related cardiomyopathy; DCCSS LATER 2 CARD, Dutch Childhood Cancer Survivor Study, LATER cohort, part 2, cardiology; DCM, dilated cardiomyopathy; LMNA, lamin A/C; LOD, limit of detection; LVEF, left ventricular ejection fraction; QC, quality control; and UMC, University Medical Center.
Ethical Approval
The investigation conforms with the principles outlined in the Declaration of Helsinki. The LATER CARD study was approved by the medical ethics board of all participating centers and included blood biobanking for future analysis. The medical ethics board of the Amsterdam University Medical Center and the University Medical Center Utrecht approved the biobanking of blood samples from patients with DCM. UNRAVEL follows the code of conduct and the use of data in health research and has been approved by the biobank board of the medical ethics committee of the University Medical Center Utrecht. 13 Informed consent was obtained from all participants.
Data Collection
Patient and cancer treatment characteristics were obtained from the central database of the LATER study (ACMP cases and controls) and from medical records (patients with DCM). Cumulative anthracycline dose was calculated as the doxorubicin equivalent dose. 12 Cardiac medication use, heart failure symptoms, and modifiable cardiovascular risk factors were obtained from questionnaires (CCSs) and medical records (patients with DCM). In ACMP cases and controls, self‐reported heart failure and cardiovascular risk factors were considered present if patients reported the use of medications for the condition. All participants underwent a physical examination at time of blood sampling to obtain body mass index and blood pressure. Fasting citrate blood samples were obtained from participants within 6 months from the qualifying echocardiogram (86% of samples were obtained at the same day). Samples were centrifuged at 3000g for 10 minutes, stored within 1 hour at −80 °C, and shipped on dry ice to the central biobank. In ACMP cases and controls, echocardiographic parameters, including biplane LVEF, were measured by a core laboratory blinded for clinical characteristics. 14 In patients with DCM, echocardiographic parameters were obtained from medical records.
Plasma Protein Measurements
Plasma levels of 276 proteins were measured with a proximity extension assay in 3 μL of citrate plasma per patient using the Cardiovascular III, Organ Damage, and Inflammation panels from Olink Proteomics (Uppsala, Sweden). We chose these 3 panels because of their known association with cardiovascular disease, apoptosis, inflammation, and remodeling. Panel validation data can be found at Olink.com. The proximity extension assay is based on pairs of antibodies that are linked to proximity probes. On binding of the antibody pair to their target protein, the probes are brought in proximity and are extended by a DNA polymerase that can subsequently be detected with real‐time polymerase chain reaction. Protein levels are expressed as normalized protein expression values, which are relative units expressed on the log2 scale, where a 1‐unit higher normalized protein expression value represents a doubling of protein concentration. Study groups were randomly distributed over the plate. Samples that did not pass Olink quality control (>0.3 normalized protein expression median deviation from the internal control) were excluded. Protein levels below the linear limit of detection were replaced with the estimated normalized protein expression value at the nonlinear part of the calibration curve if <25% was below limit of detection. Proteins with ≥25% below limit of detection were excluded (n=54 proteins). These 54 proteins were not exclusively expressed in one of the study groups. Two polymerase chain reaction readout failures were median imputed (macrophage‐capping protein in 1 ACMP case and transmembrane serine protease 15 in 1 DCM case).
Statistical Analysis
Descriptive Statistics
Continuous variables were checked for normality by visual inspection using histograms and are presented as mean±SD for normally distributed variables and as median with range for skewed variables. Categorical variables are presented as numbers and percentages. Continuous variables were compared with the t test or Wilcoxon signed‐rank test, where appropriate. Categorical variables were compared with the χ2 test or the Fisher exact test (when expected counts were <5). All analyses were conducted in R version 3.6.1.
Primary Analysis: ACMP Cases Compared With Anthracycline‐Treated Controls
Differential expression of plasma proteins in ACMP cases compared with controls was tested with multivariable linear regression models, estimating log2 fold changes. Models were adjusted for sex, time since cancer diagnosis, anthracycline/mitoxantrone dose, and chest‐directed radiotherapy dose. P values were corrected for multiple testing with the q value, which can be interpreted as a false discovery rate. 15 A q value <0.1 was considered statistically significant. In sensitivity analyses, models were adjusted for NT‐proBNP levels and were restricted to ACMP cases without self‐reported heart failure.
Elastic net logistic regression was used to identify a combination of plasma proteins best discriminating ACMP cases from controls. The elastic net simultaneously performs variable selection and shrinkage of coefficients of a large number of predictors and is relatively robust to collinear predictors. 16 Predictors entered in the elastic net were all plasma proteins and the clinical characteristics sex, age at cancer diagnosis, time since cancer diagnosis, anthracycline/mitoxantrone dose, and chest‐directed radiotherapy dose. NT‐proBNP was not subjected to selection and coefficient shrinkage, as we aimed to find proteins independent of NT‐proBNP. Predictors were standardized to have a mean of 0 and an SD of 1. We used a nested cross‐validation strategy to test performance of the elastic net on data not seen during training of the model. Matched case‐control pairs were divided into a training set and test set with 10×10‐fold cross‐validation. The elastic net parameters (α and λ) were optimized on the training set with 5‐fold cross‐validation, and the parameter combination that was within 1 SE from the optimal area under the receiver operating characteristic curve (AUC) was chosen. Median model performance over the cross‐validation folds was evaluated on the test set with the AUC, and with sensitivity and specificity at the threshold maximizing the sum of sensitivity and specificity. Proteins selected in ≥40% of the cross‐validation folds were considered important. Performance of the elastic net model, including all proteins and clinical characteristics, was compared with an elastic net model including only NT‐proBNP and clinical characteristics. AUCs were compared, and 95% CIs were calculated with the Wilcoxon signed‐rank test. In additional analysis in asymptomatic CCSs, elastic net models were also fitted in ACMP cases without heart failure and their matched controls only.
Secondary Analysis: ACMP Cases Compared With Patients With DCM
Differential expression of plasma proteins in ACMP cases compared with patients with DCM was tested with multivariable linear regression models, adjusted for sex, age at blood sample, and LVEF. A q value <0.1 was considered statistically significant. The group of proteins discriminating ACMP cases from controls was tested for their ability to also discriminate patients with DCM from controls, with the elastic net using the same modeling steps as described for the primary analysis.
Results
Patient Characteristics
After exclusion of 4 samples that did not pass quality control, we included 28 ACMP cases, 29 matched anthracycline‐treated controls, and 29 patients with DCM in this study (Figure 1). Characteristics of the participants are outlined in Table 1. ACMP cases and controls were successfully matched with respect to sex (46.4% and 48.3% men, respectively; P=1.0), time since cancer diagnosis (median, 25.4 and 29.4 years, respectively; P=0.107), and cumulative anthracycline dose (median, 360.0 and 300.0 mg/m2, respectively; P=0.626). Compared with ACMP cases, patients with DCM were older (median, 37.6 and 56.0 years, respectively; P<0.001) and were more frequently men (46.4% and 82.8%; P=0.006). Mean LVEF in ACMP cases was 40.6±5.8%, versus 58.1±3.2% in controls, and was lowest in patients with DMC (37.0±7.5%). Cardiac medications were used by all of the patients with DCM, by 16 (57.1%) of the ACMP cases, and by 3 (10.3%) of the controls. Heart failure was reported by 8 (28.6%) of the ACMP cases and 22 (75.9%) of the patients with DCM. Hypertension, diabetes, and dyslipidemia were reported by a minority of CCSs and patients with DCM. Characteristics of ACMP cases without heart failure compared with matched controls are presented in Tables S1 through S3.
Table 1.
Characteristics of the Patients With ACMP, Anthracycline‐Treated Controls, and Patients With DCM
| Characteristic | Controls (n=29) | Patients with ACMP (n=28) | Patients with DCM (n=29) | P value for ACMP‐controls | P value for DCM‐ACMP |
|---|---|---|---|---|---|
| Male sex | 14 (48.3) | 13 (46.4) | 24 (82.8) | 1 | 0.006 |
| Age at cancer diagnosis, y | 7.97 (4.03–11.82) | 8.30 (3.52– 13.11) | NA | 0.936 | NA |
| Age at blood sampling, y | 43.30 (34.71–46.97) | 37.63 (30.26–45.30) | 56.00 (39.00–64.00) | 0.271 | <0.001 |
| Time since cancer diagnosis, y | 29.44 (24.13–32.33) | 25.35 (18.85–30.21) | NA | 0.107 | NA |
| Primary cancer diagnosis | NA | 0.671 | |||
| Leukemias | 8 (27.6) | 5 (17.9) | NA | ||
| Lymphomas | 11 (37.9) | 10 (35.7) | NA | ||
| Neuroblastoma | 0 (0.0) | 1 (3.6) | NA | ||
| Renal tumors | 3 (10.3) | 2 (7.1) | NA | ||
| Bone tumors | 3 (10.3) | 7 (25.0) | NA | ||
| Soft tissue sarcomas | 3 (10.3) | 3 (10.7) | NA | ||
| Germ cell tumors | 1 (3.4) | 0 (0.0) | NA | ||
| Anthracyclines | 27 (93.1) | 23 (82.1) | NA | 0.253 | NA |
| Anthracycline cumulative dose, mg/m2 * | 300.00 (216.00–400.00) | 360.00 (169.00–462.50) | NA | 0.626 | NA |
| Mitoxantrone | 7 (24.1) | 7 (25.0) | NA | 1 | NA |
| Mitoxantrone dose, mg/m2 | 50.00 (40.00–102.00) | 120.00 (50.00–121.00) | NA | 0.299 | NA |
| Chest RT | 2 (20.0) | 3 (20.0) | NA | 0.670 | NA |
| Chest RT cumulative dose, Gy | 20.00 (20.00–20.00) | 25.00 (19.50–37.50) | NA | 0.554 | NA |
| DCM‐causing mutation | NA | NA | |||
| Titin | NA | NA | 23 (79.3) | NA | NA |
| Lamin A/C | NA | NA | 6 (21.7) | NA | NA |
| Heart failure | 0 (0.0) | 8 (28.6) | 22 (75.9) | 0.006 | 0.001 |
| Cardiac medication(s) | 3 (10.3) | 16 (57.1) | 29 (100) | 0.001 | <0.001 |
| Hypercholesterolemia | 1 (3.4) | 2 (7.1) | 9 (31.0) | 0.611 | 0.051 |
| Diabetes | 0 (0.0) | 0 (0.0) | 2 (6.9) | NA | 0.491 |
| Hypertension | 1 (3.4) | 2 (7.1) | 6 (20.7) | 0.611 | 0.253 |
| Systolic blood pressure, mm Hg | 125.5 (16.1) | 117.1 (19.8) | 114.9 (18.4) | 0.086 | 0.669 |
| Diastolic blood pressure, mm Hg | 78.8 (10.1) | 72.9 (15.5) | 72.7 (13.3) | 0.093 | 0.962 |
| Heart rate, bpm | 69.2 (14.4) | 71.2 (12.6) | 69.3 (8.3) | 0.582 | 0.509 |
| BMI, kg/m2 | 25.1 (4.6) | 25.0 (4.9) | 26.3 (4.2) | 0.926 | 0.301 |
| Biplane LVEF, % | 58.1 (3.2) | 40.6 (5.8) | 37.0 (7.5) | <0.001 | 0.045 |
| LVIDd, cm | 4.6 (0.6) | 5.2 (0.7) | 6.2 (0.8) | 0.003 | <0.001 |
Categorical values are presented as number (percentage). Continuous values are presented as median (interquartile range). ACMP indicates anthracycline‐related cardiomyopathy; BMI, body mass index; bpm, beats per minute; Chest RT, chest‐directed radiotherapy; DCM, dilated cardiomyopathy; LVEF, left ventricular ejection fraction; LVIDd, left ventricular end‐diastolic diameter; and NA, not applicable.
Doxorubicin equivalents (daunorubicin*0.6+epirubicin*0.8+idarubicin*3).
Primary Analysis: ACMP Cases Compared With Anthracycline‐Treated Controls
Differential Expression of Plasma Proteins in ACMP Cases Compared With Controls
In multivariable linear regression analyses, adjusted for sex, time since cancer diagnosis, anthracycline dose, and chest‐directed radiotherapy dose, plasma levels of NT‐proBNP, C‐C motif chemokine 19 (CCL19), pleiotrophin, C‐C motif chemokine 20 (CCL20), and PSPD (pulmonary surfactant protein D) were significantly higher in ACMP cases compared with controls (q value <0.1; Figure 2 and Table S2). When we additionally adjusted for NT‐proBNP levels, CCL19, CCL20, PSPD, and pleiotrophin remained significantly upregulated (Table S3). When we performed the analysis in 20 ACMP cases without heart failure (reflecting a surveillance population) and their matched controls, NT‐proBNP was not significantly upregulated (P=0.231), whereas the other 4 proteins remained significantly upregulated (Table S3). Biomarkers that have previously been shown to have diagnostic or prognostic value in patients with heart failure, including soluble suppression of tumorigenicity‐2, galectin‐3, troponin I, tumor necrosis factor, interleukin‐6, osteopontin, and tumor necrosis factor receptor superfamily member 6, were not differentially expressed in ACMP cases compared with controls (all q values >0.1; Table S2).
Figure 2.
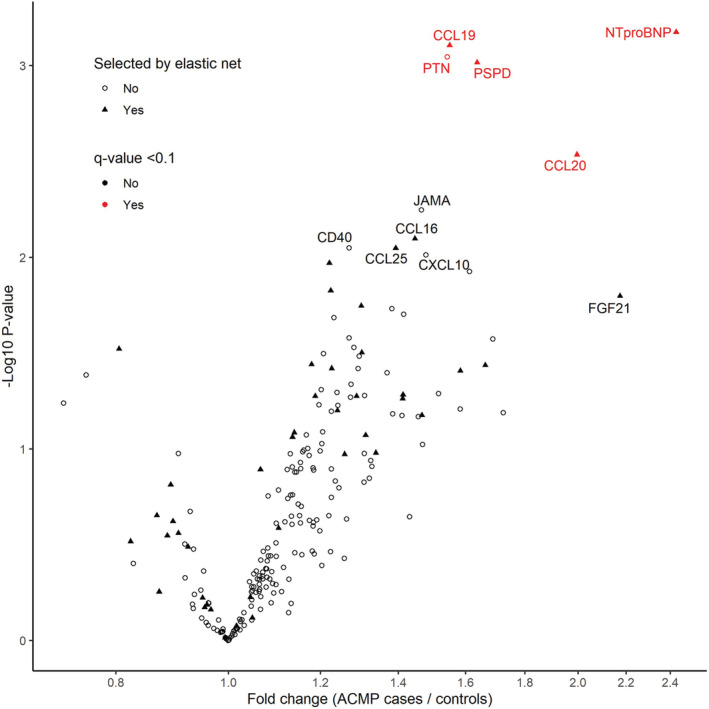
Volcano plot, showing fold changes (x axis) and P values (y axis) of 222 plasma proteins in anthracycline‐related cardiomyopathy (ACMP) compared with matched anthracycline‐treated controls.
Fold changes and P values were estimated with multivariable linear regression analysis, adjusted for sex, time since cancer diagnosis, anthracycline dose, and chest‐directed radiotherapy dose. Significantly upregulated proteins (q value <0.1) are shown in red. Proteins selected by the elastic net in >40% of the cross‐validation folds are shown as a triangle. CCL indicates C‐C motif chemokine ligand; CD40, cluster of differentiation 40; CXCL10, C‐X‐C motif chemokine ligand 10; FGF21, fibroblast growth factor 21; JAMA, junctional adhesion molecule A; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PSPD, pulmonary surfactant protein D; and PTN, pleiotrophin.
Discriminative Plasma Proteins Identified With Elastic Net
The elastic net model trained on all proteins and clinical characteristics selected 45 proteins in >40% of the elastic net cross‐validation folds, which indicates they are potentially important in discriminating ACMP cases from controls (Figure 2 and Table S2). Next to NT‐proBNP, which was not subjected to selection, this panel mainly consisted of inflammatory markers, such as CCL19, CCL20, CCL25 (C‐C‐motif chemokine ligand 25), and PSPD, and adhesion molecules, such as chitinase 3 like 1, P selectin, Ephrin type‐B receptor 4, and intracellular adhesion molecule 2. All proteins that were significantly upregulated in the multivariable linear regression analysis were also selected by the elastic net, except for pleiotrophin, which was selected in 18% of the folds, suggesting pleiotrophin does not contribute much to the discrimination when combined with other proteins.
Performance of the Elastic Net in Discriminating ACMP Cases From Controls
The elastic net model trained on all proteins and clinical characteristics had a cross‐validated AUC of 0.78, a sensitivity of 87%, and a specificity of 78% (Table 2). Discrimination of this model was slightly but not significantly higher compared with an elastic net model trained on NT‐proBNP and clinical characteristics only (AUC=0.75; P=0.766). To better reflect a surveillance population of asymptomatic CCSs, we repeated the analysis in 20 ACMP cases without self‐reported heart failure and their matched controls (n=21). In this analysis, discrimination of the elastic net model trained on all proteins and clinical characteristics retained its discriminative value better compared with the elastic net trained on NT‐proBNP and clinical characteristics only (AUC=0.75 versus AUC=0.50; P=0.022) (Table 2). More important, CCL19, CCL20, and PSPD were also selected by the elastic net in >40% of the cross‐validation folds in this analysis in ACMP cases without heart failure.
Table 2.
Cross‐Validated Performance Measures of Elastic Net Models, Including Clinical Characteristics and Plasma Proteins, to Discriminate ACMP Cases From Anthracycline‐Treated Controls
| Performance measure | NT‐proBNP+clinical characteristics* | All proteins+clinical characteristics* | Wilcoxon test P value |
|---|---|---|---|
| Main analysis in all participants (n=57) | |||
| AUC (95% CI) | 0.75 (0.71–0.80) | 0.78 (0.72–0.83) | 0.766 |
| Sensitivity (95% CI) | 0.86 (0.82–0.90) | 0.87 (0.83–0.91) | … |
| Specificity (95% CI) | 0.78 (0.82–0.90) | 0.78 (0.72–0.84) | … |
| Analysis in asymptomatic cases without heart failure (n=41) | |||
| AUC (95% CI) | 0.50 (0.50–0.62) | 0.75 (0.63–0.75) | 0.022 |
| Sensitivity (95% CI) | 0.89 (0.84–0.93) | 0.90 (0.86–0.94) | … |
| Specificity (95% CI) | 0.61 (0.54–0.68) | 0.69 (0.62–0.76) | … |
Performance measures are reported for elastic net models fitted in all participants and in asymptomatic cases without heart failure. ACMP indicates anthracycline‐related cardiomyopathy; AUC, area under the receiver operating characteristic curve; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Clinical characteristics included sex, age at cancer diagnosis, time since cancer diagnosis, anthracycline/mitoxantrone dose (doxorubicin equivalents), and chest‐directed radiotherapy dose. Sensitivities and specificities are reported at the threshold maximizing the sum of sensitivity and specificity.
Secondary Analysis: Plasma Protein Expression in ACMP Cases Compared With Patients With DCM
In multivariable linear regression analyses adjusted for sex, age, and LVEF, none of the 5 upregulated proteins in ACMP cases compared with controls (NT‐proBNP, CCL19, CCL20, PSPD, and pleiotrophin) were differentially expressed in ACMP cases compared with patients with genetically determined DCM (Table S2). Similar results were obtained when not adjusting the multivariable linear regression analysis for differences in LVEF between ACMP and DCM (NT‐proBNP, CCL19, CCL20, PSPD, and pleiotrophin all had P>0.05). In the elastic net model, the 45 discriminative proteins for ACMP, including NT‐proBNP, were also highly discriminative for DCM compared with controls (elastic net AUC=0.89), and the AUC remained high when excluding NT‐proBNP from this protein panel (elastic net AUC=0.86).
Discussion
In this discovery case‐control study, we identified 3 inflammatory proteins, CCL19, CCL20, and PSPD, as candidate plasma biomarkers for detection of ACMP in long‐term CCSs, independently of clinical characteristics, such as anthracycline dose, independently of NT‐proBNP levels, and independently of the presence of heart failure. Supporting the role of these proteins in detecting ACMP is their similarly increased presence in patients with genetically determined DCM. As our sample population is small, we regard the results as a promising finding that awaits confirmation in a larger cohort.
Previous studies in survivors of breast cancer treated with anthracyclines have also reported an association of inflammatory biomarkers with decreased LV function. 10 , 17 In one of the studies, at a mean of 11±5.5 years after treatment with anthracyclines and/or radiotherapy, 11 plasma proteins related to cardiovascular disease were associated with decreasing LVEFs that were still in the normal range (median LVEF, 58%; interquartile range, 55%–60%). 10 We confirm upregulation of one of these proteins, the inflammatory adipokine and chemokine retinoic acid receptor responder 2, which, in our study, showed an association with ACMP in CCSs that did not surpass the multiple testing threshold in our study (Table S2). In another study in patients with breast cancer with more severely depressed LVEF (ie, ≤40%; n=5) compared with anthracycline‐treated controls (n=10), a transcriptomics analysis demonstrated differential expression in genes related to lymphocyte activation and B‐cell receptor signaling, 17 which is interesting in relation to our study because CCL19 and CCL20 are chemotactic for T and B cells. In accordance with previous studies in CCSs and breast cancer survivors, we show that galectin‐3, soluble suppression of tumorigenicity‐2, interleukin‐6, tumor necrosis factor, and troponin I are not differentially expressed in ACMP cases compared with controls. 4 , 6 , 10 This finding is interesting because these biomarkers have been shown to be predictive of heart failure in the general population but may be related to other causes of heart failure. 18 , 19 , 20
Inclusion of a third group of patients with genetically determined DCM in secondary analysis allowed us to study potential overlap in biomarker profile in ACMP compared with DCM. Interestingly, we did not find significant differences between ACMP cases and patients with DCM in plasma levels of the proteins upregulated in ACMP and most proteins identified with elastic net. This overlap in upregulation strengthens the hypothesis that these proteins are associated with cardiomyopathy and do not reflect a systemic sensitivity for anthracyclines.
Despite the association of CCL19, CCL20, and PSPD with cardiomyopathy in our study, the cellular source(s) contributing to the elevated plasma levels remain uncertain. CCL19 and CCL20 are chemokines secreted by immune cells and cardiac fibroblasts in the heart under the influence of proinflammatory cytokines, but also by peripheral immune cells residing in lymph nodes. 21 PSPD is an innate immune pattern recognition collection expressed in the myocardium, but also in the lung and the vascular endothelium. 22 In addition, previous studies in patients with heart failure have demonstrated discrepancies between plasma and myocardial protein levels of other inflammatory proteins, such as galectin‐3, growth differentiation factor 15, tumor necrosis factor, and interleukin‐6. 23 , 24 It is therefore likely that the elevated plasma levels found in our study in ACMP and DCM are to a large extent produced by extracardiac sources, such as peripheral immune cells or vascular endothelial cells.
As for clinical utility, it is promising that the biomarker panel had a high sensitivity, while maintaining sufficient specificity to limit false positives, even in those patients in whom NT‐proBNP could not discriminate between ACMP and controls. However, clinical utility of the identified plasma biomarker levels for the diagnosis of LV dysfunction may be better assessed in larger cohorts and will be the subject of ongoing research.
Limitations
One may question the generalizability of our results to a surveillance population because we defined cardiomyopathy as an LVEF <45% and because 8 patients already had symptoms of heart failure. However, the LVEF thresholds of <45% for ACMP and ≥53% for controls made it possible to make a clear distinction between ACMP and controls, which was of importance in this discovery study. We also replicated the results in ACMP cases without heart failure. The patients with DCM were not matched to the patients with ACMP. However, we adjusted the analyses for differences in sex, age, and LVEF. This study should be seen as exploratory, with the purpose to select promising biomarker candidates to further study for their diagnostic value to detect asymptomatic cardiomyopathy in the DCCSS LATER 2 CARD cohort. 11
Conclusions
We identified the chemokine ligands CCL19 and CCL20 and the innate immune system marker PSPD as candidate diagnostic plasma biomarkers for anthracycline‐related cardiomyopathy in long‐term CCSs. By demonstrating overlap in expression of these biomarkers with those found in patients with genetically determined DCM, the hypothesis is strengthened that these protein markers are related to cardiac dysfunction. Further exploration and validation of the findings in a larger cohort are still needed.
Sources of Funding
This work was supported by the Dutch Heart Foundation (CVON2015‐21), Amsterdam University Funding, and Stichting Kinderen Kankervrij/Odasstichting. Dr Asselbergs is supported by University College London Hospitals National Institute for Health and Care Research Biomedical Research Centre.
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
We thank the other members of the Dutch Childhood Oncology Group (DCOG) LATER consortium (Birgitta Versluys, Martha Grootenhuis, Flora van Leeuwen, Sebastian Neggers, Lideke van der Steeg, Geert Janssens, Hanneke van Santen, Margreet Veening, Jaap den Hartogh, Saskia Pluijm, Lilian Batenburg, Hanneke de Ridder, Nynke Hollema, Lennart Teunissen, and Anke Schellekens) and all physicians, research nurses, data managers, and participating patients, parents, and siblings for their contribution.
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Feijen E, Font‐Gonzalez A, Van der Pal HJH, Kok WEM, Geskus RB, Ronckers CM, Bresters D, van Dalen EC, van Dulmen‐den BE, van den Berg MH, et al. Risk and temporal changes of heart failure among 5‐year childhood cancer survivors: a DCOG‐LATER study. J Am Heart Assoc. 2019;8:e009122. doi: 10.1161/jaha.118.009122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJ, Shankar S, Sieswerda E, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the international late effects of childhood cancer guideline harmonization group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/s1470-2045(14)70409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bossuyt PM, Irwig L, Craig J, Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ (Clin Res ed). 2006;332:1089–1092. doi: 10.1136/bmj.332.7549.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leerink JM, Verkleij SJ, Feijen EAM, Mavinkurve‐Groothuis AMC, Pourier MS, Ylänen K, Tissing WJE, Louwerens M, van den Heuvel MM, van Dulmen‐den BE, et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart (British Cardiac Society). 2019;105:210–216. doi: 10.1136/heartjnl-2018-313634 [DOI] [PubMed] [Google Scholar]
- 5. Toro‐Salazar OH, Lee JH, Zellars KN, Perreault PE, Mason KC, Wang Z, Hor KN, Gillan E, Zeiss CJ, Gatti DM, et al. Use of integrated imaging and serum biomarker profiles to identify subclinical dysfunction in pediatric cancer patients treated with anthracyclines. Cardio‐Oncol. 2018;4:4. doi: 10.1186/s40959-018-0030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armenian SH, Gelehrter SK, Vase T, Venkatramani R, Landier W, Wilson KD, Herrera C, Reichman L, Menteer JD, Mascarenhas L, et al. Screening for cardiac dysfunction in anthracycline‐exposed childhood cancer survivors. Clin Cancer Res. 2014;20:6314–6323. doi: 10.1158/1078-0432.ccr-13-3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armenian SH, Gelehrter SK, Vase T, Venkatramani R, Landier W, Wilson KD, Herrera C, Reichman L, Menteer JD, Mascarenhas L, et al. Carnitine and cardiac dysfunction in childhood cancer survivors treated with anthracyclines. Cancer Epidemiol Biomarkers Prev. 2014;23:1109–1114. doi: 10.1158/1055-9965.epi-13-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beer LA, Kossenkov AV, Liu Q, Luning Prak E, Domchek S, Speicher DW, Ky B. Baseline immunoglobulin E levels as a marker of doxorubicin‐ and trastuzumab‐associated cardiac dysfunction. Circ Res. 2016;119:1135–1144. doi: 10.1161/circresaha.116.309004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ky B, Putt M, Sawaya H, French B, Januzzi JL, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, Taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tromp J, Boerman LM, Sama IE, Maass S, Maduro JH, Hummel YM, Berger MY, de Bock GH, Gietema JA, Berendsen AJ, et al. Long‐term survivors of early breast cancer treated with chemotherapy are characterized by a pro‐inflammatory biomarker profile compared to matched controls. Eur J Heart Fail. 2020;22:1239–1246. doi: 10.1002/ejhf.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leerink JM, Feijen E, van der Pal HJH, Kok WEM, Mavinkurve‐Groothuis AMC, Kapusta L, Pinto YM, Maas A, Bellersen L, Teske AJ, et al. Diagnostic tools for early detection of cardiac dysfunction in childhood cancer survivors: methodological aspects of the Dutch late effects after childhood cancer (LATER) cardiology study. Am Heart J. 2020;219:89–98. doi: 10.1016/j.ahj.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 12. Feijen EAM, Leisenring WM, Stratton KL, Ness KK, van der Pal HJH, van Dalen EC, Armstrong GT, Aune GJ, Green DM, Hudson MM, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late‐onset cardiotoxicity. JAMA oncology. 2019;5:864–871. doi: 10.1001/jamaoncol.2018.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sammani A, Jansen M, Linschoten M, Bagheri A, de Jonge N, Kirkels H, van Laake LW, Vink A, van Tintelen JP, Dooijes D, et al. UNRAVEL: big data analytics research data platform to improve care of patients with cardiomyopathies using routine electronic health records and standardised biobanking. Neth Heart J. 2019;27:426–434. doi: 10.1007/s12471-019-1288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merkx R, Leerink JM, Feijen E, Kremer LCM, de Baat EC, Bellersen L, van Dalen EC, van Dulmen‐den Broeder E, van der Heiden‐van der Loo M, van den Heuvel‐Eibrink MM, et al. Echocardiography protocol for early detection of cardiac dysfunction in childhood cancer survivors in the multicenter DCCSS LATER 2 CARD study: design, feasibility, and reproducibility. Echocardiography. 2021;38:951–963. doi: 10.1111/echo.15081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B (Stat Methodol). 2005;67:301–320. doi: 10.1111/j.1467-9868.2005.00503.x [DOI] [Google Scholar]
- 17. Wan GX, Ji LH, Xia WB, Cheng L, Zhang YG. Bioinformatics identification of potential candidate blood indicators for doxorubicin‐induced heart failure. Exp Ther Med. 2018;16:2534–2544. doi: 10.3892/etm.2018.6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3:215–224. doi: 10.1001/jamacardio.2017.4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin‐3, a marker of cardiac fibrosis, predicts incident heart failure in the community. Journal of the American College of Cardiology. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham heart study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6 [DOI] [PubMed] [Google Scholar]
- 21. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145 [DOI] [PubMed] [Google Scholar]
- 22. Sorensen GL. Surfactant protein D in respiratory and non‐respiratory diseases. Front Med. 2018;5. doi: 10.3389/fmed.2018.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petretta M, Condorelli GL, Spinelli L, Scopacasa F, de Caterina M, Leosco D, Vicario ML, Bonaduce D. Circulating levels of cytokines and their site of production in patients with mild to severe chronic heart failure. Am Heart J. 2000;140:E28–18A. doi: 10.1067/mhj.2000.110935 [DOI] [PubMed] [Google Scholar]
- 24. Du W, Piek A, Schouten EM, van de Kolk CWA, Mueller C, Mebazaa A, Voors AA, de Boer RA, Silljé HHW. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics. 2018;8:4155–4169. doi: 10.7150/thno.26055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


