Abstract
Background
Growth differentiation factor 15 (GDF‐15) is elevated in heart failure with preserved ejection fraction and is associated with adverse outcome, but its relationship with myocardial fibrosis and other characteristics remains unclear. We sought to evaluate the effect of pirfenidone, a novel antifibrotic agent, on GDF‐15 in heart failure with preserved ejection fraction and identify characteristics that associate with GDF‐15 and with change in GDF‐15 over 1 year.
Methods and Results
Among patients enrolled (n=107) in the PIROUETTE (Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction) trial, GDF‐15 was measured at baseline and at prespecified time points in patients randomized (n=94) to pirfenidone or placebo. The response of GDF‐15 to pirfenidone and the association with baseline patient characteristics were evaluated. Pirfenidone had no impact on circulating GDF‐15 at any time point during the 52‐week trial period. In multivariable analysis, male sex, diabetes, higher circulating levels of N‐terminal pro‐B‐type natriuretic peptide, lower renal function, and shorter 6‐minute walk test distance at baseline were associated with baseline log–GDF‐15. Impaired global longitudinal strain at baseline was the strongest predictor of increased GDF‐15 over 52 weeks.
Conclusions
In patients with heart failure with preserved ejection fraction, circulating levels of GDF‐15 were unaffected by treatment with pirfenidone and do not appear to be determined by myocardial fibrosis. Circulating GDF‐15 was associated with a spectrum of important heart failure characteristics and it may represent a marker of overall physiological disruption.
Registration
URL: https://clinicaltrials.gov/ct2/show/NCT02932566; Unique identifier: NCT02932566.
Keywords: extracellular volume, heart failure, magnetic resonance imaging, myocardial fibrosis
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- ECV
extracellular volume
- GDF‐15
growth differentiation factor 15
- GLS
global longitudinal strain
- HFpEF
heart failure with preserved ejection fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- PIROUETTE
Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction
- TGF‐β
transforming growth factor β
Clinical Perspective.
What Is New?
Growth differentiation factor 15 (GDF‐15), a member of the transforming growth factor β cytokine superfamily, is elevated in heart failure with preserved ejection fraction and is associated with adverse outcome, but its relationship with myocardial fibrosis and other characteristics remains unclear. In the PIROUETTE (Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction) trial, the novel antifibrotic agent pirfenidone reduced myocardial fibrosis in patients with heart failure with preserved ejection fraction.
In the current analysis, among patients enrolled (n=107) in the PIROUETTE trial, we found that Pirfenidone had no impact on circulating GDF‐15 at any time point during the 52‐week trial period. GDF‐15 at baseline was associated with male sex, diabetes, higher circulating N‐terminal pro‐B‐type natriuretic peptide, impaired renal function, and shorter 6‐minute walk test distance.
What Are the Clinical Implications?
Our findings indicate that GDF‐15 is not involved in the myocardial fibrosis–heart failure with preserved ejection fraction disease mechanism and circulating levels of GDF‐15 are not determined by myocardial fibrotic burden.
GDF‐15 levels are associated with a spectrum of important heart failure characteristics and may therefore represent an overall biomarker of physiological disruption.
Growth differentiation factor 15 (GDF‐15) is a member of the transforming growth factor β (TGF‐β) cytokine superfamily that is expressed at low levels in most human tissues. 1 , 2 Expression of GDF‐15 in cardiomyocytes is triggered by insults such as ischemia, mechanical strain, proinflammatory cytokines, oxidative stress, and neurohormone activation, 3 , 4 although its role remains unclear. GDF‐15 is associated with adverse outcomes in patients with heart failure (HF), including those with heart failure with preserved left ventricular ejection fraction (HFpEF). 5 , 6
Myocardial fibrosis is an important pathophysiological mechanism of HFpEF. 7 Myocardial fibrosis, measured using cardiac magnetic resonance (CMR) extracellular volume (ECV), 8 , 9 is strongly associated with invasively measured load‐independent intrinsic left ventricular myocardial stiffness 10 and independently predictive of adverse outcome. 11 , 12 The PIROUETTE (Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction) study was a phase II, double‐blind, placebo‐controlled, randomized trial designed to evaluate the efficacy and mechanism of the novel antifibrotic agent, pirfenidone, in patients with HFpEF and myocardial fibrosis. 13 Pirfenidone is an orally bioavailable, small‐molecule antifibrotic agent that inhibits cardiac fibroblast synthesis and secretion of TGF‐β1, proliferation and activation of fibroblasts, and profibrotic pathways. As part of the trial protocol, participants underwent deep phenotyping, including detailed assessment of cardiac structure and function, exercise tolerance, fluid status, and quality of life.
This study aimed to evaluate the effect of pirfenidone on GDF‐15 in the PIROUETTE trial, and, utilizing deep phenotyping, identify characteristics that are associated with GDF‐15 and with change in GDF‐15 over a year. We hypothesized that GDF‐15 would be associated with myocardial fibrosis, and treatment with pirfenidone would lead to a reduction in GDF‐15.
Methods
Anonymized data will be made available in full on reasonable request in writing to the corresponding author following appropriate completion of a data sharing agreement.
Study Design and Patient Selection for the PIROUETTE Trial
Between March 7, 2017, to December 19, 2018, the PIROUETTE trial (Clinicaltrials.gov NCT02932566) randomized 94 patients with HFpEF and myocardial fibrosis to pirfenidone or placebo for 52 weeks. The trial design and results have previously been published. 13 , 14 Eligibility requirements included patients aged ≥40 years, symptoms and signs of HF, left ventricular ejection fraction of ≥45%, and elevated natriuretic peptides (brain‐type natriuretic peptide ≥100 pg/mL or N‐terminal pro‐B‐type natriuretic peptide [NT‐proBNP] ≥300 pg/mL; or brain‐type natriuretic peptide ≥300 pg/mL or NT‐proBNP ≥900 pg/mL if atrial fibrillation is present). Eligible patients underwent CMR and those with evidence of myocardial fibrosis, defined as an ECV of ≥27%, were randomized in a 1:1 ratio to treatment with either pirfenidone or matching placebo for 52 weeks using block randomization, stratified by sex. Patients without myocardial fibrosis were entered into a registry (n=13). Key exclusion criteria included alternative causes of patients' symptoms such as significant pulmonary disease, anemia, or obesity; pericardial constriction, hypertrophic cardiomyopathy, or infiltrative cardiomyopathy; and contraindication to magnetic resonance imaging. The primary outcome was change in myocardial fibrosis, measured using CMR ECV, from baseline to 52 weeks.
The trial was sponsored by Manchester University NHS Foundation Trust. Trial management, independent data management, and independent statistical analyses were performed by Liverpool Clinical Trials Centre, a United Kingdom Clinical Research Collaboration Registered Clinical Trials Unit. The study protocol was approved by a research ethics committee and trial conduct was overseen by a trial steering committee. Patients were identified at 6 hospitals in the United Kingdom. Study visits took place at Manchester University NHS Foundation Trust. All patients provided written informed consent.
Study Procedures
The protocol, trial procedures, analysis methods, and outcome measurements have been previously described. 13 , 14 In brief, CMR, echocardiography, ECG, 6‐minute walk test, laboratory tests, and the Kansas City Cardiomyopathy Questionnaire (KCCQ) were administered at baseline and repeated after 52 weeks of treatment. 31Phosphorous magnetic resonance spectroscopy, was performed at baseline and 52 weeks in a substudy (n=60) investigating the association of myocardial energetics and the impact of pirfenidone.
GDF‐15 Analysis
Patients provided written informed consent for blood samples to be stored in a central biorepository at baseline and at 13, 26, and 52 weeks postrandomization. Plasma was stored at −80‐degrees 15 and thawed to room temperature for same‐day analysis. Samples were processed on a Cobas e411 analyzer (Roche Diagnostics). The immunoassay (Elecsys GDF‐15, Roche Diagnostics) has a lower limit of detection of 400 pg/mL. The protocol for GDF‐15 analysis was approved by a research ethics committee.
Statistical Analysis
The trial was analyzed and reported according to CONSORT (Consolidated Standards of Reporting Trials) and the International Conference on Harmonization E9 guidelines. Continuous data are presented as mean±SD or median (interquartile range), as appropriate. Categorical data are presented as counts and percentages. The distribution of GDF‐15 data at baseline and at 52 weeks were non‐normal (Shapiro–Wilk tests for normality were P<0.001); therefore, log‐transformed values were used for the corresponding analyses and Spearman correlation coefficient was used for correlation analyses.
Treatment‐related analyses were conducted on an intention‐to‐treat basis, including all randomized patients retained in their randomized treatment groups. Log‐transformed GDF‐15 at week 52 was compared between treatment groups using ANCOVA, adjusting for baseline log‐transformed GDF‐15, stratification factor (sex), and treatment group. A secondary causal analysis, according to duration of pirfenidone treatment, was undertaken using instrumental variable regression to assess the causal impact of pirfenidone received on log‐transformed GDF‐15 at 52 weeks. Instrumental variable regression was used to estimate the association between pirfenidone usage and log‐transformed GDF‐15 at 52 weeks. Pirfenidone usage was summarized by the number of capsules taken, reported at visits 6, 9, 10, and 11. Zero was used for those in the placebo arm. Only participants who had full information on the number of capsules taken were included. The randomized group was chosen as the instrumental variable since it was assumed to satisfy the following criteria: (1) association with pirfenidone usage; (2) an indirect effect on GDF‐15 at 52 weeks (via pirfenidone usage); and (3) no common causes of randomization and week 52 GDF‐15. A 2‐stage least‐squares estimator was used: the first stage was to fit a model regressing pirfenidone usage on randomization and the second stage was to regress log‐transformed GDF‐15 at 52 weeks on the fitted values of pirfenidone usage predicted in the previous step. The model was adjusted for baseline GDF‐15 and sex. The impact of treatment on the association between log‐transformed GDF‐15 and over time was estimated using a repeated‐measures linear mixed model, with an unstructured covariance structure. Time (months), treatment group, sex, baseline GDF‐15, and interactions between time and treatment group were included as fixed effects.
Univariable and multivariable regression models were used to assess the relationships between baseline factors and log‐transformed baseline GDF‐15. Variables for which P values were<0.3 in the univariable analyses were included in forward stepwise selection models, with P value thresholds of 0.05 for entry and 0.1 for removal. The chosen variables were then included in multivariable regression models, where the outcome variable was log‐transformed baseline GDF‐15.
ANCOVA models were used to assess the relationship between baseline variables and change in GDF‐15 (week 52 value minus baseline value), adjusting for treatment allocation. Variables for which P values were <0.3 in the ANCOVA models were included in forward stepwise selection models, with P value thresholds of 0.05 for entry and 0.1 for removal. Treatment allocation was forced into the selection model. The chosen variables were then included in a multivariable regression model, where the outcome variable was change in GDF‐15 (week 52 value minus baseline value).
The conventional 5% significance level was used. All analyses were performed using SAS (version 9.4, SAS Institute Inc).
Results
Patients
A total of 107 patients were enrolled, including 94 who had evidence of myocardial fibrosis (ECV ≥27%) who were randomized, and 13 patients without evidence of myocardial fibrosis (ECV <27%) who were not randomized (Figure 1). Baseline characteristics are summarized in Table 1. The mean age of patients was 77 years, and 49% were women. Nearly all patients had New York Heart Association functional class II or III symptoms (94%), mean left ventricular ejection fraction was 65%, and median NT‐proBNP was 1067 pg/mL. At the end of the trial, 12 of the 94 patients who were randomized had withdrawn from the study and 2 had died. No patient was lost to follow‐up. A total of 80 patients completed the study and were therefore included in the analyses evaluating change in GDF‐15 and response to treatment.
Figure 1. Study overview and impact of pirfenidone on growth differentiation factor 15 (GDF‐15) between treatment groups at specified time points.
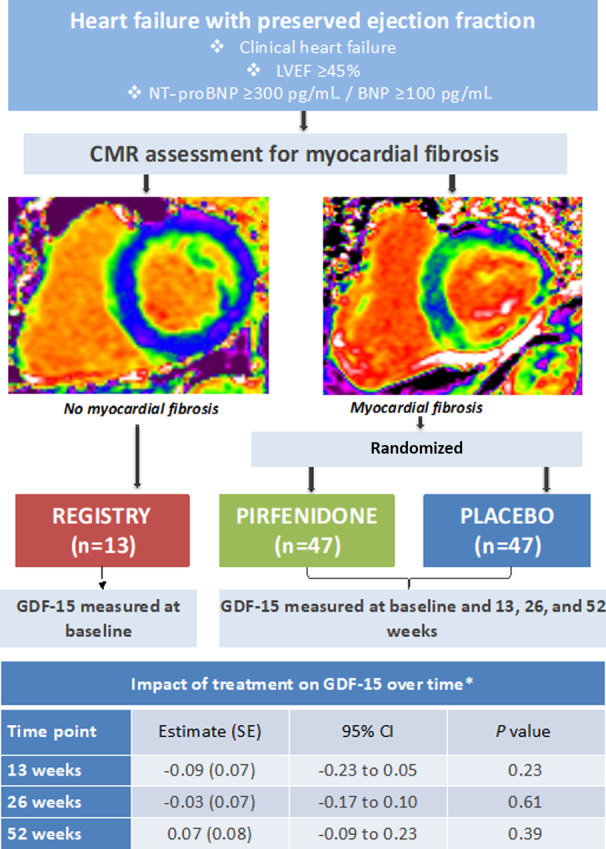
*Impact of pirfenidone on GDF‐15 between treatment groups at specified time points was assessed using a repeated‐measures mixed effects model, with time, treatment group, sex, baseline GDF‐15, and interaction between time and treatment group as fixed effects. GDF‐15 was analyzed as a log‐transformed variable. Results are presented as difference in mean GDF‐15 between treatment groups (pirfenidone vs placebo) at each visit. BNP indicates brain‐type natriuretic peptide; CMR, cardiac magnetic resonance; LVEF, left ventricular ejection fraction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Table 1.
Baseline Characteristics
| Characteristic |
Nonrandomized (n=13) |
Pirfenidone (n=47) |
Placebo (n=47) |
|---|---|---|---|
| Age, y | 74±8 | 76±8 | 80±6 |
| Women, no. (%) | 9 (69) | 22 (47) | 21 (45) |
| Hypertension, no. (%) | 10 (77) | 39 (83) | 40 (85) |
| Diabetes, no. (%) | 2 (15) | 16 (34) | 12 (26) |
| BMI, kg/m2 | 34±5 | 31±5 | 30±6 |
| eGFR, mL/min | 58±16 | 60±16 | 54±17 |
| GDF‐15, median (IQR), pg/mL | 1724 (1563–1954) | 2388 (1749–3116) | 3046 (1970–5442) |
| Log–GDF‐15, pg/mL | 7.5±0.4 | 7.8±0.6 | 8.1±0.6 |
| NT‐proBNP, median (IQR), pg/mL | 423 (324–803) | 975 (445–2064) | 1372 (626–2817) |
| Myocardial ECV, % | 24.7±0.6 | 29.5±2.5 | 30.7±2.9 |
| Absolute myocardial ECM volume, mL | 32.0±8.7 | 35.4±9.8 | 38.0±12.8 |
| Infarct LGE, no. (%) | 1 (8) | 12 (26) | 18 (38) |
| LV end‐diastolic volume index, mL/m2 | 67±11 | 63±19 | 62±18 |
| LV ejection fraction, % | 69±8 | 65±8 | 63±9 |
| Average E/e', cm/s | 12.1±3.5 | 11.4±3.0 | 13.1±3.7 |
| GLS, % | −16.6±4.2 | −15.8±4.1 | −16.2±2.9 |
| Torsion, degrees per cm | 1.6±0.7 | 1.5±0.7 | 1.4±0.6 |
| Phosphocreatine:ATP ratio | 1.3±0.3 | 1.3±0.4 | 1.2±0.4 |
| LA volume index, mL/m2 | 62±14 | 70±18 | 71±19 |
| LA strain (reservoir), % | 23.0±9.2 | 18.4±8.0 | 15.4±6.9 |
| Aortic distensibility, 10−3/mm Hg | 1.1±0.7 | 1.6±1.0 | 1.6±0.8 |
| Pulse wave velocity, m/s | 12.5±5.5 | 12.2±5.2 | 13.0±4.9 |
| Six‐min walk test, m | 298±120 | 275±124 | 255±105 |
| KCCQ Clinical Summary Score | 56±26 | 55±20 | 57±20 |
Values are expressed as mean±SD unless otherwise specified. BMI indicates body mass index; ECM, extracellular matrix; ECV, extracellular matrix volume; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; GLS, global longitudinal strain; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Effect of Pirfenidone on GDF‐15
Treatment with pirfenidone for 52 weeks was not associated with a change in log–GDF‐15 in comparison to placebo (Table 2). The causal analysis, which adjusted for duration of pirfenidone treatment, also showed that pirfenidone did not affect log–GDF‐15 (Tables S1 through S5). Pirfenidone did not significantly affect GDF‐15 at any individual time point (Figure 1); however, the association between log–GDF‐15 and time varied significantly by treatment (Table S2), ie, the effect of treatment varies over time, or equivalently the rate of change over time varies between pirfenidone and placebo. This supports the summary results shown in Figure 1, which suggest a positive (although not significant) association between treatment and GDF‐15 at 13 weeks and a negative (again not significant) association at 52 weeks.
Table 2.
Effect of Pirfenidone on GDF‐15
| Pirfenidone | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
|
Baseline (n=47) |
52 wk (n=39) |
Change from baseline to 52 wk |
Baseline (n=47) |
52 wk (n=41) |
Change from baseline to 52 wk | Between‐group difference (95% CI) | P value | |
| Log–GDF‐15, pg/mL | 7.80±0.56 | 7.80±0.68 | 0.03±0.32 | 8.06±0.63 | 7.93±0.61 | −0.07±0.32 | 0.05 (−0.12 to 0.23) | 0.53 |
ANOVA results comparing growth differentiation factor 15 (GDF‐15) at 52 weeks between treatment groups, adjusted for baseline GDF‐15 and sex. GDF‐15 was analyzed as a log‐transformed variable.
Baseline Factors Associated With Baseline GDF‐15
Univariable analyses identified positive associations between baseline factors such as age, myocardial ECV, log–NT‐proBNP, and high‐sensitivity troponin T, and negative associations with renal function, presence of atrial fibrillation, left atrial strain (reservoir), 6‐minute walk test, and KCCQ Clinical Summary Score and baseline log–GDF‐15 (Table 3 and Table S3). Correlation analyses are presented in Figure 2 and Table S4.
Table 3.
Univariable and Multivariable Associations With GDF‐15 at Baseline
| Baseline measure | Univariable Model | Multivariable Model | ||||
|---|---|---|---|---|---|---|
| β coefficient (SE) | 95% CI | P value | β coefficient (SE) | 95% CI | P value | |
| Age (per 10 y) | 0.17 (0.08) | 0.02–to 0.33 | 0.02 | |||
| Sex (women vs men) | 0.28 (0.11) | 0.06 to 0.51 | 0.01 | −0.38 (0.11) | −0.57 to −0.19 | <0.001 |
| Diabetes (yes vs no) | −0.50 (0.12) | −0.74 to −0.26 | <0.001 | 0.24 (0.10) | 0.04 to 0.44 | 0.02 |
| Atrial fibrillation (yes vs no) | −0.28 (0.11) | −0.51 to −0.05 | 0.02 | |||
| Diastolic blood pressure (per 10 mm Hg) | −0.082 (0.039) | −0.159 to −0.005 | 0.04 | |||
| eGFR (per 10 mL/min) | −0.17 (0.03) | −0.23 to −0.11 | <0.001 | −0.12 (0.03) | −0.18 to −0.07 | <0.001 |
| Hemoglobin, g/dL | −0.12 (0.04) | −0.19 to −0.04 | 0.002 | |||
| Log–NT‐proBNP, pg/mL | 0.27 (0.06) | 0.16 to 0.38 | <0.001 | 0.16 (0.05) | 0.07 to 0.25 | 0.001 |
| hs‐TnT, pg/mL | 0.008 (0.002) | 0.004 to 0.011 | <0.001 | |||
| Myocardial ECV, % | 0.05 (0.02) | 0.02 to 0.09 | 0.006 | |||
| Absolute myocardial ECM volume (per 10 mL) | 0.107 (0.052) | 0.004 to 0.209 | 0.04 | |||
| LA strain (reservoir), % | −0.02 (0.01) | −0.04 to −0.01 | 0.003 | |||
| 6‐min walk test (per 10 m) | −0.018 (0.005) | −0.027 to −0.009 | <0.001 | −0.017 (0.004) | −0.025 to −0.009 | <0.001 |
| KCCQ Clinical Summary Score (per 10 points) | −0.060 (0.028) | −0.116 to −0.004 | 0.04 | |||
All patients who underwent baseline assessment were included in the multivariable analyses (n=107). Growth differentiation factor 15 (GDF‐15) was analyzed as a log‐transformed variable. Variables for which P<0.3 in univariable regression model were included within stepwise forward selection multivariable regression model, with P value thresholds of 0.05 for entry and 0.1 for removal. ECM indicates extracellular matrix; ECV, extracellular matrix volume; eGFR, estimated glomerular filtration rate; hs‐TnT, high‐sensitivity troponin T; KCCQ, Kansas City Cardiomyopathy Questionnaire; LA, left atrial; LV, left ventricular; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide. See Table S3 for a full list of covariates.
Figure 2. Correlations with growth differentiation factor 15 (GDF‐15) at baseline.

Correlations between GDF‐15 and (A) myocardial extracellular volume (ECV), (B) log–N‐terminal pro‐B‐type natriuretic peptide (log–NT‐proBNP), (C) high‐sensitivity troponin T (hs‐TnT), and (D) 6‐minute walk test (6MWT) distance, measured in all patients (n=107) at baseline.
In multivariable analysis, male sex, diabetes, higher circulating levels of log–NT‐proBNP, lower renal function, and shorter 6‐minute walk test distance at baseline remained associated with higher baseline log–GDF‐15 (Table 3 and Table S3).
Baseline Factors Associated With Change in GDF‐15
In multivariable analysis, impaired global longitudinal strain (GLS) and higher right ventricular ejection fraction at baseline were independently associated with a greater change in GDF‐15 from baseline to 52 weeks (Table 4 and Table S5).
Table 4.
Univariable and Multivariable Associations With Change in GDF‐15 Over 1 y
| Baseline measures | Univariable model | Multivariable model | ||||
|---|---|---|---|---|---|---|
| β‐coefficient (SE) | 95% CI | P value | β‐coefficient (SE) | 95% CI | P value | |
| Hypertension (yes vs no) | 777.1 (530.7) | −279.6 to 1833.9 | 0.15 | |||
| Atrial fibrillation (yes vs no) | −506.0 (394.5) | −1291.6 to 279.6 | 0.20 | |||
| BMI, kg/m2 | −52.4 (36.0) | −124.1 to 19.3 | 0.15 | |||
| QRS duration, ms | −16.5 (11.2) | −38.7 to 5.8 | 0.14 | |||
| Infarct LGE (yes vs no) | −25.3 (19.4) | −64.1 to 13.6 | 0.20 | |||
| LV mass index, g/m2 | 17.3 (12.8) | −8.1 to 42.7 | 0.18 | |||
| GLS, % | 132.6 (53.4) | 26.4 to 238.9 | 0.02 | 183.9 (53.6) | 77.1 to 290.7 | 0.001 |
| RV ejection fraction, % | 39.4 (21.1) | −2.7 to 81.5 | 0.07 | 62.4 (20.9) | 20.8 to 104.0 | 0.004 |
| LA strain (booster), % | −121.9 (74.8) | −273.4 to 29.5 | 0.11 | |||
| LA strain (conduit), % | −87.2 (54.5) | −195.8 to 21.4 | 0.11 | |||
| KCCQ Clinical Summary Score | 14.9 (9.8) | −4.6 to 34.4 | 0.13 | |||
All patients who completed the study were included in the multivariable analyses (n=80). Variables for which P<0.3 in univariable regression model were included within stepwise forward selection multivariable regression model, with P value thresholds of 0.05 for entry and 0.1 for removal, and with treatment allocation forced within the selection model. BMI indicates body mass index; ECM, extracellular matrix; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; GLS, global longitudinal strain; KCCQ, Kansas City Cardiomyopathy Questionnaire; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; and RV, right ventricular. See Table S5 for a full list of covariates.
Correlations With Change in GDF‐15
Change in log–NT‐proBNP (r=0.22, P=0.05), right ventricular end‐diastolic volume index (r=0.27, P=0.02), and left atrial strain (conduit) (r=0.23, P=0.05) from baseline to week 52 showed positive, albeit weak, correlations with change in GDF‐15. Change in average e′ (r=−0.27, P=0.02), hemoglobin (−0.35, P=0.002), GLS (r=−0.29, P=0.01), and KCCQ Clinical Summary Score (r=−0.26, P=0.02) showed negative, albeit weak, correlations with change in GDF‐15 (Table S4).
Discussion
In this deeply phenotyped cohort of patients with HFpEF enrolled in the PIROUETTE trial, treatment with pirfenidone for 52 weeks did not lead to a change in circulating levels of GDF‐15, and GDF‐15 was not independently associated with myocardial ECV. GDF‐15 was associated with a spectrum of important HF characteristics, including diabetes, natriuretic peptides, impaired renal function, and reduced exercise tolerance. Impaired baseline GLS predicted an increase in GDF‐15 over 1 year.
GDF‐15 is expressed by most tissues in response to physiological stress. Indeed, GDF‐15 expression in the myocardium occurs in response to tissue injury, such as ischemia, pressure overload, and mechanical stretch, and it appears to have cardioprotective effects, including antihypertrophic, antiapoptotic, and anti‐inflammatory. 4 , 16 Several studies have identified the prognostic value of GDF‐15 in HF 6 , 17 , 18 , 19 ; however, the origin of the elevated circulating levels observed in HF remains unclear. 20 , 21 , 22
Pirfenidone inhibits several downstream mediators of TGF‐β (eg, Smad3, Akt, and p38), as well as inhibiting proliferation and migratory ability of cardiac fibroblasts and myofibroblast differentiation, and normalizes ratios of myocardial matrix metalloproteinases and tissue inhibitors of metalloproteinases. 23 In the main PIROUETTE study, treatment with pirfenidone for 52 weeks reduced myocardial fibrosis, and natriuretic peptide levels, providing support for the hypothesis that myocardial fibrosis has a causal role in HFpEF and is an effective therapeutic target.
Kanagala et al 24 recently demonstrated a correlation, albeit weak, between GDF‐15 and myocardial fibrosis, measured using CMR ECV (r=0.21, P=0.007), in a mixed cohort of patients with HF. In a small group of patients with end‐stage nonischemic cardiomyopathy in a study by Lok et al, 20 circulating levels of GDF‐15 were markedly elevated at baseline and reduced significantly following left ventricular assist device implantation. Circulating GDF‐15 showed a moderately strong correlation with histological collagen volume (r=0.61, P=0.01); however, GDF‐15 messenger RNA expression could not be detected in any of the myocardial biopsy specimens. In the current study, while there was a univariable association between baseline GDF‐15 and myocardial fibrosis that is in keeping with the Kanagala study, the association was no longer present on multivariable analysis. Taken together with the lack of GDF‐15 response to pirfenidone despite the observed regression of myocardial fibrosis and reduction in natriuretic peptides, our findings indicate that GDF‐15 is not involved in the myocardial fibrosis‐HF disease mechanism and circulating levels of GDF‐15 are not determined by myocardial fibrotic burden.
Baseline characteristics that were associated with circulating GDF‐15 included systemic congestion and functional limitation, as well as renal function and diabetes, both of which are associated with proinflammatory metabolic dysregulation. Furthermore, change over 1 year in multiple characteristics known to be associated with worse prognosis in HFpEF (elevated NT‐proBNP, anemia, diastolic dysfunction, and right heart dilatation 25 ) were associated with an increase in GDF‐15 over 1 year. These findings are in keeping with previous studies in HFpEF. 6 , 26 As such, while the mechanism responsible for elevated circulating levels of GDF‐15 in HF remain unknown, it may be that circulating GDF‐15 reflects overall physiological disruption, ie, a “summed” pathophysiological risk, which may explain the reportedly superior prognostic ability compared with NT‐proBNP. 7
GDF‐15 is known to be upregulated in patients with insulin resistance and type 2 diabetes and positively correlates with both glucose and hemoglobin A1c yet does not independently predict the onset of diabetes. 27 , 28 , 29 Similarly, while moderate correlations have previously been shown between circulating GDF‐15 and myocardial fibrosis (as histological collagen volume fraction) and a weak correlation was seen in this study between baseline ECV and GDF‐15, baseline ECV did not associate with circulating GDF‐15 on multivariable analysis. It is therefore plausible that diabetes and myocardial fibrosis both represent end‐organ effects of proinflammatory cytokine dysregulation for which GDF‐15 is upregulated but is not involved in the responsible disease causal pathway.
Impaired GLS was the strongest baseline predictor of an increase in GDF‐15 over 1 year. GLS was independently associated with adverse outcomes in HFpEF. 30 , 31 The mechanisms of impaired GLS in HFpEF remains undefined; however, the presence of myocardial fibrosis and concurrent cardiomyocyte dysfunction likely confers a worse prognosis than a pure “fibrotic” phenotype alone. 32 The ability of impaired GLS at enrollment to predict GDF‐15 increase at 1 year may reflect the cumulative impact of multiple disease mechanisms.
Limitations
The sample size for the PIROUETTE study was calculated based on the primary outcome. The trial was not powered for secondary outcomes; thus, the findings of this study are considered exploratory. GDF‐15 was not included in the “Statistical Analysis Plan” for the PIROUETTE trial and thus the analyses included in this study are considered post hoc. However, the analyses conducted were prespecified in an “Additional Statistical Analysis Plan” that was written before data lock and all blood samples were analyzed for GDF‐15 before data lock. The interpretation of the results of the causal analysis is also limited because of the requirement for complete compliance data, which was obtained in only 22 patients who received pirfenidone.
Conclusions
In patients with HFpEF, circulating levels of GDF‐15 were unaffected by treatment with pirfenidone and do not appear to be determined by myocardial fibrosis. Circulating GDF‐15 was associated with a spectrum of important HF characteristics and it may represent a marker of overall physiological disruption.
Sources of Funding
Dr Lewis is funded by a fellowship grant from the National Institute for Health and Care Research (NIHR). Dr Miller is funded by a Clinician Scientist Award (CS‐2015‐15‐003) from the NIHR. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
Disclosures
C.A.M. has served on advisory boards for Novartis, Boehringer Ingelheim and Lilly Alliance, and AstraZeneca; serves as an advisor for HAYA Therapeutics and PureTech Health; and has received research support from Amicus Therapeutics, Guerbet Laboratories Limited, and Univar Solutions B.V––none of these relationships are relevant to the contents of this article. The investigational medicinal product was gifted by Roche Products Limited. Immunoassay testing equipment and materials were gifted by Roche Diagnostics International Limited. Roche Products Limited and Roche Diagnostics International Limited had no role in the study design and were not involved in the preparation, drafting, or editing of this article. Roche Products Limited and Roche Diagnostics International Limited conducted a factual accuracy check of this article, but any decisions to incorporate comments were made solely at the discretion of the authors.
Supporting information
Tables S1–S5
For Sources of Funding and Disclosures, see page 8.
References
- 1. Ahmad T, Fiuzat M, Felker GM, O'Connor C. Novel biomarkers in chronic heart failure. Nat Rev Cardiol. 2012;9:347–359. doi: 10.1038/nrcardio.2012.37 [DOI] [PubMed] [Google Scholar]
- 2. Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep. 2012;9:337–345. doi: 10.1007/s11897-012-0113-9 [DOI] [PubMed] [Google Scholar]
- 3. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, et al. The transforming growth factor‐beta superfamily member growth‐differentiation factor‐15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48 [DOI] [PubMed] [Google Scholar]
- 4. Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC‐1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0 [DOI] [PubMed] [Google Scholar]
- 5. Izumiya Y, Hanatani S, Kimura Y, Takashio S, Yamamoto E, Kusaka H, Tokitsu T, Rokutanda T, Araki S, Tsujita K, et al. Growth differentiation factor‐15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol. 2014;30:338–344. doi: 10.1016/j.cjca.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 6. Chan MM, Santhanakrishnan R, Chong JP, Chen Z, Tai BC, Liew OW, Ng TP, Ling LH, Sim D, Leong KT, et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18:81–88. doi: 10.1002/ejhf.431 [DOI] [PubMed] [Google Scholar]
- 7. Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70:2186–2200. doi: 10.1016/j.jacc.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 8. Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636 [DOI] [PubMed] [Google Scholar]
- 9. Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. doi: 10.1161/CIRCIMAGING.112.000192 [DOI] [PubMed] [Google Scholar]
- 10. Rommel K‐P, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lücke C, Gutberlet M, et al. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2016;67:1815–1825. doi: 10.1016/j.jacc.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 11. Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol. 2017;2:995–1006. doi: 10.1001/jamacardio.2017.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, Gerber BL, Pouleur AC. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2018;20:55. doi: 10.1186/s12968-018-0477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis GA, Dodd S, Clayton D, Bedson E, Eccleson H, Schelbert EB, Naish JH, Jimenez BD, Williams SG, Cunnington C, et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat Med. 2021;27:1477–1482. doi: 10.1038/s41591-021-01452-0 [DOI] [PubMed] [Google Scholar]
- 14. Lewis GA, Schelbert EB, Naish JH, Bedson E, Dodd S, Eccleson H, Clayton D, Jimenez BD, McDonagh T, Williams SG, et al. Pirfenidone in heart failure with preserved ejection fraction‐rationale and design of the PIROUETTE trial. Cardiovasc Drugs Ther. 2019;33:461–470. doi: 10.1007/s10557-019-06876-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kempf T, Horn‐Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth‐differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–291. doi: 10.1373/clinchem.2006.076828 [DOI] [PubMed] [Google Scholar]
- 16. Ago T, Sadoshima J. GDF15, a cardioprotective TGF‐beta superfamily protein. Circ Res. 2006;98:294–297. doi: 10.1161/01.RES.0000207919.83894.9d [DOI] [PubMed] [Google Scholar]
- 17. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the valsartan heart failure trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846 [DOI] [PubMed] [Google Scholar]
- 18. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, et al. Prognostic utility of growth differentiation factor‐15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091 [DOI] [PubMed] [Google Scholar]
- 19. Mendez Fernandez AB, Ferrero‐Gregori A, Garcia‐Osuna A, Mirabet‐Perez S, Pirla‐Buxo MJ, Cinca‐Cuscullola J, Ordonez‐Llanos J, Roig ME. Growth differentiation factor 15 as mortality predictor in heart failure patients with non‐reduced ejection fraction. ESC Heart Fail. 2020;7:2223–2229. doi: 10.1002/ehf2.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lok SI, Winkens B, Goldschmeding R, van Geffen AJ, Nous FM, van Kuik J, van der Weide P, Klopping C, Kirkels JH, Lahpor JR, et al. Circulating growth differentiation factor‐15 correlates with myocardial fibrosis in patients with non‐ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur J Heart Fail. 2012;14:1249–1256. doi: 10.1093/eurjhf/hfs120 [DOI] [PubMed] [Google Scholar]
- 21. Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin‐3 and growth‐differentiation factor‐15 with heart failure and other non‐cardiac diseases. Clin Chim Acta. 2015;445:155–160. doi: 10.1016/j.cca.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 22. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. doi: 10.1373/clinchem.2016.255174 [DOI] [PubMed] [Google Scholar]
- 23. Aimo A, Cerbai E, Bartolucci G, Adamo L, Barison A, Lo Surdo G, Biagini S, Passino C, Emdin M. Pirfenidone is a cardioprotective drug: mechanisms of action and preclinical evidence. Pharmacol Res. 2020;155:104694. doi: 10.1016/j.phrs.2020.104694 [DOI] [PubMed] [Google Scholar]
- 24. Kanagala P, Arnold JR, Singh A, Chan DCS, Cheng ASH, Khan JN, Gulsin GS, Yang J, Zhao L, Gupta P, et al. Characterizing heart failure with preserved and reduced ejection fraction: an imaging and plasma biomarker approach. PloS One. 2020;15:e0232280. doi: 10.1371/journal.pone.0232280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stahrenberg R, Edelmann F, Mende M, Kockskamper A, Dungen HD, Luers C, Binder L, Herrmann‐Lingen C, Gelbrich G, Hasenfuss G, et al. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail. 2010;12:1309–1316. doi: 10.1093/eurjhf/hfq151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eddy AC, Trask AJ. Growth differentiation factor‐15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021;57:11–18. doi: 10.1016/j.cytogfr.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M. Increased serum concentrations of macrophage inhibitory cytokine‐1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161:397–404. doi: 10.1530/EJE-09-0417 [DOI] [PubMed] [Google Scholar]
- 29. Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, Witte DR. Macrophage inhibitory cytokine‐1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: the Whitehall II study. Eur J Endocrinol. 2010;162:913–917. doi: 10.1530/EJE-09-1066 [DOI] [PubMed] [Google Scholar]
- 30. Morris DA, Ma XX, Belyavskiy E, Aravind Kumar R, Kropf M, Kraft R, Frydas A, Osmanoglou E, Marquez E, Donal E, et al. Left ventricular longitudinal systolic function analysed by 2D speckle‐tracking echocardiography in heart failure with preserved ejection fraction: a meta‐analysis. Open Heart. 2017;4:e000630. doi: 10.1136/openhrt-2017-000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frojdh F, Fridman Y, Bering P, Sayeed A, Maanja M, Niklasson L, Olausson E, Pi H, Azeem A, Wong TC, et al. Extracellular volume and global longitudinal strain both associate with outcomes but correlate minimally. JACC Cardiovasc Imaging. 2020;13:2343–2354. doi: 10.1016/j.jcmg.2020.04.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


