Abstract
Multiple sensor systems are used to monitor physiological parameters, activities of daily living and behaviour. Digital biomarkers can be extracted and used as indicators for health and disease. Signal acquisition is either by object sensors, wearable sensors, or contact-free sensors including cameras, pressure sensors, non-contact capacitively coupled electrocardiogram (cECG), radar, and passive infrared motion sensors. This review summarizes contemporary knowledge of the use of contact-free sensors for patients with cardiovascular disease and healthy subjects following the PRISMA declaration. Chances and challenges are discussed. Thirty-six publications were rated to be of medium (31) or high (5) relevance. Results are best for monitoring of heart rate and heart rate variability using cardiac vibration, facial camera, or cECG; for respiration using cardiac vibration, cECG, or camera; and for sleep using ballistocardiography. Early results from radar sensors to monitor vital signs are promising. Contact-free sensors are little invasive, well accepted and suitable for long-term monitoring in particular in patient’s homes. A major problem are motion artefacts. Results from long-term use in larger patient cohorts are still lacking, but the technology is about to emerge the market and we can expect to see more clinical results in the near future.
Keywords: Digital biomarkers, Contact-free sensor, Heart vibration, Respiration, Facial camera, Radar, Passive infrared motion sensor, Heart rate, Heart rate variability, Vital signs, Cardiovascular disease
Graphical Abstract
Introduction
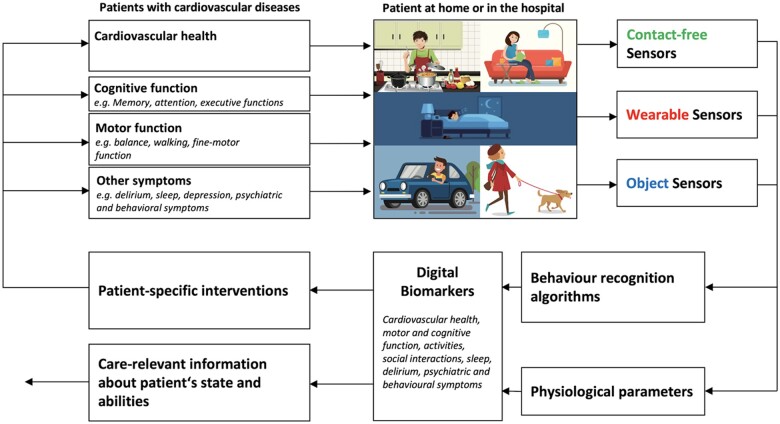
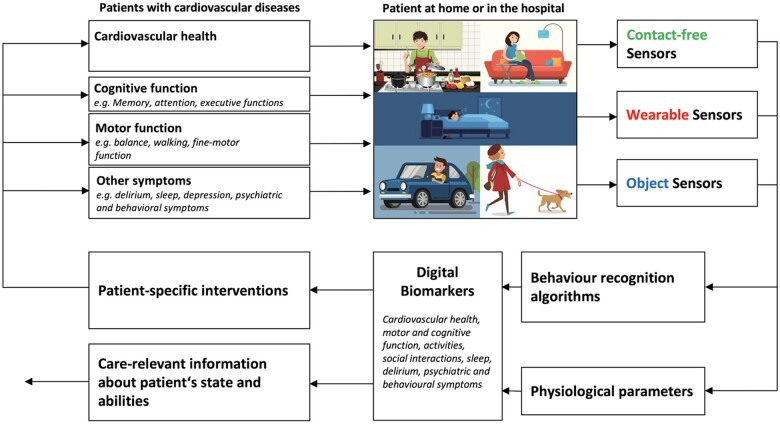
Sensor-based symptom assessment can be used in the hospital and in the homes of patients at risk for cardiovascular diseases to monitor physiological parameters (e.g. heartbeat, respiratory rate) and current activities of daily living (e.g. eating, sleeping). Based on the sensor’s reading, digital biomarkers for common cardiovascular diseases can be extracted. Digital biomarkers are defined as an ‘objective, quantifiable physiological and behavioural data that are collected and measured by means of digital devices’1 to get real-time information about the patient’s state. Digital biomarkers can be used to drive patient-specific interventions, as endpoints for clinical studies, or as information for formal and informal caregivers to help them to optimize patient care. As it is shown in Figure 1, there are mainly three types of sensors to be used in patient’s home or in the hospital.
Figure 1.
Working principle for telemonitoring of patients at risk for cardiovascular diseases. A combination of contact-free, wearable and objectattached sensors can be used to monitor behaviour and physiological parameters. From that information, digital biomarkers can be extracted to deliver care-relevant information to drive patient-specific interventions..
Object sensors (also called object-attached sensors) are connected to objects of daily living and are measuring the interaction between patient and object. For example, by measuring the movement of a computer-mouse during daily computer usage, information about the patient’s fine-motor skills and cognitive abilities can be extracted.2 Another example is a medicine box with a sensor that allows measuring the time of medicine intake.3
Wearable sensors are the most prevalent sensor type which is typically worn at the wrist of the non-dominant arm. Commercially available wearable sensors are often integrated into a smart watch. As outlined by Seshadri et al.,4 wearable sensors find their applications to monitor specific medical conditions, such as atrial fibrillation5,6 or cystic fibrosis.7,8 In a recent systematic review on wearable devices for ambulatory cardiac monitoring Sana et al.9 conclude that wearable devices are very helpful for long-term continuous monitoring in the patient’s home. Besides their advantages, wearable sensors are difficult to use in some patient populations because cooperation of the patient is needed. Patients mostly need to wear the sensor during the day and often also during the night (i.e. if sleep monitoring is desired), and they need to recharge the wearable sensor. This is feasible in patient with good cognitive abilities,10,11 but very difficult in patients with cognitive impairment (e.g. Alzheimer’s disease) where the acceptance and feasibility for the use of wearable sensors is in general very low.12
Contact-free sensors have a great advantage in that they do not need any action by the patients to work. Contact-free sensors (also called ambient sensors) are positioned in the environment of the patient and measure activities by using behaviour recognition algorithms and physiological parameters. Early work from Urwyler et al.13 uses a number of passive infrared (PIR) sensors to measure changes in infrared radiation to detect movement in the proximity of the sensor. Using machine learning algorithms to detect specific activity patterns, activities of daily living can be recognized with a sensitivity and a specificity of >90%. Since these sensors do not require patients interaction, acceptance is generally very high. Furthermore, this type of sensor is easy to use for studies in humans including patients with cognitive impairment (see Piau et al.1 for review). Recent advancements in sensor and data processing technology allow not only activity measurements but also contact-fee measurement of physiological parameters (e.g. breathing, heartbeat). Piezo-electric force sensor arrays under the bed-mattress can be used for contact-free ballistocardiography to measure heartbeat and respiration rate.14 These and other contact-free sensor principles (e.g. radar measurement) are analysed and discussed in this article. More specifically, we discuss the use of contact-free sensors as digital biomarkers for common cardiovascular diseases.
Methods
Search strategy
For this systematic review, we followed the PRISMA declaration (Preferred Reporting Items for Systematic Reviews and Meta-Analysis). We have searched PubMed, Cochrane, Scopus, and the database of the Institute of Electrical and Electronics Engineers (IEEE). Only original research in English language, published after 2010 up to 28 August 2020 were included.
For the search term, the advanced search with the following combination of terms was used:
("cardi*"[Title/Abstract] OR "Heart"[Title/Abstract]) AND ("Contactless"[All Fields] OR "Smart Home*"[All Fields] OR "Contactfree"[All Fields] OR "Contact free"[All Fields] OR "Contact-free"[All Fields] OR "Contact-less"[All Fields] OR "Contactless"[All Fields] OR “Unobtrus*”[All Fields]) OR "Nearable"[All Fields]
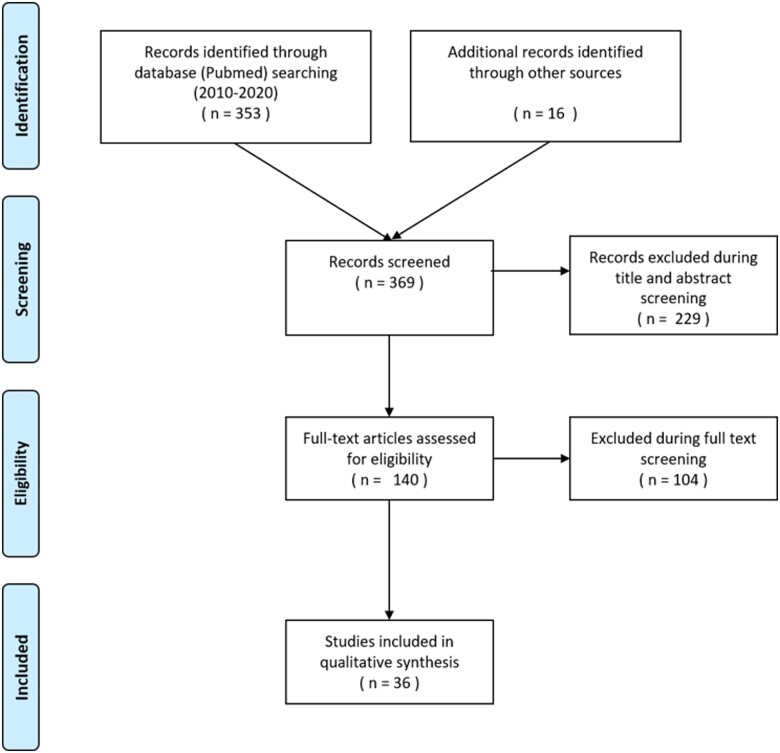
The search term has been updated after the initial search results have been reviewed. By scanning reference lists, additional references were acquired. The flow chart of the search and elimination procedures is shown in Figure 2.
Figure 2.
Flow chart of the search and elimination process.
The grading of the manuscripts was performed by two independent reviewers: one reviewer had a technical and the other reviewer a medical background. The relevance was rated as low = 1, medium = 2, or high = 3, depending on the study quality, the sample size, and study population. Studies rated as low were excluded from the final table.
The main exclusion criteria for less relevant manuscripts were either a restriction to a proof of concept or very low numbers of study participants. Another exclusion criteria was if the manuscript was focusing on the comparison of different algorithms in feature extraction.
Sensor technology principles
The following chapter briefly describes the main sensor technologies which are actually used for monitoring of physiological parameters, activities of daily living and behaviour. At the end of each subchapter, the number of reviewed articles is listed. Manuscripts describing only technical aspects of the sensors or sensor technology without clinical application have been excluded from the review. Two papers describing several technologies have been counted multiple times.
Camera based technologies
These technologies use cameras for contactless monitoring of a variety of parameters which are relevant for cardiovascular health. Camera types can be separated into two main categories: the first category includes multichannel cameras recording in the visible spectrum and the second category includes cameras which are monitoring in the infrared spectrum.15 In some cases, depth is added as an additional dimension.16 Sensor placement can be arbitrary and depends on the use case, but usually, the cameras are placed in a distance of 1–2 m from the subject.15 For cardiovascular diseases, image-based technologies have shown to allow relatively reliable recordings of heartbeats by detecting vascular pulsatile motion leading to signals which are similar in nature to signals which are acquired using photoplethysmography.15,17 Breathing rate is measurable with acceptable accuracy by monitoring chest movements—and theoretically by filtering the heartbeat signal.16,18 There have been attempts to demonstrate the possibility of analysing SpO2 and blood pressure by using camera technology. As of today, this appears still as technically challenging with limited accuracy.15 Infrared cameras have also been used to monitor skin temperature.19 In addition, cameras can also be used to infer factors such as in-home physical activity or gait parameters.20,21 Furthermore, cameras are used to track frequency of toilet visits or to monitor larger movements in bed.22
Chances
Cameras represent a widespread, established and relatively cheap technology allowing to measure a wide variety of health-relevant parameters.
Challenges
Similar to contact-based sensors, motion artefacts are a major problem also for contact-free sensor systems when measuring vital signs. Therefore, cameras are mostly useful to measure signals from subjects at rest. In addition, physical obstructions like bed sheets or lighting conditions can pose additional challenges for signal acquisition by cameras.15 However, the most obvious disadvantages of camera-based sensor technologies are privacy concerns and obtrusiveness. This concern can be tackled to a certain degree by using additional technologies such as automatic blurring (in particular faces), by extracting only silhouettes or by using edge computing—thus processing the images locally and only forwarding a predefined set of extracted parameters. However, the stigma of obtrusiveness associated with the use of cameras will likely remain a limiting factor for a widespread adoption of this technology for continuous remote monitoring applications.
Number of reviewed articles: 15
Pressure sensor-based technologies
These sensor technologies are based on the measurement of mechanical forces which are exerted by the human body to detect subtle signals like heart beats, breathing cycles or larger movements.23–26 The underlying technology is often based on piezoelectric-like sensors that convert mechanical pressure into electrical charges.27 This requires the sensors to be placed close to the human body such as below the bed mattress or on the backside of chairs, but not in direct contact with the body.27
There is substantial evidence for the potential of this technology to measure heartbeats (usually referred to as ballistocardiography) and breathing.27 More recent research even indicates the possibility of using this technology for blood pressure measurement.28
Chances
Pressure sensor-based technologies are relatively cheap and readily available. In addition, they can be completely unobtrusive and hidden inside or beneath objects. Privacy aspects are also less of a concern as the acquired data does not easily allow to identify a sensor user.
Challenges
Similar to other technologies, pressure sensors are susceptible to movements.27 In addition, any obstacle between the sensor and the heart leads to signal dampening or distortions. Therefore, a main disadvantage of this technology is that it requires the sensor to be in relatively close proximity of the upper body and the heart.
Number of reviewed articles: 16
Electromagnetic field-based technologies
The idea of non-contact capacitively coupled electrocardiogram (cECG) signal acquisition29 is to use capacitive electrodes that are placed near the human body but with a potential gap (air, furniture, sheets, cloths, etc.) inbetween, thus allowing for contact-free electrocardiogram (ECG) measurements.29–31 In theory, this technology allows the acquisition of the same signals as with regular electrode-based ECGs, including full PQRS complexes.
In addition, respiration signals can be acquired with magnetic induction (MI) based on local changes in impedance by respiration or by cardiac activity.
Chances
The big advantage of cECG and MI is the fact that it allows to actually capture electrophysiological parameters instead of just mechanical movements of the heart and the chest. The electromagnetic field technology is also relatively unobtrusive in that it can be hidden beneath everyday objects like furniture or bed.
Challenges
Compared to electrode-based ECG measurements, cECG is likely to be more prone to generate artefacts.32 In addition, due to the gap between the human body and the electrodes, the system has to deal with very high input impedance.
Similar as in cECG, the signal strength obtained from MI decreases exponentially with distance, and the sensor has to be placed very close to the subjects body. The major disadvantage of this technology is that it is still experimental and does not appear to be ready for routine clinical use. Furthermore, the technology suffers from the same location restrictions as pressure sensors in that electrodes need to be placed in closed proximity to the human body.
Number of reviewed articles: 2
Radar sensors
Radar technology is becoming more mature and a large body of literature shows evidence that this technology is ready to be used in commercial devices and beyond research test beds. Radar technology is mostly based on continuous wave, frequency modulated continuous wave or ultra wideband.33 Most commonly, the (micro) Doppler effect is used to quantify human body movements and even subtle signals such as signals from the beating heart.33,34 As a result, radar technology allows to measure heart beats and respiration beside larger movements to monitor position and physical activity.34,35
Chances
Radar-based technology’s biggest advantage is its capability to measure a wide variety of body movements, including heart and breathing. It also is less restricted to specific locations and may allow monitoring through smaller obstacles and even thin walls. Compared to camera technologies, radar is quite unobtrusive.
Challenges
Similar as for the other technologies, radar suffers from movement artefacts when it comes to quantify small body movements and to monitor persons at rest.
Availability of radars systems is still limited and prices are relatively high, but this is already changing, and we expect decreasing prices and better availability in the near future. Although radar is less obtrusive than cameras, it can still acquire a large amount of information from a monitored person’s life and actions without being limited to very specific locations and modalities. This raises concern in regard to privacy. In addition, there might be a certain stigma associated to radar-based systems as some people tend to fear wireless signals.
Number of reviewed articles: 4
Other sensors: passive infrared motion sensors, audio recordings
PIR sensors have been around for a long time and have been used in a variety of home-monitoring scenarios.36 The technology allows to detect the broader motion of a person based on changes in infrared radiation when a person moves.37 This relatively simple technology allows to primarily monitor certain risks factors like in-home physical activity, the number of toilet visits or gait speed.11,12,36,38
Audio recordings represent although a simple technology and are very easy to obtain. The most important parameters to measure using audio technology are breathing pattern and snoring.39–41
Chances
Both, PIR and audio recordings represent simple sensor technologies that are widely available, relatively unobtrusive and inexpensive. PIR sensors have the additional advantage of having little privacy concerns.
Challenges
Due to their simplicity, these types of sensors can only give limited information. When it comes to parameters being relevant for cardiovascular diseases, additional audio recordings of snoring can be of added value.
Only very broad grained basic information can be obtained through regular PIR sensors, most of which is rather behaviour based and not necessarily relevant to cardiovascular conditions. However, results may give some background information that facilitates the interpretation of other sensor data.
Number of reviewed articles: 2
Summary of the systematic review
Cardiac measurements
There are several studies which are of medium and high relevance for contactless measurement of heart-related parameters such as heart rate, heart rate variability, arrhythmias, or electrical activity (Table 1). In particular for measurements of heart rate and heart rate variability using technologies based on pressure, camera or radar, a rapid increase of accuracy and robustness of the systems can be observe over the last few years. Results from the use of these technologies are mostly accurate, unobtrusive, with good functionality and with high usability. However, most studies have been performed with healthy participants and not with patients. Therefore, more studies are required to show accuracy and usability for daily clinical practice.
Table 1.
Systematic review of relevant paper from 2010 up to date of contact less sensors measuring cardiorespiratory signals50
| References | Relevance | Study population (size) | Device | Parameter | Summary |
|---|---|---|---|---|---|
| Blood pressure | |||||
| Liu et al.42 | 2 | Healthy (128) | Cardiac vibration | High correct classification of blood pressure abnormalities | |
| Luo et al.43 | 2 | Healthy (1328) | Facial camera | Innovative machine learning approach to estimate blood pressure in normotensive adults based on facial camera | |
| Cardiac | |||||
| Yan et al.44 | 3 | Cardiologic patients (217) | Facial camera | AF | High sensibility and specificity for AF detection |
| Yan et al.45 | 2 | AF patients (20), healthy (24) | Facial camera | AF | High accuracy of AF detection with low cost approach |
| Benedetto et al.46 | 2 | Healthy (24) | Facial camera | HR | Poor accuracy compared to ECG, especially for low and high heart rate for consumer product (FaceReader™ by Noldus) |
| Brueser et al.47 | 2 | AF patients (10) | Cardiac vibration | AF | High sensitivity for AF detection |
| Brüser et al.48 | 2 | Healthy (8), insomnia patients (25) | Cardiac vibration | HR | Very low beat to beat interval error |
| Couderc et al.49 | 2 | AF patients (11) | Facial camera | AF | Abnormal pulse variability due to AF can be detected, but have to be improved |
| Hoog Antink et al.14 | 2 | Healthy (10), post-surgery patients (14) | Cardiac vibration | HR, HRV | Comparable accuracy in patients and healthy, lower accuracy than ECG |
| 2 | Healthy (21) | Doppler radar | HR | If combined with neural network high accuracy compared to ECG without heavy pre-processing | |
| McDuff et al.51 | 2 | Healthy (67) | Webcam | HR | Detection of daily patterns in cardiovascular signals |
| Paalasmaa et al.52 | 2 | Healthy (46) | Cardiac vibration | HR | Overall high accuracy compared to ECG, high interparticipant variability |
| Pino et al.53 | 2 | Healthy (54) | Cardiac vibration | HR | High accuracy compared to ECG in lying and sitting position |
| Pröll et al.54 | 2 | Hospital patients (42) | Cardiac vibration | HR | Acceptable accuracy in low quality data using EMFit Sensor |
| Sugita et al.55 | 2 | Healthy (39) | Facial camera (PPG) | HR | High accuracy while body movement |
| Wartzek et al.56 | 2 | Healthy (59) | EMF (cECG) | ECG | Robust and reliable heart rate estimation from capacitive ECG |
| Yan et al.57 | 2 | Healthy (40) | Facial camera (PPG) | HR | Acceptable accuracy compared to standard, impaired due to motion artefacts |
| Yu et al.58 | 2 | Healthy elderly (10), geriatric patients (10) | Facial camera (PPG) | HR, HRV | High accuracy compared to standard |
| Zink et al.59 | 2 | Patients with AF (22) | Cardiac vibration | HR | High accuracy compared to ECG during sinus rhythm and during AF |
| Respiration | |||||
| Hsu et al.60 | 3 | Patients with sleep disorders (63) | Cardiac vibration | High correlation of breathing parameters with PSG | |
| Castro et al.61 | 2 | Healthy (15) | EMF (cECG) | Sleep apnoea | High accuracy of respiration signals and apnoea detection derived from contactless ECG |
| Elphick et al.62 | 2 | Healthy adults (41), healthy children (20) | Facial thermal camera | High accuracy of respiration rate compared to the best standard | |
| Ermer et al.63 | 2 | Healthy (26) | Camera; cardiac vibration | Low respiration rates not reliably detectable | |
| Sleep | |||||
| Dafna et al.39 | 2 | PSG patients (150) | Audio recording | Innovative and reliably estimation of Sleep-wake activity and sleep quality parameters using audio recording | |
| Huysmans et al.64 | 2 | Sleep lab patients (114) | Cardiac vibration | Possible sensor for future sleep monitoring if correct synchronization with parameters from PSG | |
| Jung et al.65 | 2 | Healthy (10), OSA patients (10) | Cardiac vibration | Reliably detection of nocturnal awakening and sleep efficiency estimation | |
| Vital signs and other parameter | |||||
| Bennett et al.66 | 3 | Heart failure patients (29) | Cardiac vibration | Respiratory rate was the most important risk-adjusted associate of readmission for HF | |
| Negishi et al.67 | 3 | Healthy (22), influenza patients (28) | Facial thermal camera | Temperature | High accuracy compared to clinical standard |
| Saner et al.11 | 2 | Healthy Old (24) | PIR, cardiac vibration (EMFit) | Health status | Long-term monitoring using PIR and pressure-based sensors are well accepted and feasible for detection of health problems |
| Kang et al.68 | 2 | Healthy (6), patients with OSA (14) | UWB radar | High reliability and accuracy of heart and respiration rate compared to PSG in patients and healthy | |
| Tal et al.69 | 2 | Healthy (63) | Cardiac vibration | High accuracy compared to PSG | |
| Ben-Ari et al.70 | 2 | Healthy adults (16), healthy children (41) | Cardiac vibration | High accuracy compared to clinical standard | |
| Iozzia et al.71 | 2 | Healthy (26) | Facial camera (PPG) | High accuracy in sitting and standing position compared to standard | |
| Michler et al.72 | 2 | Healthy (30) | Radar (24 GHz system) | High accuracy compared to ECG | |
| Valenza et al.73 | 2 | Healthy (60) | Facial camera (PPG) | Rapid changes correctly detected | |
| Sun et al.74 | 2 | Healthy (22), influenza patients (16) | Facial thermal camera | Temperature | Higher sensitivity than conventional fever-based screening approaches |
| Diraco et al.75 | 2 | Healthy (30) | UWB radar | Falls | High accuracy compared to ECG during ADL |
If not other mentioned heart and breathing rate are combined with the term ‘vital signs’, if not other mentioned the participants were adults.
ADL, activities of daily living; AF, atrial fibrillation; cECG, capacitive electrocardiogram; ECG, electrocardiogram; EMF, electromagnetic field; HF, heart failure; HRV, heart rate variability; OSA, obstructive sleep apnoea; PIR, passive infrared sensor; PPG, photoplethysmography; PSG, polysomnography; UWB, ultra-wide band.
While heart rate is relatively easy to assess, several studies indicate also a high accuracy for heart rate variability measurements. While motion of the heart and the pulse wave can be traced relatively easy by radar or pressure-based technologies, experimental studies show also promising results for capacitive ECG technologies which have the advantage to allow to measure electrophysiological parameters. However, technologies have still to be considered as experimental and are rarely seen in clinical use. Artefacts are still a problem and rhythm disturbances are difficult to be recognized and classified with high accuracy. Another interesting but still experimental technology is contactless measurement of blood pressure, which is on the horizon but still far from clinical reality.
Respiration
Respiration seems to be one of the easiest vital sign to be recorded unobtrusively. Many studies are using contact-free sensors to measure respiration in patients and even commercial products are available for this purpose. As breathing rate is not the main focus of this review, only a few studies were included. Important indications for monitoring of respiration rate are pulmonary diseases, pulmonary embolism, cardiac disease with pulmonary congestion such as heart failure, and screening for obstructive sleep apnoea. Combined measurements of heart rate and respiration rate and thus the potential to detect an increase of both parameters over time may help to identify or exclude diseases such as COVID-19 infection in early stages.
Vital signs
Respiration rate and heart rate are characterized mainly by different frequencies in movement patterns and are therefore usually assessed simultaneously, in particular by using radar- or pressure-based technologies. Available research results show high accuracy of those parameters when compared to clinical standards.
Sleep
For the assessment of sleep parameters, sensors are usually compared to polysomnography. Several studies are looking at heart and respiration rate during the night, but only a few studies estimate the sleep stages from contactless sensors. Sensor systems which can be placed under the mattress and are based on unobtrusively measured ballistocardiography are increasingly used and show promising results for heart and respiration rate, sleep parameters, and motion in bed. Even though newer machine learning approaches show promising results for correct classification of sleep stages, the unchallenged gold standard for the assessment of sleep parameters is still the electroencephalogram.
Potential for clinical usability in cardiovascular patients
There is a great potential for the clinical application of contact-free sensors signals as digital biomarkers in particular for cardiovascular patients:
Heart rate, heart rhythm, heart rate variability, and respiration for a broad range of clinical conditions and diseases (e.g. atrial fibrillation).
Combination of heart rate and respiration rate with polyuria/bed exits at night and restlessness in bed for advanced stages of heart failure.
Combination of heart rate/rhythm and respiration rate with blood oxygen saturation for specific forms of congenital heart disease.
Combination of heart rate and respiration rate with temperature for infectious endocarditis.
Combination of heart rate and respiration rate for unobstrusive monitoring of intensive care unit patients/hospitalized patients.
Conclusion and future directions
In this article, we have reviewed the literature for studies with contact-free sensor technology for the unobtrusive measurement of blood pressure, cardiac signals, respiration and other vital signs. Most of the studies (16) used cardiac vibration recordings and evaluated the system with healthy test persons and a relatively small number of patients. Another 12 studies used a camera for signal recording in both healthy test persons and patients. A common finding is that movement artefacts are the main contributor to signal lost and signal distortion. While it is possible to detect movement and ignore the distorted signal recoding, it will not be possible to guarantee continuous recording of cardiac signal with contact-free sensors. In other words, while wrong measurements can be recognized, movement of the patient will result in an interrupted measurement. That is why these types of sensors are not well suited for time-critical recordings of the heartbeat as it is for example required for real-time monitoring in an intensive or intermediate care facility.
On the other hand, thanks to the small invasiveness of the sensors and the good to excellent patient-acceptance, these sensors are specifically well suited for long-term measurements in the patient’s homes. Especially in situations with little movement (e.g. during sleep, when sitting) good signal quality can be achieved with contact-free measurement of heart rate, heart rhythm, heart rate variability and respiration. While the feasibility has been proven, larger clinical studies should aim at investigating the potential benefit of long-term recordings for the prevention, early diagnosis and better treatment of cardiovascular diseases.
An interesting, relatively novel avenue is the use of radar sensors to measure cardiovascular signals. Recent work from Malesevic et al.50 and Kang et al.68 has shown this contact-free technology shows high accuracy of heart and respiration rate. Also here, the limitations of movement artefacts apply, but movement can be measured and affected measurements can be disregarded. The technology is about to emerge the market and we can expect to see more clinical results in the near future.
Conflict of interest: none declared.
Data availability
The data underlying this article are available in the article.
References
- 1. Piau A, Wild K, Mattek N, Kaye J.. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J Med Internet Res 2019;21:e12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seelye A, Hagler S, Mattek N, Howieson DB, Wild K, Dodge HH, Kayea J.. Computer mouse movement patterns: a potential marker of mild cognitive impairment. Alzheimers Dement (Amst) 2015;1:472. 10.1016/j.dadm.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Surya Tej M, Ameet Chavan D.. MediKit: IoT based smart healthcare system for effective supervision of patient. IJET 2018;7:1. [Google Scholar]
- 4. Seshadri DR, Li RT, Voos JE, Rowbottom JR, Alfes CM, Zorman CA, Drummond CK. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit Med, 2019;2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aliamiri A, Shen Y. Deep learning based atrial fibrillation detection using wearable photoplethysmography sensor. In: 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI). IEEE; 2018, pp. 442–445.
- 6. Steinberg BA, Piccini JP.. Screening for atrial fibrillation with a wearable device. JAMA 2018;320:139. [DOI] [PubMed] [Google Scholar]
- 7. Choi D-H, Kim JS, Cutting GR, Searson PC.. Wearable potentiometric chloride sweat sensor: the critical role of the salt bridge. Anal Chem 2016;88:12241–12247. [DOI] [PubMed] [Google Scholar]
- 8. Emaminejad S, Gao W, Wu E, Davies ZA, Nyein HYY, Challa S, Ryan SP, Fahad HM, Chen K, Shahpar Z, Talebi S, Milla C, Javey A, Davis RWet al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc Natl Acad Sci USA 2017;114:4625–4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA.. Wearable devices for ambulatory cardiac monitoring. J Am Coll Cardiol 2020;75:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Botros A, Schütz N, Camenzind M, Urwyler P, Bolliger D, Vanbellingen T, Kistler R, Bohlhalter S, Müri RM, Mosimann UP, Nef Tet al. Long-term home-monitoring sensor technology in patients with Parkinson’s disease—acceptance and adherence. Sensors 2019;19:5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saner H, Schütz N, Botros A, et al. Potential of ambient sensor systems for early detection of health problems in older adults. Front Cardiovasc Med 2020;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schütz N, Saner H, Rudin B, et al. Validity of pervasive computing based continuous physical activity assessment in community-dwelling old and oldest-old. Sci Rep 2019;9:9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urwyler P, Stucki R, Rampa L, Müri R, Mosimann UP, Nef T.. Cognitive impairment categorized in community-dwelling older adults with and without dementia using in-home sensors that recognise activities of daily living. Sci Rep 2017;7:42084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoog Antink C, Mai Y, Aalto R, et al. Ballistocardiography can estimate beat-to-beat heart rate accurately at night in patients after vascular intervention. IEEE J Biomed Health Inform 2020;24:2230–2237. [DOI] [PubMed] [Google Scholar]
- 15. Harford M, Catherall J, Gerry S, Young JD, Watkinson P.. Availability and performance of image-based, non-contact methods of monitoring heart rate, blood pressure, respiratory rate, and oxygen saturation: a systematic review. Physiol Meas 2019;40:06TR01. [DOI] [PubMed] [Google Scholar]
- 16. Procházka A, Charvátová H, Vyšata O, Kopal J, Chambers J.. Breathing analysis using thermal and depth imaging camera video records. Sensors 2017;17:1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antink CH, Lyra S, Paul M, Yu X, Leonhardt S.. A broader look: camera-based vital sign estimation across the spectrum. Yearb Med Inform 2019;28:102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakajima K, Tamura T, Miike H.. Monitoring of heart and respiratory rates by photoplethysmography using a digital filtering technique. Med Eng Phys 1996;18:365–372. [DOI] [PubMed] [Google Scholar]
- 19. Ludwig N, Formenti D, Gargano M, Alberti G.. Skin temperature evaluation by infrared thermography: comparison of image analysis methods. Infrared Phys Technol 2014;62:1–6. [Google Scholar]
- 20. Zhou Z, Chen X, Chung YC, He Z, Man TX, Keller JM.. Activity analysis, summarization, and visualization for indoor human activity monitoring. IEEE Trans Circuits Syst Video Technol 2008;18:1489–1498. [Google Scholar]
- 21. Stone E, Skubic M.. Evaluation of an inexpensive depth camera for in-home gait assessment. J Ambient Intell Smart Environ 2011;3:349–361. [Google Scholar]
- 22. Gall M, Garn H, Kohn B, et al. Automated detection of movements during sleep using a 3D time-of-flight camera: design and experimental evaluation. IEEE Access 2020;8:109144–109155. [Google Scholar]
- 23. Aubert XL, Brauers A. Estimation of vital signs in bed from a single unobtrusive mechanical Sensor: algorithms and real-life evaluation. In: Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’08 - ‘Personalized Healthcare through Technology’. IEEE Computer Society; 2008, pp. 4744–4747. [DOI] [PubMed]
- 24. Heise D, Rosales L, Skubic M, Devaney MJ. Refinement and evaluation of a hydraulic bed sensor. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS.2011, pp. 4356–4360. [DOI] [PMC free article] [PubMed]
- 25. Sadek I, Demarasse A, Mokhtari M.. Internet of things for sleep tracking: wearables vs. nonwearables. Health Technol 2020;10:333–340. [Google Scholar]
- 26. Pino EJ, Larsen C, Chavez J, Aqueveque P.. Non-invasive BCG monitoring for non-traditional settings. Conf Proc IEEE Eng Med Biol Soc 2016;2016:4776–4779. [DOI] [PubMed] [Google Scholar]
- 27. Inan OT, Migeotte PF, Park KS, et al. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Health Inform 2015;19:1414–1427. [DOI] [PubMed] [Google Scholar]
- 28. Kim CS, Carek AM, Inan OT, Mukkamala R, Hahn JO.. Ballistocardiogram-based approach to cuffless blood pressure monitoring: proof of concept and potential challenges. IEEE Trans Biomed Eng 2018;65:2384–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen M, Castro ID, Lin Q, van Hoof C, Wang G, Lian Y, van Helleputte Net al. A 400GΩ input-impedance, 220MVpp linear-input-range, 2.8Vpp CM-interference-tolerant active electrode for non-contact capacitively coupled ECG acquisition. In: IEEE Symposium on VLSI Circuits, Digest of Technical Papers. Vol. 2018. Institute of Electrical and Electronics Engineers Inc.; 2018, pp. 129–130. doi:10.1109/VLSIC.2018.8502270.
- 30. Matsuda T, Makikawa M. ECG monitoring of a car driver using capacitively-coupled electrodes. In: Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’08 - “Personalized Healthcare through Technology”. IEEE Computer Society; 2008, pp. 1315–1318. doi:10.1109/iembs.2008.4649406. [DOI] [PubMed]
- 31. Kim KK, Lim YK, Park KS. The electrically non-contacting ECG measurement on the toilet seat using the capacitively-coupled insulated electrodes. In: Annual International Conference of the IEEE Engineering in Medicine and Biology—Proceedings. 26 IV. 2004, pp. 2375–2378. doi:10.1109/iembs.2004.1403688. [DOI] [PubMed]
- 32. Ottenbacher J, Heuer S.. Motion Artefacts in Capacitively Coupled ECG Electrodes. Berlin, Heidelberg: Springer; 2009, pp. 1059–1062. [Google Scholar]
- 33. Li C, Lubecke VM, Boric-Lubecke O, Lin J.. A review on recent advances in Doppler radar sensors for noncontact healthcare monitoring. IEEE Trans Microw. Theory Tech 2013;61:2046–2060. [Google Scholar]
- 34. Li C, Cummings J, Lam J, Graves E, Wu W.. Radar remote monitoring of vital signs. IEEE Microw Mag 2009;10:47–56. [Google Scholar]
- 35. Postolache O, Girão P, Pinheiro E, Madeira R, Dias Pereira JM, Mendes J, Postolache G, Moura C. Multi-usage of microwave Doppler radar in pervasive healthcare systems for elderly. In: Conference Record- IEEE Instrumentation and Measurement Technology Conference; 2011, pp. 1–5. [Google Scholar]
- 36. Peetoom KKB, Lexis MAS, Joore M, Dirksen CD, De Witte LP.. Literature review on monitoring technologies and their outcomes in independently living elderly people. Disabil Rehabil Assist Technol 2015;10:271–294. [DOI] [PubMed] [Google Scholar]
- 37. Song B, Choi H, Lee HS. Surveillance tracking system using passive infrared motion sensors in wireless sensor network. In: 2008 International Conference on Information Networking. IEEE; 2008, pp. 1–5. doi:10.1109/ICOIN.2008.4472790.
- 38. Rana R, Austin D, Jacobs PG, Karunanithi M, Kaye J.. Gait velocity estimation using time-interleaved between consecutive passive IR Sensor Activations. IEEE Sensors J 2016;16:6351–6358. [Google Scholar]
- 39. Dafna E, Tarasiuk A, Zigel Y.. Sleep-wake evaluation from whole-night non-contact audio recordings of breathing sounds. McLoughlin I, ed. PLoS One 2015;10:e0117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan J, Rea T, Gollakota S, Sunshine JE.. Contactless cardiac arrest detection using smart devices. NPJ Digit Med 2019;2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Demir F, Sengur A, Cummins N, Amiriparian S, Schuller B.. Low level texture features for snore sound discrimination. Conf Proc IEEE Eng Med Biol Soc 2018;2018:413–416. [DOI] [PubMed] [Google Scholar]
- 42. Liu F, Zhou X, Wang Z, Cao J, Wang H, Zhang Y.. Unobtrusive mattress-based identification of hypertension by integrating classification and association rule mining. Sensors (Basel )2019;19:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo H, Yang D, Barszczyk A, Vempala N, Wei J, Wu SJ, Zheng PP, Fu G, Lee K, Feng Z-P. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging 2019;12:e008857. [DOI] [PubMed] [Google Scholar]
- 44. Yan BP, Lai WHS, Chan CKY, Chan SC, Chan LH, Lam KM, Lau HW, Ng CM, Tai LY, Yip KW, To OTL, Freedman B, Poh YC, Poh MZ. Contact-free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. J Am Heart Assoc 2018;7:e008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yan BP, Lai WHS, Chan CKY, Au ACK, Freedman B, Poh YC, Poh MZet al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol 2020;5:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benedetto S, Caldato C, Greenwood DC, Bartoli N, Pensabene V, Actis P.. Remote heart rate monitoring—assessment of the Facereader rPPg by Noldus. Mumtaz W, ed. PLoS One 2019;14:e0225592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brueser C, Diesel J, Zink MDH, Winter S, Schauerte P, Leonhardt S.. Automatic detection of atrial fibrillation in cardiac vibration signals. IEEE J Biomed Health Inform 2013;17:162–171. [DOI] [PubMed] [Google Scholar]
- 48. Brüser C, Winter S, Leonhardt S.. Robust inter-beat interval estimation in cardiac vibration signals. Physiol Meas 2013;34:123–138. [DOI] [PubMed] [Google Scholar]
- 49. Couderc J-P, Kyal S, Mestha LK, Xu B, Peterson DR, Xia X, Hall Bet al. Detection of atrial fibrillation using contactless facial video monitoring. Heart Rhythm 2015;12:195–201. [DOI] [PubMed] [Google Scholar]
- 50. Malešević N, Petrović V, Belić M, Antfolk C, Mihajlović V, Janković M.. Contactless real-time heartbeat detection via 24 GHz continuous-wave Doppler radar using artificial neural networks. Sensors 2020;20:2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McDuff D. Using non-contact imaging photoplethysmography to recover diurnal patterns in heart rate. Conf Proc IEEE Eng Med Biol Soc 2019;2019:6830–6833. [DOI] [PubMed] [Google Scholar]
- 52. Paalasmaa J, Toivonen H, Partinen M.. Adaptive heartbeat modeling for beat-to-beat heart rate measurement in ballistocardiograms. IEEE J Biomed Health Inform 2015;19:1945–1952. [DOI] [PubMed] [Google Scholar]
- 53. Pino EJ, Chavez JAP, Aqueveque P.. Noninvasive ambulatory measurement system of cardiac activity. Conf Proc IEEE Eng Med Biol Soc 2015;2015:7622–7625. [DOI] [PubMed] [Google Scholar]
- 54. Pröll SM, Hofbauer S, Kolbitsch C, Schubert R, Fritscher KD. Ejection wave segmentation for contact-free heart rate estimation from ballistocardiographic signals. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2019, pp. 3571–3576. doi:10.1109/EMBC.2019.8857731. [DOI] [PubMed]
- 55. Sugita N, Akay M, Akay YM, Yoshizawa M.. Noise reduction technique for single-color video plethysmography using singular spectrum analysis. IEEE J Biomed Health Inform 2020;24:1788–1795. [DOI] [PubMed] [Google Scholar]
- 56. Wartzek T, Eilebrecht B, Lem J, Lindner H-J, Leonhardt S, Walter M.. ECG on the road: robust and unobtrusive estimation of heart rate. IEEE Trans Biomed Eng 2011;58:3112–3120. [DOI] [PubMed] [Google Scholar]
- 57. Yan BP, Chan CK, Li CK, To OT, Lai WH, Tse G, Poh YC, Poh MZ. Resting and postexercise heart rate detection from fingertip and facial photoplethysmography using a smartphone camera: a validation study. JMIR Mhealth Uhealth 2017;5:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu X, Laurentius T, Bollheimer C, Leonhardt S, Hoog Antink C.. Noncontact monitoring of heart rate and heart rate variability in geriatric patients using photoplethysmography imaging. IEEE J Biomed Health Inform 2020;doi:10.1109/JBHI.2020.3018394. [DOI] [PubMed] [Google Scholar]
- 59. Zink MD, Brüser C, Winnersbach P, Napp A, Leonhardt S, Marx N, Schauerte P, Mischke Ket al. Heartbeat cycle length detection by a ballistocardiographic sensor in atrial fibrillation and sinus rhythm. Biomed Res Int 2015;2015:840356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hsu M-H, Fang S-C, Wang F-T, Chan H-L, Huang H-E, Yang S-C.. Sleep apnea assessment using declination duration-based global metrics from unobtrusive fiber optic sensors. Physiol Meas 2019;40:075005. [DOI] [PubMed] [Google Scholar]
- 61. Castro I, Varon C, Torfs T, Van Huffel S, Puers R, Van Hoof C.. Evaluation of a multichannel non-contact ECG system and signal quality algorithms for sleep apnea detection and monitoring. Sensors 2018;18:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Elphick HE, Alkali AH, Kingshott RK, Burke D, Saatchi R.. Exploratory study to evaluate respiratory rate using a thermal imaging camera. Respiration 2019;97:205–212. [DOI] [PubMed] [Google Scholar]
- 63. Ermer S, Brewer L, Orr J, Egan TD, Johnson K.. Comparison of 7 different sensors for detecting low respiratory rates using a single breath detection algorithm in nonintubated, sedated volunteers: Anesth Analg 2019;129:399–408. [DOI] [PubMed] [Google Scholar]
- 64. Huysmans D, Borzée P, Testelmans D, Buyse B, Willemen T, Huffel SV, Varon C. Evaluation of a commercial ballistocardiography sensor for sleep apnea screening and sleep monitoring. Sensors (Basel) 2019;19:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jung DW, Hwang SH, Yoon HN, Lee Y-JG, Jeong D-U, Park KS.. Nocturnal awakening and sleep efficiency estimation using unobtrusively measured ballistocardiogram. IEEE Trans Biomed Eng 2014;61:131–138. [DOI] [PubMed] [Google Scholar]
- 66. Bennett MK, Shao M, Gorodeski EZ.. Home monitoring of heart failure patients at risk for hospital readmission using a novel under-the-mattress piezoelectric sensor: a preliminary single centre experience. J Telemed Telecare 2017;23:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Negishi T, Abe S, Matsui T, Liu H, Kurosawa M, Kirimoto T, Sun G. Contactless vital signs measurement system using RGB-thermal image sensors and its clinical screening test on patients with seasonal influenza. Sensors 2020;20:2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kang S, Lee Y, Lim Y-H, Park H-K, Cho SH, Cho SH.. Validation of noncontact cardiorespiratory monitoring using impulse-radio ultra-wideband radar against nocturnal polysomnography. Sleep Breath 2020;24:841–848. [DOI] [PubMed] [Google Scholar]
- 69. Tal A, Shinar Z, Shaki D, Codish S, Goldbart A.. Validation of contact-free sleep monitoring device with comparison to polysomnography. J Clin Sleep Med 2017;13:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ben-Ari J, Zimlichman E, Adi N, Sorkine P.. Contactless respiratory and heart rate monitoring: validation of an innovative tool. J Med Eng Technol 2010;34:393–398. [DOI] [PubMed] [Google Scholar]
- 71. Iozzia L, Cerina L, Mainardi LT.. Assessment of beat-to-beat heart rate detection method using a camera as contactless sensor. Conf Proc IEEE Eng Med Biol Soc 2016;2016:521–524. [DOI] [PubMed] [Google Scholar]
- 72. Michler F, Shi K, Schellenberger S, Steigleder T, Malessa A, Hameyer L, Neumann N, Lurz F, Ostgathe C, Weigel R, Koelpin A. A clinically evaluated interferometric continuous-wave radar system for the contactless measurement of human vital parameters. Sensors 2019;19:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Valenza G, Iozzia L, Cerina L, Mainardi L, Barbieri R. Assessment of instantaneous cardiovascular dynamics from video plethysmography. In: 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2017, pp. 1776–1779. doi:10.1109/EMBC.2017.8037188. [DOI] [PubMed]
- 74. Sun G, Nakayama Y, Dagdanpurev S, Abe S, Nishimura H, Kirimoto T, Matsui T. Remote sensing of multiple vital signs using a CMOS camera-equipped infrared thermography system and its clinical application in rapidly screening patients with suspected infectious diseases. Int J Infect Dis 2017;55:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Diraco G, Leone A, Siciliano P.. A radar-based smart sensor for unobtrusive elderly monitoring in ambient assisted living applications. Biosensors 2017;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.