Abstract
Heart murmur, a thoracic auscultatory finding of cardiovascular origin, is extremely common in childhood and can appear at any age from premature newborn to late adolescence. The objective of this review is to provide a modern examination and update of cardiac murmur auscultation in this new era of artificial intelligence (AI) and telemedicine. First, we provide a comprehensive review of the causes and differential diagnosis, clinical features, evaluation, and long-term management of paediatric heart murmurs. Next, we provide a brief history of computer-assisted auscultation and murmur analysis, along with insight into the engineering design of the digital stethoscope. We conclude with a discussion of the paradigm shifting impact of deep learning on murmur analysis, AI-assisted auscultation, and the implications of these technologies on telemedicine in paediatric cardiology. It is our hope that this article provides an updated perspective on the impact of AI on cardiac auscultation for the modern paediatric cardiologist.
Keywords: Auscultation, Artificial intelligence, Congenital heart disease, Paediatric murmurs
Introduction
Heart murmur is a thoracic auscultatory finding of cardiovascular origin that is of greater duration than a heart sound or a click. Most practical definitions exclude pericardial friction rubs, but include the sounds of turbulent blood flow through, and harmonic vibrations of, cardiovascular structures. An innocent or functional murmur is produced by the normal activity of the heart and is not associated with any congenital or acquired cardiac abnormality. A pathologic murmur is one that is produced in association with heart disease. Seven auscultatory features of murmurs are conventionally described (Table 1).
Table 1.
Describing heart murmurs
| Feature | Points to consider |
|---|---|
| Loudness | 6 Grades |
| 1—Barely audible, softer than the heart sounds | |
| 2—Easily heard, but not extremely loud, equal to the heart sounds | |
| 3—Loud, louder than the heart sounds but no associated palpable thrill | |
| 4—Loud with thrill | |
| 5—Audible with edge of stethoscope | |
| 6—Audible without stethoscope | |
| Pitch | Usually described as low, medium, or high. Many innocent murmurs are low pitched |
| Location | Vertical axis—intercostal space |
| Horizontal axis—distance right or left of sternal border, cardiac apex may also be useful landmark | |
| Timing | Systolic, between S1 and S2 |
| Ejection peaks in intensity in mid systole | |
| Regurgitant denotes constant intensity throughout the murmur duration | |
| Holosystolic denotes lasting all of systole | |
| Late systolic (early systole is silent) | |
| Diastolic, between S2 and S1 | |
| Early vs. late | |
| Duration | |
| Radiation | If a murmur is louder than expected at a point distant from the point at which it is loudest, this is radiation. Common locations for radiation include back and neck. |
| Character | Useful descriptors for sound. Harsh, for example denotes a mixture of many frequencies. Musical denotes narrow frequency bandwidth and prominence of harmonic frequencies. Machinery-like implies the waxing and waning of intensity in some continuous murmurs. There are many other adjectives that can convey helpful comparisons to noises of common experience |
| Behavior with manoeuvres | When murmurs diminish or are augmented by change in position, Valsalva manoeuvre, of pressure with stethoscope, these can be diagnostically helpful, see also Table 3 |
Heart murmur is extremely common in childhood and can appear at any age from premature newborn to late adolescent. Murmurs can be innocent or pathologic. Sometimes, pathologic murmur is the presenting feature of cardiac disease representing a significant health risk to the child, so a key task for the clinician is to identify pathologic murmurs promptly and accurately. However, innocent murmurs are extremely common, with a cumulative prevalence of up to 70% over the duration of childhood,1 so this diagnostic task must also be accomplished cost-effectively.
There are many different types of innocent murmur, each with its own unique timing, auscultatory features, and pathogenesis (Table 2). Murmurs, especially innocent ones, are generally made more prominent by optimizing the effective transmission of sound to the stethoscope, and by conditions which increase the cardiac output (Table 3). They tend to be easier to appreciate at slower heart rates, and when there is a minimum of distracting environmental noise.
Table 2.
Timing, location, and pathogenesis of common innocent murmurs2–7
| Timing | Location | Cause | |
|---|---|---|---|
| Pulmonary flow murmur | Systolic ejection | Left upper sternal border | Normal minor systolic turbulence in the main pulmonary artery, audible because it is in such close proximity to the stethoscope through the thin chest wall of the healthy child. Geometric configuration of the chest can play a role, as in straight back syndrome |
| Innocent pulmonary branch murmur of infancy | Systolic ejection | Left and right upper sternal borders, both axillae, midback | Turbulence in the origins of the pulmonary arterial branches related to the small size and sharp angulation of the take-off of these branches from the main pulmonary artery in the first few weeks to months after birth |
| Stills murmur | Systolic ejection | Midway between left lower sternal border and cardiac apex. May transmit rightward. | Harmonic vibrations of the left ventricle as it ejects blood into the aorta |
| Venous hum | Continuous | Right infraclavicular, rarely left infraclavicular | Turbulent venous flow in the superior vena cava |
| Supraclavicular bruit | Systolic ejection | Left and/or right supraclavicular | Turbulent flow in the origins of the brachiocephalic arteries |
| Mammary souffle | Continuous usually, but can be systolic ejection | Left and right midsternal border | Turbulent flow in the chest wall arteries and veins leading to and from the engorged breasts in the setting of pregnancy or lactation |
Table 3.
An abridged differential diagnosis of paediatric heart murmur by murmur location8–11
| Timing | Location | Differential diagnosis | Key murmur features and associated physical findings | Important points to consider |
|---|---|---|---|---|
| Systolic ejection | Left upper sternal border | Innocent pulmonary flow murmur | This murmur is not harsh in character. No symptoms. | This can be accentuated by high cardiac output states such as fever, anaemia, pregnancy, and thyrotoxicosis. |
| Innocent pulmonary branch murmur of infancy | Radiates to axillae and back. No symptoms. | Characteristic time course in which the murmur can appear in the first few days postnatally, and resolves by several months of age. | ||
| Pulmonary valve stenosis (PS) | Variable systolic ejection click. Can radiate to axillae and back. When more than mild PS, there can be increased right ventricular precordial impulse. | Most cases are sporadic. Common with Noonan syndrome. The murmur of PS sometimes appears before the cyanosis, and so can be the first clinical feature recognized in tetralogy of Fallot. | ||
| Atrial septal defect (ASD) | Wide and fixed split 2nd heart sound. Diastolic flow rumble over tricuspid valve and systolic flow murmur over pulmonary valve. Increased right ventricular precordial impulse. | Most ASD is sporadic. There is a strong female predisposition. Holt-Oram syndrome and thrombocytopenia absent radius are both rare, but highly associated with ASD | ||
| Mammary souffle | Often bilateral. Disappears with firm pressure from stethoscope | Occurs during pregnancy and lactation | ||
| Right upper sternal border | Aortic stenosis | Often associated with constant early systolic ejection sound. Radiates to neck. Can be associated with palpable thrill | Common with Turner syndrome, with or without aortic coarctation. Nonsyndromic bicuspid aortic valve commonly recurs in family members. Occasionally rheumatic. | |
| Subaortic stenosis | Also can be maximal at the left upper sternal border. When obstruction is dynamic, this murmur increases with standing and during Valsalva strain, and decreases with recumbent position, squatting, or isometric handgrip exercise. | Occurs in fixed and dynamic forms. The dynamic form is generally in association with hypertrophic cardiomyopathy. | ||
| Left lower sternal border and towards apex | Stills murmur | A very musical, vibratory, low pitched sound, with multiple harmonics. Diminishes in the sitting position. | Can occur at any age, but is extremely common in the preschool and young school age population. | |
| Midback | Innocent pulmonary branch murmur of infancy | See above | See above | |
| Pulmonary branch stenosis | If severe, the right ventricular impulse on the chest wall can be increased in intensity. | Rare as an isolated lesion. Has associations with Williams syndrome and maternal rubella | ||
| Aortic coarctation | Decreased pulse intensity in the lower extremities. Arm-leg systolic BP difference. When obstruction is severe there can be heart failure symptoms in early infancy, Murmur over the back and in the left axilla. | Common with Turner syndrome. | ||
| Holosystolic | Left mid-sternal border | Ventricular septal defect (VSD) | High pitched and loud when the defect is small. Lower pitched and softer when defect is large. Large defects often have diastolic flow rumble (see below). | Defects can occur in several locations in the ventricular septum, most commonly muscular and perimembranous, but also inlet as in atrioventricular septal defect (AVSD) and subarterial. Clinical examination cannot identify location with confidence. |
| Left lower sternal border | Tricuspid valve regurgitation | Low pitched when right ventricular systolic pressure is normal. High pitched in the setting of pulmonary hypertension. Increases with inspiration. | Can occur in association with Ebstein’s anomaly or non-specific tricuspid valve dysplasia. The tricuspid valve may leak in AVSD. | |
| Apex | Mitral regurgitation | High pitched. Can have an associated mid- to late-systolic click when valve prolapses. Augmented when standing. | The mitral valve may leak in AVSD. Can be associated with Marfan syndrome. Other congenital anomalies of the mitral valve are unusual. This is the most common valve affected by rheumatic fever. | |
| Continuous | Left infraclavicular, upper- and left mid-sternal border | Patent ductus arteriosus (PDA) | Bounding pulses (wide pulse pressure) and increased left ventricular impulse when ductus is large. Late systolic accentuation. | Functional closure of the ductus arteriosus is usually complete by 3 days after birth. Premature infants are particularly prone to persistent PDA. |
| Mammary souffle | Pregnancy, lactation. | Usually identifiable in the appropriate context | ||
| Coronary artery fistula | Often no other features. If large, signs and symptoms of heart failure can be present | This is rare | ||
| Midback | Aortic coarctation | Late in the unmodifed natural history of coarctation of the aorta, the murmur becomes continuous due to growth of collateral circulation. | See above | |
| Right upper sternal border | Venous hum | Disappears when recumbent, or with ipsilateral jugular pressure. | Very common in children old enough to sit during examination; can persist into adulthood. | |
| Diastolic decrescendo | Left upper sternal border | Aortic regurgitation (AR) | High pitched, decrescendo. Increases with sudden squatting. Longer when regurgitation is more severe. Associated with bounding pulses (wide pulse pressure) when moderate or severe. | Commonly present with bicuspid aortic valve. Less commonly can be rheumatic. |
| Pulmonary regurgitation (PR) | High pitched when associated with pulmonary hypertension (Graham-Steele murmur). Low pitched and very common in the setting of surgically modified tetralogy of Fallot. | Congenital pulmonary valve disease producing audible PR is very unusual. | ||
| Diastolic rumble | Apex | VSD (large) | VSD large enough to produce a diastolic rumble will often also have increased precordial activity and signs and symptoms of heart failure. A loud P2 can mean pulmonary hypertension | Not impossible to have large muscular or subarterial VSD, but most large VSDs are either perimembranous, outlet malalignment as in double outlet right ventricle, or inlet as in AVSD. |
| Mitral valve disease | Can have opening snap, especially if rheumatic. | Can be congenital or rheumatic. A diastolic rumble can occur without true stenosis if there is severe MR. Rheumatic mitral stenosis is progressive over time. | ||
| AR | The so called Austin Flint murmur is of debatable cause, but usually indicates more than mild AR. | See above | ||
| Left lower sternal border | ASD | Also with systolic murmur (see above), fixed split S2, and increased right ventricular impulse. | See above |
Causes and differential diagnosis
The two primary mechanisms for the production of cardiac murmurs are (i) turbulent blood flow12 and (ii) harmonic vibrations13 of cardiovascular structures. They must be intense enough for the sound to transmit to the stethoscope on the chest wall. The number of conditions associated with heart murmur in children is large. One effective way of dealing with this broad and unwieldy differential diagnosis is first to determine the location at which the murmur is loudest. This narrows the possibilities to a more manageable number (Table 3).
Clinical features associated with heart murmur
History
As Table 3 reveals, the clinical context in which the murmur is identified can be diagnostically very helpful. Therefore, relevant clinical history can provide valuable context in the investigation of heart murmur. Key points include age of onset, current symptoms, such as tachypnoea, cough, respiratory distress, poor feeding, failure to thrive, colour changes, easy fatigue, chest pain, palpitations, dizziness, or syncope. Potentially, there are also clues in the past medical history, so a careful discussion of important medical and surgical conditions, the family history, and a thorough review of systems is always part of a good initial evaluation for paediatric heart murmur.
Physical examination
Diagnostic value in the physical examination is, of course, not limited to murmur description because other auscultatory features and physical findings specific to the condition can point to the diagnosis. A more general examination is also called for, including a quick inspection for the general appearance of distress or well-being, and for syndromic features is a good starting point. Height and weight plotted on a growth chart, and vital signs including right arm and lower extremity blood pressures are obtained. Observation for cyanosis can be supplemented with pulse oximetry. When evaluating a heart murmur, care should be taken to identify the character of the heart sounds, clicks, and gallops that accompany it. Palpation of the precordial impulses and the evaluation of upper and lower extremity pulses and perfusion is mandatory. The physical examination must also include pulmonary auscultation for evidence of fluid retention in the lungs, and palpation of the abdomen for fluid retention in the liver and spleen.
Evaluation of heart murmur
Although prenatal, birth, and family history are important, history taking focuses most heavily on manifestations of potentially important heart disease, including pulmonary overcirculation, heart failure, oxygen desaturation, and myocardial ischaemia. So, the history must identify infants with poor feeding, slow growth, laboured breathing, poor colour, or lethargy. Key historical features in an older child include chest pain, syncope, or shortness of breath with activity, as these may be manifestations of heart disease such as aortic stenosis or hypertrophic cardiomyopathy. The physical examination must identify any finding inconsistent with the innocent murmurs of childhood (see Table 3), such as abnormal second heart sound, palpable thrill, diastolic murmur, gallop, hyperactive precordium, or bounding, diminished, or discrepant peripheral pulse intensity.
The diagnosis of innocent murmur is established for an asymptomatic child with a systolic murmur that the examiner recognizes as one of the innocent murmurs of childhood. This is contingent on a diligent and focused evaluation that identifies no additional historical or physical red flags. The addition of an electrocardiogram or chest X-ray to the clinical assessment usually does not help differentiate an innocent murmur from a pathologic one. However, if these tests are done and are abnormal, then a paediatric cardiology evaluation, usually with an echocardiogram, is warranted.
Even when the primary care physician may be uncertain, the diagnosis of innocent murmur can often be made through a careful history and physical examination performed by an experienced paediatric cardiologist without additional testing. So, from a cost-effectiveness standpoint, referral to an experienced paediatric cardiologist is preferable to direct referral from the paediatrician for an echocardiogram. Moreover, when a minor cardiac defect is responsible for the murmur, a consultation with a paediatric cardiologist has potential advantages over a preliminary echocardiogram as it is an opportunity to provide perspective for the family, and promptly allay anxiety.
There are clinical settings, however, in which the paediatric cardiologist is likely to have a very low threshold for echocardiography to evaluate a murmur. The characteristic auscultatory features of an innocent murmur are difficult to discern in neonates and small infants, who may harbour major structural heart disease with an innocent sounding murmur and minimal or no symptoms. Therefore, paediatric cardiologists should have a high index of suspicion and high utilization of echocardiography for neonates or infants less than 3 months of age with murmur. Older children and adolescents with innocent-sounding murmur and worrisome symptoms (e.g. chest pain or syncope with exercise) is another group where a low threshold for echocardiographic evaluation is prudent.
Management and long-term monitoring
After a diagnosis is made, the physician should clearly explain the findings to the patient and family. If the murmur is innocent, the patient and the family must understand that the murmur does not imply cardiovascular disease of any sort, and that the child is not more likely to have cardiovascular issues later. The child with an innocent murmur should not be restricted from physical activities and does not require endocarditis prophylaxis for dental procedures or other invasive medical procedures. There is no need for cardiology follow-up of innocent murmur. Though most innocent murmurs disappear by late adolescence or early adulthood, the family should be assured that because the heart is normal, we are not watching for the murmur to disappear to declare the child to be in the clear.
Pathologic heart murmurs, when present, require a paediatric cardiology evaluation. The first objective in every case should be to determine the severity of the haemodynamic burden and its associated short- and long-term clinical significance. For those whose murmur is a manifestation of haemodynamically minor disease, the next objective is to arrange a rational program of clinical surveillance. Pathologic murmurs that arise from minor forms of structural heart disease may not require medical or surgical treatment and may only need outpatient cardiology follow-up. Examples are very small left to right shunts (ventricular septal defect, atrial septal defect, or patent ductus arteriosus) and mild valve disease. Though recommendations for follow-up, activity restrictions and endocarditis prophylaxis are disease-specific, most children with structural heart disease lead normal active lives.
If clinical significance is more than minor, then the next objective is to determine relative merits of available medical, catheter-mediated, or surgical options to improve outcome. A growing number of conditions can now be treated with catheter-mediated techniques (pulmonary stenosis, secundum atrial septal defect, and patent ductus arteriosus). Major structural heart diseases often require single or multiple surgical intervention(s) and lifelong cardiology follow-up. Even those successfully managed by surgical or catheter-mediated methods may have residual haemodynamic abnormalities which could benefit from medical management.
New methods for murmur diagnostics
Computer-assisted auscultation
In primary care and outpatient clinical settings, classification of a newly identified cardiac murmur as innocent or pathologic remains imperfect, with approximately 50–70% of asymptomatic children referred for specialist murmur evaluation having no heart disease.14–16 Recent declines in cardiac auscultation skills have been observed among general medicine and paediatric residents, likely promoting a pattern of over-referral. Consequently, novel diagnostic methodologies for the assessment and evaluation of cardiac murmurs are needed. Computer-assisted auscultation (CAA) was the first technologic advancement developed to address this problem. CAA includes separate algorithms for heart sound acquisition, signal processing, and automated analysis for the differentiation of pathologic from innocent murmurs.17,18 Artificial intelligence-assisted auscultation (AIAA) is another form of CAA, in which the automated classification of the murmur as benign or pathogenic employs machine learning algorithms. These algorithms can be trained using a variety of approaches including recognition of specific sequences and classification based on derived features along with a variety of machine learning architectures (e.g. support vector machines, k-nearest neighbour, random forest) or deep neural network-based algorithms. Essential to both analysis pipelines is a phonocardiogram, a high fidelity recording of heart sounds throughout the cardiac cycle. Electronic stethoscopes allow reliable acquisition of the phonocardiogram for murmur analysis and are required for both automated algorithm analysis and telemedicine.
Early algorithms for CAA-based murmur analysis utilized a combination of high pass filters for signal acquisition and phonocardiogram generation, with an electrocardiogram to identify systole and diastole in the recording.19,20 The introduction of Fourier transform-based frequency analysis,21 time-frequency analysis,20,22 and wavelet processing20 further advanced phonocardiogram algorithmic analysis and improved accuracy. However, these algorithms were limited in their application because they lacked the necessary sensitivity and specificity for use in clinical practice. In the modern era, algorithms for signal processing and low-level tasks, like identification of heart sounds and cardiac cycle timing, continue to be used; whereas artificial intelligence (AI) methods are better suited for murmur identification and classification as benign or not, and for the identification of specific pathologies.
Artificial intelligence-assisted auscultation
With the rapidly growing success and integration of AI in modern signal processing and analytics, AIAA is now more popular for murmur classification. Essentially, AIAA is a form of CAA, where machine learning or deep learning-based algorithms are used for murmur classification and diagnosis. In the case of AIAA specifically with deep neural network-based classifiers, the need for advanced signal processing of the phonocardiogram is decreased. The first machine learning-based murmur classification algorithms were integrated into larger pipelines, which included preprocessing components for feature extraction and segmentation of heart sounds. The results of these preprocessing steps were then fed into a neural network, usually a multilayer perceptron backpropagation classifier, for murmur classification, although machine learning-based methods remain popular.23–25 These networks formed the foundation for larger platforms, like eMurmur, which are currently in clinical trials.18,26 AI-based platforms include a streamlined pipeline for clinical integration, requiring only a phonocardiogram input, usually from a digital stethoscope. Following upload of the signal, these platforms have automated algorithms for signal quality assessment, heart rate determination, cardiac cycle segmentation, and importantly, AI-based murmur classification. The first pilot study of AIAA, in a cohort of 106 children, demonstrated sensitivity of 87% and specificity of 100% for the identification of a murmur as innocent or pathologic.18 More recently, Thompson et al., in a virtual clinical trial using 3180 heart sound recordings from 603 outpatients obtained from the Johns Hopkins Cardiac Auscultatory Recording Database, demonstrated sensitivity of 93%, specificity of 81%, and accuracy of 88% for classification of murmurs into one of 19 groups.26 In a recent study on a cohort of 1362 congenital heart disease patients, the AIAA platform reported high accuracy (97% sensitivity, 89% specificity, 96% accuracy) for detection and classification of heart sounds as abnormal compared to expert face-to-face auscultation.27 Remarkably, only 3.6% of patient recordings were misdiagnosed.27 A more recent study demonstrated an area under the receiver operating characteristic curve of 77% for identifying a patent ductus arteriosus using only a phonocardiogram.28 Despite these initial successes, AIAA has so far enjoyed limited clinical acceptance, in large part due to some of its limitations. First, the prediction tasks of different AIAA are heterogeneous, with some algorithms simply classifying a murmur as pathologic or not, whereas others attempt to provide a detailed classification of the murmur (e.g. systolic or diastolic, identification of the potential aetiology, etc.). This limitation is exemplified by the range of model accuracies (between 88% and 96%) in the major studies highlighted above. In addition, other sounds heard on auscultation (e.g. wheezes, crackles, rales), which physicians are trained to identify, impact the accuracy and classification ability of algorithms by adding non-contributory artefacts to the signal on which classification is based. Recently, one system has been developed using AI to identify wheezes and crackles from children and demonstrated superior performance to paediatricians (86.4 vs. 82.2% sensitivity for wheezes).29 However, algorithms to integrate both cardiac and pulmonary auscultation have yet to be developed. Finally, AIAA-based algorithms are not well integrated into the standard clinical pipeline and electronic data management systems, largely influencing their use and acceptance. As highlighted by a recent editorial, AIAA is most likely to make its impact in congenital heart disease as a clinical decision support tool as opposed to a standalone system.30
Implications for telemedicine
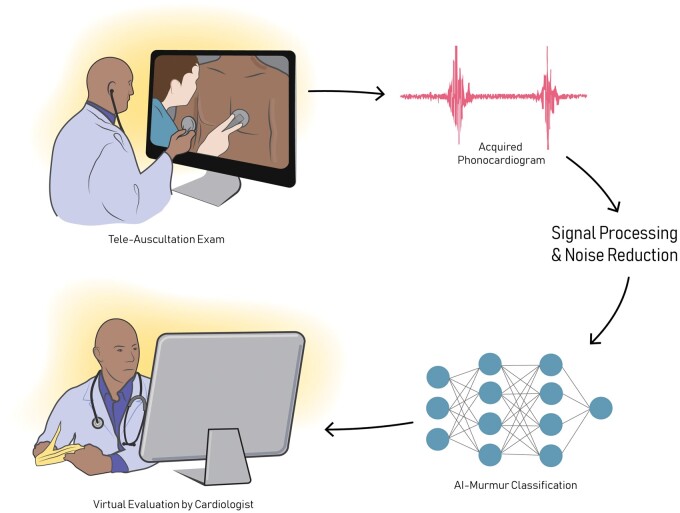
Nonetheless, both the digital stethoscope and AIAA have significant implications for applications in telemedicine. The development of the digital stethoscope has been essential to telemedicine, and more specifically, tele-auscultation, allowing for automated data acquisition and signal processing with limited input by the clinican.17,31 With this technology, a trained cardiologist can listen to and diagnose a murmur by means of a telemedicine exam, which is especially useful in geographically remote underserved areas and other circumstances which limit access to sub-specialists (Figure 1). Furthermore, tele-auscultation and AIAA can reduce variability of interpretation and decrease the burden of over-referral, allowing for more accurate and consistent identification of innocent versus pathologic murmurs. Clinical integration of these technologies utilizes a mixed reality navigation-based infrastructure, which provide detailed instructions for patient self-examinations with a digital stethoscope, combined with real-time feedback by the tele-cardiologist during the visit.32
Figure 1.
Advances in technology have allowed for completely virtual examinations of heart sounds by trained, expert cardiologists. Especially useful in geographically remote underserved areas and other circumstances which limit access to sub-specialists, these telemedicine exams have the potential to revolutionize clinical care. Here, we present a schematic of a virtual tele-medicine exam. During a visit, either video instructions, the provider, or a mobile application can provide instructions for a full auscultation exam using a digital stethoscope. The acquire phonocardiogram is then pre-processed and interpreted by the cardiologist. With deep learning, a first pass through a neural network may provide some preliminary diagnostic utility to the provider to aid in phonocardiogram interpretation.
One significant disadvantage, however, is the need for a digital stethoscope, which generally are quite expensive. While these concerns can be reduced by establishing local offsite care facilities for telemedicine and tele-transmission, new strategies will be required to further enhance healthcare accessibility. Recently, work has begun to develop smart phone-based techniques for acquisition and processing of phonocardiograms. These methods, now in their initial stages, allow for acquisition of low quality heart sound recordings using only a smart phone and have demonstrated feasibility for detection of obvious murmurs.33 Smart phone technology today is far from replacing the digital stethoscope, but its continued development could soon propel it to greater clinical significance. As we progress in the current era of virtual medicine and tele-transmission, it is expected that digital murmur recordings and AIAA will make murmur evaluation more accurate, efficient, and equitably accessible.
Conflict of interest: none declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. McLaren MJ, Lachman AS, Pocock WA, Barlow JB.. Innocent murmurs and third heart sounds in Black schoolchildren. Br Heart J 1980;43:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hurst JW, Staton J, Hubbard D.. Precordial murmurs during pregnancy and lactation. N Engl J Med 1958;259:515–517. [DOI] [PubMed] [Google Scholar]
- 3. Groom D, Boone JA, Jenkins M.. Venous hum in cardiac auscultation. J Am Med Assoc 1955;159:639–641. [DOI] [PubMed] [Google Scholar]
- 4. Miyake T, Yokoyama T.. Evaluation of transient heart murmur resembling pulmonary artery stenosis in term infants by Doppler and M-mode echocardiography. Jpn Circ J 1993;57:77–83. [DOI] [PubMed] [Google Scholar]
- 5. Nelson WP, Hall RJ.. The innocent supraclavicular arterial bruit—utility of shoulder maneuvers in its recognition. N Engl J Med 1968;278:778. [DOI] [PubMed] [Google Scholar]
- 6. Fogel DH. The innocent systolic murmur in children: a clinical study of its incidence and characteristics. Am Heart J 1960;59:844–855. [DOI] [PubMed] [Google Scholar]
- 7. Harvey WP. Heart sounds and murmurs. Circulation 1964;30:262–271. [DOI] [PubMed] [Google Scholar]
- 8. Danford DA, Martin AB, Fletcher SE, Gumbiner CH.. Echocardiographic yield in children when innocent murmur seems likely but doubts linger. Pediatr Cardiol 2002;23:410–414. [DOI] [PubMed] [Google Scholar]
- 9. Danford DA. Effective use of the consultant, laboratory testing, and echocardiography for the pediatric patient with heart murmur. Pediatr Ann 2000;29:482–488. [DOI] [PubMed] [Google Scholar]
- 10. Danford DA. Cost‐effectiveness of echocardiography for evaluation of children with murmurs. Echocardiography 1995;12:153–162. [DOI] [PubMed] [Google Scholar]
- 11. Biancaniello T. Innocent murmurs. Circulation 2005;111:e20–e22. [DOI] [PubMed] [Google Scholar]
- 12. Sabbah HN, Stein PD.. Turbulent blood flow in humans: its primary role in the production of ejection murmurs. Circ Res 1976;38:513–525. [DOI] [PubMed] [Google Scholar]
- 13. Wennevold A. The origin of the innocent “vibratory” murmur studied with intracardiac phonocardiography. Acta Med Scand 1967;181:1–5. [DOI] [PubMed] [Google Scholar]
- 14. Watrous RL, Thompson WR, Ackerman SJ.. The impact of computer-assisted auscultation on physician referrals of asymptomatic patients with heart murmurs. Clin Cardiol 2008;31:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haney I, Ipp M, Feldman W, McCrindle BW.. Accuracy of clinical assessment of heart murmurs by office based (general practice) paediatricians. Arch Dis Child 1999;81:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Etchells E, Bell C, Robb K.. Does this patient have an abnormal systolic murmur? JAMA 1997;277:564–571. [PubMed] [Google Scholar]
- 17. Leng S, San Tan R, Chai KTC, Wang C, Ghista D, Zhong L.. The electronic stethoscope. Biomed Eng Online 2015;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai LSW, Redington AN, Reinisch AJ, Unterberger MJ, Schriefl AJ.. Computerized automatic diagnosis of innocent and pathologic murmurs in pediatrics: a pilot study. Congenit Heart Dis 2016;11:386–395. [DOI] [PubMed] [Google Scholar]
- 19. Ninova PP, Dascalov IK, Dimitrova MI.. Automated phonocardiographic screening for heart disease in children. Cardiology 1978;63:5–13. [DOI] [PubMed] [Google Scholar]
- 20. Hayek CS, Thompson WR, Tuchinda C, Wojcik RA, Lombardo JS.. Wavelet processing of systolic murmurs to assist with clinical diagnosis of heart disease. Biomed Instrum Technol 2003;37:263–270. [DOI] [PubMed] [Google Scholar]
- 21. Lehner RJ, Rangayyan RM.. A three-channel microcomputer system for segmentation and characterization of the phonocardiogram. IEEE Trans Biomed Eng 1987;485–489. [DOI] [PubMed] [Google Scholar]
- 22. Bentley EMP, McDonnell E. Wavelet analysis of cardiovascular signals. In: Signal Processing VII, Theories and Applications. Proceedings of EUSIPCO-94 Seventh European Siganl Processing Conference. 1994. pp. 78–81.
- 23. Sepehri AA, Gharehbaghi A, Dutoit T, Kocharian A, Kiani A. A. novel method for pediatric heart sound segmentation without using the ECG. Comput Methods Programs Biomed 2010;99:43–48. [DOI] [PubMed] [Google Scholar]
- 24. Gupta CN, Palaniappan R, Swaminathan S, Krishnan SM.. Neural network classification of homomorphic segmented heart sounds. Appl Soft Comput 2007;7:286–297. [Google Scholar]
- 25. Ahmad MS, Mir J, Ullah MO, Shahid MLUR, Syed MA.. An efficient heart murmur recognition and cardiovascular disorders classification system. Australas Phys Eng Sci Med 2019;42:733–743. [DOI] [PubMed] [Google Scholar]
- 26. Thompson WR, Reinisch AJ, Unterberger MJ, Schriefl AJ.. Artificial intelligence-assisted auscultation of heart murmurs: validation by virtual clinical trial. Pediatr Cardiol 2019;40:623–629. [DOI] [PubMed] [Google Scholar]
- 27. Lv J, Dong B, Lei H, Shi G, Wang H, Zhu F, Wen C, Zhang Q, Fu L, Gu X.. Artificial intelligence-assisted auscultation in detecting congenital heart disease. Eur Heart J Digit Heal 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gómez-Quintana S, Schwarz CE, Shelevytsky I, Shelevytska V, Semenova O, Factor A, Popovici E, Temko A.. A framework for AI-assisted detection of patent ductus arteriosus from neonatal phonocardiogram. Healthcare 2021;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Wang H-S, Zhou H-Y, Dong B, Zhang L, Zhang F, Liu S-J, Wu Y-F, Yuan S-H, Tang M-Y.. Real-world verification of artificial intelligence algorithm-assisted auscultation of breath sounds in children. Front Pediatr 2021;9:627337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ou Y. Can artificial intelligence-assisted auscultation become the Heimdallr for diagnosing congenital heart disease? Eur Heart J Digit Health 2021;2:117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakhe A, Sodhi I, Warrier J, Sinha V.. Development of digital stethoscope for telemedicine. J Med Eng Technol 2016;40:20–24. [DOI] [PubMed] [Google Scholar]
- 32. Hori K, Uchida Y, Kan T, Minami M, Naito C, Kuroda T, Takahashi H, Ando M, Kawamura T, Kume N. Tele-auscultation support system with mixed reality navigation. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2013. pp. 4646–4649. [DOI] [PubMed]
- 33. Kang S-H, Joe B, Yoon Y, Cho G-Y, Shin I, Suh J-W.. Cardiac auscultation using smartphones: pilot study. JMIR mHealth uHealth 2018;6:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.