Abstract
Aim
Atrial fibrillation (AF) management guidelines advise using risk tools to optimize AF treatment. This study aims to develop a dynamic and clinically applicable digital device to assess stroke and bleeding risk, and to facilitate outcome improvements in AF patients. The device will provide tailored treatment recommendations according to easily attainable individual patient data.
Methods and Results
This Universal Clinician Device (UCD) was created using the GARFIELD-AF registry using a split sample approach. The GARFIELD-AF risk tool was adapted with two modifications. First, predictors with ≥1000 missing data points were separated, allowing expected risks estimation. Second, recommendations for modifiable risk factors and associated 2-year outcome estimates were incorporated. Outcomes of interest were all-cause mortality, non-haemorrhagic stroke/systemic embolism (SE), and major bleeding. All patients were randomized to a derivation (n = 34853) and validation cohort (n = 17165). In the derivation cohort, predictors were identified using least absolute shrinkage and selection operator regression. Cox models were fitted with the selected parameters. The UCD demonstrated superior predictive power compared with CHA2DS2VASc for all-cause mortality [0.75(0.75–0.76) vs. 0.71(0.70–0.72)] and non-haemorrhagic stroke/SE [0.68(0.66–0.70) vs. 0.65(0.63–0.67)], and with HAS-BLED for major bleeding [0.69(0.67–0.71) vs. 0.64(0.62–0.65)]. Universal Clinician Device recommendations reduced all-cause mortality (8.45–5.42%) and non-haemorrhagic stroke/SE (2.58–1.50%). Patients with concomitant diabetes and chronic kidney disease benefitted further, reducing mortality risk from 13.15% to 8.67%. One-third of patients with a CHA2DS2VASc score of >1 had the lowest risk of stroke.
Conclusion
The UCD simultaneously predicts mortality, stroke, and bleeding risk in patients using easily attainable individual clinical data and guideline-based optimized treatment plans.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier for GARFIELD-AF: NCT01090362
Keywords: Atrial fibrillation, Universal Clinician Device, Management guidelines, Diabetes, Personalized medicine, Risk prediction
Graphical Abstract
Graphical Abstract.
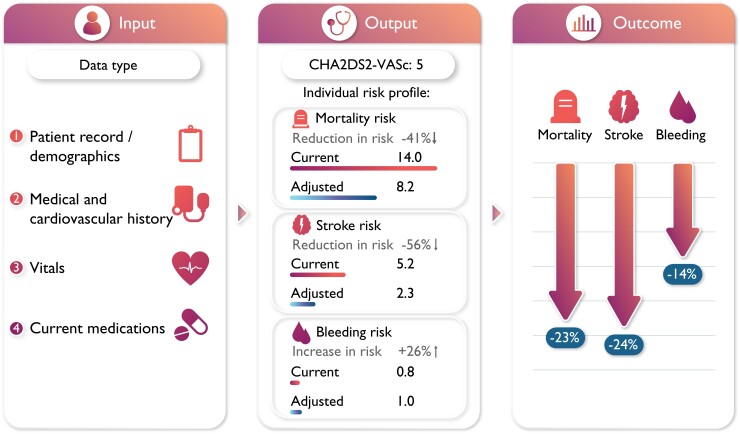
The UCD uses patient data, clinical and current treatment characteristics to provide an individual risk assessment of the current risk and the risk with optimal therapy. Applying this device in clinical practice provides significant improvement potential vs. current practice.
Introduction
Atrial fibrillation (AF) is one of the most common forms of sustained cardiac arrhythmia worldwide, impacting over 37 million people.1 Atrial fibrillation is most notably connected with an elevated risk of ischaemic stroke, estimated to affect 5–7% of AF patients each year2 with rates varying substantially according to the patient demographic and clinical background.3,4 Thrombo-prophylaxis for stroke prevention is primarily achieved via oral anticoagulant (OAC) therapy, encompassing both Vitamin K antagonists (VKAs)5,6 and non-vitamin K antagonist oral anticoagulants (NOACs).7–10
Current AF-management guidelines advise the use of risk scores to stratify stroke and bleeding risk according to individual characteristics, to aid appropriate therapy choice.11 The CHA2DS2VASc score, although widely utilized, has shown imprecision in patients both with a high stroke risk or at a lower stroke risk.11 Recently, the GARFIELD-AF risk calculator advanced risk stratification substantially by providing an integrated tool that simultaneously assesses the risk of mortality, stroke, and bleeding in AF patients.12,13 As indicated by the ESC 2020 AF-management guidelines, the GARFIELD-AF risk tool was predictively superior to both the CHA2DS2VASc and HAS-BLED score.12–14 However, it is suggested that risk scores should balance simplicity and practicality against precision, to optimize risk stratification.
This study aimed to develop a simple, dynamic, and translatable clinician assistant device to assess stroke and bleed risk and provide tailored treatment recommendations according to easily attainable individual patient data.
Methods
Universal Clinician Device design
The Universal Clinician Device (UCD) (The UCD (OptiCorTM) is a proprietary and protected tool of tenac.io GmbH) was designed by assessing clinically accessible predictors for the outcomes of AF, all-cause mortality, non-haemorrhagic stroke/systemic embolism (SE), and major bleeding, within 2 years of follow-up in GARFIELD-AF (Table 1). Predictors associated with each outcome were incorporated into the risk device. The design of the UCD included examining the impact of adjustable characteristics associated with adverse AF outcomes: heart rate, blood pressure, BMI, smoking status, and OAC use. To account for the most recent treatment options, this UCD also incorporated the impact of sodium–glucose co-transporter 2 (SGLT2) inhibitor/glucagon-like peptide 1 receptor antagonists (GLP-1RA) with proven cardiovascular (CV) benefits used in AF patients with concomitant diabetes mellitus Type 2 and ≥1 diagnosis of established CV disease [heart failure, coronary artery disease, history of stroke, chronic kidney disease (CKD), peripheral artery disease] and/or ≥2 risk factors for CV disease (hypertension, smoking status, BMI >30, and age ≥75) based on the result of recent randomized controlled trials.15 Under the assumption that these two classes of diabetes treatment do not interact with other factors in the models, the risks of death and stroke decreased by 13% and 6%, respectively. Furthermore, no change in bleeding risk was recorded following the addition of either treatment. Adjustable characteristics associated with achievable risk reduction were incorporated into the UCD evidence-based treatment recommendations.
Table 1.
Potential predictiors considered for each of the three Universal Clinician Device models
| Category | Predictors |
|---|---|
| Demographic characteristics | Sex, age, race/ethnicity |
| Medical and CV history | Hypertension, diabetes, moderate-to-severe CKD, history of bleeding, CHF, ACS, carotid occlusive disease, VTE, vascular disease, prior stroke, prior TIA, prior SE, hypercholesterolemia, cirrhosis, hyperthyroidism, hypothyroidism, dementia |
| Lifestyle factors | Current smoker, heavy alcohol consumption |
| Vital signs | Weight (kg), pulse (b.p.m.), SBP (mmHg), DBP (mmHg) |
| Atrial fibrillation | New-onset AF, permanent AF, persistent AF, paroxysmal AF |
| Treatment at diagnosis | NOAC, VKA, AP |
ACS, acute coronary syndrome; AF, atrial fibrillation; AP, antiplatelet; CHF, chronic heart failure; CKD, chronic kidney disease; DBP, dyastolic blood pressure; NOAC, non-oral anticoagulants; SBP, systolic blood pressure; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Effective risk discrimination at 2 years of follow-up was compared between the UCD models and the CHA2DS2VASc (all-cause mortality and stroke) and HAS-BLED (major bleeding) risk models by calculating and comparing their c-statistics (95% CIs). Furthermore, the predictive power of the UCD was examined in comparison to the performance of the CHA2DS2VASc score for stroke occurrence in the 1st year.
The UCD was applied to the GARFIELD-AF population, and the results were compared with the observed real-world event rates for two scenarios: first, using the CHA2DS2VASc score to determine the need for anticoagulation therapy and second, using the UCD for this purpose. For specific high-risk patient profiles, estimated event rates were compared with no treatment.
Registry design and population
The UCD is a clinical device developed for the individualised risk prediction of mortality, stroke, and bleeding in AF patients and for providing individual, specific recommendations for treatment optimization. It was developed using patients who were recruited into cohorts 1–5 of the GARFIELD-AF registry between March 2010 and August 2016 as well as the most recent AHA/ACC guidelines for the management of patients with AF and CV risk factors. Data were extracted from the registry database on 30 June 2019. The design of GARFIELD-AF has been published previously.16,17 Briefly, eligible patients (≥18 years) must have had a recent diagnosis of new onset, non-valvular AF (within 6 weeks prior to enrolment), as well as one additional stroke risk factor. Participating sites were computationally selected at random from a list of representative care settings in each of the 35 participating countries.
Data for demographic, clinical, OAC (NOACs, VKAs), and antiplatelet (AP) therapy, as well as components of the CHA2DS2VASc18 and HAS-BLED19 risk stratification scores, were collected by electronic case report form (eCRF). Follow-up was carried out over 2 years, and data were collected at 4-month intervals. An audit and quality control programme was ensured by the coordinating centre [The Thrombosis Research Institute (TRI), London, UK] by examining data for completeness and accuracy. In accordance with the registry protocol, 20% of all eCRFs were monitored against source documentation. Events that occurred after the 2 years of follow-up were censored. Patients with unavailable follow-up information were excluded from the analyses.
Ethics statement
Independent ethics committee and hospital-based institutional review board approvals were obtained. The registry is conducted in accordance with the principles of the Declaration of Helsinki, local regulatory requirements, and the International Conference on Harmonization–Good Pharmacoepidemiological and Clinical Practice guidelines. Written informed consent was obtained from all study participants, and their confidentiality and anonymity were maintained throughout.
Statistical modelling
The GARFIELD-AF risk models had been previously developed12,13 and updated.20 For the UCD, the factors of the GARFIELD-AF process were replicated with two important differences while keeping the validity of the algorithm intact. Firstly, patients were not excluded due to missing information. For predictors that contained missing information for ≥1000 study patients, a separate category (i.e. unknown) was created to estimate the effect in patients with unavailable information. This allows the models to provide estimates of the patients’ expected risks, despite unavailable characteristic values. The covariates for which the additional ‘unknown’ category was included were ethnicity, BMI, moderate to severe CKD, diastolic blood pressure (DBP), pulse, and smoking status. The remaining predictors, containing only a small missingness proportion, were imputed using single imputation by fully conditional specification methods. The models were refit with these new variables for missing data, and coefficients from the new models were applied in the device. Single imputation was utilized as previously, with 52 000 patients, single and multiple imputation provided comparable values in this population when discussing the point estimate.
Secondly, as previously stated, the modelled risk for death and stroke were further reduced by 13% and 6%, respectively, if the use of SGLT2 or GLP-1RA with proven CV benefit is selected in the tool for diabetic Type 2 patients with at least 1 additional CV disease or ≥2 CV risk factors. Average estimated outcome probabilities (%) within 2 years follow-up according to the UCD recommendations were calculated by inputting specific characteristics (e.g. current smoking status combined with a DBP of ≥110 mmHg) and calculating the estimated risk according to other measured characteristics.
For the derivation and validation of the UCD, all patients were randomly divided into derivation cohort and validation cohort with a theoretical ratio of 2:1. A full list of potential predictors included within each of these categories can be found in Table 1. For each outcome, the predictors were identified using the least absolute shrinkage and selection operator regression in the derivation cohort. A Cox model was fitted with the selected parameters. Thirty-fold cross-validation was applied during the modelling process. Both a Kolmogorov-type supremum statistical test and a graphical examination of the Schoenfeld residuals were used to assess the Cox model proportional hazards assumption. In both the derivation and validation cohorts, C-indices and calibration curves were used to assess the accuracy of the prediction model compared to the observed results. To compare results with CHA2DS2VASc and HAS-BLED scores, c-indices for the scores and for models that refit the components of these scores were generated.
Risk reductions were calculated for each outcome by applying specific recommendations in the whole GARFIELD-AF population. More specifically, two sets of models were generated and compared with the observed event rates in the clinical practice, i.e. in the non-interventional registry. Firstly, in Scenario 1, patients were assigned to all applicable treatments as per current guidelines whereby decisions regarding initiation of oral anticoagulation therapy were defined at a CHA2DS2VASc score >1 in males and >2 in females.21 Second, in Scenario 2, all applicable treatment recommendations were applied as in Scenario 1, except decisions regarding oral anticoagulation were defined by a 2-year stroke risk of >1% as per the UCD.
Results
Baseline characteristics
Of the 52 057 patients enrolled into the GARFIELD-AF registry, 38 were omitted due to unavailable follow-up information. Of eligible patients, 44.2% were female, the median age was 71 years [interquartile range (IQR): 63–78], and the median BMI was 26.9 kg/m (IQR: 23.9–30.7 kg/m2). Most of the cohort was comprised of Caucasian (63.1%) or Asian (28.1%) patients. Full details of baseline characteristics are displayed in Table 2. Missing data were more frequent in data obtained during physical examination including BMI (22%), blood pressure (6.4%), and heart rate (7.3%). The association between these variables suggests that this missingness was not random, and therefore, including missing data as a predictor was deemed of value. The percentage of missing values for all variables is provided in Table 3. For the split sample analysis for model development, 34 853 patients were randomly allocated to the derivation set and 17 165 were allocated to the validation set. Baseline characteristics were comparable between the two sample groups (see Supplementary material online, Table S1).
Table 2.
Baseline characteristics for the full study population according to 2 years outcomes (mortality, non-hemhorragic stroke/SE, and major bleeding)
| Variable, n (%) | All patients (n = 52 018) | Outcomes (within 2 years) | ||
|---|---|---|---|---|
| All-cause mortality (n = 3708) | Non-haemorrhagic stroke/SE (n = 966) | Major bleeding (n = 942) | ||
| Female, n (%) | 22 986 (44.2) | 1687 (45.5) | 483 (50.0) | 447 (47.5) |
| Age, median (Q1; Q3) | 71.0 (63.0; 78.0) | 78.0 (71.0; 84.0) | 75.0 (68.0; 81.0) | 76.0 (69.0; 82.0) |
| Age, years, n (%) | ||||
| ȃ<65 | 15691 (30.2) | 459 (12.4) | 167 (17.3) | 131 (13.9) |
| ȃ65–69 | 8015 (15.4) | 360 (9.7) | 119 (12.3) | 110 (11.7) |
| ȃ70–74 | 8931 (17.2) | 535 (14.4) | 175 (18.1) | 163 (17.3) |
| ȃ≥75 | 19381 (37.3) | 2354 (63.5) | 505 (52.3) | 538 (57.1) |
| Race/ethnicity, n (%) | ||||
| ȃCaucasian | 31997 (63.1) | 2509 (69.5) | 604 (64.2) | 650 (71.6) |
| ȃHispanic/Latino | 3393 (6.7) | 310 (8.6) | 74 (7.9) | 56 (6.2) |
| ȃAsian | 14276 (28.1) | 685 (19.0) | 230 (24.4) | 182 (20.0) |
| ȃBlack/mixed/other | 1071 (2.1) | 107 (3.0) | 33 (3.5) | 20 (2.2) |
| BMI, kg/m2 median (Q1; Q3) | 26.9 (23.9; 30.7) | 26.0 (22.8; 30.1) | 26.7 (23.7; 30.1) | 26.5 (23.3; 30.7) |
| SBP, mmHg, median (Q1; Q3) | 130.0 (120.0; 145.0) | 130.0 (119.0; 143.0) | 135.0 (120.0; 150.0) | 134.0 (120.0; 145.0) |
| DBP, mmHg, median (Q1; Q3) | 80.0 (70.0; 88.0) | 79.0 (70.0; 85.0) | 80.0 (70.0; 90.0) | 80.0 (70.0; 88.0) |
| Pulse, b.p.m., median (Q1; Q3) | 84.0 (70.0; 105.0) | 88.0 (73.0; 110.0) | 86.0 (72.0; 108.0) | 87.0 (72.0; 110.0) |
| Type of AF, n (%) | ||||
| ȃPermanent | 6630 (12.7) | 629 (17.0) | 138 (14.3) | 112 (11.9) |
| ȃPersistent | 7753 (14.9) | 506 (13.6) | 147 (15.2) | 124 (13.2) |
| ȃParoxysmal | 14304 (27.5) | 734 (19.8) | 225 (23.3) | 228 (24.2) |
| ȃNew onset (unclassified) | 23325 (44.8) | 1839 (49.6) | 456 (47.2) | 478 (50.7) |
| Care setting specialty at diagnosis, n (%) | ||||
| ȃInternal medicine/neurology/geriatrics | 10443 (20.1) | 977 (26.3) | 276 (28.6) | 235 (24.9) |
| ȃCardiology | 34173 (65.7) | 2230 (60.1) | 547 (56.6) | 549 (58.3) |
| ȃPrimary care/general practice | 7396 (14.2) | 501 (13.5) | 143 (14.8) | 158 (16.8) |
| Care setting location at diagnosis, n (%) | ||||
| ȃHospital | 30335 (58.3) | 2359 (63.6) | 606 (62.7) | 530 (56.3) |
| ȃOffice/anticoagulation clinic/thrombosis centre | 15918 (30.6) | 948 (25.6) | 254 (26.3) | 258 (27.4) |
| ȃEmergency room | 5758 (11.1) | 401 (10.8) | 106 (11.0) | 154 (16.3) |
| Medical history, n (%) | ||||
| ȃHeart failure | 11739 (22.6) | 1470 (39.6) | 277 (28.7) | 220 (23.4) |
| ȃAcute coronary syndromes | 5533 (10.7) | 655 (17.8) | 156 (16.2) | 158 (16.8) |
| ȃVascular disease | 12815 (24.8) | 1369 (37.3) | 313 (32.6) | 301 (32.2) |
| ȃCarotid occlusive disease | 1538 (3.0) | 158 (4.3) | 37 (3.9) | 54 (5.8) |
| ȃVTE | 1355 (2.6) | 148 (4.0) | 34 (3.5) | 29 (3.1) |
| ȃPrior stroke/TIA/SE | 5839 (11.3) | 596 (16.2) | 220 (22.9) | 152 (16.2) |
| ȃHistory of bleeding | 1315 (2.5) | 202 (5.5) | 43 (4.5) | 55 (5.9) |
| ȃHypertension | 39604 (76.4) | 2858 (77.3) | 788 (81.7) | 746 (79.5) |
| ȃHypercholesterolaemia | 20955 (41.6) | 1428 (40.1) | 428 (46.3) | 412 (44.5) |
| ȃDiabetes | 11542 (22.2) | 1022 (27.6) | 257 (26.6) | 257 (27.3) |
| ȃCirrhosis | 293 (0.6) | 48 (1.3) | 4 (0.4) | 9 (1.0) |
| ȃModerate to severe CKD | 5354 (10.7) | 833 (23.5) | 172 (18.5) | 194 (21.2) |
| ȃDementia | 764 (1.5) | 187 (5.1) | 40 (4.2) | 15 (1.6) |
| Heavy alcohol use, n (%) | 1028 (2.3) | 75 (2.4) | 24 (2.9) | 20 (2.6) |
| Current smoker, n (%) | 5202 (11.0) | 337 (10.0) | 109 (12.1) | 84 (9.8) |
| Treatment, n (%) | ||||
| ȃNOAC ± AP | 14112 (27.5) | 838 (23.0) | 205 (21.6) | 230 (25.0) |
| ȃVKA ± AP | 20183 (39.3) | 1463 (40.1) | 353 (37.2) | 474 (51.5) |
| ȃAP only | 10761 (21.0) | 871 (23.9) | 271 (28.6) | 131 (14.2) |
| ȃNone | 6240 (12.2) | 474 (13.0) | 120 (12.6) | 86 (9.3) |
| AP treatment, n (%) | 18103 (35.3) | 1503 (41.2) | 420 (44.3) | 348 (37.8) |
| CHA2DS2VASc score, median (Q1; Q3) | 3.0 (2.0; 4.0) | 4.0 (3.0; 5.0) | 4.0 (3.0; 5.0) | 4.0 (3.0; 5.0) |
| HAS-BLED score, median (Q1; Q3)a | 1.0 (1.0; 2.0) | 2.0 (1.0; 2.0) | 2.0 (1.0; 2.0) | 2.0 (1.0; 2.0) |
AF, atrial fibrillation; AP, antiplatelet; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; NOAC, non-oral anticoagulant; VKA, vitamin K antagonist; SE, systemic embolism; SBP, systolic blood pressure; TIA, transient ischaemic attack; VTE, venous thromboembolism.
The risk factor ‘Labile INRs’ is not included in the HAS-BLED score as it is not collected at baseline. As a result, the maximum HAS-BLED score at baseline is 8 points (not 9).
Table 3.
Missing data distribution in the GARFIELD-AF study population
| Variable | GARFIELD-AF (n = 52 018) N (%) |
|---|---|
| Sex | 1 (0.0) |
| Age | 0 (0.0) |
| Race/ethnicity | 1281 (2.5) |
| Type of AF | 6 (0.01) |
| Care setting speciality in diagnosis | 6 (0.01) |
| Care setting location in diagnosis | 7 (0.01) |
| Heart failure | 8 (0.02) |
| Acute coronary syndromes | 217 (0.4) |
| Vascular disease | 389 (0.7) |
| Carotid occlusive disease | 733 (1.4) |
| VTE | 311 (0.6) |
| Prior stroke/TIA/SE | 400 (0.8) |
| History of bleeding | 235 (0.4) |
| Hypertension | 151 (0.3) |
| Hypercholesterolaemia | 1643 (3.2) |
| Diabetes | 8 (0.02) |
| Cirrhosis | 772 (1.5) |
| Moderate to severe CKD | 1833 (3.5) |
| Dementia | 288 (0.5) |
| Alcohol use | 8015 (15.4) |
| Smoking status | 4608 (8.9) |
| Antithrombotic treatment | 722 (1.4) |
| BMI | 11 443 (22.0) |
| SBP | 3312 (6.4) |
| DBP | 3312 (6.4) |
| Pulse | 3789 (7.3) |
AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; SE, systemic embolism; TIA, transient ischaemic attack; VTE, venous thromboembolism.
Treatment patterns and clinical outcomes
NOACs (±AP), VKAs (±AP), or AP alone were initiated for 87.8% of patients. Overall, VKAs were the most prescribed treatment (39.3%), followed by NOACs (27.5%) (Table 2 and Supplementary material online, Table S1).
Within the full GARFIELD-AF population, all-cause mortality was the primary adverse outcome with 3708 events over 2 years of follow-up [3.83 (3.71–3.95), per 100 person years) (Table 4). A total of 966 non-haemorrhagic stroke/SE events [1.01 (0.94–1.07), per 100 person years] and 942 major bleeds [0.98 (0.92–1.05), per 100 person years] were recorded. There was negligible difference observed when comparing event rates between the derivation and validation sets (Table 4). Of specific high-risk patients, 1284 had baseline comorbid diabetes and moderate CKD: 202 (15.7%) of these patients died, 51 (4.0%) experienced a non-haemorrhagic stroke/SE, and 42 (3.3%) experienced a major bleed within 2 years follow-up. A total of 1165 patients had a combination of diabetes, a BMI of ≥30 kg/m2, and were aged ≥75 years: 173 (14.8%) died, 35 (3.0%) experienced a non-haemorrhagic stroke/SE, and 45 (3.9%) experienced a major bleed within 2 years follow-up.
Table 4.
Event rates (per 100 person years) at 2 years of follow-up by sampling group
| Study population Outcomes |
Events | Rate (95% CI) |
|---|---|---|
| Full GARFIELD-AF population (n = 52 108) | ||
| ȃAll-cause mortality | 3708 | 3.83 (3.83–3.95) |
| ȃNon-haemorrhagic stroke/SE | 966 | 1.01 (0.94–1.07) |
| ȃMajor bleeding | 942 | 0.98 (0.92–1.05) |
| Derivation set (n = 34853) | ||
| ȃAll-cause mortality | 2453 | 3.78 (3.63–3.93) |
| ȃNon-haemorrhagic stroke/SE | 636 | 0.99 (0.91–1.07) |
| ȃMajor bleeding | 611 | 0.95 (0.88–1.03) |
| Validation set (n = 17165) | ||
| ȃAll-cause mortality | 1255 | 3.93 (3.72–4.15) |
| ȃNon-haemorrhagic stroke/SE | 330 | 1.04 (0.94–1.16) |
| ȃMajor bleeding | 331 | 1.05 (0.94–1.17) |
Development of the UCD risk models
The following characteristics were identified as important predictors for each of the UCD models: (i) all-cause mortality: age, female sex, ethnicity, BMI, DBP, pulse, OAC treatment, vascular disease, CHF, moderate-to-severe CKD, diabetes, dementia, prior stroke, history of bleeding, smoking status; (ii) non-haemorrhagic stroke/SE: Age, DBP, OAC treatment, CHF, moderate-to-severe CKD, diabetes, prior stroke, smoking status; and (iii) major bleeding: age, OAC treatment, AP treatment, pulse, moderate-to-severe CKD, history of bleeding. Forest plots indicating the resulting hazard ratio for each component of the UCD are shown in Supplementary material online, Figures S1–S3, as calculated using the derivation set.
Performance of UCD risk models
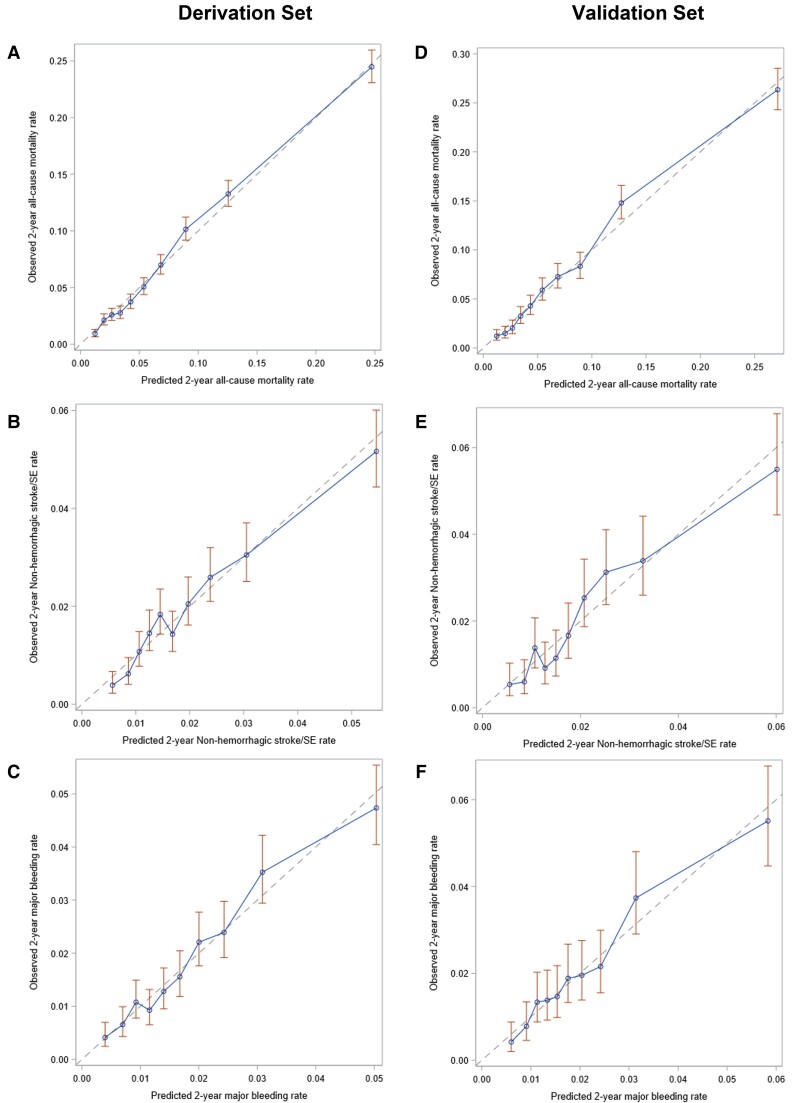
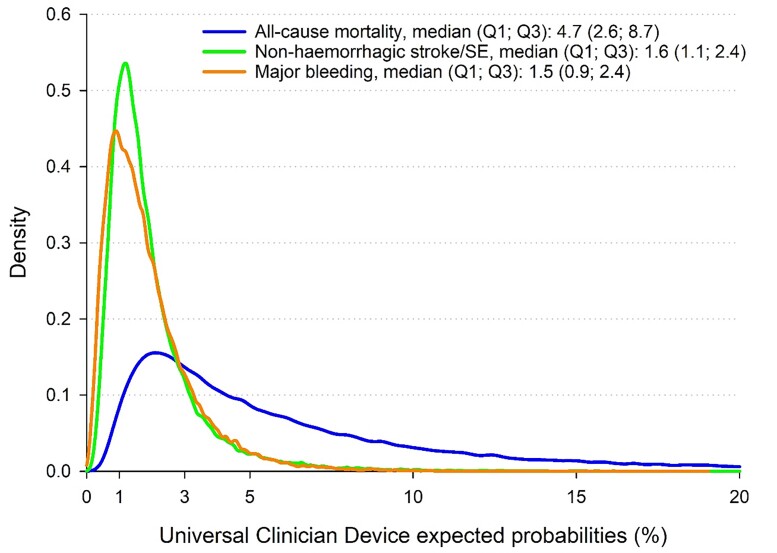
Within the derivation set, close calibration was indicated between the observed and the predicted risk of all-cause mortality (Figure 1A) and major bleeding (Figure 1C). A lower but good calibration was shown for non-haemorrhagic stroke/SE (Figure 1B), with a slight overestimation of patients at very high risk. Comparable results were calculated for the validation set, as close calibration was indicated between the observed and predicted outcomes (Figure 1 D–F). The median percentage risk (Q1; Q3) of all-cause mortality, non-haemorrhagic stroke/SE, and major bleeding were 4.7 (2.6–8.7), 1.6 (1.1–2.4), and 1.5 (0.9–2.4), respectively (Figure 2).
Figure 1.
Calibration of the Universal Clinician Device withing the derivation set for (A) all-cause mortality, (B) non-haemorrhagic stroke/systemic embolism, and (C) major bleeding, and within the vadlidation set for (A) all-cause mortality, (B) non-haemorrhagic stroke/systemic embolism, and (C) major bleeding at 2 years of follow-up in the GARFIELD-AF population. The dashed line represents the predicted risk over 2 years. The dots represent the observed rate of outcomes over 2 years by decile.
Figure 2.
Density function of the Universal Clinician Device risk models for all-cause mortality, non-haemorrhagic stroke/systemic embolism, and major bleeding at 2 years in the full GARFIELD-AF population (not imputed). The X-axis has been truncated at 20%.
Comparison of the UCD new innovative models and the CHA2DS2VASc/HAS-BLED scores
Within the derivation sample set, the UCD demonstrated superior discrimination at 2 years of follow-up for all-cause mortality [0.75 (0.74–0.76) vs. 0.66 (0.65–0.67)] and non-haemorrhagic stroke/SE [0.68 (0.66–0.70) vs. 0.64 (0.62–0.66)] compared with the CHA2DS2VASc model. Similarly, the UCD showed superior discrimination than the HAS-BLED model for major bleeding [0.69 (0.67–0.71) vs. 0.61 (0.59–0.63)]. These results persisted having refit the CHA2DS2VASc and HAS-BLED models for better discrimination (Table 5). Comparable results were indicated for the validation sample set: all-cause mortality [0.75 (0.74–0.77) vs. 0.66 (0.65–0.67)], non-haemorrhagic stroke/SE [0.68 (0.66–0.71) vs. 0.64 (0.61–0.67)], and major bleeding [0.68 (0.66–0.71) vs. 0.61 (0.59–0.64)].
Table 5.
C-index with 95% confidence intervals for the UCD models vs. CHA2DS2VASc (all-cause mortality and stroke/SE) or HAS-BLED (major bleeding) at 2 years of follow-up in the GARFIELD-AF population and selected subgroup
| Population outcome |
Derivation set | Validation set | ||
|---|---|---|---|---|
| UCD risk models | CHA2DS2VASc/HAS-BLED score | UCD risk models | CHA2DS2VASc/HAS-BLED score | |
| Overall | ||||
| ȃAll-cause mortality | 0.75 (0.75–0.76) | 0.66 (0.65–0.67) | 0.75 (0.74–0.77) | 0.66 (0.65–0.67) |
| ȃNon-haemorrhagic stroke/SE | 0.68 (0.66–0.70) | 0.64 (0.62–0.66) | 0.68 (0.66–0.71) | 0.64 (0.61–0.67) |
| ȃMajor bleeding | 0.69 (0.67–0.71) | 0.61 (0.59–0.63) | 0.68 (0.66–0.71) | 0.61 (0.59–0.64) |
| OAC at baseline | ||||
| ȃAll-cause mortality | 0.74 (0.73–0.75) | 0.65 (0.64–0.66) | 0.74 (0.73–0.75) | 0.65 (0.63–0.67) |
| ȃNon-haemorrhagic stroke/SE | 0.68 (0.65–0.70) | 0.64 (0.61–0.66) | 0.68 (0.65–0.72) | 0.66 (0.63–0.70) |
| ȃMajor bleeding | 0.68 (0.66–0.71) | 0.63 (0.60–0.65) | 0.68 (0.64–0.71) | 0.62 (0.59–0.65) |
| No OAC at baseline | ||||
| ȃAll-cause mortality | 0.77 (0.76–0.78) | 0.68 (0.67–0.70) | 0.77 (0.75–0.79) | 0.68 (0.66–0.71) |
| ȃNon-haemorrhagic stroke/SE | 0.67 (0.65–0.71) | 0.64 (0.62–0.67) | 0.67 (0.63–0.71) | 0.62 (0.58–0.66) |
| ȃMajor bleeding | 0.70 (0.66–0.74) | 0.61 (0.56–0.65) | 0.70 (0.66–0.76) | 0.63 (0.58–0.69) |
| Very low to low riska | ||||
| ȃAll-cause mortality | 0.74 (0.70–0.78) | 0.53 (0.49–0.57) | 0.72 (0.65–0.79) | 0.51 (0.44–0.58) |
| ȃNon-haemorrhagic stroke/SE | 0.71 (0.64–0.78) | 0.52 (0.46–0.59) | 0.71 (0.64–0.79) | 0.54 (0.45–0.63) |
| ȃMajor bleeding | 0.72 (0.66–0.78) | 0.54 (0.45–0.61) | 0.72 (0.65–8.0) | 0.59 (0.47–0.71) |
OAC, oral anticoagulant; SE, systemic embolism; UCD, universal clinician device.
CHA2DS2VASc score of 0 or 1 (men) and 1 or 2 (women); HAS-BLED 0 or 1 for major bleeding/haemorrhagic stroke.
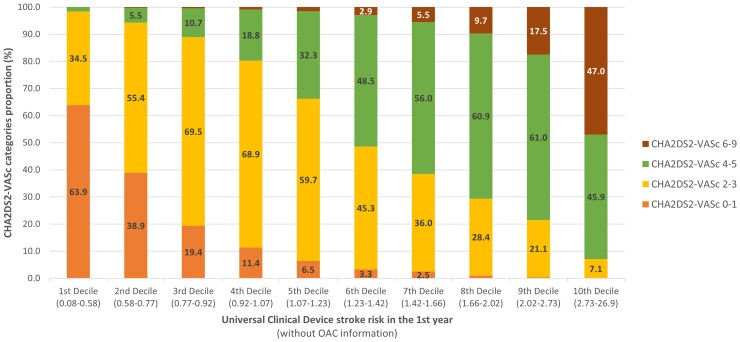
Patients within in the 1st decile of the UCD stroke risk tool (0.08–0.58%) rarely exceeded a CHA2DS2VASc score of 3; most patients within the 10th decile of the UCD (2.73–26.9%) exceeded a CHA2DS2VASc score of 2 (Figure 3). Approximately 34.5% of patients categorized with a CHA2DS2VASc score of >1 were predicted to have the lowest risk of stroke according to the UCD; approximately 11.4% of patients in the 4th decile of the UCD (0.92–1.07% 1-year stroke risk) had a CHA2DS2VASc score of 0–1.
Figure 3.
Distribution of the CHA2DS2VASc score categories according to the deciles of Universal Clinician Device 1 year stroke risk device in the full GARFIELD-AF population.
Increasing UCD quartiles provided a superior prediction of changing average rates of stroke compared with increasing CHA2DS2VASc categories (Table 6).
Table 6.
Non-haemorrhagic stroke/SE incidence estimates (1 — Kaplan–Meier) and corresponding 95% CIs at 1 year follow-up by CHA2DS2VASc score categories by UCD stroke in 1st year quartiles in the GARFIELD-AF population
| CHA2DS2VASc score | New innovativeb stroke risk in the 1st year | |||
|---|---|---|---|---|
| Min to 1st quartile (0.08–0.84) | 1st quartile to median (0.84–1.23) | Median to 3rd quartile (1.23–1.81) | 3rd quartile to max (1.81–26.9) | |
| 0–1 | 0.34 (0.21–0.54) | 0.67 (0.37–1.20) | 1.97 (0.40–2.31) | NAa |
| 2–3 | 0.40 (0.27–0.58) | 0.86 (0.67–1.11) | 1.12 (0.86–1.45) | 1.92 (1.42–2.58) |
| 4–5 | NAa | 0.69 (0.46–1.03) | 1.30 (1.04–1.62) | 2.30 (1.96–2.69) |
| 6–9 | NAa | NAa | NAa | 2.95 (2.42–3.59) |
UCD, universal clinician device.
Not enough patients available in this category to compute a survival estimate.
In order to conduct a fair comparison between UCD and the CHA2DS2VASc score, the UCD model was analysed assuming a lack of baseline OAC treatment.
Risk reduction according to UCD model recommendations
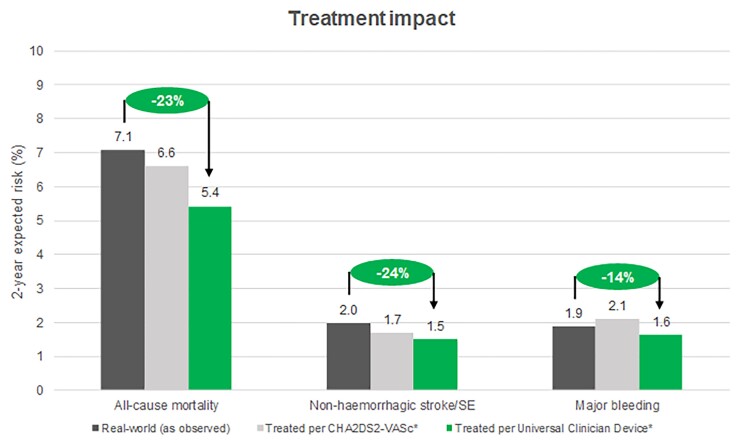
Within the GARFIELD-AF population, the average observed 2-year rates for all-cause mortality, stroke/SE, and major bleeding were 7.1%, 1.7%, and 1.9%, respectively (Figure 4). Implementation of treatment recommendations using CHA2DS2VASc for OAC therapy (Scenario 1) and therapies for other clinical risk factors reduced estimated event rates of mortality and stroke to 6.6%, 1.7%, and increased major bleeding to 2.1%. The use of the UCD for stroke risk, to determine the need for OAC therapy and therapies for other clinical risk factors, further reduced the estimates for all-cause mortality and stroke/SE to 5.4% and 1.5%. The major bleed rate was 1.6%, exhibiting both a lower rate compared with both Scenario 1 and the real-world (Figure 4, Table 7).
Figure 4.
Estimated average rate for outcomes over 2 years of follow up according to the observed real-world GARFIELD-AF population, if all patients followed CHA2DS2VASc guidelines for treatment with oral anticoagulant, and if all patients followed treatment guidelines according to the Universal Clinician Device.
Table 7.
Average estimated outcome probabilities (%) within 2 years follow-up by new innovative recommendations
| Outcomes | Following no recommendationsa | Following non-OAC related recommendations | Following OAC and non-OAC related recommendations | Following OAC and non-OAC recommendations and SGLT2 inhibitors/GLP-1RAs |
|---|---|---|---|---|
| Original GARFIELD-AF population | ||||
| ȃAll-cause mortality | 8.45 | 8.02 | 5.42 | — |
| ȃNon-haemorrhagic stroke/SE | 2.58 | 2.40 | 1.50 | — |
| ȃMajor bleeding | 1.19 | 1.13 | 1.62 | — |
| Current smoker and DBP of 110 mmHg | ||||
| ȃAll-cause mortality | 9.55 | 7.11 | 5.51 | — |
| ȃNon-haemorrhagic stroke/SE | 8.62 | 2.71 | 2.05 | — |
| ȃMajor bleeding | 1.19 | 1.13 | 1.45 | — |
| Diabetes and moderate CKD | ||||
| ȃAll-cause mortality | 13.15 | 12.50 | 9.97 | 8.67 |
| ȃNon-haemorrhagic stroke/SE | 4.40 | 4.08 | 3.11 | 2.93 |
| ȃMajor bleeding | 1.76 | 1.67 | 2.12 | 2.12 |
| Diabetes, BMI: 32 kg/m2, age: 80 years | ||||
| ȃAll-cause mortality | 13.08 | 12.25 | 10.20 | 8.87 |
| ȃNon-haemorrhagic stroke/SE | 4.23 | 3.85 | 3.07 | 2.88 |
| ȃMajor bleeding | 1.84 | 1.74 | 2.12 | 2.12 |
CKD, chronic kidney disease; DBP, diastolic blood pressure; OAC, oral anticoagulant; SE, systemic embolism; UCD, universal clinician device.
Assuming no oral anticoagulation at baseline.
In patients with diabetes and comorbid conditions such as CKD, implementation of applicable UCD recommendations reduced the estimated probability of all-cause mortality from 13.15% (no treatment) to 8.67% (recommended treatments provided). Similarly, stroke/SE was reduced from 4.40 to 2.93%. Major bleeding increased slightly from 1.76% to 2.12% (Table 7).
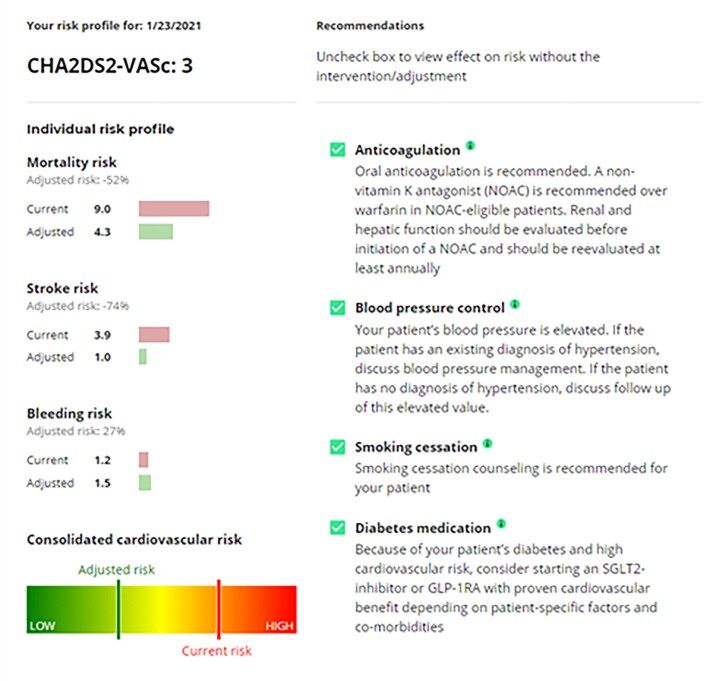
In elderly patients with concomitant diabetes, a BMI of 32 kg/m2 and of 80 years of age, the UCD recommendations improved the estimated probability of all-cause mortality from 13.08% to 8.87%. Stroke risk was similarly improved (4.23–2.88%). Major bleeding increased slightly due to the OAC therapy (1.84–2.12%). An example of the output from the UCD is illustrated in Figure 5.
Figure 5.
Sample output from the Universal Clinician Device, providing individualized recommendations and the adjusted outcomes risks.
Discussion
The design of the UCD combines easily attainable patient data with curated guideline-based recommendations into a dynamic and translatable outcome risk tool. It directly applies an algorithm to data available in routine clinical care and generates appropriate tailored treatment recommendations. Importantly, the UCD aids treatment choices by illustrating outcome risk changes associated with modification or treatment of key AF risk factors. In addition, the UCD benefits from an ability to account for missing data.
The GARFIELD-AF risk model has previously indicated that both OAC use and the type of OAC used to play an important role in improving outcomes.13 However, successful thrombo-prophylaxis for AF patients requires a careful balance of improved stroke risk and the increased bleeding risk associated with OAC therapy. The ability of the UCD to both predict individual risks for mortality, stroke, and major bleeding and to demonstrate the impact of OAC initiation, therefore, allows for better informed treatment decision. This is particularly important for high-risk patients such as those with concomitant diabetes. Cardiovascular disease and risk factors are primary causes of death in diabetic patients.22 Therefore, specialised treatment strategies to alleviate CV outcomes in this sub-population are paramount. Sodium–glucose cotransporter 2 inhibitors/GLP-1RAs significantly reduce the risk of CV outcomes in diabetic patients and thus were incorporated into the device’s recommendations.23–30 Two recently published meta-analyses have detailed the extensive association of SGLT2 inhibitors and GLP-1RA’s with a reduced risk of CV outcomes in patients with diabetes.31,32 Patient populations incorporated into each of these meta-analyses demonstrated a substantial over-lap of baseline patient characteristics with the GARFIELD-AF population, suggesting that the estimated risk reduction associated with SGLT2/GLP-1RA’s is applicable for the current study. Furthermore, there has been no evidence within these meta-analyses to indicate treatment interactions with these baseline factors. The overall treatment effect should, therefore, apply to the GARFIELD-AF patients. Incorporation of SGLT2/GLP-1RA into the UCD allowed optimized treatment choice in this high-risk cohort. Similarly, it provided valuable information for patients with a low risk of stroke.
A number of risk scores are available for stroke risk prediction according to OAC choices such as ATRIA, the ABC-stroke score, the GARFIELD-AF stroke model, and the most commonly utilized, the CHA2DS2VASc score. However, as described by the European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) consensus of 2020, there is no one perfect predictive stroke risk score, and therefore it is important to consider the most appropriate tool for the right circumstances.33 Although the UCD demonstrated superior predictive power compared with CHA2DS2VASc for all-cause mortality, the absolute difference in c-indexes was small. Similarly, the c-indexes for stroke/SE and bleeding were significantly improved compared with simple clinical scores, but did not reach perfect prediction. However, it is important to note that the UCD aims to guides clinicians to recognize the risk zone for individual patients and to link risk reduction potential with guideline-based treatment recommendations rather than striving for perfection in prediction.
The ESC 2020 AF-management guidelines suggest optimization of shared decision making between the treating physician and patient.11 In addition, due to the dynamic nature of AF-related risk factors, the guidelines recommend regular assessment of stroke and bleeding risk at each clinical review. In contrast to the CHA2DS2VASc score, the UCD facilitates these requirements by providing easy-to-assess tailored information for the annual treatment burden associated with each treatment choice. As an adjustable and dynamic risk device, it accounts for fluctuating risk factors, allowing long-term anticoagulation to be consistently re-considered at each clinical review. The use of a multifunction, single individualized risk tool has been shown to better the rates of mortality, stroke, and bleeding events in comparison to multiple single-purpose risk tools.34 Recently, the use of an integrated mobile health device for dynamic management of AF has been investigated as a potential route to improving patient outcome. The mobile AF application (mAFA) described by Guo et al.35, which provides a range of clinical decisions support tools, including CHA2DS2VASc and HAS-BLED, demonstrated a significant improvement in rates of stroke/SE, mortality, and re-hospitalization over a follow-up of almost 9 months. Interestingly, the use of the mAFA also lead to a substantial increase in OAC administration, particularly for high-risk patients in comparison to those receiving usual care. Interestingly, the use of the mAFA tool in combination with the HAS-BLED score was capable of dynamically monitoring modifiable bleeding risks and led to a significant reduction in bleeding events and increased OAC use.36
The UCD allows simultaneous analyses of the risk of all-cause mortality, stroke/SE, and major bleeding, while also accounting for high-risk sub-populations. It is clinically advantageous as it allows for simple individualised risk assessment. Future work is required to carry out a prospective study to establish the predicted benefits of the UCD in clinical practice. In addition, due to the simple nature of the UCD, future studies will include a mixed-methods approach such as human factor testing with health care professionals (cardiologists, GPs, physician assistants, and nurse practitioners). Indeed, recent human factor tests have indicated that users found the UCD intuitive, and individualised CV risk-based treatment recommendations to be beneficial. It was also of educational value for patients to increase treatment adherence.
Strengths
Strengths of the UCD include its development using the large global GARFIELD-AF registry of over 52 000 patients with long-term follow-up of 2 years. The device was internally validated using a split sample approach and demonstrated comparable results throughout for both the derivation and validation sets. Use within routine care is simple, as it requires readily available patient data and does not necessitate specialized information such as genomic data or laboratory testing. In addition, it capably maintains accurate risk assessment when key data are missing, a critical advantage within routine clinical care where patient data is often incomplete.
Limitations
The limitations of this study include that an external dataset was not available for additional validation and thus these results have not been validated in an independent population; however, we anticipate that further research groups will perform external validation as they utilize the UCD. Some parameters within the algorithm had very little missing data making estimation of missingness for these factors difficult. Finally, this analysis includes some assumed benefits, determined according to published clinical trial data, which is not an optimal substitute for RCT evidence.
Conclusion
The UCD provides a new user-friendly algorithm to simultaneously predict the risk of mortality, stroke, and bleeding using easily attainable individual patient clinical data and guideline-based optimized treatment plans. It generates tailored treatment recommendations and illustrates the outcome risks associated with each treatment choice, as well as provides risk adjustments associated with modification of key AF risk factors. The simple and dynamic nature of the UCD would allow translation into routine clinical practice and care. Future work is required to carry out a prospective study to establish the predicted benefits of the UCD in clinical practice.
Lead author biography
 Georg van Husen, MD, PhD Georg is a medical doctor by training and started his professional career in cardiology. He then worked as a management consultant with McKinsey & Co, from where he joined the pharmaceutical industry. He held various roles at the global headquarters, in the USA, and as General Manager in South Africa before becoming global SVP and franchise head for CardioMetabolism with Boehringer Ingelheim. In 2019, he became co-founder and CEO of tenacio, a digital health start-up focusing on cardiovascular diseases.
Georg van Husen, MD, PhD Georg is a medical doctor by training and started his professional career in cardiology. He then worked as a management consultant with McKinsey & Co, from where he joined the pharmaceutical industry. He held various roles at the global headquarters, in the USA, and as General Manager in South Africa before becoming global SVP and franchise head for CardioMetabolism with Boehringer Ingelheim. In 2019, he became co-founder and CEO of tenacio, a digital health start-up focusing on cardiovascular diseases.
Supplementary Material
Acknowledgements
We thank the physicians, nurses, and patients involved in the GARFIELD-AF registry. Medical writing support was provided by Rebecca Watkin (TRI, London, UK). SAS programming support by Madhusudana Rao (TRI, London, UK).
Contributor Information
Georg van Husen, tenac.io, Oberwallstrasse 6, 10117 Berlin, Germany.
Saverio Virdone, Department of Statistics, The Thrombosis Research Institute, London, UK.
Karen Pieper, Department of Statistics, The Thrombosis Research Institute, London, UK.
Gloria Kayani, Department of Statistics, The Thrombosis Research Institute, London, UK.
Keith A A Fox, Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, UK.
Supplementary material
Supplementary material is available at European Heart Journa—Digital Health.
Funding
Funding for the development of the UCD was funded by tenac.io. The funding sources had no involvement in the data collection, data analysis, or data interpretation.
Conflict of interest: G.v.H.: Employee and shareholder of tenac.io GmbH. K.P.: Consultancies from Johnson & Johnson, Artivion, and Element Science. S.V., and G.K. report no conflict of interests. K.A.A.F. has received grants and personal fees from Bayer/Janssen and AstraZeneca and personal fees from Sanofi/Regeneron and Verseon.
Data availability
The data are not currently available for external use.
References
- 1. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16(2):217–221. [DOI] [PubMed] [Google Scholar]
- 2. Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study). Eur Heart J 2006;27:1954–1964. [DOI] [PubMed] [Google Scholar]
- 3. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 4. Christiansen CB, Gerds TA, Olesen JB, Kristensen SL, Lamberts M, Lip GYH, Gislason GH, Køber L, Torp-Pedersen C. Atrial fibrillation and risk of stroke: a nationwide cohort study. Europace 2016;18:1689–1697. [DOI] [PubMed] [Google Scholar]
- 5. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators, Singer DE, Hughes RA, Gress DR, Sheehan MA, Oertel LB, Maraventano SW, Blewett DR, Rosner B, Kistler JP. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 1990;323:1505–1511. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thromb Res 2006;118:321–333. [DOI] [PubMed] [Google Scholar]
- 7. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 9. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan Pt, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 11. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 12. Fox KAA, Lucas JE, Pieper KS, Bassand JP, Camm AJ, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Kayani G, Oto A, Mantovani LG, Misselwitz F, Piccini JP, Turpie AGG, Verheugt FWA, Kakkar AK, GARFIELD-AF Investigators . Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 2017;7:e017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fox KAA, Virdone S, Pieper KS, Bassand JP, Camm AJ, Fitzmaurice DA, Goldhaber SZ, Goto S, Kayani G, Oto A, Misselwitz F, Piccini JP, Dalgaard F, Turpie AGG, Verheugt FWA, Kakkar AK, GARFIELD-AF Investigators . GARFIELD-AF risk score for mortality, stroke, and bleeding within 2 years in patients with atrial fibrillation. Eur Heart J Qual Care Clin Outcomes 2022;8:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalgaard F, Pieper K, Verheugt F, Camm AJ, Fox KAA, Kakkar AK, Pallisgaard JL, Rasmussen PV, van Weert H, Lindhardt TB, Torp-Pedersen C, Gislason GH, Ruwald MH, Harskamp RE. GARFIELD-AF model for prediction of stroke and major bleeding in atrial fibrillation: a Danish nationwide validation study. BMJ Open 2019;9:e033283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL, Kalyani RR, Kosiborod M, Magwire M, Morris PB, Neumiller JJ, Sperling LS. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:1117–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GYH, Mantovani LG, Verheugt FWA, Jamal W, Misselwitz F, Rushton-Smith S, Turpie AGG. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13–19.e1. [DOI] [PubMed] [Google Scholar]
- 17. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas a, Hacke W, Lip GYH, Mantovani LG, Turpie AGG, van Eickels M, Misselwitz F, Rushton-Smith S, Kayani G, Wilkinson P, Verheugt FWA, Hernandez AV. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol 2010;56:827–837. [DOI] [PubMed] [Google Scholar]
- 19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 20. QxMD G-A . GARFIELD-AF Risk Calculator 2019 Available from: https://qxmd.com/calculate/calculator_685/garfield-af-risk-calculator.
- 21. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care 2020;43:S111–S134. [DOI] [PubMed] [Google Scholar]
- 23. Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2016;374:1094––1096.. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 25. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 26. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 28. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 29. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu Ne, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WHH, Temelkova-Kurktschiev Theodora, REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 30. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 31. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 32. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra SG, Masiukiewicz U, Cannon CP. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, Sepehri Shamloo A, Alfie A, Boveda S, Dagres N, Di Toro D, Eckhardt LL, Ellenbogen K, Hardy C, Ikeda T, Jaswal A, Kaufman E, Krahn A, Kusano K, Kutyifa V, S. Lim H, Lip GYH, Nava-Townsend S, Pak HN, Rodríguez Diez G, Sauer W, Saxena A, Svendsen JH, Vanegas D, Vaseghi M, Wilde A, Bunch TJ, Buxton AE, Calvimontes G, Chao TF, Eckardt L, Estner H, Gillis AM, Isa R, Kautzner J, Maury P, Moss JD, Nam GB, Olshansky B, Molano LFP, Pimentel M, Prabhu M, Tzou WS, Sommer P, Swampillai J, Vidal A, Deneke T, Hindricks G, Leclercq C. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm 2020;36:553–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med 2011;154:627–634. [DOI] [PubMed] [Google Scholar]
- 35. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH, mAF-App II Trial Investigators . Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 36. Guo Y, Lane DA, Chen Y, Lip GYH. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-II randomized trial. Am J Med 2020;133:1195–1202.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not currently available for external use.