Abstract
Aims
Activity trackers for clinical trials and remote monitoring are appealing as they provide objective data outside of the clinic setting. Algorithms determine physical activity intensity and count steps. Multiple studies show physical inactivity in pulmonary arterial hypertension (PAH). There are no studies comparing different activity trackers worn on different parts of the body in PAH. We had patients with PAH simultaneously wear two different accelerometers, compared measures between the two devices, and correlated the measures with standard clinical metrics in PAH.
Methods and results
This was a single-centre, prospective observational study. Daily physical activity and daily total steps were measured using Actigraph GT9X Link and MC10 Biostamp nPoint for 5–10 days. Actigraph was worn on the non-dominant hand and the MC10 Biostamp nPoint was worn on the chest and leg with disposable adhesives. Twenty-two participants wore both accelerometers >12 h/day for an average 7.8 days. The average activity time measured by Actigraph was significantly higher than that measured by MC10 (251 ± 25 min vs. 113 ± 18 min, P = 0.0001). Actigraph’s algorithm reported more time in light activity than moderate (190 ± 62 min vs. 60 ± 56 min, P = 0.0001). REVEAL 2.0 scores correlated highly with activity time measured using either device. Invasively measured haemodynamics within 7 days did not correlate with activity time or daily steps.
Conclusion
Different activity trackers yield discordant results in PAH patients. Further studies are needed in determining the best device, optimal wear time, and different thresholds for activities in chronic diseases.
Keywords: Activity tracker, Wearable, PAH, Steps, Activity time
Graphical abstract
Introduction
The COVID-19 pandemic has highlighted an urgent need for remote patient monitoring; of course, home monitoring minimizes exposure risk to patient and providers but could also decrease cost and improve management of chronic disease.1 Multiple clinical trials have also started to include exploratory, or even primary,2 endpoints using wearable technology.3 Activity trackers are appealing as they provide objective data outside of the clinic setting not reliant on patient recall.4 Proprietary algorithms can determine intensity (light, moderate, and vigorous) and segregate sedentary vs. active time. The initial validation studies of activity trackers monitored physical activity levels in healthy controls using wrist-5,6 and hip-based sensors.7 There are multiple studies using different accelerometers showing physical inactivity in chronic pulmonary diseases8 and specifically pulmonary arterial hypertension (PAH).9–11 Multiple different devices are available and can be worn on different parts of the body. At this stage, there are few studies comparing data quality and algorithm analysis for different devices worn on different parts of the body at the same time.12–15 Activity trackers placed on the ankle likely offer the most accurate step count.14,15 Wearing them on the ankle is not as aesthetically appealing as the wrist, heart failure patients with oedema or compression stockings may have difficulty wearing them, and new devices have other features (heart rate monitoring) that would be difficult to use on the ankle. There are none comparing activity data quality in PAH a disease for which we know activity is remarkably low. Total wear time is also an important factor when evaluating physical activity time or total steps.
To address the question of how well two different accelerometers compare in a chronic cardiopulmonary disease with decreased activity time, we had patients with PAH simultaneously wear a research grade accelerometer on their non-dominant hand and a chest/leg-based accelerometer. We compared total physical activity time measurements and total daily steps between the two devices. We also compared physical activity measurements to established metrics in PAH [risk assessment, N-terminal pro B-type natriuretic peptide (NT-pro-BNP), and haemodynamics]. We hypothesized that wrist-based devices would artefactually measure more physical activity time and steps compared to the chest/leg-based devices because of less relevant arm movements. We also hypothesized that chest-based devices would correlate more tightly with PAH clinical metrics because they would be less by irrelevant movements measured with the wrist-based device.
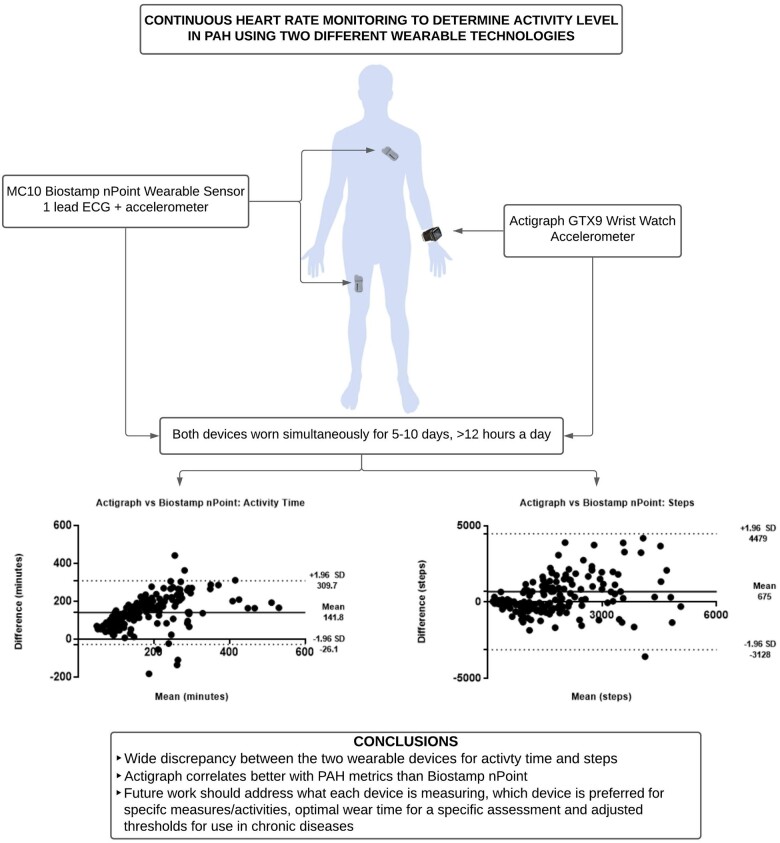
Methods
This was a single-centre prospective observational study completed at the University of Rochester Medical Center Pulmonary Hypertension Association’s accredited Comprehensive Care Center. IRB approval was obtained before consenting any participants. We offered participation to interested WHO Group 1 PAH patients16 at the time of a routine clinic visit during the recruitment period (March 2020–2021). We only excluded those who endorsed severe immobility unrelated to PAH. Patients were not required to have a smart device or internet. Daily physical activity and daily total steps were measured using Actigraph GT9X Link and MC10 Biostamp nPoint for 5-10 days. Seven days has been shown to be sufficient when monitoring stable PAH patients using a wrist-based activity tracker.9 Given the added work of wearing MC10 Biostamp nPoint in this chronically ill group, we included an option for participants to return the device early if it was too burdensome or have flexibility on when they could return the device based on their schedule. At the minimum participants needed to have the device for 5 days. Participants were asked to wear the Actigraph on the non-dominant hand; the MC10 Biostamp nPoint requires two small sensors (chest and thigh) affixed to the skin with disposable adhesives.17 We downloaded Actigraph data after the unit was returned; MC10 Biostamp data must be downloaded and re-charged daily which takes roughly 60 min on a dock. During this docking time, we encouraged participants to shower or do activities that would not require much physical activity, and we asked them to remove the Actigraph when the MC10 device was charging (for parity). We reviewed Actigraph data to verify that minimal activity occurred during MC10 charging. Participants were encouraged to wear the devices nearly continuously or at least during waking hours. Proprietary algorithms prevented direct comparisons between select periods of activity or time. If participants had difficulty sleeping with either device on they were asked to take both off to minimize time where direct comparisons could not be made. After consent at the initial visit, a 6-min walk test (6MWT) was performed according to the American Thoracic Society 6MWT guidelines,18 and, using the MC10 Biostamp nPoint affixed to the chest, we also measured continuous heart rate to calculate cardiac effort during the 6MWT (heart beats/walk distance) using previously reported methods.19,20 Actigraph is capable of measuring heart rate when paired with Polar chest strap. We did not provide subjects with chest straps. Participants completed an emphasis 10 questionnaire21 at the initial visit when an investigator recorded baseline demographics and clinical information to calculate a Reveal 2.0 score.22 No changes were made to medications within 30 days of enrolment. Participants were encouraged to continue their baseline activity during the monitoring period. During the remote monitoring period, participants completed a daily survey documenting on a scale of 1–10 (10 being the most active) their perceived level of activity for the day, duration of activity (any time spent moving around during the day), duration of time spent out of the house, degree of breathlessness with activity and rest, duration of inactivity, energy level for the day, and duration of sleep.
Statistical analysis
We included data for analysis if both accelerometers were worn for >12 h per day during the same time and then used Bland–Altman to compare total activity time and total daily steps between the two devices. Using Pearson and Spearman correlation coefficient, we compared these activity metrics to age, 6MWT distance, cardiac effort during 6MWT, NT-pro-BNP, Functional Class, Emphasis 10 score, Reveal 2.0, and remote daily activity survey. We compared haemodynamic data to activity data if right heart catheterization was performed within 7 days of the start of activity monitoring. Right heart catheterization was only performed if clinically indicated. Categorical variables are reported as counts and percentages. Continuous variables are reported as mean with standard deviation or median with interquartile ratio. Student’s paired t-test or Mann–Whitney test was performed as appropriate for group comparisons using SAS 9.4.
Results
Twenty-two participants were enrolled and wore both accelerometers >12 h/day (720 min) for an average of 7.8 days (173 days of paired measurements). No participants were excluded because of inadequate data collection. The Actigraph was worn on average for 1249 ± 139 min per day while the MC10 was worn on average for 1220 ± 99 min per day. Demographics are listed separately in Table 1 for participants who were treatment-naïve; for those who were starting additional PAH therapy because of intermediate or high Reveal 2.0 scores (treatment intensification); and for those whom the provider and patient agreed were satisfactory on current PAH therapy (stable).
Table 1.
Disease characteristics at the time of activity measures
| Treatment Naïve (n = 6) | Treatment intensification (n = 6) | Stable (n = 10) | |
|---|---|---|---|
| Age | 61 ± 13 | 53 ± 17 | 53 ± 15 |
| Female sex | 5 (83%) | 3 (50%) | 7 (70%) |
| PAH Aetiology | |||
| Idiopathic | 4 (67%) | 3 (50%) | 6 (60%) |
| Connective tissue disease | 2 (33%) | 2 (33%) | 1 (1%) |
| Vasodilator therapy | |||
| None | 6 (100%) | 0 | 0 |
| Monotherapy | 0 | 2 (33%) | 1 (10%) |
| Combination | 0 | 4 (67%) | 5 (50%) |
| Prostacyclin | 0 | 0 | 4 (40%) |
| 6MWD (m) | 395 (229, 429) | 377 (152, 498) | 381 (352, 459) |
| Cardiac effort (beats/m) | 1.9 (1.4, 2.9) | 1.9 (1.3, 4.4) | 1.6 (1.4, 2.0) |
| NT-pro-BNP | 1827 (112, 3852) | 2029 (50, 3845) | 214 (137, 360) |
| Functional class (I/II/III) | 0/3/3 | 0/1/5 | 0/10/0 |
| Reveal 2.0 | 7.3 ± 4.5 | 9.3 ± 3.5 | 4.2 ± 2.2 |
| Emphasis 10 | 33 ± 5 | 31 ± 12 | 24 ± 9 |
| RHC | N = 6 | N = 4 | N = 6 |
| RA, mmHg | 9 ± 3 | 12 ± 6 | 10 ± 3 |
| mPAP, mmHg | 45 ± 13 | 48 ± 5 | 38 ± 11 |
| PVR, Woods units | 11.8 ± 6.7 | 8.2 ± 3.1 | 5.7 ± 2.9 |
| CI, L/min/m2 | 1.7 ± 0.5 | 2.2 ± 0.5 | 2.3 ± 0.5 |
Physical activity time
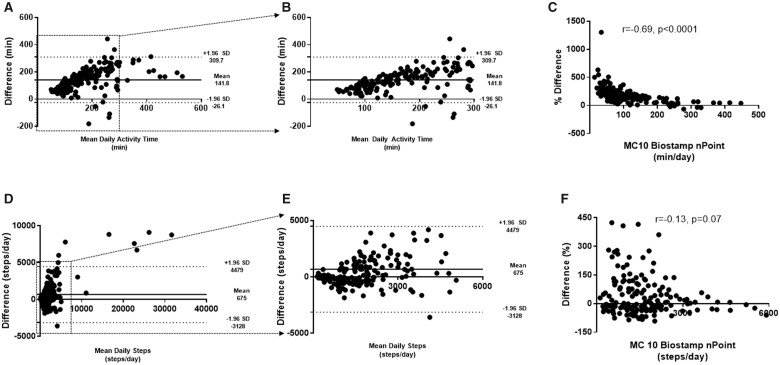
The average activity time measured by Actigraph was significantly higher than what was measured by the chest and thigh MC10 sensors (251 ± 25 min vs. 113 ± 18 min, P = 0.0001). Of the activity time, Actigraph’s algorithm reported that participants spent more time in light activity than moderate (190 ± 62 min vs. 60 ± 56 min, P = 0.0001). MC10 does not attempt such as segregation. Bland–Altman shows that the wrist-based Actigraph almost always measures more ‘activity’ than the chest/leg-based MC10, but this is most pronounced for those with more than 150 min of activity daily (Figure 1).
Figure 1.
Bland–Altman plot and comparison of daily physical activity time and daily total steps between Actigraph and MC10 Biostamp. (A) The Actigraph’s wrist-based location measures markedly more activity time than MC10’s chest and thigh sensors. (B) Bland–Altman plot showing physical activity time <300 min (boxed region in panel A). Twelve points are missing. (C) Using MC10 as the baseline, there is significant difference between Actigraph and MC10 measurement <150 min. (D) There is wide range and agreement between step count measured between the two devices. (E) Bland–Altman plot showing <6000 daily steps (boxed region in panel D). Ten points are missing. (F) Using MC10 as the baseline, there is wide variability between step counts measured between the two devices, specifically at <3000 steps/day.
Total daily steps
There was a numeric difference in the average number of daily steps measured between the Actigraph and MC10 (3254 ± 5781 steps vs. 2448 ± 3990 steps, P = 0.59). Bland–Altman plots show very wide limits of agreement without a clear bias in steps measured by the two devices. Actigraph appears to overestimate daily steps at lower daily step counts (<3000 steps/day) when using MC10 as the reference (Figure 1).
Activity, steps, and other pulmonary arterial hypertension metrics
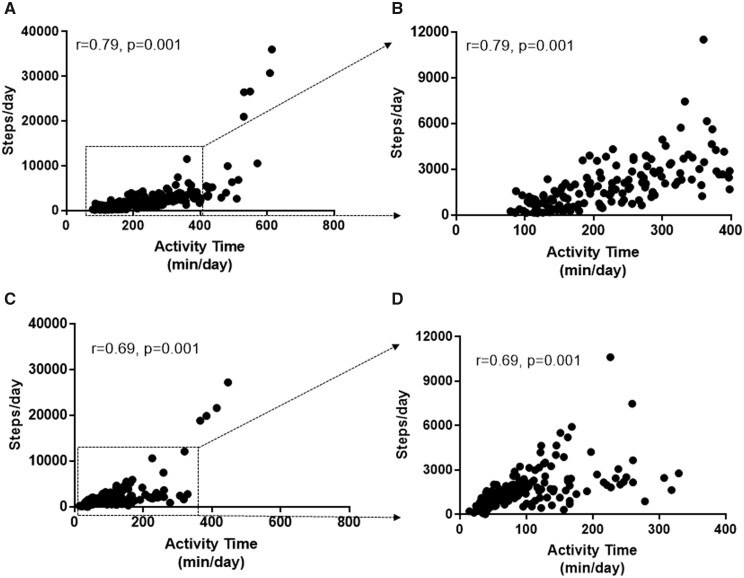
Actigraph had a stronger correlation between its own measure of daily activity time and steps than MC10 (Figure 2). REVEAL 2.0 scores correlated highly with daily activity times measured using either device but much less so with daily steps (Table 2). Similarly, 6MWT distance and NT-pro-BNP correlated strongly with activity time (both devices) but not with daily steps (either device). Invasively measured haemodynamics measured by right heart catheterization within 7 days of initiating activity measures as well as cardiac effort during 6MWT did not correlate with activity time or daily steps measured by either device (Table 2). Investigator assessed FC (before the activity trackers were issued) correlated strongly with light and total activity on the Actigraph but much less strongly with the MC-10 device; Emphasis-10 quality of life scores did not correlate with activity.
Figure 2.
Internal correlations between steps/day and physical activity time measurement with Actigraph and MC10 Biostamp nPoint. (A) There was stronger internal correlation between steps/day and activity time measured by Actigraph. (B) Comparison with activity time <400 min/day with steps/day measured by Actigraph (boxed region in panel A). Twelve points are missing. (C) Correlation between steps/day and activity time measured by MC10 Biostamp nPoint. (D) Comparison with activity time <400 min/day with steps/day measured by MC10 Biostamp nPoint (boxed region in panel C). Five points are missing.
Table 2.
Pearson correlation between activity metrics and other measures/characteristics
| Average daily activity time |
Average daily steps |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actigraph |
MC 10 |
Actigraph |
MC10 |
|||||||||
| Light |
Moderate |
Total |
Total |
|||||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| REVEAL 2.0 | −0.76 | 0.0001 | −0.61 | 0.002 | −0.71 | 0.0002 | −0.62 | 0.002 | −0.42 | 0.051 | −0.35 | 0.10 |
| FC | −0.68 | 0.0004 | −0.47 | 0.02 | −0.60 | 0.002 | −0.37 | 0.08 | −0.26 | 0.23 | −0.24 | 0.26 |
| NT-pro-BNP | −0.55 | 0.007 | −0.41 | 0.05 | −0.50 | 0.01 | −0.46 | 0.02 | −0.27 | 0.20 | −0.23 | 0.29 |
| 6MWD (m) | 0.55 | 0.007 | 0.45 | 0.03 | 0.52 | 0.01 | 0.52 | 0.01 | 0.29 | 0.17 | 0.24 | 0.26 |
| Age (years) | −0.44 | 0.03 | −0.30 | 0.16 | −0.39 | 0.07 | −0.42 | 0.04 | −0.16 | 0.45 | −0.11 | 0.61 |
| Emphasis 10 | −0.35 | 0.10 | −0.14 | 0.51 | −0.26 | 0.23 | −0.07 | 0.74 | 0.04 | 0.85 | 0.09 | 0.67 |
There was no correlation with sex, cardiac effort, body mass index, mPAP, PVR, and SV with any measure of activity.
Remote survey
Eighteen subjects returned their daily activity survey (Table 3). Age and body mass index were not associated with any of the survey parameters (Table 4). REVEAL 2.0 score and NT-pro-BNP correlated strongly with activity level and estimated inactivity time. Different Actigraph parameters correlated with activity level and estimated activity and inactivity time. MC10 correlated with estimated activity time. Interestingly, cardiac expenditure correlated the strongest with energy level. Borg score after 6MWT correlated strongly with their reported degree of breathless during remote activities (r = 0.69, P = 0.001). We did not find any correlation with time spent out of the house or estimated sleep duration.
Table 3.
Daily remote survey
| N = 18 | |
|---|---|
| Activity perception (1–10) | 5.3 (3, 7.3) |
| Estimated exertion time (min) | 45 (22, 91) |
| Estimated time out of the house (min) | 137 (45, 248) |
| Breathlessness with activity (1–10) | 3.9 (1.5, 5.8) |
| Breathlessness with rest (1–10) | 1(1, 1.2) |
| Estimated inactivity time (h) | 5.6 (4, 7.7) |
| Estimated sleep duration (h) | 6.9 (6.3, 7.7) |
| Energy level (1–10) | 5.7 (4.3, 6.6) |
Table 4.
Correlation with daily remote survey
| Activity level |
Estimated activity time |
Estimated inactivity time |
Energy level |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| REVEAL 2.0 | −0.63 | 0.005 | −0.26 | 0.29 | 0.70 | 0.001 | −0.52 | 0.03 |
| NT-pro-BNP | −0.64 | 0.004 | −0.25 | 0.31 | 0.76 | 0.0002 | −0.45 | 0.06 |
| Actigraph | ||||||||
| Total activity time (min) | 0.58 | 0.01 | 0.45 | 0.06 | −0.63 | 0.005 | 0.22 | 0.36 |
| Light activity time (min) | 0.56 | 0.01 | 0.39 | 0.10 | −0.65 | 0.003 | 0.31 | 0.21 |
| Moderate activity time (min) | 0.56 | 0.01 | 0.49 | 0.03 | −0.55 | 0.01 | 0.12 | 0.68 |
| Daily steps | 0.44 | 0.06 | 0.57 | 0.01 | −0.43 | 0.07 | −0.03 | 0.88 |
| MC10 Biostamp | ||||||||
| Total activity time (min) | 0.43 | 0.07 | 0.48 | 0.04 | −0.53 | 0.02 | 0.12 | 0.64 |
| Daily steps | 0.41 | 0.08 | 0.62 | 0.006 | −0.42 | 0.08 | −0.11 | 0.67 |
| 6MWD (m) | 0.42 | 0.08 | 0.25 | 0.30 | −0.44 | 0.06 | −0.64 | 0.004 |
| Cardiac expenditure (beats/m) | −0.41 | 0.09 | 0.25 | 0.31 | 0.39 | 0.10 | −0.66 | 0.003 |
| Emphasis 10 | −0.15 | 0.54 | −0.10 | 0.68 | 0.27 | 0.26 | −0.57 | 0.01 |
| Age (years) | −0.27 | 0.27 | −0.02 | 0.94 | 0.32 | 0.20 | −0.36 | 0.14 |
| BMI (kg/m2) | 0.28 | 0.25 | 0.03 | 0.90 | −0.28 | 0.26 | 0.08 | 0.73 |
Discussion
We report significant differences between the metrics (especially activity time) measured with wrist as compared to chest-based activity trackers in a diverse group of PAH patients. The chest-based MC10 nPoint is less likely to be influenced by otherwise sedentary arm movements compared to the wrist-based Actigraph, but interestingly, light activity and total activity measured by the Actigraph correlated strongly with Reveal 2.0 and functional class (more so than the MC10). Especially for the subjective measure of functional class (symptoms), it may be that near-sedentary activity involving the arms ‘counts’ as activity in this impaired group of individuals with PAH. The MC10 algorithm focusing on torso and leg movements is apparently more stringent in counting activity time especially for inactive participants (Figure 1A), but then the correlation with symptom reports is weaker. As our field considers novel trial endpoints and remote markers of clinical status, it is important to understand what wearable devices are capable of measuring and how those metrics correlate with validated clinical measures.
Activity trackers offer continuous monitoring in the home setting without patient recall or bias.4 They are capable of measuring different types of movement, with walking being the major component. Ankle worn activity trackers are likely the most accurate way to obtain step counts in the remote setting.14,15 Multiple reasons including oedema, difficulty affixing the device, or aesthetics could limit compliance with an ankle worn activity tracker. No single device is comprehensive, because an ankle monitor would be insensitive to upper torso activity. When comparing hip and wrist-based Actigraph in healthy young and old adults, the wrist-worn Actigraph counted significantly more steps, specifically for those whose counts were <6000 steps/day.23 The authors cautioned using total steps per day as an outcome.23 This is similar to what we observed comparing MC10 and Actigraph (Actigraph counted substantially more activity time and somewhat more steps in this more sedentary range). Another study compared different commercial accelerometers worn on the wrist, shirt, waist, and ankle/foot and measured variable accuracy in an older population with abnormal gaits24; this observation suggests that the proprietary algorithms may not be useful in those with illness. Our study hypothesized potential benefits of MC10 Biostamp nPoint: (i) sensors are lightweight and worn under clothes; (ii) activity time and step measurements are based off dynamic activities (i.e. those typically needed for health benefits); and (iii) there is less noise introduced by arm or leg swing when at rest. In contrast with our hypothesis, we found that the wrist-based Actigraph correlated better with commonly used clinical metrics in PAH better than the MC10 Biostamp nPoint. One key unknown is whether a less stringent Biostamp algorithm for movement would have shown more activity and therefore stronger correlations.
Over the past decade, there have been multiple studies showing decreased physical activity time in PAH patients using multiple activity trackers worn on different parts of the body (arm, wrist, hip, and clothes).9–11,25–28 We do not know how well the activity segregation algorithms (for the Actigraph) perform with variable gaits and walking speeds seen in chronic diseases, such as PAH. In our study, three participants wore an Actigraph during their 6MWT. Actigraph classified the majority of the 6MWT as light activity, even with a walk distance of ∼500 m. Our patients uniformly regard hallway walking for 6MWT as moderate (or heavy) activity. This is supported by the Borg score correlating with perceived breathlessness at home. The current algorithms may be disconnected from the functional abilities in those impaired by chronic disease. Although an activity may be classified as light for a healthy individual, it might actually be more moderate in someone with chronic disease. Development of different thresholds for light or moderate activity are needed for chronically ill patients, as the current algorithms are likely misclassifying activity types.
We found very little moderate activity time measured by Actigraph and relatively little activity time using the apparently more stringent MC10 nPoint. Our data suggest that, in PAH, physical activity time seems to be a much more meaningful measurement than daily steps as it correlated better with REVEAL 2.0, functional class, 6MWT distance, and NT-pro-BNP (Table 2). Daily steps (with either device) did not correlate at all with validated PAH disease measures. Interestingly, for those participants with a recent invasive study, none of the activity metrics (either device) correlated with haemodynamic measures; this finding mirrors previous reports.10,26,27 It seems odd that the correlation between total activity (both devices) and NT-pro-BNP was relatively strong but relationships with invasive measures (even stroke volume) were nil. This could be a function of power (all had NT-pro-BNP and only seven had recent invasive data), but it is intriguing that NT-pro-BNP stands far apart from invasive data in correlating with activity. Cardiac effort, which is the number of heart beats used during the 6MWT divided by the walk distance which we propose as an indirect measure of right ventricular function because it correlates with stroke volume in two previous reports19,20; interestingly, this measure did not correlate with activity measures. The contrast between the NT-pro-BNP (as a barometer of right ventricular strain/dysfunction) correlating well with activity and these other measures of right ventricular function not correlating with overall activity requires further study in larger groups. However, one possible explanation is that, like with 6MWT distance, factors other than right ventricular function can have substantial influence on daily activity time and steps (e.g., medication side effects, comorbidities, depression, fatigue, and deconditioning). Nonetheless, because the Actigraph measure of daily activity correlated strongly with validated measures of PAH disease activity like NT-pro-BNP, Reveal score, and functional class, our data support this tool for remote research and clinical monitoring. We did not find an advantage for the apparently more stringent MC10 device with its current algorithms (and, in fact, our data suggest that recording arm activity as such is important).
There are limitations to our study. This was a small study of 22 PAH patients, although we think the wide range of disease severity and different types of therapies was a strength. The data was collected during the pandemic, which likely further constrained activity in this already sedentary population. Participants could have changed their activity behaviour while being monitored, although this seems less of an issue given the extremely low activity time measured. We did not obtain granular details about activities performed remotely. We did not try to ‘prescribe’ specific activity (e.g. climbing steps, mopping the floor) nor did we look for changes in activity with new or intensified therapy in this first study. Further studies are needed to determine what each device is actually measuring by prescribing specific activities and assessing the best device for a given activity. We also need to study the optimal wear time (as this could significantly influence measurements) and whether the categorical thresholds (e.g. low intensity, moderate intensity) should be adjusted for chronic diseases.
In summary, our data add to the growing body of literature which suggests that activity monitoring can provide useful information in routine clinical care and as novel endpoints in research. There is still much to be learned about what activity trackers are measuring (cardiopulmonary health and conditioning vs. environmental or behavioural confounds), and our data indicate that different devices can yield discordant results. Further studies are needed in determining what each device is actually measuring through controlled manipulation, the best type of device for a given measure, optimal wear time for assessment (as this could significantly influence measurements), and adjusted thresholds for chronic diseases.
Funding
The project described in this publication was supported by the University of Rochester, Clinical and Translational Science Award number KL2 TR001999 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
United Therapeutics (Investigator Initiated) provided funding for this study but had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Conflict of interest: DJL has received payment for speaking on behalf of United Therapeutics. The University of Rochester receives payments for clinical trial work supported by United Therapeutics. The remaining authors have no conflicts.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Daniel Lachant, Division of Pulmonary and Critical Care Medicine, University of Rochester Medical Center, 601 Elmwood Ave, Box 692, Rochester, NY 14620, USA.
Allison Light, Division of Pulmonary and Critical Care Medicine, University of Rochester Medical Center, 601 Elmwood Ave, Box 692, Rochester, NY 14620, USA.
Kevin Hannon, Department of Internal Medicine, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14620, USA.
Farrukh Abbas, Division of Pulmonary and Critical Care Medicine, University of Rochester Medical Center, 601 Elmwood Ave, Box 692, Rochester, NY 14620, USA.
Michael Lachant, Division of Pulmonary and Critical Care Medicine, University of Rochester Medical Center, 601 Elmwood Ave, Box 692, Rochester, NY 14620, USA.
R James White, Division of Pulmonary and Critical Care Medicine, University of Rochester Medical Center, 601 Elmwood Ave, Box 692, Rochester, NY 14620, USA.
References
- 1. Mecklai K, Smith N, Stern AD, Kramer DB. Remote patient monitoring—overdue or overused? N Engl J Med 2021;384:1384–1386. [DOI] [PubMed] [Google Scholar]
- 2.REBUILD Study. https://clinicaltrialsgov/ct2/show/NCT03267108 (6 November 2021, date last accessed).
- 3. Krittanawong C, Rogers AJ, Johnson KW, et al. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol 2021;18:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith MP, Horsch A, Standl M, Heinrich J, Schulz H. Uni- and triaxial accelerometric signals agree during daily routine, but show differences between sports. Sci Rep 2018;8:15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Napolitano MA, Borradaile KE, Lewis BA, et al. Accelerometer use in a physical activity intervention trial. Contemp Clin Trials 2010;31:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyden K, Keadle SK, Staudenmayer J, Freedson PS. The activPALTM accurately classifies activity intensity categories in healthy adults. Med Sci Sports Exerc 2017;49:1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 2011;32:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cordova-Rivera L, Gardiner PA, Gibson PG, Winkler EAH, Urroz PD, McDonald VM. Sedentary time in people with obstructive airway diseases. Respir Med 2021;181:106367. [DOI] [PubMed] [Google Scholar]
- 9. Matura LA, Shou H, Fritz JS, et al. Physical activity and symptoms in pulmonary arterial hypertension. Chest 2016;150:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pugh ME, Buchowski MS, Robbins IM, Newman JH, Hemnes AR. Physical activity limitation as measured by accelerometry in pulmonary arterial hypertension. Chest 2012;142:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakazato L, Mendes F, Paschoal IA, Oliveira DC, Moreira MM, Pereira MC. Association of daily physical activity with psychosocial aspects and functional capacity in patients with pulmonary arterial hypertension: a cross-sectional study. Pulm Circ 2021;11:2045894021999955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfister T, Matthews CE, Wang Q, Kopciuk KA, Courneya K, Friedenreich C. Comparison of two accelerometers for measuring physical activity and sedentary behaviour. BMJ Open Sport Exerc Med 2017;3:e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dibben GO, Gandhi MM, Taylor RS, et al. Physical activity assessment by accelerometry in people with heart failure. BMC Sports Sci Med Rehabil 2020;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karabulut M, Crouter SE, Bassett DR Jr. Comparison of two waist-mounted and two ankle-mounted electronic pedometers. Eur J Appl Physiol 2005;95:335–343. [DOI] [PubMed] [Google Scholar]
- 15. Storm FA, Buckley CJ, Mazzà C. Gait event detection in laboratory and real life settings: accuracy of ankle and waist sensor based methods. Gait Posture 2016;50:42–46. [DOI] [PubMed] [Google Scholar]
- 16. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sen-Gupta E, Wright DE, Caccese JW, et al. A pivotal study to validate the performance of a novel wearable sensor and system for biometric monitoring in clinical and remote environments. Digit Biomark 2019;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 19. Lachant DJ, Light AN, Mackin ML, Schwartz RG, White RJ. Heart rate expenditure correlates with right ventricular function. Ann Am Thorac Soc 2020;17:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lachant DJ, Light A, Offen M, Adams J, White RJ. Heart rate monitoring improves clinical assessment during 6-min walk. Pulm Circ 2020;10:2045894020972572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yorke J, Corris P, Gaine S, et al. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J 2014;43:1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019;156:323–337. [DOI] [PubMed] [Google Scholar]
- 23. Mandigout S, Lacroix J, Perrochon A, Svoboda Z, Aubourg T, Vuillerme N. Comparison of step count assessed using wrist- and hip-worn actigraph GT3X in free-living conditions in young and older adults. Front Med 2019;6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hergenroeder AL, Barone Gibbs B, Kotlarczyk MP, Perera S, Kowalsky RJ, Brach JS. Accuracy and acceptability of commercial-grade physical activity monitors in older adults. J Aging Phys Act 2019;27:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mainguy V, Provencher S, Maltais F, Malenfant S, Saey D. Assessment of daily life physical activities in pulmonary arterial hypertension. PLoS One 2011;6:e27993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sehgal S, Chowdhury A, Rabih F, et al. ; STep-count using an Accelerometer for Monitoring PAH—STAMP Study group. Counting steps: a new way to monitor patients with pulmonary arterial hypertension. Lung 2019;197:501–508. [DOI] [PubMed] [Google Scholar]
- 27. González-Saiz L, Santos-Lozano A, Fiuza-Luces C, et al. Physical activity levels are low in patients with pulmonary hypertension. Ann Transl Med 2018;6:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okumus G, Aslan GK, Arseven O, Ongen G, Issever H, Kiyan E. The role of an activity monitor in the objective evaluation of patients with pulmonary hypertension. Clin Respir J 2018;12:119–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.