Abstract
Aims
Atrial fibrillation (AF) is a major cause of morbidity and mortality. Current guidelines support performing electrocardiogram (ECG) screenings to spot AF in high-risk patients. The purpose of this study was to validate a new algorithm aimed to identify AF in patients measured with a recent FDA-cleared contact-free optical device.
Methods and results
Study participants were measured simultaneously using two devices: a contact-free optical system that measures chest motion vibrations (investigational device, ‘Gili’) and a standard reference bed-side ECG monitor (Mindray®). Each reference ECG was evaluated by two board certified cardiologists that defined each trace as: regular rhythm, AF, other irregular rhythm or indecipherable/missing. A total of 3582, 30-s intervals, pertaining to 444 patients (41.9% with a history of AF) were made available for analysis. Distribution of patients with active AF, other irregular rhythm, and regular rhythm was 16.9%, 29.5%, and 53.6% respectively. Following application of cross-validated machine learning approach, the observed sensitivity and specificity were 0.92 [95% confidence interval (CI): 0.91–0.93] and 0.96 (95% CI: 0.95–0.96), respectively.
Conclusion
This study demonstrates for the first time the efficacy of a contact-free optical device for detecting AF.
Keywords: Contact free, Vital signs, Heart rate, Respiratory rate, Laser, Camera
Graphical abstract
Introduction
The prevalence of atrial fibrillation (AF) is increasing and is expected to double by 2050.1 The cost of care for AF is expected to be around 2% of total healthcare expenditure in high-income countries.2 Diagnosing AF is of paramount importance since anticoagulation has been shown to reduce the incidence of stroke by up to 65%.3,4 In patients presenting with ischaemic stroke, about 10% will be diagnosed with AF for the first time.5 The current European Society of Cardiology recommends screening by opportunistic pulse palpation or electrocardiogram (ECG) in all patients ≥65 years when in contact with health service.6 It was shown to be cost effective in this age group.7 The existing gold standard for arrhythmia diagnosis is the ECG, done and interpreted by a trained medical professional.
Currently, there are several self-monitoring devices that can detect cardiac arrhythmia including AF; however, they all require contact and may result in discomfort to the user thereby limiting their use. Repetitive screening has been showed to increase the sensitivity of AF detection—the Swedish STROKESTOP Study8 screened 7173 participants aged 75–76 years from the general population with a single-lead ECG and found that 0.5% had previously undiagnosed AF and up to 3% if repeated ECGs are performed in a 2-week period. Recently, this same study showed a positive net clinical benefit including stroke and bleeding suggesting that is safe and beneficial.9 Another study suggested even higher rate of undiagnosed AF to be higher, i.e. closer to 10%.10 In patients with a recent stroke, extended screening for AF led to higher rates of AF being discovered.11 Screening patients with hypertension, led to a 10-fold increase in AF diagnosis and the initiation of anticoagulant therapy.12
Clearly, patient adherence to monitoring protocols is key to increasing the chances for identifying episodes of AF. In addition, making the screening process easier has been shown to be associated with higher usage.13
In this sense, contact-free measurement solutions offer simplified and non-intrusive user-experience, potentially enhancing long-term adherence to screening protocols. Such contact-free screening tools may also be of particular interest amid the recent COVID-19 pandemic.
The purpose of this study was to validate a new algorithm aimed to identify AF in patients measured with a recent FDA-cleared contact-free optical device.
Methods
Ethics and study population
Patients presenting to the cardiology ward and/or outpatient clinics at the Tel Aviv Sourasky Medical Center for various indications were prospectively recruited to this study (IRB approvals # TLV-0168-19, TLV-0438-18, and TLV-0692-20). All study subjects provided written informed consent before their participation. For all subjects, the following information was recorded: demographics, height, weight, medical history, concomitant medications and components of the CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes mellitus, previous stroke, vascular disease, and sex category) score.3 Assignment of patients into active arrhythmia/control groups were based on blinded analysis of their respective ECG traces as recorded during study procedure (see below).
Study devices
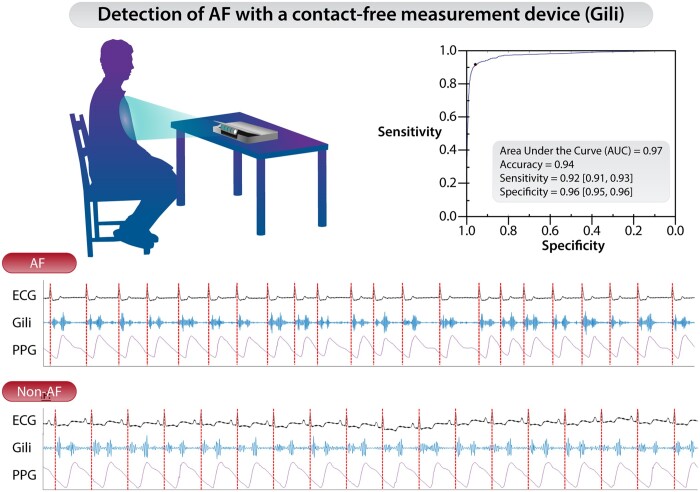
Study participants were measured simultaneously using two devices: (i) Gili Pro BioSensor (investigational device, ‘Gili’) and (ii) a standard reference bed-side monitor, inclusive of a 6-lead ECG (Mindray® Benevision™ N1 or Somnomedics® SOMNOtouch™ NIBP). The Gili device is an FDA-cleared, contact-free optical system consisting of an optical sensing unit controlled by a tablet. The device consists of an illuminating light source (Class 1, eye safe laser), digital image sensor, range meter, and firmware to facilitate data processing (Figure 1). The system functions on the principle that subtle movements of a given surface (e.g. the thoracic wall) are recognized when illuminated via the light source while the device simultaneously captures the back reflected light pattern via the image sensor. Temporal changes in the reflected light pattern (known as speckle pattern) are coupled with the motions of the illuminated surface, which are affected by heart and breathing motions. Analysis of these motion-coupled light patterns enables correlation of these motions with different physiological manifests (e.g. seismo/phonocardiography) of which specific vital signs, like heart and respiratory rates, may be derived.14–17 The device is placed on a rigid surface in front of the inspected subject and captures only motion-vibration data, without any physical contact. No full-scale images of the inspected subject are taken at any time via the Gili device, essentially maintaining the subject’s privacy. The device has been validated on, and capable of measuring patients with different BMIs, chest shapes, skin pigmentation, and multiple clothing layers17 and is cleared for use in different ambient lighting conditions for spot-measurement of heart rate and respiratory rate (FDA authorization # DEN200038). Of note—the Gili device is intended for spot-measurement purposes only, on a patient compliant with the measurement procedure, and not for continuous measurement of physiological data.
Figure 1.
Gili Pro BioSensor. (A) Gili sensing unit. (B) Measurement setup.
Study endpoints
The two co-primary endpoints of this study were sensitivity and specificity, i.e. estimates of the Gili AF algorithm to correctly detect or rule out AF compared to standard cardiologist overread ECG (CORE), with point estimates of at least 0.9 each endpoint separately.
Procedure and test methods
Following signing of informed consent, patients were asked to sit calmly either in a chair in front of a table on which the Gili device was placed, or in a bed with the backrest raised in front of a cardiac table on which the device was positioned. For comparative reference measurements, ECG electrodes were placed on the patient’s chest according to the manufacturer’s guidelines. The Gili and reference devices were prompted to take a synchronized measurement session of up to 5 min. During this time, patients were asked to remain still and refrain from talking. Following completion, data were exported from both devices for offline comparisons. Representative comparative signals derived from the ECG, Gili, and photoplethysmogram (PPG) are depicted in Figure 2.
Figure 2.
Comparative synchronized signals derived from electrocardiogram, Gili (investigational device), and photoplethysmogram for patients with and without atrial fibrillation. The red dotted lines mark the R-waves on the electrocardiogram.
Annotation of reference data
All analyses were based on 30-s measured intervals, similar to other marketed spot-measurement devices e.g. the AliveCor® KardiaMobile ECG device.18 Verification of reference ECG waveform data quality was done via manual inspection by two board certified cardiologists blinded to each other. Main criteria for verifying waveform validity was based on confirming the absence of: low-quality signals/artifacts/missing data. In order for an interval to be considered valid, both cardiologists were to agree as to the validity of each interval. In case either cardiologist deemed an interval as non-valid, it was excluded from further downstream analysis. Intervals with an active pacemaker rhythm were also excluded as they could bias the results obtained via downstream analyses. For intervals that were deemed valid by both cardiologists, in case their interpretation was not precisely agreed upon, a third cardiologist was sought and a decision was made based on the majority rule. Ultimately, ECG traces were annotated via one of the following options: (i) regular rhythm (normal rhythm), (ii) AF, (iii) other irregular rhythm [i.e. ventricular premature beat, atrial premature beat (APC), sinus arrhythmia, 2nd/3rd degree atrio-ventricular (AV) block], and (iv) indecipherable (low quality, artefacts, missing, etc.).
Development and evaluation of the Gili AF algorithm’s diagnostic accuracy
Algorithm development was conducted on the basis of a classifier that provides an output on a scale of zero (0) to one (1), essentially representing the likelihood that the measured interval is deemed to be a case (i.e. close to 1) or a control (i.e. close to 0). As such, a borderline score close to 0.5 is deemed inconclusive and is thus omitted from being displayed as part of the device’s outputs. Above-mentioned annotated data were used for algo-training and validation purposes based on a cross-validation approach19–21 (see below). Algo-training was done to: (i) define a machine learning model which includes parameters applied to a set of descriptors relating to periodicity and time-frequency properties of the processed signal and (ii) specify thresholds used to define the best cut-offs for discriminating between a predicted case, control and inconclusive measurements with borderline scores (i.e. to maximize the performance accuracy of the algorithm).
Cross-validation19–21 was used to estimate the preliminary accuracy of the algorithm. Our implementation of cross-validation was based on the ‘leave one user out’ approach: we assume our training database contains N users with one or multiple measurements each; the ‘leave one user out’ process is repeated N times, with one iteration per every user; at each iteration, all measurements from a specific user are to be used for testing, while the data from the rest of N − 1 users is used to optimize (train) the algorithm’s parameters. Eventually, the scores for all measurements are computed based on an algorithm trained on data that did not contain a single measurement of a tested user per each iteration. The overall method sensitivity and specify were computed at all possible classification thresholds and were visualized via the receiver-operator characteristics curve. Ultimately. the recommended classification threshold was selected at the point of maximal balanced accuracy ([sensitivity + specificity]/2). Lastly, positive and negative predictive values (PPV and NPV) were also estimated—once on the prevalence of AF within the studied sample set, and second based on an expected AF prevalence of 10% in the population aged ≥65 years.10 Positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were also estimated. The data underlying this article will be shared on reasonable request to the corresponding author.
Covariate effects and additional statistical considerations
Potential effects of covariates on device performance were assessed by evaluating the impact of different covariates on the classification accuracy. Specifically with respect to Gili, since the device measures motion-vibration data, potential effects of conditions with acoustical manifestations and/or manifestations on breathing patterns were also examined (i.e. presence of valvular disorders and known respiratory conditions). For continuous and non-binary covariates (age, height, weight, BMI, and CHA2DS2-VASc score), the Kolmogorov–Smirnov test was applied; for all other binary covariates, the Fisher’s exact test was applied. Descriptive statistics for continuous variables are expressed as means and standards deviations if normally distributed, or otherwise by medians with interquartile ranges. Categorical variables were expressed using number of observations and percentages. 95% confidence intervals (CIs) were constructed using a 1000 bootstrap resampling with replacement approach. Since there are multiple measurement per each patient, the bootstrap resampling was done as follows: (i) randomly pick a patient, (ii) randomly pick a measurement for a selected patient, and (iii) repeat i–ii as the total number of patients. A P-value of <0.05 was considered statistically significant. All analyses were conducted using MATLAB (MathWorks®) release 2021b.
Results
Study population and distribution of cardiac rhythms
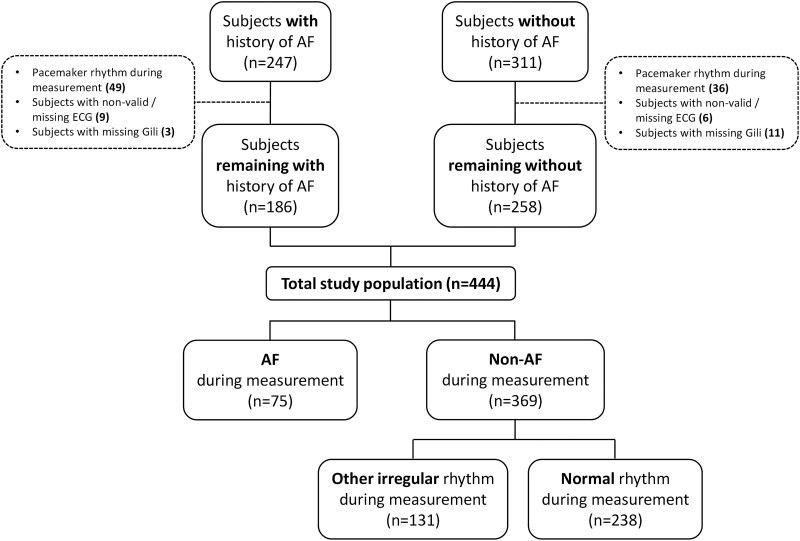
A total of 558 patients were recruited. One hundred and fourteen measured subjects were excluded due to the following reasons: 85 had pacemaker rhythm, 15 had non-valid or missing reference ECG data, and 14 were missing valid Gili data. Ultimately, a final set comprising 444 patients (41.9% with a history of AF) encompassing 3582, 30-s intervals, was used for downstream analyses. Of the 3582 30-s intervals, only 10 (0.3%) required a third cardiologist for determining their final interpretation based on the majority rule. Of all evaluable 30-s intervals, prevalence of AF and other arrhythmias was 16.6% and 15.5% (premature beats, etc.), respectively. Study flowchart is depicted in Figure 3. Characteristics of the final set of analysed study subjects are summarized in Table 1. Distribution of types of rhythms present across all evaluable measurements is summarized in Table 2.
Figure 3.
Study flowchart.
Table 1.
Characteristics of analysed study subjects (n = 444)
| Characteristics | Total population (n = 444) | History of AF |
Active AF during measurement |
||
|---|---|---|---|---|---|
| Yes (n = 186) | No (n = 258) | Yes (n = 75) (68 with previous AF history) | No (n = 369) | ||
| Age (years), mean (SD) | 69 (13) | 73 (10) | 66 (14) | 73 (10) | 68 (13) |
| Gender (males), n (%) | 291 (66%) | 117 (63%) | 174 (67%) | 48 (64%) | 243 (66%) |
| Gender (female), n (%) | 153 (34%) | 69 (37%) | 84 (33%) | 27 (36%) | 126 (34%) |
| Height (cm), mean (SD) | 168 (10) | 168 (11) | 168 (9) | 168 (11) | 168 (10) |
| Weight (kg), mean (SD) | 80 (16) | 79 (15) | 80 (17) | 79 (16) | 80 (17) |
| BMI (kg/m2), mean (SD) | 28 (6) | 28 (5) | 28 (6) | 28 (5) | 28 (6) |
| BMI ≥30, n (%) | 131 (30%) | 53 (28%) | 78 (30%) | 23 (31%) | 108 (29%) |
| Smoking, n (%) | 67 (15%) | 22 (12%) | 45 (17%) | 9 (12%) | 58 (16%) |
| Risk factors | |||||
| Heart failure, n (%) | 354 (80%) | 157 (84%) | 197 (76%) | 67 (89%) | 287 (78%) |
| Hypertension, n (%) | 256 (58%) | 126 (68%) | 130 (50%) | 49 (65%) | 207 (56%) |
| Diabetes, n (%) | 148 (33%) | 68 (37%) | 80 (31%) | 27 (36%) | 121 (33%) |
| Stroke/TIA/thromboembolism, n (%) | 39 (9%) | 20 (11%) | 19 (7%) | 7 (9%) | 32 (9%) |
| Vascular disease, n (%) | 197 (44%) | 81 (44%) | 116 (45%) | 31 (41%) | 166 (45%) |
| CHA2DS2-VASc score, median (IQR) | 4 (3–5) | 4 (3–5) | 4 (2–5) | 4 (3–5) | 4 (3–5) |
| CHA2DS2-VASc score ≥2, n (%) | 402 (91%) | 180 (97%) | 222 (86%) | 75 (100%) | 327 (89%) |
| Other medical conditions | |||||
| Respiratory conditions (COPD, asthma, pulmonary HTN, pulmonary embolism, lung cancer, etc.) | 76 (17%) | 40 (22%) | 36 (14%) | 16 (21%) | 60 (16%) |
| Valvular disorders (any type; mild, moderate or severe) | 210 (47%) | 121 (65%) | 89 (34%) | 57 (76%) | 153 (41%) |
| Medications | |||||
| Anticoagulant, n (%) | 191 (43%) | 165 (89%) | 26 (10%) | 63 (84%) | 128 (35%) |
| Antiplatelet, n (%) | 229 (52%) | 64 (34%) | 165 (64%) | 21 (28%) | 208 (56%) |
| ACEi, n (%) | 128 (29%) | 47 (25%) | 81 (31%) | 16 (21%) | 112 (30%) |
| ARB, n (%) | 86 (19%) | 46 (25%) | 40 (16%) | 17 (23%) | 69 (19%) |
| CCB, n (%) | 100 (23%) | 50 (27%) | 50 (19%) | 20 (27%) | 80 (22%) |
| Beta-blocker, n (%) | 299 (67%) | 153 (82%) | 146 (57%) | 67 (89%) | 232 (63%) |
| Diuretic (any), n (%) | 195 (44%) | 114 (61%) | 81 (31%) | 52 (69%) | 143 (39%) |
| Antiarrhythmic, n (%) | 77 (17%) | 72 (39%) | 5 (2%) | 24 (32%) | 53 (14%) |
ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes mellitus, previous stroke, vascular disease, and sex category; COPD, chronic obstructive pulmonary disease; HTN, hypertension; IQR, interquartile range; SD, standard deviation; TIA, transient ischaemic attack.
Table 2.
Distribution of types of rhythms evident across the 30-s intervals acquired during study measurements (n = 3582)
| Type of rhythm | # of segments | % of segments |
|---|---|---|
| Normal rhythm | 2433 | 67.9 |
| APC (<4) | 130 | 15.5 |
| APC—multiple (≥4) | 126 | |
| VPC (<4) | 153 | |
| VPC—multiple (≥4) | 69 | |
| 2nd/3rd degree AV block | 2 | |
| Sinus arrhythmia | 76 | |
| Atrial fibrillation | 593 | 16.6 |
| Total | 3582 | 100 |
Number of occurrences per 30-s intervals.
APC, atrial premature beat; AV, atrio-ventricular; VPC, ventricular premature beat.
Diagnostic accuracy
Five percent of the measured segments were deemed inconclusive by the algorithm, thus ensuing 95% of the segments with valid predictions by the algorithm (which is comparable and even exceeds performances reported for other marketed devices).22,23 Subsequently, the observed sensitivity and specificity were 0.92 (95% CI: 0.91–0.93) and 0.96 (95% CI: 0.95–0.96), respectively (Figure 4 and Table 3) accounting for a balanced accuracy of 0.94. In all instances, the observed estimates of PPVs and NPVs were at least 0.7 and 0.98, respectively (Table 3), which are similar to those reported for other marketed devices.23,24 Lastly, estimates of PLR and NLR were promising, with a minimum observed PLR of 21 and a maximum observed NLR of 0.087 (Table 3), essentially denoting a high probability of correctly identifying or refuting the presence of AF (respectively) based on device outputs.
Figure 4.

Receiver-operator characteristics curve depicting potential performance of the Gili atrial fibrillation algorithm when compared to cardiologist over-read electrocardiogram.
Table 3.
Diagnostic accuracy of the Gili atrial fibrillation algorithm when compared to cardiologist overread electrocardiogram
| Variable | Point estimates [95% CI] |
|---|---|
| Sensitivity | 0.92 [0.91, 0.93] |
| Specificity | 0.96 [0.95, 0.96] |
| PPVSample | 0.81 [0.79, 0.82] |
| NPVSample | 0.98 [0.98, 0.99] |
| PPV10% | 0.7 [0.69, 0.72] |
| NPV10% | 0.99 [0.99, 0.99] |
| PLR | 21 [18, 25] |
| NLR | 0.087 [0.078, 0.098] |
Sample: based on prevalence from study sample set; 10%: Based on expected prevalence of 10% (9).
CI, confidence interval; NLR: negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
Covariate effects
Covariate analysis (Table 4) revealed a significant difference/effect with respect to the presence of other active arrhythmias on device performance (P-value = 0.00101; constituting 15.5% of the measured intervals). A more detailed assessment across specific types of arrhythmias revealed that specifically multiple APCs are probably accountable for this significant effect (P-values = 0.00731; constituting 3.5% of the measured intervals), whereas others do not. Lastly, although a trend was identified with respect to effect of age on device performance (P-values = 0.0861), this trend is most likely attributed to the inherent age difference between patients presenting without AF/other arrhythmias and those with active AF, with the latter being higher at an average of 5 years compared to the former. No effects of disease conditions with acoustical manifestations and/or potential manifestations of breathing patterns on device performance were observed.
Table 4.
Covariate effects on device performance (i.e. misclassifications)
| Covariate | P-Value |
|---|---|
| Gender | 0.638 |
| Age | 0.0861 |
| Height | 0.466 |
| Weight | 0.554 |
| BMI | 0.596 |
| BMI ≥30 | 0.629 |
| Smoking | 0.62 |
| Risk factors | |
| Heart failure | 0.598 |
| Hypertension | 0.494 |
| Diabetes | 0.568 |
| Stroke/TIA/thromboembolism | 0.714 |
| Vascular disease | 0.649 |
| CHA2DS2-VASc | 0.615 |
| CHA2DS2-VASc score ≥2 | 0.658 |
| Other medical conditions | |
| Respiratory condition (COPD, asthma, pulmonary HTN, pulmonary embolism, lung cancer, etc.) | 0.521 |
| Valvular disorder (any type; mild, moderate, or severe) | 0.614 |
| Medications | |
| Anticoagulant | 0.417 |
| Antiplatelet | 0.584 |
| ACEi | 0.661 |
| ARB | 0.615 |
| CCB | 0.349 |
| Beta-blocker | 0.536 |
| Diuretic | 0.37 |
| Antiarrhythmic | 0.603 |
| Arrhythmias | |
| Active other arrhythmias | 0.00101 |
| APC | 0.637 |
| APC—multiple | 0.00731 |
| VPC | 0.629 |
| VPC—multiple | 0.216 |
| 2nd/3rd degree AV block | 1a |
| Sinus arrhythmia | 0.171 |
ACEi, angiotensin-converting enzyme inhibitor; APC, atrial premature beat; ARB, angiotensin receptor blocker; AV, atrio-ventricular; CCB, calcium channel blocker; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes mellitus, previous stroke, vascular disease, and sex category; COPD, chronic obstructive pulmonary disease; HTN, hypertension; TIA, transient ischaemic attack; VPC, ventricular premature beat.
Available sample size is small.
Complementary analysis: potential effects of inconclusive measurements
For completeness, to assess potential worse-case scenario, the diagnostic accuracy of the Gili AF algorithm was assessed once more, only this time without excluding the fraction (5%) of 30-s intervals, which were deemed with inconclusive scores by the algorithm. In this case, as expected, the observed diagnostic accuracy was slightly impeded, with an estimated sensitivity and specificity of 0.9 (95% CI: 0.89–0.91) and 0.94 (95% CI: 0.93–0.95), but still met the requirements set-forth via the co-primary endpoints (see Supplementary material online, Table S1). No new significant covariate effects were observed with respect to this broader sample set (see Supplementary material online, Table S2).
Discussion
This study analysed a total of 444 subjects, 75 with active AF (of which 91% had a previous history of AF) and 369 controls (a third of which included intervals with other irregular rhythms). Overall, the AF algorithm of the Gili device (an FDA-cleared contact-free optical device) was able to detect AF with a 92% sensitivity and a 96% specificity accounting for a diagnostic accuracy of 94% meeting the study's co-primary endpoints.
The performance of the algorithm was only affected by the presence of other arrhythmias, with multiple premature beats showing the most influential bias. Such a phenomenon is not uncommon and has been documented in other devices/mobile applications analysing non-ECG signals via algorithms relying on properties of periodicity in the heart rhythm.25 In this sense, previous clinical investigations have identified a strong correlation between the presence of APCs and the risk for future AF development.26 Analysis from the REGARDS study27,28 reported an increased risk of 92% for future AF among patients with APCs. In the appropriate clinical context, it might be worth to continue screening these groups for AF and/or presence of multiple APCs.
When considering the short measurement duration required by the device (30-s intervals), the diagnostic accuracy of Gili’s AF algorithm is very much comparable to other marketed mobile applications and devices (assessing signals acquired via single-lead ECG, PPG, or peripheral pulsations), that also commonly utilize short measurement durations, or multiple measurements as means for providing a single output.3,18,29–31 That said, it should be noted that for detecting paroxysmal AF, longer measurement durations are preferred, in which continuous measurement devices are advantageous.
AF has been implicated in heart failure admissions,32 ischaemic stroke,5 and death.33 Screening for AF has been recognized to improve patient care and overall outcomes,4,34 ultimately leading to reduction in healthcare expenditures, and as such has been incorporated as a recommendation in recent guidelines.6,8,35
Connected personal devices, such as wearables in the form of watches, on-skin patches, chest belts, necklaces, etc., enable remote monitoring of subjects for various vital signs. The advantage of using mobile technologies to monitor and detect AF is well recognized.3,12,31 Most of these are wearables that require close-contact with the patient’s skin.36 The most well-recognized technology is based on the measurement of single-lead ECG via smartwatches/chest straps/patches. In recent years, attempts to use PPG technology to identify AF have been sought, mainly via use of a device with the classical light-emitting diode and a receiving photodiode, or via use of smartphone cameras.37,38 Although results are promising, this technology has two drawbacks: (i) those that require contact are prone to bias by other factors such as skin colour, and motion artefacts, and (ii) those that operate remotely are also highly dependent on ambient lighting conditions and temperature.39–41
However, such devices become redundant if patients or clinicians are reluctant to use them for various reasons (discomfort, known allergic reactions/irritations to ECG patches, technological limitations, etc.).42 Such acceptance issues are becoming increasingly important if one seeks to increase screening rates to diagnose AF, which is commonly a paroxysmal medical condition. In this sense, a recent review across 31 studies showed that >60% of elderly people were interested in using connected devices, but also stressed the need to consider human-factors associated challenges so to increase long-term acceptance rate.43 Contact-free and accurate monitoring solutions will most probably serve to ameliorate a lot of caveats associated with wearable devices, thereby potentially advancing adoption and patient adherence, not to mention ameliorating the risk for nosocomial infections contracted at the in-clinic setting. In future context, such systems may even be incorporated into personal computers, televisions, etc.
In recent years, amid the COVID-19 pandemic, contact-free medical data acquisition has transformed from a nice-to-have technology to a must-have technology, especially outside the clinical setting. Although the Gili Pro device is limited with respect to its usefulness at the in-patient setting (where other established methods are more readily available), with proper usability adjustments (currently being deployed in a future model of the device), such a device can be incorporated into current telemedicine/remote patient care services for use on or even by patients at risk or with confirmed arrhythmias for periodic measurements. Screening for arrhythmia as part of the daily vital signs measurement already done by patients (part of which the Gili Pro can also identify) could serve as means for preventing future exacerbations and hospitalizations, especially among high-risk patients.
Although this study includes a large and diverse cohort of patients, it has four main limitations: (i) The number of patients with active AF made available for this study was relatively small compared to the control set. (ii) The performance of the AF algorithm was based on a cross-validation approach, which, although recognized as a valid method for validating algorithmic performance, is not considered routinely acceptable for true clinical validation purposes. Owing to the above, a follow-up clinical validation study on a new and blinded cohort of patients is mandated to re-validate this study’s outcomes. (iii) The Gili Pro device in its current form is intended for spot-measurement by a professional and thus requires the patient to sit still, similar to other contemporary cardiovascular screening tools (12-lead ECG, impedance cardiography, etc.). (4) The Gili Pro device requires the patient to comply with the measurement procedure and as such its duration is short (30 s). Future models will enable passive screening while watching television or working on a computer.
In conclusion, this is the first study to report the ability of a contact-free optical system, Gili Pro BioSensor, to identify AF with a high degree of accuracy (Graphical Abstract).
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Funding
Donisi Health (no grant numbers).
Conflict of interest: O.G., J.C.S., R.R.-O., J.R.-R., Z.Z., J.G.-M., M.S., S.P., and Y.A. hold equity with Donisi Health. The other authors report no conflict of interest.
Supplementary Material
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart S, Murphy NF, Murphy N, Walker A, McGuire A, McMurray JJV.. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hindricks G, Potpara T, Dagres N, et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 4. López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friberg L, Rosenqvist M, Lindgren A, Terént A, Norrving B, Asplund K.. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke 2014;45:2599–2605. [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 7. Hobbs FD, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9:iii, ix–x, 1–74. [DOI] [PubMed] [Google Scholar]
- 8. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M.. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 9. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M.. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–1506. [DOI] [PubMed] [Google Scholar]
- 10. Turakhia MP, Shafrin J, Bognar K, et al. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One 2018;13:e0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buck BH, Hill MD, Quinn FR, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA 2021;325:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, et al. ; SCREEN-AF Investigators and Coordinators. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Nielsen K, Goldberg J, et al. Association of wearable device use with pulse rate and health care use in adults with atrial fibrillation. JAMA Netw Open 2021;4:e215821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zalevsky Z, Beiderman Y, Margalit I, et al. Simultaneous remote extraction of multiple speech sources and heart beats from secondary speckles pattern. Opt Express 2009;17:21566–21580. [DOI] [PubMed] [Google Scholar]
- 15. Ozana N, Margalith I, Beiderman Y, et al. Demonstration of a remote optical measurement configuration that correlates with breathing, heart rate, pulse pressure, blood coagulation, and blood oxygenation. Proc IEEE 2015;103:248–262. [Google Scholar]
- 16. Zeev Zalevsky JG. Motion detection system and method. Israeli Patent Application No. 184868; 2007.
- 17. Havakuk O, Sadeh B, Merdler I, et al. Validation of a novel contact-free heart and respiratory rate monitor. J Med Eng Technol 2021;45:344–350. [DOI] [PubMed] [Google Scholar]
- 18. Desteghe L, Raymaekers Z, Lutin M, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 19. Hastie T, Tibshirani R, Friedman JH.. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed.New York: Springer; 2009. [Google Scholar]
- 20. Kennedy A, Finlay DD, Guldenring D, Bond RR, Moran K, McLaughlin J.. Automated detection of atrial fibrillation using R-R intervals and multivariate-based classification. J Electrocardiol 2016;49:871–876. [DOI] [PubMed] [Google Scholar]
- 21. Raghunath S, Pfeifer JM, Ulloa-Cerna AE, et al. Deep neural networks can predict new-onset atrial fibrillation from the 12-lead ECG and help identify those at risk of atrial fibrillation-related stroke. Circulation 2021;143:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brasier N, Raichle CJ, Dörr M, et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019;21:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B.. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: diagnostic accuracy study of the FibriCheck App. JMIR Mhealth Uhealth 2019;7:e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez MV, Mahaffey KW, Hedlin H, et al. ; Apple Heart Study Investigators. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B.. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: diagnostic accuracy study of the FibriCheck App. JMIR Mhealth Uhealth 2019;7:e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soliman EZ, Howard G, Judd S, Bhave PD, Howard VJ, Herrington DM.. Factors modifying the risk of atrial fibrillation associated with atrial premature complexes in patients with hypertension. Am J Cardiol 2020;125:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 28. O'Neal WT, Kamel H, Judd SE, et al. Usefulness of atrial premature complexes on routine electrocardiogram to determine the risk of atrial fibrillation (from the REGARDS Study). Am J Cardiol 2017;120:782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Sullivan JW, Grigg S, Crawford W, et al. Accuracy of smartphone camera applications for detecting atrial fibrillation: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e202064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mairesse GH, Moran P, Van Gelder IC, et al. ; ESC Scientific Document Group. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017;19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 31. Jones NR, Taylor CJ, Hobbs FDR, Bowman L, Casadei B.. Screening for atrial fibrillation: a call for evidence. Eur Heart J 2020;41:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmed A, Ullah W, Hussain I, et al. Atrial fibrillation: a leading cause of heart failure-related hospitalizations; a dual epidemic. Am J Cardiovasc Dis 2019;9:109–115. [PMC free article] [PubMed] [Google Scholar]
- 33. Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E.. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016;68:2508–2521. [DOI] [PubMed] [Google Scholar]
- 34. Hart RG, Pearce LA, Aguilar MI.. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 35. Freedman B, Camm J, Calkins H, et al. ; AF-Screen Collaborators. Screening for atrial fibrillation: a report of the AF-SCREEN international collaboration. Circulation 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 36. Cheung CC, Krahn AD, Andrade JG.. The emerging role of wearable technologies in detection of arrhythmia. Can J Cardiol 2018;34:1083–1087. [DOI] [PubMed] [Google Scholar]
- 37. Krivoshei L, Weber S, Burkard T, et al. Smart detection of atrial fibrillation†. Europace 2017;19:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee JS, Lin KW, Syue JL.. Smartphone-based heart-rate measurement using facial images and a spatiotemporal alpha-trimmed mean filter. Technol Health Care 2016;24 Suppl 2:S777–83. [DOI] [PubMed] [Google Scholar]
- 39. Benedetto S, Caldato C, Greenwood DC, Bartoli N, Pensabene V, Actis P.. Remote heart rate monitoring - assessment of the facereader rPPg by Noldus. PLoS One 2019;14:e0225592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ni A, Azarang A, Kehtarnavaz N.. A review of deep learning-based contactless heart rate measurement methods. Sensors (Basel) 2021;21:3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamura T. Current progress of photoplethysmography and SPO(2) for health monitoring. Biomed Eng Lett 2019;9:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergmann JH, McGregor AH.. Body-worn sensor design: what do patients and clinicians want? Ann Biomed Eng 2011;39:2299–2312. [DOI] [PubMed] [Google Scholar]
- 43. Kekade S, Hseieh CH, Islam MM, et al. The usefulness and actual use of wearable devices among the elderly population. Comput Methods Programs Biomed 2018;153:137–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.