Abstract
Aims
Within the TeleCheck-AF project, numerous centres in Europe used on-demand photoplethysmography (PPG) technology to remotely assess heart rate and rhythm in conjunction with teleconsultations. Based on the TeleCheck-AF investigator experiences, we aimed to develop an educational structured stepwise practical guide on how to interpret PPG signals and to introduce typical clinical scenarios how on-demand PPG was used.
Methods and results
During an online conference, the structured stepwise practical guide on how to interpret PPG signals was discussed and further refined during an internal review process. We provide the number of respective PPG recordings (FibriCheck®) and number of patients managed within a clinical scenario during the TeleCheck-AF project. To interpret PPG recordings, we introduce a structured stepwise practical guide and provide representative PPG recordings. In the TeleCheck-AF project, 2522 subjects collected 90 616 recordings in total. The majority of these recordings were classified by the PPG algorithm as sinus rhythm (57.6%), followed by AF (23.6%). In 9.7% of recordings, the quality was too low to interpret. The most frequent clinical scenarios where PPG technology was used in the TeleCheck-AF project was a follow-up after AF ablation (1110 patients) followed by heart rate and rhythm assessment around (tele)consultation (966 patients).
Conclusion
We introduce a newly developed structured stepwise practical guide on PPG signal interpretation developed based on presented experiences from TeleCheck-AF. The present clinical scenarios for the use of on-demand PPG technology derived from the TeleCheck-AF project will help to implement PPG technology in the management of AF patients.
Keywords: Atrial fibrillation, Heart rhythm disorders, Mobile health, Photoplethysmography, Structured stepwise approach
Introduction
Heart rhythm disorders are frequently encountered and treated in cardiology outpatient clinics. Traditionally, an electrocardiogram (ECG) is recommended to assess heart rate and rhythm and to diagnose potential cardiac arrhythmias. In addition to ECG, novel mobile health (mHealth) technologies have been introduced to assist in the detection of arrhythmias as well as to support remote management of patients with arrhythmias.1 Some of these mHealth devices use (single/poly-lead) ECGs, whereas others base their assessment on photoplethysmography (PPG).
The differences in technologies have important implications. Current AF guidelines2 state that ECG documentation, either on standard 12-lead ECG or on a single-lead ECG tracing, is required to establish the diagnosis of AF. Although PPG technology thus cannot be used to diagnose AF, it displays an excellent accuracy to detect AF in patients with diagnosed AF.3 Therefore, PPG technology can be of value in heart rate and rhythm assessment to support the management of patients with a diagnosis of a typical arrhythmia such as AF.
During the coronavirus disease 2019 (COVID-19) pandemic, we initiated the TeleCheck-AF project. TeleCheck-AF is an international, multicentre project to provide an infrastructure to maintain AF management through the combination of teleconsultation with on-demand heart rate and rhythm monitoring using a CE-marked mobile phone app incorporating PPG technology (FibriCheck®, Qompium, Hasselt, Belgium).4,5
Despite the rapid uptake and wide use of PPG-based technology in current clinical practice, physicians and allied healthcare professionals are not introduced to this new technology in a structured way. In contrast to ECG courses, PPG analysis and interpretation are not yet part of the medical curricula. The 2020 ESC core curriculum for the cardiologist states that a physician should be able to ‘use modalities of heart rhythm monitoring’, but also stresses the limitations of consumer devices, particularly in patients with palpitations.6 In addition, surveys distributed within the TeleCheck-AF project7 and the result of the recent wEHRAbles survey8 showed that physicians are less likely to take clinical actions based on a PPG recording alone, as compared to a single-lead ECG alone. Unfamiliarity with the technology might lead to a lack of confidence, therewith making physicians less likely to use it in clinical practice.
This PPG dictionary aims to provide an overview on PPG technology and describe an educational structured stepwise practical guide on how to analyse and interpret PPG signals recorded within the TeleCheck-AF project. By introducing clinical scenarios where the use of on-demand app-based PPG heart rate and rhythm monitoring can be of clinical benefit and presenting several representative PPG recordings, the task of this manuscript also covers the creation of an educational tool and support to implement PPG technology in clinical practice.
Methods
The TeleCheck-AF project
The TeleCheck-AF approach4 and the onboarding of centres as well as the centre- and patient-experiences7 are described elsewhere.
Photoplethysmography technology and accuracy to detect atrial fibrillation
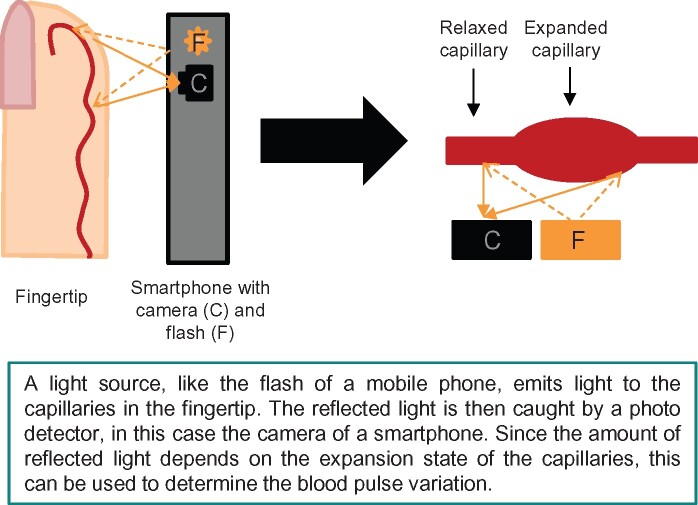
In the TeleCheck-AF project, a CE-marked mobile phone app incorporating PPG technology (FibriCheck®, Qompium, Hasselt, Belgium) was used.4,5 Photoplethysmography is a technology which determines blood volume pulse variation in the local arterioles of the fingertip by measuring the amount of reflected light in the built-in camera of smart devices.9,10 The application then converts the 60 Hz video data to raw signals.11 In this way, each individual pulse wave can be detected. The time intervals between consecutive pulse signals can be used to determine the heart rhythm and differentiate normal sinus rhythm from AF.9Figure 1 depicts an overview of the PPG mechanism.
Figure 1.
Overview of photoplethysmography mechanisms.
Several studies have been performed to determine the accuracy of PPG-based devices to detect ECG-confirmed AF.3,12,13 A meta-analysis by O’Sullivan et al. including four PPG-based mHealth devices presented an overall sensitivity and specificity of 94.2 and 95.8%, respectively.3 The FibriCheck® algorithm involving artificial intelligence shows an overall sensitivity and specificity for AF detection of 95.6% and 96.6%, respectively, when compared to a standard 12-lead ECG.9 A study comparing the beat-to-beat detection by the FibriCheck® algorithm with wearable ECG recorders showed a high correlation of 0.993.11 This correlation was strong, both for patients with regular rhythms as well as for patients with AF. Importantly, within the TeleCheck-AF project, the treating healthcare providers have access to the raw data of the PPG signals together with the RR-tachogram and Poincaré/Lorenz plot. Additionally, certified technicians review all irregular PPG recordings and the results are integrated in the cloud dashboard. This may even further increase the accuracy to detect AF episodes.
Visual presentation of the raw photoplethysmography traces
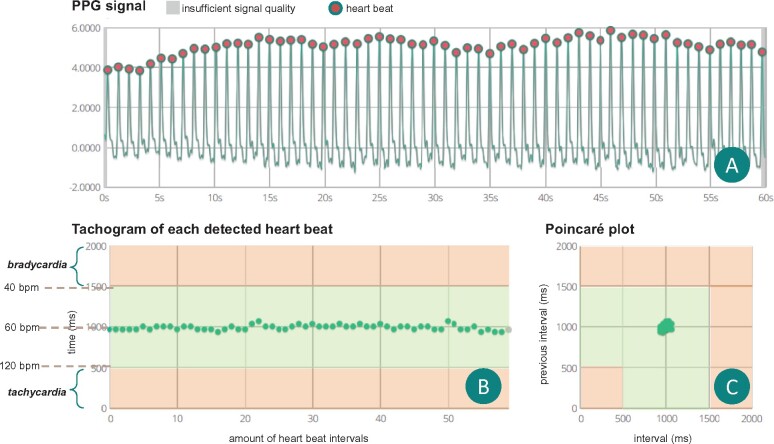
Figure 2A shows the result of a representative 60- PPG recording of a regular rhythm (sinus rhythm) (FibriCheck® application). In this graph, each peak indicates one pulse wave. The number of peaks in this trace represents the pulse rate in beats per minute. The cycle length is determined by measuring the time difference between two consecutive peaks. Figure 2B illustrates a tachogram representing the time intervals (y-axis) of consecutive pulse signals (x-axis). Thus, the tachogram can visualize signal-to-signal time interval variability. In this figure, limits for bradycardia and tachycardia are indicated in amber. Figure 2C shows a Lorenz or Poincaré plot representing the pulse signal interval as a function of the preceding interval. The structured evaluation of a tachogram pattern or Poincaré/Lorenz plot cluster pattern can support in detecting and identifying certain arrhythmias. Additional examples are presented in Supplementary material online, Figure S1.
Figure 2.
Overview of a regular (sinus rhythm) recording. (A) Raw photoplethysmography signals of a 60-s recording. The equal distance between consecutive peaks indicates a regular rhythm. (B) The tachogram showing all consecutive pulse signal intervals. In this case the interval is regular around 1000 ms, indicating a heart rate of 60 b.p.m. (C) The Poincaré or Lorenz plot shows a condensed cluster of points, which corresponds to a regular rhythm. Small variations in all graphs are caused by respiratory variation, suggestive of sinus rhythm. b.p.m., beats per minute; PPG, photoplethysmography.
Development of the structured stepwise practical guide on how to evaluate and interpret photoplethysmography signals
On 22 December 2020, an online conference on Zoom was organized to discuss a structured stepwise practical guide to evaluate and interpret PPG signals. The TeleCheck-AF investigators of the best including centres of the TeleCheck-AF project and three experienced experts in the fields of PPG monitoring were invited. The result of this meeting was used to design the first draft of this stepwise practical guide. The practical guide was further refined during an internal review process. After appropriate revisions, the practical guide was approved by all the TeleCheck-AF investigators involved. All TeleCheck-AF investigators were invited to describe typical clinical scenarios which they practice in their centre. Of note, the current practical guide was designed as an educational tool to satisfy a need for structural education on PPG technology and its interpretation. The impact of this practical guide on clinical practice has not yet been assessed and validation of this tool is beyond the scope of the current manuscript.
Data collection
The total number of heart rate and rhythm measurements, the number of AF, sinus rhythm, and low-quality recordings identified by the FibriCheck® algorithm and the representative PPG recordings were obtained anonymously from the FibriCheck® cloud. Data are presented as numbers or percentages.
Results
Structured stepwise practical guide on how to evaluate and interpret photoplethysmography signals
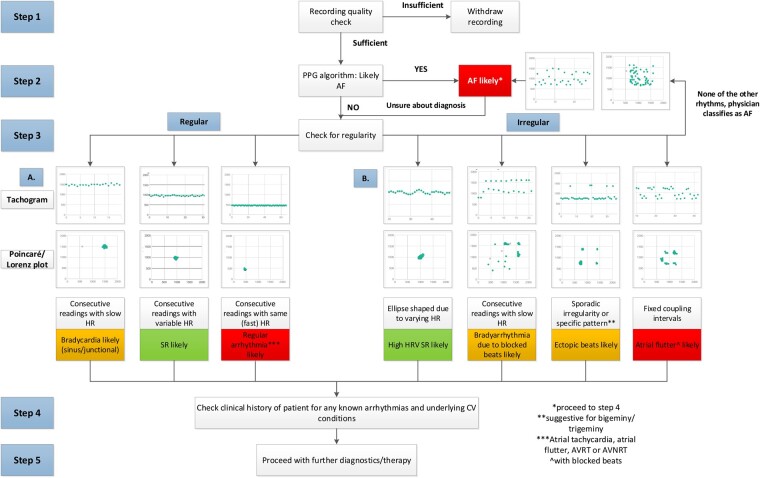
Figure 3 provides a flowchart for the systematic interpretation of PPG tracings. Within the TeleCheck-AF project, 2522 subjects collected in total 90 616 recordings. Supplementary material online, Figure S2 displays the incidence of respective recordings in the TeleCheck-AF project.
Figure 3.
Structured stepwise practical guide on photoplethysmography signal interpretation. AF, atrial fibrillation; AVNRT, atrioventricular nodal re-entrant tachycardia; CV, cardiovascular; HR, heart rate; HRV, heart rate variability; SR, sinus rhythm.
Step 1: Check for the quality of the PPG tracings. The quality of a signal can be rated as good when there are clear distinguishable peaks. If the signal is noisy with erratic peaks, the quality of the signal might be too low to interpret. If more than 50% of the recording time (>30 s) is of insufficient quality, the measurement cannot be interpreted correctly. In general, the FibriCheck® PPG algorithm already identifies sections with low-quality PPG signals and marks them in grey. If the quality of the measurement is too low, this recording is labelled in blue. Whenever this happens, the app will automatically notify the user and ask for a new recording. Within the TeleCheck-AF project, 9.7% (8814) recordings were rated as low quality. Some examples of recordings with low quality are presented in Supplementary material online, Figure S3.
Step 2: Check the output of the PPG FibriCheck® algorithm. The FibriCheck® algorithm has been validated to detect AF with high sensitivity and specificity.9 Within the TeleCheck-AF project, 21 404 recordings were classified as AF (23.6%). However, visual confirmation is recommended. AF typically presents with irregularly varying intervals between the peaks in the PPG tracing, randomly distributed points on the tachogram, and the absence of a distinct cluster of points in the Poincaré/Lorenz plot. If there is substantial doubt about the diagnosis or if the FibriCheck® algorithm does not classify a recording as AF, proceed to Step 3.
Step 3: Check for regularity. Equal intervals between the peaks in the raw PPG signal, a single line without deviations in the tachogram, and one dense clustered cloud of points in the Poincaré/Lorenz plot are indicative of a regular rhythm. Anything deviating from this pattern, can be labelled as ‘irregular’, in which the most likely diagnosis is dependent on the specific pattern.
Step 3a: Evaluate the regular rhythm by checking the heart rate in consecutive recordings. As RR-intervals in heart rhythm disorders like an atrial flutter, atrial tachycardia, AVRT, AVNRT or haemodynamically tolerated VT are usually regular, it is easy to confuse these rhythm disorders with normal sinus rhythm when measured by PPG.9 This can be especially challenging in the case of a heart rate within normal ranges, as could be the case in atrial flutters with low ventricular rates. In the TeleCheck-AF project, 57.6% of all recordings were classified as sinus rhythm with a heart rate between 40 and 110 b.p.m., whereas 1.4% were classified as tachycardia with a heart rate above 110 b.p.m. For these recordings, interpretation and confirmation by a healthcare professional are of importance. In the case of sinus rhythm, consecutive measurements typically do not show exactly the same heart rate. If the heart rate in consecutive recordings is identical (strictly regular), an underlying regular arrhythmia should be suspected. Typical heart rates for atrial flutter are 120–160/min (2:1 conduction), 100/min (3:1 conduction), or 75/min (4:1 conduction). However, the availability of consecutive recordings is dependent on the initial instruction of the patient. Additionally, respiratory sinus arrhythmias are typically present during sinus rhythm but are often absent during regular tachycardias. Attention should be given to the occurrence of blocked beats during variable conduction with similar coupling intervals. In this case, proceed to Step 3b. Furthermore, the presence of symptoms during the recording period, particularly newly developed palpitations or dyspnoea, may increase suspicion for the presence of a regular rhythm disorder. Indeed, in TeleCheck-AF, in 21.6% of recordings classified as sinus rhythm the patient indicated symptoms. Sometimes, a definitive diagnosis cannot be made based on the PPG signals only. If suspicion for the presence of a regular arrhythmia is high, an ECG should be performed to further characterize the arrhythmia.
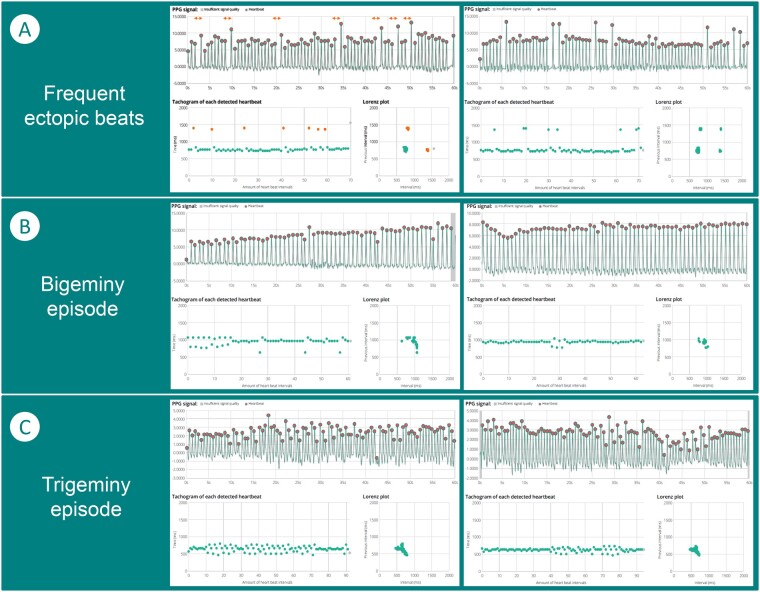
Step 3b: Evaluate the irregular rhythm by checking the pattern of the recordings. The first consideration in case of an irregular rhythm is to check whether the irregularity is solely caused by increased heart rate variability (HRV). In a small number of recordings, the FibriCheck® algorithm might also indicate this. However, in other instances visual confirmation is necessary. Increased HRV presents as a wave pattern on the tachogram in combination with an ellipse-shaped figure in the Poincaré/Lorenz plot. If the irregularity is based on occasional beats with fixed coupling intervals, it is important to differentiate between a sporadic irregularity and a repeated pattern. Sporadic irregularity is usually caused by ectopic atrial or ventricular beats and could present as shorter intervals (premature beats) followed by longer intervals (pauses) with fixed coupling intervals in the raw PPG signal or the tachogram. In the Poincaré/Lorenz plot, beats with similar coupling intervals will result in additional dense clusters. In case of a repeated pattern, multiple options are possible. If a low heart rate is present, bradyarrhythmia based on a blocked beats due variable conduction of an underlying supraventricular tachycardia should be considered. In contrast, when the heart rate is normal, a bigeminy or trigeminy episode could be present. This can be seen by identifying a series of peaks that follow the previous one too quickly in the raw PPG signal, two or three distinct lines in the tachogram due to green points alternately deviating up and down or high-middle-low, respectively, and different dense clusters of green points in the Poincaré/Lorenz plot. Figure 4 shows examples of ectopic beats and associated patterns. Of all recordings in the TeleCheck-AF project, the FibriCheck® algorithm classified 4.7% as extrasystoles, 1.8% as bigeminy (episodes), and 0.6% as trigeminy (episodes). Especially in recordings with these classifications and in case of the presence of fixed coupling intervals, attention should be paid to the possibility of atrial flutter with variable conduction as underlying cause of the pattern seen on the PPG recordings. The FibriCheck® algorithm itself classifies just 0.2% of all recordings as atrial flutter, mainly because of the difficult distinction. If none of the above patterns are present and the PPG waveform shows a completely irregular pattern, the possibility of AF should be re-evaluated.
Figure 4.
Ectopic beats. (A) Two recordings with frequent ectopic beats. This can be seen by the bigger pauses between two consecutive beats, indicated in amber in the graphs. In the photoplethysmography signal, the distance between consecutive peaks is larger, which leads to a longer RR-interval in the tachogram and multiple clusters in the Poincaré/Lorenz plot. (B) Two examples of bigeminy episodes, in which the tachogram shows fixed coupled intervals. (C) Two examples of trigeminy episodes, in which the tachogram shows three alternating lines during the episode.
Step 4: Check for known, previously documented arrhythmias and underlying cardiovascular conditions. As discussed in Steps 3a and 3b, some arrhythmias may cause similar patterns on the PPG recordings making a differentiation difficult. The clinical history of a patient may increase the likeliness of a certain arrhythmia and can be used to increase the pre-test probability. In Case Box 1, an example for this assessment is provided.
Case box 1: Assessment of PPG recordings in a patient with regular rhythm disorders
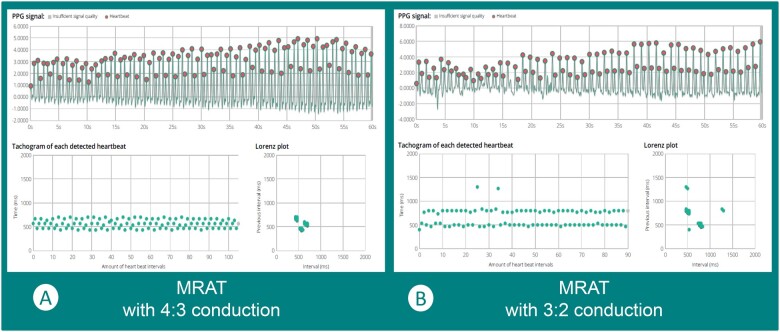
Medical history: A 65-year-old woman is treated at the rhythm outpatient clinic for different atrial arrhythmias. She has a history of AF, cavotricuspid isthmus (CTI)-dependent atrial flutter, atypical atrial flutter, and atrial tachycardia. In 2018, she underwent a combined mitral valve repair and Maze procedure. Due to frequent symptomatic episodes of atrial flutter and atrial tachycardia she underwent an endocardial CTI ablation procedure in April 2019. In January 2020, she underwent another procedure in an attempt to treat an atypical superior vena cava-dependent atrial flutter, which was unsuccessful. After repeated electrical cardioversion, she developed another regular atrial tachyarrhythmia, suspect for macro re-entrant atrial tachycardia (MRAT), upon which a rate control strategy was adopted. PPG-monitoring was used to evaluate the rate control strategy.
Recordings and MRAT with variable conduction: The patient recorded 28 registrations within 7 days. The recordings demonstrated various heart rhythms. Two examples are provided in Figure 5. Figure 5A presumably depicts MRAT (focal atrial tachycardia and MRAT including typical and atypical atrial flutter are differential diagnoses) with 4:3 conduction to the ventricles, given the repetitive pattern of two subsequent increasing intervals before a shorter interval occurs. Figure 5B illustrates a pattern which most frequently appears in sinus rhythm with premature atrial complexions in bigeminy. However, as sinus rhythm is very unlikely in this patient with persistent atrial arrhythmias, the probable explanation for this pattern is similar to the left figure: macro re-entrant atrial tachycardia or atrial flutter with variable conduction, although now occurring in a 3:2 ratio.
Figure 5.
Macro re-entrant atrial tachycardia with variable conduction. (A) Presumably macro re-entrant atrial tachycardia with 4:3 conduction. (B) Presumably macro re-entrant atrial tachycardia with 3:2 conduction.
Step 5: Proceed with further diagnostics or therapy as indicated. Depending on the clinical scenario for which PPG monitoring was implemented, further steps can be taken. In case of diagnostic uncertainties, PPG recordings should prompt further diagnostic ECG recordings via a 12-lead resting ECG in the case of ongoing arrhythmias, extended Holter monitoring in the case of regularly (daily) occurring paroxysmal arrhythmias or ECG-based wearable devices in the case of rarely occurring paroxysmal arrhythmias.
Typical clinical scenarios for photoplethysmography monitoring practiced within the TeleCheck-AF project
Photoplethysmography monitoring is predominantly useful in patients with a diagnosis of AF but may also be used for patients in whom other arrhythmias are suspected or necessitate monitoring. In addition, it might also be a valuable screening tool to guide further (ambulatory) ECG monitoring in high-risk patients. Several clinical scenarios for PPG monitoring are summarized in Table 1 and in Case Box 2–5. In addition, we provide the number of AF patients managed in the respective clinical scenario within the TeleCheck-AF project. Of note, patients could use the app more than once or for various indications and thereby can be included in more than one group.
Table 1.
Possible clinical scenarios for PPG monitoring
| Atrial fibrillation | Other arrhythmias/palpitations |
|---|---|
|
|
AAD, antiarrhythmid drugs; AF, atrial fibrillation; CV, cardioversion; ECG, electrocardiogram.
Case box 2: rate control in a patient with atrial fibrillation
A 75-year-old man who has been diagnosed with paroxysmal AF in 2008, without recurrences since 2010, presented to the cardiology outpatient clinic in autumn 2019 because of a recurrent AF episode. A follow-up appointment combined with echocardiography was scheduled. Because of the COVID-19 pandemic, this consultation was converted into a teleconsultation. During echocardiography and the patient’s remote heart rhythm monitoring using PPG prior to teleconsultation, paroxysms of AF with the high ventricular rate (130–140) were observed. Digoxin was added to the rate control medication. Thereafter, he was again remotely monitored using PPG to evaluate the effect of the medication. His resting heart rate during AF was now 80–90 b.p.m., which was accepted, taken into account that PPG recordings might underestimate the heart rate in AF by approximately 10 b.p.m. due to a pulse deficit. Therefore, it is advised to aim for stricter rate control compared to the ECG-based cut-off of a heart rate of 110 b.p.m. Precise cut-off values for PPG-based rate control are currently determined in ongoing studies.
Case box 5: PPG-monitoring as step up for Holter recording
A 58-year-old woman with a history of AF was seen at the outpatient clinic because of persistent palpitations despite pharmacological heart rate and rhythm control attempts. Therefore, she was now scheduled for pulmonary vein isolation (PVI). Awaiting the procedure, she used the FibriCheck app to examine her heart rate and rhythm control. The first 3 days she mainly had sinus bradycardia, alternated with short episodes of AF with normal ventricular frequencies. However, the fourth day she had an episode of a very fast (190 b.p.m), regular rhythm, later turning into AF. Upon contact, she explained that she had been experiencing these fast palpitations alternating with the slower, irregular palpitations in the previous months, not realizing these might be due to another arrhythmia other than AF. To determine the cause of these regular tachyarrhythmias, Holter monitoring was scheduled within 1 week. However, 1 day after the teleconsultation she experienced the fast palpitations again, this time accompanied by chest pain. She contacted the emergency medical services and upon arrival of the ambulance, an ECG suggestive of AVNRT was acquired. After coughing, the rhythm first converted to AF, and several minutes later she spontaneously converted to sinus rhythm. She had not been previously diagnosed with any other arrhythmias than AF, nor had her history been very suggestive of it.
Since the episodes of AVNRT were associated with more severe symptoms than the AF episodes, and episodes of AVNRT seemed to provoke AF, the intended PVI was converted to a supraventricular tachycardia ablation first. The PVI was postponed and, depending on the results of the planned procedure, might even be dispensable.
On-demand remote heart rate and rhythm assessment: Within the TeleCheck-AF project,4,5 PPG monitoring was primarily used to acquire heart rate and rhythm information prior to a scheduled teleconsultation and to guide follow-up management of AF patients. Remote monitoring can also be used prior to an outpatient face-to-face consultation. In total, 966 patients with diagnosed AF were managed this way, with recruitment both during lockdown restrictions and after easing the lockdown restrictions.7
Post-ablation follow-up: Similar to the use of Holter monitoring post-ablation, PPG technology can be used in patients who have undergone AF catheter ablation, to monitor the heart rhythm in order to check for recurrences during the post-ablation period. Within the TeleCheck-AF project, 1110 patients were followed up by PPG technology after ablation. A survey conducted in the participating centres7 suggested that physicians see an additional value of using PPG technology particularly in these patients.
Photoplethysmography-guided remote adaptation of heart rate and rhythm control medication: PPG technology can also be used to guide the remote adaption of heart rate and rhythm control medication. Case examples in a patient with persistent AF (Case Box 2) and a patient with atrial flutter (Case Box 3) are described below. In 78 patients, rate or rhythm control was adopted during the teleconsultation.
Photoplethysmography monitoring for spontaneous conversion: Recently, a study comparing early cardioversion to delayed cardioversion within 48 h after symptom-onset showed that delayed cardioversion is a safe and efficient treatment for patients with acute episodes of paroxysmal AF.14 Up to 70% of the patients in the delayed cardioversion group spontaneously converted to sinus rhythm within 48 h. PPG monitoring can be applied to monitor rate control and spontaneous conversion in these patients in whom a delayed conversion approach is implemented (Case Box 4).15
Other scenarios: On-demand PPG monitoring could potentially be used to support decision-making whether patients with specific palpitations would require Holter monitoring. Moreover, PPG may support the assessment of symptom–rhythm correlation. If no heart rhythm disorders are captured by PPG, a symptom-rhythm correlation can likely be excluded. If some signs of heart rhythm disorders are present without a diagnosis yet, this could prompt additional Holter monitoring. Furthermore, ectopic beats as underlying cause for palpitations can be captured by PPG monitoring. However, Holter monitoring will still be required to determine whether ectopic beats are predominantly supraventricular or ventricular and to locate its origin (Case Box 5). In addition, PPG can also be used for symptom–rhythm assessment around rhythm interventions in patients with known rhythm disorders (i.e. planned electrical cardioversion). Establishing the presence of a symptom-rhythm correlation is of importance to guide treatment decisions, since poor symptom–rhythm correlation might negatively affect outcomes of rhythm interventions. During the TeleCheck-AF project, 275 were followed around cardioversion using PPG technology. Similar to heart rate assessment in patients with AF or other sustained arrhythmias, PPG monitoring can also be used for heart rate monitoring during up-titration of negatively chronotropic medication in patients with heart failure.
Screening: PPG screening for AF in high-risk patients has the benefit of being easily available and convenient for the patient.16 Photoplethysmography can easily be used for a longer period of time without the necessity of an implantable device. Suspect AF on PPG recordings may then prompt further intensive ECG monitoring. However, AF screening was not goal of the TeleCheck-AF project.
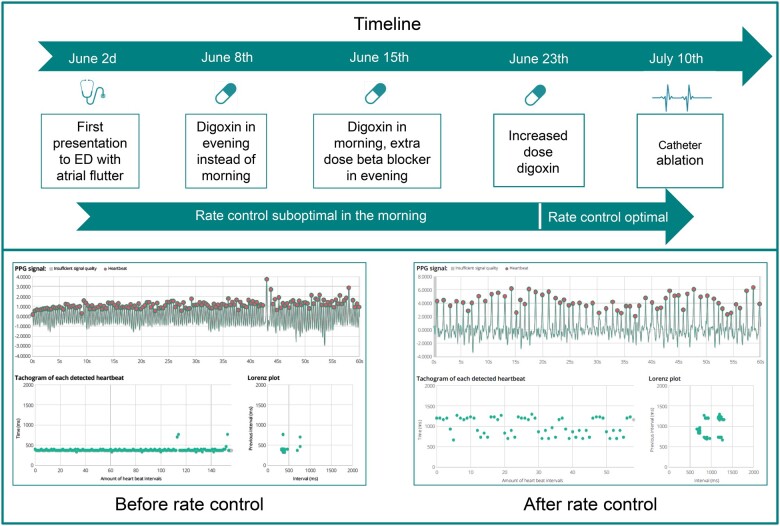
Case box 3: rate control in a patient with atrial flutter
A 59-year-old man presented to the emergency department (ED) because of palpitations and tachycardia which had started that morning. He experienced palpitations once before, a few weeks ago. He had no other complaints. ECG showed a typical atrial flutter with a heart rate of 165 b.p.m. He was given a beta-blocker and digoxin at the ED. In addition, he was scheduled for a catheter ablation in 4 weeks. To optimize rate control in the meantime, the patient was discharged with a prescription for the FibriCheck app. After discharge from the ED, his heart rate showed the same pattern every day: adequately controlled after daily ingestion of beta-blockers but suboptimal in the mornings. Therefore, his heart rate control medication was first distributed over the day by subscribing beta-blocker in the morning and digoxin in the evening. However, this did not result in the desired effect, so digoxin was reverted to mornings, and an extra dose of beta-blocker was added in the evenings. Because therapy was still suboptimal in the morning, the dose of digoxin was increased, after which the therapy regime stayed optimal until catheter ablation. A timeline for remote heart rate control adaption, together with PPG examples, is provided in Figure 6.
Case box 4: delayed cardioversion in a patient with atrial fibrillation and atrial tachycardia
A 51-year-old man with a history of paroxysmal AF and atrial tachycardia presented to the ED due to tachycardia and dyspnoea. Electrocardiogram showed AF with a heart rate of 120 b.p.m. Before arrival at the ED, the patient had already taken flecainide (pill in the pocket), which had not led to conversion to sinus rhythm. At the ED, verapamil was given to optimize rate control and alleviate symptoms. Afterwards, the patient was discharged to await spontaneous conversion at home. The FibriCheck app was prescribed to the patient to monitor whether spontaneous conversion occurred, or whether the patient should return the next day for delayed cardioversion. The next day, FibriCheck showed conversion to sinus rhythm. A teleconsultation with the patient took place, in which he was informed of the findings. No cardioversion was necessary.
Supplementary material online, Figure S4 presents an overview of PPG and ECG recordings for the arrhythmias described in Case Box 2–5. Of note, these recordings are not taken simultaneously but could have some time in between.
Figure 6.
Timeline remote rate control adaption in a patient with atrial flutter. Upper panel: Timeline presenting the different adaptions to rate control. Lower panel: two examples of photoplethysmography recordings. Left: photoplethysmography recording taken in the morning, before administration of rate control medication. Right: photoplethysmography recording taken in the afternoon, after administration of rate control medication.
Discussion
Over the last years, novel mHealth applications using PPG technology have been developed for the screening and management of AF. Studies suggest that mHealth devices have some advantages for users compared to standard 12-lead ECG or Holter monitoring in terms of convenience, portability, and flexibility.10 In addition, they enable longer monitoring which makes capturing of rhythm disorders more likely, without the need for implantable devices.13 Interestingly, a recent mHealth study found that with long-term (4 weeks) intermittent heart rhythm monitoring after ablation, significantly more recurrences were captured compared to short-term Holter monitoring.17 Additionally, data show that PPG technology is nearly as accurate as ECG to detect AF.18,19
Despite the good accuracy of PPG-based devices for the detection of AF,9,18,20 the PPG algorithms are not validated to detect arrhythmias other than AF, such as atrial flutter, atrial tachycardia, AVNRT, AVRT, or others. Regular rhythms can be easily mistaken for normal sinus rhythm, leading to false negative results. In this manuscript, we developed a structured stepwise guide on how to analyse and interpret PPG signals. Combining the PPG raw data with the tachogram and Poincaré/Lorenz plot as shown in this dictionary may improve the readability of PPG recordings. The representative PPG recordings may provide a dictionary for PPG documentations of typical cardiac arrhythmias. Although it may seem that the knowledge of PPG analysis is superfluous, PPG-based devices can, like any algorithm, provide a misdiagnosis. Therefore, in our opinion a physician’s own interpretation of the record together with the knowledge of the patient's medical history can improve the diagnosis and even extend it to arrhythmias other than AF. Of note, the interpretation of PPG tracings in the TeleCheck-AF project was only based on the FibriCheck app. Other PPG apps might present the data in slightly different ways, but PPG waveforms, tachograms, and the Poincaré/Lorenz plot should constitute the basis for interpretation of PPG.
Additionally, we introduced clinical scenarios encountered during the TeleCheck-AF project to demonstrate how on-demand PPG technology within this approach may be implemented in clinical practice.21 Results from the TeleCheck-AF project showed that on-demand mHealth combined with teleconsultation is feasible and was received positively by both physicians and patients.7 This is in line with other studies reporting good adherence to mHealth, also when used long term (>1 year).22 Reduced risk of rehospitalization and clinical adverse events was previously demonstrated for an integrated AF care approach supported by mHealth.22,23 However, clinical outcomes have not been evaluated in the TeleCheck-AF project, yet.
Accelerated through the COVID-19 pandemic and the initiation of the TeleCheck-AF project, more and more centres in Europe have been using PPG technology on-demand around teleconsultations to allow remote heart rate and rhythm assessment of consulted patients. However, the implementation in existing hospital infrastructures remains a challenge. One important step is that adaption of existing care coordination and the set-up of clinical pathways is necessary. Due to the lack of PPG technology validation, many patients and health care professionals are not convinced about introducing PPG-based devices in daily routine. Initiatives such as the TeleCheck-AF project will help step-by-step to implement this technology in clinical care pathways by providing information on the effectiveness of this technology and education on PPG analysis to expand the level of patient arrhythmia diagnostics and management.
Up until now, implementation of PPG-based devices into clinical practice mostly occurred through patient-initiated paying-models,21 probably due to several concerns with mHealth devices such as fear of data overload and a call for practical guidance. Although this educational manuscript may help to familiarize cardiologists and allied health specialists with the PPG technology, various other research questions still need to be answered. To this end, the data from the TeleCheck-AF project are currently retrospectively collected. Several mHealth trials, such as RACE 9 OBSERVE-AF (NCT04612335), are currently conducted and will help to implement PPG technology into clinical practice and to determine whether on-demand mHealth use increases health care efficiency. In addition, further research focusing on the application of PPG technology for the assessment of haemodynamic changes is warranted.
Limitations
An important limitation that should be mentioned is that the validation of this structured guide and its subsequent clinical impact is beyond the scope of this manuscript. In addition, several limitations of the use of PPG-based mHealth devices should be mentioned. Photoplethysmography cannot differentiate between regular tachycardias such as fast sinus rhythm, atrial tachycardia, AVNRT, AVRT, VT, and fast atrial flutter. A final diagnosis still requires an ECG documentation or an electrophysiological study. However, PPG recordings can be indicative for the presence of these arrhythmias, and can therefore be used as a step-up for Holter monitoring. Caution should also be taken when implementing PPG technology for rate control in patients with very fast irregular rhythms, because of the possible occurrence of a pulse deficit. However, an often mentioned limitation of smartphone applications for remote monitoring not being eligible for older patients has proven not to be a large concern within the TeleCheck-AF project.
Conclusions and future perspectives
Herein, we provide a structured stepwise practical guide on how to analyse and interpret PPG signals. Additionally, we introduce clinical scenarios, where the use of PPG-based heart rate and rhythm monitoring will be of clinical benefit. Further research and validation studies, including educational approaches for both healthcare professionals and patients on its use and possible indications, are warranted.
Conflict of interest: D.D. has received speaker honoraria and/or travel grants from Abbott, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Medtronic, Pfizer, Zoll. MM has received speaker honoraria and/or travel grants from Biosense Webster, Abbott, Biotronik, Zoll, Boston Scientific, Daiichi Sankyo, Bayer, Pfizer, Amomed as well as research grants from Biosense Webster. A.S. has received speaker honoraria and/or travel grants from Abbott, Biosense Webster, Bayer, Boston Scientific, Medtronic, Pfizer. E.S. has received speaker honoraria from Bayer, Bristol-Myers Squibb-Pfizer, Boehringer- Ingelheim, Merck Sharp & Dohme, and Sanofi, as received institutional research grants from Carl Bennett Ltd and Roche Diagnostics. K.V. has a consultancy agreements with Medtronic, Abbott and Boston scientific and received research grants from Medtronic, Biotronik, and Abbott. J.E. has 0.5% virtual shares of Preventicus, received research grants from Preventicus, Ada Health, speaker honoraria from Bayer, Roche, Pfizer, travel grants from Bayer and investigator honoraria from Bayer and BMS. The other authors declare that there is no conflicts of interests.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Varma N, Marrouche NF, Aguinaga L, Albert CM, Arbelo E, Choi J-I, Chung MK, Conte G, Dagher L, Epstein LM, Ghanbari H, Han JK, Heidbuchel H, Huang H, Lakkireddy DR, Ngarmukos T, Russo AM, Saad EB, Saenz Morales LC, Sandau KE, Sridhar ARM, Stecker EC, Varosy PD.. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic: developed in partnership with and endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2020;23:313. [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL.. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020. [DOI] [PubMed] [Google Scholar]
- 3. O'Sullivan JW, Grigg S, Crawford W, Turakhia MP, Perez M, Ingelsson E, Wheeler MT, Ioannidis JPA, Ashley EA.. Accuracy of smartphone camera applications for detecting atrial fibrillation: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e202064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pluymaekers N, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes S, Vernooy K, Crijns H, Hendriks JM, Linz D.. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linz D, Pluymaekers N, Hendriks JM.. TeleCheck-AF for COVID-19. Eur Heart J 2020;41:1954–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanner FC, Brooks N, Fox KF, Gonçalves L, Kearney P, Michalis L, Pasquet A, Price S, Bonnefoy E, Westwood M, Plummer C, Kirchhof P; ESC Scientific Document Group. ESC core curriculum for the cardiologist. Eur Heart J 2020;41:3605–3692. [DOI] [PubMed] [Google Scholar]
- 7. Gawalko M, Duncker D, Manninger M, Van der Velden RMJ, Hermans ANL, Verhaert DVM, Pison L, Pisters R, Hemels M, Sultan A, Steven D, Gupta D, Heidbuchel H, Sohaib A, Wijtvliet P, Tieleman R, Gruwez H, Chun J, Schmidt B, Keaney JJ, Müller P, P. L, Svennberg E, Hoekstra O, Jansen W, Desteghe L, De Potter T, Tomlinson DR, Neubeck L, Crijns HJGM, Pluymaekers NAHA, Hendriks JM, Linz D.. The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace 2021;23:1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manninger M, Kosiuk J, Zweiker D, Njeim M, Antolic B, Kircanski B, Larsen JM, Svennberg E, Vanduynhoven P, Duncker D.. Role of wearable rhythm recordings in clinical decision making—the wEHRAbles project. Clin Cardiol 2020;43:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B.. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: diagnostic accuracy study of the FibriCheck App. JMIR Mhealth Uhealth 2019;7:e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castaneda D, Esparza A, Ghamari M, Soltanpur C, Nazeran H.. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelectron 2018;4:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandenberk T, Stans J, Mortelmans C, Van Haelst R, Van Schelvergem G, Pelckmans C, Smeets CJ, Lanssens D, De Cannière H, Storms V, Thijs IM, Vaes B, Vandervoort PM.. Clinical validation of heart rate apps: mixed-methods evaluation study. JMIR Mhealth Uhealth 2017;5:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasitlumkum N, Cheungpasitporn W, Chokesuwattanaskul A, Thangjui S, Thongprayoon C, Bathini T, Vallabhajosyula S, Kanitsoraphan C, Leesutipornchai T, Chokesuwattanaskul R.. Diagnostic accuracy of smart gadgets/wearable devices in detecting atrial fibrillation: a systematic review and meta-analysis. Arch Cardiovasc Dis 2021;114:4–16. [DOI] [PubMed] [Google Scholar]
- 13. Giebel GD, Gissel C.. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth 2019;7:e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pluymaekers NAHA, Dudink EAMP, Luermans JGLM, Meeder JG, Lenderink T, Widdershoven J, Bucx JJJ, Rienstra M, Kamp O, Van Opstal JM, Alings M, Oomen A, Kirchhof CJ, Van Dijk VF, Ramanna H, Liem A, Dekker LR, Essers BAB, Tijssen JGP, Van Gelder IC, Crijns HJGM.. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med 2019;380:1499–1508. [DOI] [PubMed] [Google Scholar]
- 15. Pluymaekers NAHA, Van der Velden RMJ, Hermans ANL, Gawalko M, Buskes S, J.H.M.W. K, Vorstermans B, Crijns HJGM, Hendriks JM, Linz D.. On-demand mHealth infrastructure for remote rhythm monitoring within a wait-and-see strategy for recent-onset atrial fibrillation: TeleWAS-AF. Cardiology 2021;146:392–396. [DOI] [PubMed] [Google Scholar]
- 16. Linz D, Hermans ANL, Tieleman R.. Early atrial fibrillation detection and the transition to comprehensive management. Europace 2021;23:ii46–ii51. [DOI] [PubMed] [Google Scholar]
- 17. Hermans ANL, Gawalko M, Pluymaekers N, Dinh T, Weijs B, van Mourik MJW, Vorstermans B, den Uijl DW, Opsteyn L, Snippe H, Vernooy K, Crijns H, Linz D, Luermans J.. Long-term intermittent versus short continuous heart rhythm monitoring for the detection of atrial fibrillation recurrences after catheter ablation. Int J Cardiol 2021;329:105–112. [DOI] [PubMed] [Google Scholar]
- 18. Chan PH, Wong CK, Poh YC, Pun L, Leung WW, W ong YF, Wong MM, Poh MZ, Chu DW, Siu CW.. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eerikainen LM, Bonomi AG, Schipper F, Dekker LRC, Vullings R, de Morree HM, Aarts RM.. Comparison between electrocardiogram- and photoplethysmogram-derived features for atrial fibrillation detection in free-living conditions. Physiol Meas 2018;39(8):084001. [DOI] [PubMed] [Google Scholar]
- 20. Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S, Bulacher F, Salomon L, Noah T, Birkemeyer R, Eckstein J.. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019;21:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hermans ANL, van der Velden RMJ, Gawalko M, Verhaert DVM, Desteghe L, Duncker D, Manninger M, Heidbuchel H, Pisters R, Hemels M, Pison L, Sohaib A, Sultan A, Steven D, Wijtvliet P, Tieleman R, Gupta D, Dobrev D, Svennberg E, Crijns H, Pluymaekers N, Hendriks JM, Linz D.. On-demand mobile health infrastructures to allow comprehensive remote atrial fibrillation and risk factor management through teleconsultation. Clin Cardiol 2020;43:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo Y, Guo J, Shi X, Yao Y, Sun Y, Xia Y, Yu B, Liu T, Chen Y, Lip GYH, m AFAIITi.. Mobile health technology-supported atrial fibrillation screening and integrated care: a report from the mAFA-II trial long-term extension cohort. Eur J Internal Med 2020;82:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH.. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.