Abstract
Aims
Interoception is the sensing function of physiological conditions and is crucial in self-regulation and decision-making. We examined the association of heartbeat tracking task performance, an indicator of interoceptive accuracy, with the degree of improvement in exercise tolerance in patients undergoing home-based cardiac rehabilitation.
Methods and results
Participants underwent baseline peak oxygen uptake (VO2) measurements and a heartbeat tracking task. The heartbeat tracking task score varies between 0 and 1, with higher scores indicating a better heartbeat perception. After 6 months of home-based exercise training, peak VO2 was measured again, and the percentage change (%Δ peak VO2) relative to the peak VO2 at baseline was calculated. Univariate regression analysis was performed to examine the association between %Δ peak VO2 and the heartbeat tracking task score. Multiple regression analysis was performed to determine the predictors of %Δ peak VO2. Of 120 participants, 100 patients (age 65.9 ± 11.9 years; 86% male) were included. There was a significant positive association between %Δ peak VO2 and the heartbeat tracking task score at baseline (R2 = 0.236, P < 0.001). In multiple regression analysis, the percentage of measured peak VO2 to the predicted value (%predicted peak VO2) (β = −0.248, P = 0.002), exercise adherence (β = 0.364, P < 0.001), and heartbeat tracking task score at baseline (β = 0.372, P < 0.001) were significantly associated with %Δ peak VO2.
Conclusions
Heartbeat tracking task performance, an indicator of interoceptive accuracy, at baseline is associated with the degree of improvement in exercise tolerance.
Keywords: Heart disease, Home-based cardiac rehabilitation, Interoception, Interoceptive accuracy, Heartbeat tracking task, Exercise tolerance
Graphical Abstract
Graphical Abstract.
Introduction
Cardiac rehabilitation (CR) is a valuable treatment that reduces recurrence rates and mortality in patients with a broad spectrum of heart diseases.1,2 The degree of improvement in exercise tolerance is associated with prognosis in patients with heart disease.3–5 Although CR can significantly improve exercise tolerance,6 there are many patients in clinical practice whose exercise tolerance is reduced or does not improve well. Only physiological indices have been examined in previous reports on factors associated with the degree of improvement in exercise tolerance.7–9 Anxiety and depression have been reported to be associated with exercise tolerance in patients with heart disease.10,11 A recent report found a negative association between depressive tendencies and changes in exercise capacity in patients taking part in inpatient CR.11 However, to the best of our knowledge, no previous study has examined factors associated with the degree of improvement in exercise tolerance, including psychological and neurological factors other than anxiety and depression.
Recently, interoception has been attracting attention in psychology and neurology as the sensing function of internal bodily changes and physiological conditions, which are composed of signals transmitted from internal organs and blood vessels to the brain.12–15 Interoception is associated with anxiety and depression.16 Interoceptive mechanisms appear central to somatic disorders of brain–body interactions, including functional digestive disorders, chronic pain, and comorbid conditions.15 Furthermore, interoception plays an integral role in self-regulation, decision-making, consciousness, and motivational representations.13,15 Interoceptive accuracy (IA) is defined as the objective accuracy in detecting internal bodily sensations.17 The heartbeat tracking task (HTT) proposed by Schandry18 is a reliable method for evaluating IA.17,19–23 It has been suggested that people who perform well on HTT show a more finely tuned behavioural self-regulation of physical load in exercise,19 which may influence the effectiveness of exercise training. Since home-based CR is performed under unsupervised conditions, self-regulating abilities, such as willingness to engage in exercise, recognition of one’s physical condition, and adjustment of the exercise load, are likely to be necessary. Interoceptive accuracy has been shown to increase significantly after 2–3 months of exercise training in healthy individuals.23 However, the association between IA and the improvement of exercise tolerance by exercise training is not clear. Furthermore, the influence of interoception in patients undergoing CR has not been investigated.
Identifying factors associated with improvement of exercise tolerance that also affects prognosis is vital for better optimization of interventions at the initiation of exercise training in patients with heart disease and effective CR. Therefore, we conducted an observational study to determine the association of HTT performance, an indicator of IA, with the degree of improvement in exercise tolerance.
Methods
Ethics statements
The present study was conducted according to the principles outlined in the Declaration of Helsinki and was approved by the Ethics committee of KKR Takamatsu Hospital (approval no.: E165). Written informed consent was obtained from all patients.
Study design and population
This prospective observational study screened 171 patients with heart disease who first visited the CR outpatient clinic at the KKR Takamatsu Hospital between April 2019 and December 2020. Of these, 120 participants were enrolled, after excluding 51 patients with atrial fibrillation, physical comorbidities precluding cardiopulmonary exercise testing (CPX), pacemaker implantation, cognitive impairment, or those who refused to participate in this study. In addition, participants in the study did not include patients with cancer, dysgnosia, cardiac and non-cardiac surgeries, or severe mental illness.
The period of observation or intervention in previous studies of home-based CR varies significantly, ranging from 2 to 12 months.24 Here, the observation period was set at 6 months considering the follow-up period set in many previous studies.24 Participants underwent CPX at baseline to measure their peak oxygen uptake (VO2) as an indicator of exercise tolerance and were prescribed exercise for home-based exercise training. The HTT was performed to evaluate IA. In addition, anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS),25 which was self-administered by the participants. The handgrip strength was measured using a Smedley-type hand dynamometer (T.K.K.5401, Grip D, Takei Scientific Instruments Co., Niigata, Japan) to indicate muscle strength. Other information such as echocardiography parameters, laboratory data, medication status, and comorbidities were collected from each patient’s most recent medical records. Peak VO2 was measured again after 6 months of home-based exercise training.
Cardiopulmonary exercise testing
The protocol of CPX followed that of a previous study.26 Cardiopulmonary exercise testing was performed using a cycle ergometer (Well Bike BE-260; Fukuda Denshi Co., Tokyo, Japan) with breath-by-breath respiratory gas measurements using a computerized metabolic cart (AE-310S; Minato Medical Science, Osaka, Japan). After a 3-min rest on the ergometer, the exercise began with a 3-min warm-up at 10 W and 50 repetitions/min, followed by 10-W ramp loading every minute. During CPX, the 12-lead electrocardiogram was continuously monitored with an exercise stress test device (MLX-1000; Fukuda Denshi), and blood pressure (BP) was measured every minute. The test was terminated when the patient showed (i) maximal volitional fatigue, (ii) VO2 levelling off, (iii) excessively high BP (i.e. systolic BP >250 mmHg), or (iv) electrocardiogram abnormalities. The highest value of VO2 during exercise was calculated as peak VO2; it was corrected by body weight (mL/kg/min). The percentage of measured peak VO2 to the predicted peak VO2 (%predicted peak VO2) was calculated using the predicted values by age and sex for healthy Japanese subjects.27 The anaerobic threshold (AT) was measured by the V-slope method.28 Cardiopulmonary exercise testing was performed by a different measurer than the HTT examiner.
Heartbeat tracking task
The HTT was performed according to the measurement method by Schandry.18–21,23 This task was conducted in a quiet room without any noise. Participants were instructed to silently count their heartbeats by concentrating on their heart activity in a resting sitting position. Participants were not permitted to take their pulse or to attempt any other physical manipulations (e.g. holding their breath) that could facilitate the detection of heartbeats. Participants were asked to count the number of times they felt a heartbeat in each of the three intervals of 25, 35, and 45 s and verbally report the number of counted heartbeats at the end of each counting phase. The beginning and the end of the counting intervals were indicated by the examiner's start and stop cue. The order of measurements in the three intervals was determined arbitrarily by the examiner so as not to keep it constant among participants; the participants received no information about the length of these intervals or on the quality of their performance at the end of each trial. The examiner used a three-lead electrocardiogram (lead II) with electrodes attached under the middle of the right and left clavicles and on the left lib to count and record the actual heartbeats for each interval. The HTT score was calculated as the average score of the three heartbeat perception intervals according to the following formula:
The HTT score can vary between 0 and 1, with higher scores indicating a better heartbeat perception and consequently better IA.18–23
Home-based exercise training
After being assessed by CPX at baseline, the participants were fully informed about the benefits of CR. According to guidelines,29 they were individually instructed to perform exercise training at home. Each participant was required to perform at least 30 min of aerobic exercise per day, at least 3 days per week. Exercise intensity was prescribed at an AT level intensity based on the results of their initial CPX. The heart rate at the AT level was used as the target heart rate, and 11 (light)–13 (somewhat hard) on the Borg scale was used as the subjective intensity. Participants were instructed to check their heart rate using a pulse rate measuring device or to measure their own pulse. The type of exercise was walking or riding a bicycle ergometer. Participants were given a diary to keep track of their exercise and were instructed to write down the days and times of exercise training. During the 6 months of the study, participants underwent a regular examination by a physician and received direct guidance on the safety of exercise and continued training by CR specialists at least once every 2 months. During exercise training, the target heart rate was not re-prescribed, but the participants were instructed to adjust the exercise intensity based on their subjective intensity using the Borg scale. At the final evaluation, participants’ records and interviews were used to assess exercise adherence. Participants were categorized into four groups with reference to the statement on exercise adherence30: those whose adherence both to the number of exercise training sessions prescribed (≥30 min, 3 times/week) and to the duration of the prescribed cycle (24 weeks) was at least 80% (high adherence group), those whose adherence was between 50% and 80% (somewhat high adherence group), those whose adherence was between 20% and 50% (somewhat low adherence group), and those whose adherence was less than 20% (low adherence group).
Outcomes
The outcome was the percentage change in peak VO2 (%Δ peak VO2) and indicated the degree of improvement in exercise tolerance. The %Δ peak VO2 was calculated as the degree of change in peak VO2 after 6 months relative to the value at baseline according to the following formula:
Statistical analysis
Variables are expressed as mean ± standard deviation or median (interquartile range) for continuous variables and as the number of patients (percentage) for categorical variables. Univariate regression analysis was performed to examine the explanatory power of the HTT score for %Δ peak VO2. Univariate and multiple regression analyses were performed to determine the predictors of %Δ peak VO2. In the univariate regression analyses, variables with a P-value <0.10 were included in the multiple regression analysis. The multi-collinearity between the independent variables was checked using the variance inflation factor. Univariate ordinal logistic regression analysis was applied to evaluate the association between the HTT score and exercise adherence, with exercise adherence (low adherence: 0, somewhat low adherence: 1, somewhat high adherence: 2, high adherence: 3) as the dependent variable and the HTT score as the independent variable; odds ratios (ORs) along with 95% confidence intervals (95% CIs) are reported. Spearman’s rank correlation coefficient was used to evaluate the association between the HTT score and HADS score at baseline and between exercise adherence and baseline HADS score. For an effect size as f2 = 0.22 in reference to the study by Suzuki et al.8 and with α = 0.05, 1 − β = 0.8, and the number of predictors = 10, the appropriate sample size for multiple regression analysis estimated using G*Power software (version 3.1.9.7)31 was 84 participants. The target sample size was set at 120 participants while assuming a withdrawal rate of 30% during the 6-month observation period so that at least 84 participants were included in the final analyses. This calculation was based on the actual treatment results observed at our facility, wherein the withdrawal rate of new CR patients was 30% within 6 months.
All statistical analyses were performed using EZR version 1.52 (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), a graphical user interface for R version 4.02 (The R Foundation for Statistical Computing, Vienna, Austria).32 A P-value <0.05 was considered statistically significant.
Results
Baseline characteristics
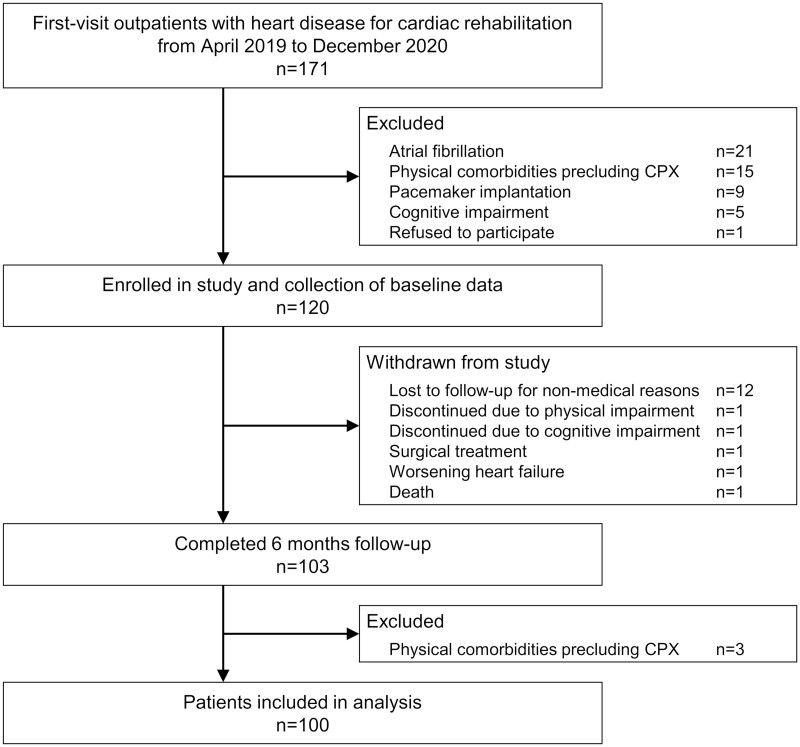
Figure 1 shows a flow diagram of the study participants. In total, 120 participants were enrolled and assessed at baseline. During the follow-up, 17 patients were withdrawn because of loss to follow-up for non-medical reasons (12 patients) and discontinued because of physical impairment, cognitive impairment, surgical treatment, worsening heart failure, and death (1 patient each). Of 103 patients who completed the 6-month follow-up, 100 patients were included in the final analysis, excluding the 3 patients with physical comorbidities precluding CPX at the final evaluation. The characteristics of the patients included in the final analysis are shown in Table 1. The average patient age was 65.9 ± 11.9 (40–93) years, and 86.0% of patients were male. The median peak VO2 was 15.0 (7.4–34.6) mL/kg/min, and the average %predicted peak VO2 was 63.5 ± 14.6 (35.6–116.2) %. The median HTT score was 0.75 (0.17–0.98).
Figure 1.
Flow diagram of the study participants. CPX, cardiopulmonary exercise testing.
Table 1.
Baseline characteristics of patients and results of univariate regression analyses between the percentage of change of peak VO2 (%Δ peak VO2) after 6 months of home-based exercise training and each variable
| Variable | Patients included in the final analysis | Patients excluded from the final analysis | R 2 | P-value |
|---|---|---|---|---|
| (n = 100) | (n = 20) | |||
| Age (years) | 65.9 ± 11.9 | 70.6 ± 11.8 | 0.046 | 0.033 |
| Male sex, n (%) | 86 (86.0) | 16 (80.0) | <0.001 | 0.88 |
| BMI (kg/m2) | 24.7 (22.4–26.7) | 23.8 (20.3–27.8) | <0.001 | 0.99 |
| Heart rate (beats/min) | 68.9 ± 11.6 | 67.7 ± 12.1 | 0.003 | 0.60 |
| Systolic blood pressure (mmHg) | 126.7 ± 16.9 | 131.9 ± 13.8 | 0.014 | 0.24 |
| Diastolic blood pressure (mmHg) | 75.5 ± 11.6 | 74.3 ± 12.5 | 0.005 | 0.49 |
| Current smoker, n (%) | 25 (25.0) | 3 (15.0) | 0.006 | 0.46 |
| Current drinker, n (%) | 38 (38.0) | 10 (50.0) | 0.001 | 0.73 |
| Diagnostic category, n (%) | ||||
| Angina pectoris | 48 (48.0) | 9 (45.0) | 0.009 | 0.35 |
| Acute myocardial infarction | 31 (31.0) | 4 (20.0) | 0.013 | 0.25 |
| Old myocardial infarction | 12 (12.0) | 3 (15.0) | <0.001 | 0.91 |
| Valvular disease | 2 (2.0) | 4 (20.0) | 0.002 | 0.66 |
| Others | 7 (7.0) | 0 (0.0) | <0.001 | 0.88 |
| Heart failure (%) | 26 (26.0) | 6 (30.0) | <0.001 | 0.84 |
| Echocardiographic findings | ||||
| LVEF (%) | 58.0 (53.0–64.8) | 61.0 (54.5–65.3) | <0.001 | 0.90 |
| LVDd (mm) | 47.0 (42.0–51.0) | 47.0 (41.8–51.3) | <0.001 | 0.86 |
| LVDs (mm) | 31.5 (28.0–36.0) | 32.6 (27.0–37.3) | <0.001 | 0.95 |
| E/A | 0.8 (0.7–1.1) | 0.9 (0.8–1.0) | 0.020 | 0.17 |
| E/E′ | 10.7 (9.1–13.0) | 12.7 (10.2–18.0) | 0.001 | 0.81 |
| LAVI (mL/m2) | 20.0 (13.0–26.0) | 31.0 (22.5–37.8) | 0.001 | 0.47 |
| Laboratory values | ||||
| BNP (pg/dL) | 41.6 (18.8–93.3) | 40.6 (25.2–123.1) | 0.012 | 0.28 |
| Haemoglobin (g/dL) | 14.2 (13.2–15.3) | 14.0 (12.8–15.1) | 0.007 | 0.40 |
| Creatinine (mg/dL) | 0.88 (0.78–1.03) | 0.83 (0.72–0.96) | 0.010 | 0.33 |
| eGFR (mL/min/1.73 m2) | 63.5 ± 13.9 | 65.9 ± 20.9 | 0.016 | 0.21 |
| HDL-cholesterol (mg/dL) | 52.9 ± 15.1 | 51.9 ± 14.9 | 0.004 | 0.52 |
| LDL-cholesterol (mg/dL) | 89.5 (68.0–104.0) | 78.0 (63.5–76.5) | 0.026 | 0.11 |
| L/H ratio | 1.70 (1.36–2.31) | 1.69 (1.18–2.00) | 0.001 | 0.71 |
| Haemoglobin A1c (%) | 5.9 (5.6–6.5) | 6.2 (5.8–7.1) | <0.001 | 0.89 |
| Medications, n (%) | ||||
| ARB or ACEI | 68 (68.0) | 14 (70.0) | 0.051 | 0.024 |
| Ca2+ antagonist | 29 (29.0) | 10 (50.0) | 0.006 | 0.44 |
| β-Blocker | 55 (55.0) | 14 (70.0) | 0.020 | 0.16 |
| Diuretic | 20 (20.0) | 5 (25.0) | 0.032 | 0.076 |
| Statin | 87 (87.9) | 17 (85.0) | 0.004 | 0.51 |
| Comorbidities, n (%) | ||||
| Chronic kidney disease | 21 (21.0) | 5 (25.0) | 0.044 | 0.037 |
| Diabetes mellitus | 35 (35.0) | 10 (50.0) | <0.001 | 0.78 |
| Chronic respiratory disease | 7 (7.0) | 1 (5.0) | 0.007 | 0.41 |
| Anaemia | 10 (10.0) | 5 (25.0) | <0.001 | 0.84 |
| Hand grip strength (kgf) | 33.9 ± 9.4 | 30.7 ± 9.8 | 0.017 | 0.20 |
| HADS | ||||
| Anxiety score | 4.0 (2.0–6.0) | 6.5 (4.5–8.0) | 0.051 | 0.023 |
| Depression score | 4.0 (2.0–7.0) | 6.5 (4.5–8.0) | 0.001 | 0.71 |
| Total score | 8.0 (4.0–13.0) | 13.0 (6.5–15.3) | 0.011 | 0.30 |
| CPX parameters | ||||
| Peak RER | 1.19 ± 0.06 | 1.16 ± 0.08 | 0.014 | 0.24 |
| VO2 at AT (mL/kg/min) | 9.3 (7.9–10.4) | 8.5 (7.5–9.4) | 0.024 | 0.13 |
| Peak VO2 (mL/kg/min) | 15.0 (12.3–17.8) | 13.7 (11.1–15.5) | 0.021 | 0.15 |
| %Predicted peak VO2 (%) | 63.5 ± 14.6 | 61.0 ± 11.9 | 0.067 | 0.010 |
| HTT score | 0.75 (0.60–0.86) | 0.71 (0.55–0.82) | 0.236 | <0.001 |
| Exercise adherence | — | — | 0.258 | <0.001 |
Data are presented as mean ± standard deviation, median (with lower and upper quartiles), or number (percentage). R2 and P-value reported the results of univariate regression analyses between the %Δ peak VO2 and each variable for the 100 patients included in the final analysis.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AT, anaerobic threshold; BMI, body mass index; BNP, B-type natriuretic peptide; CPX, cardiopulmonary exercise testing; eGFR, estimated glomerular filtration rate; HADS, Hospital Anxiety and Depression Scale; HDL, high-density lipoprotein; HTT, heartbeat tracking task; LAVI, left atrial volume index; LDL, low-density lipoprotein; L/H, low-density lipoprotein-cholesterol/high-density lipoprotein-cholesterol; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; RER, respiratory exchange ratio; VO2, oxygen uptake.
Cardiac history
There were 91 (91.0%) patients with ischaemic heart disease such as angina pectoris or myocardial infarction, and all patients had undergone percutaneous coronary intervention. Participants in the study included a few patients with non-ischaemic heart disease such as valvular disease, but none of these participants had comorbid coronary artery disease. There were 26 patients (26.0%) with heart failure. The median left ventricular ejection fraction was 58.0 (22.0–73.0)%.
Outcomes
In 100 patients included in the final analysis, the degree of adherence to home-based exercise training for 6 months was as follows: high adherence, 47 patients = 47%; somewhat high adherence, 23 patients = 23%; somewhat low adherence, 10 patients = 10%; and low adherence, 20 patients = 20%. The overall median %Δ peak VO2 was 4.7 (−27.6 to 54.0) %. The median %Δ peak VO2 by exercise adherence was 12.9 (−17.4 to 42.1) % for the high adherence group, 6.0 (−9.4 to 54.0) % for the somewhat high adherence group, −6.4 (−12.7 to 10.6) % for the somewhat low adherence group, and −4.8 (27.6–17.8) % for the low adherence group, respectively. In the univariate regression analysis, there was a significant positive association between %Δ peak VO2 and the HTT score at baseline (R2 = 0.236, P < 0.001) (Figure 2). Among the candidate variables for multivariable analysis shown in Table 1, age, angiotensin II receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI) use, diuretic use, chronic kidney disease, anxiety score of the HADS, %predicted peak VO2, exercise adherence, and HTT score showed a P-value <0.10, and they were selected as independent variables. In the multiple regression analysis, %predicted peak VO2 at baseline (P = 0.002), exercise adherence (P < 0.001), and HTT score at baseline (P < 0.001) were statistically significant predictors of %Δ peak VO2. The standardized partial regression coefficients were −0.248 for %predicted peak VO2, 0.364 for exercise adherence, and 0.372 for HTT score. The adjusted R2 value was 0.478 (Table 2). The variance inflation factors for each independent variable were all <5.0. Stepwise multiple regression analyses using the Bayesian information criterion (BIC) showed an increase in the adjusted R2 and a decrease in the BIC and residual standard error in the model with HTT score compared with the model with HTT score excluded from the independent variables, indicating an improvement in the model fit (Supplementary material online, Table S1). There was a significant positive association between exercise adherence and the HTT score at baseline (OR = 22.80, 95% CI 2.67–209.00; P = 0.005). There were no significant associations between anxiety and depression subscales and total scores on HADS at baseline and HTT score or exercise adherence.
Figure 2.
Association between %Δ peak VO2 and the heartbeat tracking task score at baseline. %Δ peak VO2 was significantly positively associated with the heartbeat tracking task score at baseline. HTT, heartbeat tracking task; VO2, oxygen uptake.
Table 2.
Results of multiple regression analysis of the percentage of change of peak VO2 (%Δ peak VO2) after 6 months of home-based exercise training (n = 100)
| Dependent variable: %Δ peak VO2 after 6 months of home-based exercise training | |||||
|---|---|---|---|---|---|
| Independent variable | B | Standard error | β | t statistic | P-value |
| Age (years) | −0.021 | 0.100 | −0.017 | −0.207 | 0.84 |
| ARB or ACEI usea | 2.458 | 2.462 | 0.078 | 0.999 | 0.32 |
| Diuretic usea | 4.670 | 2.847 | 0.128 | 1.640 | 0.10 |
| Chronic kidney diseasea | −4.726 | 2.859 | −0.132 | −1.653 | 0.10 |
| Anxiety score | 0.395 | 0.350 | 0.086 | 1.130 | 0.26 |
| %Predicted peak VO2 | −0.250 | 0.080 | −0.248 | −3.112 | 0.002 |
| Exercise adherenceb | 4.556 | 0.989 | 0.364 | 4.604 | <0.001 |
| HTT score | 31.531 | 6.713 | 0.372 | 4.697 | <0.001 |
| R 2 = 0.520, Adjusted R2 = 0.478, ANOVA P < 0.001 | |||||
Yes: 1, No: 0.
High: 3, Somewhat high: 2, Somewhat low: 1, Low: 0.
ACEI, angiotensin-converting enzyme inhibitor; ANOVA, analysis of variance; ARB, angiotensin II receptor blocker; B, partial regression coefficient; β, standardized partial regression coefficient; HTT, heartbeat tracking task; VO2, oxygen uptake.
Discussion
The present observational study showed that the degree of improvement in exercise tolerance was significantly negatively associated with %predicted peak VO2 at baseline and significantly positively associated with exercise adherence and the HTT score at baseline in patients with heart disease who underwent home-based CR. In clinical practice, adding the evaluation of IA by HTT may provide a better predictor of the degree of improvement in exercise tolerance and contribute to the delivery of personalized medicine. To the best of our knowledge, this is the first report to show that HTT performance is an independent factor associated with the degree of improvement in exercise tolerance. In patients with heart disease, the greater the improvement in peak VO2, the better the prognosis.3–5 Before initiating CR, it is crucial to predict which patients will improve or worsen their exercise tolerance during home-based exercise training, as this information will lead to a more optimized CR programme for each patient.
Interoception is the sensing function of internal changes and physiological conditions of the body.12 Neuroanatomical and neuroimaging studies have shown that brain networks, including the insular cortex and anterior cingulate cortex, are involved in the brain mechanisms that support interoception.12,13,16,33 There are various measurement methods of interoception.14,17 The exact method for evaluating IA remains controversial, and there are problems regarding the use of the HTT scores—it fails to correctly differentiate the IA of the top 60% individuals with high HTT scores, it is structurally bound to actual heart rates, and the scores vary across the time intervals used in the task.16,34 Nevertheless, there is limited evidence for these challenges related to HTT, and the HTT has been very widely used and well-validated as a measure of IA in previous clinical studies.17,19–23 A significant positive correlation was found between the heartbeat discrimination task, another measure of IA, and the HTT.17 Presently, there is not a more objective or a more scientifically reliable method that uses a device to evaluate IA. Therefore, the HTT was used to evaluate IA in the present study. HTT scores have not been previously reported in patients with heart disease. The mean or median HTT scores reported in other populations have ranged from 0.52 to 0.76,17,19–21,23,35 which may be similar to those of HTT scores of the participants in this study. The indications and accuracy of the HTT in patients with atrial fibrillation have not been adequately investigated in previous studies, and therefore, they were excluded from the present study. The HTT scores are reportedly negatively correlated with the resting heart rate.34 However, the present study was unable to address the association between factors that may affect heart rate (e.g. sex, smoking, drinking, caffeine/theine, and medications such as β-blockers and inhaled β2 agonists), and heart rate and HTT performance. Therefore, further studies are required.
There are three possible pathways for the association between IA as expressed by HTT performance and the degree of improvement of peak VO2 in home-based CR patients. The first is the interrelationship with exercise adherence. The rate of high exercise adherence among participants in the present study was similar to previous reports.24,30 The high exercise adherence group showed a sufficient improvement in peak VO2, and exercise adherence was an independent factor associated with %Δ peak VO2. In this context, it is interesting to note that there was a significant positive association between exercise adherence and HTT performance. It has been shown that people in the high IA group take actions that are likely to benefit themselves in the long term.36 In contrast, people in the low IA group have difficulty making rational decisions.35 The better the IA, the greater the ability to be adherent to one’s physical condition and illness, which may contribute to improved exercise adherence as a rational action to improve or prevent deterioration. In healthy individuals, the ability to accurately perceive one’s heartbeat is negatively correlated with depression symptoms, an effect that manifests itself only when coupled with high anxiety levels.16 In the present study, there was no significant association between the HTT score and anxiety and depression at baseline. Anxiety and depression were also not significantly associated with exercise adherence. Previous studies have shown different results in patients with depression, with increased,37 decreased,38 or no association39 with exercise adherence. The association between anxiety and exercise adherence is also unclear and is currently being studied.40 The association between IA and anxiety and depression in exercise adherence in patients undergoing home-based CR needs to be further clarified in future studies.
The possible second pathway includes self-regulation of the load during exercise training. The degree of improvement in exercise tolerance in patients with heart disease is related to the frequency and intensity characteristics of training,9 and it is crucial to perform exercise training with an appropriate load. Usually, home-based CR involves unsupervised exercise training based on professional exercise prescriptions. The exercise intensity is targeted at the instructed heart rate and load, but since the exercise load adjustment is done by the patient alone, the ability to fine-tune the appropriate exercise load is required. Although reported in healthy subjects, it has been suggested that interoception plays an important role in self-regulating physical load during exercise.19,20 Patients with higher IA were able to perceive the physical load during exercise accurately and may have been able to perform exercise training while adjusting the exercise load to a more appropriate level.
Finally, there is the possibility of a pathway for physical activity in daily life. Physical activity is important in patients with heart disease to improve functional capacity, quality of life, and prognosis and is a Class 1A recommendation in the European Society of Cardiology guidelines.41 There is a positive correlation between physical activity during the daytime and HTT performance, although the previous study that reported this was conducted in healthy children.22 Although it is unclear whether this tendency is similar in adult patients with heart disease, the association between IA and physical activity may affect %Δ peak VO2. In a previous study of patients with chronic musculoskeletal pain, the differences between objective measures of moderate to vigorous physical activity and self-reported levels in daily life showed a significantly negative correlation with HTT performance, suggesting that IA affects the ability to self-monitor one’s physical activity.21 In patients with heart disease, kinesiophobia reduces physical activity42 and participation in CR.43 It has been suggested that patients with high levels of kinesiophobia are more prone to anxiety44 and often have difficulties in distinguishing between harmful and harmless body signals.45 Interoceptive accuracy is associated with anxiety and is the sensing function of the body's internal changes and physiological conditions.16 The association between kinesiophobia and IA is an important topic for future research.
In the present study, %predicted peak VO2 at baseline before initiating CR was also a significant factor associated with %Δ peak VO2. Previous studies have shown that heart disease patients with low exercise capacity are more likely to improve their exercise tolerance,7–9 and the present study of home-based CR patients showed reasonable results to support these findings. There is a significant negative association between age and %Δ peak VO2.7,9 The improvement in exercise tolerance is poor in patients with comorbid kidney dysfunction,46 and ARB and ACEI use improve skeletal muscle function and contribute to the improvement of exercise tolerance in patients with heart failure.47 Drugs such as calcium antagonists, β-blockers, and diuretics were not significantly associated with %Δ peak VO2. This may be due to the heterogeneity in the diagnostic category of the participants in this study. A recent study suggested that the higher depression scores on HADS at the initiation of CR were associated with less improvement in exercise capacity during rehabilitation in hospitalized patients.11 In the present study, there was no association between depression scores on HADS and the degree of improvement in exercise tolerance. This difference in results may be due to the difference between inpatients and home-based patients and the difference in the observation period.
Our findings revealed that exercise tolerance and HTT performance at baseline are more important indicators for predicting the effect of home-based CR on exercise tolerance. In particular, if patients have poor HTT performance, it may be necessary to strengthen measures to improve exercise tolerance, including the selection of supervised exercise training, frequent monitoring for continued exercise training, counselling, and the use of remote systems. Home-based CR using Information and Communication Technology, such as mobile devices, will become more widespread in the future.24,48 The findings of our study could be one of the personal markers for patients undergoing home-based CR who do not receive direct supervision and may deliver personalized treatment.
Limitations
The current study has several limitations. First, the implementation of exercise training at home was an evaluation based on self-reports from the participants and may lack objectivity because actual heart rate monitoring during exercise training and daily physical activity was not directly measured. Further investigations using a blinded activity tracker are required to determine the relationship of exercise adherence, ability to self-regulate physical load, and physical activity with HTT performance and %Δ peak VO2. Second, the study participants were patients undergoing home-based CR; hence, further investigation is required to determine the generalizability of our results to patients undergoing centre-based CR. Third, in the present study, IA, anxiety, and depression have each been assessed on a single scale only. There was no association between IA and anxiety or depression, which may conflict with some previous studies. Further studies should evaluate the present findings according to different interoception scales14,17 and other psychological scales, including scales measuring anxiety and depression.10,49 Finally, the sample size was small. Although the statistically valid sample size was met, 17% of participants were lost to follow-up, and the participants were from only one hospital. Most of the participants were older men, a group who had a high proportion of ischaemic heart disease, particularly angina pectoris. In addition, the participants also included a group who had low exercise adherence and had a less relevant clinical improvement in peak VO2 performance, thus limiting the generalizability of the results. In the future, the number of participants should be increased. The results of this study should be further validated by subgroup analysis according to diagnostic categories, analysis using multiple regression prediction models adjusted for other clinical factors, and the evaluation of the prediction model.
Future directions
Although it has been demonstrated that IA can be trained,14,50 the present study cannot address causality, such as whether enhancing HTT performance will significantly improve exercise tolerance. Improvement in HTT performance in patients with heart disease may further improve exercise tolerance, although further intervention studies are required to confirm this. The present study endpoint was set as %Δ peak VO2, a surrogate marker for prognosis. However, the lack of clinical outcome data (e.g. all-cause mortality, cardiovascular mortality, and hospital admissions) is a significant limitation of this study. Further investigations are required to determine the association between the HTT score and clinical outcomes to support the use of HTT scores for patients with heart disease in clinical practice.
Conclusions
In patients with heart disease undergoing home-based CR, %predicted peak VO2 at baseline, exercise adherence, and HTT performance at baseline were associated with the degree of improvement in exercise tolerance. In clinical practice, evaluation of IA by HTT may help better predict the degree of improvement in exercise tolerance. Further investigations are required to clarify the causal relationship between HTT performance and improvement in exercise tolerance.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary Material
Acknowledgements
The authors would like to thank all participants, the cardiac rehabilitation staff, and the cardiovascular care team at KKR Takamatsu Hospital.
Declaration of Helsinki
The present study was conducted according to the principles outlined in the Declaration of Helsinki and was approved by the ethical committee of KKR Takamatsu Hospital (approval no.: E165). Written informed consent was obtained from all patients.
Conflict of interest: none declared.
Contributor Information
Shinjiro Miyazaki, Rehabilitation Center, KKR Takamatsu Hospital, 4-18 Tenjinmae, Takamatsu City, Kagawa Prefecture 760-0018, Japan.
Kenji Kanbara, Psychosomatic Medicine, Department of Clinical Psychology, Faculty of Medicine, Kagawa University, 1750-1 Ikenobe, Miki-cho, Kita-gun, Kagawa Prefecture 761-0793, Japan.
Jun Kunikata, Clinical Research Support Center, Kagawa University Hospital, 1750-1 Ikenobe, Miki-cho, Kita-gun, Kagawa Prefecture 761-0793, Japan.
Atsushi Tobiume, Department of Cardiorenal and Cerebrovascular Medicine, Faculty of Medicine, Kagawa University, 1750-1 Ikenobe, Miki-cho, Kita-gun, Kagawa Prefecture 761-0793, Japan.
Shusei Hayashino, Rehabilitation Center, KKR Takamatsu Hospital, 4-18 Tenjinmae, Takamatsu City, Kagawa Prefecture 760-0018, Japan.
Tsunetatsu Namba, Department of Cardiology, KKR Takamatsu Hospital, 4-18 Tenjinmae, Takamatsu City, Kagawa Prefecture 760-0018, Japan.
Ichiro Matsumoto, Department of Cardiology, KKR Takamatsu Hospital, 4-18 Tenjinmae, Takamatsu City, Kagawa Prefecture 760-0018, Japan.
Yuichiro Takagi, Department of Cardiology, KKR Takamatsu Hospital, 4-18 Tenjinmae, Takamatsu City, Kagawa Prefecture 760-0018, Japan.
Tetsuo Minamino, Department of Cardiorenal and Cerebrovascular Medicine, Faculty of Medicine, Kagawa University, 1750-1 Ikenobe, Miki-cho, Kita-gun, Kagawa Prefecture 761-0793, Japan.
References
- 1. Ambrosetti M, Abreu A, Corrà U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;30:2047487320913379.. [DOI] [PubMed] [Google Scholar]
- 2. McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med 2017;27:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 2008;156:292–300. [DOI] [PubMed] [Google Scholar]
- 4. Swank AM, Horton J, Fleg JL, et al. HF-ACTION Investigators . Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 2012;5:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakanishi M, Nakao K, Kumasaka L, et al. Improvement in exercise capacity by exercise training associated with favorable clinical outcomes in advanced heart failure with high B-type natriuretic peptide level. Circ J 2017;81:1307–1314. [DOI] [PubMed] [Google Scholar]
- 6. Oldridge N, Pakosh M, Grace SL. A systematic review of recent cardiac rehabilitation meta-analyses in patients with coronary heart disease or heart failure. Future Cardiol 2019;15:227–249. [DOI] [PubMed] [Google Scholar]
- 7. Witvrouwen I, Pattyn N, Gevaert AB, et al. Predictors of response to exercise training in patients with coronary artery disease - a subanalysis of the SAINTEX-CAD study. Eur J Prev Cardiol 2019;26:1158–1163. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki Y, Ito K, Yamamoto K, et al. Predictors of improvements in exercise capacity during cardiac rehabilitation in the recovery phase after coronary artery bypass graft surgery versus acute myocardial infarction. Heart Vessels 2018;33:358–366. [DOI] [PubMed] [Google Scholar]
- 9. Vanhees L, Stevens A, Schepers D, Defoor J, Rademakers F, Fagard R. Determinants of the effects of physical training and of the complications requiring resuscitation during exercise in patients with cardiovascular disease. Eur J Cardiovasc Prev Rehabil 2004;11:304–312. [DOI] [PubMed] [Google Scholar]
- 10. Kazukauskiene N, Burkauskas J, Macijauskiene J, et al. Mental distress factors and exercise capacity in patients with coronary artery disease attending cardiac rehabilitation program. Int J Behav Med 2018;25:38–48. [DOI] [PubMed] [Google Scholar]
- 11. Bermudez T, Bierbauer W, Scholz U, Hermann M. Depression and anxiety in cardiac rehabilitation: differential associations with changes in exercise capacity and quality of life. Anxiety Stress Coping 2021;1–15. [DOI] [PubMed] [Google Scholar]
- 12. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–666. [DOI] [PubMed] [Google Scholar]
- 13. Khalsa SS, Adolphs R, Cameron OG, et al. Interoception Summit 2016 Participants . Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khoury NM, Lutz J, Schuman-Olivier Z. Interoception in psychiatric disorders: a review of randomized, controlled trials with interoception-based interventions. Harv Rev Psychiatry 2018;26:250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonaz B, Lane RD, Oshinsky ML, et al. Diseases, disorders, and comorbidities of interoception. Trends Neurosci 2021;44:39–51. [DOI] [PubMed] [Google Scholar]
- 16. Quadt L, Critchley HD, Garfinkel SN. The neurobiology of interoception in health and disease. Ann N Y Acad Sci 2018;1428:112–128. [DOI] [PubMed] [Google Scholar]
- 17. Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol 2015;104:65–74. [DOI] [PubMed] [Google Scholar]
- 18. Schandry R. Heart beat perception and emotional experience. Psychophysiology 1981;18:483–488. [DOI] [PubMed] [Google Scholar]
- 19. Herbert BM, Ulbrich P, Schandry R. Interoceptive sensitivity and physical effort: implications for the self-control of physical load in everyday life. Psychophysiology 2007;44:194–202. [DOI] [PubMed] [Google Scholar]
- 20. Tabor A, Vollaard N, Keogh E, Eccleston C. Predicting the consequences of physical activity: an investigation into the relationship between anxiety sensitivity, interoceptive accuracy and action. PLoS One 2019;14:e0210853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shizuma H, Abe T, Kanbara K, et al. Interoception and alexithymia are related to differences between the self-reported and the objectively measured physical activity in patients with chronic musculoskeletal pain. J Psychosom Res 2021;140:110324. [DOI] [PubMed] [Google Scholar]
- 22. Georgiou E, Matthias E, Kobel S, et al. Interaction of physical activity and interoception in children. Front Psychol 2015;6:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amaya Y, Abe T, Kanbara K, Shizuma H, Akiyama Y, Fukunaga M. The effect of aerobic exercise on interoception and cognitive function in healthy university students: a non-randomized controlled trial. BMC Sports Sci Med Rehabil 2021;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol 2019;74:133–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 26. Miyazaki S, Hayashino S, Matsumoto I, et al. Mid-arm muscle circumference as an indicator of exercise tolerance in chronic heart failure. Geriatr Gerontol Int 2021;21:411–415. [DOI] [PubMed] [Google Scholar]
- 27. Itoh H, Ajisaka R, Koike A, et al. ; Committee on Exercise Prescription for Patients (CEPP) Members . Heart rate and blood pressure response to ramp exercise and exercise capacity in relation to age, gender, and mode of exercise in a healthy population. J Cardiol 2013;61:71–78. [DOI] [PubMed] [Google Scholar]
- 28. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027. [DOI] [PubMed] [Google Scholar]
- 29. JCS Joint Working Group . Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014;78:2022–2093. [DOI] [PubMed] [Google Scholar]
- 30. Conraads VM, Deaton C, Piotrowicz E, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2012;14:451–458. [DOI] [PubMed] [Google Scholar]
- 31. Faul F, Erdfelder E, Lang AG, Buchner A. GPower 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 32. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci 2015;16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zamariola G, Maurage P, Luminet O, Corneille O. Interoceptive accuracy scores from the heartbeat counting task are problematic: evidence from simple bivariate correlations. Biol Psychol 2018;137:12–17. [DOI] [PubMed] [Google Scholar]
- 35. Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in major depressive disorder. J Affect Disord 2013;151:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Werner NS, Jung K, Duschek S, Schandry R. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology 2009;46:1123–1129. [DOI] [PubMed] [Google Scholar]
- 37. Krishnamurthi N, Schopfer DW, Shen H, Whooley MA. Association of mental health conditions with participation in cardiac rehabilitation. J Am Heart Assoc 2019;8:e011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McGrady A, McGinnis R, Badenhop D, Bentle M, Rajput M. Effects of depression and anxiety on adherence to cardiac rehabilitation. J Cardiopulm Rehabil Prev 2009;29:358–364. [DOI] [PubMed] [Google Scholar]
- 39. Ge C, Ma J, Xu Y, et al. Predictors of adherence to home-based cardiac rehabilitation program among coronary artery disease outpatients in China. J Geriatr Cardiol 2019;16:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Foldes-Busque G, Dionne CE, Turcotte S, et al. Epidemiology and prognostic implications of panic disorder and generalized anxiety disorder in patients with coronary artery disease: rationale and design for a longitudinal cohort study. BMC Cardiovasc Disord 2021;21:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piepoli MF, Hoes AW, Agewall S, et al. ; ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Knapik A, Dąbek J, Brzęk A. Kinesiophobia as a problem in adherence to physical activity recommendations in elderly polish patients with coronary artery disease. Patient Prefer Adherence 2019;13:2129–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bäck M, Cider Å, Herlitz J, Lundberg M, Jansson B. Kinesiophobia mediates the influences on attendance at exercise-based cardiac rehabilitation in patients with coronary artery disease. Physiother Theory Pract 2016;32:571–580. [DOI] [PubMed] [Google Scholar]
- 44. Keessen P, den Uijl I, Visser B, et al. Fear of movement in patients attending cardiac rehabilitation: a validation study. J Rehabil Med 2020;52:jrm00021. [DOI] [PubMed] [Google Scholar]
- 45. Keessen P, Latour CHM, van Duijvenbode ICD, et al. Factors related to fear of movement after acute cardiac hospitalization. BMC Cardiovasc Disord 2020;20:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Irzmański R, Kapusta J, Obrębska-Stefaniak A, Urzędowicz B, Kowalski J. Cardiac rehabilitation in patients with ST-segment elevation myocardial infarction: can its failure be predicted? Ther Adv Cardiovasc Dis 2017;11:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail 2010;3:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindman BR, Gillam LD, Coylewright M et al. Effect of a pragmatic home-based mobile health exercise intervention after transcatheter aortic valve replacement: a randomized pilot trial. Eur Heart J Digit Health 2021;2:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorini A, Giuliani M, Marton G, et al. Spontaneous participation in secondary prevention programs: the role of psychosocial predictors. Int J Environ Res Public Health 2020;17:6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weng HY, Feldman JL, Leggio L, Napadow V, Park J, Price CJ. Interventions and manipulations of interoception. Trends Neurosci 2021;44:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.