This research-article refers to ‘ECG machine learning for detection of cardiovascular disease in African Americans: the Jackson Heart Study’ by J.D. Pollard et al., on page 137.
Cardiovascular disease (CVD) is often a slow progressing disease and when neglected for a longer time could cause severe heart failure or even a fatal heart attack. The early detection of CVD is crucial for prevention beneficial for both the patient and the healthcare system. Regular screening would be fundamental to reach this goal. However, access to healthcare is sometimes limited which is emphasized currently with the COVID-19 pandemic, even resulting in a new class of patients at increased CVD risk.1,2 One of the most used screening tests to detect CVD is the electrocardiogram (ECG). The ECG as a diagnostic tool provided an instantaneous image of the electrical function of the heart in which also possible mechanical problems are reflected. In the study performed by Pollard et al.,3 the potential added value of machine learning analysing ECGs for the screening of CVD in a community setting is investigated.
That the ECG can be collected and analysed in a local community setting might have a significant impact on the early detection of CVD, although the best model showed a modest 70% detection accuracy. To apply this at the local barber shop provides patients with a low-cost easy access for which you normally must go to a hospital. This study also shows that using an easily derived ECG parameter like the QRS-T-wave angle is useful for the screening of CVD. However, to compute the QRST angle you must perform a 12-lead ECG recording. To apply the 12-leads following the standard procedure can be challenging. Misplacement of the electrodes will result in measurement errors reducing its accuracy. Even in the day-to-day clinical practice acquiring 12-lead ECG’s by dedicated staff, the electrodes are frequently misplaced.4 To enable the full diagnostic potential of the ECGs at a low-cost non-hospital setting, the data collection needs to be performed by non-experts. To make sure that the electrodes are accurately placed or that the ECGs are analysed by algorithms that can correct for this, the following solutions could possibly be implemented:
Applying a mobile phone.5,6 The camera of the phone can monitor the electrodes positioning process and guide the local person for the correct placement. This reduces the training of personnel and improving the diagnostic accuracy of the ECG.
Additional algorithms might be able to detect and correct for lead misplacements. For this purpose, machine learning algorithms can be applied, Rjoob et al.7
The ultimate solution would be to develop diagnostic algorithms that can withstand measurement errors or by taking into account the patient's physique. Age can be used as an indication to define the physique used in the CVD risk prediction model described in the study by Pollard et al.3 Other factors that can increase accuracy and sensitivity may be related to optimal electrode placement on the chest while taking body build into account. This could be extracted if cameras were used in the data collection process. Because of the different nature of the patient's data sources, ECG signals, and image data, machine learning algorithms are ideally suited to extract the accurate data to screen for CVD risk.
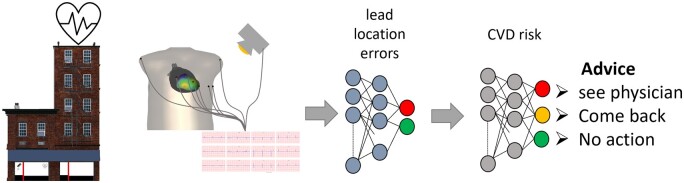
Ultimately, the screening process within a community setting should result in an advice to see a cardiologist or maybe to support a pharmacist-led CVD management. Because of the low threshold to get a diagnostic ECG measurement within the community environment, they can also collect the ECGs over time. We can again use machine learning algorithms to accurately track patients based on the acquired ECG and image data,8 see the community setting workflow figure (Figure 1).
Figure 1.
Community setting workflow of the cardiovascular risk assessment. The electrocardiogram recording can be monitored by a camera. The recording process is evaluated by machine learning algorithms taking the input from multiple inputs, like the electrocardiogram and derived parameters like a QRST angle, camera, but also weight might be added here. Once verified the cardiovascular disease risk is estimated by a machine learning network using all input data, resulting in an advice on the cardiovascular disease risk.
Machine learning thus might be essential to guard and improve the screening process of CVD at an easy accessible level within a community. The last step of how such a diagnostic path would fit within the current healthcare systems needs to be further evaluated once we have established the accuracy of the screening methods. The study by Pollard et al.3 provides a first step.
Conflict of interest: Peter M van dam is an owner of ECG Excellence.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal – Digital Health or of the European Society of Cardiology.
References
- 1. Srivastava K. Association between COVID-19 and cardiovascular disease. Int J Cardiol Heart Vasc 2020;29:100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shchendrygina A, Nagel E, Puntmann VO, Valbuena-Lopez S.. COVID-19 myocarditis and prospective heart failure burden. Expert Rev Cardiovasc Ther 2021;19:5–14. [DOI] [PubMed] [Google Scholar]
- 3. Pollard JD, Haq KT, Lutz KJ, Rogovoy NM, Paternostro KA, Soliman EZ, Maher J, Lima JAC, Musani SK, Tereshchenko LG.. ECG machine learning for detection of cardiovascular disease in African Americans: the Jackson Heart Study. Eur Heart J Digital Health 2021;2:137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook DA, Oh SY, Pusic MV.. Accuracy of physicians' electrocardiogram interpretations: a systematic review and meta-analysis. JAMA Intern Med 2020;180:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulze WH, Mackens P, Potyagaylo D, Rhode K, Tulumen E, Schimpf R, Papavassiliu T, Borggrefe M, Dossel O.. Automatic camera-based identification and 3-D reconstruction of electrode positions in electrocardiographic imaging. Biomed Eng 2014;59:515–528. [DOI] [PubMed] [Google Scholar]
- 6. van Dam PM, Gordon JP, Laks M.. Sensitivity of CIPS-computed PVC location to measurement errors in ECG electrode position: the need for the 3D camera. J Electrocardiol 2014;47:788–793. [DOI] [PubMed] [Google Scholar]
- 7. Rjoob K, Bond R, Finlay D, McGilligan V, Leslie SJ, Rababah A, Guldenring D, Iftikhar A, Knoery C, McShane A, Peace A.. Machine learning techniques for detecting electrode misplacement and interchanges when recording ECGs: a systematic review and meta-analysis. J Electrocardiol 2020;62:116–123. [DOI] [PubMed] [Google Scholar]
- 8. Jekova I, Krasteva V, Leber R, Schmid R, Twerenbold R, Müller C, Reichlin T, Abächerli R.. Intersubject variability and intrasubject reproducibility of 12-lead ECG metrics: implications for human verification. J Electrocardiol 2016;49:784–789. [DOI] [PubMed] [Google Scholar]