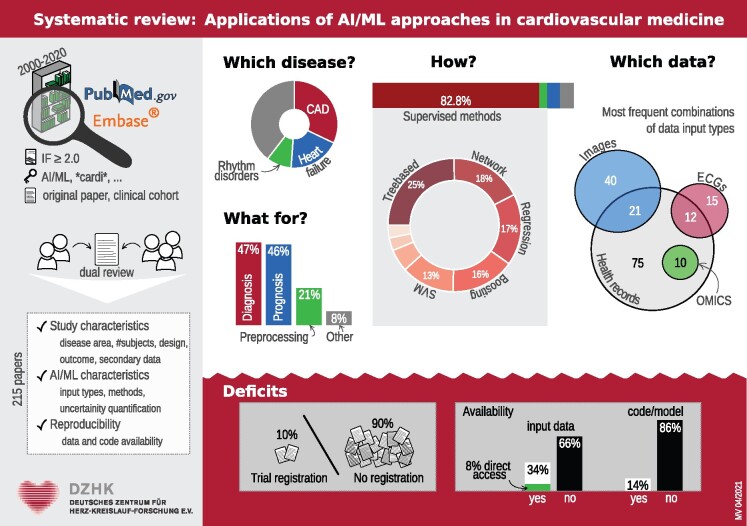
Abstract
Aims
Artificial intelligence (AI) and machine learning (ML) promise vast advances in medicine. The current state of AI/ML applications in cardiovascular medicine is largely unknown. This systematic review aims to close this gap and provides recommendations for future applications.
Methods and results
Pubmed and EMBASE were searched for applied publications using AI/ML approaches in cardiovascular medicine without limitations regarding study design or study population. The PRISMA statement was followed in this review. A total of 215 studies were identified and included in the final analysis. The majority (87%) of methods applied belong to the context of supervised learning. Within this group, tree-based methods were most commonly used, followed by network and regression analyses as well as boosting approaches. Concerning the areas of application, the most common disease context was coronary artery disease followed by heart failure and heart rhythm disorders. Often, different input types such as electronic health records and images were combined in one AI/ML application. Only a minority of publications investigated reproducibility and generalizability or provided a clinical trial registration.
Conclusions
A major finding is that methodology may overlap even with similar data. Since we observed marked variation in quality, reporting of the evaluation and transparency of data and methods urgently need to be improved.
Keywords: Artificial intelligence, Machine learning, Cardiology, Cardiovascular disease
Graphical Abstract
Introduction
Despite significant improvements over the last decades, cardiovascular diseases remain the leading cause of morbidity and mortality in Europe and the USA.1,2 Due to complex disease pathways and heterogeneity, disease diagnostic and prognostic assessment remain a challenging task. On the other hand, modern technologies are constantly increasing the ability to collect large quantities of data, which require implementation of comprehensive automated analytical methods to improve the understanding of the underlying disease complexity and ultimately increase the quality of healthcare.
Artificial intelligence (AI) is an overarching term that describes the use of algorithms and software which demonstrate human-like cognition in analysing, interpreting, and understanding complicated medical and health data. An algorithm is simply a set of actions to be followed to get a solution. Algorithms are trained to learn how to process information. The term AI may also be applied to any machine that exhibits traits associated with a human mind, such as learning and problem-solving. When machines can extract information from data, improve their function or make predictions about future events, they are referred to as machine learning (ML), a subset of AI.3 The overall objective of these approaches is to learn from samples and to generalize to new, yet unseen cases. Machine learning includes a range of advanced sub-branches, such as deep learning (DL) and neural networks.
AI/ML methods achieved remarkable progress, and their use has increased significantly over the last years in cardiovascular medicine, as indicated by recently published reviews.3–10
Compared to other reviews such as Johnson et al.,8 we chose a different approach: our intention was to investigate what is currently published under the label ‘AI/ML’ in cardiovascular medicine as opposed to providing specific examples of AI/ML applications in a given disease context or comparing the predictive ability of various AI/ML methods in a meta-analysis.10 As Nagendran et al.5 note, there is a danger that the ‘[…] public and commercial appetite for healthcare AI outpaces the development of a rigorous evidence base to support this comparatively young field'. Many authors also criticize the lack of details published on AI/ML methods, which hinder reproducibility and transparency.11,12 Lopez-Jimenez et al.9 provide a list of key aspects for evaluating AI literature. Moreover, there is still ‘[…] a scarcity of external validation studies and randomised trials […]’ to evaluate the superiority of using these methods.13 Hence, the CONSORT-AI guidelines were published very recently to improve transparency and completeness in reporting clinical trials for AI interventions.14 In parallel, the SPIRIT-AI extension15 was developed as a new reporting guideline for clinical trial protocols evaluating interventions with an AI component. A relevant issue concerning AI applications is the current overemphasis on the technical aspects, which sometimes leaves less attention to their interaction with the human users, see the DECIDE-AI statement for a discussion of this issue.16
In this systematic review, we provide an overview of the literature on applications of AI/ML methods in cardiovascular research. In the following, we describe the exact search strategy. We provide our results including descriptions of the specific methods applied in different research settings. Additionally, we evaluate whether the methods used were appropriately described and if code/data availability statements were provided. We conclude with some recommendations regarding the reporting and evaluation of methods as well as improving data and methods transparency. Our broad focus on all methods described by the respective authors as AI or ML methods without restriction with regards to specific disease areas or study designs allows for a clear view on the current state of AI/ML applications in cardiovascular medicine. As such, we aim to provide clear recommendations on how AI/ML studies should be conducted in contrast to describing criteria for the evaluation of AI/ML literature as provided by Vollmer et al.12 or Lopez-Jimenez et al.9
Methods
In this systematic review, clinical studies applying AI/ML approaches in cardiovascular medicine without limitations regarding study design or study population were included. To specifically focus on clinical application, we excluded animal studies as well as publications reporting only the methodological aspect of an AI/ML approach without presentation of clinical data of the study population. The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement17 and is registered in PROSPERO (registration number CRD42020196696).
Systematic literature search and study selection
We performed a literature search using PubMed and EMBASE to identify relevant publications. A first search in PubMed using the search terms ‘(artificial intelligence [Title/Abstract] OR machine learning [Title/Abstract]) AND (cardiac OR cardiology OR cardiosurgical OR cardiology[MeSH] OR heart OR heart[MeSH] OR *cardia* OR *cardio* OR *infarct* OR *valve*)’ and considering publications from the year 2000 onwards resulted in 2410 abstracts, see the PRISMA flow chart in Supplementary material online, Figure S1. In order to restrict our search to applied clinical studies and to exclude purely methodological publications, we limited our search to journals listed on the Web of Science in the categories ‘CARDIAC & CARDIOVASCULAR SYSTEMS’, ‘MEDICINE, GENERAL & INTERNAL’, ‘MEDICINE, RESEARCH & EXPERIMENTAL’, as well as ‘MULTIDISCIPLINARY SCIENCES’. To furthermore restrict the analysis to articles with potential clinical impact, we only included journals with an impact factor of at least two in 2018 as given on the Web of Science (https://jcr.clarivate.com/JCRJournalHomeAction.action). This resulted in 228 distinct journals. Articles included in our analyses were published in 65 distinct journals, see Supplementary material online, Table S1 for the complete list of journals. Within the cardiovascular journals, the search terms used were ‘(artificial intelligence [Title/Abstract] OR machine learning [Title/Abstract])’, while in the other journals we searched for ‘(artificial intelligence [Title/Abstract] OR machine learning [Title/Abstract]) AND (cardiac OR cardiology OR cardiosurgical OR cardiology [MeSH] OR heart OR heart [MeSH] OR *cardia* OR *cardio* OR *infarct* OR *valve*)’. The date of the last search was 5 March 2020.
Studies discussed in review papers and commentaries or editorials were also screened, but no additional studies fulfilling our inclusion criteria (see below) were found.
The list of abstracts was independently screened for inclusion by dual review of overall 16 reviewers using the following inclusion criteria: (i) an application of AI/ML methods, (ii) cardiovascular application, and (iii) the study has to present a clearly described clinical cohort. In particular, we excluded animal studies and review papers. Any disagreements were resolved in discussion or rescreened by a third reviewer (S.F., T.F., C.H., and J.R.).
Data extraction
We extracted data on study characteristics and study population (number of subjects included, study design, outcome scale, use of secondary data), characteristics of the applied AI/ML techniques such as uncertainty quantification and comparison to traditional statistical methods, reproducibility, disease area, and type of input data. Information was collected by at least two independent reviewers using a predefined data extraction form. Secondary data were specified as data originally collected for a different purpose. Studies were classified to quantify uncertainty of the AI/ML methods if they provided any measure of uncertainty such as confidence intervals for area under the curve estimates. AI/ML methods were extracted as defined by the authors of the corresponding study and the superiority to classical methods was defined based on the authors’ claims.
To investigate the increase of publications on AI/ML methods we also extracted the number of articles published per year in the journals considered in our literature search by counting the findings of the assigned journal IDs (using the ID of the bibliographic database of the National Library of Medicine: NlmId) in PubMed as of 10 December 2020. Moreover, we extracted the number of citations of the reviewed papers (searched by its PMID in PubMed) as of 10 December 2020.
Since the investigations’ aim was not always clearly stated in the articles, we applied a rather strict definition of prognostic and diagnostic approaches: we defined an approach as diagnostic when patients were classified or divided into subgroups without any time reference (e.g. publications 8 and 91 from Supplementary material online, Table S1), and as prognostic when there was a time reference (e.g. longitudinal outcomes in publications 50 and 167 from Supplementary material online, Table S1).
Data analysis
Descriptive summaries are used to describe study characteristics. Metric variables are characterized by median and interquartile range, while discrete variables are summarized by providing absolute and relative frequencies.
In order to enable comparisons and study interactions, the cardiovascular context is categorized into ten types, namely coronary artery disease (CAD), valvular heart disease, cardiomyopathies, heart rhythm disorders, peripheral vascular disease, hypertension, heart failure (HF), congenital heart disease, cardiometabolic, and other entities. These categories were chosen according to the disease context mentioned by the authors of the corresponding publication in title or abstract.
Similarly, the applied AI/ML methods are categorized into supervised, unsupervised and unspecified methods. The latter category refers to publications where the AI/ML approach was not mentioned or explained by the authors, e.g. since a commercial software was used or due to statements such as ‘we used a machine learning approach’. Additionally, the unsupervised methods are categorized into three and the supervised methods into eight sub-categories. A brief description of the methods along with some references is provided in Supplementary material online, Table S2. Supplementary material online, Table S3 shows an overview of the allocation of methods found in our search to the different sub-categories.
Interactions between categorical variables are presented as graphs displaying relative frequencies. All statistical analyses are performed in R 3.6.3 (R Foundation for statistical computing, Vienna, Austria).
Results
Included studies
The literature review identified 524 distinct publications that were screened for eligibility. A total of 215 studies were included in the final analysis, see also PRISMA flow chart in Supplementary material online, Figure S1 as well as Supplementary material online, Table S1 for the complete list of references. The study populations of the included publications are summarized in Table 1, see Supplementary material online, Table S4 for a stratified summary by disease area.
Table 1.
Study characteristics
| Variable | Level | Total |
|---|---|---|
| Subjects | Median (IQR) | 1083.0 (213.5–10 757.0) |
| Subject categories | <100 | 31 (14.4) |
| 100–1000 | 73 (34.0) | |
| 1000–10 000 | 53 (24.7) | |
| 10 000–100 000 | 45 (20.9) | |
| 100 000–1 000 000 | 11 (5.1) | |
| >1 000 000 | 2 (0.9) | |
| Design | Prospective cohort study | 48 (22.3) |
| Retrospective cohort study | 138 (64.2) | |
| Case-control study | 20 (9.3) | |
| RCT | 9 (4.2) | |
| Outcome | Binary | 153 (71.2) |
| Categorical | 13 (6.0) | |
| Continuous | 27 (12.6) | |
| Time to event | 22 (10.2) | |
| Secondary data | No | 64 (29.8) |
| Yes | 151 (70.2) |
Values are n (%) unless otherwise stated.
Time trends
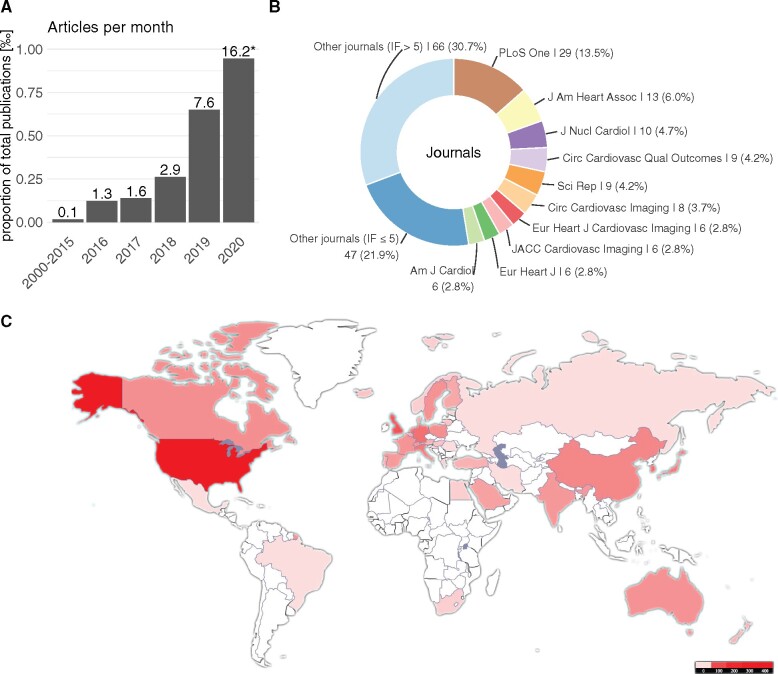
Temporal trends in AI/ML applications in cardiovascular research were explored. We observed a relative and absolute increase in publications with AI/ML applications in the last years. Figure 1A shows the proportion of papers (in ‰) included in our study with respect to the total number of studies published in the considered journals. Note that the number for 2020 is based on the studies published until 5 March 2020 and is thus likely to be incomplete. A similar trend was observed for the articles we did not screen due to the restrictions of our search strategy (Supplementary material online, Figure S2). Figure 1B displays the journals in which the identified articles were published most frequently, while Figure 1C shows the geographic distribution of the authors. With respect to the different AI/ML methods, no specific time trend could be observed (Supplementary material online, Figure S3).
Figure 1.
Meta-data of articles included in our systematic review. Panel (A) shows the increase in publications with artificial intelligence/machine learning application per month in relation to the total number published in the journals we included in our search. Panel (B) shows the proportion of journals of the reviewed papers. Panel (C) displays the geographic distribution of the authors’ affiliations.
Popular AI/ML methods and their areas of application
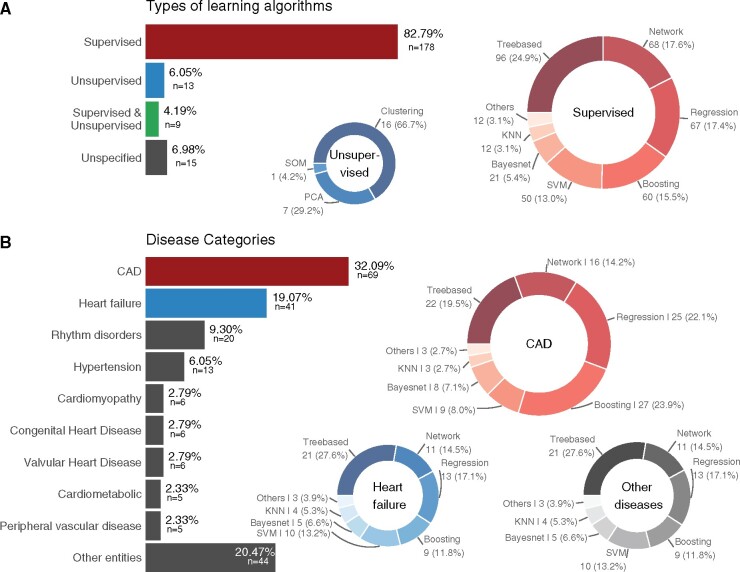
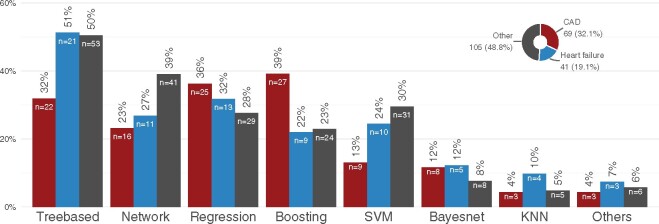
The majority (87%) of methods applied belonged to the context of supervised learning, see Figure 2A. Within this group, tree-based methods were most commonly used, followed by network and regression analyses as well as boosting approaches. In 15 articles (7%), the authors did not describe the AI/ML approach in detail. In most of these cases, a commercial software was used. Among the unsupervised methods clustering was the most popular and included 67% of the unsupervised methods (Figure 2A). We also found that unsupervised and unspecified methods are more common when the AI/ML method is used for pre-processing than in other applications. In particular, supervised methods were applied in 60% of the studies that used AI/ML for pre-processing only as opposed to 92% in the other studies.
Figure 2.
Overview of the methods and disease areas presented in the articles. Panel (A) shows the types of artificial intelligence/machine learning algorithms applied. Panel (B) displays the distribution of disease areas as well as which supervised methods are most commonly applied in which disease area.
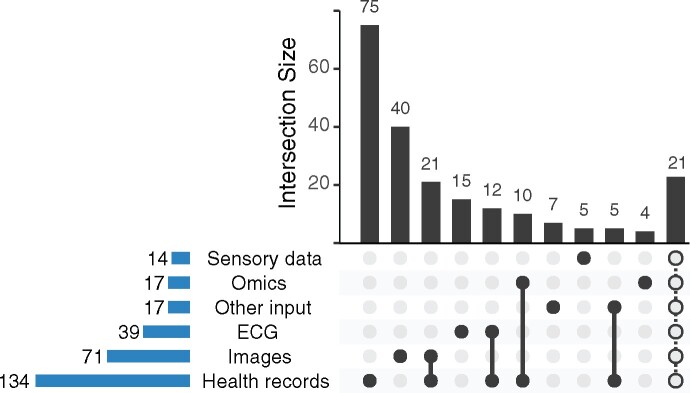
Concerning the areas of application, the most common disease context was CAD followed by HF and heart rhythm disorders as depicted in Figure 2B. The most common input for the AI/ML methods were health records (Figure 3). Often, different types of input data were combined in one AI/ML-application. For example, 10 (5%) studies used omics data in combination with health records and 21 (10%) studies combined health records with images. Figure 4 shows the distribution of the supervised methods applied in the most common disease areas (CAD, HF and all other diseases than CAD or HF) in more detail. In CAD, for example, boosting and regression methods are the most common methods of choice. In HF, on the other hand, tree-based methods are often used.
Figure 3.
Input type used for the artificial intelligence/machine learning algorithms. Displayed are the absolute number a respective input type was used (lower left bars) and the most common combinations of input types (upper bar plot). The last bar summarizes all other combinations that occurred less than four times.
Figure 4.
Overview of the distribution of the applied supervised methods stratified by disease area (coronary artery disease, heart failure, and all other diseases) in more detail. The bars will not sum up to 100% because multiple methods were used in the reviewed papers.
Comparison to non-AI/ML methods
Uncertainty of the AI/ML estimates was reported in 133 studies (62%). Results of AI/ML were compared to ‘classical’ methods (according to the authors’ definition) in 111 (52%) studies. The majority of these, 94 (85%) decided in favour of the AI/ML approach, for example the work by Commandeur et al.18 (publication number 50 in Supplementary material online, Table S1) or the work by Leha et al.19 (publication number 91 in Supplementary material online, Table S1). Sample sizes reported in these studies are displayed in Supplementary material online, Figure S4.
A positive example concerning the investigation of reproducibility and generalizability is the work from Bhuva et al.20 (publication number 122 in Supplementary material online, Table S1) which describes a multicentre, scan–rescan cardiac magnetic resonance study to test generalizability for imaging biomarkers.
Trial registration and reproducible research
Only 21 studies (10%) provided a clinical trial registration; these were mostly randomized clinical trials. Only 3 (6%) of the prospective cohort studies and 11 (8%) of the retrospective cohort studies were registered. Of the case-control studies, 2 (10%) were registered.
Of the studies analysed, 73 (34%) stated that the data used for the analysis was available. However, only 17 studies (8%) provided direct access to the data. With respect to code, only 31 studies (14%) had made their code publicly available.
Prognostic vs. diagnostic analyses
According to the definition described above, the aim of the AI/ML approach was diagnostic in 91 articles (42%). Another 93 articles (43%) used the AI/ML approach to build prognostic models. Both diagnostic and prognostic aims were considered in only 4 (2%) of the studies. A total of 27 (13%) articles applied AI/ML algorithms for neither diagnostic nor prognostic models, but rather as part of the pre-processing, e.g. to extract features from images.
We found that methods such as clustering, k-nearest neighbour and network analyses were mainly used for diagnostic purposes, whereas prognostic models rather used tree-based approaches and regression models (data not shown).
Typical cases of AI/ML algorithms
Given the larger number of different methods and their applications in cardiovascular medicine, inspection of specific examples is helpful to understand how the use of AI/ML algorithms might have a potential benefit for clinical practice. Therefore, Table 2 lists typical cases, which were selected based on their high number of overall citations. Interestingly, the number of trial subjects differed widely in the top-cited publications. Furthermore, there was no general preference for one AI/ML method. Of note, none of the highly cited examples were randomized controlled trials.
Table 2.
Examples of AI/ML applications to cardiovascular medicine
| Authors/title/doi | Disease area | AI/ML method | Trial design | No. of subjects | Aim | Citations | Abstract no. |
|---|---|---|---|---|---|---|---|
Weng et al.
|
Other entities | Random forest, logistic regression, gradient boosting machines, neural networks | Prospective cohort study | 378 256 | Cardiovascular risk prediction | 125 | 427 |
Zahid et al.
|
Rhythm disorders | Supervised machine learning algorithm | Prospective cohort study | 20 | Testing the hypothesis that atrial fibrillation re-entrant drivers persist only in regions with specific fibrosis patterns | 80 | 464 |
Motwani et al.
|
CAD | Gradient boosting | Retrospective cohort study | 10 030 | Prediction of all-cause mortality in patients with suspected coronary artery disease | 78 | 460 |
McConnell et al.
|
Other entities | Unsupervised | Prospective cohort study | 40 017 | Assessing the feasibility of obtaining measures of lifestyle from smartphones | 53 | 443 |
Ambale-Venkatesh et al.
|
Other entities | Random survival forests | Retrospective cohort study | 6814 | Prediction of six cardiovascular outcomes in comparison to standard cardiovascular risk scores | 50 | 410 |
Narula et al.
|
Cardiomyopathy | Support vector machines, random forests, and artificial neural networks | Retrospective cohort study | 139 | Automated discrimination of hypertrophic cardiomyopathy from physiological hypertrophy seen in athletes | 49 | 446 |
Attia et al.
|
Heart failure | Convolutional neural network | Retrospective cohort study | 44 959/52 870 | Identification of cardiac contractile dysfunction by ECG | 48 | 156 |
Frizzell et al.
|
Heart failure | Tree-augmented naive Bayesian network, random forest, gradient-boosted, logistic regression, least absolute shrinkage, and selection operator models | Retrospective cohort study | 56 477 | Prediction of 30-day readmission rate in patients discharged following hospitalization for heart failure | 48 | 447 |
Mortazavi et al.
|
Heart failure | Random forests, boosting, random forests, support vector machines, logistic regression, Poisson regression | Retrospective cohort study | 1004 | Prediction of readmission after hospitalization for heart failure | 46 | 436 |
Attia et al.
|
Rhythm disorders | Convolutional neural network | Retrospective cohort study | 180 922 | Identification of patients with atrial fibrillation during sinus rhythm by ECG | 37 | 156 |
The examples were chosen according to the overall number of citations. The table depicts the disease area, the ML method(s) considered in the paper, the trial design, the sample size, and the aim of the study. Abstract number refers to Supplementary material online, Table S1.
Recommendations
The choice of a specific AI/ML method is complex and depends on various parameters that are specific to the individual problem to be solved. From this extensive review, a few broad recommendations on this choice can be derived. In feature selection with, e.g. tabular data such as health records, tree-based, regression or boosting methods are most commonly applied. The application of DL to tabular data is generally possible and might perform similarly or even outperform other methods especially on larger data sets,21 but specific adaptions of DL to tabular data are still an area of active research.22,23 For image (and similarly electrocardiogram signal or omics) data, network-based DL methods are the most commonly used method. Besides the excellent performance of DL on image data, the possibility to do transfer-learning easily with DL methods is one of the key components that make DL the go-to method for image data, if the number of patients is sufficiently large.
We explicitly restricted our search to clinical applications of AI/ML methods, excluding methodological publications from our literature search. However, some recent reviews provide nice overviews for specific methodologies. For example, Chen et al.24 reviewed the use of DL in image segmentation, while Bizopoulos and Koutsouris4 provide an overview of DL applications in structured data, signal and imaging modalities. In line with Chen et al.,24 we recommend that future research explicitly targets the deployment of novel methodology such as DL in real-world clinical applications.
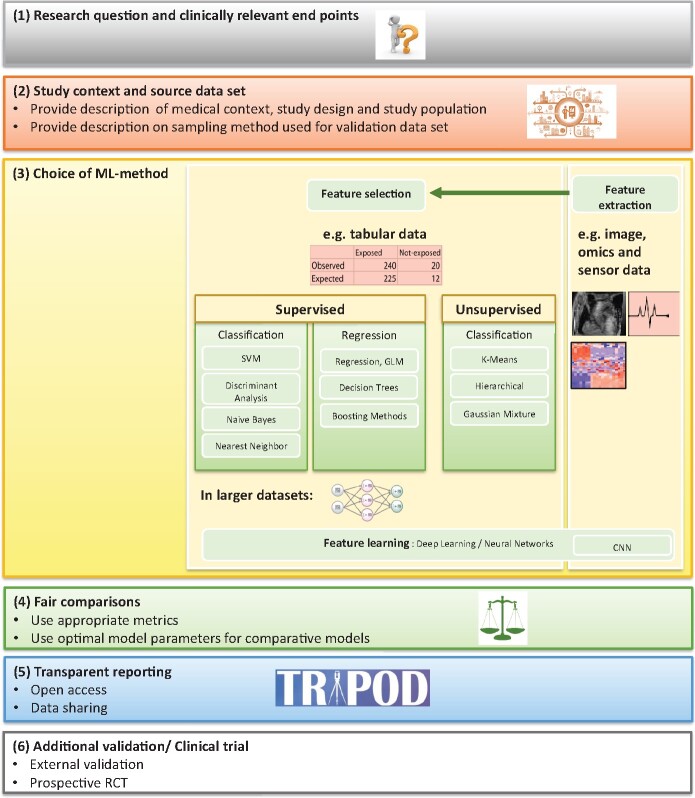
Furthermore, we recommend that some points are considered with regard to the (i) evaluation of an ML algorithm in itself and in comparison to alternative algorithms; (ii) reporting of the evaluation; and (iii) transparency of data and methods. The recommendations are summarized in Figure 5.
Figure 5.
Recommended steps to be taken into account when using artificial intelligence/machine learning methods in cardiovascular research. Feature selection (selecting the most relevant subset of features, e.g. a biomarker, age or sex of a patient or image information), feature extraction (finding a minimalistic representation of a larger data set, e.g. an image), and feature learning (the algorithm chooses/learns relevant features from the data).
The evaluation of an ML algorithm requires that a model has been developed and validated in a carefully designed study. This includes, among other aspects, that predictor variables were assessed independently from outcome variables, and that sample sizes were sufficient for stable model building and a precise estimation. Vollmer et al.12 provide a list of critical questions to assess the quality of AI/ML applications in medical applications. Moreover, tools to assess data quality in observational studies have recently been proposed and implemented.25 The metrics used to assess algorithms should generally cover aspects of calibration as well as discrimination and specifically need to match the aim of the study. For instance, the focus could be on an overall performance in all study participants versus in specific subgroups (sensitivity or specificity); the absolute performance of the algorithm could be of interest versus its incremental value above established methods; the focus could be on the statistical performance or the clinical utility of applying the algorithm in practice. Comparisons to classical regression methods or established predictive models should be ‘fair’: model parameters for classical regression methods should be tuned to the best performance possible in the sample under investigation for AI/ML. Established predictive models have to be re-calibrated to the sample used for AI/ML to have the best possible performance. Ideally, method comparison studies should be conducted by independent research groups.26,27
Where possible, the reporting of methods’ comparisons and applications should adhere to available guidelines. Oftentimes, AI/ML is used to train predictive models (for prognostic or diagnostic settings) which are usually multivariable models. A first important step in this context is to highlight the aim of the AI/ML approach, i.e. whether a longitudinal prognostic (incidence, risk for future event) or cross-sectional diagnostic/classification outcome is of interest. With the TRIPOD statement28 well established guidelines on the reporting of such models are available. While an explicit adaptation to AI/ML methods is still under development,29 the statement is overall well applicable to predictive AI/ML models. The 22 items on the checklist relate to all parts of a typical academic report from the title to the supplement and cover areas as the data source (items 4 and 5), the outcome (item 6), the model building (item 10), and the clinical implications (item 20).
Most challenging to address is item 15, which asks to present the full model and to explain how the trained model is applied and how to interpret the results. Complex AI/ML models involving many variables (e.g. random forest, deep neural network) are not easily shared in printed form and other means to make the trained model available have to be found in these cases, see the Discussion section for possibilities.
Finally, we recommend a high level of transparency throughout the process of model development and validation with regard to the utilized data, the specific final model(s) built, and the program code that was used. While performing the extensive literature review, we often recognized a lack of reporting in the exact methods used, which limits comparability and reproducibility of the proposed approaches. To overcome this shortcoming, we recommend that source code should be openly available, e.g. in a web-based version control system such as git/github (https://github.com/). The respective URL should be included in the manuscript.
It is of crucial importance to further encourage data sharing. Safe and trusted open data initiatives such as Zenodo (https://zenodo.org/) are recommended for sharing data. The platform provides a DOI to each upload to make data citable and traceable. It also offers a sophisticated data access model to restrict data access only to certain groups. Google Cloud provides a suite of tools as part of their AI platform offering (https://cloud.google.com/ai-platform) to build, validate, and explain models. As for proprietary data sharing, Triple Blind (https://tripleblind.ai/ai-in-healthcare/) is an example of a platform to reproduce results using the same datasets and models while maintaining privacy.
If data sharing is not possible due to legal issues, it is recommended to make the trained model openly available such that other research groups can re-use model weights or estimated model coefficients. This is a very common approach in traditional computer vision, where network models like VGG16 are shared with the public for re-usage. To allow for an easy integration of such models into novel applications, such pre-trained networks are now even built-in common libraries, such as Keras (https://keras.io/api/applications/). However, possible issues of model inversion30 need to be taken into account, i.e. Zhu et al.30 could show that it is possible to recover the (private) training data from the publicly shared models.
Discussion
In this article, we have reviewed the current state of AI/ML applications in cardiovascular medicine. We provide a comprehensive overview of the spectrum of the various different AI/ML methods and illustrate the context in which these were applied to address questions in a variety of cardiovascular diagnostic applications and diseases. Since a major finding is that methodology may overlap even in similar data and since we observed marked variation in quality we also provide some recommendations with respect to applying AI/ML methods in practice. This methodological overlap may be explained by the fact that to date no consensus exists as to which method should best be applied in which disease context. Therefore, many publications included in our review investigated and compared several methods simultaneously. Indeed, the choice of a specific AI/ML method is complex as are the various parameters and pitfalls that determine appropriate use. We found that AI/ML-based work frequently lacks aspects of quality such as transparency regarding methodology and data as well as validation of the methods. Other important aspects of AI/ML research include data partition and cross-validation. Krittanawong et al.10 found a large heterogeneity with respect to these aspects in their meta-analysis. Therefore, after a period of rather intense AI/ML research, which we document herein, we advocate a more vigorous approach to scientific standards, which should be a prerequisite for clinical application.
Our review is limited by our literature search, where we explicitly required the search terms ‘artificial intelligence’ or ‘machine learning’ mentioned in title or abstract. Moreover, we have focused on clinical applications and thus not considered publications in methodological journals, since we specifically wanted to focus on AI/ML applications to real-world clinical data. In contrast, methodological papers often demonstrate the usefulness or applicability of new methods on freely available benchmark data sets. Thus, they play an important role with respect to proof of concept and feasibility of newly developed methods and can be seen as an important intermediate step between method development and widespread clinical use.
A potential source of bias in our study is the exclusion of journals with an impact factor less than 2. The rationale behind this approach was to limit our analyses to articles with potential clinical impact. The threshold of 2 was chosen since it lies between the median impact factor in cardiology (median IF 2.3) and general internal medicine (median IF 1.6) according to the Web of Science.31 In total, 228 journals were searched and articles from 65 distinct journals were included in our analyses. The effect of this restriction is also displayed in the PRISMA flow chart, see Supplementary material online, Figure S1.
A further limitation results from the categorization of AI/ML approaches and disease categories. Here we used the terms primarily used by the authors of the articles included, but a potential overlap, e.g. between HF and cardiomyopathies, cannot be ruled out completely.
The broad scope of our publication limits in-depth discussion with respect to specific disease areas or data types. However, we deliberately chose this approach in order to give a broad overview. In this, our systematic review complements previously published work that focused on particular applications. Finally, we provide only descriptive analyses with respect to superiority of the AI/ML methods as compared to ‘classical’ methods, therein relying solely on the authors’ definition of superiority. As already mentioned in the recommendations above, however, these comparisons are often not conducted fairly.32 From a clinical perspective, there is still a lack of randomized controlled trials as the mainstay of evidence-based medicine in the cardiovascular field of AI/ML. Comparison of AI/ML-incorporated algorithms to standard of care by means of clinically relevant endpoints and validation in prospective studies are prerequisites for further integration and acceptance. Only the minority of publications investigated reproducibility and generalizability. However, such studies are necessary to foster large-scale clinical implementation of novel AI/ML approaches. But even if prospective validation is not implemented at this stage, now is the time for advancing quality of AI/ML-based work, given an increasing body of practical recommendations. For example, the essential TRIPOD guidelines have been extended by additional important work, such as recommendations for proper reporting of AI prediction models.29 Likewise, standards for avoiding bias and fostering reproducibility have been communicated and should be demanded, ultimately, to avoid harm to patients.14,15 Our review suggests that most of the time, standards were set too low. On the other hand, demanding that any data used to train AI/ML models must be(come) open source, while certainly ideal, might significantly preclude important hypothesis generating work. Given the shortage of open-source training data, work on closed source data, such as some registries, is indeed important for hypothesis generation and, provided it is labelled as such, deserves attention. However, at a stage where routine clinical decision making takes place, we consider external validation essential.
Our extensive review also showed that some promising AI/ML methods are currently underutilized in clinical practice. To encourage wider use of potentially superior AI/ML methods and to push such research on urgent, clinically relevant problems, one promising approach is to conduct medical challenges, as has been done frequently in various research areas. Linked to this is the definition of the task and appropriate metrics to evaluate the incoming results. Participants, mostly volunteers, can register and are asked to upload their code and/or results before the predefined deadline. Platforms like Grand Challenge (https://grand-challenge.org/challenges/) or Kaggle (https://www.kaggle.com/) provide options for data upload, participant registration and leaderboard visualization. The main benefit is that the developed methods are directly comparable, because they were, unlike in many other works, trained and tested on the same data sets. Moreover, a spill of training data into validation datasets, a problem that is hard to control for in several AI/ML settings, is excluded by design. To allow for such challenges, grants supporting the purpose of data acquisition, including an incentive to provide open or closed source data to such a challenge should be promoted.
Another possible path for future directions entails the use of federated learning. Federated learning means ‘to let the algorithms travel and not the data’. Rieke et al.33 propose to use federated learning to avoid the complexity of data sharing that is associated from a legal point of view. This may be realized by linking the data infrastructure of the hospital to an in-house computational node that trains models and sends the trained model weights to a central node outside the hospital, where the models are aggregated to create a novel powerful approach, which better accounts for more variants of data.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health online.
Supplementary Material
Acknowledgements
The authors would like to thank the members of the DZHK project group AI/ML.
Funding
M.K. is a fellow in the BIH Charité Digital Clinician Scientist Program funded by DFG. We acknowledge the support of the German Center for Cardiovascular Research funded by the Bundesministerium für Bildung und Forschung, grant 81Z1700102 (to I.R.K.), 81Z0300108 (to T.F.), 81Y0400120 (to S.G.), 81X3400108 (to S.G.), 81Z0400101 (to M.B.), 81X2400123 (to M.B.), and 81X2400143 (to M.B.).
Conflict of interest: T.F. reports personal fees from Novartis, personal fees from Bayer, personal fees from Janssen, personal fees from Roche, personal fees from Boehringer Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Galapagos, personal fees from Penumbra, personal fees from Parexel, personal fees from Vifor, personal fees from BiosenseWebster, personal fees from CSL Behring, personal fees from Fresenius Kabi, personal fees from Coherex Medical, personal fees from LivaNova, personal fees from Minoryx, outside the submitted work. I.R.K. reports grants from German Center for Cardiovascular Research, grants from German Center for Lung Research, grants from Deutsche Forschungsgemeinschaft, grants from Deutsche Krebshilfe, grants from Federal Ministry of Education and Research, outside the submitted work. J.S. reports grants from DZHK e.V., during the conduct of the study. All other authors have declared no conflict of interest.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P.. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J 2020;41:12–85. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3. Kilic A. Artificial intelligence and machine learning in cardiovascular health care. Ann Thorac Surg 2020;109:1323–1329. [DOI] [PubMed] [Google Scholar]
- 4. Bizopoulos P, Koutsouris D.. Deep learning in cardiology. IEEE Rev Biomed Eng 2018;12:168–193. [DOI] [PubMed] [Google Scholar]
- 5. Nagendran M, Chen Y, Lovejoy CA, Gordon AC, Komorowski M, Harvey H, Topol EJ, Ioannidis JPA, Collins GS, Maruthappu M.. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ 2020;368:m689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP.. Machine learning in cardiovascular medicine: are we there yet? Heart 2018;104:1156–1164. [DOI] [PubMed] [Google Scholar]
- 7. Seetharam K, Shrestha S, Sengupta PP.. Artificial intelligence in cardiovascular medicine. Curr Treat Options Cardiovasc Med 2019;21:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson KW, Torres Soto J, Glicksberg BS, Shameer K, Miotto R, Ali M, Ashley E, Dudley JT.. Artificial intelligence in cardiology. J Am Coll Cardiol 2018;71:2668–2679. [DOI] [PubMed] [Google Scholar]
- 9. Lopez-Jimenez F, Attia Z, Arruda-Olson AM, Carter R, Chareonthaitawee P, Jouni H, Kapa S, Lerman A, Luong C, Medina-Inojosa JR, Noseworthy PA, Pellikka PA, Redfield MM, Roger VL, Sandhu GS, Senecal C, Friedman PA.. Artificial intelligence in cardiology: present and future. Mayo Clin Proc 2020;95:1015–1039. [DOI] [PubMed] [Google Scholar]
- 10. Krittanawong C, Virk HUH, Bangalore S, Wang Z, Johnson KW, Pinotti R, Zhang HJ, Kaplin S, Narasimhan B, Kitai T, Baber U, Halperin JL, Tang WHW.. Machine learning prediction in cardiovascular diseases: a meta-analysis. Sci Rep 2020;10:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haibe-Kains B, Adam GA, Hosny A, Khodakarami F; Massive Analysis Quality Control (MAQC) Society Board of Directors, Waldron L, Wang B, McIntosh C, Goldenberg A, Kundaje A, Greene CS, Broderick T, Hoffman MM, Leek JT, Korthauer K, Huber W, Brazma A, Pineau J, Tibshirani R, Hastie T, Ioannidis JPA, Quackenbush J, Aerts HJWL.. Transparency and reproducibility in artificial intelligence. Nature 2020;586:E14–E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vollmer S, Mateen BA, Bohner G, Király FJ, Ghani R, Jonsson P, Cumbers S, Jonas A, McAllister KSL, Myles P, Granger D, Birse M, Branson R, Moons KGM, Collins GS, Ioannidis JPA, Holmes C, Hemingway H.. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ 2020;368:l6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wynants L, Smits LJM, Van Calster B.. Demystifying AI in healthcare. BMJ 2020;370:m3505. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Lancet Digit Health 2020;2:e537–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cruz Rivera S, Liu X, Chan AW, Denniston AK, Calvert MJ; SPIRIT-AI and CONSORT-AI Working Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Lancet Digit Health 2020;2:e549–e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DECIDE-AI Steering Group. DECIDE-AI: new reporting guidelines to bridge the development-to-implementation gap in clinical artificial intelligence. Nat Med 2021;27:186–187. [DOI] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D.. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Commandeur F, Slomka PJ, Goeller M, Chen X, Cadet S, Razipour A, McElhinney P, Gransar H, Cantu S, Miller RJH, Rozanski A, Achenbach S, Tamarappoo BK, Berman DS, Dey D.. Machine learning to predict the long-term risk of myocardial infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: a prospective study. Cardiovasc Res 2020, 116:2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leha A, Hellenkamp K, Unsöld B, Mushemi-Blake S, Shah AM, Hasenfuß G, Seidler T.. A machine learning approach for the prediction of pulmonary hypertension. PLoS One 2019;14:e0224453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhuva AN, Bai W, Lau C, Davies RH, Ye Y, Bulluck H, McAlindon E, Culotta V, Swoboda PP, Captur G, Treibel TA, Augusto JB, Knott KD, Seraphim A, Cole GD, Petersen SE, Edwards NC, Greenwood JP, Bucciarelli-Ducci C, Hughes AD, Rueckert D, Moon JC, Manisty CH.. A multicenter, scan-rescan, human and machine learning CMR study to test generalizability and precision in imaging biomarker analysis. Circ Cardiovasc Imaging 2019;12:e009214. [DOI] [PubMed] [Google Scholar]
- 21. Klambauer G, Unterthiner T, Mayr A, Hochreiter S.. Self-normalizing neural networks. arXiv preprint arXiv:1706.02515; 2017.
- 22. Arik SO, Pfister T.. Tabnet: attentive interpretable tabular learning. arXiv preprint arXiv:1908.07442; 2019.
- 23. Popov S, Morozov S, Babenko A.. Neural oblivious decision ensembles for deep learning on tabular data. arXiv preprint arXiv:1909.06312; 2019.
- 24. Chen C, Qin C, Qiu H, Tarroni G, Duan J, Bai W, Rueckert D.. Deep learning for cardiac image segmentation: a review. Front Cardiovasc Med 2020;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt CO, Struckmann S, Enzenbach C, Reineke A, Stausberg J, Damerow S, Huebner M, Schmidt B, Sauerbrei W, Richter A.. Facilitating harmonized data quality assessments. A data quality framework for observational health research data collections with software implementations in R. BMC Med Res Methodol 2021;21:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boulesteix AL, Wilson R, Hapfelmeier A.. Towards evidence-based computational statistics: lessons from clinical research on the role and design of real-data benchmark studies. BMC Med Res Methodol 2017;17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weber LM, Saelens W, Cannoodt R, Soneson C, Hapfelmeier A, Gardner PP, Boulesteix AL, Saeys Y, Robinson MD.. Essential guidelines for computational method benchmarking. Genome Biol 2019;20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins GS, Reitsma JB, Altman DG, Moons KG.. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Circulation 2015;131:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collins GS, Moons KGM.. Reporting of artificial intelligence prediction models. Lancet 2019;393:1577–1579. [DOI] [PubMed] [Google Scholar]
- 30. Zhu L., Han S.. Deep leakage from gradients. In Yang Q, Fan L, Yu H, eds. Federated Learning. Lecture Notes in Computer Science, vol. 12500. Cham: Springer, 2020, pp. 17–31. 10.1007/978-3-030-63076-8_2. [DOI] [Google Scholar]
- 31.Journal Impact Factor 2020, Journal Citation Reports Science Edition, Clarivate Analytics. https://jcr.clarivate.com/JCRHomePageAction.action?
- 32. Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B.. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol 2019;110:12–22. [DOI] [PubMed] [Google Scholar]
- 33. Rieke N, Hancox J, Li W, Milletarì F, Roth HR, Albarqouni S, Bakas S, Galtier MN, Landman BA, Maier-Hein K, Ourselin S, Sheller M, Summers RM, Trask A, Xu D, Baust M, Cardoso MJ.. The future of digital health with federated learning. NPJ Digit Med 2020;3:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.