Abstract
Aims
Mobile, portable ECG-recorders allow the assessment of heart rhythm in out-of-hospital conditions and may prove useful for monitoring patients with cardiovascular diseases. However, the effectiveness of these portable devices has not been tested in everyday practice.
Methods and results
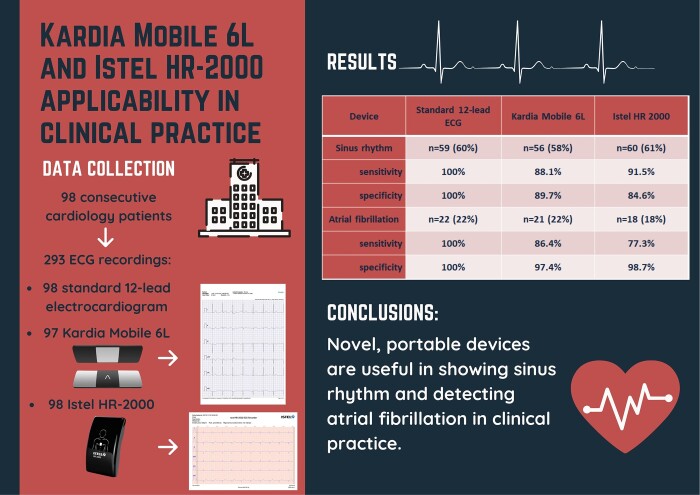
A group of 98 consecutive cardiology patients [62 males (63%), mean age 69 ± 12.9 years] were included in an academic care centre. For each patient, a standard 12-lead electrocardiogram (SE), as well as a Kardia Mobile 6L (KM) and Istel (IS) HR-2000 ECG were performed. Two groups of experienced physicians analysed obtained recordings. After analysing ECG tracings from SE, KM, and IS, quality was marked as good in 82%, 80%, and 72% of patients, respectively (P < 0.001). There were no significant differences between devices in terms of detecting sinus rhythm [SE (60%, n = 59), KM (58%, n = 56), and IS (61%, n = 60); SE vs. KM P = 0.53; SE vs. IS P = 0.76) and atrial fibrillation [SE (22%, n = 22), KM (22%, n = 21), and IS (18%, n = 18); (SE vs. KM P = 0.65; SE vs. IS = 0.1)]. KM had a sensitivity of 88.1% and a specificity of 89.7% for diagnosing sinus rhythm. IS showed 91.5% and 84.6% sensitivity and specificity, respectively. The sensitivity of KM in detecting atrial fibrillation was higher than IS (86.4% vs. 77.3%), but their specificity was comparable (97.4% vs. 98.7%).
Conclusion
Novel, portable devices are useful in showing sinus rhythm and detecting atrial fibrillation in clinical practice. However, ECG measurements concerning conduction and repolarization should be clarified with a standard 12-lead electrocardiogram.
Keywords: Atrial fibrillation, Mobile devices, Arrhythmia, Telemedicine
Graphical Abstract
Introduction
Novel telemedicine solutions are required to overcome the current leading healthcare-related challenge: the spread of cardiovascular disease. Portable devices have been developed to improve the diagnostic process, including the Kardia Mobile 6L (KM; AliveCor Inc., USA) and Istel HR-2000 (IS; Istel, Poland) wireless electrocardiogram (ECG) devices, which are compatible with iOS and Android operating systems. KM consists of a small plate equipped with three electrodes (one on the bottom and two on the top) that enables six-lead ECG recording. The device has been approved by the Food and Drug Administration (FDA) in the USA and was designed to identify atrial fibrillation (AF) or other cardiac arrhythmias, as well as normal heart rhythm. IS is a quadrate six-lead ECG recorder with four built-in conducting plates. It is registered as class IIa in Europe and holds CE marking. The outcomes of the tests conducted with these devices are immediately transmitted to the connected smartphone via Bluetooth technology.
This study aimed to evaluate the usability of portable ECG recorders by comparing rhythm and basic ECG parameters (PQ, RR and QT intervals, duration of QRS complexes, etc.) obtained with KM/IS to standard 12-lead ECG tracings.
Methods
Recruitment
Consecutive patients were included from the cardiology ward in a tertiary cardiovascular care centre. The patients were admitted to the hospital between November and December 2019. On ECG recording, all of the recruited patients were clinically stable.
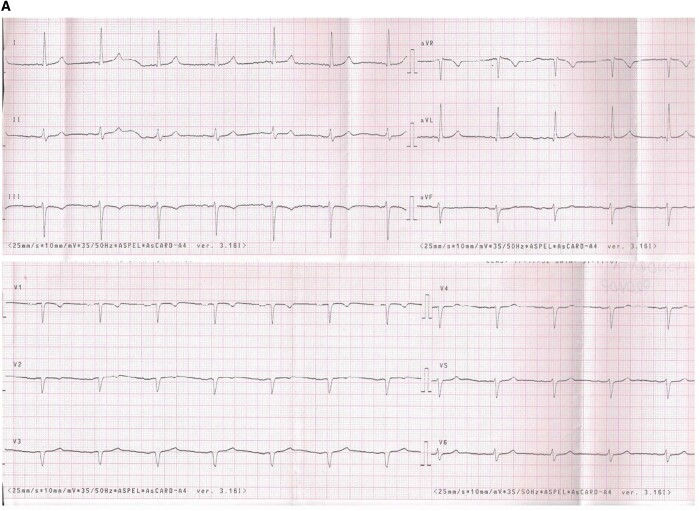
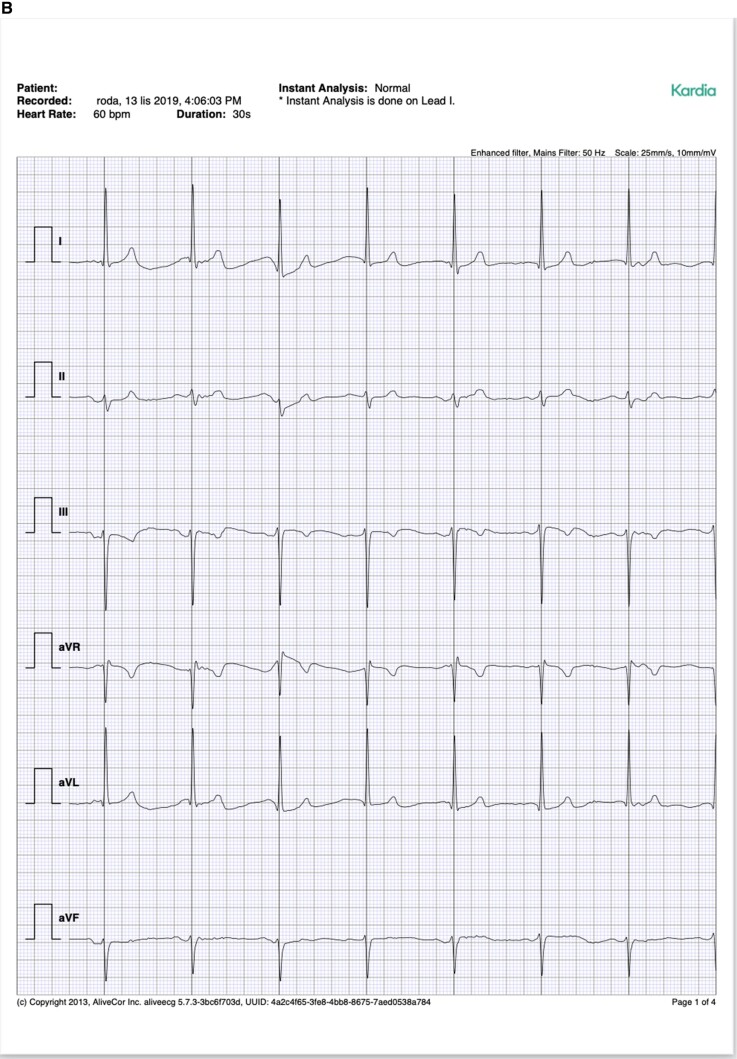
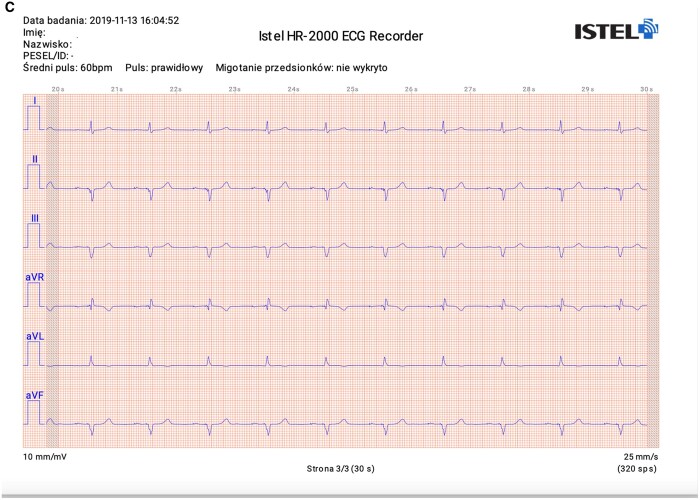
All patients were instructed on study’s aim and flow and gave informed consent for their participation. Experienced technicians performed all recording, both 12-lead ECG and KM/IS recording. All recordings were made one by one, which took up to 5 min including data collection. The devices are shown in Figure 1 and exemplary ECG tracings are shown in Figure 2.
Figure 1.
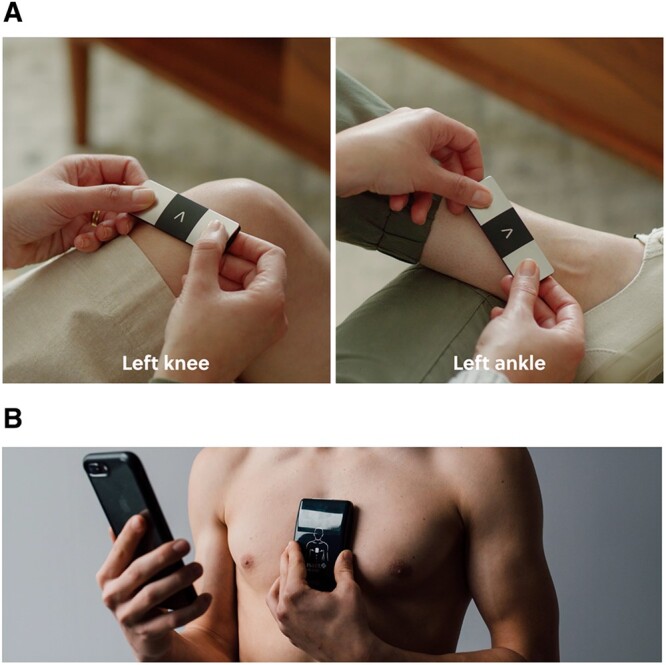
Kardia Mobile 6L (A) and Istel HR 2000 (B) placed on a patient. *Both images come from promotional materials.
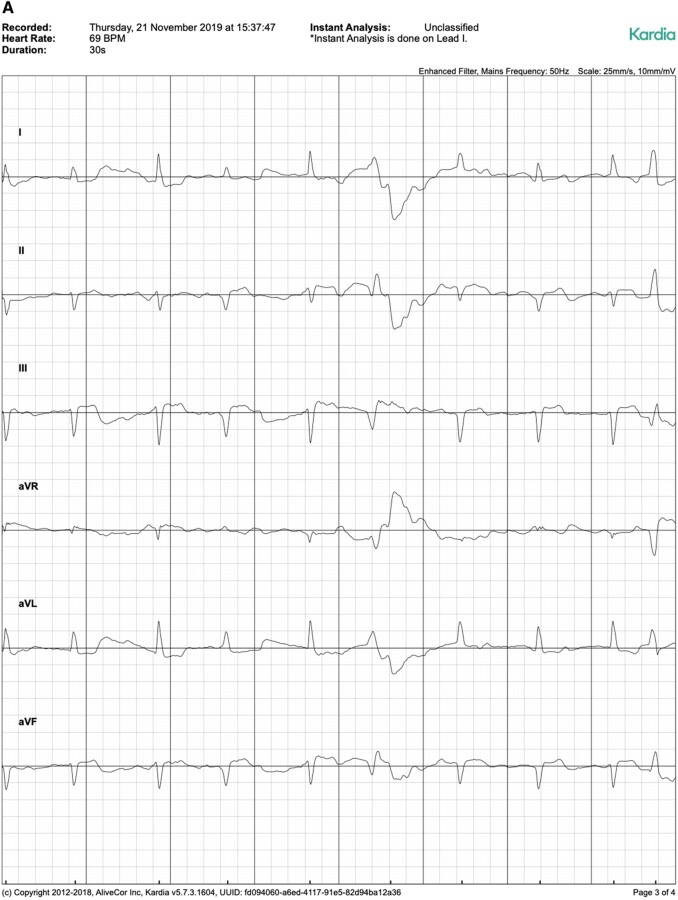
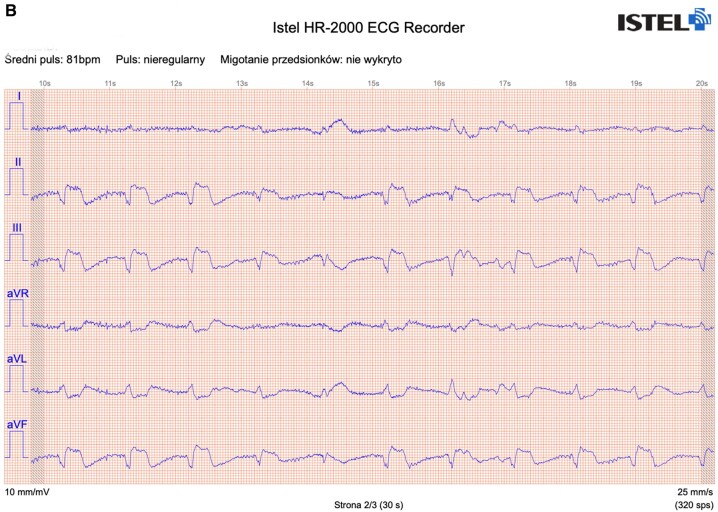
Figure 2.
Exemplary ECG tracings from standard 12-lead ECG (A), Kardia Mobile (B), and Istel HR (C).
During the examination with a KM, the patient put the device on bare skin on their left leg and placed two fingers of each hand on the electrodes on the top. Afterwards, an IS device was placed in the middle of the patient’s chest. In one case, KM recording was not performed due to Parkinson’s disease-related tremors and inability to obtain ECG tracing.
This was an observational, non-invasive, and non-randomized study. The local bioethics committee was notified. Every patient signed an informed consent form on admission to the clinic for personal and medical data administration and analysis, and verbally agreed to participate in this project.
ECG assessment
All ECG recordings were blinded and assessed by one of the two independent groups of experienced physicians. Every ECG was analysed by a younger cardiologist, which a senior physician subsequently checked. In case of disagreement the discussion began, and the final verdict was agreed upon on the guidelines. ECG assessment was carried out according to the Polish ECG Reporting Guidelines.1 The interpretation included an assessment of the recording quality, rhythm, P wave, QRS complexes, and ST-segment morphologies. Moreover, the heart axis, presence of atrioventricular blocks, pathologic Q waves, intraventricular conduction delays, previous myocardial infarction, and current ischaemia were analysed. The measurements of intervals, such as PQ, RR, and QT, were also performed. The doctors’ responses were collected in an encrypted online form (Typeform, Spain). Assessments of the 12-lead ECGs were defined as the referral, and interpretations of KM and IS tracings were compared to them.
Statistical analysis
The database data were analysed using appropriate statistical tests to determine the portable devices’ diagnostic accuracy. Categorical variables, expressed as frequency and percentages, were compared using McNemar test for 2 × 2 tables, Wilcoxon signed-rank test for 2 × 3 tables, and McNemar–Bowker test for 3 × 3 tables. In the case of multiple comparisons, Bonferroni correction was considered to calculate statistical significance level.
Continuous data were described as mean ± standard deviation and were compared using Student’s unpaired t-test. A statistical significance was defined as a two-sided P-value of <0.05 and <0.0167 after Bonferroni correction. Statistical analysis was calculated using SAS® software (Cary, NC, USA), version 9.4.
Results
In total, 98 patients were included in the study; 62 (63%) were male. The essential characteristics of the study population are shown in Table 1.
Table 1.
Patient characteristics
| Baseline demographics | N (%) or mean ± SD |
|---|---|
| Gender (male) | 62 (63%) |
| Age (years) | 69 ± 12.9 |
| Body mass (kg) | 82.4 ± 17.3 |
| Height (cm) | 169.4 ± 9.0 |
| Body mass index | 28.7 ± 5.4 |
| Medical history | |
| Nicotinism (history) | 47 (48%) |
| Diabetes mellitus | 40 (40.8%) |
| Hypertension | 72 (73.4%) |
| Dyslipidaemia | 72 (73.4%) |
| Chronic kidney disease | 25 (25.5%) |
| eGFR | 68.5 ± 21 |
| Thyroid dysfunction | 19 (19.4%) |
| Hypothyroidism | 15 (15.3%) |
| Hyperthyroidism | 4 (4.1%) |
| Asthma | 4 (4.1%) |
| Chronic obstructive pulmonary disease | 9 (9.2%) |
| Cardiovascular history: | |
| Stable angina (angina pectoris) | 31 (31.6%) |
| ACS (admission) | 15 (15.3%) |
| Myocardial infarction (history) | 31 (31.6%) |
| PCI/CABG (history) | 41 (41.8%) |
| Heart failure | 55 (56.1%) |
| NYHA II | 30 (30.6%) |
| NYHA III | 22 (22.4%) |
| NYHA IV | 3 (3.1%) |
| LVEF (%) | 49.7 ± 12.6 |
| Atrial fibrillation | 43 (43.9%) |
| Paroxysmal | 17 (17.3%) |
| Persistent | 12 (12.2%) |
| Chronic | 14 (14.3%) |
| CIED implanted | 34 (34.7%) |
| Pacemaker | 22 (22.4%) |
| AAI | 1 (1.0%) |
| VVI | 10 (10.2%) |
| DDD | 11 (11.2%) |
| ICD | 9 (9.2%) |
| CRT | 3 (3.1%) |
| Ablation | 6 (6.1%) |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CIED, cardiac implantable electronic devices; CRT, cardiac resynchronization; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
A total of 293 ECG recordings from 98 patients were analysed (98 SE, 97 KM, 98 IS). There were significant differences between devices in terms of ECG quality, with IS showing the lowest quality (SE 82% good, n = 80; KM 72% good, n = 70; IS 44% good, n = 43; SE vs. KM P = 0.046; SE vs. IS P < 0.001). However, there were no significant differences in detecting sinus rhythm between SE (60%, n = 59), KM (58%, n = 56), and IS (61%, n = 60) (SE vs. KM P = 0.53; SE vs. IS P = 0.76). The sensitivity and specificity of KM for sinus rhythm detection were 88.1% and 89.7%, respectively; IS showed 91.5% sensitivity and 84.6% specificity. Moreover, the effectiveness of AF detection was similar among all three devices: SE (22%, n = 22), KM (22%, n = 21), and IS (18%, n = 18) (SE vs. KM P = 0.65; SE vs. IS = 0.1) (Table 2). The sensitivity of KM for diagnosing AF was higher than that of IS (86.4% vs. 77.3%), but the specificity was slightly lower (97.4% vs. 98.7%). According to the device used the results of the sinus rhythm and AF detection according to the device used are summarized in Table 3.
Table 2.
Sensitivity and specificity of AF and sinus rhythm diagnosis with Kardia Mobile 6L and Istel HR 2000 against standard 12-lead ECG
| Device | Standard 12-lead ECG | Kardia Mobile 6L | Istel HR 2000 |
|---|---|---|---|
| Sinus rhythm | n = 59 (60%) | n = 56 (58%) | n = 60 (61%) |
| Sensitivity | 100% | 88.1% | 91.5% |
| Specificity | 100% | 89.7% | 84.6% |
| Atrial fibrillation | n = 22 (22%) | n = 21 (22%) | n = 18 (18%) |
| Sensitivity | 100% | 86.4% | 77.3% |
| Specificity | 100% | 97.4% | 98.7% |
Table 3.
Comparison of ECG parameters among a standard 12-lead ECG, Kardia Mobile 6L, and Istel HR 2000
| Analysed variables | 12-lead ECG | Kardia Mobile | Istel HR-2000 | P value | P value | P value | P value | |
|---|---|---|---|---|---|---|---|---|
| 12-lead ECG vs. Kardia Mobilea | 12-lead ECG vs. Istel HR-2000a | Kardia Mobile vs. Istel HR-2000a | ||||||
| Quality | Good | n = 80 (82%) | n = 70 (72%) | n = 43 (44%) | <0.001 | 0.046 | <0.001 | <0.001 |
| acceptable | n = 17 (17%) | n = 22 (23%) | n = 49 (50%) | |||||
| Poor | n = 1 (1%) | n = 5 (5%) | n = 6 (6%) | |||||
| Normal sinus rhythm | n = 59 (60%) | n = 56 (58%) | n = 60 (61%) | 0.53 | 0.76 | 0.44 | ||
| Atrial fibrillation | n = 22 (22%) | n = 21 (22%) | n = 18 (18%) | 0.65 | 0.1 | 0.26 | ||
| Normal heart axis | n = 68 (69%) | n = 59 (61%) | n = 18 (18%) | 0.06 | <0.001 | <0.001 | ||
| Block exclusion | n = 57 (58%) | n = 55 (57%) | n = 57 (58%) | 0.81 | 1 | 0.82 | ||
| Pathologic Q waves | n = 51 (52%) | n = 61 (63%) | n = 27 (28%) | 0.06 | <0.001 | <0.001 | ||
| Normal QRS morphology | n = 72 (73%) | n = 58 (60%) | n = 76 (78%) | 0.0158 | 0.39 | 0.002 | ||
| Previous MI | Excluded | n = 56 (57%) | n = 45 (46%) | n = 80 (82%) | <0.001 | 0.13 | <0.001 | <0.001 |
| Recognized | n = 19 (19%) | n = 20 (21%) | n = 7 (7%) | |||||
| Suspected | n = 23 (23%) | n = 32 (33%) | n = 11 (11%) | |||||
| Current ischaemia | Excluded | n = 77 (79%) | n = 81 (84%) | n = 44 (45%) | <0.001 | 0.14 | <0.001 | <0.001 |
| ST depression | n = 11 (11%) | n = 12 (12%) | n = 7 (7%) | |||||
| ST elevation | n = 10 (10%) | n = 4 (4%) | n = 47 (48%) | |||||
| Normal T wave | n = 60 (61%) | n = 74 (76%) | n = 74 (76%) | 0.011 | 0.04 | 0.86 | ||
MI, myocardial infarction.
After Bonferroni correction P < 0.0167 should be considered as statistically significant.
Normal heart axis was diagnosed less often in IS (18%, n = 18) than in SE (69%, n = 68) and KM (61%, n = 59) (SE vs. KM P = 0.06; SE vs. IS P < 0.001). Normal QRS morphology was described with different frequency in SE (73%, n = 72), KM (60%, n = 58), and IS (78%, n = 76) (SE vs. KM P = 0.0158; SE vs. IS P = 0.39). Significantly less pathologic Q-waves were observed in IS (28%, n = 27) compared to SE (52%, n = 51) or KM (63%, n = 61) (SE vs. KM P < 0.06; SE vs. IS P < 0.001). The SE ruled out previous myocardial infarction (57%, n = 56) with lower frequency than with IS (82%, n = 80) but higher when compared with KM [46%, n = 45 (SE vs. KM P = 0.13); SE vs. IS P < 0.001]. Similarly, significant differences in current ischaemia assessment (ST segment elevation in ≥2 contiguous leads) were observed in IS (48%, n = 47) in comparison with SE (10%, n = 10) and KM (4.1%, n = 4) (SE vs. KM P = 0.14; SE vs. IS P < 0.001). These device comparisons are summarized in Table 3.
There were no significant differences in the mean PQ interval measurements between SE and KM (171 vs. 168 ms, P = 0.63) and between SE and IS (171 vs. 174 ms, P = 0.27). Measurements of the RR interval performed by KM were shorter than those obtained with SE (815 vs. 860 ms, P = 0.033) and IS (815 vs. 877 ms, P = 0.001). There were no significant differences in RR interval measurements between SE and IS (860 vs. 877 ms, P = 0.27). QT intervals measured using KM were shorter than those measured with SE (366 vs. 403 ms, P = 0.0017) and IS (366 vs. 404 ms, P = 0.003). The QT interval measurements performed by SE and IS were similar (403 vs. 404 ms, P = 0.84). The QRS duration analysis showed differences between SE and KM (98 vs. 103 ms, P = 0.007). In comparison, the results obtained with SE and IS devices were not significantly different (98 vs. 99 ms, P = 0.77).
Finally, both devices were synchronized with dedicated mobile applications and after every recording, an information regarding AF presence was displayed. AF diagnosis was made based on algorithms, which were supposed to help in arrhythmia screening. We have compared the results from the applications with those from our expert analysis. The AF diagnoses were consistent in 100% (21/21) and 89% (16/18) for KM and IS, respectively.
Discussion
As cardiovascular disease continues to pose new challenges, innovative tools are needed to improve the diagnostic process. Atrial fibrillation is the most prevalent sustained cardiac arrhythmia in adults, with an estimated occurrence of between 2% and 4% in the general population.2 However, more adults are expected to suffer from AF in the future due to increased longevity, more frequent diagnosing, and other cardiac comorbidities.3 As AF is usually associated with stroke,4 and can increase the morbidity and mortality among patients with dementia or other diseases,5–7 there are sufficient reasons to improve the diagnosis and treatment of cardiac arrhythmias and provide more effective prophylaxis.
Polish and international cardiac societies recommend using telemedicine solutions in medical practice.8,9 Additionally, the 2020 European Society of Cardiology (ESC) guidelines for the diagnosis and management of AF declared that single-lead 30s ECG tracing is diagnostic of clinical AF.10 Therefore, modern, mobile ECG devices may be useful telemedicine tools, particularly for AF diagnosis. However, despite the variety of ECG monitoring solutions on the market, their efficiency is still to be established in everyday practice.
In the conducted study, the adult patients were examined on the cardiology ward with a standard 12-lead ECG and the KM and IS devices. The study showed that KM and IS provided sufficient sensitivity and specificity of ECG tracing for detecting AF and sinus rhythm (Table 2). Previous analysis on another group of patients by Kołtowski et al.11 comparing single-lead KM with standard ECG showed that although KM provided satisfactory results in detecting AF, the ECG tracings were of lower quality. However, the ECG tracings produced by the KM device in our study were of good quality.
Transient ischaemic attacks and strokes can be the first visual symptoms of earlier unrecognized arrhythmias. In these cases, the trigger has to be particularly identified to implement secondary prevention. Therefore, due to its potential asymptomatic nature, it is crucial to search for other ways to diagnose AF. Indeed, a frequent ECG examination is advised in high-risk patients. As it is impossible for specialists to perform ECGs on a daily basis in a medical centre, mobile devices could be beneficial in the early detection of AF. Although other studies on different populations have confirmed that modern mobile devices could be used to screen AF,12,13 head-to-head studies comparing the various devices are lacking.
Chan et al.14 claimed that AF screening using modern solutions was feasible in a general practitioner setting. Using a handheld ECG device, both newly diagnosed and known cases of AF could be identified as suitable for preventative stroke therapy.14 However, the authors stated that an improved awareness of AF is required to maximize the screening benefits.14,15 Indeed, patient involvement in the diagnostic process through self-recording of ECG tracings may help raise AF awareness and encourage them to seek further, health-related knowledge.
This study indicates KM and IS are suitable tools for diagnosing sinus rhythm and AF. These modern solutions are an attractive alternative for diagnosing both symptomatic and asymptomatic arrhythmias due to their ease of use and mobility. Similarly, the use of personal handheld devices in the ongoing monitoring of patients with acute palpitations and pre-syncope was heralded by Reed et al. They claimed that adding the wireless ECG monitor to standard care is beneficial. In particular, the use of a smartphone-based ECG monitoring event recorder increased the number of patients in whom heart rhythm disturbance could be recorded (i.e. the number of patients in whom an ECG was obtained during the appearance of the symptoms quintupled). In the study by Reed et al.,16 87.0% of participants who filled in the survey described the KM as easy to use. This solution was also cost-effective; namely, the costs of symptomatic rhythm diagnosis were £921 lower than in the control group. However, it is essential to mention that the initial cost of a new smartphone device may limit this solution’s uptake, as some patients simply cannot afford them. Moreover, some people (e.g. the elderly) do not feel comfortable with smartphone software and thus, may not fully benefit from this telemedicine solution.
This study found no significant differences between SE and IS in QT interval measurements, although QT measurements were shorter (on average by 37 ms) in KM ECGs. Haberman et al.17 also reported a difference in QT measurements of 33 ± 44 ms using a single-lead KM across a group of elite athletes, healthy subjects, and cardiology clinic patients. Although discrepancies’ aetiology is multifactorial, a major limitation of both KM and IS is the presence of ECG artefacts (Figure 3), which often arise from body movements.
Figure 3.
Exemplary ECG tracings from (A) Kardia Mobile and (B) Istel HR showing possible artefacts.
Gropler et al.18 showed single-lead KM accurately assessed ECG intervals of healthy children and other young patients with various arrhythmias. The average difference in QTc measurements between the mobile device and SE was 15.6 ± 12.7 ms. Indeed, KM recordings have proven to be credible for the accurate detection of QT intervals in children for screening purposes. Similarly, Garabelli et al.19 confirmed that KM could detect QTc prolongation in healthy volunteers and in-patients receiving sotalol or dofetilide; the I-lead KM was the most accurate in measuring QTc <500 ms.
Overall, this study shows that modern held–held devices have limited applicability in cardiac telemedicine, which comes from several differences shown in our analysis and lack of precordial leads. The perspective of the heart’s electrical activity is narrowed. Additionally, the quality of ECG tracings from mobile devices was lower. However, they are sufficient to make an AF diagnosis. Therefore, electrocardiographic telemonitoring should be added to the standard care to complement and improve diagnostics or screening capabilities. A possible solution is to supply patients with immediate, personalized care, and information, as well as remote contact with specialists. For example, Koole et al.20 recently examined a new mobile health program for telemonitoring of symptomatic adults with congenital heart disease, which was found to improve healthcare quality.
Additionally, mobile applications using photoplethysmography (PPG) have been recently developed and adopted for AF screening. Their undisputed advantage is the lack of need for additional devices and almost every patient with a smartphone can use it. Currently used algorithms are as effective in AF diagnosing as single-lead ECG. The reported sensitivity and specificity were 96% and 97%, respectively and were very similar to single-lead ECG.21 However, even the authors of the application stated that PPG should be measured simultaneously with ECG.22 Additionally, PPG is not sufficient method to make AF diagnosis according to ESC guidelines, whereas even single-lead 30s ECG tracing of good quality is.
According to the authors’ knowledge, this is the first study to directly compare electrocardiographic parameters received from KM and IS devices with those from SE, and ascertain their diagnostic capability for arrhythmias. The results regarding rhythm diagnosis are encouraging. As more and more devices enter the market, it will be crucial to determine their relative effectiveness in a similar manner.
Limitations
There are some limitations to this study that should be mentioned. First, this was a single-centre study, and a larger group of patients would provide more dependable results. Second, only patients who were in a stable condition were examined, which restricted the study range. More ECG tracings in unstable patients would be more of a challenge for the mobile devices. Furthermore, ECG assessments were made by only two groups of specialists, and according to their expertise, which could have caused bias in the study results. When looking at the RR interval analysis it should be noted that ECGs were recorded one by one and therefore the heart rate might have changed in the meantime. Additionally, a disturbing limitation of electrocardiographic telemonitoring, albeit not of the study itself, are artefacts produced on the ECG record. Such artefacts are most often associated with body movements and can cause difficulties in ECG interpretation.
Conflict of interest: none declared.
Lead author biography

Dr Bartosz Krzowski is a first-year general cardiology fellow at 1st Department of Cardiology, Medical University of Warsaw. He is following his PhD, which concerns mobile application use in patients after myocardial infarction. His research interests include mobile devices and arrhythmia. Dr Krzowski is also training to become clinical cardiac electrophysiology operator.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Baranowski RBK, Kozłowski D.. Zalecenia dotyczące stosowania rozpoznań elektrokardiograficznych. Kardiol Pol 2010;68:335–390. [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528.30700139 [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oladiran O, Nwosu I.. Stroke risk stratification in atrial fibrillation: a review of common risk factors. J Community Hosp Intern Med Perspect 2019;9:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf PA, Abbott RD, Kannel WB.. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 6. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015;72:1288–1294. [DOI] [PubMed] [Google Scholar]
- 7. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE.. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 8. Piotrowicz R, Krzesinski P, Balsam P, Kempa M, Glowczynska R, Grabowski M, et al. Cardiology telemedicine solutions - opinion of the experts of the Committee of Informatics and Telemedicine of Polish Society of Cardiology, Section of Non-invasive Electrocardiology and Telemedicine of Polish Society of Cardiology and Clinical Sciences C. Kardiol Pol 2018;76:698–707. [DOI] [PubMed] [Google Scholar]
- 9. Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017;14:e55–e96. [DOI] [PubMed] [Google Scholar]
- 10. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020. [DOI] [PubMed] [Google Scholar]
- 11. Koltowski L, Balsam P, Gllowczynska R, Rokicki JK, Peller M, Maksym J, et al. Kardia Mobile applicability in clinical practice: a comparison of Kardia Mobile and standard 12-lead electrocardiogram records in 100 consecutive patients of a tertiary cardiovascular care center. Cardiol J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Godin R, Yeung C, Baranchuk A, Guerra P, Healey JS.. Screening for atrial fibrillation using a mobile, single-lead electrocardiogram in Canadian primary care clinics. Can J Cardiol 2019;35:840–845. [DOI] [PubMed] [Google Scholar]
- 13. Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 14. Chan LL, Chan SC, Yan BP.. Feasibility and acceptability of atrial fibrillation screening using a hand-held ECG device in general practice setting in Hong Kong. Value Health 2017;20:A599. [Google Scholar]
- 15. Cunha S, Antunes E, Antoniou S, Tiago S, Relvas R, Fernandez-Llimos F, et al. Raising awareness and early detection of atrial fibrillation, an experience resorting to mobile technology centred on informed individuals. Res Social Adm Pharm 2020;16:787–792. [DOI] [PubMed] [Google Scholar]
- 16. Reed MJ, Grubb NR, Lang CC, O'Brien R, Simpson K, Padarenga M, et al. Multi-centre randomised controlled trial of a smartphone-based event recorder alongside standard care versus standard care for patients presenting to the emergency department with palpitations and pre-syncope: the IPED (Investigation of Palpitations in the ED) study. EClinicalMed 2019;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haberman ZC, Jahn RT, Bose R, Tun H, Shinbane JS, Doshi RN, et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol 2015;26:520–526. [DOI] [PubMed] [Google Scholar]
- 18. Gropler MRF, Dalal AS, Van Hare GF, Silva JNA.. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS One 2018;13:e0204403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garabelli P, Stavrakis S, Albert M, Koomson E, Parwani P, Chohan J, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016;27:827–832. [DOI] [PubMed] [Google Scholar]
- 20. Koole MAC, Kauw D, Winter MM, Dohmen DAJ, Tulevski II, de Haan R, et al. First real-world experience with mobile health telemonitoring in adult patients with congenital heart disease. Neth Heart J. 2019;27:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B.. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: diagnostic accuracy study of the FibriCheck app. JMIR Mhealth Uhealth 2019;7:e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandenberk T, Stans J, Mortelmans C, Van Haelst R, Van Schelvergem G, Pelckmans C, et al. Clinical validation of heart rate apps: mixed-methods evaluation study. JMIR Mhealth Uhealth 2017;5:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.