Abstract
Aims
Patients with a systemic right ventricle (sRV) in the context of transposition of the great arteries (TGA) after atrial switch or congenitally corrected TGA are prone to heart failure and arrhythmias. This study evaluated feasibility, patient adherence, and satisfaction of a smart technology-based care pathway for heart failure treatment optimization in these patients.
Methods and results
Patients with symptomatic sRV failure eligible for initiation of sacubitril/valsartan were provided with four smartphone compatible devices (blood pressure monitor, weight scale, step counter, and rhythm monitor) and were managed according to a smart technology-based care pathway. Biweekly sacubitril/valsartan titration visits were replaced by electronical visits, patients were advised to continue measurements at least weekly after titration. Data of 24 consecutive sRV patients (median age 47 years, 50% female) who participated in the smart technology-based care pathway were analysed. Median home-hospital distance was 65 km (maximum 227 km). Most patients (20, 83.3%) submitted weekly measurements; 100% submitted prior to electronical visits. Titration conventionally occurs during a hospital visit. By implementing eHealth smart technology, 68 such trips to hospital were replaced by virtual visits facilitated by remote monitoring. An eHealth questionnaire was completed by 22 patients (92%), and 96% expressed satisfaction. After titration, 30 instances of remote adjustment of heart failure medication in addition to scheduled outpatient clinic visits occurred, one (4%) heart failure admission followed, despite ambulant adjustments. Five patients (21%) sent in rhythm registrations (n = 17), of these 77% showed sinus rhythm, whereas supraventricular tachycardia was detected in the remaining four registrations.
Conclusion
These data suggest that implementation of a smart technology-based care pathway for optimization of medical treatment sRV failure is feasible with high measurement adherence and patient satisfaction.
Keywords: Transposition of the great arteries, Systemic right ventricle, Heart failure, Congenital heart disease, eHealth, Smart technology-based care pathway
Graphical Abstract
Introduction
The number of patients with congenital heart disease (CHD) is growing, with currently over 90% survival to adulthood. Despite the improved surgical outcomes, these patients remain at risk for long-term complications such as heart failure, arrhythmias, and pulmonary hypertension and require lifelong follow-up.1 In the 21st century, there has been a significant rise in the development and implementation of telemonitoring with smart technology devices and eHealth. The use of telemonitoring via smart technology has shown conflicting results in patients with acquired heart disease, especially in terms of reducing mortality and hospitalizations,2–5 yet studies in adult CHD have been carefully optimistic.6–10
The adult CHD population seems particularly eligible for smart technology since they are generally younger than the average cardiac patient, are more likely to be in the possession of a smartphone device, and have a high degree of digital literacy.9 Previous studies have also shown that they are highly motivated for telemonitoring and eHealth.9,11 Care for these patients requires a high degree of specialization and structural follow-up in an expertise centre is indicated for patients with heart failure in the context of CHD.1 The concentration of care in these tertiary centres often leads to longer travel time and interference with daily (work-) obligations for patients. In addition, patients with CHD have shown to have high utilization of emergency care resources.1 These factors could make this population particularly fit for telemonitoring via smart technology.9–11
Patients with a systemic right ventricle (sRV)—a morphological right ventricle in a subaortic position supporting the systemic circulation—in the context of transposition of the great arteries (TGA) after atrial switch or congenitally corrected TGA are particularly prone to long-term complications, including sRV heart failure and arrhythmias.1,12–15 Although there are no specific recommendations in the current guidelines for the use of heart failure medication in this population, it is carefully and pragmatically prescribed in sRV failure based on expert opinion, small trials, and lack of alternatives.1 A recent analysis of 150 adult atrial switch patients in the Netherlands showed decreased mortality in symptomatic patients treated with renin–angiotensin–aldosterone system (RAAS) inhibitors and β-blockers16 and beneficial effects of sacubitril/valsartan treatment in adults with sRV failure on N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and echocardiographic function have recently been reported.17 The aim of the current study was to evaluate the feasibility, patient adherence, and appreciation of a smart technology-based care pathway for heart failure treatment optimization and monitoring in sRV failure. The secondary outcome of this study was to evaluate the potential of the smart technology-based care in detection of arrhythmias in this group.
Methods
Design and inclusion/exclusion criteria
In this single-centre, non-blinded cohort study performed at the Department of Cardiology at the Leiden University Medical Center, data on all adult patients with a failing sRV in a biventricular circulation who were started on sacubitril/valsartan according to the smart technology-based care pathway in February 2019—February 2020, were reported. Patients who had an (estimated) sRV ejection fraction of ≤40% and had persisting symptomatic heart failure (NYHA class ≥ II) despite treatment with highest tolerated doses of a β-blocker and/or an angiotensin-converting enzyme inhibitor/angiotensin II receptor-blocker for a period of at least 3 months were advised to start treatment with sacubitril/valsartan.17 Patients who were started on sacubitril/valsartan (n = 25) were offered four smartphone compatible devices (blood pressure monitor, weight scale, step counter, and rhythm monitor). If they accepted (n = 24), they were treated and monitored according to a smart technology-based care pathway (Figure 1). One patient declined participation in digital monitoring due to personal preference for face-to-face meetings with the cardiologist in the outpatient clinic. The conventional pathway consisted of a biweekly visit to the outpatient clinic with either the treating cardiologist or physician assistant specialized in heart failure. Check-up consisted of assessment of complaints, physical examination including blood pressure and weight monitoring and laboratory workup. The smart technology-based pathway was designed based on the previously validated care pathway in post-myocardial infraction patients and consisted of measurements performed by the patients twice a week with the blood pressure monitor and weight scale and biweekly (every 2nd week) consultations by telephone, including assessment of complaints, smart technology measurements, and laboratory workup.18 Patients were instructed to perform laboratory workup at their general practitioner and to perform the above measurements and a single-lead electrocardiogram (ECG) registration in case of complaints of palpitations (Figure 1). Patients were stimulated to use the step counter daily.
Figure 1.
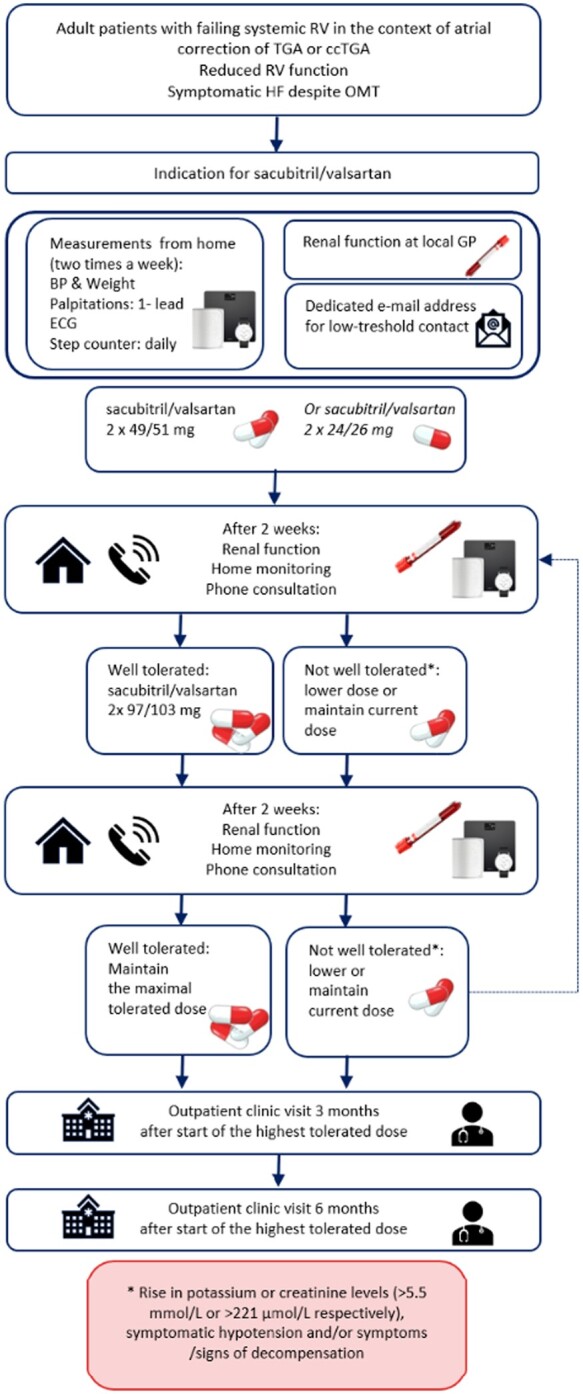
Sacubitril/valsartan titration according to the smart technology care pathway. BP, blood pressure; ccTGA, congenitally corrected transposition of the great arteries; GP, general practitioner; HF, heart failure; OMT, optimal medical treatment; RV, right ventricle; TGA, transposition of the great arteries.
Materials
The blood pressure monitor (Connect, Wi-Fi Smart Blood Pressure Monitor), step counter (Move, activity and sleep watch), and weight scale (Body, Weight and BMI Wi-Fi Scale) were from the brand Withings and compatible with the Health Mate app for iOS and Android. The blood pressure monitor is an oscillometric device, not requiring assistance from health-care personnel. The rhythm monitor used was from Alivecor KardiaMobile (mobile single-lead ECG) and was compatible with the Kardia app for iOS and Android (Figure 2). The rhythm monitor generates a single-lead ECG rhythm strip after manual initiation by the patient. All devices used have European Conformity (CE) marking and have been previously clinically validated and/or tested.19–21 A dedicated eHealth team instructed and assisted patients during installation and use of these devices and the corresponding apps. A dedicated email address and telephone number were available for ‘on demand’ assistance.
Figure 2.
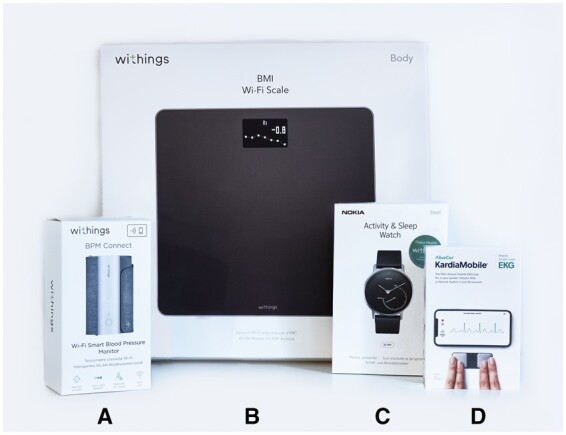
eHealth devices, including weight scale, blood pressure monitor, step counter, and rhythm monitor. (A) Blood pressure monitor (Withings Wi-Fi Smart Blood Pressure Monitor); (B) weight scale (Withings Body, Weight and BMI Wi-Fi Scale); (C) step counter (Withings Move, activity and sleep watch); and (D) rhythm monitor (Alivecor KardiaMobile).
Treatment and follow-up
The smart technology-based care pathway is summarized in Figure 1. A dedicated email address was made available for low threshold contact for patients during the titration process and follow-up.
After 2 weeks of treatment with the starting dose or after dosage modification, blood pressure, weight, complaints, and laboratory findings (renal function, potassium level) were evaluated in a telephone consultation. Data from telemonitoring devices and laboratory values (blood samples drawn at the local general practitioner’s office) were collected from the hospitals patient information systems (EPD-Vision, Leiden, the Netherlands and Hix, Chipsoft, Amsterdam, the Netherlands). If the medication was well tolerated, the dose of sacubitril/valsartan was increased in a stepwise fashion according to clinical protocol. These steps were repeated until the highest tolerated dose or maximal dose of 97/103 mg was reached. Rise in potassium or creatinine levels (>5.5 mmol/L or >221 µmol/L, respectively), symptomatic hypotension, and/or symptoms or signs of decompensation (e.g. weight gain) were followed by a step-down or termination of treatment.
After 3 and 6 months of treatment with the optimal tolerated dose, blood pressure, weight, symptoms, and laboratory investigations (including haemoglobin levels, kidney function, electrolytes, and NT-pro-BNP) were repeated, followed by a consultation in the hospital.
Outcomes
Feasibility (technical and practical consideration of the pathway making it fit for implementation in daily clinical practice), potential to reduce physical hospital visits, patient adherence and satisfaction, and detection of arrhythmias were measured and evaluated by manual assessment of patient information and a questionnaire.
Patient information was derived through assessment of the electronic patient information systems, distance from home to hospital was assessed using the quickest route as indicated in Google Maps route planning. Minimal one-way travel time was assessed using the quickest travel time by car without traffic in Google Maps. Patients were instructed to measure weight and blood pressure twice a week and were stimulated to wear the step counter daily. Patients were instructed to make a single-lead ECG registration if they experienced palpitations or other rhythm-related symptoms. Data were automatically and safely imported into the electronic patient health record file and were only accessible to the medical team. The single-lead ECGs were evaluated by the treating cardiologist and followed-up with a telephone or email contact. Patients were instructed to continue measurements once to twice a week.
Patient satisfaction was assessed by an anonymous questionnaire. Patients were asked to evaluate the use of smart technology in the process of titration of new heart failure medication 3 months after optimal dose. This was done by a self-developed dedicated questionnaire (Supplementary material online) consisting of 19 questions and evaluates feasibility, user satisfaction, usability, and overall rating. The results were anonymized and analysed by the study investigators. The treating physician did not have access to the results of the questionnaire during the study period.
Ethics statement
All tests and procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 2013 Helsinki declaration or comparable ethical standards and according to Dutch legislation for handling of personal data.
Consent
Appropriate local scientific board approval was obtained and the need for written informed consent was waived by the institutional medical ethical board. All patients provided consent for registration of their data and publication.
Statistical analysis
All statistical analyses were performed in IBM SPSS version 25. Normally distributed continuous data are displayed as mean ± standard deviation and non-normally distributed continuous data are displayed as median [quartile 1, quartile 3]. Proportions are displayed as numbers (percentages).
Results
Patient characteristics
In total, 24 sRV patients (median age 47 years, 50.0% female) were included in the analysis of the primary outcome (Table 1). Median follow-up time was 17 months [14; 19]. Sixteen patients (66.7%) had TGA corrected with the Mustard or Senning atrial switch procedure, and eight patients (33.3%) had ccTGA. 87.5% had a moderately to severely reduced sRV function. The NYHA functional class was II in 17 patients (70.8%) and class III–IV in 29.2%. The median home-hospital distance was 65 km [28; 121], with a maximal distance of 227 km. Median minimal travel time (one-way trip) was 52 min [29; 93], with a maximal time of 158 min.
Table 1.
Patient characteristics at baseline (n = 24)
| Patient characteristics | Baseline (n = 24) |
|---|---|
| Age (years) (median [Q1; Q3]) | 47 [44; 50] |
| Female (n, %) | 12 (50.0%) |
| Anatomy (n, %) | |
| TGA with atrial switch procedure | 16 (66.7%) |
| ccTGA | 8 (33.3%) |
| sRV function (n, %) | |
| Mildly reduced | 3 (12.5%) |
| Moderately reduced | 16 (66.7%) |
| Severely reduced | 5 (20.8%) |
| Tricuspid regurgitation (n, %) | |
| Grade I | 11 (45.8%) |
| Grade II | 12 (50.0%) |
| Grade III | 0 (0%) |
| Grade IV | 1 (4.2%) |
| NYHA (n, %) | |
| NYHA II | 17 (70.8%) |
| NYHA III–IV | 7 (29.2%) |
| History of SVT (n, %) | 14 (58.3%) |
| Atrial fibrillation | 9 (37.5%) |
| Atrial flutter | 8 (33.3%) |
| Atrial tachycardia | 10 (41.7%) |
| AVNRT | 4 (16.7%) |
| SVT ablation (n, %) | 10 (41.7%) |
| Pacemaker/ICD (n, %) | 13 (54.2%) |
| Distance to hospital (km) (median [Q1; Q3]) | 65 [28; 121] |
AVNRT, atrioventricular nodal re-entrant tachycardia; ccTGA, congenital corrected transposition of the great arteries; ICD, Implantable cardioverter-defibrillator; NYHA, New York Heart Association Functional Classification; Q1, quartile 1; Q3, quartile 3; sRV, systemic right ventricle; SVT, supraventricular tachycardia; TGA, transposition of the great arteries.
Titration process and feasibility
Of all 24 patients, 12 (50.0%) had two contact moments for the titration of the medication before the final dose was achieved. In the other patients, a maximum of five contact moments was required to titrate to the maximum tolerated dose. This saved a total of 68 trips to the hospital compared with the conventional outpatient clinic titration process. Thirteen patients (54.1%) could be titrated to the maximal dose of sacubitril/valsartan (97/103 mg). No episodes of hyperkalaemia or deterioration of renal function were observed. One patient (4.2%) discontinued sacubitril/valsartan treatment 2 months after initiation. Other patients (n = 10, 41.7%) did not reach the maximal dose due to (symptomatic) hypotension.
Patient adherence and experience
Of 24 patients, 83.3% of patients submitted measurements twice a week during the titration process. All patients (100%) submitted measurements prior to visits as requested. After titration, 50.0% continued weekly measurements and 70.8% continued (at least) monthly measurements. Twelve patients (50.0%) wore the step counter daily. On average, patients took 429 ± 1976 steps a day. Twenty-two patients completed the eHealth questionnaire (response rate 91.7%, Figures 3 and 4).
Figure 3.
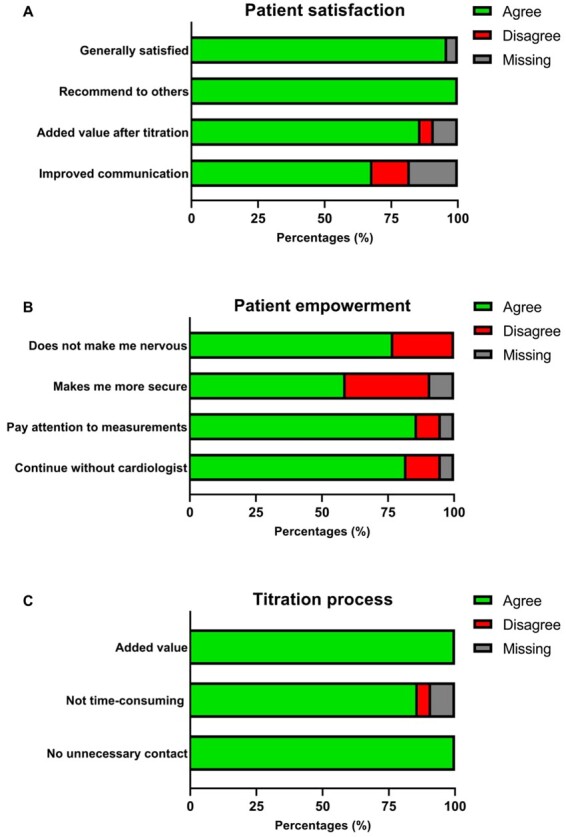
Outcome questionnaire patient experience (n = 22). (A) General patient satisfaction. (B) Patient empowerment. (C) Experience titration process.
Figure 4.
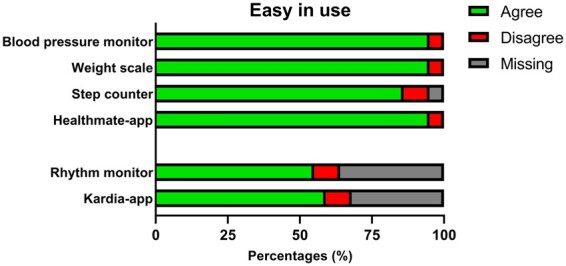
Outcome questionnaire assessment of usability (n = 22).
Patient satisfaction
Twenty-one patients (95.5%) expressed satisfaction with the smart technology intervention. All patients would recommend the smart technology intervention to others. Nineteen patients (86.4%) considered the smart technology intervention to be of a continued added value after titration and 15 (68.2%) thought it improved their communication with the cardiologist (Figure 3A).
Patient empowerment
The smart technology intervention made 59.1% (n = 13) of the patients feel more secure and 22.7% (n = 5) of the patients more nervous. Nineteen patients (86.4%) looked at and paid attention to their own measurements and 18 (81.8%) would continue even if the cardiologist would not look at the measurements (Figure 3B).
Titration process
All patients considered the smart technology intervention of value during the titration process, most (86.4%, n = 19) did not think it was too time-consuming. None of the patients thought the cardiologist reached out to them unnecessarily because of the measurements (Figure 3C). Fourteen patients (63.6%) found this the best way for the titration of medication, while four patients would have preferred a different way of titration. Two patients (8.3%) would have preferred titration through physical outpatient visits, one (4.2%) patient would have preferred titration through the general practitioner’s office, and one other (4.2%) would have preferred a combination of both smart technology and physical visits.
Usability
Twenty-one (95.5%) patients thought that the blood pressure monitor, scale, and Healthmate App were easy to use and 86.4% considered the step counter to be easy in use. The rhythm monitor and Kardia App was considered easy to use by 54.5% (36.4% missing), and 59.1% (31.8% missing), respectively (Figure 4).
Heart failure
After the titration to the highest tolerated dose, there was a total of 30 contact moments by telephone or email in six patients (25%) in response to home measurements and symptoms, resulting in adjustment of heart failure medication.
One patient discontinued treatment of sacubitril/valsartan and measurements through telemonitoring after a heart failure-related admission. Despite the patient having sent in measurements and repeated contact moments with the treatment team due to congestion-related symptoms and subsequent ambulant medication adjustments, heart failure-related admission was not prevented. (n = 1, 4.2%). No other heart failure-related admissions were observed during the study period.
Detection of arrhythmia
A total of 14 patients (58.3%) had a history of supraventricular arrhythmia’s, 12 patients experienced more than one arrhythmia. Nine patients (37.5%) had a history of atrial fibrillation, 8 (33.3%) of atrial flutter, 10 (41.7%) of atrial tachycardia, and 4 (16.7%) of atrioventricular (AV) nodal re-entry tachycardia. In total, 10 patients (41.7%) had undergone at least one ablation procedure of a supraventricular arrhythmia prior to the study period.
Five patients (20.8%) sent in a single-lead ECG registration using the provided smart technology rhythm monitor (Figure 5). One patient sent in an ECG due to chest discomfort, all other registrations were made due to palpitations. All patients who sent in an ECG due to palpitation symptoms had a history of supraventricular arrhythmias. Of the 17 single-lead ECG registrations made during complaints, 13 (76.5%) showed sinus rhythm, consequently reassuring the patients and potentially saving a trip to the emergency department or general practitioner’s office. Four registrations (23.5%) showed a supraventricular arrhythmia, of which one was an atrial flutter with alternating conductivity, and the three other ones were atrial tachycardias. These registrations resulted in three admissions for electrical cardioversion, one patient was already listed for electrophysiological study and ablation and decided not to present to healthcare services awaiting the already planned procedure. Classification of the exact type of supraventricular arrhythmia was done at admission based on a 12-lead ECG. Although the single-lead ECG registration provides information on the frequency and regularity and can help differentiate between supra- and ventricular origin of an arrhythmia, it has not been validated to diagnose the exact type of arrhythmia in a patient group with complex anatomy and intrathoracic cardiac position. No cases of novel arrhythmias were diagnosed through the smart technology rhythm monitor.
Figure 5.
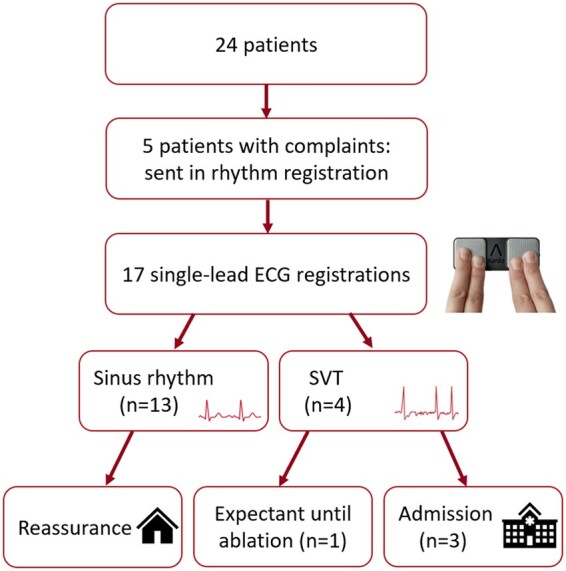
Detection of arrhythmia during 6 months of follow-up. ECG, electrocardiogram; SVT, supraventricular tachycardia.
Discussion
This is, to the best of our knowledge, the first study assessing feasibility of telemonitoring and titration of heart failure medication in adult patients with a failing systemic right ventricle in a biventricular circulation. The main findings of this study are1 eHealth can be effectively implemented as a part of a clinical care pathway to support medication optimization and titration in this patient group2; patient adherence and satisfaction is high; and3 eHealth can be of potential value in the assessment of arrhythmias and avoiding unnecessary utilization of (emergency) health-care resources in this patient group.
Current results in light of previous studies
A review by Treskes et al.9,22 showed that studies on telemedicine solutions for medication adherence are conflicting in the general population, yet a recent pilot showed that symptomatic CHD patients are suitable and highly motivated for eHealth implementation. Kauw and colleagues also reported promising results of eHealth used for telemonitoring in the CHD population.6,10 Although several randomized controlled trials in acquired heart failure showed no benefit of eHealth in comparison to regular healthcare or treatment, eHealth was found feasible with a positive tendency to reduce readmissions and contacts with the treatment team.3,5,23 Prospective studies on the use of eHealth and telemonitoring in CHD patients reported high adherence rates (97% and >70%, respectively) and patient satisfaction (84%), comparable to the results in the current patient group.7,8 This can be explained by the high digital literacy of relatively young adult CHD patients.9 The results of this study therefore corroborate findings of previous studies regarding feasibility of eHealth in adult CHD (ACHD) patients. Based on the literature and the results of this study, specific patient eligibility characteristics for effective remote monitoring can be defined (Table 2). To avoid ‘data overload’,24 we suggest that eHealth is used with an approach integrated into a care pathway tailored to specific predefined ACHD patient groups.
Table 2.
Patient eligibility characteristics for complete remote monitoring
| Patients who can be managed remotely | Patients who require hospital visits |
|---|---|
| Language proficiency in Dutch/English | Not fulfilling any of the criteria listed in the left column |
| Digital literacy | Episodes of congestion during titration process that require hospitalization |
| Adequate Wifi connection | (Near-) syncopal arrhythmia |
| Possession of a smart phone device | |
| Commitment and personal motivation to perform structural measurements in a timely manner | |
| No episodes of congestion/ deterioration of heart failure that would require hospitalization |
Optimization of medication
Titration of medication in sRV patient population can be challenging due to relatively low baseline blood pressures and poorly understood mechanism of action of heart failure medication in this group, combined with a busy lifestyle with work and personal commitments of these often relatively young patients, and long travel distance to expert clinical centres. By means of structural monitoring of the telemonitoring data and a dedicated email address for low threshold contact, it was possible to rapidly and accurately adjust heart failure medication during titration and follow-up. Several studies and case series on the effects of sacubitril/valsartan in ACHD patients are published to date.17,25–27 Although sacubitril/valsartan was generally well tolerated, Maurer et al. did see a reduction in systolic and diastolic blood pressure and a deterioration of renal function, suggesting close monitoring during titration is essential. Our results suggest that eHealth guided titration process is feasible and is of additional value in this young patient group, saving 68 potential outpatient clinic visits. This way of titration was also positively valued by the patients, as 63.6% found this to be the best way for titration. It also allowed for swift therapy changes to be made as a result of telemonitoring measurements in a substantial number of patients (25%).
Arrhythmia detection
Patients with an sRV in a biventricular circulation are prone to arrhythmias1 which are a substantial cause of emergency department presentations and morbidity in this patient group.11,28 We report a high prevalence of atrial arrhythmias in our study population (58.3%). The failing sRV is prone to further deterioration during atrial tachycardia and arrhythmias are a potential cause of sudden cardiac death in these patients.1,29,30 This makes early detection and aggressive treatment of these arrhythmias of great importance. In our population, 10 out of 14 patients with known supraventricular arrhythmias had a history of ablation procedures. Telemonitoring has previously been shown to successfully detect supraventricular arrhythmias, and atrial fibrillation in particular.31 Our data furthermore show that rhythm registrations obtained through the smart technology care pathway were predominantly normal sinus rhythm registrations, showing no arrhythmia (76.5%). These data are in line with previous work of Kauw et al.,7 who reported 55% of rhythm registrations performed by 80 CHD patients to show sinus rhythm. This reflects on the high patient awareness of potential tachyarrhythmias and the ability of this technology to create remote patient assurance, self-management, and empowerment, and prevention of unnecessary emergency health-care utilization.
COVID-19 pandemic
Although the COVID-19 pandemic was beyond the scope of this study, it clearly demonstrated the need of non-contact care delivery and self- and telemonitoring of symptomatic patients.32 Especially, in this vulnerable patient population, it is absolutely necessary to curtail hospital visits to avoid potential infections. Due to the COVID-19 pandemic, telemonitoring through smart technology has been rapidly and widely implemented as the ‘new healthcare standard’ allowing for the provision of the right care for the right patient at the right place. This study illustrates that if embedded in a regulated care pathway, eHealth can play an important role in care for ACHD patients in the 21st century.
Study limitations
This study is limited by its single-arm, non-blinded design, and the small study population. Although this is reflective of the rarity of the condition and the inability to blind patients for performing eHealth measurements, the findings reported should be interpreted taking into account the open nature of the study. Another limitation is the lack of internationally validated questionnaires to assess patient satisfaction of eHealth. Ideally, results should be confirmed in a larger, randomized multicentre trial setting.
Conclusion
These data suggest that implementation of a smart technology-based care pathway for optimization of medical treatment for sRV failure is feasible with high measurement adherence and patient satisfaction. If implemented as a part of a regulated care pathway, eHealth has potential in improving healthcare and patient empowerment in the field of ACHD.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health online.
Supplementary Material
Acknowledgements
The authors would like to thank Ronald Slagter (Department of Anatomy and Embryology, Leiden University Medical Center, the Netherlands) for his assistance with the Graphical abstract.
Funding
The work was funded by the general funds of the Department of Cardiology of the Leiden University Medical Center, Leiden, the Netherlands. A.D.E. received additional funding support from the Johanna Zaaijer Foundation of the Leiden University Medical Center. M.R.M.J. is funded by Organization for Scientific Research (NOW) research grant [projectnr. 016.196.346]. R.W.T. has received a speaker’s honorarium from Boston Scientific in the past 5 years.
Conflict of interest: none declared.
Author biography
Marieke Nederend (1993) is currently a PhD candidate at the Leiden University Medical Center, the Netherlands. The main focus of her thesis is clinical, long term, outcome in congenital heart disease, centralizing on systemic right ventricle failure.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563–645. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA,, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM.. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buys R, Claes J, Walsh D, Cornelis N, Moran K, Budts W, Woods C, Cornelissen VA.. Cardiac patients show high interest in technology enabled cardiovascular rehabilitation. BMC Med Inform Decis Mak 2016;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP, TEHAF investigators. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail 2012;14:791–801. [DOI] [PubMed] [Google Scholar]
- 6. Kauw D, Koole MAC, van Dorth JR, Tulevski II, Somsen GA, Schijven MP, Dohmen DAJ, Bouma BJ, Mulder BJM, Schuuring MJ, Winter MM.. eHealth in patients with congenital heart disease: a review. Expert Rev Cardiovasc Ther 2018;16:627–6. [DOI] [PubMed] [Google Scholar]
- 7. Kauw D, Koole MAC, Winter MM, Dohmen DAJ, Tulevski II, Blok S, Somsen GA, Schijven MP, Vriend JWJ, Robbers-Visser D, Mulder BJM, Bouma BJ, Schuuring MJ.. Advantages of mobile health in the management of adult patients with congenital heart disease. Int J Med Inform 2019;132:104011. [DOI] [PubMed] [Google Scholar]
- 8. Koole MAC, Kauw D, Winter MM, Dohmen DAJ, Tulevski II, de Haan R, Somsen GA, Schijven MP, Robbers-Visser D, Mulder BJM, Bouma BJ, Schuuring MJ.. First real-world experience with mobile health telemonitoring in adult patients with congenital heart disease. Neth Heart J 2019;27):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treskes RW, Koole M, Kauw D, Winter MM,, Monteiro M, Dohmen D, Abu-Hanna A, Schijven MP, Mulder BJ, Bouma BJ, Schuuring MJ.. Adults with congenital heart disease: ready for mobile health? Neth Heart J 2019;27:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuuring MJ, Kauw D.. How to initiate eHealth in congenital heart disease patients? Eur Heart J - Digital Health 2020;1:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schuuring MJ, Backx AP, Zwart R, Veelenturf AH, Robbers-Visser D, Groenink M, Abu-Hanna A, Bruining N, Schijven MP, Mulder BJ, Bouma BJ.. Mobile health in adults with congenital heart disease: current use and future needs. Neth Heart J 2016;24:647–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filippov AA, Del Nido PJ, Vasilyev NV.. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation 2016;134:1293–1302. [DOI] [PubMed] [Google Scholar]
- 13. Vejlstrup N, Sorensen K, Mattsson E, Thilen U, Kvidal P, Johansson B, Iversen K, Sondergaard L, Dellborg M, Eriksson P.. Long-term outcome of mustard/senning correction for transposition of the great arteries in Sweden and Denmark. Circulation 2015;132:633–638. [DOI] [PubMed] [Google Scholar]
- 14. van Dissel AC, Winter MM, van der Bom T, Vliegen HW, van Dijk APJ, Pieper PG, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJM, Bouma BJ.. Long-term clinical outcomes of valsartan in patients with a systemic right ventricle: Follow-up of a multicenter randomized controlled trial. Int J Cardiol 2019;278:84–87. [DOI] [PubMed] [Google Scholar]
- 15. Woudstra OI, Zandstra TE, Vogel RF, van Dijk APJ, Vliegen HW, Kiès P, Jongbloed MRM, Egorova AD, Doevendans PAFM, Konings TC, Mulder BJM, Tanck MWT, Meijboom FJ, Bouma BJ.. Clinical course long after atrial switch: a novel risk score for major clinical events. J Am Heart Assoc 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woudstra OI, Kuijpers JM, Jongbloed MRM, van Dijk APJ, Sieswerda GT, Vliegen HW, Egorova AD, Kies P, Duijnhouwer AL, Robbers-Visser D, Konings TC, Zwinderman AH, Meijboom FJ, Mulder BJM, Bouma BJ.. Medication in adults after atrial switch for transposition of the great arteries: clinical practice and recommendations. Eur Heart J Cardiovasc Pharmacother 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zandstra TE, Nederend M,, Jongbloed MRM, Kies P, Vliegen HW, Bouma BJ, Tops LF, Schalij MJ, Egorova AD.. Sacubitril/valsartan in the treatment of systemic right ventricular failure. Heart 2021; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Treskes RW, van Winden LAM, van Keulen N, van der Velde ET, Beeres S, Atsma DE, Schalij MJ.. Effect of smartphone-enabled health monitoring devices vs regular follow-up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Netw Open 2020;3:e202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Topouchian J, Agnoletti D, Blacher J, Youssef A, Chahine MN, Ibanez I, Assemani N, Asmar R.. Validation of four devices: Omron M6 Comfort, Omron HEM-7420, Withings BP-800, and Polygreen KP-7670 for home blood pressure measurement according to the European Society of Hypertension International Protocol. Vasc Health Risk Manag 2014;10:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Himmelreich JCL, Karregat EPM, Lucassen WAM, van Weert H, de Groot JR, Handoko ML, Nijveldt R, Harskamp RE.. Diagnostic accuracy of a smartphone-operated, single-lead electrocardiography device for detection of rhythm and conduction abnormalities in primary care. Ann Fam Med 2019;17:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Au-Yeung WM, Kaye JA, Beattie Z.. Step count standardization: validation of step counts from the withings activite using PiezoRxD and wGT3X-BT. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:4608–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Treskes RW, Van der Velde ET, Schoones JW, Schalij MJ.. Implementation of smart technology to improve medication adherence in patients with cardiovascular disease: is it effective? Expert Rev Med Devices 2018;15:119–126. [DOI] [PubMed] [Google Scholar]
- 23. Kotooka N, Kitakaze M, Nagashima K, Asaka M, Kinugasa Y, Nochioka K, Mizuno A, Nagatomo D, Mine D, Yamada Y, Kuratomi A, Okada N, Fujimatsu D, Kuwahata S, Toyoda S, Hirotani SI, Komori T, Eguchi K, Kario K, Inomata T, Sugi K, Yamamoto K, Tsutsui H, Masuyama T, Shimokawa H, Momomura SI, Seino Y, Sato Y, Inoue T, Node K, HOMES-HF study investigators. The first multicenter, randomized, controlled trial of home telemonitoring for Japanese patients with heart failure: home telemonitoring study for patients with heart failure (HOMES-HF). Heart Vessels 2018;33:866–876. [DOI] [PubMed] [Google Scholar]
- 24. Clark P, Capuzzi K, Harrison J.. Telemedicine: medical, legal and ethical perspectives. Med Sci Monit 2010;16:261–272. [PubMed] [Google Scholar]
- 25. Lluri G, Lin J, Reardon L, Miner P, Whalen K, Aboulhosn J.. Early experience with sacubitril/valsartan in adult patients with congenital heart disease. World J Pediatr Congenit Heart Surg 2019;10:292–295. [DOI] [PubMed] [Google Scholar]
- 26. Maurer SJ, Pujol Salvador C, Schiele S, Hager A, Ewert P, Tutarel O.. Sacubitril/valsartan for heart failure in adults with complex congenital heart disease. Int J Cardiol 2020;300:137–140. [DOI] [PubMed] [Google Scholar]
- 27. Appadurai V, Thoreau J, Malpas T, Nicolae M.. Sacubitril/valsartan in adult congenital heart disease patients with chronic heart failure—a single centre case series and call for an international registry. Heart Lung Circ 2020;29:137–141. [DOI] [PubMed] [Google Scholar]
- 28. Yang H, Kuijpers JM, de Groot JR, Konings TC, van Dijk A, Sieswerda GT, Post MC, Mulder BJM, Bouma BJ.. Impact of atrial arrhythmias on outcome in adults with congenital heart disease. Int J Cardiol 2017;248:152–154. [DOI] [PubMed] [Google Scholar]
- 29. Roca-Luque I, Rivas Gandara N, Dos Subira L, Francisco Pascual J, Perez-Rodon J, Pijuan Domenech A, Subirana MT, Miranda B, Santos Ortega A, Casaldaliga Ferrer J, Garcia-Dorado Garcia D, Moya Mitjans A.. Intra-atrial re-entrant tachycardia in patients with congenital heart disease: factors associated with disease severity. Europace 2018;20:1343–1351. [DOI] [PubMed] [Google Scholar]
- 30. Kammeraad JA, van Deurzen CH, Sreeram N, Bink-Boelkens MT, Ottenkamp J, Helbing WA, Lam J, Sobotka-Plojhar MA, Daniels O, Balaji S.. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol 2004;44:1095–1102. [DOI] [PubMed] [Google Scholar]
- 31. Shacham J, Birati EY, Malov N, Yanay Y, Steinberg DM, Tamari M, Golovner M, Roth A.. Telemedicine for diagnosing and managing paroxysmal atrial fibrillation in outpatients. The phone in the pocket. Int J Cardiol 2012;157:91–95. [DOI] [PubMed] [Google Scholar]
- 32. Mann DM, Chen J, Chunara R, Testa PA, Nov O.. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc 2020;27:1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


