Abstract
Aims
Previous research has shown the possibility to use the pre-operative period to improve a patient’s tolerance for surgery. However, there is limited experience with prehabilitation in cardiac surgery. The aim of this study is to evaluate the effect of a comprehensive personalized teleprehabilitation programme on major adverse cardiac events (MACE) in patients scheduled for elective cardiac surgery. Secondary outcomes are post-operative complications, cardiovascular risk factors, quality of life, and cost-effectiveness.
Methods and results
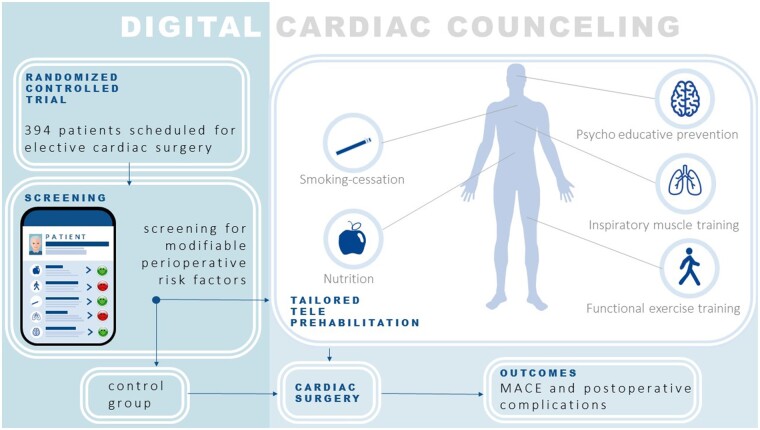
In this single-centre randomized controlled trial, patients are eligible for inclusion when they are ≥18 years of age and cardiac surgery is scheduled at least 8 weeks from informed consent. Participants will be randomized to the teleprehabilitation group or the control group. After a digital baseline screening for perioperative risk factors, patients in the intervention arm can pre-operatively be referred to one or more of the prehabilitation modules (functional exercise training, inspiratory muscle training, psychological support, nutritional support, and/or smoking cessation). The programme is targeted at a duration of at least 6 weeks. It is executed by a multidisciplinary team using (video)calls and supported by a custom-made digital platform. During the pre-operative period, the platform is also used to inform patients about their upcoming surgery and for telemonitoring.
Conclusion
Reducing perioperative risk factors might result in a reduction of MACE, post-operative complications, length of stay, and cardiovascular risk factors, as well as improved quality of life. Cost-effectiveness will be evaluated.
Keywords: Pre-operative assessment, Prehabilitation, Teleprehabilitation, Telehealth, Cardiac events, Physical fitness
Graphical Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been a disruptive event for the organization of elective care.1 Concern has been raised about the effect of a prolonged pre-operative period on morbidity and mortality in cardiac surgery.2 Previous studies suggest that an increased pre-operative waiting time for elective cardiac surgery is associated with an increase in morbidity and mortality.3–5 In our hospital, the expected pre-operative time period for elective surgery doubled during the COVID-19 crisis.
In light of the growing population of older, more comorbid, and less physically fit patients, previous research in the field of prehabilitation in cardiac surgery has shown the possibility to use the pre-operative period to improve a patient’s tolerance to surgery by optimizing the patient’s physical fitness and mental health. Pre-operative assessment can help to identify patients with a high risk for perioperative complications and employ preventive interventions (prehabilitation), including physical exercise training, nutritional, pharmacological, and mental support, reducing the risk of post-operative morbidity, mortality, and adverse functional outcomes.6 A systematic review by Hulzebos et al.7 demonstrated that pre-operative physical exercise training reduces post-operative pulmonary complications and length of hospital stay in patients undergoing elective cardiac surgery. Reduced intensive care unit stay and a reduced hospital length of stay were seen in a randomized controlled trial, whereas patients scheduled for elective coronary artery bypass surgery were randomized to an 8-week prehabilitation programme consisting of hospital-based supervised aerobic interval training, education, and monthly nurse-initiated telephone calls.8 Two recent systematic reviews demonstrated the beneficial effects of prehabilitation in cardiac surgery, specifically in the domains of physical exercise training, inspiratory muscle training, psychological support, nutritional support, and smoking cessation on post-operative complications, intensive care unit stay, and a reduced hospital length of stay.9,10 Pre-operative preventive interventions tailored to an individual patient’s risk factors will not only result in optimization for the perioperative phase but also could result in optimization of general health and reduce cardiovascular risk factors on the long term.
Although pre-operative physical exercise training for patients at risk is ideally home- or community-based and directly supervised by a competent and trained physiotherapist,11 public health legislation during the COVID-19 pandemic has resulted in forced transitioning to the use of remote care in order to continue the provision of (p)rehabilitation worldwide. Moreover, although a prolonged pre-operative period gives the opportunity to provide prehabilitation, it also carries the risk of progression of symptoms. As such, telemonitoring during this period provides the possibility to detect patients who need medical consultation or prioritizing on the waiting list. As telemedicine becomes widely adopted, especially during a pandemic in which physical contact is undesirable, prehabilitation interventions may be delivered using this technology.12,13 Telerehabilitation has already found its way into cardiac rehabilitation14,15; however, evidence on the feasibility and effectiveness of teleprehabilitation in patients planned for cardiac surgery is lacking. Therefore, the primary aim of this randomized controlled trial is to investigate whether a comprehensive individualized teleprehabilitation programme (Digital Cardiac Counselling) personalized to an individual patient’s risk factors (intervention group) reduces the cumulative incidence of major adverse cardiac events (MACE) from inclusion until 1-year post-operatively when compared with patients without prehabilitation (control group). The secondary aim of this trial is to evaluate the effects of cardiac teleprehabilitation on post-operative complications, cardiovascular risk factors, quality of life, and cost-effectiveness.
Methods
Study design
This study is a single-centre, non-blinded, randomized controlled clinical trial. The study has started in June 2020 and will run until patient inclusion is completed (probably medio 2022) at the Maastricht University Medical Centre (Maastricht UMC+) in Maastricht, The Netherlands. The study was approved by the Medical Ethics Committee Maastricht in April 2020 (NL73754.068.20/METC20-028) and is registered on clinical trials.gov (NCT04393636). Protocol amendments need to be approved by the Medical Ethics Committee of the Maastricht UMC+.
Participants
All patients scheduled for any elective cardiac surgery at the Maastricht UMC+ (a tertiary referral centre) ≥18 years of age and in which the surgery is scheduled at least 8 weeks from informed consent to ensure a minimal prehabilitation time of 6 weeks, are eligible for inclusion. During the daily multidisciplinary heart team meeting, a cardiologist and cardiothoracic surgeon agree on the indication, urgency, and timing for surgery (emergency surgery, surgery within 7 days, surgery within 6 weeks, or elective surgery). The category of elective patients is deemed eligible for our trial. Patients who cannot use a digital platform with help of an informal caregiver (e.g. not having access to the internet and/or having a lack of digital literacy), patients with a language barrier (not able to speak or read the Dutch language at a sufficient level), and patients with interfering physical, psychiatric, or neurological conditions making it impossible to complete the study procedures, will be excluded.
Recruitment
Patients who are accepted for elective surgery will be screened for eligibility. Eligible patients will be contacted by telephone by the department of Cardiothoracic Surgery to ask for their permission to be approached by the investigators. All eligible patients will be registered, and reasons for not being eligible and reasons for non-participation will be recorded. After being informed by the investigators, patients receive a digital patient information letter and informed consent form per e-mail, as well as by mail. At the third contact, eligible patients will be asked to provide informed consent for participation using a video-consultation (using FaceTalk™, Qconferencing, Diemen, The Netherlands). After returning the signed informed consent form by e-mail or mail, patients will be randomized and signed-up for the digital platform.
Randomization
The investigators will use stratified block randomization with random permuted block sizes of 4, 6, and 8. Strata will be based on invasive- vs. minimal-invasive surgery and three groups based on the EUROII score (0 to <2%, 2 to <5%, and ≥5%).Participants will be randomized to the teleprehabilitation group or to the control group. The randomization will be computer-based and will generate two groups in a 1:1 ratio. Both groups will get access to the Digital Cardiac Counselling platform and both groups will complete the same set of validated questionnaires at the same time intervals. The intervention groups will get additional training modules and e-consulting based on risk assessment retrieved from the completed questionnaires.
Sample size calculation
The primary outcome is the difference in percentage of patients that experienced a MACE from inclusion until 1-year follow-up post-operatively. It is expected that ∼20% of the patients in the control group will experience an event.16–18 A total of 197 patients will be included per group, or 394 in total, to be able to have 80% power to detect an absolute difference in MACE of 10% between groups in favour of the intervention group, using an alpha of 0.05. The percentage of patients experiencing a MACE during the waiting period will be compared between groups using Pearson’s χ2 statistic. In case of baseline imbalance determined by the presence of clinically meaningful differences between groups, a secondary analysis on the primary endpoint will be performed using logistic regression analyses adjusting for potential confounders. Possible confounders are EuroSCORE II,19 type of the operation, operation time, cross-clamp time, and unforeseen intraoperative complications. The investigators will use Kaplan–Meier plots to illustrate the cumulative incidence of overall and cardiovascular-related, and COVID-19-related mortality over the 1-year follow-up period. Cox proportional hazards regression will be used to test for differences between groups. This model will also be extended with potential confounding variables in case of baseline imbalance. Differences between groups in the average New York Heart Association (NYHA) Class, CSS, SF-36, EQ-5D-5L, cardiovascular risk factors, and cost-effectiveness at hospital admission for surgery and 3, 6, and 12 months afterwards will be tested using the independent samples t-test, or linear regression in case of baseline imbalances.
Safety monitoring
The study is monitored by the Clinical Trial Centre Maastricht (CTCM). The investigators will use an electronic case report form (eCRF) for storage of anonymized patient data.
Digital platform
For this study, a customized digital environment was created on an existing software framework (Medify B.V., Amsterdam, The Netherlands). The platform will be used to present audio-visual information (e.g. addressing COVID-19, hospital admission, surgical procedure), to monitor patients throughout the programme (e.g. New York Heart Association class and Canadian Cardiovascular Society class for worsening cardiac symptoms, and to screen for COVID-19 symptoms according to the Dutch National Institute for Public Health and the Environment20). All patients will receive login codes for their personal account. The platform will automatically send notifications asking the patients to fill in questionnaires for gathering patient- and outcome-related study data. When the weekly monitoring questionnaires show progression of symptoms, the investigators will contact the patients to objectivate the symptoms and refer patients to referring cardiologist when indicated. Before surgery, patients are tested for COVID-19 if indicated by the local hospital protocol (this hospital protocol might change over time) or when COVID-19 symptoms are detected via the questionnaires on the platform. The platform will also be used to deliver a personalized teleprehabilitation programme to the intervention group.
Personalized teleprehabilitation intervention
After randomization, relevant components of the teleprehabilitation programme are accessible to the patients in the intervention arm of this study, personalized to their specific pre-operative risk factors. The teleprehabilitation programme consists of five modules: inspiratory muscle training, functional exercise training, psychological support (anxiety and depression reduction), nutritional support, and smoking cessation. The outcomes of this pre-operative risk screening will be discussed by the patient and case manager; using shared decision-making, the teleprehabilitation programme will be tailored to the needs and preferences of the individual patient. The actual prehabilitation programme is led by a multidisciplinary team, including a cardiologist, nurses, physiotherapists, a counselling psychologist, and dietitians. The team meets once a week during an interdisciplinary meeting to inform each other about new patients entering the prehabilitation programme and to discuss best treatment options for more complex patients (e.g. patients who are eligible for more than two modules, patients with significant comorbidities interfering with our prehabilitation programme like neurologic dysfunction, orthopaedic problems, cardiac symptoms, or patients who fail to show progression in their prehabilitation). In Table 1, an overview of the study procedures is depicted, whereas the prehabilitation programme is explained in more detail in Table 2.
Table 1.
Schedule of enrolment, interventions, and assessments
| Study period | Pre-prehabilitation |
Prehabilitation | Hospital admission | Planned surgery | Follow-up |
|||
|---|---|---|---|---|---|---|---|---|
| Timepoint from surgery | Week-12 | Week-10 | Week-9 to -1 | Day-1 | Day 0 | Month +3 | Month +6 | Month +12 |
| Enrolment | ||||||||
| Screening patients for participation | • | |||||||
| Feasibility | • | • | • | |||||
| Patient information letter for eligible patients | • | |||||||
| Informed consent, feasibility, and randomization | • | |||||||
| Assessments | ||||||||
| Baseline characteristics | • | |||||||
| Quality of life questionnaire, SF-36 | • | • | • | • | • | |||
| Health status questionnaire, EQ-5D-5L | • | • | • | • | • | |||
| Pulmonary risk scorea | • | • | • | • | • | |||
| Three questions about experiencing physical limitations | • | • | • | • | • | |||
| Anxiety and depression screening with HADS | • | • | • | • | • | |||
| Nutritional status with MUST* and BMI | • | • | • | • | • | |||
| Smoking status | • | • | • | • | • | |||
| Resource use, adapted versions of iMCQ/iPCQ | • | • | • | • | ||||
| Blood sampling | • | |||||||
| Physical tests | ||||||||
| Handgrip strength (kg) | • | |||||||
| 30-s chair stand test (AMRAP) | • | |||||||
| Maximal inspiratory pressure (cm H2O) | • | |||||||
| Monitoring | ||||||||
| NYHA* classification of heart failure (during pre-operative period weekly) | • | • | • | • | • | • | ||
| CCS* class of angina pectoris (during pre-operative period weekly) | • | • | • | • | • | • | ||
| COVID-19 (during pre-operative period weekly) symptoms/signs | • | • | • | |||||
| Serious adverse events patient questionnaire | • | • | • | • | ||||
| Information | ||||||||
| COVID-19 | • | • | ||||||
| Video’s and animations about scheduled hospital admission and surgery | • | • | ||||||
| Intervention-arm | ||||||||
| Personalized teleprehabilitationb | • | |||||||
| Inspiratory muscle training | • | |||||||
| Functional exercise training | • | |||||||
| Psychological support | • | |||||||
| Nutritional support | • | |||||||
| Smoking cessation | • | |||||||
| Adherence to intervention | • | |||||||
AMRAP, as many repetitions as possible; BMI, body mass index; EQ-5D-5L, EuroQol five-dimensional five-level questionnaire; CCS, Canadian Cardiovascular Society; HADS, Hospital Anxiety and Depression Scale; iMCQ, iMedical consumption questionnaire; iPCQ, iProductivity cost questionnaire, pulmonary risk score; MUST, malnutrition universal screening tool; NYHA, New York Heart Association; SF-36: 36-item short form health survey.
The pulmonary risk score is based on several risk factors for post-operative pulmonary complications, see the section addressing inspiratory muscle training (IMT).
Explained in more detail in Table 2, only for patients randomized to the intervention arm.
Table 2.
Overview of the teleprehabilitation programme
| Module | Indication for participating in a specific teleprehabilitation modulea | Intake-9 | Week-8 | Week-7 | Week-6 | Week-5 | Week-4 | Week-3 | Week-2 |
|---|---|---|---|---|---|---|---|---|---|
| Inspiratory muscle training | Pulmonary risk score ≥2b | ||||||||
| Digital information | • | • | • | • | • | • | • | ||
| Digital consultation | • | • | • | • | |||||
| MIP prediction | • | ||||||||
| Sending IMT device and training schedule | • | ||||||||
| IMT training | • | • | • | • | • | • | • | ||
| Submaximal test | • | • | • | • | |||||
| Functional exercise training | Using SF-36 and VAS questionnaire c | ||||||||
| Digital information | • | • | • | • | • | • | • | ||
| Digital consultation | • | • | • | • | |||||
| Exercise training | • | • | • | • | • | • | • | ||
| Psychological support | HADS anxiety or depression score ≥8 | ||||||||
| Digital information | • | • | • | • | • | • | • | ||
| Consultation | • | • | • | ||||||
| Nutritional support | |||||||||
| Obesity | BMI >30 kg/m2 | ||||||||
| Digital information | • | • | • | • | • | • | • | ||
| Digital consultation | • | • | • | ||||||
| Malnutrition | MUST score ≥2 | ||||||||
| Digital information | • | • | • | • | • | • | • | ||
| Digital consultation | • | • | • | • | |||||
| Protein enriched supplements | • | • | • | • | • | • | • | ||
| Smoking cessation | Current smoker | ||||||||
| Digital information | • | • | • | • | • | • | • | ||
| Digital consultation | • | • | • | • | |||||
| Medication support | • | • | • | • | • | • | • | ||
| Stop smoking | • | • | • | • | • |
BMI, body mass index; HADS, hospital anxiety and depression scale; IMT, inspiratory muscle training; MIP, maximal inspiratory pressure; MUST, malnutrition universal screening tool; SF-36, 36-item short form health survey; VAS, visual analogue scale.
When a patient meets the criteria for a module, he or she will be referred after shared decision-making.
The pulmonary risk score is based on several risk factors for post-operative pulmonary complications, see the section addressing IMT.
A subset from the physical domain of the SF-36 and three VAS questionnaires are used to screen patients for an indication for functional exercise training, see Supplementary material online, Appendix and the section addressing functional exercise training.
Inspiratory muscle training
After inclusion, the pulmonary risk score21 will be assessed in all patients to screen for an elevated risk on post-operative pulmonary complications like extended intubation and pneumonia. The score contains age, body mass index (BMI), smoking status, and a history of chronic obstructive pulmonary disease. Patients who have a score ≥2 will be called by the case manager to verify their motivation for inspiratory muscle training (IMT). The goal of this programme is to increase inspiratory muscle strength/endurance using the POWERbreathe® device (Gaiam Ltd; Southam, Warwickshire, UK). On the digital platform, additional information about IMT, instruction videos on how to use the POWERbreathe® device, and a digital training log can be found. Training sessions are scheduled five times a week for two times a day. A training session consists of 3 sets of 10 inspirations against a resistance using the POWERbreathe®. With this device, patients inspire against a threshold load, whereas expiration is unimpeded. The inspiratory load is calibrated in cmH2O and training intensity is changed according to the patient’s 6–20 Borg score for rate of perceived exertion.22 To personalize training intensity, a predicted maximal inspiratory pressure (MIP) was calculated based on a regression formula including sex, age, weight, and height created by POWERbreathe®, based on two large databases.23,24 Patients start training at 30% of their predicted MIP, which increases to 55% after 6–8 weeks. If the rate of perceived exertion is <13 or based on clinical experience of the physiotherapist, the resistance of the inspiratory threshold trainer can be increased incrementally by 4 cmH2O. Video calls are scheduled at baseline, and after 1, 3, and 6 weeks to monitor progress. On an individual basis, the frequency of the video calls can be increased. Maximal inspiratory pressure will be assessed on the day of admission as a maximal isometric inspiratory manoeuvere25 using the MicroRPM™ (Micro Medical, Kent, UK), a handheld non-invasive tool to measure maximal inspiratory pressures.
Functional exercise training
After inclusion, patients will be screened for a reduced physical fitness using a subset of questions from the SF-36 questionnaire (Supplementary material online, Appendix). Three questions using a visual analogue scale (VAS) will evaluate (i) whether patients experience difficulties in accepting their physical limitations in daily life activities, (ii) whether patients know their physical limits, and (iii) whether patients experience anxiety or fear for physical exercise. Patients with restrictions in their daily activities according to the SF-36 questionnaire and/or a score >5 on one of the three VAS questions will be called by the case manager to assess their motivation for consultation by a physiotherapist. The goal of physiotherapy consultation is to promote pre-operative physical activity and physical fitness, increase knowledge and acceptance, and to reduce anxiety in relation to physical exercise. The programme consists of video call counselling by a physiotherapist and a digital platform with additional information related to the importance of pre-operative physical activity and physical fitness, as well as instruction videos of home-based functional exercises. Additionally, an informal caregiver (buddy) will be involved in the functional exercise training to motivate, help, and join the patient during the training sessions without further supervision. Video calls are scheduled at the start of the programme and every 2–4 weeks until surgery to monitor progression and to ensure that functional exercise training consists of activities of relevance to a patient.
For strength, coordination, and mobility training, there are four exercises with video instructions. Each exercise can be done on three different levels, which correspond with a maximum metabolic equivalent (MET) score of <2, 2–4, and >4. The advised level is based on the current activity level of the patient using a MET score list at intake by the physiotherapist and a 30-s chair stand test.26,27 Patients are advised to train three times a week, until the scheduled surgery. To monitor training intensity, patients fill in the 6–20 Borg score for rate of perceived exertion after each training session in a digital raining log. To maintain an adequate training stimulus, training intensity or frequency can be increased when the rate of perceived exertion is <13; however, functional task exercises can also progress in difficulty in terms of making exercises more complex (e.g. dual tasks) and by adding variation. At hospital admission, handgrip strength using the Martin Vigori™ metre (Firma Gebrüder Martin, Tuttlingen, Germany), and the 30-s chair stand test will be measured.
Psychological support
Patients with a hospital anxiety and depression scale (HADS)28 anxiety or depression score ≥8 will be called by the case manager to assess their motivation for a psycho-educational prevention (PEP) module. The goal of this module is to relieve symptoms of anxiety and depression. The PEP-module is based on acceptance commitment therapy philosophy and mindfulness. The programme consists of at least three phone calls by a psychological counsellor, one at the start of the programme and then every 2–4 weeks until the scheduled surgery. At the digital platform, additional support is available using psycho-education and tools to improve coping skills. We created four chapters on the platform consisting of information and practical exercises regarding four important themes in relation to our patients (stress and anxiety, ways of mindful relaxation, acceptance of thoughts and emotions, and discovering core values). Every 2 weeks, a new module is introduced by our psychological counsellor and evaluated in the next appointment.
Nutritional support
Obese patients (BMI >30 kg/m2) and/or patients with a malnutrition universal screening tool (MUST)29 score ≥2 will be called by the case manager to assess their motivation for nutritional support. This module aims to improve pre-operative nutritional status. The programme consists of phone calls by a dietician (at baseline and every 2–4 weeks) and a digital platform with additional information about a healthy diet. Patients with an elevated MUST will be screened for malnutrition and low-protein intake and treated with (protein)-enriched supplements according to the treating dietician. It is not intended to promote extreme weight loss pre-operatively in patients with obesity.
Smoking cessation
Following inclusion, smoking status will be assessed for all participants. Patients that smoke will receive a motivational enhancement module where after the case manager will assess their motivation for smoking cessation. The goal of the programme is to stop smoking before the planned surgery. The programme is led by a pulmonary care nurse, qualified to support smoking cessation. The first intake will be supported by video calls to facilitate a successful behavioural change. Motivational interviewing is used to explore and resolve ambivalence about behaviour change.30 The Fagerstrom questionnaire is used to assess nicotine dependence31 and to aid the decision to start nicotine replacement therapy32 or the use of anti-depressants.33 The programme consists of regular phone calls at intake, within 2 weeks after receiving medicine (if applicable), 1 day after the planned stop date, and at 1, 2, 4, 12, 26, and 52 weeks after the planned stop date,34 which will be supported by the digital platform with additional information about the general effects of smoking, the costs of smoking, and the improvement of perioperative outcomes in cardiac surgery after smoking cessation.
Feasibility
All patients who are eligible for the study are contacted to screen them for participation. Reasons for exclusion are registered, especially non-participation due to lack of digital skills or language barriers. Adherence to the modules will also be logged by measuring time spent in each module on the platform (this is automatically recorded) and the number of contact moments with the team.
Blood samples
At the outpatient clinic or at admission on the day before surgery, a routine laboratory test is performed. Relevant parameters for this study will be, haemoglobin, creatinine, albumin, and c-reactive protein levels.
Safety
The urgency and planning for surgery are decided during the daily multidisciplinary heart team meeting. Patients are only eligible for inclusion in our trial if there is no medical reason (e.g. NYHA IV, left main stenosis) to schedule the surgery within 6 weeks. Patients who are scheduled for elective surgery at least 8 weeks from informed consent are eligible for participation in our trial. All participants are monitored for progression of cardiac symptoms with an online questionnaire on a weekly base. When there is progression of symptoms, this will be discussed with the treating cardiologist in which the planning for surgery will be reconsidered. Safety for patients participating in the functional exercise training programme is guaranteed by a digital video intake before the start of the training programme. A physiotherapist with experience in cardiac rehabilitation will assess physical capacity and prescribe a low-intensity physical exercise training programme according to the physical fitness of the patient. Patients are advised to train with an informal caregiver (buddy) to increase safety, to increase motivation, and to increase compliance. Patients and their informal caregivers are instructed to seek medical advice in case of any cardiac symptoms.
Study outcomes
The primary objective is to investigate the effect of a personalized telemonitored pre-operative integrated care programme in patients scheduled for elective cardiac surgery on the cumulative incidence of MACE from inclusion until 1-year after the surgery. Major adverse cardiac events is defined as a composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and hospitalization for heart failure or for hospitalization earlier than initial planned due to increasing cardiac symptoms. Major adverse cardiac events will also be analysed pre- and post-operative separately. Secondary objectives are to investigate all-cause, cardiovascular, and COVID-19-related mortality. These endpoints will be analysed by comparing the two groups the day before the scheduled surgery, 30-day post-operatively, and 3, 6, and 12 months after surgery. Post-operative complications will be measured at discharge from hospital and 30 days after surgery using a standard list of complications as listed by the Dutch Netherlands Heart registry35. Other endpoints which will focus on cardiac symptoms [NYHA and Canadian Cardiovascular Society (CCS)], quality of life [EuroQol five-dimensional five-level questionnaire (EQ-5D-5L36) and 36-item short form health survey (SF-3637)], cardiovascular risk factors (smoking status and BMI), anxiety and depression scores (HADS), societal costs (iMedical consumption questionnaire (iMCQ38) and iProductivity cost questionnaire (iPCQ39), unplanned visits to the emergency department, post-operative length of stay, and participation in post-operative cardiac rehabilitation. All individual intervention modules will be evaluated on their effectiveness.
Cost-effectiveness analyses
While the primary aim of the study is to assess the effectiveness of prehabilitation using a personalized telemonitored pre-operative integrated care programme, it is relevant to relate the difference between the costs of this programme and the costs of usual care (see ‘cost analysis’ below) to the (potential) health gains of the programme. Therefore, the cost-effectiveness of the prehabilitation programme compared to usual care will be evaluated alongside the clinical trial, following the Dutch guidelines for health economic evaluations.40 The economic evaluation will be conducted from a societal perspective and will have a time horizon of ∼15 months.
Total costs over the study period will be calculated by multiplying individual resource use with the costs per unit. Resource use in the hospital (e.g. teleprehabilitation programme, control visits, hospitalization, diagnostic tests, surgery, other medical procedures) will be obtained from hospital records. Costs and resource use outside the hospital (e.g. out-of-pocket payments for over the-counter medication, visits to the general practitioner or other health care professionals, travel time, informal care, and productivity losses) will be collected using patient surveys (iMCQ and iPCQ) filled out pre-operatively, and at 3, 6, and 12 months after surgery. Sources for valuation will be cost prices of the Dutch costing manual and cost prices from the Pharmacotherapeutic compass.40
The quality-adjusted life year (QALY) is the health outcome of choice for the economic evaluation.41 The QALY is a measure of life expectancy weighted by health-related quality of life, the latter being presented as a utility score. The EQ-5D-5L provides the (Dutch) utility scores and is completed at baseline, pre-operatively, and at 3, 6, and 12 months after surgery. The utilities at the various time‐points were used to compute QALYs by means of the area under the curve method, where the utility of a particular health state is multiplied by the time spent in this specific health state.41 Cost-effectiveness is expressed in an incremental cost effectiveness ratio (ICER): the incremental costs divided by the incremental QALYs. Non-parametric bootstrapping with 5000 replicates of the joint distribution of costs and QALYs will estimate the probability of the intervention being cost-effective, for various ceiling ratios for the ICER, presented in a cost-effectiveness acceptability curve (CEAC). Ceiling ratios reflect the maximum price health policymakers are willing to pay for an additional QALY. Several one-way sensitivity analyses will be performed to assess the robustness of results.
Discussion
This manuscript addresses the study protocol of a randomized controlled trial to study the effect of prehabilitation using a personalized telemonitored pre-operative integrated care programme in patients scheduled for elective cardiac surgery. An integrated care programme will be utilized, using five different modules which were proven effectively in previous studies in cardiac surgery. As this study should be representative for all patients waiting for cardiac surgery, only patients under the age of 18 years or patients scheduled for urgent cardiac surgery will not be screened for eligibility. Patients will only be excluded for participation if they refuse informed consent or if patients cannot use our platform due to a lack of digital literacy, langue barrier, or interfering medical condition. We chose the cumulative incidence of MACE to evaluate the effect of our programme on the pre-operative incidence of MACE (including unplanned hospitalization), as well on the post-operative incidence at 1-year. We expect that our intervention reduces the negative impact of a prolonged pre-operative period and improves patient outcomes on the long term.
As the prehabilitation programme is fully digital and supported by (video) calls, it provides the opportunity to start the current trial despite the social-distancing measures during the COVID-19 pandemic. Important reasons for non-participation in cardiac rehabilitation (e.g. transportations problems due to health issues or participants who live further away from the hospital42) might be overcome with the digital programme. As such, however, the programme might not be accessible for patients with limited access to the Internet, patients with a lack of digital skills, or patients with language barriers. Although there is little direct evidence, it seems obvious that there is a close correlation between digital literacy, general health literacy, and cardiovascular health outcomes.43,44 It is important to find smart solutions to ensure that vulnerable patients, who might benefit the most from cardiac (p)rehabilitation, are willing and able to participate in digital health solutions. Unfortunately, the required digital screening makes it impossible to use performance tests or pulmonary function testing to stratify patients for our teleprehabilitation programme. Physical performance tests may result in a better risk assessment for adverse perioperative events and also facilitates an optimal personalized exercise training prescription. Instead of performance tests, questionnaires will be used to screen patients for risk factors and subsequent relevant modules of the teleprehabilitation programme. The advantage of these online questionnaires with predefined cut-off points is their ability to be used remotely at a large scale.
As patient recruitment starts once they are placed on the waiting list, the actual pre-operative period and duration of prehabilitation might differ between individual patients, for which a statistical correction will be applied. Due to the weekly evaluation of cardiac symptoms, it is also possible that progression of symptoms will be observed in patients, warranting a more urgent scheduling of surgery. Post-operatively, all patients are referred for cardiac rehabilitation, for which we expect that patients who participated in our prehabilitation programme will have a higher motivation to participate in the post-operative cardiac rehabilitation programme. This might even increase the effect of our intervention on long-term outcomes.
What we expect to learn from this study
The primary endpoint of this study is the difference in MACE at 1-year after surgery. However, other trial outcomes are of interest as well, such as its feasibility by investigating the number of patients who cannot participate due to a lack of digital literacy. To access the safety of the digital prehabilitation programme, the study will be monitored by our local clinical trial centre. Important secondary outcomes are the effectiveness of the individual modules, as well as the effect on post-operative length of hospital stay and perioperative complications. The effect on quality of life and analyses of the cost-effectiveness of our programme are two other important research questions.
Future perspectives
In the future, a multidisciplinary pre-operative assessment at the outpatient clinic is performed when patients are referred for cardiac surgery, in order to assess their readiness for cardiac surgery. Ideally, this will be done using physical performance tests to objectively assess physical capacity of the patients and to screen on other domains like sarcopenia and frailty. Patients who seem fit for surgery can be accepted and scheduled in 1–2 weeks. Patients with a very high surgical risk might be rejected for surgery. A third group with an elevated but modifiable risk profile might be accepted for surgery after a cardiac prehabilitation programme. This last group might be divided in a group who can attend a digital programme and a second group that might benefit most from supervised (home- or community-based) prehabilitation. Patients participating in a functional exercise training programme will be closely monitored using wearable devices like heart rate monitors and a pedometer. Close monitoring of patients during their pre-operative period may justify postponement of scheduled surgery in patients with little cardiac symptoms in favour of patients showing rapid progression of cardiac complaints. Finally, participation in our post-operative rehabilitation programme might be stimulated by creating a continuum of care from prehabilitation to rehabilitation. Pre-operatively, there will be a focus on optimizing patients for their scheduled surgery. Post-operatively, we will focus on participation in social activities and work and also promote a healthy lifestyle to reduce future cardiovascular events.
Registration
The study was approved by the Medical Ethics Committee Maastricht and is registered on clinicaltrials.gov (NCT04393636).
Lead author biography

Bart Scheenstra is a cardiologist at the Maastricht University Medical Centre+, The Netherlands. Recently, he started a PhD and a fellowship at the Department of Cardiothoracic Surgery. His research focuses on improving perioperative care of patients scheduled for cardiothoracic surgery. By means of digital solutions, he aims to improve patient experiences and patient outcomes.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health online.
Conflict of interest: none declared.
Data availability
No new data were generated or analysed as part of this paper.
Supplementary Material
References
- 1. Wiseman SM, Crump RT, Sutherland JM.. Surgical wait list management in Canada during a pandemic: many challenges ahead. Can J Surg 2020;63:e226–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basman C, Kliger CA, Pirelli L, Scheinerman SJ.. Management of elective aortic valve replacement over the long term in the era of COVID-19. Eur J Cardiothorac Surg 2020;57:1029–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgan CD, Sykora K, Naylor CD.. Analysis of deaths while waiting for cardiac surgery among 29,293 consecutive patients in Ontario, Canada. The Steering Committee of the Cardiac Care Network of Ontario. Heart 1998;79:345–349. [PMC free article] [PubMed] [Google Scholar]
- 4. Malaisrie SC, McDonald E, Kruse J, Li Z, McGee EC Jr, Abicht TO, Russell H, McCarthy PM, Andrei AC.. Mortality while waiting for aortic valve replacement. Ann Thorac Surg 2014;98:1564–1570; discussion 1570–1. [DOI] [PubMed] [Google Scholar]
- 5. Elbaz-Greener G, Masih S, Fang J, Ko DT, Lauck SB, Webb JG, Nallamothu BK, Wijeysundera HC.. Temporal trends and clinical consequences of wait times for transcatheter aortic valve replacement: a population-based study. Circulation 2018;138:483–493. [DOI] [PubMed] [Google Scholar]
- 6. Carli F, Scheede-Bergdahl C.. Prehabilitation to enhance perioperative care. Anesthesiol Clin 2015;33:17–33. [DOI] [PubMed] [Google Scholar]
- 7. Hulzebos EH, Smit Y, Helders PP, van Meeteren NL.. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev 2012;11:CD010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arthur HM, Daniels C, McKelvie R, Hirsh J, Rush B.. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med 2000;133:253–262. [DOI] [PubMed] [Google Scholar]
- 9. McCann M, Stamp N, Ngui A, Litton E.. Cardiac prehabilitation. J Cardiothorac Vasc Anesth 2019;33:2255–2265. [DOI] [PubMed] [Google Scholar]
- 10. Marmelo F, Rocha V, Moreira-Gonçalves D.. The impact of prehabilitation on post-surgical complications in patients undergoing non-urgent cardiovascular surgical intervention: systematic review and meta-analysis. Eur J Prev Cardiol 2018;25:404–417. [DOI] [PubMed] [Google Scholar]
- 11. Bongers B, Dronkers J, Hulzebos E, Hoogeboom T, Buhre W, van Meeteren N et al. Optimizing perioperative physical therapy care in major elective surgery to improve surgical outcome in high-risk patients: the Better in, Better out™ concept. Ned Tijdschr Anesthesiol 2016:134–139. [Google Scholar]
- 12. Silver JK. Prehabilitation could save lives in a pandemic. BMJ 2020;369:m1386. [DOI] [PubMed] [Google Scholar]
- 13. Lambert G, Drummond K, Ferreira V, Carli F.. Teleprehabilitation during COVID-19 pandemic: the essentials of "what" and "how". Support Care Cancer 2021;29:551–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS.. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol 2017;4:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brouwers RWM, van Exel HJ, van Hal JMC, Jorstad HT, de Kluiver EP, Kraaijenhagen RA, Kuijpers P, van der Linde MR, Spee RF, Sunamura M, Uszko-Lencer N, Vromen T, Wittekoek ME, Kemps HMC.. Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth Heart J 2020;28:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy SK, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Pogue J, Chrolavicius S, Yusuf S.. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013;368:1179–1188. [DOI] [PubMed] [Google Scholar]
- 17. Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr, Moskowitz AJ, Voisine P, Ailawadi G, Bouchard D, Smith PK, Mack MJ, Acker MA, Mullen JC, Rose EA, Chang HL, Puskas JD, Couderc JP, Gardner TJ, Varghese R, Horvath KA, Bolling SF, Michler RE, Geller NL, Ascheim DD, Miller MA, Bagiella E, Moquete EG, Williams P, Taddei-Peters WC, O'Gara PT, Blackstone EH, Argenziano M.. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG.. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 19. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U.. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–744; discussion 744–5. [DOI] [PubMed] [Google Scholar]
- 20.Rijksinstituut-voor-Volksgezondheid-en-Milieu. https://www.rivm.nl/coronavirus-covid-19 (26 December 2020).
- 21. Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL.. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006;296:1851–1857. [DOI] [PubMed] [Google Scholar]
- 22. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 23. Carpenter MA, Tockman MS, Hutchinson RG, Davis CE, Heiss G.. Demographic and anthropometric correlates of maximum inspiratory pressure: the Atherosclerosis Risk in Communities Study. Am J Respir Crit Care Med 1999;159:415–422. [DOI] [PubMed] [Google Scholar]
- 24. Wilson SH, Cooke NT, Edwards RH, Spiro SG.. Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax 1984;39:535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- 26. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, Paffenbarger RS. Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 27. Jones CJ, Rikli RE, Beam WC.. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- 28. Zigmond AS, Snaith RP.. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 29. Scott A. Screening for malnutrition in the community: the MUST tool. Br J Community Nurs 2008;13:406–408, 410–412. [DOI] [PubMed] [Google Scholar]
- 30. Lindson-Hawley N, Thompson TP, Begh R.. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2015;3:CD006936. [DOI] [PubMed] [Google Scholar]
- 31. Svicher A, Cosci F, Giannini M, Pistelli F, Fagerström K.. Item response theory analysis of Fagerström test for cigarette dependence. Addict Behav 2018;77:38–46. [DOI] [PubMed] [Google Scholar]
- 32. Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T.. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11:CD000146. [DOI] [PubMed] [Google Scholar]
- 33. Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T.. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2014;2014:CD000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stead LF, Hartmann-Boyce J, Perera R, Lancaster T.. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013;8:CD002850. [DOI] [PubMed] [Google Scholar]
- 35.Nederlandse Hart Registratie (NHR). Handboek Nederlandse Hart Registratie2020. https://nederlandsehartregistratie.nl/wp-content/uploads/2020/04/NHR_HANDBOEK_versie-2020v0.3_DEFINITIEF.pdf (26 December 2020).
- 36. Versteegh M, Vermeulen K, Evers S, de Wit GA, Prenger R, Stolk E.. Dutch tariff for the five-level version of EQ-5D. Value Health 2016;19:343–352. [DOI] [PubMed] [Google Scholar]
- 37. Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E.. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–1068. [DOI] [PubMed] [Google Scholar]
- 38.Institute for Medical Technology Assessment (IMTA). Medical Consumption Questionnaire. https://docplayer.nl/60086739-Medical-consumption-questionnaire-productivity-and-health-research-group.html (26 December 2020).
- 39.Institute for Medical Technology Assessment (IMTA). Productivity Cost Questionnaire. https://docplayer.nl/59287785-Handleiding-productivity-cost-questionnaire-institute-for-medical-technology-assessment-productivity-and-health-research-group.html (26 December 2020).
- 40.Institute for Medical Technology Assessment (IMTA). Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. www.zorginstituutnederland.nl (26 December 2020).
- 41. Drummond MF, Claxton K, Stoddart GL, Torrance GW.. Methods for the Economic Evaluation of Health Care Programmes, 4th edn. Oxford: Oxford University Press; 2015. [Google Scholar]
- 42. Ruano-Ravina A, Pena-Gil C, Abu-Assi E, Raposeiras S, van't Hof A, Meindersma E, Bossano Prescott EI, González-Juanatey JR.. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol 2016;223:436–443. [DOI] [PubMed] [Google Scholar]
- 43. Magnani JW, Mujahid MS, Aronow HD, Cené CW, Dickson VV, Havranek E, Morgenstern LB, Paasche-Orlow MK, Pollak A, Willey JZ.. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation 2018;138:e48–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Conard S. Best practices in digital health literacy. Int J Cardiol 2019;292:277–279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed as part of this paper.