Abstract
Aims
Underutilization of guideline-directed heart failure with reduced ejection fraction (HFrEF) medications contributes to poor outcomes.
Methods and results
A pilot study to evaluate the safety and efficacy of a home-based remote monitoring system for HFrEF management was performed. The system included wearable armband monitors paired with the smartphone application. An HFrEF medication titration algorithm was used to adjust medication daily. The primary endpoint was HFrEF medication utilization at 120 days. Twenty patients (60.5 ± 8.2 years, men: 85%) with HFrEF were recruited. All received angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB)/angiotensin receptor-neprilysin inhibitor (ARNI) at recruitment; 45% received ≥50% maximal targeted dose (MTD) with % MTD of 44.4 ± 31.7%. At baseline, 90 and 70% received beta-adrenergic blocker and mineralocorticoid receptor antagonist (MRA), 35% received ≥50% MTD beta-adrenergic blocker with % MTD of 34.1 ± 29.6%, and 25% received ≥50% MTD MRA with % MTD of 25.0 ± 19.9%. At 120 days, 70% received ≥50% MTD ACEI/ARB/ARNI (P = 0.110) with % MTD increased to 64.4 ± 33.5% (P = 0.060). The proportion receiving ≥50% MTD ARNI increased from 15 to 55% (P = 0.089) with % MTD ARNI increased from 20.6 ± 30.9 to 53.1 ± 39.5% (P = 0.006*). More patients received ≥50% MTD MRA (65 vs. 25%, P = 0.011*) with % MTD MRA increased from 25.0 ± 19.9 to 46.2 ± 28.8% (P = 0.009*). Ninety-five per cent of patients had reduced NT-proBNP with the percentage reduction of 26.7 ± 19.7%.
Conclusion
Heart failure with reduced ejection fraction medication escalation with remote monitoring appeared feasible.
Keywords: Heart failure, Telemonitoring, Drug escalation
Graphical Abstract
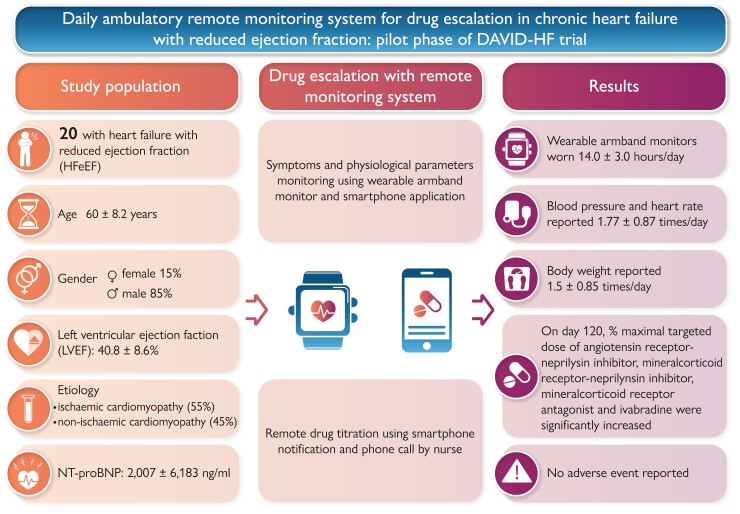
Graphical Abstract.
Daily ambulatory remote monitoring system for drug escalation in chronic heart failure with reduced ejection fraction.
Introduction
Heart failure (HF) is an emerging public health challenge that affects an estimated 38 million people worldwide with a prevalence of 1–2% in the adult population.1,2 Over the past few decades, randomized controlled trials have demonstrated that HF medications, including angiotensin-converting enzyme inhibitors (ACEIs),3,4 angiotensin receptor blockers (ARBs), beta-adrenergic blockers,5–7 mineralocorticoid receptor antagonists (MRAs),8,9 ivabradine,10 angiotensin receptor-neprilysin inhibitor (ARNI),11 and more recently sodium glucose cotransporter (SGLT) inhibitors,12,13 can significantly improve the outcomes of patients with HF with reduced ejection fraction (HFrEF). However, in real-world clinical practice, the prognosis of patients with HFrEF remains poor. In a recent European registry of patients hospitalized for HF, 16.4% of patients with newly diagnosed HF died within 1 year of diagnosis.14 Similarly, 1-year mortality of HF in Asia was reported to range between 8.9 and 19.5%.15–17
Failure to translate favourable results observed in landmark HFrEF clinical trials into real-world practice is partly attributed to the underutilization and underdosing of guideline-directed HF medications.18,19 Observational studies consistently demonstrated a positive correlation between the dosage of HFrEF medication and clinical outcomes.20–22 In fact, international guidelines for HF management strongly emphasize the importance of up-titrating evidence-based HFrEF medication to the target dose, in addition to initiating the drugs in concern.23,24 However, underdosing of guideline-directed HF medications remains to be very common in real-world practice. An international prospective observational longitudinal survey involving 7092 patients with chronic HFrEF from 36 countries revealed a disappointingly low proportion of patients receiving guideline-directed HF medications at the target dose: 27.9% for ACEIs, 24.8% for beta-adrenergic blocker, and 6.9% for ARB.19 More recently, in the CHange the Management of Patients With Heart Failure (CHAMP-HF) registry involving 3095 patients with HFrEF,18 despite a high proportions of patients receiving guideline-directed HF medication with 75.1% taking ACEI/ARB/ARNI, 82.7% taking beta-adrenergic blocker, and 32.5% taking MRA, only <20% of these HF medications were taken at target doses. Obviously, true medication intolerance may limit up-titration of guideline-directed HF medications in some patients. However, in some scenario, clinicians are hesitant towards up-titrating HF medication because they cannot confidently assess patient’s blood pressure, heart rate, and drug tolerability while they are at home.
Remote acquisition of symptomatology and physiological parameters such as blood pressure, heart rate, and body weight has been explored to optimize HF management. Conceptually, these information could facilitate home-based HF management and improve clinical outcomes. However, previously, a large-scale randomized control trial that utilized telephone-based interactive voice-response system to collect daily information about symptoms and body weight for clinician review failed to improve the clinical outcomes in terms of hospitalization and/or mortality.25 It has been postulated that patient non-adherence to daily reporting, clinician non-compliance to data review and action implementation due to information overload, and lack of a systematic intervention strategy undermine the effectiveness of telemonitoring for remote HF management.26–28 Over the past decade, mobile technology has revolutionized the way people communicate, allowing instantaneous, multi-directional, and massive data transfer. In addition, wearable technology nowadays can continuously monitor multiple physiological parameters in the ambulatory setting in a fully automated manner. The potentials of these technologies in the management of patients with HF have nonetheless not been fully explored. The Daily Ambulatory remote monitoring system Vs conventIonal therapy for Drug escalation in chronic Heart Failure with reduced ejection fraction study (DAVID-HF Study) is a multi-centre, open-label, randomized controlled trial to explore the potentials of utilizing mobile and wearable technologies to remotely manage HF patients, particularly for remote escalation of evidence-based HF medications. The present report described results from the pilot phase of the DAVID-HF study.
Methods
Study design
The present study is the pilot phase of the DAVID-HF study performed in a single-centre setting to evaluate the feasibility of the remote monitoring system for home-based HF medication escalation. The subsequent randomization phase of the DAVID-HF Study will be performed using an open-label, multi-centre, and randomized design to explore the potentials of mobile technology for the optimization of HF management in a home-based remote setting. The investigation conforms to the principles outlined in the Declaration of Helsinki. The study protocol has been approved by the Institutional Review Board of The University of Hong Kong, and Hong Kong West Cluster, Hospital Authority, Hong Kong. The study is registered with ClinicalTrials.gov (NCT03072693).
Study participants
In the pilot phase, 20 patients with chronic HFrEF from the outpatient cardiac clinic at Queen Mary Hospital, Hong Kong, were recruited. Patients aged ≥ 18 years with chronic HFrEF, defined as having stabilized conditions for >3 months, were eligible for the study. Patients were excluded if they had recent hospitalization for HF, acute coronary syndrome, and/or other cardiovascular events within 6 months; had a history of complex congenital heart disease, or significant valvular stenosis; were unable to operate simple electronic devices; or did not have a mobile network service in the place of residence.
Home-based remote heart failure management system
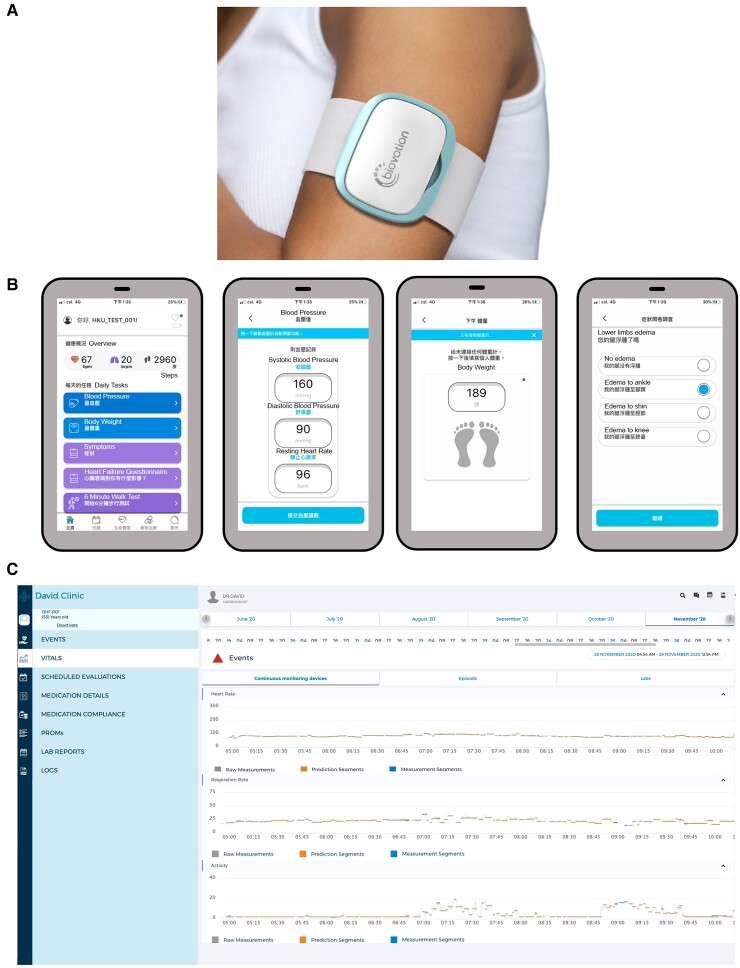
The home-based remote HF management system comprises a multi-sensor-based wearable armband monitor, Everion (Biofourmis, Boston, MA, USA),29,30 a handheld single-lead electrocardiogram (ECG) recorder (Comfit limited, Hong Kong SAR, China), an electronic blood pressure device, an electronic bath scale, a customized smartphone application BiovitalsHF (Biofourmis), a web-based clinician dashboard BiovitalsHF platform (Biofourmis), and a cloud-based data analytical algorithm, Biovitals Analytics Engine (Biofourmis) (Figure 1). The wearable monitor was capable of tracking heart rate, heart rate variability, blood pulse wave, oxygen saturation, respiration rate, skin temperature, electrodermal activity, and steps count. The wearable monitor was worn during daytime and recharged while bathing or sleeping. The handheld single-lead ECG recorder was incorporated with artificial intelligence-based AF detection.31 Patients were instructed to document blood pressure in the morning and evening, record body weight in the evening, and report symptoms using the smartphone application (Figure 1). All devices were connected to the study smartphone via Bluetooth. All data were automatically transferred in real time through the smartphone application to a secured cloud storage for processing using Biovitals Analytics Engine. Results were then displayed on the web-based clinician dashboard for daily review and action implementation (Figure 1).
Figure 1.
The home-based remote heart failure management system. (A) Wearable armband monitor, Everion (Biofourmis), capable of tracking heart rate, heart rate variability, blood pulse wave, oxygen saturation, respiration rate, skin temperature, electrodermal activity, and steps count. The wearable monitor was worn during daytime and recharged while bathing or sleeping. (B) Screenshots of patient smartphone application, and (C) clinician dashboard.
Home-based remote drug escalation
The objective was to maximize the utilization of guideline-directed HF medications including ACEI, ARB, ARNI, beta-adrenergic blocker, MRA, ivabradine, and SGLT inhibitor using remotely obtained physiological parameters of the patients. Based on individual patient’s daily physiological parameters, recommendations for HF medication adjustment were automatically generated by the remote monitoring platform according to the predefined drug escalation algorithm (see Supplementary material online, Methods and Figure S1). Cardiologists involved in the clinical trial reviewed the algorithm-generated recommendations and granted endorsement if they agree. Patients were notified for any medication adjustment through both text messages in the smartphone application and phone calls from the research nurse. Drug records were updated if both cardiologists and patients in concern agreed with the proposed medication change. The hierarchy of HF medication escalation was based on the European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure, i.e. first line: ACEI/ARB, MRA, and beta-adrenergic blocker; and second line: ivabradine and sacubitril/valsartan.32 The dosage was stepwise adjusted to the maximally tolerated dose according to patient status. The drug escalation period was 17 weeks (119 days). During drug escalation, blood tests were required to ensure tolerance and safety. Patients were instructed to directly visit phlebotomists for blood taking without the need to arrange additional clinical visits to be assessed by clinicians or nurses.
BiovitalsHF
The BiovitalsHF is a software platform designed to monitor and manage medication for patients with heart failure. The platform comprises a set of clinician tools available in a mobile application or web dashboard, a patient mobile application, and a web-based software application responsible for data storage and management along with containing the clinical rules and decision logic that encapsulate the clinical practice guidelines.
Biovitals Analytics Engine
The Biovitals Analytics Engine was a US Food and Drug Administration 510(k) cleared algorithm which provides machine learning-derived health index (Biovitals Index) that reflects the derivatives in physiology parameters or changes in symptoms. The higher Biovitals Index reflected larger deviations of physiology parameters from baseline or new symptoms, potentially indicating the worsening of health status. The machine learning and statistical methods for the derivation of the Biovitals Index were previously described.29,33
Study measures
The primary measures were the proportion of patients receiving ≥ 50% of maximal target dose (MTD) of guideline-directed HF medications and the percentage MTD of guideline-directed HF medications at the end of the study. The secondary measures included wearable device and monitoring adherence, New York Heart Association (NYHA) functional class, unscheduled medical visit, NT-proBNP, renal function, and 6 min walk test.
Statistical analysis and data safety monitoring
Data are expressed as mean ± standard deviation, median [interquartile range (IQR)], or numbers and percentage of patients. The paired sample t-test or Mann–Whitney U-test was used to identify the effects of home-based remote HF management system on the study measures and other variables of interest between baseline and the end of the study. Calculations were performed using SPSS software (version 26.0, SPSS, Chicago, IL, USA). A P-value of <0.05 was considered statistically significant. The Data Safety Monitoring Board specializing in HF management and one independent statistician reviewed the study progress and adverse event rates and determine whether the study should be stopped early because of excessive risk or benefits of study procedures.
Results
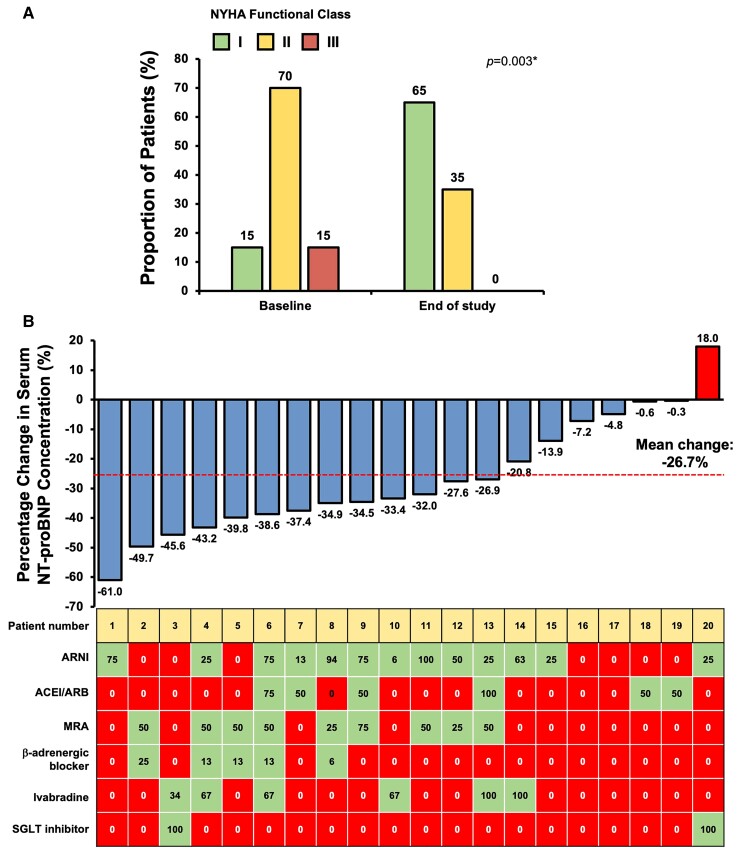
Thirty-one patients with HFrEF were referred to our clinic for consideration of enrolment. Five (16.1%) were excluded because of the inability to independently operate the wearable armband and smartphone application, 1 (3.22%) was excluded because of advanced chronic renal failure, and 5 (16.1%) declined enrolment after explanation of trial details. Finally, a total of 20 patients with chronic HFrEF on stable medications for 3 months or more followed up in our cardiac clinic were recruited. During the study period, the wearable armband monitors were worn 14.0 ± 3.0 h/day. Blood pressure and heart rate were reported 1.77 ± 0.87 times daily and body weight was reported 1.5 ± 0.85 times daily. Table 1 summarizes the baseline clinical characteristics of the recruited patients. The median age was 60.2 (IQR: 55.0, 65.6) years and 85% were men. The median age of HFrEF diagnosis was 52.6 (IQR: 48.5, 61.1) years and the median duration from diagnosis of HFrEF was 72.6 (IQR: 16.3, 130.1) months. The mean LVEF at recruitment was 40.8 ± 8.6%. Eleven patients (55.0%) had underlying coronary artery disease and the remaining nine patients (45.0%) had non-ischaemic aetiologies. Fifty per cent of patients had hypertension and 50% had diabetes mellitus. At recruitment, after being stabilized with HFrEF medication for > 3 months, the proportion of patients classified as NYHA function Class I, II, and III was 15, 70, and 15%, respectively. The baseline systolic and diastolic blood pressure were 122.0 (IQR: 108.0, 130.5) and 77.0 (IQR: 70.0, 81.0) mmHg with the resting heart rate of 63.0 (IQR: 58.5, 69.0) b.p.m. The mean and median serum NT-proBNP concentration were 2007 ± 6183 and 402 ng/mL (IQR: 150, 970 ng/mL), respectively. The mean serum creatinine was 99.7 ± 25.8 μmol/L.
Table 1.
Baseline characteristics
| (n = 20) | |
|---|---|
| Age (years) | 60.5 ± 8.2 |
| Male, n (%) | 17 (85.0) |
| Hypertension, n (%) | 50 (50.0) |
| Diabetes mellitus, n (%) | 10 (50.0) |
| Ischaemic, n (%) | 11 (55.0) |
| Non-ischaemic, n (%) | 9 (45) |
| Atrial fibrillation, n (%) | 3 (15.0) |
| Previous stroke, n (%) | 1 (5.0) |
| Left ventricular ejection fraction (%) | 40.8 ± 8.6 |
| Implantable cardioverter defibrillator, n (%) | 7 (35) |
| Cardiac resynchronization therapy, n (%) | 3 (15) |
| NYHA function class | |
| ȃI, n (%) | 3 (15) |
| ȃII, n (%) | 14 (70) |
| ȃIII, n (%) | 3 (15) |
| Systolic blood pressure, mmHg | 119.8 ± 17.3 |
| Diastolic blood pressure, mmHg | 74.6 ± 11.5 |
| Resting heart rate, b.p.m. | 65.1 ± 9.2 |
| Serum NT-proBNP, ng/mL | 2007 ± 6183 |
| 402 (150, 970) | |
| Serum creatinine, µmol/L | 99.7 ± 25.8 |
| Serum sodium, mmol/L | 139.8 ± 2.7 |
| Serum potassium, mmol/L | 4.3 ± 0.5 |
| Haemoglobin, g/dL | 14.7 ± 1.8 |
The majority of patients received ACEI/ARB/ARNI (100%), beta-adrenergic blocker (90%), and MRA (70%) at baseline; however, the proportion of patients who received ≥ 50% MTD of these guideline-directed HF medications was only 45, 35, and 25%, respectively (Table 2). Specifically, the mean percentage MTD of ACEI/ARB/ARNI was 44.4 ± 31.7%, beta-adrenergic blocker was 34.1 ± 29.6%, and MRA was 25.0 ± 19.9%. In addition, 55% of patients received SGLT inhibitor and 20% received ivabradine. Thirty-five per cent of patients had implantable cardioverter defibrillator and 15% had cardiac resynchronization therapy.
Table 2.
Utilization of heart failure medications at baseline
| HF medications | Patients on HF medications (%) | Patients on ≥ 50% MTD HF medication (%) | Mean % MTD |
|---|---|---|---|
| ACEI/ARB/ARNI | 100 | 45 | 44.4 ± 31.7 |
| ARNI | 55 | 15 | 20.6 ± 30.9 |
| ACEI/ARB | 45 | 30 | 23.8 ± 32.9 |
| MRA | 70 | 25 | 25.0 ± 19.9 |
| Beta-blocker | 90 | 35 | 34.1 ± 29.6 |
| Ivabradine | 20 | 5 | 8.3 ± 4.1 |
| SGLT inhibitor | 55 | 55 | 55.0 ± 51.0 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor and neprilysin inhibitor; HF, heart failure; MRA, mineralocorticoid receptor antagonist; MTD, maximal target dose; SGLT, sodium glucose cotransporter.
Heart failure medication escalation
Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor
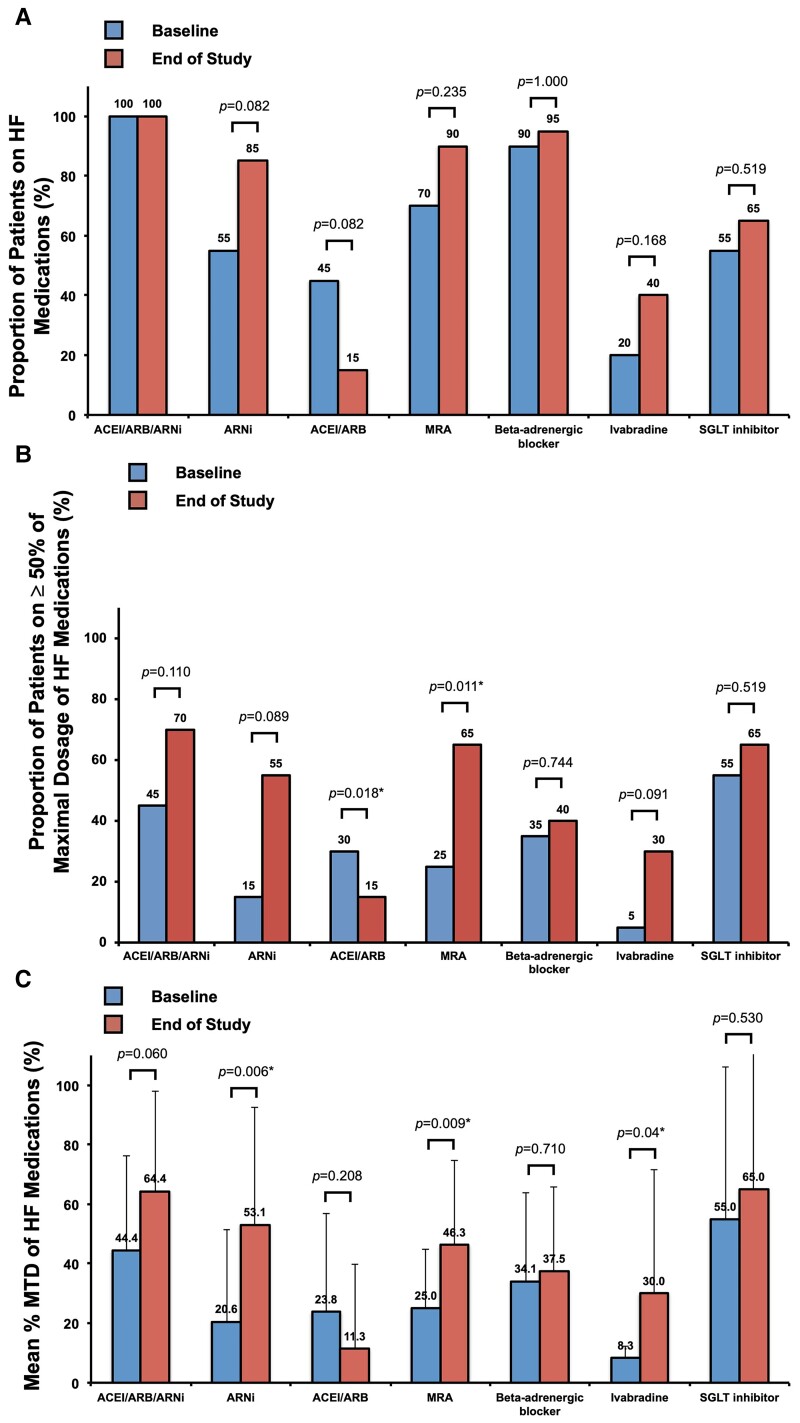
The proportion of patients receiving ≥ 50% MTD ACEI/ARB/ARNI increased from 45% at baseline to 70% at the end of the study (P = 0.110), with an insignificant increase in percentage MTD (64.4 ± 33.5 vs. 44.4 ± 31.7%, P = 0.060). Thirty per cent of patients were escalated from ACEI/ARB to ARNI and the proportion of patients receiving ≥ 50% MTD ARNI increased from 15 to 55% (P = 0.089) with the mean percentage MTD of ARNI increased from 20.6 ± 30.9 to 53.1 ± 39.5% (P = 0.006*) (Figures 2 and 3A).
Figure 2.
Utilization of guideline-directed heart failure medications. (A) Proportion of patients on heart failure medications, (B) proportion of patients on ≥50% maximal target dose of heart failure medications, and (C) mean percentage heart failure medications, at baseline and the end of the study period. *P < 0.05. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor and neprilysin inhibitor; HF, heart failure; MRA, mineralocorticoid receptor antagonist; MTD, maximal target dose; SGLT, sodium glucose cotransporter.
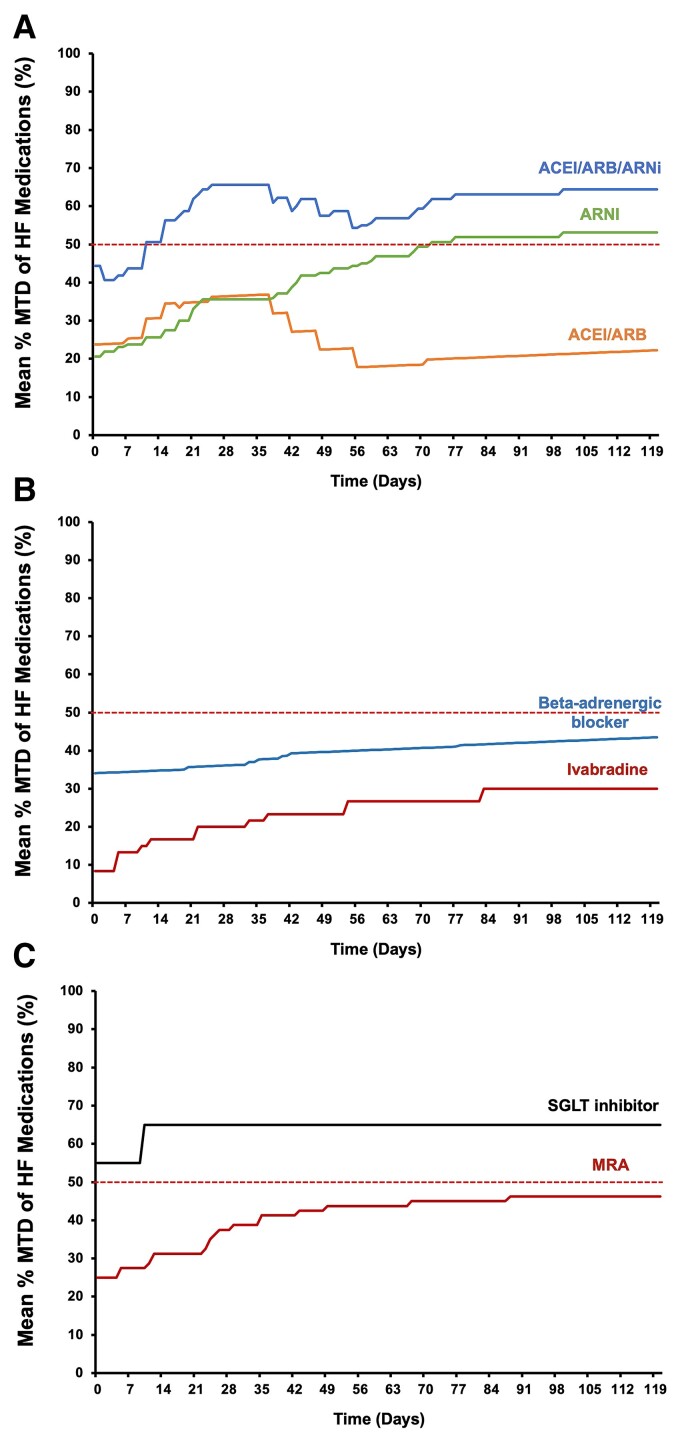
Figure 3.
Change in the mean percentage maximal target dose of heart failure medications over the study period. (A) Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor and neprilysin inhibitor, angiotensin receptor and neprilysin inhibitor, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, (B) beta-adrenergic blocker and ivabradine, and (C) mineralocorticoid receptor antagonist and sodium glucose cotransporter inhibitor. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor and neprilysin inhibitor; HF, heart failure; MRA, mineralocorticoid receptor antagonist; MTD, maximal target dose; SGLT, sodium glucose cotransporter.
Beta-adrenergic blocker and ivabradine
Compared with baseline, there was no statistically significant difference in the proportion of patients receiving ≥ 50% MTD of beta-adrenergic blocker (40 vs. 35%, P = 0.744), or the mean percentage MTD of beta-adrenergic blocker (34.1 ± 29.6 vs. 37.5 ± 28.4%, P = 0.710). On the other hand, the proportion of patients receiving ivabradine increased from 20 to 40% (P = 0.168) and the proportion of patients receiving ≥ 50% MTD of ivabradine from 5 to 30% (P = 0.091) with the mean percentage MTD of ivabradine from 8.3 ± 4.1 to 30.0 ± 41.7% (P = 0.04*) (Figures 2 and 3B). The escalation of ivabradine was only performed if there were instances of blood pressure below the predefined threshold of systolic blood pressure ≤ 100 mmHg (see Supplementary material online, Appendix and Figure S1). As a result of low blood pressure, ivabradine was escalated instead of beta-adrenergic blocker in a subgroup of the recruited patients. The inability to escalate beta-adrenergic blockers to the maximal target dose for HFrEF was similarly observed in certain contemporary multi-centre registries.34
Mineralocorticoid receptor antagonist and sodium glucose cotransporter inhibitor
The proportion of patients receiving MRA increased from 70% at baseline to 90% at the end of the study (P = 0.235). The proportion of patients receiving ≥ 50% maximal targeted dose of MRA increased from 25 to 65% (P = 0.011*) and the mean percentage MTD of MRA increased from 25.0 ± 19.9 to 46.2 ± 28.8% (P = 0.009*) (Figures 2 and 3C). On the other hand, there was no statistically significant change in the utilization of SGLT inhibitor over the study period.
NYHA functional class and serum NT-proBNP concentration
At Week 17, 70% patients had improved NYHA function class, 25% remained unchanged, and 5% worsened. Compared with baseline, the proportion of patients with NYHA function Class I increased from 15 to 65% at the end of the study (P = 0.003*) (Figure 4A). As depicted in Figure 4B, all except one patient (95%) had reduction in serum NT-proBNP concentration at the end of the study compared with baseline, and the mean percentage reduction in serum NT-proBNP concentration was 26.7%. Thirteen patients (65%) had serum NT-proBNP concentration reduced ≥25% from baseline.
Figure 4.
(A) New York Heart Association Function class at baseline and the end of study, and (B) percentage change in serum NT-proBNP concentration and increase in the percentage MTD of HF medications of individual patients from baseline to the end of study. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor and neprilysin inhibitor; HF, heart failure; MRA, mineralocorticoid receptor antagonist; MTD, maximal target dose; SGLT, sodium glucose cotransporter.
Haemodynamic changes, kidney function, and adverse events
Compared with baseline, there were no statistically significant differences in systolic [122.0 (IQR: 108.0, 130.5) vs. 123.0 (IQR: 102.5, 130.0) mmHg, P = 0.38] and diastolic blood pressure [77.0 (IQR: 70.0, 81.0) vs. 72.0 (IQR: 59.5, 81.5) mmHg, P = 0.23], and the resting heart rate [63.0 (IQR: 58.5, 69.0) vs. 63.0 (IQR: 58.0, 71.5) b.p.m., P = 0.44] at the end of study measured in clinic (see Supplementary material online, Figure S2). Likewise, serum creatinine concentration did not change significantly from baseline towards the end of the study. There was no symptomatic hypotension, worsening of renal function, or worsening of HF symptoms necessitating unscheduled medical visit during the study period.
Six-minute walk test
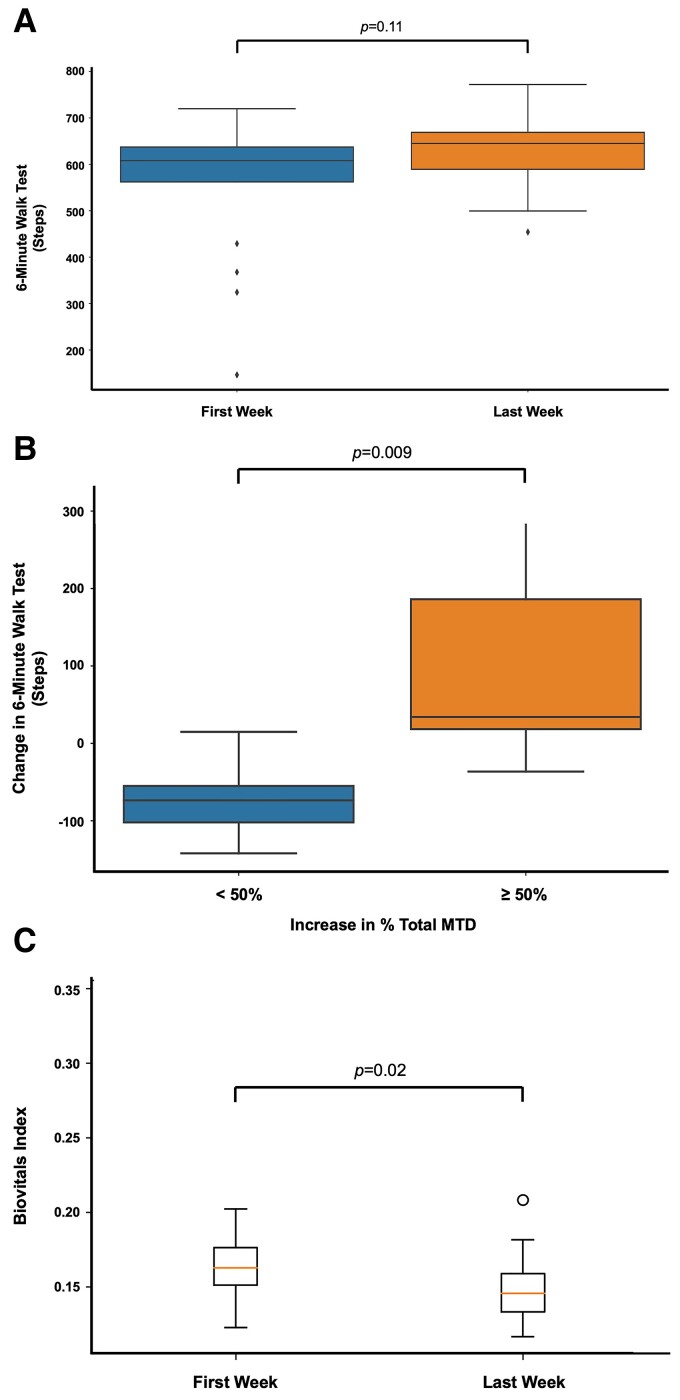
The 6 min walk test has been commonly used to assess functional capacities of HF patients.35 Using wearable armband monitors, the number of steps taken by patients within any 6 min periods could be measured. As the wearable armband monitors did not have Global Positioning System (GPS) modules for tracking distance, the maximum number of steps taken within 6 min periods was used as proxies for 6 min walk test. It was previously shown that the maximum daily total steps count highly correlated with NYHA functional status.36 There was no statistically significant difference in 6 min walk test at the end of the study comparing to baseline (P = 0.11) (Figure 5A). Nonetheless, when stratified by change in % total MTD, patients with at least 50% increase in % total MTD showed significantly larger improvements in 6 min walk test in the last week of the study when compared with the first week of the study (P = 0.009*) (Figure 5B).
Figure 5.
(A) The maximum number of steps taken within 6 min periods were used as proxies for 6 min walk test. There was no statistically significant difference in 6 min walk test in the first week of study (P = 0.1). (B) When stratified by change in % total maximal target dose, patients with at least 50% increase in % total maximal target dose showed significantly larger improvements in 6 min walk test in the last week of study when comparing with the first week of study (P = 0.009*). (C) Overall patients’ daily mean Biovitals Index was significantly lower in the last week of study when compared with the first week of study (P = 0.02*), indicating more stable physiological parameters and fewer symptoms. MTD, maximal target dose.
Biovitals Index for heart failure patient monitoring
The Biovitals Index was a US Food and Drug Administration 510(k) cleared machine learning-derived health index that reflects the derivatives in physiology parameters or changes of symptoms. The higher Biovitals Index reflected larger deviations of physiology parameters from baseline or new symptoms, potentially indicating the worsening of health status29,33 (see Supplementary material online, Appendix). The overall patients’ daily mean Biovitals Index was significantly lower in the last week of the study when compared with the first week of the study (P = 0.02*), indicating more stable physiological parameters and fewer symptoms (Figure 5C).
Discussion
Using a single-arm, open-label approach, we evaluated the safety and feasibility home-based HFrEF medication escalation using the wearable armband monitor and remote monitoring system. Following 17 weeks of home-based remote HF management, the proportion of patients receiving ≥50% MTD of ARNI increased from 15 to 40% (P = 0.077*) with % MTD from 20.6 ± 30.9 to 53.1 ± 39.5% (P = 0.006*). Likewise, the proportion of patients receiving ≥50% MTD of MRA increased from 25 to 65% (P = 0.011*) and the % MTD increased from 25.0 ± 19.9 to 46.2 ± 28.8% (P = 0.009*). The proportion of patients receiving ivabradine increased from 5 to 30% (P = 0.091) with % MTD from 8.3 ± 4.1 to 30.0 ± 41.7% (P = 0.04*). However, there was no statistically significant change in the dosage of ACEI/ARB, beta-adrenergic blockers, and SGLT inhibitors. The proportion of patients in NYHA functional Class I increased from 15 to 65% (P = 0.003*). The majority of patients (95%) had reduced serum NT-proBNP concentration with a mean percentage reduction of 26.7 ± 19.7%. There were no unscheduled medical visits, or worsening of renal function during the drug escalation period.
Heart failure is a public health challenge affecting 38 million people worldwide with increasing prevalence.37,38 Remote monitoring has been explored as a means to reduce HF-associated mortality, hospitalization, and healthcare burden. The main objective of most of these remote monitoring systems is to facilitate fluid status management, thereby reducing the risk of HF decompensation. The implantation pulmonary artery pressure sensor has been utilized to remotely monitor fluid status and guide heart failure medication adjustment. In the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in the NYHA Class III Heart Failure Patients (CHAMPION) trial, the implanted pressure sensor was shown to reduce HF-related hospitalization by 37% comparing to control.39,40 Alternatively, reporting of blood pressure, pulse, body weight, and blood oximetry through the Internet using smartphones, tablet computers, and desktop computers has been used to guide HF management with some randomized controlled trials demonstrating the reduction in the risk of HF hospitalization.41–44 In addition to fluid status management, DAVID-HF took a step further by empowering remote escalation of guideline-directed medical therapy for HFrEF. Despite the overwhelming evidence that the usage of guideline-directed medical therapy results in the lower risk of HF hospitalization and mortality, there is underutilization because of various barriers such as infrequent clinic visits and lack of self-monitored physiological parameters by patients. The remote drug escalation approach adopted in DAVID-HF potentially benefit patients and healthcare providers in a number of ways: first, HFrEF medication titration in DAVID-HF was guided by automatic analysis of the collected physiological parameters and predefined drug escalation algorithms. As a result, the amount of time and effort clinical staff need to spend on filtering medical record to identify patients requiring drug titration is reduced. Second, from the perspective of hospital administers, remote titration of medications reduced the usage of resources by reducing the number of clinic sessions required. Third, it reduces the number of commutes patients have to make between home and hospital during the drug escalation period. Fourth, continuous passive measurement by wearable armband ensures clinicians to have access to physiological parameters without requiring patient to perform manual measurement and documentation.
Another important feature that distinguishes DAVID-HF from other HF remote monitoring trials is the inclusion of wearable armband monitor for monitoring a wide-range of physiological parameters. Parameters such as photoplethysmogram-based heart rate and oxygen saturation could be continuously measured in a 24/7 manner. Previous clinical trials revealed that only 62% of the required physiological parameters measurement were completed43 and the expected compliance in real-life setting is similarly suboptimal. Wearable armband-based data collection allows more consistent and reliable data collection. Moreover, there are a wide array of parameters that are not otherwise available in previous HF remote monitoring trials. As demonstrated by us and others,45,46 assessment of 6 min walk test could be performed by measuring steps count using an accelerometer in wearable armband monitors to serve as objective indicators of exerciser tolerance. Furthermore, the wearable armband monitor was capable of tracking blood pulse wave, respiration rate, skin temperature, and electrodermal activity. The multi-dimensional data streams are suitable for developing machine learning-based prediction and classification models. In a previously published Multisensor Non-invasive Remote Monitoring for Prediction of Heart Failure Exacerbation (LINK-HF) study, multivariate physiological parameters acquired using wearable sensors allowed prediction of HF hospitalization with 76–88% sensitivity and 85% specificity. 47 In the study, a multivariate change index was devised to assess for change in physiological parameters comparing with the baseline state. Similarly, a machine learning-derived health index that reflects the derivatives in physiology parameters or changes of symptoms was used in the currently reported study.33 We previously demonstrated that such machine learning-derived health index was superior to National Early Warning Score 2 (NEWS2) in predicting clinical worsening events (sensitivity 94.1% and specificity 88.9%) and prolonged hospitalization (sensitivity 66.7% and specificity 72.7%) among Coronavirus Disease 2019 patients.29 The accuracy in predicting HF hospitalization will be further explored in the randomized controlled phase of the DAVID-HF trial.
Limitations
This study had several limitations. First, the generalizability of results from this pilot phase study was limited by basing on a single centre, having a small sample size, and not having random allocation of treatment groups. Nevertheless, it demonstrated that remote drug escalation is safe among HFrEF patients and a randomization phase will follow to provide further insight regarding its efficacy. Second, the remote monitoring platform for HFrEF management used in this study is not yet available for use or validation by other clinicians. Third, as this study was performed before the release of the 2021 ESC guideline for HFrEF, some of the latest societal recommendation were not reflected in the original study protocol. Study protocol will be updated accordingly before initiating the randomization phase of the study. Fourth, as this study had an open-label design, the Hawthorne effect and other biases could not be entirely eliminated. Fifth, patients who could not independently operate the wearable armband and smartphone application, or lack Internet access, could not readily utilize the remote monitoring system without assistance from caregivers and derive benefit from it. Sixth, data regarding disagreements between cardiologists and the algorithm-generated drug titration recommendations would provide useful insights. Nonetheless, the database system used in this pilot phase study was not designed to document the occurrence and reasons for such discrepancies. The updated database system will be utilized in the subsequent randomization phase to record the relevant information.
Conclusion
Home-based, remote HF monitoring using a wearable armband monitor and dedicated smartphone application appeared feasible for HF medication escalation in chronic HFrEF.
Supplementary Material
Acknowledgements
Everion armband monitors were donated by Biofourmis Singapore Pte Ltd, Singapore.
Contributor Information
Chun Ka Wong, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Ka Chun Un, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Mi Zhou, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Yangyang Cheng, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Yuk Ming Lau, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Puigi Catherine Shea, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Hin Wai Lui, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Ming Liang Zuo, Department of Echocardiography & Non-invasive Cardiology Laboratory, Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, Chengdu, China.
Li Xue Yin, Department of Echocardiography & Non-invasive Cardiology Laboratory, Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, Chengdu, China.
Esther W Chan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Ian C K Wong, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Simon Wai Ching Sin, Respiratory Division, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Pauline Pui Ning Yeung, Respiratory Division, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Hao Chen, Biofourmis Singapore Pte Ltd, Singapore, Singapore.
Sandi Wibowo, Biofourmis Singapore Pte Ltd, Singapore, Singapore.
Tong Li Nikki Wei, Biofourmis Singapore Pte Ltd, Singapore, Singapore.
Sze Ming Lee, Harmony Medical Inc., Hong Kong SAR, China.
Augustine Chow, Harmony Medical Inc., Hong Kong SAR, China.
Raymond Cheuk Fung Tong, Harmony Medical Inc., Hong Kong SAR, China.
Jojo Hai, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Frankie Chor Cheung Tam, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Chung Wah Siu, Division of Cardiology, Department of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Authors’ contributions
C.-K.W., K.-C.U., J.H., F.C.-C.T., and C.-W.S. contributed to the conception and design of the study. C.-K.W., K.-C.U., M.Z., Y.C., Y.-M.L., P.C.S., H.-W.L., M.-L.Z., L.-X.Y., E.W.C., I.C.K.W., S.W.-C.S., P.P.-N.Y., H.C., S.W., T.L.N.W., S.-M.L., A.C., R.C.-F.T., J.H., F.C.-C.T. and C.-W.S. contributed to the acquisition of data. Data analysis and interpretation will be conducted by C.-K.W. and C.-W.S. C.-K.W. and C.-W.S. wrote the first draft of the protocol. K.-C.U., M.Z., Y.C., H.C., S.W., T.L.N.W., J.H., and F.C.-C.T. revised the manuscript critically for important intellectual content. All authors have read and approved the final version of the manuscript to be published.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Funding
None.
Conflict of interest: H.C., S.W., and T.L.N.W. are employed by Biofourmis Singapore Pte Ltd, which donated Everion wearable biosensors. S.-M.L., A.C., and R.C.-F.T. are employed by Harmony Medical Inc.
Data availability
Deidentified data will be available from the corresponding author on reasonable request after obtaining approval by the investigators and signing data access agreement, from date of publication to 1 year after publication.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, Task Force for the Diagnosis, Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 2. Reyes EB, Ha JW, Firdaus I, Ghazi AM, Phrommintikul A, Sim D, Vu QN, Siu CW, Yin WH, Cowie MR. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol 2016;223:163–167. [DOI] [PubMed] [Google Scholar]
- 3. The SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 4. The CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 5. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 6. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P, MERIT-HF Study Group . Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). JAMA 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 7. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 8. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 9. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 10. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 14. Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Hochadel M, Komajda M, Lopez-Sendon JL, Ponikowski P, Tavazzi L. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail 2010;12:239–248. [DOI] [PubMed] [Google Scholar]
- 15. Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H, JCARE-CARD Investigators . Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009;73:1893–1900. [DOI] [PubMed] [Google Scholar]
- 16. Youn YJ, Yoo BS, Lee JW, Kim JY, Han SW, Jeon ES, Cho MC, Kim JJ, Kang SM, Chae SC, Oh BH, Choi DJ, Lee MM, Ryu KH, Kor HFR. Treatment performance measures affect clinical outcomes in patients with acute systolic heart failure: report from the Korean Heart Failure Registry. Circ J 2012;76:1151–1158. [DOI] [PubMed] [Google Scholar]
- 17. Hai JJ, Chan PH, Huang D, Ho MH, Ho CW, Cheung E, Lau CP, Tse HF, Siu CW. Clinical characteristics, management, and outcomes of hospitalized heart failure in a Chinese population-the Hong Kong heart failure registry. J Card Fail 2016;22:600–608. [DOI] [PubMed] [Google Scholar]
- 18. Peri-Okonny PA, Mi X, Khariton Y, Patel KK, Thomas L, Fonarow GC, Sharma PP, Duffy CI, Albert NM, Butler J, Hernandez AF, McCague K, Williams FB, DeVore AD, Patterson JH, Spertus JA. Target doses of heart failure medical therapy and blood pressure: insights from the CHAMP-HF registry. JACC Heart Fail 2019;7:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L, QUALIFY Investigators . Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail 2016;18:514–522. [DOI] [PubMed] [Google Scholar]
- 20. Fiuzat M, Wojdyla D, Kitzman D, Fleg J, Keteyian SJ, Kraus WE, Pina IL, Whellan D, O'Connor CM. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol 2012;60:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Ryden L, Thygesen K, Uretsky BF, ATLAS Study Group . Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation 1999;100:2312–2318. [DOI] [PubMed] [Google Scholar]
- 22. Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N, MOCHA Investigators . Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 1996;94:2807–2816. [DOI] [PubMed] [Google Scholar]
- 23. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 24. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members and Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 25. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swedberg K, Wolf A, Ekman I. Telemonitoring in patients with heart failure. N Engl J Med 2011;364:1078. author reply 1079–1080. [DOI] [PubMed] [Google Scholar]
- 27. Inglis SC, Clark RA, Cleland JG, Cochrane Systematic Review T . Telemonitoring in patients with heart failure. N Engl J Med 2011;364:1078–1080; author reply 1079–1080. [DOI] [PubMed] [Google Scholar]
- 28. Everett W, Kvedar JC, Nesbitt TS. Telemonitoring in patients with heart failure. N Engl J Med 2011;364:1079; author reply 1079–1080. [DOI] [PubMed] [Google Scholar]
- 29. Un KC, Wong CK, Lau YM, Lee JC, Tam FC, Lai WH, Lau YM, Chen H, Wibowo S, Zhang X, Yan M, Wu E, Chan SC, Lee SM, Chow A, Tong RC, Majmudar MD, Rajput KS, Hung IF, Siu CW. Observational study on wearable biosensors and machine learning-based remote monitoring of COVID-19 patients. Sci Rep 2021;11:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong CK, Ho DTY, Tam AR, Zhou M, Lau YM, Tang MOY, Tong RCF, Rajput KS, Chen G, Chan SC, Siu CW, Hung IFN. Artificial intelligence mobile health platform for early detection of COVID-19 in quarantine subjects using a wearable biosensor: protocol for a randomised controlled trial. BMJ Open 2020;10:e038555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poh MZ, Poh YC, Chan PH, Wong CK, Pun L, Leung WW, Wong YF, Wong MM, Chu DW, Siu CW. Diagnostic assessment of a deep learning system for detecting atrial fibrillation in pulse waveforms. Heart 2018;104:1921–1928. [DOI] [PubMed] [Google Scholar]
- 32. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members and Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016. [DOI] [PubMed] [Google Scholar]
- 33. Chen J, Yan M, Chin Howe RL, Tong NW, Chee CS, Niu W, Rajput KS, Majmudar M, Chen G. Biovitals: a personalized multivariate physiology analytics using continuous mobile biosensors. Annu Int Conf IEEE Eng Med Biol Soc 2019;2019:3243–3248. [DOI] [PubMed] [Google Scholar]
- 34. Bots SH, Onland-Moret NC, Tulevski II, van der Harst P, Cramer MJM, Asselbergs FW, Somsen GA, den Ruijter HM. Heart failure medication dosage and survival in women and men seen at outpatient clinics. Heart 2021;107:1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, Collins E, Fletcher G, American Heart Association Committee on Exercise Rehabiliation, Prevention of the Council on Clinical Cardiology and American Heart Association Council on Cardiovascular Nursing . Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007;116:329–343. [DOI] [PubMed] [Google Scholar]
- 36. Baril JF, Bromberg S, Moayedi Y, Taati B, Manlhiot C, Ross HJ, Cafazzo J. Use of free-living step count monitoring for heart failure functional classification: validation study. JMIR Cardio 2019;3:e12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 38. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 40. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 2016;387:453–461. [DOI] [PubMed] [Google Scholar]
- 41. Frederix I, Vanderlinden L, Verboven AS, Welten M, Wouters D, De Keulenaer G, Ector B, Elegeert I, Troisfontaines P, Weytjens C, Mullens W, Dendale P. Long-term impact of a six-month telemedical care programme on mortality, heart failure readmissions and healthcare costs in patients with chronic heart failure. J Telemed Telecare 2019;25:286–293. [DOI] [PubMed] [Google Scholar]
- 42. Comin-Colet J, Enjuanes C, Verdu-Rotellar JM, Linas A, Ruiz-Rodriguez P, Gonzalez-Robledo G, Farre N, Moliner-Borja P, Ruiz-Bustillo S, Bruguera J. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: results of a randomized controlled trial. J Telemed Telecare 2016;22:282–295. [DOI] [PubMed] [Google Scholar]
- 43. Pedone C, Rossi FF, Cecere A, Costanzo L, Antonelli Incalzi R. Efficacy of a physician-led multiparametric telemonitoring system in very old adults with heart failure. J Am Geriatr Soc 2015;63:1175–1180. [DOI] [PubMed] [Google Scholar]
- 44. Kashem A, Droogan MT, Santamore WP, Wald JW, Bove AA. Managing heart failure care using an internet-based telemedicine system. J Card Fail 2008;14:121–126. [DOI] [PubMed] [Google Scholar]
- 45. Prescher S, Schoebel C, Koehler K, Deckwart O, Wellge B, Honold M, Hartmann O, Winkler S, Koehler F. Prognostic value of serial six-minute walk tests using tele-accelerometry in patients with chronic heart failure: a pre-specified sub-study of the TIM-HF-Trial. Eur J Prev Cardiol 2016;23:21–26. [DOI] [PubMed] [Google Scholar]
- 46. Jehn M, Prescher S, Koehler K, von Haehling S, Winkler S, Deckwart O, Honold M, Sechtem U, Baumann G, Halle M, Anker SD, Koehler F. Tele-accelerometry as a novel technique for assessing functional status in patients with heart failure: feasibility, reliability and patient safety. Int J Cardiol 2013;168:4723–4728. [DOI] [PubMed] [Google Scholar]
- 47. Stehlik J, Schmalfuss C, Bozkurt B, Nativi-Nicolau J, Wohlfahrt P, Wegerich S, Rose K, Ray R, Schofield R, Deswal A, Sekaric J, Anand S, Richards D, Hanson H, Pipke M, Pham M. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ Heart Fail 2020;13:e006513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data will be available from the corresponding author on reasonable request after obtaining approval by the investigators and signing data access agreement, from date of publication to 1 year after publication.