Abstract
Aims
Increased complexity in cardiac surgery over the last decades necessitates more precise preoperative planning to minimize operating time, to limit the risk of complications during surgery and to aim for the best possible patient outcome. Novel, more realistic, and more immersive techniques, such as three-dimensional (3D) virtual reality (VR) could potentially contribute to the preoperative planning phase. This study shows our initial experience on the implementation of immersive VR technology as a complementary research-based imaging tool for preoperative planning in cardiothoracic surgery. In addition, essentials to set up and implement a VR platform are described.
Methods
Six patients who underwent cardiac surgery at the Erasmus Medical Center, Rotterdam, The Netherlands, between March 2020 and August 2020, were included, based on request by the surgeon and availability of computed tomography images. After 3D VR rendering and 3D segmentation of specific structures, the reconstruction was analysed via a head mount display. All participating surgeons (n = 5) filled out a questionnaire to evaluate the use of VR as preoperative planning tool for surgery.
Conclusion
Our study demonstrates that immersive 3D VR visualization of anatomy might be beneficial as a supplementary preoperative planning tool for cardiothoracic surgery, and further research on this topic may be considered to implement this innovative tool in daily clinical practice.
Lay summary
Over the past decades, surgery on the heart and vessels is becoming more and more complex, necessitating more precise and accurate preoperative planning. Nowadays, operative planning is feasible on flat, two-dimensional computer screens, however, requiring a lot of spatial and three-dimensional (3D) thinking of the surgeon. Since immersive 3D virtual reality (VR) is an upcoming imaging technique with promising results in other fields of surgery, we aimed in this study to explore the additional value of this technique in heart surgery. Our surgeons planned six different heart operations by visualizing computed tomography scans with a dedicated VR headset, enabling them to visualize the patient’s anatomy in an immersive and 3D environment. The outcomes of this preliminary study are positive, with a much more reality-like simulation for the surgeon. In such, VR could potentially be beneficial as a preoperative planning tool for complex heart surgery.
Keywords: Virtual reality, Preoperative planning, Cardiothoracic surgery, Minimally invasive cardiac surgery, Innovation
Introduction
Over time, the field of cardiothoracic surgery has gradually evolved into the development of better and more advanced surgical strategies, leading to improved patient outcomes and has enabled surgeons to perform more complex surgery. Also, the introduction of minimally invasive cardiac surgical techniques has increased the possibility to adopt less invasive strategies to perform cardiac surgery, while reducing the postoperative recovery time, improving perioperative outcomes and the quality of life after cardiac surgery.1,2
Besides changes in the surgical approach, progress in the development of novel technology has resulted in the clinical implementation of innovative imaging techniques to enhance preoperative planning for both complex and minimally invasive cardiac surgery (MICS). Preoperative awareness of (ab)normal anatomy is essential in both minimally invasive and complex (e.g. redo operations) surgery, since direct assessment and visual analysis of anatomical structures is not always possible. Even though conventional imaging strategies such as computed tomography (CT) and echocardiography are essential tools for preoperative patient selection and (peri)operative planning, recent articles on various novel supplementary (imaging) modalities [e.g. three-dimensional (3D) CT, 3D printing] have demonstrated to aid cardiothoracic surgeons in both preoperative surgical planning and intraoperative guidance for minimally invasive and complex cardiovascular interventions and surgery.3–5
In this regard, recent technological advances in extended reality (XR) [e.g. virtual and augmented reality (AR)] have enabled the application of more state-of-the-art XR equipment to render 3D volumetric, CT- or ultrasound-derived images of anatomy to support patient selection, preoperative procedural planning, and intraoperative guidance.6–8 By using head mounted displays (HMD’s), dedicated medical software, and powerful computers, the user is able to review images and environments in a totally virtual reality (VR). On the other hand, in AR, the user is able to project virtual 3D images onto the real world.
Over the last years, the technology of XR-guided visualization of medical images is gradually gaining more attention in a broad number of surgical and medical specialties, including neurosurgery, paediatric surgery, and urology.9–11 These techniques come with the added advantage that they enable the user to engage with the anatomy of the patient in a fully immersive and reality-like way. However, in conventional CT-guided planning, the surgeons need to create a mental 3D reconstruction of the images, which could provide inaccurate information. Consequently, XR potentially augments the understanding of spatial anatomical relationships and therefore contributes to a better optimization of surgical strategy.
Even though the broad implementation of VR in cardiothoracic surgery is actively being explored,6,12,13 literature is scarce on the feasibility, efficacy, and application of VR in minimally invasive and complex cardiac surgery. This preliminary study demonstrates the results of our initial experience on the implementation of an immersive 3D VR technology as a supplementary research-based imaging tool for preoperative planning of complex adult and minimally invasive cardiothoracic surgery. The performance of preoperative VR-based surgical planning depends mainly on the development and maintenance of a dedicated ‘3D-VR surgery team’. Therefore, based on our experience, we will also highlight and discuss the necessary and essential elements of such a team and setup to provide directions for clinical implementation.
Methods
Patient selection
A total of six patients that underwent (complex) MICS or complex conventional cardiac surgery at the Erasmus Medical Center, between March 2020 and August 2020 were included. Patients were included when a reconstruction of CT images in VR was requested by the surgeon for preoperative planning. The patients were selected by surgeons based on the complexity of surgery (e.g. due to expected challenges with surgical access, cannulation strategy, etc.). Patients were eligible for inclusion when conventional CT scans were available. The study passed the local medical ethical committee (MEC-2020-0702). Written informed consent was obtained from all participants undergoing surgery before inclusion.
Computed tomography
To create volumetric 3D VR rendering of the CT scans, preoperative scans were essential. Besides a maximum of 1000 CT images, there was no specific technical requirement for the CT scan, however, appropriate quality for conventional two-dimensional (2D) review was necessary. Three patients underwent preoperative electrocardiography-gated multidetector and contrast-enhanced CT scans of the thorax. Two patients underwent a (contrast-enhanced) CT scan of the thorax and abdomen (including peripheral arteries) and one patient underwent contrast-enhanced CT of the thorax. CT scans were made according to local protocol. When necessary, CT scans were acquired from referring hospitals and loaded into our patient archiving and communication system (PACS). Initially, all surgeons reviewed the scans of the patients by conventionally viewing the 2D CT scans. After that, a 3D VR reconstruction was requested by the surgeon.
3D image segmentation
Overall, 3D image segmentation was not essential for surgical planning due to the standard features or our VR equipment. In particular cases, 3D segmentation was performed to highlight anatomic structures (in colour) within the VR environment. For 3D segmentation, the digital imaging and communications in medicine (DICOM) files of CT scans were extracted from the PACS and, after anonymization, loaded into ITK-SNAP14 software by a surgical resident physician. 3D segmentations of specific structures were manually generated (based on ITK-SNAP tutorials) and exported as a NIfTI (Neuroimaging informatics Technology Initiative (nii.gz.)) file (Figure 1). The segmentation of a structure took about 5 min, depending on the number and accuracy of segmentations. Afterwards, the segmentation was checked by the surgeon who reviewed the VR reconstruction.
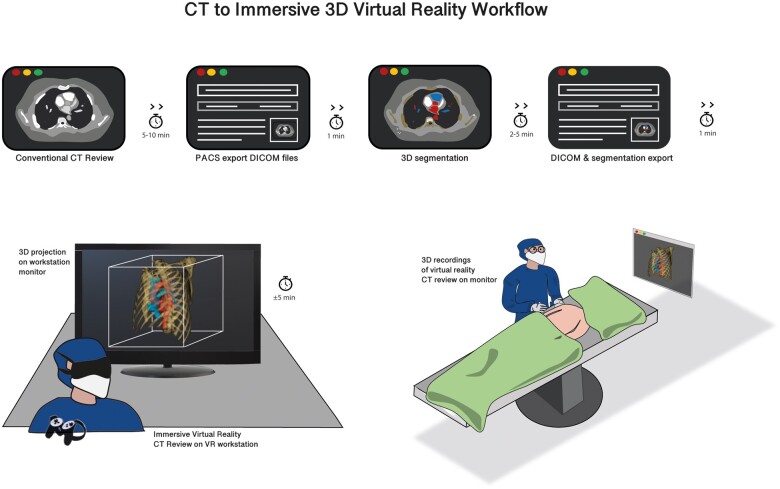
Figure 1.
Three-dimensional immersive virtual reality workflow. After evaluation of the computed tomography (CT) scan in two-dimensional (2D), the digital imaging and communications in medicine (DICOM) files of the CT scan are exported from the patient archiving and communication system (PACS) and used for 3D segmentation in dedicated software. Hereafter, the DICOM files are rendered together with optional segmentation files into a virtual reality workstation. The virtual reality (VR) images can be evaluated in immersive 3D VR, on a computer screen, and on a monitor in the operating room.
Immersive 3D virtual reality rendering, preoperative planning, and intraoperative guidance
Anonymized DICOM files, together with 3D segmentation files, were loaded into our CardioVR surgical planning tool. CardioVR was developed in collaboration with MedicalVR (Amsterdam, The Netherlands). CardioVR software enables immediate automatic CT to 3D VR rendering and also provides the user additional editing tools to view the conventional 2D-CT scan images, change visual scan settings (e.g. opacity), highlight structures by brushing (colouring) and erasing parts of the scan, and add/remove/highlight the colour of additional 3D segmentations. All scans can be accurately reviewed with an HMD (Oculus Rift S, Oculus VR, Irvine, CA, USA) and controllers while 3D projections of the VR view are provided on a computer screen (Figure 1, Supplementary material online, Video S1). After initial assessment of the scans by a resident, the surgeon reviewed the scans in immersive VR. The surgeon analysed the VR reconstruction one day before or on the same day of the procedure (when the procedure was planned in the afternoon). During review of the CT scan by the surgeon, a recording of the analysis was performed and saved to provide (optional) visualization and display of the recordings during surgery, on a monitor (Figure 1).
Questionnaire design and data analysis
All first operators reviewed the CT scans (first in 2D-CT and then in immersive 3D-VR) of their patients individually. After surgery, a questionnaire (Supplementary material online, S1) was filled out by all participants (five surgeons) to evaluate user-friendliness, ease of learning, and attitude towards future use regarding VR as a supplementary preoperative planning tool.
Results
Patients and preoperative planning
A total of six patients with various indications for cardiac surgery were included as a case series to study and present the feasibility of reviewing CT scans in 3D VR for preoperative cardiac surgery planning (Table 1). All patients underwent successful cardiac surgery. No intraoperative complications occurred. Four out of six patients underwent MICS consisting of minimally invasive direct coronary artery bypass (MIDCAB) (n = 2), tricuspid valve repair (n = 1), and totally thoracoscopic ablation (n = 1). The remaining two cases were complex redo operations: left thoracotomy to remove a left ventricular assist device (LVAD) (n = 1) and redo aortic surgery through median sternotomy (n = 1) (Table 1). Three-dimensional VR rendering of all CT scans was performed successfully for preoperative surgical planning. All CT scans were rendered, post-processed, segmented in our VR workstations (if needed) and analysed by the surgeon within 15 min for each case. Immersive 3D review of all scans was performed either on Day 1 before surgery or on the day of surgery. In Table 2, a list of performed operations is presented including the specific surgeon’s requests and motivation for preoperative evaluation in VR. Next, a summary of all operations and highlights on the execution of preoperative VR review is provided.
Table 1.
Patient characteristics
| Surgery | Diagnosis | Sex | Age | Previous cardiac surgery | Surgical approach | Cannulation strategy | Preoperative contrast-enhanced CT scan |
|---|---|---|---|---|---|---|---|
| MICS: Thoracoscopic ablation and LAA occlusion for AF | Paroxysmal atrial fibrillation | Female | 71 | No | MICS | N/A | Yes |
| MICS: MIDCAB | 1 vessel coronary artery disease | Male | 18 | No | MICS | N/A | Yes |
| MICS: MIDCAB | 1 vessel coronary artery disease | Male | 45 | No | MICS | N/A | Yes |
| MICS: Tricuspid valve repair MICS | Severe tricuspid valve regurgitation | Male | 56 | Yes | MICS | Right femoral artery and vein + right jugular vein | Yes |
| Ascending aorta and partial arch replacement | Aortic aneurysm formation | Female | 78 | Yes | Sternotomy | Ascending aorta, right atrium | Yes |
| LVAD (Heartmate 3) extraction | Non compaction cardiomyopathy | Male | 27 | Yes | Thoracotomy | Femoral | No |
AF, atrial fibrillation; CT, computed tomography; LAA, left atrial appendage; LAD, left anterior descending artery; LVAD, left ventricular assist device; MICS, minimally invasive cardiac surgery; MIDCAB, minimally invasive direct coronary artery bypass. N/A, not applicable
Table 2.
List of surgeon’s specific preoperative questions and motivation for preoperative 3D immersive VR review of CT scans
| Operation | Surgeon’s specific questions |
|---|---|
| MIDCAB #1 |
|
| MIDCAB #2 |
|
| Tricuspid valve repair |
|
| Aortic surgery |
|
| LVAD extraction |
|
| Thoracoscopic ablation and left atrial appendage occlusion |
|
Cx, circumflex; LAA, left atrial appendage; LAD, left anterior descending; LM, left main; LVAD, left ventricular assist device; MIDCAB, minimally invasive direct coronary artery bypass; RIMA, right internal mammary artery.
MICS: thoracoscopic ablation and left atrial appendage exclusion
A patient with paroxysmal atrial fibrillation underwent a fully thoracoscopic ablation and left atrial appendage (LAA) exclusion. Segmentation of the left atrium and pulmonary vein ostia, the coronary arteries, and pulmonary artery in the VR environment was performed. Hereafter, the LAA base was measured in VR, which aided the surgeon with choosing a suitable clip size preoperatively (Figure 2A). The base was measured 28 mm (from different angles) in the VR environment and was measured roughly 30 mm during surgery, after which the surgeon decided to use the smallest Atriclip (Atricure, Mason, OH, USA) of 35 mm available (Figure 3A). Compared to conventional CT scans, LAA base sizing was performed more easily due to instant and reality-like 3D evaluation. In addition, the software enables to reduce opacity, which aids the surgeon to evaluate the LAA better as a 3D structure. The pulmonary veins on both sides were simulated via the same thoracoscopic view to determine the approach for the pulmonary vein isolation. Furthermore, the close spatial anatomy between the intended clip location and the left coronary artery was visualized, which increased the awareness of this close relationship to the surgeon.
Figure 2.
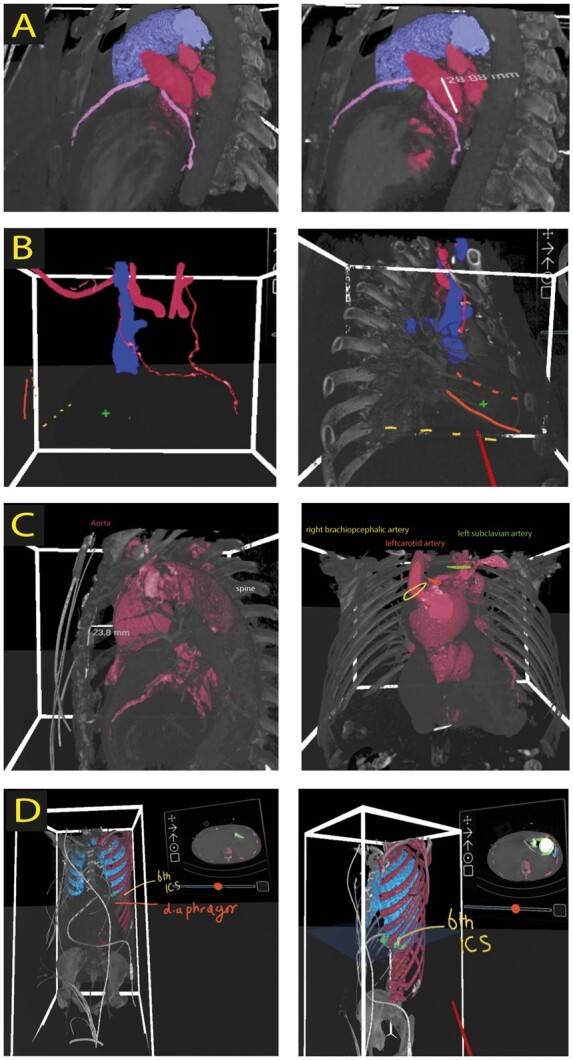
Screenshots of 3D virtual reality rendered images. (A) Planning of thoracoscopic ablation and left atrial appendage exclusion. Screenshots represent the anatomical orientation and relationship of coronary arteries (pink), pulmonary artery (purple), and left atrial appendage together with pulmonary veins (red). In addition, measurement of the base of left atrial appendage is depicted (right panel). (B) VR planning for redo tricuspid valve repair. Both left (LIMA) and right (RIMA) internal mammary arteries are marked red. 4th (dotted orange line) and 5th rib (orange line), right atrium (plus sign), and diaphragm level (yellow dotted line) is depicted to define surgical entry site. The relation between superior vena cava (blue) and RIMA (red) can be seen clearly in left and right panel. ICS, intercostal space. (C) Redo aortic surgery planning in virtual reality (VR) including measurements of the distance between sternum and aneurysm (left panel). Offspring of aortic arch vessels is depicted in the right panel. (D) Left ventricular assist device (green) extraction planning in VR. The 6th intercostal space (yellow), lungs (blue), and diaphragm are marked as a landmark for determining surgical access through thoracotomy.
Figure 3.
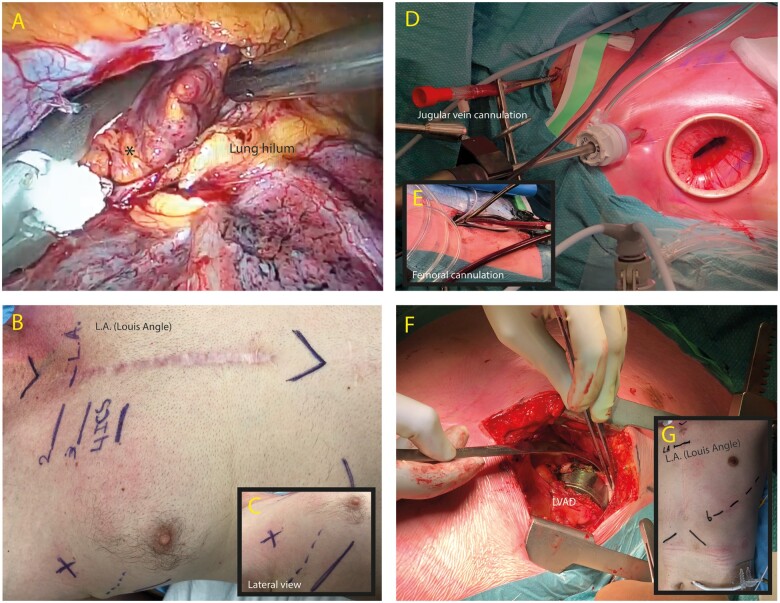
Perioperative images of virtual reality planned operations. (A) Application of left atrial appendage (*) exclusion device (Atriclip 35 mm). (B/C) Determination and intraoperative view of surgical site for mini thoracotomy in the 4th intercostal space (between dotted and solid line in the anterior axillary line). (D/E) Intraoperative images depicting double venous cannulation strategy for redo minimally invasive tricuspid valve repair. (F/G) Exposure of LVAD (left ventricular assist device) after thoracotomy through the 6th intercostal space as planned by virtual reality.
MICS: redo tricuspid valve repair
A 56-year-old patient was referred to our hospital for a redo tricuspid valve repair due to severe isolated tricuspid valve regurgitation, after total arterial [in situ left (LIMA) and in situ right (RIMA) internal mammary artery arteries] coronary artery revascularization 4 years earlier. The CT scan (thorax and peripheral arteries) of this patient was reviewed in VR preoperatively to choose cannulation strategy and optimal surgical entry location for a utility incision (Figures 2B and 3B and C). The course of the RIMA along the superior caval vein (SVC) was visualized by a post-processing brushing technique in VR. Given the close proximity of the RIMA and the SVC, safe snaring of the SVC could not be performed due to the risk of damaging the RIMA. Consequently, a double venous cannulation strategy through right internal jugular vein and right common femoral vein was performed (Figure 3D and E). This strategy was also chosen for a maximal venous drainage during the operation. Intraoperatively, with a modified cardiopulmonary bypass strategy (reduced venous drainage, reduced flow, and associated moderate hypothermia) a balance was found between preventing air entrapment (in the heart lung machine cannulas) and obscuring of the surgical field. The VR preoperative planning provided significant insights in anatomy and chosen strategy.
MICS: MIDCAB
Two patients were preoperatively planned for MIDCAB surgery with VR. We have recently published on this topic and the added value for preoperative planning.6 The course of the left anterior descending (LAD) coronary artery on the heart and the LIMA on the thoracic cavity wall were visualized in VR. Specifically, in one of the cases, this was important due to possible intramyocardial LAD course on coronary angiography. In addition, the location for the LIMA to LAD anastomosis was selected in VR, with the coronary angiography in mind. By combining the aforementioned information, the most optimal thoracoscopic entry points to harvest LIMA and the small thoracotomy incision location for bypass grafting were determined. Due to better preoperative planning in VR, the operation plan was modified and thoracoscopic port placement locations were changed according to the insights provided by VR-guided planning.
Redo ascending aorta and partial aortic arch replacement
A patient with a history of a Bentall procedure and aortic arch replacement after Stanford type A dissection was accepted for ascending aorta and partial aortic arch replacement due to aneurysm formation and degeneration of the arch prosthesis. Preoperative VR-guided planning for this reoperation showed the associated anatomy between the ascending aorta, aortic arch aneurysm, and the sternum and exact localization of the arch branches. The 3D VR reconstruction visualized the most optimal location for distal anastomosis, considering the diameter of the aorta and the branching of the carotid and left subclavian artery (Figure 2C).
Heartmate 3 extraction
In 2016, a 24-year-old male patient with dilated cardiomyopathy associated with a non-compaction cardiomyopathy and MYH7 gene mutation underwent LVAD [Heartmate 3™ (Abott, North Chicago, IL, USA)] implantation and a concomitant aortic valve replacement intended as a bridge to heart transplantation. In 2020, however, the patient could be weaned from his LVAD and explantation of the assist device was planned and prepared for in VR. After 3D segmentation of the Heartmate 3, lungs, and thoracic ribcage, VR rendering of the CT scan enabled immediate selection of the best suitable (6th) intercostal space for thoracotomy and subsequent removal of the LVAD (Figures 2D and 3F and G).
Evaluation of virtual reality guided cardiac surgery planning
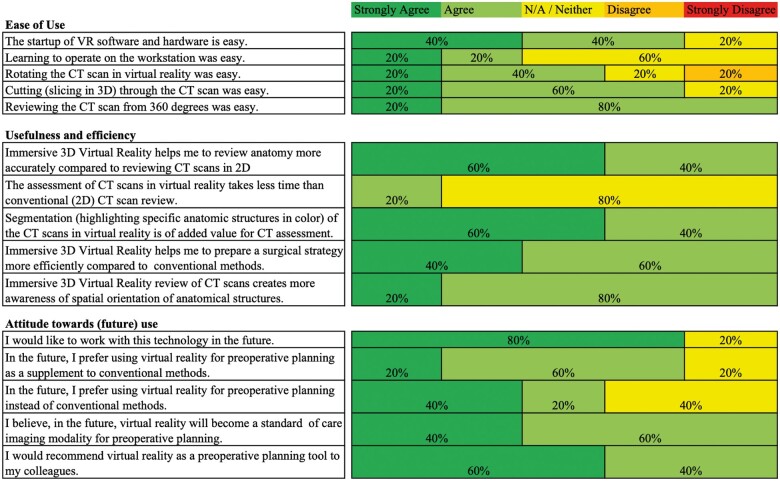
To determine the rating of the users (cardiothoracic surgeons) on the user interface, the hardware, and other VR experiences, questionnaires were filled out by all participants. All surgeons evaluated individually the user-friendliness, usefulness and efficiency, and attitude towards (future) use by rating a set of 15 questions on a Likert-scale (Figure 4, Supplementary material online, S1). Besides the five sub questions on each topic, the general user-friendliness, usefulness and efficiency, and attitude towards (future) use was rated through the questionnaire (Supplementary material online, S1, yellow marking). The participants rated the aforementioned three topics with an average of 4, 4.4, and 4, respectively. Due to small numbers of study participants, no statistical analysis was carried out.
Figure 4.
Questionnaire results on the usability of virtual reality as a preoperative planning tool for cardiothoracic surgery.
Discussion
In this preliminary study, we present our results and early experience on the implementation of an immersive VR platform for preoperative planning of adult cardiac surgery. This platform enables immersive 3D assessment of CT scans in a VR environment. In addition, the user is able to add segmentations of specific anatomic structures, which could potentially ease the visual analysis of CT scans for surgical planning. In this article, we present VR-guided preoperative planning of six patients undergoing cardiac surgery through either conventional (median sternotomy and thoracotomy) or minimally invasive surgical access. Overall, cardiothoracic surgeons were predominantly interested in determining the ideal location for surgical access and to review spatial anatomical orientation of structures in 3D VR. Incidentally, for redo operations, some surgeons requested VR rendering of CT scans to determine the ideal cannulation strategy for cardiopulmonary bypass and the distance between important structures (e.g. ascending aorta, right ventricle) and sternum. In one of the MIDCAB cases, the surgeon was specifically interested in VR reconstructions to establish whether the LAD coronary artery had an intramural course. Also in the MIDCAB cases, the initial surgical plan was modified to ensure adequate locations for utility port incisions. This underlines the need for such immersive technologies that enable in-depth, 3D, and spatial evaluation of anatomy. Additionally, it shows that the currently described method may be useful for complex surgical situations, especially for MICS.
The application of conventional 3D CT imaging has already demonstrated its added value in cardiothoracic surgical planning in different settings.4,15 However, a drawback of this method is that the assessment of these 3D reconstructions needs to be done on a 2D screen, which could partly eliminate the feeling of in-depth perception as well as the immersive involvement for the surgeon. In addition, manual 3D segmentation for 3D rendering of CT scans can be time consuming and might require assistance from radiologists or imaging technicians. Other novel technologies, such as 3D printing, have proven their effect on determination of surgical strategy,16 however, being time consuming (requires segmentation and printing), often costly, and consequently not always a cost-effective solution.17 Additionally, a solid 3D printed model is static and does not allow for dynamic modulation of anatomic structures possibly preventing a good view inside the model.
Over the past years, there has been a growing interest in the application of mixed reality technology (such as VR, AR, and mixed reality) for surgical planning in the field of cardiovascular surgery.6,18–20 Previously surgeons needed to convert 2D CT images mentally into 3D images in order to create a proper anatomical understanding and surgical planning. The VR platform presented in this study allowed for fast (<15 min) 3D visualization of CT scans. This resulted in higher usefulness and efficiency in the context of preoperative planning. Moreover, the results showed that the participants rated the hardware and software of the VR platform as an easy-to-use tool for reviewing CT scans.
So far, several groups have reported on the utilization of various XR modalities to create patient-specific 3D models of cardiac pathology for diagnostic and interventional application.7,8,20 However, most articles do not elaborate on the necessities and requirements to implement such an XR platform in clinical practice. In our experience, the performance and clinical implementation of a VR platform mainly depends on the availability of some essential elements such as: (i) the initiation and maintenance of a dedicated and multidisciplinary 3D-surgery team (consisting of at least a surgeon, a technician, a software engineer or information technology specialist, and when available a (cardiovascular) radiologist), (ii) a VR platform (e.g. VR headset or VR rooms), (iii) powerful computers with high performance graphic cards, (iv) an interface between the PACS and the electronic patient record system that enables fast extraction of DICOM files, and finally (v) 3D image segmentation software. In our centre, we have a dedicated 3D-VR surgery team that works with a VR software platform (MedicalVR, Amsterdam, The Netherlands) that enables fast conversion of DICOM files into 3D VR. We use an Oculus Rift S (Oculus VR, Irvine, CA, USA) VR headset together with a high-performance laptop and graphic card to visualize VR content effectively. Even though the essential hardware elements have an initial cost (approximately €4.000–€6.000), after purchasing the necessary components, the rendering and reconstruction of CT scans have (apart from manual labour) relatively low costs, depending on the software platforms that will be used.
Our study demonstrates that immersive 3D VR visualization of anatomy is a potentially beneficial supplementary tool providing a real-life format for preoperative planning tool for cardiothoracic surgery. However, we do recognize the limitations of this preliminary study. Firstly, a small sample size was used to demonstrate feasibility of our VR platform. In the near future, we aim to confirm the proof-of-concept with complex lung surgery and paediatric cardiac surgery as well. Secondly, we did not compare the application of 3D VR visualization to conventional 2D CT analysis for determining surgical strategy. Instead, we used VR as a supplementary method for planning. Another drawback of our VR platform is that currently, only visualization of CT scans is possible. In the future, VR platforms could have a broader impact by enabling immersive visualization of magnetic resonance imaging and ultrasound images. Furthermore, visualization of the 3D images via a monitor instead of an HMD in the operating room is a drawback. One of our future goals is to make AR and perioperative VR experience possible. Lastly, image segmentation still is a time-consuming process that mainly depends on medical and technical expertise of the user. Future development of more advanced and (semi-) automatic segmentation could hopefully standardize these essential steps with the ultimate goal to automatically visualize patient-specific anatomy in 3D and VR.
Conclusion
In the current feasibility study, we present our initial results on the implementation of VR technology to visualize patient-specific anatomy for preoperative planning in six adult patients undergoing conventional or MICS. This high-throughput translational platform provides the possibility for careful preoperative planning and allows the surgeon to create a mental map of the surgery at hand. Furthermore, the easy-to-use interface and low financial costs could prove this tool to be superior to traditional 3D CT. More (comparative) future research is needed to define the benefits of VR in preoperative cardiothoracic surgery planning. Additionally, the development of more advanced software to render other imaging modalities (such as echocardiography, magnetic resonance imaging, etc.) could be valuable.
Acknowledgements
We would like to thank Ms Jet Peek for providing assistance in preparation of the supplementary video.
Data availabilty
Data that support the findings of this study are available on request from the corresponding author.
Consent: Informed written consent was obtained from all patients.
Conflict of interest: none declared.
Supplementary Material
Author
Lead author biography: Amir H. Sadeghi is a surgical resident at the Department of Cardiothoracic Surgery, Erasmus Medical Center in Rotterdam (The Netherlands). Besides his clinical activities, Sadeghi is currently involved in various research projects related, but not limited, to preoperative planning in cardiothoracic surgery and extended reality applications in this field.
References
- 1. Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943–952. [DOI] [PubMed] [Google Scholar]
- 2. Grossi EA, Galloway AC, Ribakove GH, Zakow PK, Derivaux CC, Baumann FG, Colvin SBet al. Impact of minimally invasive valvular heart surgery: a case-control study. Ann Thorac Surg 2001;71:807–810. [DOI] [PubMed] [Google Scholar]
- 3. Yoo JS, Reddy YNV, Kim KH. Heart transplantation for dextrocardia: preoperative planning using 3D printing. Eur Heart J Cardiovasc Imaging 2020;21:346. [DOI] [PubMed] [Google Scholar]
- 4. Gasparovic H, Rybicki FJ, Millstine J, Unic D, Byrne JG, Yucel K, et al. Three dimensional computed tomographic imaging in planning the surgical approach for redo cardiac surgery after coronary revascularization. Eur J Cardiothorac Surg 2005;28:244–249. [DOI] [PubMed] [Google Scholar]
- 5. Ooms J, Minet M, Daemen J, Van Mieghem N. Pre-procedural planning of transcatheter mitral valve replacement in mitral stenosis with multi-detector tomography-derived 3D modeling and printing: a case report. Eur Heart J Case Rep 2020;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadeghi AH, Taverne Y, Bogers A, Mahtab EAF. Immersive virtual reality surgical planning of minimally invasive coronary artery bypass for Kawasaki disease. Eur Heart J 2020;41:3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kasprzak JD, Pawlowski J, Peruga JZ, Kaminski J, Lipiec P. First-in-man experience with real-time holographic mixed reality display of three-dimensional echocardiography during structural intervention: balloon mitral commissurotomy. Eur Heart J 2020;41:801. [DOI] [PubMed] [Google Scholar]
- 8. Mendez A, Hussain T, Hosseinpour AR, Valverde I. Virtual reality for preoperative planning in large ventricular septal defects. Eur Heart J 2019;40:1092. [DOI] [PubMed] [Google Scholar]
- 9. Incekara F, Smits M, Dirven C, Vincent A. Clinical feasibility of a wearable mixed-reality device in neurosurgery. World Neurosurg 2018;118:e422–e427. [DOI] [PubMed] [Google Scholar]
- 10. Shirk JD, Thiel DD, Wallen EM, Linehan JM, White WM, Badani KK, et al. Effect of 3-dimensional virtual reality models for surgical planning of robotic-assisted partial nephrectomy on surgical outcomes: a randomized clinical trial. JAMA Netw Open 2019;2:e1911598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wellens LM, Meulstee J, van de Ven CP, Terwisscha van Scheltinga CEJ, Littooij AS, van den Heuvel-Eibrink MM, et al. Comparison of 3-dimensional and augmented reality kidney models with conventional imaging data in the preoperative assessment of children with Wilms tumors. JAMA Netw Open 2019;2:e192633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cacau Lde A, Oliveira GU, Maynard LG, Araujo Filho AA, Silva WM Jr, Cerqueria Neto ML, et al. The use of the virtual reality as intervention tool in the postoperative of cardiac surgery. Rev Bras Cir Cardiovasc 2013;28:281–289. [DOI] [PubMed] [Google Scholar]
- 13. Mosso J, Gao K, Wiederhold B, Wiederhold M. Virtual reality for pain management in cardiac surgery. Cyberpsychol Behav Soc Netw 2014;17:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116–1128. [DOI] [PubMed] [Google Scholar]
- 15. Heuts S, Maessen JG, Sardari Nia P. Preoperative planning of left-sided valve surgery with 3D computed tomography reconstruction models: sternotomy or a minimally invasive approach? Interact Cardiovasc Thorac Surg 2016;22:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vukicevic M, Mosadegh B, Min JK, Little SH. Cardiac 3D printing and its future directions. JACC Cardiovasc Imaging 2017;10:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau I, Wong YH, Yeong CH, Abdul Aziz YF, Md Sari NA, Hashim SA, et al. Quantitative and qualitative comparison of low- and high-cost 3D-printed heart models. Quant Imaging Med Surg 2019;9:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ender J, Koncar-Zeh J, Mukherjee C, Jacobs S, Borger MA, Viola C, et al. Value of augmented reality-enhanced transesophageal echocardiography (TEE) for determining optimal annuloplasty ring size during mitral valve repair. Ann Thorac Surg 2008;86:1473–1478. [DOI] [PubMed] [Google Scholar]
- 19. Ong CS, Krishnan A, Huang CY, Spevak P, Vricella L, Hibino N, et al. Role of virtual reality in congenital heart disease. Congenit Heart Dis 2018;13:357–61. [DOI] [PubMed] [Google Scholar]
- 20. Brun H, Bugge RAB, Suther LKR, Birkeland S, Kumar R, Pelanis E, et al. Mixed reality holograms for heart surgery planning: first user experience in congenital heart disease. Eur Heart J Cardiovasc Imaging 2019;20:883–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.