Abstract
Microbes have disproportionate impacts on the macroscopic world. This is in part due to their ability to grow to large populations that collectively secrete massive amounts of secondary metabolites and alter their environment. Yet, the conditions favoring secondary metabolism despite the potential costs for primary metabolism remain unclear. Here we investigated the biosurfactants that the bacterium Pseudomonas aeruginosa makes and secretes to decrease the surface tension of surrounding liquid. Using a combination of genomics, metabolomics, transcriptomics, and mathematical modeling we show that the ability to make surfactants from glycerol varies inconsistently across the phylogenetic tree; instead, lineages that lost this ability are also worse at reducing the oxidative stress of primary metabolism on glycerol. Experiments with different carbon sources support a link with oxidative stress that explains the inconsistent distribution across the P. aeruginosa phylogeny and suggests a general principle: P. aeruginosa lineages produce surfactants if they can reduce the oxidative stress produced by primary metabolism and have excess resources, beyond their primary needs, to afford secondary metabolism. These results add a new layer to the regulation of a secondary metabolite unessential for primary metabolism but important to change physical properties of the environments surrounding bacterial populations.
Research organism: Other
Introduction
Microorganisms touch practically every human activity, in harmful and beneficial ways: they cause infections (Morens et al., 2004) and help us digest food (Rowland et al., 2018), make bioterrorism weapons (Henderson, 1999) and synthesize life-saving drugs (Demain and Fang, 2000), contribute to climate change (Bardgett et al., 2008), and clean our wastewater (Kartal et al., 2010). The outsize impact of microbes on the macroscopic world that surrounds them comes from their ability to multiply to populations of billions that cooperate to transform chemicals in vast amounts. Many of the chemicals that they release are compounds unessential to growth, development, and division: they are secondary metabolites. But despite their importance, it remains unclear why microbes invest in secondary metabolites (Cavalier-Smith, 1992; Kell et al., 1995).
Secondary metabolites enable collective traits like cell-cell signaling (Dufour and Rao, 2011), microbial warfare (Demain and Fang, 2000), pathogenesis (Nicas and Iglewski, 1985), and nutrient scavenging (Albelda-Berenguer et al., 2019). These collective traits bring fitness advantages to a population (Maplestone et al., 1992) but by diverting precursors, cofactors, and energy away from primary metabolism they can also bring a cost to the individual cell (Yan et al., 2018). How did secondary metabolites evolve and how are they maintained by natural selection?
Social evolution theory provides an answer. A secondary metabolite that brings a fitness cost C to the cell and a benefit B to the population can be favored by natural selection if rB >C, where r is the relatedness among different genotypes across the population (West et al., 2006). This means that secondary metabolite with a high cost can still be evolved if relatedness r is very high, such as in monoclonal populations. But population mixing and mutation, which are frequent in a microbial world (Andersen et al., 2015; Andersen et al., 2018), mean that microbial populations can have a low relatedness, and a low r limits the maximum cost allowed for secondary metabolites.
Social evolution theory therefore predicts that secondary pathways should have a low cost (Cavalier-Smith, 1992). But many secondary products need multiple enzymes for their biosynthesis (Osbourn, 2010), and their burden on primary metabolism can be hard to avoid. Microorganisms have sophisticated molecular networks that can sense environmental stimuli and regulate the expression of secondary pathways to lower their cost (Brakhage, 2013; Bibb, 2005; Bayram et al., 2016; Lind et al., 2015). Experiments in laboratory culture show, for example, that microbes can regulate the production of secondary metabolites depending on environmental conditions such as the type and quantity of the nutrients provided, especially the carbon source (Ruiz et al., 2010), that they can restrict production to specific growth phases, typically the stationary phase (Malik, 1980), and that many secondary metabolites are controlled by quorum sensing (Whiteley et al., 1999), a type of cell-cell communication that regulates genes in a density-dependent way. Understanding how microbes regulate their secondary metabolites complements the evolutionary explanation by providing a different level of explanation—molecular mechanism—of the same biological phenomenon (Bateson and Laland, 2013).
Here we investigate the evolution and regulation of biosurfactants that the bacterium Pseudomonas aeruginosa secretes to decrease the surface tension of the surrounding liquid (Abdel-Mawgoud et al., 2010). These biosurfactants are secondary metabolites, and they enable spectacular collective behaviors like swarming (Tremblay et al., 2007; Deforet, 2022), climbing over walls (Yang et al., 2021), killing microbial competitors (Sotirova et al., 2008), and breaching the epithelial barriers of a host (Zulianello et al., 2006). These collective feats, impossible to achieve by a single bacterium, can benefit the population. But a P. aeruginosa cell produces 20% or more of its dry weight in biosurfactants (Guerra-Santos et al., 1984), which is potentially a huge cost (Xavier et al., 2011). A full understanding of P. aeruginosa surfactant secretion requires both evolutionary and molecular explanations (Yan et al., 2019).
The first step in the biosynthesis of rhamnolipid surfactants is catalyzed by the RhlA enzyme, which takes a metabolic intermediate from fatty acid synthesis, β-hydroxyacyl-acyl carrier protein (β-hydroxyacyl-ACP), and produces 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs) (Zhu and Rock, 2008). The RhlB and RhlC enzymes each add one unit of rhamnose to the HAAs to produce mono-rhamnolipids and di-rhamnolipids (Chong and Li, 2017). The expression of the rhlAB operon is regulated by a network that includes at least three quorum sensing signals (Mukherjee et al., 2018; Latifi et al., 1996) and is conditional to the nutrients in the media, requiring a high carbon-to-nitrogen or carbon-to-iron ratio (Xavier et al., 2011; Boyle et al., 2015; Mellbye and Schuster, 2014). This regulatory strategy—called metabolic prudence—delays the expression of the secondary biosynthetic genes to times when the population is large enough, but also checks if the individual cell has sufficient carbon source to make the product at low or no cost to its fitness (Xavier et al., 2011). Metabolic prudence regulates the expression of other secondary metabolites in P. aeruginosa besides surfactants (Mellbye and Schuster, 2014) and may provide a general evolutionary explanation for secondary metabolites in other microbes as well (Bruger and Waters, 2015). Compared to the non-regulated constitutive production, metabolic prudence lowers the C/B ratio and stabilizes biosurfactant synthesis—even when r is low—by preventing cheaters from increasing in frequency (de Vargas Roditi et al., 2013). But this evolutionary explanation alone cannot explain why the surfactant production varies widely among strains, even when they are closely related (Müller et al., 2011), and even though the genes encoding the secondary pathway—rhlA, rhlB, and rhlC—are highly conserved (Germer et al., 2020). A better understanding of the molecular processes driving surfactant secretion can shed light on the evolution of regulation of this secondary metabolite.
Here we investigate surfactant secretion among diverse P. aeruginosa strains that we isolated from infected patients. We start by focusing on the role of glycerol as a carbon source, a relevant nutrient for P. aeruginosa infection (Scoffield and Silo-Suh, 2016; Abdel-Mawgoud et al., 2014) that puts a substantial strain on Pseudomonas primary metabolism (Poblete-Castro et al., 2020). Then, we investigate other carbon sources to search for a general explanation: we show that the lineages that lost their ability to make the surfactants tend to grow not less but slower compared to lineages that can make surfactants. Using metabolomics and mathematical modeling, we link the inability to reduce oxidative stress produced by primary metabolism with the lack of surfactant secretion. Our results add a new layer to the regulation of surfactant biosynthesis and help explain the inconsistent variation of this secondary metabolite that P. aeruginosa secretes to alter the surface tension of its surrounding environment.
Results
Surfactant production varies inconsistently across the phylogenetic tree
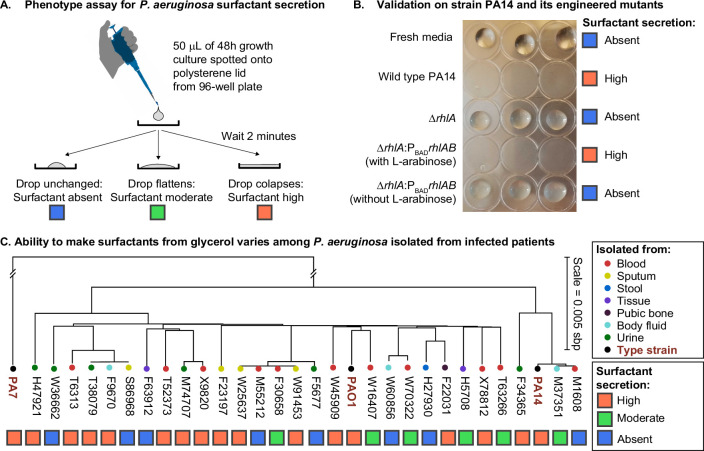
We investigated the phenotypic variability among clinical isolates in a diverse panel of 31 P. aeruginosa strains, including 28 isolates from infected patients (Yan et al., 2017) and the 3 types of strains PAO1, PA14, and PA7, all of which have their genomes sequenced. We grew each strain in synthetic media using glycerol as the sole carbon source and ammonium sulfate as the sole nitrogen source, producing a carbon-to-nitrogen molar ratio of 7.0. Then we used the drop collapse assay (Figure 1A) to classify each strain as surfactant absent, moderate, or high. Previous work had shown that glycerol enables the biosynthesis and secretion of surfactants in the strain PA14 (Boyle et al., 2015), a finding that we confirmed here with the wild-type PA14 and its isogenic mutants in the rhamnolipid synthesis. As expected, the wild type caused drop collapse indicating surfactant secretion, the ΔrhlA mutant did not, indicating loss of surfactants, and the complementation of the ΔrhlA with the L-arabinose inducible PBADrhlAB restored the drop collapse but only when L-arabinose was added, as expected (Figure 1B). Among the clinical isolates, however, there was a wide variability of surfactant phenotypes. The strongest producers (n=17) showed phenotypes similar to the wild-type PA14, while non-producers (n=8) showed no surfactant secretion similar to the ΔrhlA mutant; moderate producers (n=6) showed a phenotype in between. There was no obvious relation between the body site infected in the patient and the surfactant phenotype (Figure 1C). There was also no coherent distribution with the isolates’ phylogeny, which we built using the sequence variation in core genes, also supported by a poor correlation in Moran’s I test of p=0.14. Ancestral reconstruction of the surfactant secretion trait supports that the ability to make surfactants from glycerol was present in the common ancestor of all strains (Figure 1—figure supplement 2). Importantly, the genes rhlA, rhlB, and rhlC, the genes encoding the secondary biosynthesis pathway, were present in all 31 isolates, and their sequence was 100% conserved. This indicates that the absence of surfactant secretion was not due to a loss of the biosynthetic pathway. Instead, we hypothesized that the phenotypic variation was due to the life histories of the strains: some lineages may have lost their ability to rewire their regulatory network to overcome the specific selective pressures that they had faced during their evolution.
Figure 1. The ability to make surfactants from glycerol and reduce the surface tension of its liquid medium varies widely among clinical isolates of Pseudomonas aeruginosa, is uncorrelated with phylogeny, and is not associated with the tissue of origin.
(A) The drop collapse assay assesses the ability of P. aeruginosa to reduce the surface tension of its media by looking at the shape of a drop placed onto polystyrene. (B) The phenotypic assay in the wild-type PA14 (which can make surfactants from glycerol) is compared with the PA14 ΔrhlA isogenic mutant (which does not produce the rhamnolipid biosurfactants because it lacks a key biosynthetic gene). We also included the complementation mutant PA14 ΔrhlA PBADrhlA with and without the inducer of the PBAD promoter, L-arabinose. As expected, the ability to reduce surface tension is only present in the PA14 wild type and in the induced PBAD mutant. The ΔrhlA and the PBAD mutant in the absence of L-arabinose show high surface tensions like the fresh media. (C) A phylogenetic tree built from the core genome of 28 clinical isolates obtained from patients with cancer at Memorial Sloan Kettering Cancer Center (Lind et al., 2015; Cai et al., 2017) and the three type strains PAO1, PA14, and PA7. The tissue where each isolate was originally obtained from is indicated by the colored circles. The surfactant phenotype was assessed for all strains using the drop collapse assay after growth in glycerol minimal medium. The phenotype shows no obvious pattern with phylogeny or tissue of origin.
Figure 1—figure supplement 1. Phylogenetic ancestor state reconstruction of surfactant secretion from glycerol minimal media.
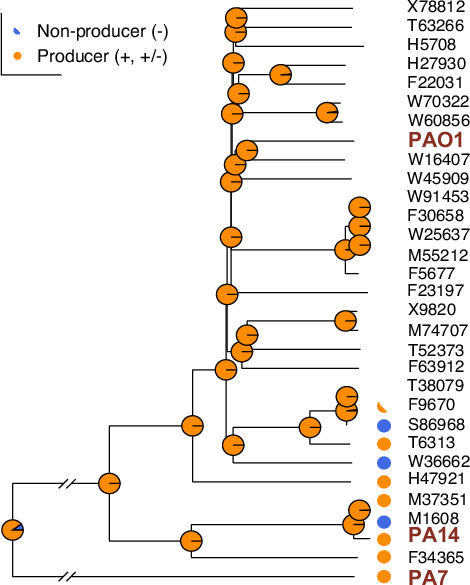
Figure 1—figure supplement 2. A principal component analysis (PCA) of the matrix of presence/absence of genes in each strain.
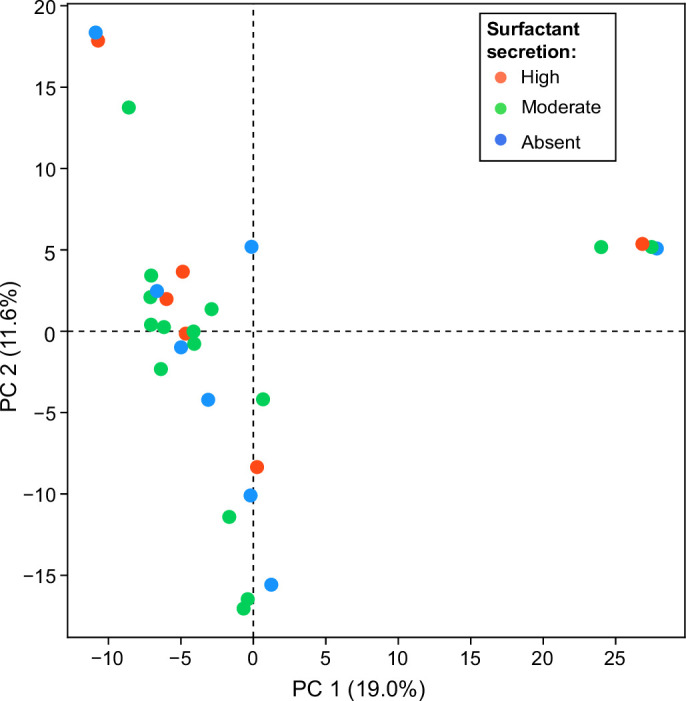
We further investigated whether variation elsewhere in the genome—particularly in the accessory genome (the set of genes missing in at least one strain)—could explain the phenotype differences. Among the 8290 accessory genes, 354 are only absent in those non-producers but present in all mild and strong producers (Supplementary file 1). In a principal component analysis (PCA) of the presence-absence profiles, there was no visible grouping of isolates by their surfactant phenotype (Figure 1—figure supplement 2), which is consistent with the lack of association with the phylogenetic tree built from the core genome.
Surfactant secretion lost in strains with slower exponential growth
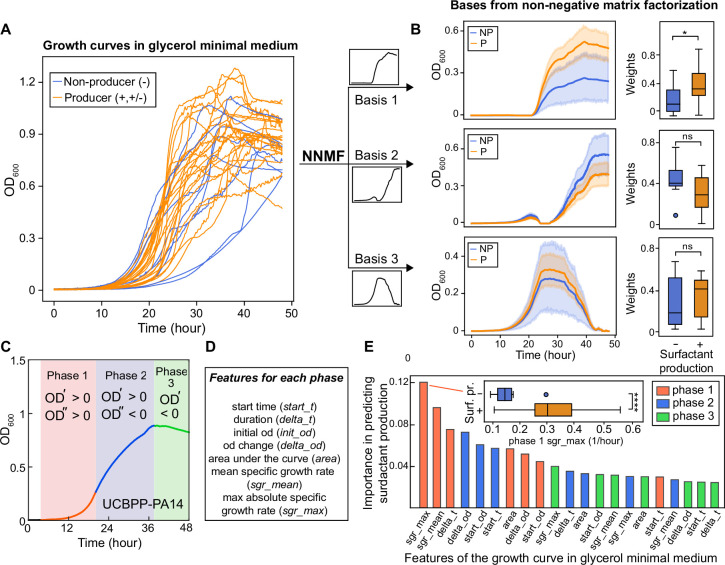
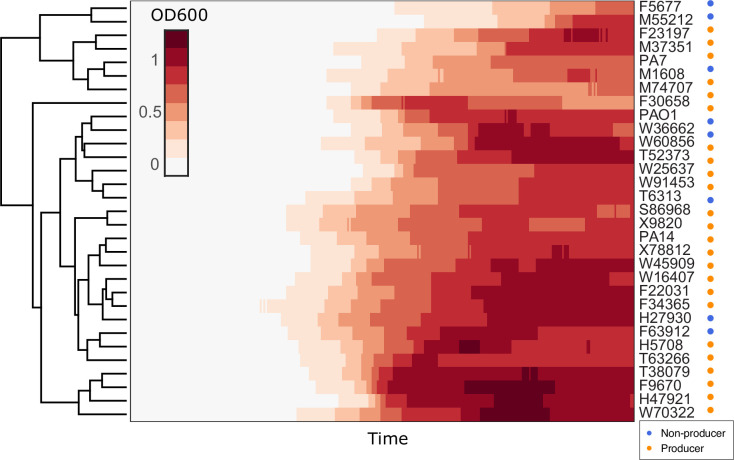
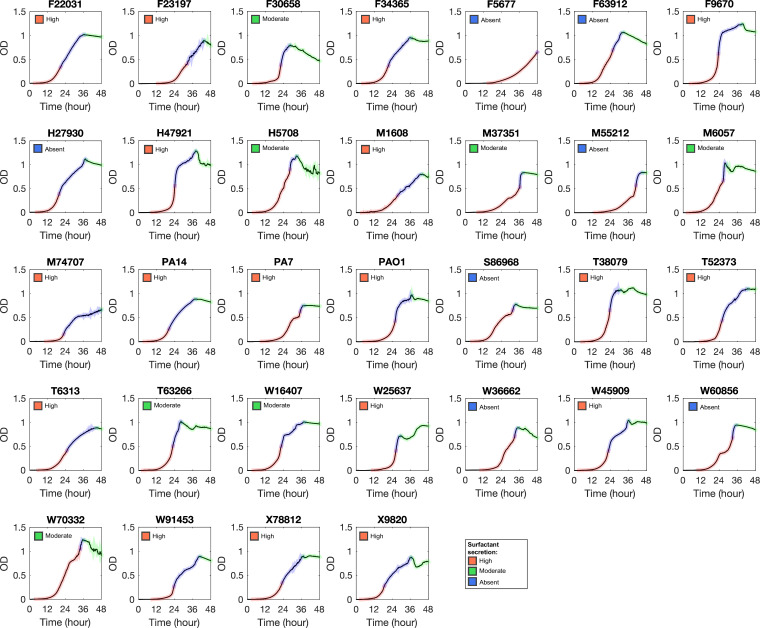
To search for a general explanation, we conducted analyses that showed producers have faster primary metabolisms in the same media of drop collapse assay. This finding was unexpected because, if anything, secondary metabolism should divert resources from primary metabolism (Yan et al., 2018): strains that produce surfactants would grow slower, not faster. We started by noting differences among the growth curves of the 31 strains, as assessed by the dynamics of the population optical density at 600 nm (OD600). The maxima that the population densities reached showed no obvious difference in producers vs non-producers, but the cultures did vary widely in the shape of their lag phase and the exponential phase (Figure 2A). The shape of the growth curve, as a whole, was not predictive of surfactant secretion because hierarchical clustering using the entire time series failed to separate producers and non-producers (Figure 2—figure supplement 1). Instead, the slope of the exponential growth phase was steeper in producers. This was also shown using non-negative matrix factorization (Lee and Seung, 1999), which could decompose each growth curve as a weighted sum of three basis functions (i.e. features). This analysis showed that—although the growth curves of the producers (orange lines; including mild and strong producers) and non-producers (blue lines) largely overlapped (Figure 2A)—producers had higher weights for base 1, which agrees with a faster growth rate in the exponential phase (Figure 2B). This was also confirmed by dividing each growth curve into three phases (Figure 2C and Figure 2—figure supplement 2) and defining seven quantitative features to characterize each growth phase (Figure 2D and Supplementary file 2). Random forest classification revealed that the top two features associated with surfactant production were the maximum and the average specific growth rates in phase I, the exponential growth phase (Figure 2E). Features of phase III (the decay phase), such as the starting value of OD600 in phase III which represents the final biomass produced by different strains in phase II, were among the least significant.
Figure 2. The shape of the growth curve in glycerol minimal media distinguishes surfactant producers from non-producers.
(A) Growth curves obtained in glycerol minimal media for producers (high and moderate, in orange) and non-producers (in blue). (B) We used two types of analysis: first, non-negative matrix factorization (NNMF) was used to decompose the growth curves into three additive basis functions (features). Each growth curve can be approximately represented by the weighted sum of these functions. The components 1 and 2 (basis function multiplied by weights; left panels) and weights (right panels) from NNMF of surfactant producers differ from non-producers. The shaded areas represent 95% bootstrap confidence intervals of the mean. (C) Second, we used supervised feature selection by random forest classifier, first divides each growth curve (excluding the initial lag phase) into three phases. (D) Each phase is described by seven quantitative features. (E) The random forest analysis quantifies the importance of each feature in distinguishing producers from non-producers. Inset: boxplot of maximum specific growth rate of phase I grouped by surfactant production. Welch’s t-test was used in (D) and (G) for significance testing. ****, p-value ≤0.0001; *, p-value ≤0.05; ns, p-value >0.05.
Figure 2—figure supplement 1. Hierarchical clustering of growth curves of Pseudomonas aeruginosa clinical isolates and three type strains PA14, PAO1, and PA7 in glycerol minimal medium.
Figure 2—figure supplement 2. Growth curve of Pseudomonas aeruginosa strains in glycerol minimal medium.
These analyses indicate that the ability to make surfactants, which typically peak when growth slows down from exponential (Latifi et al., 1996; Boyle et al., 2015; Mellbye and Schuster, 2014), is associated with the speed of the exponential phases, and hence, an efficient primary metabolism on glycerol. This suggests that the non-producer lineages had particular life histories that are selected for specific metabolic adaptations, and that those adaptations had the collateral damage of impacting their ability to grow on glycerol as a sole carbon source.
Metabolomics shows differences in intracellular metabolomes associated with loss of surfactant biosynthesis
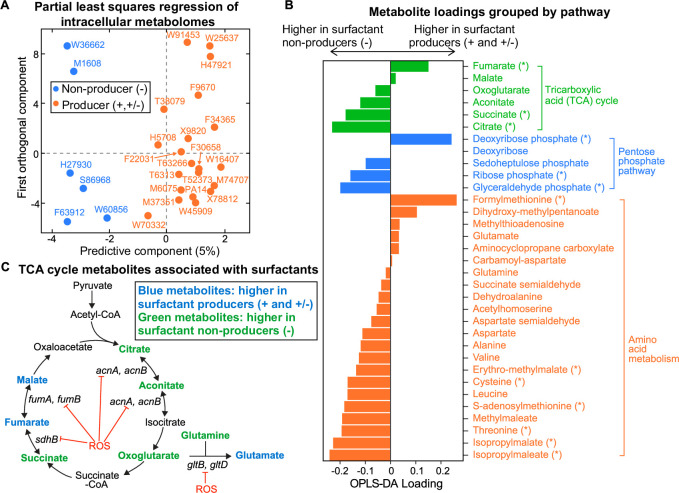
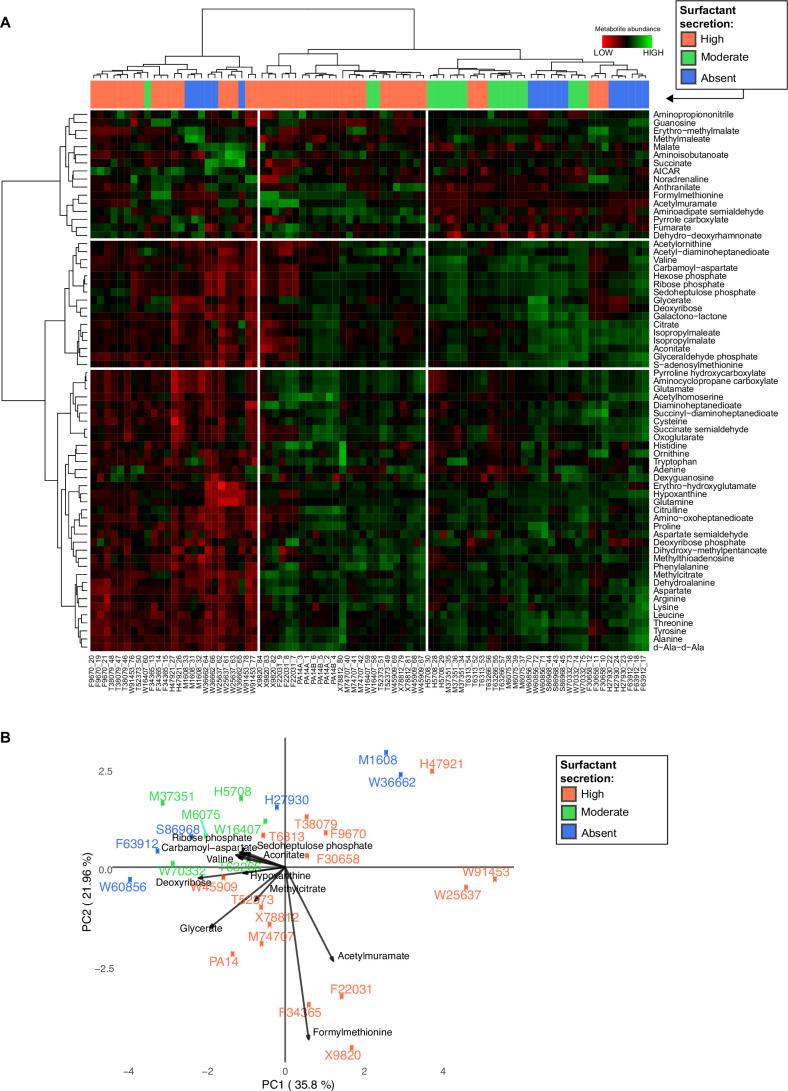
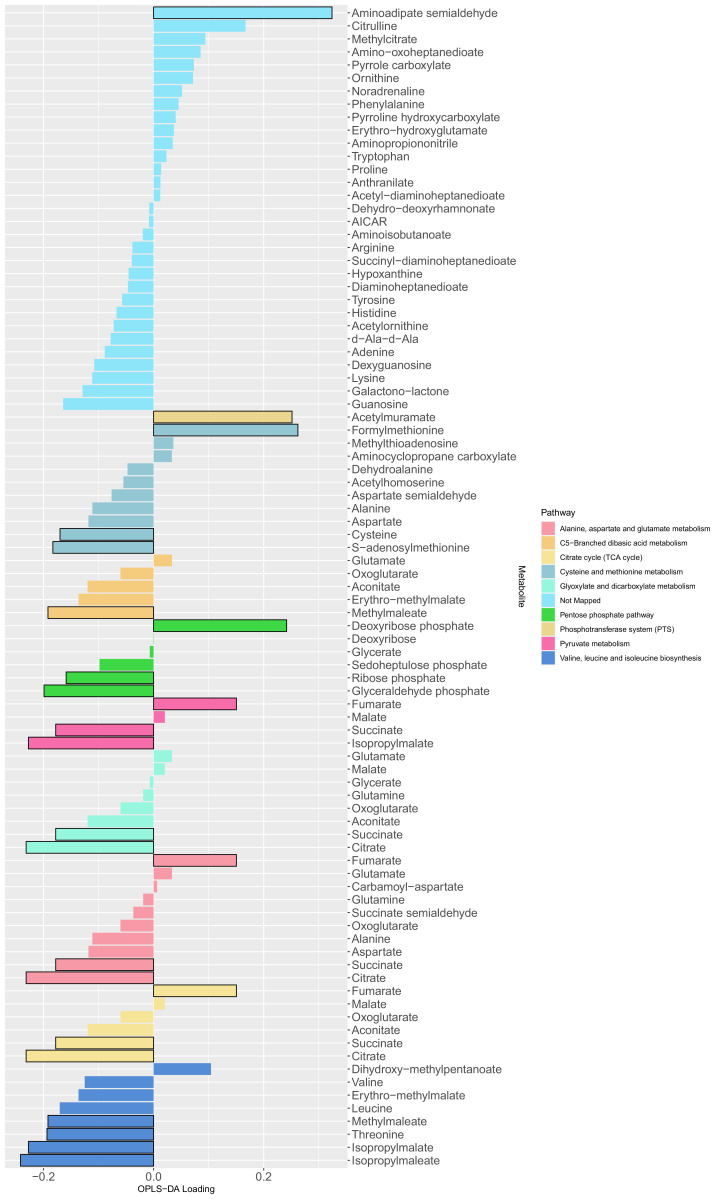
We then searched the composition of the intracellular metabolome for differences that distinguish producer and non-producer lineages. We extracted the metabolites from all our strains except for two non-producers (M55212 and F23197), which grew too slowly during the transition between phase I and phase II when rhamnolipid production begins (Materials and methods). Liquid chromatography-mass spectrometry (LC-MS)revealed 67 metabolites whose identities are known, and their abundances varied significantly across the tested strains (Figure 3—figure supplement 1A–B). Hierarchical clustering and PCA (Figure 3—figure supplement 2A–B) showed a reasonable separation between producers and non-producers. Fitting the data using the orthogonal projections to latent structures-discriminant analysis (OPLS-DA) (Cloarec et al., 2005) confirmed that there is a significant association between the broad metabolome and surfactant secretion (Figure 3A, R2=0.82, Q2=0.66, p-value = 5e−4). A Mann-Whitney U test revealed the 15 metabolites whose abundances differed most significantly between producers and non-producers. Then, we used those 15 metabolites to identify pathways perturbed in non-producers using the FELLA algorithm for pathway enrichment analysis (Picart-Armada et al., 2018; Figure 3—figure supplement 3, Supplementary file 3). The most perturbed pathways were the TCA cycle and amino acid metabolism (Figure 3B). Three out of six metabolites in the TCA cycle—fumarate, succinate, and citrate—were significantly changed: fumarate and malate had positive loadings, which indicated higher levels in producers. Citrate, succinate, and to a lesser extent cis-aconitate and alpha-ketoglutarate had negative loadings, which indicated higher levels in non-producers (Figure 3B). Pyruvate remained relatively constant across all the strains (Figure 3—figure supplement 1A), implying that the differential responses in the TCA cycle were independent from the changes in its upstream central carbon metabolism.
Figure 3. Intracellular metabolites measured in glycerol minimal media differ between surfactant producers and non-producers.
(A) OPLS-DA (orthogonal partial least squares discriminant analysis) was used to compare the intracellular metabolomes of producers (high and moderate) and non-producers. The predictive component is high in producers and low in non-producers. (B) OPLS-DA loading values for the predictive component of a selected number of metabolites. Metabolites with asterisk (*) are significantly different between producers and non-producers (adjusted p-value of a Mann-Whitney test <0.05). (C) Differential abundance of metabolites involved in reactions catalyzed by some Fe-S-containing enzymes whose activities are inhibited by reactive oxygen species (ROS). Abbreviations: acnA: aconitate hydratase A, acnB: aconitate hydratase B, sdhB: succinate dehydrogenase subunit, fumA: fumarase A; fumB: fumarase B; gltB: glutamate synthase subunit; gltD: glutamate synthase subunit.
Figure 3—figure supplement 1. Metabolites identified in this study were examined and imputed to represent the profile of all 31 strains for further analysis.
Figure 3—figure supplement 2. Variance and clustering analysis of metabolomics across Pseudomonas aeruginosa strains.
Figure 3—figure supplement 3. The loading values of all predictive metabolites of the orthogonal partial least squares discriminant analysis model.
Figure 3—figure supplement 4. Volcano plot of metabolomics data between wildtype (WT) Pseudomonas aeruginosa PA14 strain and its ΔrhlA mutant grown in glycerol minimal medium (replotted with permission from Bayram et al., 2016).
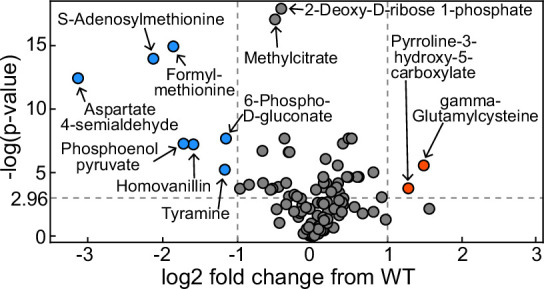
Besides the TCA cycle metabolites, most of the annotated compounds in the metabolism of branched chain amino acids (leucine/isoleucine, valine) and sulfur-containing amino acids (cysteine/methionine) were more abundant in non-producers than producers (Figure 3—figure supplement 3). A notable exception was formylmethionine (fMet), which was lower in non-producers. To place this observation in context, we reanalyzed previous metabolomics data (Boyle et al., 2017) from an experiment where we had compared PA14 and its ΔrhlA mutant. Interestingly, the ΔrhlA mutant also had lower levels of fMet compared with the wild type (Figure 3—figure supplement 4). The ΔrhlA mutant grows just as fast in glycerol as the wild type (van Ditmarsch and Xavier, 2011), which means that the link between lower fMet and lack of surfactant secretion is not simply due to a growth defect.
Theory formalizes the link between oxidative stress, slower growth, and lack of surfactants
Several theoretical arguments support that non-producers might have slower primary metabolisms because they suffer from higher oxidative stress than the producers. First, the major differences include TCA intermediates (Figure 3B). The TCA cycle harbors five enzymes with Fe-S clusters, aconitase A, aconitase B, succinate dehydrogenase (SDH), fumarase A, and fumarase B (Py and Barras, 2010), which make them especially vulnerable to oxidative stress (Figure 3C). If non-producers are less successful at removing the oxidative stress from growth in glycerol, then the stress accumulated could reduce flux through the TCA cycle, which would slow down growth. The accumulation of succinate and depletion of fumarate in non-producers could be due to a reduced activity of SDH under oxidative stress: SDH is a membrane-bound dehydrogenase linked to the respiratory chain—a major site of ROS production in the cell—and also a member of the TCA cycle that catalyzes the oxidation of succinate into fumarate (Hederstedt and Rutberg, 1981). Since SDH contains [2Fe-2S], [3Fe-4S], and [4Fe-4S] clusters (Ayala-Castro et al., 2008), ROS that damages Fe-S clusters could decrease SDH activity in vivo.
Second, we saw that non-producers also included intermediate metabolites in amino acid biosynthetic pathways which are also substrates of Fe-S containing enzymes (Figure 3B). For example, the glutamate synthetase has a large chain (encoded by gltB) and a small chain (encoded by gltD), and both subunits contain Fe-S clusters that catalyze the production of glutamate from oxoglutarate and glutamine (Curti et al., 1996). Non-producers had slightly higher oxoglutarate and glutamine as well as lower glutamate than producers.
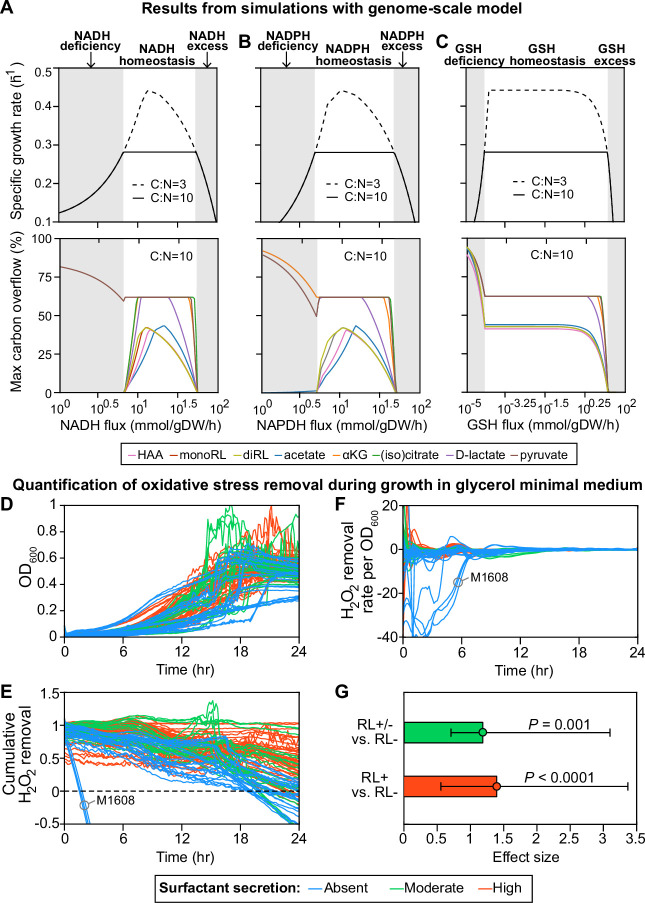
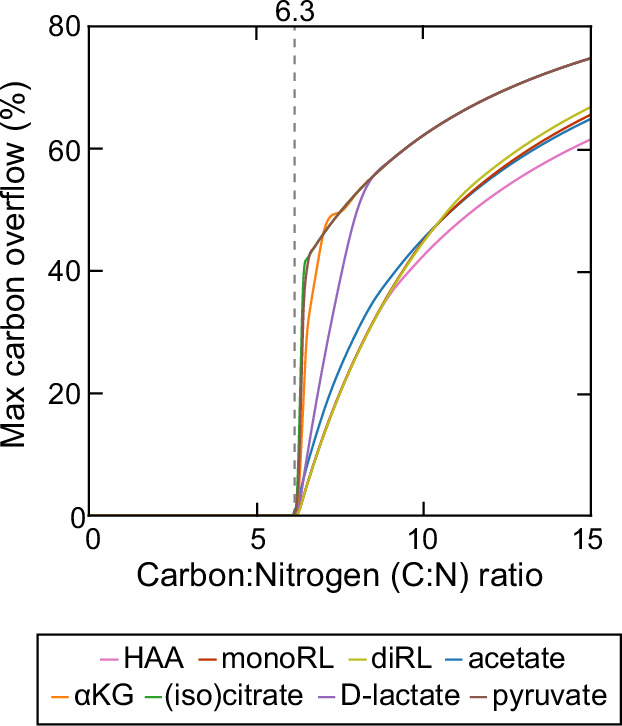
Finally, we used a whole-genome reconstruction to model the intracellular metabolic fluxes during growth in the glycerol medium (Nogales et al., 2017; Nogales et al., 2020). The rhamnolipids are produced when carbon is in excess (Boyle et al., 2015; Mellbye and Schuster, 2014) and, in the simulations, rhamnolipids secretion occurred when C:N flux ratio >6.3 (Figure 4—figure supplement 1). We then set C:N to 10.0, which exceeds the minimum threshold, and we simulated various levels of redox stress level by changing the flux through three redox molecules—NADH (reduced nicotinamide adenine dinucleotide), NADPH (reduced nicotinamide adenine dinucleotide phosphate), and GSH (reduced glutathione)—responsible for the bulk of cellular electron transfer and the main sources of reactive oxygen species (ROS) (Xiao and Loscalzo, 2020). Although redox stress is ultimately caused by high ROS levels, not fluxes, and ROS was not an explicit variable in our model, we assumed that ROS could perturb intracellular metabolic fluxes, particularly the fluxes of redox molecules, and we then asked how cell growth and surfactant synthesis responded to these ROS-mediated indirect perturbations.
For all three redox molecules, we found that the maximum growth rate was maintained at an intermediate flux range (redox homeostasis; Figure 4A–C, upper panels, white area). The growth rate decreased gradually as the flux of redox molecules changed in either direction away from this homeostatic interval (gray shading). The reason for the gradual decrease in growth rate is that deficiency or excess of these redox molecules which participate in the redox control of a great variety of biological processes disturbs the primary metabolism for growth. Importantly, before the growth starts slowing down due to an excess in redox species there was an abrupt shutdown in the overflow on carbon into many central carbon metabolites and—importantly— in the secretion of the HAAs, mono- and di-rhamnolipids that makeup the surfactants (Figure 4A–C, lower panels). The shutdown of overflow reactions under excess of redox molecules mimics the metabolic state in the non-producer strains who lose metabolic plasticity to overflow metabolites without compromising growth (Vemuri et al., 2006). When we forced these secretion fluxes to zero—to simulate a ΔrhlA mutant—the simulation did not predict a growth benefit. This result agrees with data showing that the ΔrhlA mutant has no growth benefit in glycerol media compared with wild type (van Ditmarsch and Xavier, 2011).
Figure 4. Computer simulations using flux-balance analysis and experiments with H2O2 indicate that the ability to produce surfactants in glycerol media depends on a strain’s ability to reduce oxidative stress.
(A-C) Computer model: the simulations vary the redox balance of Pseudomonas metabolism by altering the fluxes of (A) NADH (reduced nicotinamide adenine dinucleotide), (B) NADPH (reduced nicotinamide adenine dinucleotide phosphate), and (C) GSH (reduced glutathione). The upper panels show the predicted maximum growth rates, and lower panels are predicted maximum byproduct secretion fluxes. C:N indicates the carbon-to-nitrogen influx ratio provided by the culture medium. C:N=3 and C:N=10 represent carbon- and nitrogen-limiting conditions, respectively. Abbreviations: HAA: 3-(3-hydroxyalkanoyloxy) alkanoate; monoRL: monorhamnolipid; diRL: dirhamnolipid; aKG: alpha-ketoglutarate. (D–G). Experimental comparison of the ability to remove hydrogen peroxide (H2O2) among strong surfactant producers (+), weak producers (±), and non-producers (−). (D) Population density (OD600). (E) The total amount of hydrogen peroxide removed from the environment. Negative values indicate net cellular production of hydrogen peroxide released to the environment. (F) The specific hydrogen peroxide removal rate. In both (E) and (F), each trajectory of H2O2 fluorescence intensity was normalized to the averaged trajectory of the wild-type PA14 strain. (G) Effect size of surfactant production as a predictor of H2O2 removal rate per unit of OD600 determined by a linear mixed-effect model shows that producers can reduce oxidative stress better than non-producers.
Figure 4—figure supplement 1. Theoretical estimation of threshold carbon (glycerol):nitrogen (ammonium) ratio above which carbon is in excess in the sense that carbon release through rhamnolipids and central carbon metabolites does not compromise biomass production.
This also suggests that regaining surfactant synthesis should not restore fast growth in glycerol to non-producers, which agrees with the idea that loss of surfactant secretion is a consequence—not a cause—of the slower growth in non-producers. Notably, most of the overflowed metabolites are carbon-rich molecules, including HAA, mono-, and di-rhamnolipid (Xavier, 2011). Collectively, these theoretical arguments support a link between the ability to make surfactants and oxidative stress: fast growth and surfactant secretion are both impacted by the accumulation of ROS produced by primary metabolism.
Non-producers are worse at reducing oxidative stress
Our model suggested that non-producers suffer from higher oxidative stress. Pseudomonas have a variety of antioxidant enzymes including catalases, glutathione reductases, NADH peroxidases, and cysteine-based peroxidases (Mishra and Imlay, 2012), which in PA14 and PAO1 were only made when the growth rate maintained its maximum value under excess carbon (Xavier et al., 2011; Boyle et al., 2015; Mellbye and Schuster, 2014). Isogenic mutants lacking some of those could make surfactants (Vinckx et al., 2010). If the slower growth in the clinical non-producer strains is due to impairments in some of those mechanisms to reduce oxidative stress, then this could affect surfactant biosynthesis.
To test this hypothesis, we compared the dynamics of hydrogen peroxide (H2O2) during growth in glycerol (Figure 4D and E). H2O2 is a representative ROS that can diffuse freely between cells and the environment; we can access its dynamics by measuring the fluorescence by the Amplex assay, where the difference between cell cultures and baseline reveals each strain’s net ability to reduce H2O2 (Zhao et al., 2012). All strains except for M1608, one of the non-producers, quenched the signal over the first 18 hr (Figure 4E). Consistent with our hypothesis, the non-producers (blue lines) removed less H2O2 than moderate producers (green lines) and strong producers (red lines). To factor out the possibility that the non-producers performed worse due to lower cell density, we calculated the H2O2 removal rate per cell. We observed a similar relative trend of H2O2 degradation between non-producers and producers (Figure 4F): a linear mixed-effect model quantified the difference that mild and strong producers have in per-cell H2O2 removal rates, which was in both cases significantly higher than in non-producers (p<0.001; Figure 4G). This is consistent with the notion that non-producers suffer more from oxidative species, which can be caused by high production or slow degradation.
To identify the genes that would explain the varying tolerance to oxidative stresses, we performed a Spearman correlation between gene presence or absence and the time averaged H2O2 removal rate per cell. No gene passed the significance threshold (p<0.05) after Benjamini-Hochberg correction for multiple hypothesis testing. It is possible that oxidative stress tolerance is a system-level phenotype that depends on the interactions among many genes in multiple pathways. Alternatively, the phenotype can be determined by a small number of, but different genes for different strains. For example, the worst strain in this assay, M1608, lacks katE (Supplementary file 1), a catalase that degrades H2O2 (Mulvey et al., 1988), and another non-producer, F5677, lacks the redox sensor soxR (Hidalgo et al., 1998).
RNAseq reveals oxidative stress response in non-producers
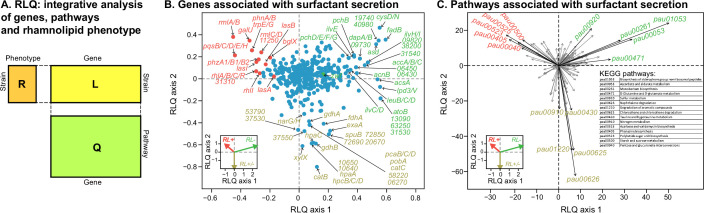
To investigate transcriptomic differences associated with the surfactant phenotype, we selected 11 representative strains (6 strong producers, 3 mild producers, and 2 non-producers), in proportion to the number in each category. We then assessed their transcriptomics in Phase II, which is typically when producers start their surfactant biosynthesis (van Ditmarsch and Xavier, 2011). A PCA of the gene expression profiles could not cluster the strains according to their phenotypes (Figure 5—figure supplement 1). We then turned to RLQ analysis (R, L, and Q are explained in Figure 5A) to find both genes (Figure 5B) and pathways (Figure 5C) associated with rhamnolipid production.
Figure 5. Transcriptomic data, analyzed using the RLQ analysis, reveals genes and metabolic pathways with expression associated with the ability to produce surfactants in glycerol minimal media.
(A) RLQ provides a way to analyze simultaneously associations from three tables. The results reveal not only the genes (B) but also the pathways (C) whose expression is associated with the rhamnolipid phenotype. The insets in both (B) and (C) represent the surfactant secretion phenotype (assessed in glycerol) in the two principal RLQ axes. Genes and pathways align with the direction of each phenotypic category: in panel (C), the top five pathways (‘pau’ are KEGG pathway IDs) associated with each phenotype are highlighted in black. The five-digit number in panel (C) represents the PA14 locus ID.
Figure 5—figure supplement 1. Principal component analysis (PCA) plot of RNA expression colored by surfact production phenotypes in glycerol minimal media.
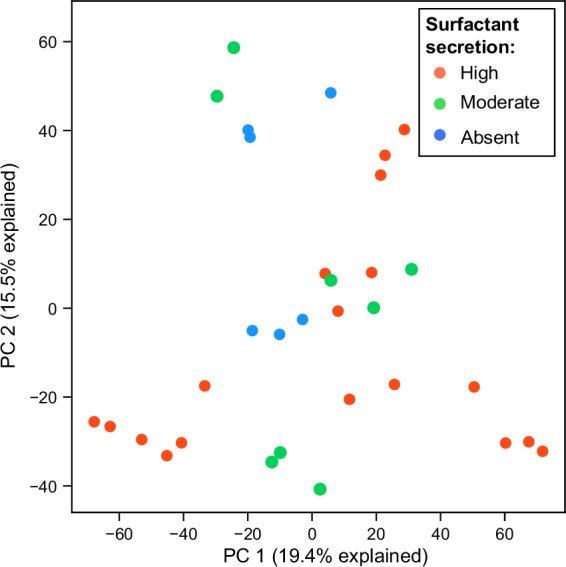
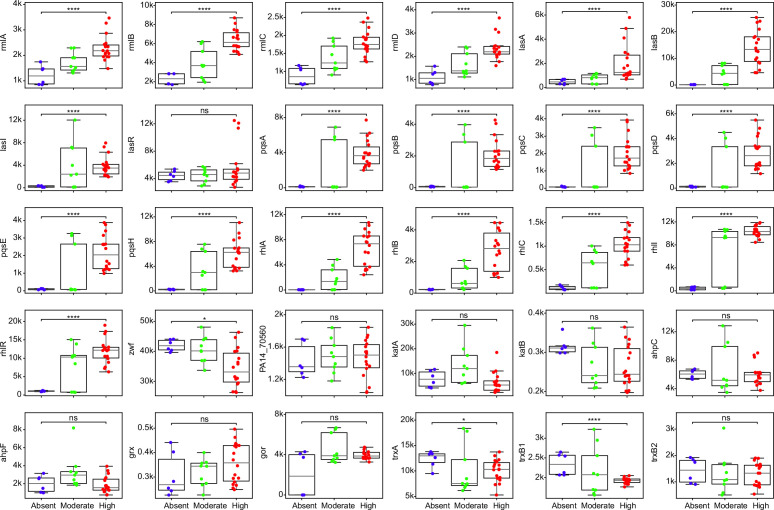
Figure 5—figure supplement 2. Comparisons of expression for selected genes across strains with different abilities of surfactant biosynthesis from glycerol.
As expected, the genes in the biosynthesis of rhamnose (rmlA, rmlB, rmlC, rmlD), rhamnolipid biosynthesis (rhlA, rhlB, rhlC, rhlR, rhlI), and quorum sensing (lasA, lasB, lasI, pqsABCDEH) were significantly less expressed in the non-producers (Figure 5B, Figure 5—figure supplement 2). Interestingly, in vitro data shows that the expression of genes from the above list, including lasI, lasR, rhlI, rhlR, pqsA, pqsR, in P. aeruginosa PAO1, can be significantly reduced by quorum sensing quencher and oxidative stress by exogenous H2O2 (Mohamed et al., 2020), supporting that the loss of the phenotype is linked to oxidative stress. More importantly, the zwf gene encoding glucose 6-phosphate dehydrogenase (G6PDH) was expressed higher in non-producers (Figure 5—figure supplement 2). G6PDH, a major source of NADPH that contributes to antioxidant defenses by reducing oxidized glutathione, was expressed higher, supporting our hypothesis that non-producers experience higher oxidative stress. Other genes, including the peroxide-sensing transcriptional regulator (oxyR), catalases (katA, katB), alkyl hydroperoxide reductases (ahpC, ahpF), glutaredoxin (grx), glutathione reductase (gor), and thioredoxins (trxA, trxB1, trxB2) were not highly expressed in the producers (Figure 5—figure supplement 2). We speculate that the stress levels may occur locally within the cells (e.g. within SDH) so that the oxidative stress response is also local. Another possibility is that the antioxidant defense may be regulated at the metabolic level. A notable example is biosynthesis of phenazine, whose genes (e.g. phzA1/B1/B2) are highly expressed in the producers but not in the non-producers. It was previously shown that the phenazine pyocyanin plays a role in the reduction of oxidative stress as it can restore growth of the oxyR mutant in rich medium (LB) and undergoes H2O2 oxidation (Vinckx et al., 2010).
The RLQ analysis also revealed the top five pathways enriched specifically in each phenotype group: strong producers were enriched in acarbose and validamycin biosynthesis (pau00525), phenazine biosynthesis (pau00405), polyketide sugar unit biosynthesis (pau00523), starch and sucrose metabolism (pau00500), and pentose and glucuronate interconversions (pau00040) (Supplementary file 4). By contrast, non-producers were enriched in the biosynthesis of siderophore group nonribosomal peptides (pau01053), ascorbate and aldarate metabolism (pau00053), monobactam biosynthesis (pau00261), D-glutamine and D-glutamate metabolism (pau00471), and sulfur metabolism (pau00920).
Exogenous oxidative stress impacts expression of surfactant biosynthetic genes
So far, we have shown data supporting that surfactant producers can reduce ROS better than non-producers. This explains why strains that grow slower in glycerol also tend to produce less surfactants from that carbon source, likely due to the impact of ROS on growth. It is even possible that the ability to direct metabolic fluxes to surfactant secretion is part of a multifarious response to oxidative stress. Previous work in an engineered strain of P. putida showed that inducing surfactant synthesis reduces production of the final electron acceptor CO2, suggesting that directing metabolic flux to surfactant secretion could indeed serve as an alternative electron sink (Tiso et al., 2016).
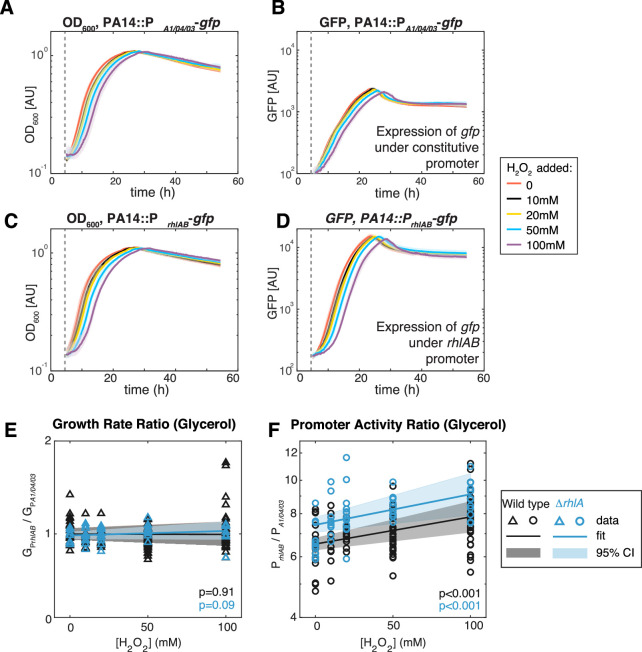
To investigate the link with oxidative stress further, we measured the expression of the rhamnolipid synthesis operon rhlAB to extrinsic oxidative stress using PA14 genetically engineered with a reporter fusion (PrhlABgfp). This strain allows tracking the expression of rhlAB through a dynamic readout of green fluorescence (Figure 6). A PA14 carrying gfp under a constitutive promoter, PA1/04/03gfp (Lambertsen et al., 2004), was used in parallel to account for growth effects on the gfp dynamics. We then added H2O2 at different doses always at the time when the culture started entering the exponential phase of growth in glycerol. In both PA14::PrhlABgfp and PA14::PA1/04/03gfp, the start of the exponential phase was increasingly delayed as the concentration of H2O2 was larger (Figure 6A and C), but once the exponential growth started there was no difference in the growth rate under different H2O2 concentrations (Figure 6E). This indicates that PA14 can restore normal operation of its primary metabolism after removal of the extrinsic stress.
Figure 6. Extrinsic oxidative stress by adding H2O2 increases PrhlAB activity without impacting cell growth in the stain PA14 in glycerol medium.
(A–D) Growth (A, C) and gfp expression (B, D) of PA14::PA1/04/03-GFP (constitutive promoter) and PA14::PrhlAB-GFP (rhlAB promoter) with increasing levels of H2O2 added to the media at the beginning of exponential growth (gray dash line). The shaded area represents the range of all replicates from the same experiment. (E, F) The ratio of growth rate (E) and promoter activity (F) of PA14::PrhlAB-GFP to PA14::PA1/04/03-GFP in phase II under different levels of H2O2 in glycerol minimal media. The p-value quantifies the significance of the coefficient (slope) obtained from linear fitting.
The apparent removal of H2O2 during the lag phase may be achieved by metabolic rewiring that affects rhamnolipid production in the late exponential phase (phase II). Similar to growth dynamics, the promoter activity of both mutants was also delayed, and the time delay is longer at higher H2O2 concentration (Figure 6B and D). We determined that the ratio of the median PrhlAB expression relative to the median PA1/04/03 increased linearly with the H2O2 concentration (Figure 6F, orange). Since PA1/04/03 measures the growth dependence of promoter activity (e.g. changes in number of RNA polymerases and ribosomes), the increased ratio showed that extrinsic redox stress can induce rhlAB expression via a growth-independent mechanism that likely involves transcriptional activators.
We next compared the above results obtained using PA14 wild-type strain and the ΔrhlA mutant. The gfp dynamics showed that in the absence of H2O2 the ΔrhlA strain still attempts to express rhlAB at a similar level as the wild type (p=0.3). We observed no decrease in the growth rate with increasing H2O2 concentration (Figure 6E), which indicates that the lack of surfactant biosynthesis had no detrimental impacts on the primary metabolism. Similar to the PA14 wild-type strain, H2O2 increased the expression of the rhlAB promoter relative to the constitutive promoter in the ΔrhlA mutant (Figure 6F, blue), but the baseline promoter activity increased significantly compared to the wild type (1.14×, p<0.01). Taken together, these results indicate that the genes for surfactant biosynthesis can be induced by extrinsic redox stress, and that the induction is stronger if surfactant synthesis fails to start.
Mathematical model predicts impact of carbon sources on surfactant production
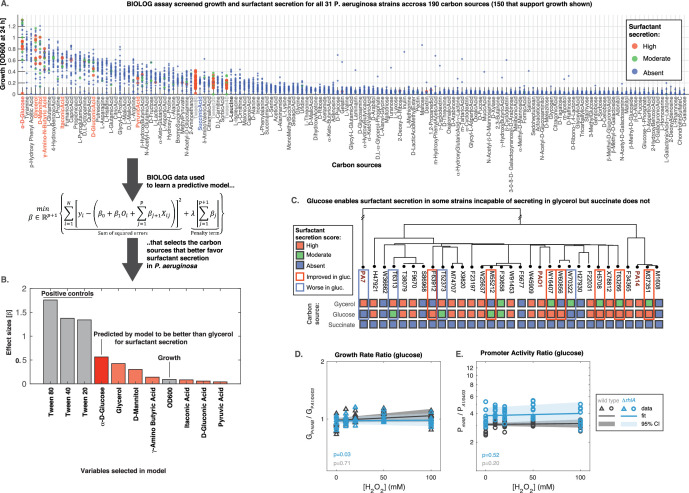
All analyses so far were done in glycerol, a carbon source relevant for P. aeruginosa rhamnolipid production in bioremediation (Zhao et al., 2021) and clinical settings (Scoffield and Silo-Suh, 2016; Abdel-Mawgoud et al., 2014). But could different environments lead strains that are non-producers in glycerol to produce surfactants, and vice-versa? To address this question, we developed a predictive model from data and then sought out to test its predictions experimentally.
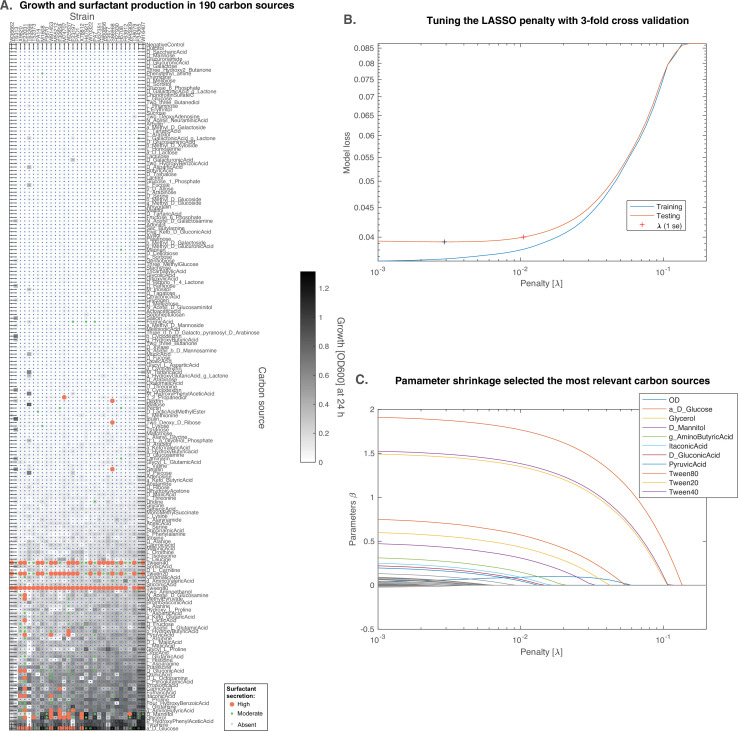
The model is a function that takes as input a given carbon source and predicts whether a strain produces surfactants from that source. To parameterize the model we obtained a dataset using the BIOLOG phenotype microarrays PM1 and PM2a; these two arrays together contain 190 distinct carbon sources in separate wells of two 96-well microtiter plates. We used the same composition as in the glycerol media (minus the glycerol). Then, we grew each of the 31 strains in each of the 190 nutrients and negative controls (wells without carbon source) for 24 hr (shorter than 48 hr used previously to facilitate high throughput). We measured the final absorbance (OD600) to quantify the ability to grow in that nutrient (Figure 7A; Figure 7—figure supplement 1A). The data showed that D-glucose (also known as dextrose) was the best carbon source for growth. Also, we saw 40 carbon sources (including the non-metabolizable isomer of D-glucose, L-glucose) in which none of the strains could grow on (OD600 <0.05).
Figure 7. Glucose can make some strains secrete surfactants that could not do it from glycerol, further linking primary metabolism, oxidative stress, and surfactant secretion.
(A) The 31 strains were profiled for growth and surfactant secretion in 190 carbon sources (see supporting Figure 8 for full dataset), here ranked by their potential for growth (highest on top; the carbon sources selected by model highlighted in red; succinate in blue; and the tween positive controls in gray). (B) We trained a predictive model using the LASSO (least absolute shrinkage and selection operator), a supervised learning approach that shrinks linear regression parameters using an L1-penalty. The parameter fitting minimizes the sum of the squared errors of the model (left-hand term in the mathematical expression) and the sum of the absolute values of the betas, the model parameters (right-hand term in the mathematical expression) The values obtained for the parameters beta reveal D-glucose as the only carbon source (besides the positive controls) better than glycerol at inducing surfactant secretion. (C) Validation using glucose minimal medium reveals seven strains that improved surfactant secretion compared to glycerol, and four strains that worsened. This is in sharp contrast to succinate, a carbon source that imposes more oxidative stress and where no strain produces surfactants. (D) Similar experiment as in Figure 6 but now adding H2O2 to PA14 growing in glucose. The extrinsic stress had no significant impact on the growth rate, even in the mutant lacking rhlA. (E) The rhlAB expression was also unaffected by H2O2, indicating that glucose places a lower burden on primary metabolism, enabling cells to both cope with oxidative stress and keep their level of the genes needed to make the surfactants.
Figure 7—figure supplement 1. D-glucose is predicted to induce surfactant secretion better than glycerol.
Then we assessed the ability of each strain to secrete surfactants from each carbon source and gave a score of 0 (absent), 1 (moderate), or 2 (high) using the drop collapse assay. To avoid experimentalist bias, the drop collapse was done by an experimentalist blind to the surfactant secretion score that we had determined previously in glycerol. The final dataset totaled N=6014 data points (31 strains grown on 194 conditions each, including the 190 carbon sources and 4 negative controls)—a sizeable dataset that we used to parameterize the model.
As with any high throughput approach, the BIOLOG data came with a loss of precision. We could assess that imprecision first by looking at three positive controls—tween20, tween40, and tween80—which come in the PM1 BIOLOG plate and are themselves surfactants, which means that they should produce a score of 3 regardless of surfactant secretion. The error of failing to identify collapse in these compounds was 4.2%. Then, we collected additional BIOLOG data on the PA14 ΔrhlA, which cannot produce surfactants. The rate of false identification of collapse in this strain was 1.3%. Finally, we used the fact that the PM1 plates contain glycerol in one of the wells to compare those results with our previous surfactant secretion scores (Figure 1). The assay correctly determined 8/8 non-producers, which amount to a 100% specificity rate. It failed to detect 10/23 producers, which amount to a 43% sensitivity rate. Taken together, these results show that BIOLOG assay was mildly sensitive but highly specific: while it may miss some surfactant secretion cases, it is unlikely to overestimate the ability of a specific sugar to induce secretion.
In the model, we encoded each carbon source as a set of hot-encoded binary variables . We included another covariate important to determine the surfactant production outcome: the OD600 after at 24 hr (covariate ), which quantifies whether that strain could grow on that nutrient. The model therefore had parameters, each represented by the Greek letter with an index () where is the number of nutrient conditions tested ( represents the parameter, or effect size, for the OD600, and each to represents the parameters or effect sizes for each nutrient). We used the LASSO (Tibshirani, 1996) to impose an L1-penalization for the parameter λ (see equation in Figure 7). Then we used threefold cross-validation, which splits the dataset into training and testing in repeated iterations and uses the test loss (the error rate of the model) to tune the penalty parameter. As common practice with LASSO, we choose the penalty value that was one standard error above the one that produced the minimum loss in the test: λ=9 × 10–3 (Figure 7—figure supplement 1B). This penalty shrank all but 11 parameters to 0 (Figure 7—figure supplement 1C). The covariates with non-zero effect sizes included OD600 and 10 carbon sources (Figure 7D). The top three compounds were—as expected—the positive controls tween80, tween40, and tween20. This provided a sanity check that the model worked as intended. D-glucose came in fourth place: the only carbon source that the model deemed better than glycerol at favoring P. aeruginosa surfactant production.
To validate the glucose result, we grew all the 31 strains in minimal medium using D-glucose as the sole carbon source, now using media that we made ourselves from scratch as opposed to the high throughput—but less precise—BIOLOG plates and we did each test in triplicate for added rigor. Consistent with our model, glucose improved surfactant secretion overall, showing better secretion seven strains relatively to the secretion we had seen in glycerol (Figure 7C); this included three strains that were non-producers in glycerol but turned into producers in glucose. Interestingly, the switch also went in the opposite direction: four strains had worse surfactant scores in D-glucose than in glycerol. This suggests a complex strain-to-strain variability in the metabolic features of P. aeruginosa. Nonetheless, the data showed that—at least for some strains—glucose, a carbon source which should impose less burden on primary metabolism (Guerra-Santos et al., 1984), could free up resources for secondary metabolism.
To test this idea further, we tested another carbon source, succinate, which P. aeruginosa can grow on (Figure 7A) but which—according to our model—should not improve secretion relatively to glycerol. Succinate enters the carbon metabolism directly through the TCA cycle, and compared to glycerol and glucose, the initial TCA cycle flux would be faster and generate more ROS level that would burden primary metabolism and impair surfactant synthesis. Indeed, none of the strains secreted surfactants in succinate (Figure 7C).
To investigate how oxidative stress might impact expression rhlAB in glucose we added H2O2 and monitored PrhlABgfp fluorescence in the PA14 wild type and ΔrhlA strains. As described previously (Figure 6), we used the same constitutive PA1/04/03gfp as the baseline for comparison. Phase I growth rate was unaffected, indicating that both strains could still remove oxidative stress effectively (Figure 7D). As in glycerol, the rhlAB expression levels showed the same higher baseline level in the ΔrhlA compared to the wild type. But, contrasting with the results from glycerol, D-glucose did not increase rhlAB expression with increasing H2O2 in either strain (Figure 7E). Taken together, these results support that carbon sources that place a lower burden on primary metabolism leave resources available to both deal with extrinsic oxidative stress and sustain surfactant production.
Discussion
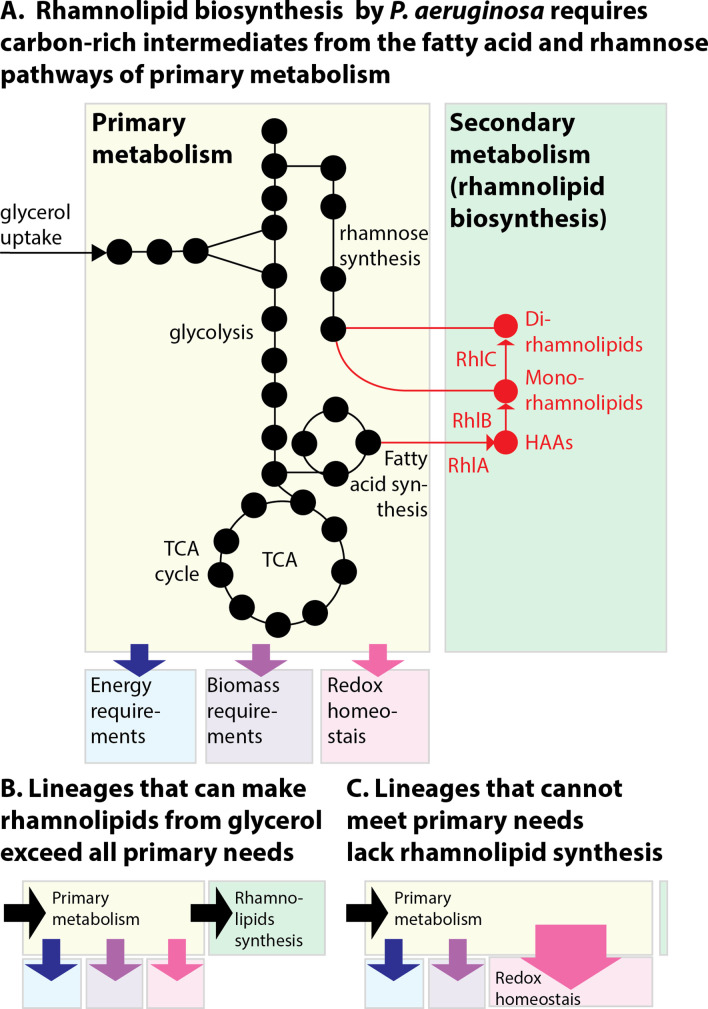
Here we investigated the evolution and regulation of surfactant secretion using diverse isolates of P. aeruginosa. Our results suggest a generalizable principle: that a strain can use its secondary metabolic pathways to produce surfactants if it can meet its primary requirements of energy production, biomass synthesis, and reduction of oxidative stress (Figure 8A). The lineages that retained their ability to make surfactants from glycerol were also those capable of reducing the oxidative stress created by primary metabolism and could overflow any excess carbon to the secondary metabolic pathway (Figure 8B). The inconsistent distribution across the phylogenetic tree was possibly due to the life histories of each lineage. Non-producing strains showed impaired abilities to reduce oxidative stress, a slower exponential growth in that carbon source (indicating a deficient primary metabolism), and perturbed levels of TCA cycle metabolites consistent with oxidative damage and accumulated intermediates of amino acid synthesis (also consistent with a slower primary metabolism). Those burdens may be what prevented the strains from making surfactants as their resources were prioritized for primary metabolism (Figure 8C). This was consistent with the observation the OD600 at the end of exponential growth was not a good distinguisher for producers and non-producers, indicating that their final yield of biomass production was unaffected, while their exponential growth was.
Figure 8. The allocation of metabolic resources made from glycerol in primary vs secondary metabolism.
(A) Growth and rhamnolipid biosynthesis on glycerol as the sole carbon source place a strong burden on Pseudomonas aeruginosa: The bacterial cell has to make all the molecules needed for energy, biomass, and redox homeostasis; surfactant biosynthesis—a secondary metabolic pathway—competes for those resources. (B) P. aeruginosa lineages that retain the ability to make surfactants from glycerol meet their primary needs and use excess resources for secondary metabolism. (C) P. aeruginosa uncapable of making surfactants from glycerol was also worse at reducing the oxidative stress produced from growth in glycerol; the needs imposed on primary metabolism, such as maintaining redox homeostasis, may leave insufficient resources for secondary metabolism, explaining the loss of surfactant secretion.
What were the selective pressures that led to the diversity of surfactant secretion phenotypes? The answer to this question is less clear. The strains uncapable of making surfactants from glycerol-lacked genes (Supplementary file 1) that suggested a particular path which led to their loss of phenotype. Importantly, though, all three genes of the secondary biosynthetic pathway (rhlA, rhlB, rhlC) were intact, and some of those strains could make surfactants in D-glucose. Absence of a selective pressure to produce surfactants from glycerol in the life histories of non-producer lineages could account for these results. But another explanation is that the impaired ability to reduce oxidative stress in glycerol is a maladaptive consequence of specific adaptations experienced by those lineages. Since the strains were isolated from hospitalized patients—a host-associated environment where we may expect oxidative stresses imposed by the immune system in their fight against pathogens (Zhu et al., 2019) and by antibiotic treatment (Mohamed et al., 2020)—it is unlikely that the selection was for a worse response to oxidative stress.
Experiments in PAO1 and clinical isolates had shown previously that P. aeruginosa decreases expression of the key quorum-sensing genes (lasI, lasR, rhlI, rhlR, pqsA, and pqsR) in response to oxidative stress caused by exogenous H2O2 (Mohamed et al., 2020). Considering that quorum sensing regulates many genes (Whiteley et al., 1999), shutting down surfactant biosynthesis could be part of a broader response to reduce oxidative stress and save resources for primary metabolism. For PA14, a strain capable of reducing the stress produced by glycerol metabolism, adding an extrinsic oxidative stress induced expression of rhlAB in glycerol, though not in glucose. ΔrhlA increased the baseline expression in both glycerol and glucose but did not impact the rate of rhlAB increase with H2O2, suggesting an intricate regulation. Also, the lack of rhlA did not impact growth—in glycerol or glucose—compared to the wild type, even in the highest H2O2 concentrations tested. This is consistent with our flux-balance modeling of Pseudomonas metabolism, where shutting down rhamnolipid secretion did not impact the growth rate because the excess carbon could be alternatively secreted in other forms such as acetate (Figure 4A).
We did detect nuanced consequences of deleting surfactant biosynthesis in PA14: previous data shows that the ΔrhlA isogenic mutant of PA14 has lower levels of fMet (Boyle et al., 2017) which, interestingly, also happened in non-producer isolates analyzed here. fMet plays a role in translation initiation, and in the quality control mechanisms that degrade misfolded proteins (Piatkov et al., 2015). Bacillus subtilis lacking formyl-methionine transferase—the enzyme attaching a formyl group to methionine loaded on tRNAfmet—is more sensitive to H2O2 and defective in swarming (Cai et al., 2017). This link between fMet, oxidative stress, and the loss of rhamnolipid synthesis is intriguing and warrants further research. Also, the ΔrhlA mutant had higher levels of gamma-glutamylcysteine (Figure 3—figure supplement 4), the immediate precursor of glutathione. Since glutathione is a well-known antioxidant that protects cells from oxidative damage (Ezraty et al., 2017), the result could indicate a mild oxidative stress. The finding that ΔrhlA expressed rhlAB at a higher level than the wild type—in glycerol and in glucose—suggests a futile attempt by this mutant to synthesize more surfactants. The fatty acid biosynthesis pathway that provides precursors for surfactant biosynthesis regenerates NAD(P)+ from NAD(P)H. ΔrhlA could perturb this pathway causing minor increases in oxidative stress.
Our analysis adds to the model of metabolic prudence, which provided a social evolutionary explanation for biosurfactant secretion. The model of metabolic prudence says that the microbial cell can lower the cost of a cooperative public good by delaying expression to times when it has excess carbon source (Xavier et al., 2011). A detailed study suggested that the strain PA14, needs to regulate rhlAB expression to carefully split the flux of carbon between biomass production and rhamnolipid synthesis (Boyle et al., 2015). Here, comparing diverse strains reminds us that heterotrophs like P. aeruginosa use organic compounds like glycerol, glucose, and succinate as carbon source for biomass, but also as an energy source. That energy fuels essential processes, including the reduction of oxidative stress, which ends up competing with secondary metabolism. This adds molecular detail to complement the evolutionary explanation and advance our understanding of P. aeruginosa surfactants.
More generally, our analysis highlights the importance of balancing the individual costs and population benefits of secondary metabolism. There are many products of secondary metabolism that contribute to the outsize impact that microbes have on the macroscopic world. Products of one microbial species can impact other species—cooperatively or competitively—potentially amplifying microbial functions in diverse communities such as the human microbiome (Xavier, 2011). The surfactants of P. aeruginosa are only one instance of the multitude of microbial secondary metabolism (Demain, 2014). But their analysis suggests a general principle: natural selection will favor sophisticated regulation that conciliates the individual-level costs and the population-level benefits of secondary metabolism.
Materials and methods
Media
Glycerol synthetic media were prepared with 800 ml of Milipore water, 200 ml of 5× minimal salts buffer, 1 ml of 1 M magnesium sulphate, 0.1 ml of calcium sulphate with 0.5 gN/l nitrogen (ammonium sulphate), iron at 5 μM (iron III sulphate), and 3.0 gC/l glycerol, respectively. 5× stock minimal salts buffer was prepared with 12 g of Na2HPO4 (anhydrous), 15 g of KH2PO4 (anhydrous), and 2.5 g of NaCl into 1 l water.
Growth curve assays
The clinical isolates were inoculated in 3 mL of lysogen broth (LB) and incubated at 37°C overnight with shaking at 250 rpm. 500 μL of cell culture was pelleted and washed three times with PBS. 0.0025 units were inoculated into 3.0 g of carbon/liter of medium in glycerol minimal medium (7.7 g/l of glycerol) in BD Falcon (BD Biosciences, San Jose, CA) 96-well flat-bottom plates, with 150 μL of suspension per well. The plate was incubated during 48 hr at 37°C in a Tecan Infinite M1000 or Tecan Infinite M1000 Pro plate reader (Männedorf, Switzerland), with an orbital shaking of 4 mm of amplitude. OD600 was measured in 10 min intervals.
Rhamnolipid production quantification
The production of rhamnolipids was assessed by drop collapse assay (Jain et al., 1991; Chen et al., 2007). We placed 50 μL of the culture’s supernatant on a polystyrene surface (the lid of a 96-well plate). The presence of rhamnolipids decreases the surface tension of the liquid, making the drop collapse. We considered a strain rhamnolipid non-producer if the drop does not spread, a mild-producer if spreads, but not enough to pass over the lid’s circle that corresponds to the wells, and producer if spreads over it.
Pangenome analysis
The pangenomes of our clinical P. aeruginosa isolates were identified using the roary tool (Page et al., 2015) using the output of genome annotation from prokka (Seemann, 2014). The core genes need to have at least 95% identity using blastp and to be present in at least 99% of all genomes, and then were aligned with the MAFFT method (Katoh et al., 2002).
Phylogenetic analysis
The phylogenetic tree of clinical P. aeruginosa strains was constructed from core genomes as previously described (Yan et al., 2017). Moran’s I test was carried out using the ape package in R (Paradis and Schliep, 2019). The ancestral state of swarming and rhamnolipid production was reconstructed using corHMM package (Beaulieu et al., 2013).
Growth curve analysis
We first normalized the growth curves conducted in different experimental batches (different microtiter plates) using the PA14 inoculated in the same plate as the reference for normalization. The plate-specific coefficients were obtained by solving a linear regression model ‘log(OD of PA14)~Plate + Time’ (the Wilkinson notation) using Matlab function fitlm, where Plate is plate ids and Time is time points (both are categorical variables). Then the growth curves in each plate were normalized by multiplying the adjustment coefficient of the plate. These normalized growth curves were then smoothed by Savitzky-Golay filter (sgolayfilt function in Matlab). The growth phases (phase I, II, III) were determined by the following rules: (phase I) first-order derivative >0 and second-order derivative >0; (phase II) first-order derivative >0 and second-order derivative <0; (phase III) first-order derivative <0. The mean and maximum specific growth rate for each growth phase were estimated from the best-fit model among three models of bacterial growth curves: (Morens et al., 2004) exponential model ; (Rowland et al., 2018) Zwietering-Logistic model ; (Henderson, 1999) Zwietering-Gompertz model , where are the parameters to fit. The goodness of fit for each model was evaluated by the value of the coefficient of determination, , where is the growth curve data observed at time point t and . Both non-negative factorization and random forest regression were performed in python using sklearn.decomposition.NMF and sklearn.ensemble.RandomForestClassifier, respectively.
Metabolite extraction
All P. aeruginosa strains were grown until the end of exponential phase of growth in glycerol minimal medium. Bacteria was then loaded into 0.25-μm nylon membranes (Millipore) using vacuum, transferred to pre-warmed hard agar plates with the same medium composition and incubated at 37°C during 2.5 hr. The filters were then passed to 35-mm polystyrene dishes (Falcon) with 1 mL of 2:2:1 methanol:acetonitrile:H2O quenching buffer and incubated there during 15 min on dry ice. Cells were removed by scraping, and the lysate containing quenching buffer was transferred to 1.5 mL tubes and centrifuged at 16,000 rpm for 10 min at 4°C. Supernatant transferred to fresh tubes and stored at –80°C.
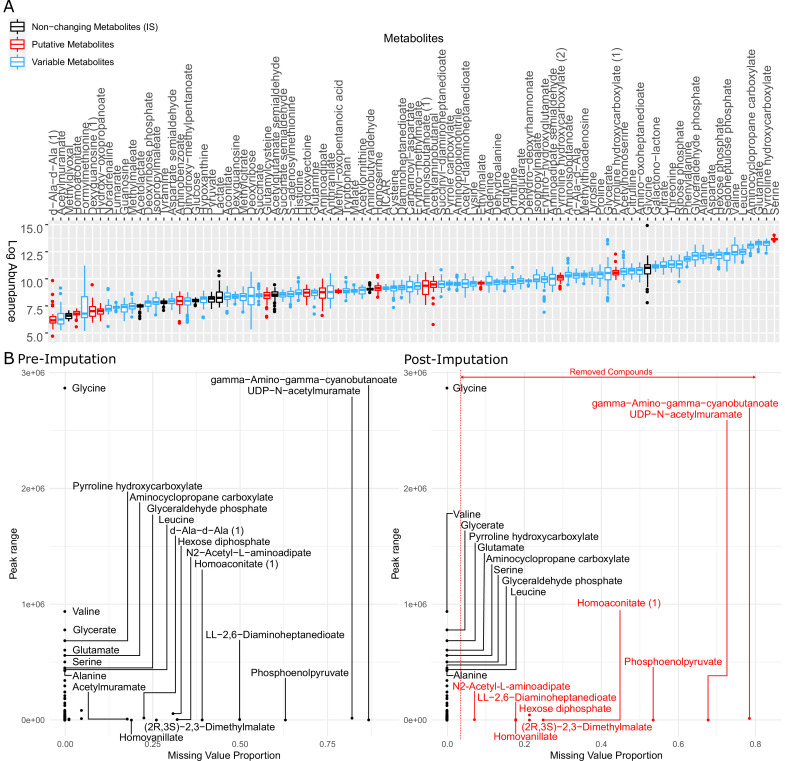
Metabolomic data preprocessing
The extracts were profiled using LC-MS, identifying a total of 92 compounds (Supplementary file 5). Some compounds contained missing values. These missing values in metabolite abundance can be (Morens et al., 2004) truly missing; (Rowland et al., 2018) present in a sample but its level is below detection limit; (Henderson, 1999) present in a sample at a level above the detection limit but missing due to failure of algorithms in data processing. Here we assume that a metabolite with missing values in all three replicates is truly missing in the sample and removed from our analysis (Supplementary file 5). However, if the missing values were only found in one or two replicates, the missing values were imputed by the average of the non-missing values. After that imputation, all compounds remaining with missing values were removed (Supplementary file 5).
The peak areas were normalized using cross-contribution compensating multiple standard normalization (Redestig et al., 2009) with NormalizeMets R package (De Livera et al., 2018). This method relies on the use of multiple internal standards. Since LC-MS lacks such internal standards, we used instead a set of metabolites assumed to be constant across all the strains. They were selected with a Kuskal-Wallis test, adjusting the p-value with Benjamini-Hochberg method. The ones with a p-value above 0.05 were considered constant (pyruvate, methylglyoxal, (S)–2-acetolactate, tyramine, D-glucose, (S)-lactate, N-acetyl-L-glutamate 5-semialdehyde, 4-aminobutyraldehyde, and glycine), therefore after the normalization step they were removed (indicated in red, Supplementary file 5A). The processed area peaks for all metabolites are included in Supplementary file 5.
Unsupervised analysis of metabolomic data
The hierarchical clustering of the normalized metabolomic data was performed using gplots R package (Warnes et al., 2015), with Euclidean distance and Ward’s aggregation method. The clustering was performed with all metabolites, including 16 unidentified metabolites that we could not identify (indicated in black, Figure 2—figure supplement 2). We included these 16 unidentified compounds in the clustering analysis because they were detected in all strains and therefore were not artifacts of the LC-MS. Nonetheless, because they were unidentified, we removed them from Figure 3—figure supplement 2 and from the downstream analyses. Two other compounds, fumarate and guanosine, which were only putative initially, were bioinformatically confirmed as all other compounds with the same molecular weight are produced by enzymatic reactions missing in our clinical isolates. The PCA was done with R’s base method, using also the normalized data and previously removing the 16 metabolites with unknown identity. The plot was obtained using ggplot2 R package (Wickham, 2016).
Metabolic pathway enrichment
The differential metabolites between rhamnolipid producers and non-producers were determined by a Mann-Whitney test, with p-values adjusted with Benjamini-Hochberg method and a significance level of 0.05. These compounds were fed to FELLA algorithm (Picart-Armada et al., 2018; Picart-Armada et al., 2017). FELLA retrieves a graph describing the relationships among pathway, module, enzyme, reaction, and compounds of P. aeruginosa strain PA14 from the KEGG database. The graph was then used as an input network of differential compounds for its diffusion algorithms (Vandin et al., 2011). The output of FELLA consists of all subnetworks of the entries predicted to have a high connectivity with the differential compounds. We filtered the subnetwork entries to keep only metabolic pathways. The entries shown in Supplementary file 3 are the ones with a significant probability of receiving part of the simulated flux.
OPLS-DA model
OPLS-DA model of metabolomics data was built using ropls R package (Thévenot et al., 2015), fixing the number of orthogonal components to 3. R2 and Q2, key parameters for assessing the validity of the model, were assessed with sevenfold cross-validation. The significance of the model was determined by permutation test (n=2000). The p-value corresponds to the proportion of Q2perm above Q2. With a p-value below 0.05 we considered the model significant. The loadings of the predictive component of the model were extracted to determine how each metabolite contributes to the separation according to the phenotype.
Genome-scale modeling
Custom Python codes were developed with the COBRApy package (Ebrahim et al., 2013) to carry out all metabolic flux modeling and simulations in the paper. Since iJN1411 model was developed for P. putida, we removed genes and associated reactions that are missing in all our strains but present in the iJN1411 model. The futile cycles involving NADH, NADPH, and GSH were also removed. The modified iJN1411 model was further expanded by adding rhamnolipid biosynthesis pathway involving 9 new metabolites and 12 new reactions.
The boundary fluxes of the model were set to mimic the composition of the glycerol minimum medium. For C:N=10, the lower bounds of glycerol and ammonium fluxes were set to –10 and –3, respectively. For C:N=3, the lower bounds were set to –3 and –3, respectively. The flux unit is mmol/gDW/hr throughout the paper. To constrain the total producing flux of NADH (the same for NADPH and GSH) at a certain value , we first defined a binary variable for each NADH-involving reaction to indicate whether NADH is produced by this reaction. Given the stoichiometric coefficient of NADH in this reaction () and its flux value (), the mathematical constraints for was set by for and otherwise. Therefore, the constraint that equalizes the total NADH producing flux and a constant is simply . However, both and are variables and such quadratic constraint has not yet been supported by COBRApy. We overcame this difficulty by defining and linearized the product with the following two inequalities: and , where and are the lower and upper bounds of . The two constraints ensure that when and when . The minimum/maximum flux values of byproduct secretion were simulated by flux variability analysis at maximum growth rate.
Detection of hydrogen peroxide (H2O2)
The H2O2 level in the extracellular medium was quantified with Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen, Carlsbad, USA, Catalog no. A22188). 500 μL of overnight growth in LB medium cell suspension were spun down, washed twice in PBS, and resuspended into glycerol medium at OD 1. Each reaction was done in a final volume of 100 μL, with a final concentration of 50 μM of Amplex Red reagent, 0.1 U/mL of HRP (Horseradish Peroxidase), and 0.2 cell OD, in glycerol synthetic medium, in BD Falcon (BD Biosciences, San Jose, CA) 96-well flat-bottom plates. The first column of the plate corresponded to the reaction without cells (the volume was substituted by PBS), and the last column instead of cells contained H2O2 (final concentration 10 μM). OD600 and fluorescence 530/590 nm were measured in 10 min intervals (48 hr 37°C).
RNA-seq and data analysis
The P. aeruginosa strains were inoculated into LB and grown with agitation for 16 hr. The overnight culture was diluted in glycerol synthetic medium the next day and grew to late exponential phase to harvest RNA. Cells were harvested by centrifuge and then immediately resuspended in RNAprotect (QIAGEN). The RNA was extracted using the RNeasy Mini Kit from QIAGEN. The standard RNA sequencing was done by Genewiz (Genewiz, Inc; South Plainfield, NJ) using pair-end indices of 150 bp read length on an Illumina HiSeq platform. The raw fastq files first went through quality check by fastQC (Andrews, 2010), and aligned with STAR (Dobin et al., 2013). The final output was generated using DEseq2 (Love et al., 2014) package in R. The read counts for each gene are included in Supplementary file 6. The SRA accession number for the RNAseq data is listed in Supplementary file 7.
RLQ analysis
To test for the functional associations between genes and rhamnolipid production, we adopted the RLQ analysis (ade4 package in R) that was developed for identifying the associations between traits and environmental variables. Here we applied it to RNAseq analysis to understand the main co-structures between gene functions and phenotypes mediated gene expression. The theory of RLQ analysis is described in detail elsewhere (Thioulouse et al., 2018). Briefly, it builds on the simultaneous ordination of three tables: an R table (categorical) describing rhamnolipid production (strong-, mild-, and non-producers) for 11 selected isolates (each replicate was treated as an independent observation), an L table (continuous) describing the RNAseq counts of 1474 core genes shared among the 11 isolates, and a Q table (binary) describing 120 KEGG pathways for the 1474 genes. We first applied corresponding analysis (CA) to table L and Q and PCA to table R. In the CA of R and Q, each strain and gene were weighted by their total RNA expression levels, respectively. The three separate ordinations are then used as inputs to the rlq function. The output of the function includes coefficients for the gene functions and phenotypes (loadings) as well as the scores of strains (out of phenotypes) and scores of genes (out of gene functions). RLQ analysis maximizes the squared cross-covariances between the two sets of scores weighted by gene expressions.
Hydrogen peroxide (H2O2) degradation
H2O2 was removed from environment by cells which degrade H2O2 intracellularly. The cumulative H2O2 removal curve was calculated by subtracting the values of emission of the wells containing each strain from the values of emission of the wells without cells or H2O2. Then they were normalized to the relative scale by dividing their values by the corresponding values (at the same time points) of the control strain (PA14) in the same experiment (plate). These normalized curves were then smoothed by the Savitzky-Golay filter (sgolayfilt function in Matlab). The H2O2 removal rate per cell was calculated by dividing the first-order derivative of the cumulative removal curves by OD values. To estimate the effect of rhamnolipid production on per-cell H2O2 removal rate, we developed a linear mixed-effect model that extends the simple linear regression model by considering both fixed and random effects. The model was specified by the Wilkinson notation ‘RemovalRate ~Time + RHL + (1|CurveID)’, where RemovalRate is per-cell H2O2 removal rate, Time is time point (categorical variable), RHL is categorical rhamnolipid production (non-producer, mild-producer, and strong-producer), and CurveID is the unique ID for each strain and replicate. The expression ‘(1|CurveID)’ indicates that CurveID was modeled as random effects. The model was solved by fitlme in Matlab.
Promoter activity and growth rate comparison
Two P. aeruginosa strains carrying chromosomal insertions of the reporter fusion PrhlABgfp (Lequette and Greenberg, 2005) and PA1/04/03gfp (a constitutive promoter, to be used as internal standard) (Lambertsen et al., 2004) in a PA14 background (Xavier et al., 2011), respectively, were inoculated into LB, and overnight cultures were harvested and washed with PBS three times. Cells were diluted to OD600=0.1 in synthetic media and monitored using TECAN plate reader for both OD600 and GFP fluorscence. Upon cells entering the exponential phase, H2O2 was added to the cells at a concentration in the assay of 0, 10, 20, 50, and 100 mM, continuing growth monitoring over 48 hr. After identifying growth phase II, the promoter activity at each timepoint within this interval was calculated as: . The ratio of promoter activity in each experiment was extracted as Rp = . Similarly, the growth rate was calculated as , and the ratio of strains with different promoters was calculated as . The effect of H2O2 was estimated by fitglme in Matlab as: log(R)~a0+ [H2O2]+, where R is the measured ratio of either promoter activity or growth rate, and is denoted as the random effect of experiments. This procedure was used too for performing the promoter activity and growth rate analyses in PA14 ∆rhlA mutant.
BIOLOG assay to obtain data for predictive modeling
We grew each of the P. aeruginosa stains in the BIOLOG phenotype microarrays PM1 and PM2a from Biolog Inc (Hayward, California, USA). We prepared minimal media without any carbon source added (800 ml of Milipore water, 200 ml of 5× minimal salts buffer, 1 ml of 1 M magnesium sulphate, 0.1 ml of calcium sulphate with 0.5 gN/l nitrogen [ammonium sulphate], iron at 5 μM [iron III sulphate]; 5× stock minimal salts buffer was prepared with 12 g of Na2HPO4 [anhydrous], 15 g of KH2PO4 [anhydrous], and 2.5 g of NaCl into 1 l water). We grow 5 mL bacterial cultures for 12–16 hr at 250 rpm and 37 ℃, then aliquoted washed 1.5 mL in media without carbon source and adjusted the initial OD by further dilution. We added 150 ul of inoculated media onto each well of a BIOLOG plate and incubated for 24 hr overnight shaking at 250 rpm at 37℃. After that we measured the endpoint OD and assessed surfactant secretion using the drop collapse assay.
Acknowledgements
This work was supported by NIH grants U01 AI124275 and R01 AI137269-01 to JBX, FRP and GS were partially supported by UIDB/04046/2020 and UIDP/04046/2020 Centre grants from FCT, Portugal (to BioISI) and by the LungCARD project (Grant Agreement n°: 734790, H2020-MSCA-RISE-2016). GS is the recipient of a fellowship from BioSys PhD programme PD65-2012 (Ref SFRH/BD/142899/2018) from FCT (Portugal). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Jinyuan Yan, Email: jinyuanyan@gmail.com.
Joao B Xavier, Email: xavierj@mskcc.org.
Sara Mitri, University of Lausanne, Switzerland.
Naama Barkai, Weizmann Institute of Science, Israel.
Funding Information
This paper was supported by the following grants:
National Institutes of Health U01 AI124275 to Joao B Xavier.
National Institutes of Health R01 AI137269 to Joao B Xavier.
Fundação para a Ciência e a Tecnologia UIDB/04046/2020 to Francisco Rodrigues Pinto.
Fundação para a Ciência e a Tecnologia UIDP/04046/2020 to Francisco Rodrigues Pinto.
European Research Council 734790 to Francisco Rodrigues Pinto.
Fundação para a Ciência e a Tecnologia SFRH/BD/142899/2018 to Guillem Santamaria.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Author contributions
Conceptualization, Software.
Conceptualization, Resources, Data curation, Software, Validation, Visualization, Methodology, Writing – original draft, Writing – review and editing.
Investigation, Writing – review and editing.
Formal analysis, Writing – review and editing.
Investigation, Methodology.
Supervision, Methodology.
Conceptualization, Supervision.
Conceptualization, Resources, Data curation, Software, Investigation, Methodology, Writing – original draft, Project administration, Writing – review and editing.
Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing – original draft, Project administration, Writing – review and editing.
Additional files
For each rhamnolipid production category (strong-, mild-, and non-producers), its correlation value with a single gene or a functional pathway was computed as the dot products between the arrow of phenotypic category and the arrow of the gene or pathway in RLQ axes.
Data availability
Sequencing data have been deposited in SRA, in the bioproject accession number PRJNA253624. Each individual sample has a file accession number listed in Supplementary file 7. The additional dataset is provided through Dryad. The source code for generating the figures of this study is available from: https://github.com/guisantagui/code_PA_paper (copy archived at swh:1:rev:cf190909e0f87db785ec4803bdd6e1a7cb854ad9); https://github.com/liaochen1988/Source_code_for_Pseudomonas_Metabolomics_Paper (copy archived at swh:1:rev:dcc50627d31d66a9f25a4d6b60294321087edf50); https://github.com/Jinyuan1998/PA_metabolomics_rhamnolipids_SourceCode (copy archived at swh:1:rev:9c8f53d6d21bd089c605f8b55f72f04774fd31bd).
The following datasets were generated:
Yan J. 2021. RNAseq files. NCBI BioProject. PRJNA253624
Xavier J, Pinto FR, Wang Z, Rhee K, Liao C, Santamaria G, Yan J. 2021. Evolution and regulation of microbial secondary metabolism. Dryad Digital Repository.
References
- Abdel-Mawgoud AM, Lépine F, Déziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Applied Microbiology and Biotechnology. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Mawgoud AM, Lépine F, Déziel E. A stereospecific pathway diverts β-oxidation intermediates to the biosynthesis of rhamnolipid biosurfactants. Chemistry & Biology. 2014;21:156–164. doi: 10.1016/j.chembiol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Albelda-Berenguer M, Monachon M, Joseph E. Siderophores: from natural roles to potential applications. Advances in Applied Microbiology. 2019;106:193–225. doi: 10.1016/bs.aambs.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Andersen SB, Marvig RL, Molin S, Krogh Johansen H, Griffin AS. Long-term social dynamics drive loss of function in pathogenic bacteria. PNAS. 2015;112:10756–10761. doi: 10.1073/pnas.1508324112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SB, Ghoul M, Marvig RL, Lee Z-B, Molin S, Johansen HK, Griffin AS. Privatisation rescues function following loss of cooperation. eLife. 2018;7:e38594. doi: 10.7554/eLife.38594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. FastQC: A quality control tool for high throughput sequence data (QUBES) v3Babraham Bioinformatics. 2010 http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Ayala-Castro C, Saini A, Outten FW. Fe-S cluster assembly pathways in bacteria. Microbiology and Molecular Biology Reviews. 2008;72:110–125. doi: 10.1128/MMBR.00034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett RD, Freeman C, Ostle NJ. Microbial contributions to climate change through carbon cycle feedbacks. The ISME Journal. 2008;2:805–814. doi: 10.1038/ismej.2008.58. [DOI] [PubMed] [Google Scholar]
- Bateson P, Laland KN. Tinbergen’s four questions: an appreciation and an update. Trends in Ecology & Evolution. 2013;28:712–718. doi: 10.1016/j.tree.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Bayram Ö, Feussner K, Dumkow M, Herrfurth C, Feussner I, Braus GH. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genetics and Biology. 2016;87:30–53. doi: 10.1016/j.fgb.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, O’Meara BC, Donoghue MJ. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Systematic Biology. 2013;62:725–737. doi: 10.1093/sysbio/syt034. [DOI] [PubMed] [Google Scholar]
- Bibb MJ. Regulation of secondary metabolism in streptomycetes. Current Opinion in Microbiology. 2005;8:208–215. doi: 10.1016/j.mib.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Boyle KE, Monaco H, van Ditmarsch D, Deforet M, Xavier JB, Reed JL. Integration of metabolic and quorum sensing signals governing the decision to cooperate in a bacterial social trait. PLOS Computational Biology. 2015;11:e1004279. doi: 10.1371/journal.pcbi.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle KE, Monaco HT, Deforet M, Yan J, Wang Z, Rhee K, Xavier JB. Metabolism and the evolution of social behavior. Molecular Biology and Evolution. 2017;34:2367–2379. doi: 10.1093/molbev/msx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage AA. Regulation of fungal secondary metabolism. Nature Reviews. Microbiology. 2013;11:21–32. doi: 10.1038/nrmicro2916. [DOI] [PubMed] [Google Scholar]
- Bruger E, Waters C. Sharing the sandbox: evolutionary mechanisms that maintain bacterial cooperation. F1000Research. 2015;4:F1000 Faculty Rev-1504. doi: 10.12688/f1000research.7363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Chandrangsu P, Gaballa A, Helmann JD. Lack of formylated methionyl-tRNA has pleiotropic effects on Bacillus subtilis. Microbiology. 2017;163:185–196. doi: 10.1099/mic.0.000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Origins of secondary metabolism. Ciba Foundation Symposium; 1992. [DOI] [PubMed] [Google Scholar]
- Chen CY, Baker SC, Darton RC. The application of a high throughput analysis method for the screening of potential biosurfactants from natural sources. Journal of Microbiological Methods. 2007;70:503–510. doi: 10.1016/j.mimet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chong H, Li Q. Microbial production of rhamnolipids: opportunities, challenges and strategies. Microbial Cell Factories. 2017;16:0753-2. doi: 10.1186/s12934-017-0753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloarec O, Dumas M-E, Craig A, Barton RH, Trygg J, Hudson J, Blancher C, Gauguier D, Lindon JC, Holmes E, Nicholson J. Statistical total correlation spectroscopy: an exploratory approach for latent biomarker identification from metabolic 1H NMR data sets. Analytical Chemistry. 2005;77:1282–1289. doi: 10.1021/ac048630x. [DOI] [PubMed] [Google Scholar]
- Curti B, Vanoni MA, Verzotti E, Zanetti G. Glutamate synthase: a complex iron-sulphur flavoprotein. Biochemical Society Transactions. 1996;24:95–99. doi: 10.1042/bst0240095. [DOI] [PubMed] [Google Scholar]
- Deforet M. The Rhamnolipids of Pseudomonas aeruginosa Change the Mechanical Properties of Biogels. bioRxiv. 2022 doi: 10.1101/2022.06.29.498166. [DOI]
- De Livera AM, Olshansky G, Simpson JA, Creek DJ. NormalizeMets: assessing, selecting and implementing statistical methods for normalizing metabolomics data. Metabolomics. 2018;14:54. doi: 10.1007/s11306-018-1347-7. [DOI] [PubMed] [Google Scholar]
- Demain AL, Fang A. The natural functions of secondary metabolites. Advances in Biochemical Engineering/Biotechnology. 2000;69:1–39. doi: 10.1007/3-540-44964-7_1. [DOI] [PubMed] [Google Scholar]
- Demain AL. Importance of microbial natural products and the need to revitalize their discovery. Journal of Industrial Microbiology & Biotechnology. 2014;41:185–201. doi: 10.1007/s10295-013-1325-z. [DOI] [PubMed] [Google Scholar]
- de Vargas Roditi L, Boyle KE, Xavier JB. Multilevel selection analysis of a microbial social trait. Molecular Systems Biology. 2013;9:684. doi: 10.1038/msb.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. Star: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour N, Rao RP. Secondary metabolites and other small molecules as intercellular pathogenic signals. FEMS Microbiology Letters. 2011;314:10–17. doi: 10.1111/j.1574-6968.2010.02154.x. [DOI] [PubMed] [Google Scholar]
- Ebrahim A, Lerman JA, Palsson BO, Hyduke DR. COBRApy: constraints-based reconstruction and analysis for python. BMC Systems Biology. 2013;7:74. doi: 10.1186/1752-0509-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezraty B, Gennaris A, Barras F, Collet JF. Oxidative stress, protein damage and repair in bacteria. Nature Reviews. Microbiology. 2017;15:385–396. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- Germer A, Tiso T, Müller C, Behrens B, Vosse C, Scholz K, Froning M, Hayen H, Blank LM. Exploiting the natural diversity of rhla acyltransferases for the synthesis of the rhamnolipid precursor 3- (3-hydroxyalkanoyloxy) alkanoic acid. Applied and Environmental Microbiology. 2020;86:e02317-19. doi: 10.1128/AEM.02317-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Santos L, Käppeli O, Fiechter A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Applied and Environmental Microbiology. 1984;48:301–305. doi: 10.1128/aem.48.2.301-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L, Rutberg L. Succinate dehydrogenase -- a comparative review. Microbiological Reviews. 1981;45:542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DA. The looming threat of bioterrorism. Science. 1999;283:1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- Hidalgo E, Leautaud V, Demple B. The redox-regulated SoxR protein acts from a single DNA site as a repressor and an allosteric activator. The EMBO Journal. 1998;17:2629–2636. doi: 10.1093/emboj/17.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain DK, Collins-Thompson DL, Lee H, Trevors JT. A drop-collapsing test for screening surfactant-producing microorganisms. Journal of Microbiological Methods. 1991;13:271–279. doi: 10.1016/0167-7012(91)90064-W. [DOI] [Google Scholar]
- Kartal B, Kuenen JG, van Loosdrecht MCM. Engineering: sewage treatment with anammox. Science. 2010;328:702–703. doi: 10.1126/science.1185941. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB, Kaprelyants AS, Grafen A. Pheromones, social behaviour and the functions of secondary metabolism in bacteria. Trends in Ecology & Evolution. 1995;10:126–129. doi: 10.1016/S0169-5347(00)89013-8. [DOI] [PubMed] [Google Scholar]
- Lambertsen L, Sternberg C, Molin S. Mini-tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environmental Microbiology. 2004;6:726–732. doi: 10.1111/j.1462-2920.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and rhlr (vsmr) to expression of the stationary-phase sigma factor rpoS. Molecular Microbiology. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401:788–791. doi: 10.1038/44565. [DOI] [PubMed] [Google Scholar]
- Lequette Y, Greenberg EP. Timing and localization of rhamnolipid synthesis gene expression in Pseudomonas aeruginosa biofilms. Journal of Bacteriology. 2005;187:37–44. doi: 10.1128/JB.187.1.37-44.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AL, Wisecaver JH, Smith TD, Feng X, Calvo AM, Rokas A. Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus aspergillus. PLOS Genetics. 2015;11:e1005096. doi: 10.1371/journal.pgen.1005096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with deseq2. Genome Biology. 2014;15:12. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS. Microbial secondary metabolism. Trends in Biochemical Sciences. 1980;5:68–72. doi: 10.1016/0968-0004(80)90071-7. [DOI] [Google Scholar]
- Maplestone RA, Stone MJ, Williams DH. The evolutionary role of secondary metabolites -- a review. Gene. 1992;115:151–157. doi: 10.1016/0378-1119(92)90553-2. [DOI] [PubMed] [Google Scholar]
- Mellbye B, Schuster M. Physiological framework for the regulation of quorum sensing-dependent public goods in Pseudomonas aeruginosa. Journal of Bacteriology. 2014;196:1155–1164. doi: 10.1128/JB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Imlay J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Archives of Biochemistry and Biophysics. 2012;525:145–160. doi: 10.1016/j.abb.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed FA, Shaker GH, Askoura MM. Oxidative stress influences Pseudomonas aeruginosa susceptibility to antibiotics and reduces its pathogenesis in host. Current Microbiology. 2020;77:479–490. doi: 10.1007/s00284-019-01858-7. [DOI] [PubMed] [Google Scholar]
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Moustafa DA, Stergioula V, Smith CD, Goldberg JB, Bassler BL. The pqse and rhlr proteins are an autoinducer synthase-receptor pair that control virulence and biofilm development in Pseudomonas aeruginosa. PNAS. 2018;115:E9411–E9418. doi: 10.1073/pnas.1814023115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Hörmann B, Kugel M, Syldatk C, Hausmann R. Evaluation of rhamnolipid production capacity of Pseudomonas aeruginosa PAO1 in comparison to the rhamnolipid over-producer strains DSM 7108 and DSM 2874. Applied Microbiology and Biotechnology. 2011;89:585–592. doi: 10.1007/s00253-010-2901-z. [DOI] [PubMed] [Google Scholar]
- Mulvey MR, Sorby PA, Triggs-Raine BL, Loewen PC. Cloning and physical characterization of katE and KatF required for catalase HPII expression in Escherichia coli. Gene. 1988;73:337–345. doi: 10.1016/0378-1119(88)90498-2. [DOI] [PubMed] [Google Scholar]
- Nicas TI, Iglewski BH. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Canadian Journal of Microbiology. 1985;31:387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- Nogales J, Gudmundsson S, Duque E, Ramos JL, Palsson BO. Expanding the Computable Reactome in Pseudomonas Putida Reveals Metabolic Cycles Providing Robustness. bioRxiv. 2017 doi: 10.1101/139121. [DOI]
- Nogales Juan, Mueller J, Gudmundsson S, Canalejo FJ, Duque E, Monk J, Feist AM, Ramos JL, Niu W, Palsson BO. High-Quality genome-scale metabolic modelling of Pseudomonas putida highlights its broad metabolic capabilities. Environmental Microbiology. 2020;22:255–269. doi: 10.1111/1462-2920.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn A. Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends in Genetics. 2010;26:449–457. doi: 10.1016/j.tig.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Schliep K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- Piatkov KI, Vu TTM, Hwang CS, Varshavsky A. Formyl-methionine as a degradation signal at the N-termini of bacterial proteins. Microbial Cell. 2015;2:376–393. doi: 10.15698/mic2015.10.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picart-Armada S, Fernández-Albert F, Vinaixa M, Rodríguez MA, Aivio S, Stracker TH, Yanes O, Perera-Lluna A. Null diffusion-based enrichment for metabolomics data. PLOS ONE. 2017;12:e0189012. doi: 10.1371/journal.pone.0189012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picart-Armada S, Fernández-Albert F, Vinaixa M, Yanes O, Perera-Lluna A. FELLA: an R package to enrich metabolomics data. BMC Bioinformatics. 2018;19:538. doi: 10.1186/s12859-018-2487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblete-Castro I, Wittmann C, Nikel PI. Biochemistry, genetics and biotechnology of glycerol utilization in Pseudomonas species. Microbial Biotechnology. 2020;13:32–53. doi: 10.1111/1751-7915.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nature Reviews. Microbiology. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- Redestig H, Fukushima A, Stenlund H, Moritz T, Arita M, Saito K, Kusano M. Compensation for systematic cross-contribution improves normalization of mass spectrometry based metabolomics data. Analytical Chemistry. 2009;81:7974–7980. doi: 10.1021/ac901143w. [DOI] [PubMed] [Google Scholar]
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz B, Chávez A, Forero A, García-Huante Y, Romero A, Sánchez M, Rocha D, Sánchez B, Rodríguez-Sanoja R, Sánchez S, Langley E. Production of microbial secondary metabolites: regulation by the carbon source. Critical Reviews in Microbiology. 2010;36:146–167. doi: 10.3109/10408410903489576. [DOI] [PubMed] [Google Scholar]
- Scoffield J, Silo-Suh L. Glycerol metabolism promotes biofilm formation by Pseudomonas aeruginosa. Canadian Journal of Microbiology. 2016;62:704–710. doi: 10.1139/cjm-2016-0119. [DOI] [PubMed] [Google Scholar]
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Sotirova AV, Spasova DI, Galabova DN, Karpenko E, Shulga A. Rhamnolipid–biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Current Microbiology. 2008;56:639–644. doi: 10.1007/s00284-008-9139-3. [DOI] [PubMed] [Google Scholar]
- Thévenot EA, Roux A, Xu Y, Ezan E, Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. Journal of Proteome Research. 2015;14:3322–3335. doi: 10.1021/acs.jproteome.5b00354. [DOI] [PubMed] [Google Scholar]
- Thioulouse J, Dray S, Dufour AB, Siberchicot A, Jombart T, Pavoine S. Multivariate Analysis of Ecological Data with Ade4. New York, NY: Springer; 2018. [DOI] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the LASSO. Journal of the Royal Statistical Society. 1996;58:267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- Tiso T, Sabelhaus P, Behrens B, Wittgens A, Rosenau F, Hayen H, Blank LM. Creating metabolic demand as an engineering strategy in Pseudomonas putida-rhamnolipid synthesis as an example. Metabolic Engineering Communications. 2016;3:234–244. doi: 10.1016/j.meteno.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay J, Richardson AP, Lépine F, Déziel E. Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environmental Microbiology. 2007;9:2622–2630. doi: 10.1111/j.1462-2920.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- Vandin F, Upfal E, Raphael BJ. Algorithms for detecting significantly mutated pathways in cancer. Journal of Computational Biology. 2011;18:507–522. doi: 10.1089/cmb.2010.0265. [DOI] [PubMed] [Google Scholar]
- van Ditmarsch D, Xavier JB. High-resolution time series of Pseudomonas aeruginosa gene expression and rhamnolipid secretion through growth curve synchronization. BMC Microbiology. 2011;11:140. doi: 10.1186/1471-2180-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA. Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Applied and Environmental Microbiology. 2006;72:3653–3661. doi: 10.1128/AEM.72.5.3653-3661.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckx T, Wei Q, Matthijs S, Cornelis P. The Pseudomonas aeruginosa oxidative stress regulator oxyr influences production of pyocyanin and rhamnolipids: protective role of pyocyanin. Microbiology. 2010;156:678–686. doi: 10.1099/mic.0.031971-0. [DOI] [PubMed] [Google Scholar]
- Warnes G, Bolker B, Bonebakker L, Gentleman R, Liaw W, Lumley T. Gplots: Various R Programming Tools for Plotting Data. Springer; 2015. [Google Scholar]
- West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nature Reviews. Microbiology. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. PNAS. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Ggplot2: Elegant Graphics for Data Analysis. Springer; 2016. [DOI] [Google Scholar]
- Xavier JB. Social interaction in synthetic and natural microbial communities. Molecular Systems Biology. 2011;7:483. doi: 10.1038/msb.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Molecular Microbiology. 2011;79:166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Loscalzo J. Metabolic responses to reductive stress. Antioxidants & Redox Signaling. 2020;32:1330–1347. doi: 10.1089/ars.2019.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Deforet M, Boyle KE, Rahman R, Liang R, Okegbe C, Dietrich LEP, Qiu W, Xavier JB. Bow-tie signaling in c-di-GMP: machine learning in a simple biochemical network. PLOS Computational Biology. 2017;13:e1005677. doi: 10.1371/journal.pcbi.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Lopes LD, Shaffer BT, Kidarsa TA, Vining O, Philmus B, Song C, Stockwell VO, Raaijmakers JM, McPhail KL, Andreote FD, Chang JH, Loper JE, Lindow SE. Secondary metabolism and interspecific competition affect accumulation of spontaneous mutants in the gacs-gaca regulatory system in pseudomonas protegens. MBio. 2018;9:e01845-17. doi: 10.1128/mBio.01845-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Monaco H, Xavier JB. The ultimate guide to bacterial swarming: an experimental model to study the evolution of cooperative behavior. Annual Review of Microbiology. 2019;73:293–312. doi: 10.1146/annurev-micro-020518-120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JQ, Sanfilippo JE, Abbasi N, Gitai Z, Bassler BL, Stone HA. Evidence for biosurfactant-induced flow in corners and bacterial spreading in unsaturated porous media. PNAS. 2021;118:e2111060118. doi: 10.1073/pnas.2111060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Summers FA, Mason RP. Photooxidation of Amplex red to resorufin: implications of exposing the Amplex red assay to light. Free Radical Biology & Medicine. 2012;53:1080–1087. doi: 10.1016/j.freeradbiomed.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wu Y, Wang Q, Zheng M, Cui Q. Glycerol or crude glycerol as substrates make Pseudomonas aeruginosa achieve anaerobic production of rhamnolipids. Microbial Cell Factories. 2021;20:185. doi: 10.1186/s12934-021-01676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Rock CO. RhlA converts beta-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the beta-hydroxydecanoyl-beta-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. Journal of Bacteriology. 2008;190:3147–3154. doi: 10.1128/JB.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Chen S, Sysoeva TA, You L, Balaban N. Universal antibiotic tolerance arising from antibiotic-triggered accumulation of pyocyanin in Pseudomonas aeruginosa. PLOS Biology. 2019;17:e3000573. doi: 10.1371/journal.pbio.3000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulianello L, Canard C, Köhler T, Caille D, Lacroix JS, Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infection and Immunity. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]