Abstract
Background
Evidence suggests that the SARS-CoV-2 omicron (B.1·1.529) is associated with lower risks of adverse outcomes than the delta (B.1.617.2) variant among the general population. However, little is known about outcomes after omicron infection in pregnancy. We aimed to assess and compare short-term pregnancy outcomes after SARS-CoV-2 delta and omicron infection in pregnancy.
Methods
We did a national population-based cohort study of women who had SARS-CoV-2 infection in pregnancy between May 17, 2021, and Jan 31, 2022. The primary maternal outcome was admission to critical care within 21 days of infection or death within 28 days of date of infection. Pregnancy outcomes were preterm birth and stillbirth within 28 days of infection. Neonatal outcomes were death within 28 days of birth, and low Apgar score (<7 of 10, for babies born at term) or neonatal SARS-CoV-2 infection in births occurring within 28 days of maternal infection. We used periods when variants were dominant in the general Scottish population, based on 50% or more of cases being S-gene positive (delta variant, from May 17 to Dec 14, 2021) or S-gene negative (omicron variant, from Dec 15, 2021, to Jan 31, 2022) as surrogates for variant infections. Analyses used logistic regression, adjusting for maternal age, deprivation quintile, ethnicity, weeks of gestation, and vaccination status. Sensitivity analyses included restricting the analysis to those with first confirmed SARS-CoV-2 infection and using periods when delta or omicron had 90% or more predominance.
Findings
Between May 17, 2021, and Jan 31, 2022, there were 9923 SARS-CoV-2 infections in 9823 pregnancies, in 9817 women in Scotland. Compared with infections in the delta-dominant period, SARS-CoV-2 infections in pregnancy in the omicron-dominant period were associated with lower maternal critical care admission risk (0·3% [13 of 4968] vs 1·8% [89 of 4955]; adjusted odds ratio 0·25, 95% CI 0·14–0·44) and lower preterm birth within 28 days of infection (1·8% [37 of 2048] vs 4·2% [98 of 2338]; 0·57, 95% CI 0·38–0·87). There were no maternal deaths within 28 days of infection. Estimates of low Apgar scores were imprecise due to low numbers (5 [1·2%] of 423 with omicron vs 11 [2·1%] of 528 with delta, adjusted odds ratio 0·72, 0·23–2·32). There were fewer stillbirths in the omicron-dominant period than in the delta-dominant period (4·3 [2 of 462] per 1000 births vs 20·3 [13 of 639] per 1000) and no neonatal deaths during the omicron-dominant period (0 [0 of 460] per 1000 births vs 6·3 [4 of 626] per 1000 births), thus numbers were too small to support adjusted analyses. Rates of neonatal infection were low in births within 28 days of maternal SARS-CoV-2 infection, with 11 cases of neonatal SARS-CoV-2 in the delta-dominant period, and 1 case in the omicron-dominant period. Of the 15 stillbirths, 12 occurred in women who had not received two or more doses of COVID-19 vaccination at the time of SARS-CoV-2 infection in pregnancy. All 12 cases of neonatal SARS-CoV-2 infection occurred in women who had not received two or more doses of vaccine at the time of maternal infection. Findings in sensitivity analyses were similar to those in the main analyses.
Interpretation
Pregnant women infected with SARS-CoV-2 were substantially less likely to have a preterm birth or maternal critical care admission during the omicron-dominant period than during the delta-dominant period.
Funding
Wellcome Trust, Tommy's charity, Medical Research Council, UK Research and Innovation, Health Data Research UK, National Core Studies—Data and Connectivity, Public Health Scotland, Scottish Government Health and Social Care, Scottish Government Chief Scientist Office, National Research Scotland.
Introduction
The SARS-CoV-2 omicron (B.1·1.529) variant rapidly became dominant in Scotland in December, 2021, as it has done in most other high-income countries.1 Emerging evidence suggests that omicron is associated with lower risks of adverse outcomes than previous variants, particularly delta (B.1.617.2), among the general population.2, 3 However, little is known about outcomes after omicron infection in pregnancy, although there have been reports of less severe disease with the omicron variant when compared with the delta variant in a selected population of pregnant women attending prenatal care.4
Research in context.
Evidence before this study
SARS-CoV-2 infection in pregnancy is associated with an increased risk of complications for women (critical care admission and preterm birth) and babies (stillbirth and neonatal mortality). Observational data suggest that the delta (B.1.617.2) variant is associated with particularly severe outcomes, but scarce data exists on the effects of the omicron (B.1.1.529) variant, which is currently dominant in many settings, in pregnancy. We searched PubMed for observational studies including pregnant women exposed to SARS-CoV-2 as of June 8, 2022, with the search terms pregnan* and COVID-19 or SARS-CoV-2, and Delta or Omicron or B.1.1.529 or B.1.617.2, with no language restrictions. A single study from the USA reported less severe maternal disease with omicron variant than with delta in a population of pregnant women attending prenatal care (2641 women). A small study in two maternity units, one in Turkey and one in the UK, found that outcomes of pregnant women admitted to hospital with omicron infection (n=77) were similar to those of pregnant women admitted during the pre-delta period, but the statistical power was low. We searched medRxiv for relevant preprint articles from Jan 01 to June 15, 2022, using the same search terms. We found a preprint study examining outcomes in 1561 women hospitalised with a positive SARS-CoV-2 PCR test up to 7 days before admission or during admission up to 2 days after giving birth, during the period when omicron was dominant. The authors found that the risk of severe respiratory disease among unvaccinated pregnant women admitted with symptomatic SARS-CoV-2 infection during the omicron-dominant period was similar to that observed during the period when the wildtype variant was dominant.
Added value of this study
To our knowledge, our study is the first to report population-level data of pregnant women exposed to the omicron variant, compared with delta, including those in early pregnancy. We found that the risk of maternal critical care, and COVID-19-related critical care, associated with SARS-CoV-2 infection in pregnancy during the omicron-dominant period was lower than that during the delta-dominant period. We found that SARS-CoV-2 infection in pregnancy during the omicron-dominant period was associated with substantially lower rates of preterm birth in the month after infection, compared with infection during the delta-dominant period. The numbers of stillbirths and neonatal deaths after SARS-CoV-2 infection during the omicron-dominant period were low.
Implications of all the available evidence
Our national population-based study shows that SARS-CoV-2 infection in pregnancy during the omicron-dominant period was associated with a lower risk of short-term maternal and perinatal adverse outcomes than infection during the delta-dominant period. Pregnant women, health-care professionals, and policy makers should be aware of this information as it can inform mitigation measures, such as guidance on partners attending hospital and provision of assisted reproduction treatments for unvaccinated women. Further studies are needed to assess longer-term pregnancy outcomes and rare outcomes such as stillbirth.
Understanding the impact of omicron and any future variants in pregnancy is important to inform appropriate public health measures to prevent infection in pregnant populations. Updated information is also crucial to inform policy around maternity and neonatal care provision, for example, for guidance on partners attending hospital and provision of assisted reproduction treatments for unvaccinated women.5, 6
In this study, we aimed to assess and compare short-term pregnancy-related outcomes after SARS-CoV-2 infection in pregnancy during omicron-dominant and delta-dominant periods, using whole population data from a national cohort.7, 8
Methods
Study design and population
For this population-based cohort study, we followed a pre-published protocol and statistical analysis plan with methods described in detail previously,7, 8, 9 and we report results according to STROBE guidelines.
Briefly, pregnant women were identified for inclusion through the COVID-19 in Pregnancy in Scotland Study (COPS) database.7, 8 COPS is a substudy of EAVE II (Early Pandemic Evaluation and Enhanced Surveillance of COVID-19).10 COPS comprises a dynamic database based on linkage of routine electronic health records, which includes all women aged 11–55 years at the time of conception who were known to be pregnant in Scotland from Jan 1, 2015, to the present date.
For the analyses presented here, we included the cohort of women who had SARS-CoV-2 infection in pregnancy during the period between May 17, 2021 (the start of the period in which the delta variant was dominant in Scotland), and Jan 31, 2022. We examined maternal and pregnancy outcomes occurring up to 28 days after SARS-CoV-2 infection in pregnancy. For liveborn babies born within 28 days of maternal infection, we also examined the babies' outcomes up to the end of the neonatal period (up to 28 days of life).
To allow for adequate follow-up and outcome ascertainment from health-care records,7, 8 we limited cases to those occurring up to Jan 31, 2022, given source data latency of up to 3 months.8
COPS has ethical approval from the National Research Ethics Service Committee, South East Scotland 02 (REC 12/SS/0201: SA 2) and information governance approval from the Public Benefit and Privacy Panel for Health and Social Care (2021–0116); written consent from participants was not required or obtained. All data were housed within Public Health Scotland and accessed only by approved researchers.
Exposures
SARS-CoV-2 infection in pregnancy was generally defined as infection diagnosed at any point from the date of conception (2+0 weeks of gestation) to the date the pregnancy ended inclusive (censoring infections occurring at 44+0 weeks of gestation or more, because it was very likely that these women would have completed their pregnancy, but the end of pregnancy record had not yet been received by Public Health Scotland). More specific exposure periods for individual outcomes are described in the appendix (p 2). The date of first positive sample collection was taken as the date of onset of the first episode of SARS-CoV-2 infection. Subsequent episodes were recorded if a positive sample was taken 90 days or more after a first positive sample. Throughout the study period, confirmed infections (maternal or neonatal) were determined by a positive RT-PCR test. From Jan 6, 2022, onwards, confirmed infections were also determined by a positive lateral flow device (provided it was not followed by a negative RT-PCR within 48 h).11
We used period of infection to designate SARS-CoV-2 variants. We defined two study exposure periods indicating times during which delta or omicron variants were dominant. On the basis of the S gene status of positive RT-PCR samples taken from the general population in Scotland (available for 95% of RT-PCR tests during the study period), we defined the delta period as May 17, 2021, to Dec 14, 2021, (>50% of samples were S-gene positive) and the omicron period as Dec 15, 2021, to Jan 31, 2022 (>50% of samples were S-gene negative; appendix p 10). We chose a 50% threshold to define dominance to align to other studies of SARS-CoV-2 variants in pregnancy.4, 12 We tested the impact of using this cutoff in a prespecified sensitivity analysis of infections during periods when 90% of SARS-CoV-2 infections were S-gene positive (delta; June 19, 2021, to Dec 7, 2021) or after the point when 90% of SARS-CoV-2 infections became S-gene negative again (omicron; Dec 27, 2021, to Jan 31, 2022). We were unable to directly use viral sequencing data for our analyses as this was only done on a small proportion (about 20%) of all population tests in Scotland.13
Outcomes
Outcome definitions, data sources, and relevant exposure periods are summarised in the appendix (p 2). The primary maternal outcome was admission to critical care or maternal death. Critical care admission was defined as any admission to critical care in which the date of onset of infection occurred during a critical care admission or within the 21 days before admission, using critical care discharge records from the Scottish Intensive Care Society Audit Group (SICSAG). Completed admissions to all intensive care units and general (non-obstetric) high-dependency units across Scotland were included. Completed admissions to the seven obstetric high-dependency units that contribute data to SICSAG (collectively covering about 60% of deliveries in Scotland) were also included. Maternal death was defined as any death within 28 days of the date of infection.
We also examined the more stringent outcome of admission to critical care in which COVID-19 was recorded as a primary or secondary diagnostic code (but not coded as an incidental infection), or in which COVID-19 was recorded as the primary cause of maternal death.
Pregnancy outcomes included stillbirths within 28 days of infection, and preterm birth (20+0 weeks to 36+6 weeks of gestation) in livebirths within 28 days of infection. We used a broader definition of stillbirth than the one normally used in the UK, including late fetal losses from 20+0 weeks to 23+6 weeks of gestation, as well as statutorily registerable stillbirths from 24+0 weeks onwards. This definition aligns to one used in other countries such as the USA and Australia.14 Late fetal losses share similar causes with stillbirths that occur after 24 weeks of gestation and are included in stakeholder developed core outcome sets for stillbirth prevention.15
Neonatal outcomes examined among liveborn babies born within 28 days of maternal infection included neonatal death (death from any cause within 28 days of birth), low 5-min Apgar score (<7 of 10), and confirmed neonatal infection with SARS-CoV-2 within 28 days of birth in livebirths within 28 days of maternal infection. Examination of Apgar score was restricted to babies born at term (≥37+0 weeks of gestation) as preterm birth is recognised to be strongly associated with low Apgar scores.16
Covariates
We included the following potentially confounding variables within our models: maternal age, deprivation quintile (based on the Scottish index of multiple deprivation17), maternal ethnicity (White; South Asian; Black, Caribbean, or African; mixed or other ethnic group, and unknown), week of gestation at time of infection, and vaccination status at time of infection (categorised as unvaccinated [no COVID-19 vaccination before the date of onset of SARS-CoV-2 infection, or with one dose of vaccination ≤21 days before the date of onset]; one dose [one dose of vaccination >21 days before the date of onset of infection, or two doses with the second dose ≤14 days before the date of onset]; two doses [two doses with the second dose >14 days before infection, or three doses with the third dose ≤14 days before the date of onset]; or three or more doses [three or more doses, with the third dose >14 days before infection]). In a post-hoc additional analysis, we also included clinical vulnerability status (ascertained from the national shielding list maintained by Public Health Scotland for extreme clinical vulnerability18 and general practitioner records for clinical vulnerability19) and smoking status. We were not able to adjust for body-mass index (BMI; which is ascertained from completed birth records) because of imbalances in missing data due to a higher proportion of ongoing pregnancies in the omicron-dominant period.
We did not adjust for previous SARS-CoV-2 infection, but we explored the effect of previous infection in a pre-specified sensitivity analysis restricting the cohort to women who had not had a previous confirmed SARS-CoV-2 infection recorded within national records.
Statistical analysis
Monthly infection rates were calculated as the number of pregnant women infected during the month divided by the number of women with ongoing pregnancies at the start of the month of interest. Infection rates were stratified by trimester at the time of infection.
We compared maternal, pregnancy, and neonatal outcomes in the delta and omicron pregnant cohorts using logistic regression, with adjustments for maternal age, deprivation, ethnicity, vaccination status at the time of infection, and gestation at the time of infection (except for in the analysis of Apgar score, as this outcome was only considered in babies born at term and thus was restricted to infections ≥33+0 weeks of gestation). Clustering was used to account for pregnancies with multiple babies (as babies from the same pregnancy are more likely to have the same outcome as each other). Odds ratios (ORs) with 95% CIs were produced for each comparison. In post-hoc additional analyses, we explored a potential interaction between SARS-CoV-2 variant and vaccination status at the time of infection by including an interaction term between these predictors within the models examining critical care admission or death and preterm birth. In response to a suggestion from reviewers on submission of the manuscript to this journal, we also did two additional post-hoc analyses for the outcomes of any critical care admission or death, critical care admission or death due to COVID-19, and preterm birth. Firstly, we restricted the analysis to women who were unvaccinated at the time of infection in pregnancy (ie, had not received a first dose of a COVID-19 vaccine >21 days before infection). Secondly, we included additional covariates in the model to adjust for any potential confounding effect from clinical vulnerability or smoking status.
Analyses were done in R, version 3.6.1. We used the package named survey (version 4.1-1) for conditional logistic regression to account for clustering. The code is available online.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
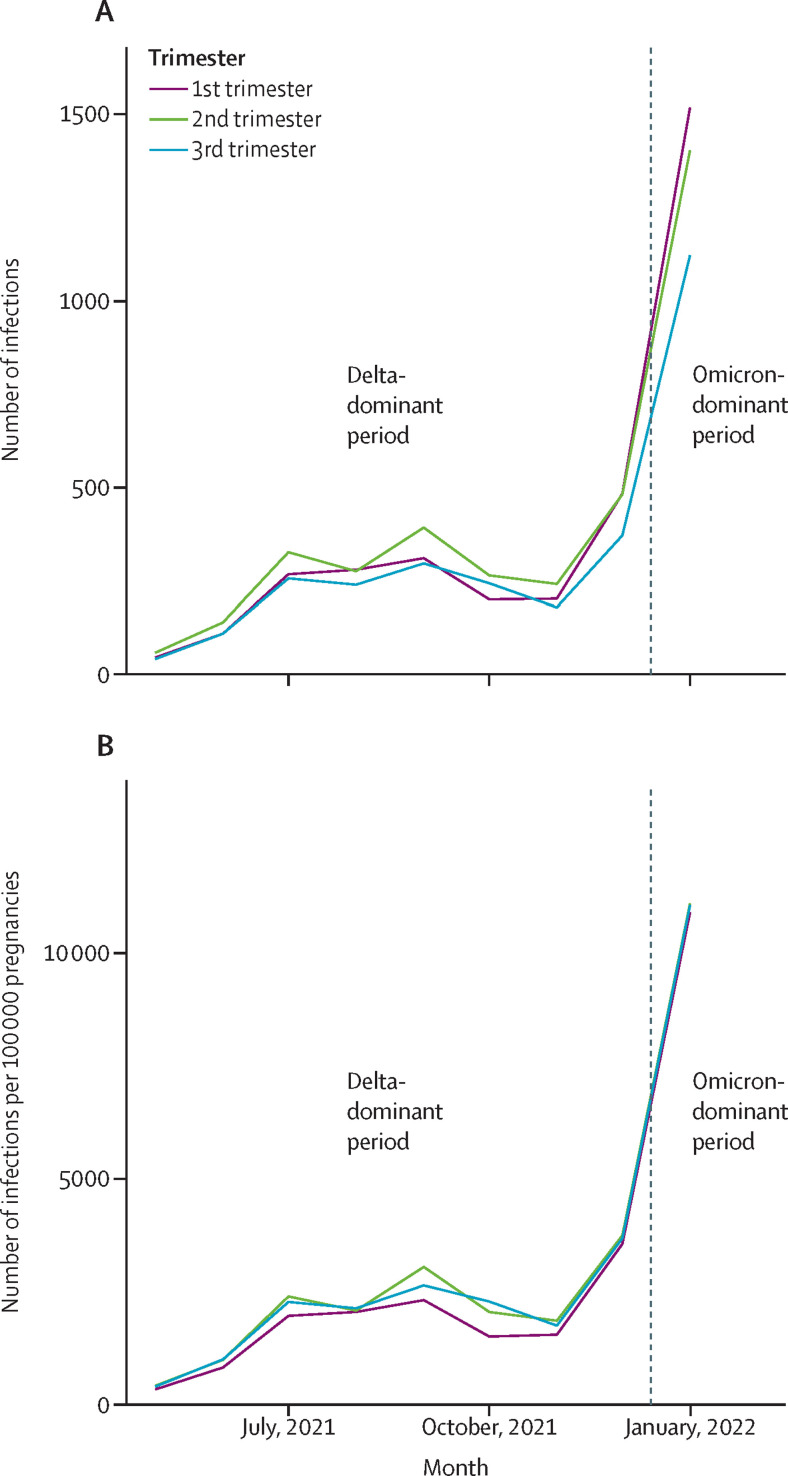
Between May 17, 2021, and Jan 31, 2022, there were 9923 SARS-CoV-2 infections in 9823 pregnancies, in 9817 women in Scotland. Disregarding May and December (the months in which delta was dominant for only part of the month), the monthly infection rate during the delta-dominant period ranged from 1710 per 100 000 pregnant women (November, 2021) to 2658 per 100 000 pregnant women (September, 2021). In the omicron-dominant period (January, 2022) the infection rate was 11 011 per 100 000 pregnancies. The number and rate of SARS-CoV-2 infections confirmed by RT-PCR in pregnant women in Scotland over the study period are shown in figure 1 .
Figure 1.
SARS-CoV-2 infection in women who were pregnant from May, 2021, to January, 2022
(A) Number of infections. (B) Infection rate per 100 000 pregnant women.
In the delta-dominant period, two women were infected in two different pregnancies and eight women were infected twice during the same pregnancy (4955 infections in 4947 pregnancies, in 4945 women). In the much shorter omicron-dominant period, there were no recorded repeat infections (4968 infections in 4968 pregnancies, in 4968 women). However, 96 of the women infected in the omicron-dominant period had been previously infected in pregnancy during the delta-dominant period.
Maternal demographics of pregnant women infected with SARS-CoV-2 were similar over the two time periods (table ). Of note, a higher proportion of women with SARS-CoV-2 infection in pregnancy in the omicron-dominant period had received one or more doses of COVID-19 vaccine than in the delta-dominant period, which reflects the rollout of the vaccination programme.
Table.
Characteristics of pregnant women with SARS-CoV-2 infection during pregnancy included in the study
| Delta-dominant period | Omicron-dominant period | ||
|---|---|---|---|
| Infections | 4955 | 4968 | |
| Trimester 1 (2+0 to 13+6 weeks of gestation) | 1579 (31·9% [30·3–33·4]) | 1869 (37·6% [36·1–39·2]) | |
| Trimester 2 (14+0 to 27+6 weeks gestation) | 1870 (37·7% [36·2–39·3]) | 1724 (34·7% [33·2–36·3]) | |
| Trimester 3 (≥28+0 weeks gestation) | 1506 (30·4% [28·9–32·0]) | 1375 (27·7% [26·1–29·2]) | |
| Age group, years | |||
| ≤19 | 224 (4·5% [3·0–6·0]) | 194 (3·9% [2·4–5·4]) | |
| 20–24 | 836 (16·9% [15·4–18·3]) | 802 (16·1% [14·7–17·6]) | |
| 25–29 | 1478 (29·8% [28·4–31·3]) | 1495 (30·1% [28·6–31·6]) | |
| 30–34 | 1521 (30·7% [29·2–32·2]) | 1579 (31·8% [30·3–33·3]) | |
| 35–39 | 747 (15·1% [13·6–16·6]) | 754 (15·2% [13·7–16·7]) | |
| ≥40 | 149 (3·0% [1·5–4·5]) | 144 (2·9% [1·4–4·4]) | |
| Missing | 0 | 0 | |
| SIMD quintile | |||
| 1 (most deprived) | 1330 (26·8% [25·4–28·3]) | 1241 (25·0% [23·6–26·4]) | |
| 2 | 1155 (23·3% [21·9–24·8]) | 1058 (21·3% [19·9–22·8]) | |
| 3 | 892 (18·0% [16·6–19·5]) | 891 (17·9% [16·5–19·4]) | |
| 4 | 880 (17·8% [16·3–19·2]) | 977 (19·7% [18·2–21·1]) | |
| 5 (least deprived) | 691 (13·9% [12·5–15·4]) | 781 (15·7% [14·3–17·2]) | |
| Missing or unknown | 7 (0·1% [0–1·6]) | 20 (0·4% [0–1·9]) | |
| Ethnicity | |||
| White | 4535 (91·5% [90·8–92·2]) | 4532 (91·2% [90·5–92·0]) | |
| South Asian | 133 (2·7% [2·0–3·4]) | 129 (2·6% [1·9–3·3]) | |
| Black, Caribbean, or African | 61 (1·2% [0·5–1·9]) | 80 (1·6% [0·9–2·3]) | |
| Other or mixed ethnicity | 140 (2·8% [2·1–3·5]) | 121 (2·4% [1·7–3·2]) | |
| Missing or unknown | 86 (1·7% [1·0–2·4]) | 106 (2·1% [1·4–2·9]) | |
| Vaccination status at time of infection | |||
| Unvaccinated | 3006 (60·7% [59·3–62·1]) | 1227 (24·7% [23·2–26·2]) | |
| One dose | 720 (14·5% [13·1–16·0]) | 385 (7·7% [6·3–9·2]) | |
| Two doses | 1198 (24·2% [22·8–25·6]) | 2375 (47·8% [46·3–49·3]) | |
| Three or more doses | 31 (0·6% [0–2·1]) | 981 (19·7% [18·3–21·2]) | |
Data are n (% [95% CI]). The delta (B.1.617.2)-dominant period was defined as the period between May 17, 2021, and Dec 14, 2021. The omicron (B.1.1.529)-dominant period was defined as the period between Dec 15, 2021, and Jan 31, 2022. SIMD=Scottish index of multiple deprivation.
We observed one maternal death after COVID-19 in pregnancy in the delta-dominant period. However, this occurred more than 28 days after the first SARS-CoV-2-positive test, and thus it is not included in our outcome definition. Therefore, all models for critical care admission or death included only critical care admissions.
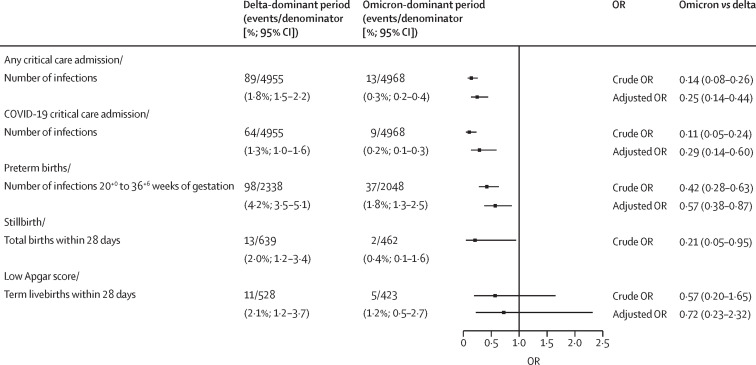
Compared with the delta-dominant period, SARS-CoV-2 infection in pregnancy during the omicron-dominant period was associated with a reduction in any critical care admission (0·3% [13 of 4968] vs 1·8% [89 of 4955]; adjusted OR 0·25, 95% CI 0·14–0·44) and a reduction in critical care admission with COVID-19 (0·2% [9 of 4968] vs 1·3% [64 of 4955]; 0·29, 0·14–0·60; figure 2 ). There were no maternal deaths within 28 days of infection.
Figure 2.
Crude and adjusted ORs for all outcomes
“Within 28 days” means within 28 days of a positive maternal RT-PCR test. OR=odds ratio.
There were 4386 livebirths occurring within 28 days of SARS-CoV-2 infection between 20 and 36+6 weeks of gestation. Infections in the omicron-dominant period were also associated with a reduction in preterm birth within 28 days of infection at 20+0 to 36+6 weeks of gestation (1·8% [37 of 2048] vs 4·2% [98 of 2338]; 0·57, 95% CI 0·38–0·87) compared with infections in the delta-dominant period. Full models for critical care admission and preterm birth are shown in the appendix (p 3).
There was imprecision surrounding estimates of low Apgar scores due to low numbers (5 [1·2%] of 423 with omicron vs 11 [2·1%] of 528 with delta; adjusted OR 0·72, 95% CI 0·23–2·32). We observed fewer stillbirths in the omicron-dominant period versus delta-dominant period (4·3 [2 of 462] per 1000 births vs 20·3 [13 of 639] per 1000; crude OR 0·21, 95% CI 0·05–0·95) and no neonatal deaths during the omicron-dominant period (0 [0 of 460] per 1000 births vs 6·3 [4 of 626] per 1000 births), so numbers were too small to support adjusted analyses.
Rates of neonatal infection were low in births within 28 days of maternal SARS-CoV-2 infection, with 11 cases of neonatal SARS-CoV-2 after maternal infection in the delta-dominant period (rate of 17·6 per 1000 livebirths within 28 days of maternal infection), and one case of neonatal SARS-CoV-2 after maternal infection in the omicron-dominant period (2·1 per 1000 livebirths).
Regarding the sensitivity analyses restricted to women with infections at periods with 90% or more of delta and omicron predominance (appendix pp 4, 11), including women with first infections only (appendix pp 5, 12), or including women who were unvaccinated at the time of SARS-CoV-2 infection in pregnancy (appendix pp 6, 13), the findings were similar to those in the main analyses, although numbers were lower, and thus 95% CIs were wider around estimates. The inclusion of smoking and clinical vulnerability status in models did not materially change the results (appendix pp 7–8, 14). The inclusion of a term for an interaction between variant and vaccination status at the time of infection was not significant (appendix p 9), although it was imprecisely estimated.
Discussion
We have used whole population data from Scotland to show for the first time, to our knowledge, that the risk of preterm birth in the 28 days after SARS-CoV-2 infection in pregnancy was substantially lower in the omicron-dominant period compared with the delta-dominant period. We also show that the risk of maternal critical care admission was lower after infection in the omicron-dominant period than in the delta-dominant period. These risk reductions persisted after adjustment for maternal age, deprivation, ethnicity, vaccination status, and gestation at the time of infection and were robust to sensitivity analyses. These data suggest that, as in non-pregnant adults, the omicron variant is less commonly associated with severe COVID-19 than the delta variant.13
We saw some evidence of a reduced risk of stillbirth and neonatal deaths in association with infection in the omicron-dominant period compared with the delta-dominant period. However, the numbers of deaths in the omicron-dominant period were very small, and thus we were unable to meaningfully adjust for potential confounding factors in our comparisons. Only three of 15 stillbirths, and no neonatal infections occurred in women who had two or more doses of vaccine at the time of maternal infection. This supports evidence that maternal COVID-19 vaccination is protective against severe pregnancy and neonatal outcomes.9
Our findings on critical care admission after SARS-CoV-2 infection in whole pregnancy and whole population data substantiate those from a 2022 study looking at outcomes of pregnant women with COVID-19 attending prenatal care at a single centre in the USA.4 Another USA hospital-based study found higher rates of asymptomatic COVID-19 in pregnant women admitted to hospital who were COVID-19-positive in the omicron-dominant period versus delta-dominant period, with lower rates of maternal respiratory support use. However, analyses were unadjusted and vaccination rates were higher in women admitted in the omicron-dominant period than in the delta-dominant period.20 We found that the reduction in maternal critical care admissions in association with SARS-CoV-2 infection in the omicron-dominant period persisted even when only first SARS-CoV-2 infections were considered or only unvaccinated women were included in analyses. This suggests that differences were not due to maternal immunity to SARS-CoV-2 and are most likely to be associated with the variant.
The key strengths of our study are that we used whole population data, with a prespecified protocol and analysis plan. Our study also had some limitations that need to be considered. Firstly, we did not have sequencing for all samples, so we used the dominant variant in the wider population at the time of SARS-CoV-2 infection as a proxy for variant identification. Secondly, at the time of analysis, the omicron-dominant period was relatively short. We restricted our study period to infections occurring up to the end of January, 2022, to ensure that sufficient time was allowed to reliably ascertain outcome events after infections occurring across the study period.8 Thirdly, we had an insufficient sample size for the adjusted analysis of rare, but important outcomes such as stillbirth and neonatal death. Fourthly, there is the potential for unmeasured or residual confounding. We specified a priori a small number of covariates within models, given that we anticipated a low number of women and babies with outcomes of interest. We adjusted for potential biases arising from group imbalances in maternal age, ethnicity, deprivation quintile, vaccination status, and week of gestation at the time of infection, which are important potential confounders.21 In a post-hoc additional analysis including smoking status and comorbidities, the results were similar to those of the main analysis. However, we could not account for all potential confounders. For example, BMI is ascertained from completed maternity hospital discharge records generated at the end of pregnancy. We were unable to include BMI in our models because of imbalances in missing data, as there were a higher proportion of ongoing pregnancies in the omicron-dominant period. We were also unable to account for seasonal variation in outcomes. Finally, it is possible that there was a change in pregnancy outcomes more generally over the periods studied, independent of SARS-CoV-2 variant circulation. However, this seems unlikely given no such change has been seen in routine surveillance data for pregnancy outcomes such as preterm birth, stillbirth, and Apgar score.22
In conclusion, our data suggest that SARS-CoV-2 infection in pregnancy during the omicron-dominant period was associated with reduced risk of complications compared with the delta-dominant period, with substantially decreased risk for critical care admission and preterm birth. Future work should capture pregnancy outcomes with a longer timeframe than 28 days, and meta-analyses with data from other sources is important to determine effects on rarer outcomes such as stillbirth. Additionally, the development and validation of prediction models to identify women at highest risk of complications from SARS-CoV-2 infection would be clinically useful when sufficient size cohorts allow adequately powered analyses.
Data sharing
Aggregate data files of infections among pregnant women are available online (https://www.opendata.nhs.scot/organization/health_protection). Patient-level data underlying this article cannot be shared publicly due to data protection and confidentiality requirements. Public Health Scotland and the Chief Medical Officer for Scotland are the data holders for the data used in this study. Data can be made available to approved researchers for analysis after securing relevant permissions from the data holders through the Public Benefit and Privacy Panel. Enquiries regarding data availability should be directed to phs.edris@phs.scot.
Declaration of interests
AS and CR were members of the Scottish Government's COVID-19 Advisory Group and are members of the New and Emerging Respiratory Virus Threats Advisory Group risk stratification subgroup and the Scottish Government's Committee on Pandemic Preparedness (unremunerated roles). CR is a member of the Scientific Pandemic Influenza Group on Modelling (unremunerated role). AS is a member of AstraZeneca's Thrombotic Thrombocytopenic Advisory Group (unremunerated role). SVK was co-chair of Scottish Government's Expert Reference Group on Ethnicity and COVID-19 (unremunerated role). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Our thanks to the EAVE II Patient Advisory Group and Sands charity for their support. COPS is a substudy of EAVE II, which is funded by the Medical Research Council (MR/R008345/1) with the support of BREATHE—The Health Data Research Hub for Respiratory Health (MC_PC_19004), which is funded through the UK Research and Innovation Industrial Strategy Challenge Fund and delivered through Health Data Research UK. Additional support has been provided through Public Health Scotland and Scottish Government DG Health and Social Care and the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation. COPS has received additional funding from Tommy's charity. SJS is funded by a Wellcome Trust Clinical Career Development Fellowship (209560/Z/17/Z). SVK acknowledges funding from a NRS Senior Clinical Fellowship (SCAF/15/02), the Medical Research Council (MC_UU_00022/2), and the Scottish Government Chief Scientist Office (SPHSU17).
Contributors
SJS, CC, RW, CR, and AS conceived and designed the study. SJS, EM, CR, and RW drafted the protocol. JC, CD, JD, LEMH, LH, AG, LL, TM, and EM prepared the dataset for analysis. EM, LL, and JC accessed and verified the data and performed data analysis. SJS and EM prepared the first draft of the manuscript. All authors provided critical input to drafts of the manuscript. SJS, CC, RW, and AS gave final approval of the version to be published. SJS and RW acts as guarantors for the study. All authors had the final responsibility to submit for publication.
Supplementary Material
References
- 1.Sheikh A, Kerr S, Woolhouse M, et al. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis. 2022;22:959–966. doi: 10.1016/S1473-3099(22)00141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhi SA, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with omicron variant in South Africa. N Engl J Med. 2022;386:1314–1326. doi: 10.1056/NEJMoa2119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhikari EH, MacDonald L, SoRelle JA, Morse J, Pruszynski J, Spong CY. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with delta (B.1.617.2) and omicron (B.1.1.529) variant predominance. JAMA. 2022;327:1500–1502. doi: 10.1001/jama.2022.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scottish Government Coronavirus (COVID-19): fertility treatment for unvaccinated patients. 2022. https://www.gov.scot/publications/coronavirus-covid-19-fertility-treatment-for-unvaccinated-patients/
- 6.Black M, Farre A, Gray NM, Kynn M, Gavine AAS. Public Heath Scotland; Edinburgh: 2022. Perinatal experiences during the COVID-19 pandemic in Scotland: exploring the impact of changes in maternity services on women and staff. [Google Scholar]
- 7.Stock SJ, McAllister D, Vasileiou E, et al. COVID-19 in Pregnancy in Scotland (COPS): protocol for an observational study using linked Scottish national data. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock SJ, Carruthers J, Denny C, et al. Cohort profile: the COVID-19 in Pregnancy in Scotland (COPS) dynamic cohort of pregnant women to assess effects of viral and vaccine exposures on pregnancy. Int J Epidemiol. 2022 doi: 10.1093/ije/dyab243. published online Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28:504–512. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulholland RH, Vasileiou E, Simpson CR, et al. Cohort profile: early pandemic evaluation and enhanced surveillance of COVID-19 (EAVE II) database. Int J Epidemiol. 2021;50:1064–1074. doi: 10.1093/ije/dyab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health Scotland Further changes to COVID-19 reporting. 2022. https://publichealthscotland.scot/news/2022/january/further-changes-to-covid-19-reporting/
- 12.Vousden N, Ramakrishnan R, Bunch K, et al. Severity of maternal infection and perinatal outcomes during periods of SARS-CoV-2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. BMJ Med. 2022;1 doi: 10.1136/bmjmed-2021-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr S, Robertson C, Hillman S, Grange Z, Sullivan C, Sheikh A. Severity of BA.2 variant and vaccine effectiveness against symptomatic disease in Scotland. Lancet Reg Health Eur (in press). [DOI] [PMC free article] [PubMed]
- 14.Reinebrant HE, Leisher SH, Coory M, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth. BJOG. 2018;125:212–224. doi: 10.1111/1471-0528.14971. [DOI] [PubMed] [Google Scholar]
- 15.Kim BV, Aromataris EC, Middleton P, et al. Development of a core outcome set for interventions to prevent stillbirth. Aust N Z J Obstet Gynaecol. 2021;61:658–666. doi: 10.1111/ajo.13369. [DOI] [PubMed] [Google Scholar]
- 16.Iliodromiti S, Mackay DF, Smith GC, Pell JP, Nelson SM. Apgar score and the risk of cause-specific infant mortality: a population-based cohort study. Lancet. 2014;384:1749–1755. doi: 10.1016/S0140-6736(14)61135-1. [DOI] [PubMed] [Google Scholar]
- 17.Scottish Government Scottish index of multiple deprivation. https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/
- 18.Public Health Scotland Search criteria for highest risk patients for inclusion to the shielding list. 2021. https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3008/documents/1_covid-19-search-criteria-highest-risk-patients.pdf
- 19.Clift AK, Coupland CAC, Keogh RH, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371 doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seasely AR, Blanchard CT, Arora N, et al. Maternal and perinatal outcomes associated with the omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Obstet Gynecol. 2022;140:262–265. doi: 10.1097/AOG.0000000000004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fell DB, Dimitris MC, Hutcheon JA, et al. Guidance for design and analysis of observational studies of fetal and newborn outcomes following COVID-19 vaccination during pregnancy. Vaccine. 2021;39:1882–1886. doi: 10.1016/j.vaccine.2021.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health Scotland COVID-19 wider impacts on the health care system. Available at https://scotland.shinyapps.io/phs-covid-wider-impact/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregate data files of infections among pregnant women are available online (https://www.opendata.nhs.scot/organization/health_protection). Patient-level data underlying this article cannot be shared publicly due to data protection and confidentiality requirements. Public Health Scotland and the Chief Medical Officer for Scotland are the data holders for the data used in this study. Data can be made available to approved researchers for analysis after securing relevant permissions from the data holders through the Public Benefit and Privacy Panel. Enquiries regarding data availability should be directed to phs.edris@phs.scot.