Introduction
The pathophysiology linking Chiari I malformation to syringomyelia development and progression remains controversial. Neurosurgeons and scientists generally agree that obstruction of the normal circulation of cerebrospinal (CSF) flow in and around the foramen magnum is a critical component of Chiari I-associated syringomyelia pathogenesis. This article will review experimental and clinical observations about Chiari I malformation-associated syringomyelia and scientific investigations providing insights into how obstruction of normal CSF flow at the foramen magnum could, by a hydrodynamic mechanism, lead to syringomyelia development and progression.
History
In 1891, Hans Chiari originally described his type I malformation as involving tonsillar ectopia and hydrocephalus, but not syringomyelia.1 Earlier, the German pathologist Theodor Langhans published a case of cerebellar tonsillar extension inferior to the foramen magnum associated with an intramedullary cystic fluid collection in the cervical spinal cord. The title of his 1881 article, Über Höhlenbildung im Rückenmark als Folge Blutstauung (translated as “Regarding cavity creation in the spinal cord as a consequence of obstruction to blood flow”), implied an ischemic etiology for syringomyelia. However, the text noted cerebrospinal (CSF) flow obstruction as another factor in syrinx formation, “The increase in pressure in the cerebellar cavity will hinder or greatly impede the outflow of blood and cerebral spinal fluid.” 2 Later, neurosurgeons recognized that syringomyelia resolved after decompressing the Chiari I malformation at the foramen magnum and re-establishing normal CSF flow there.3,4 Classic theories of syringomyelia pathogenesis associated with Chiari I malformation included Gardner’s Theory, in which the fourth ventricle created a ′water-hammer″ pulse wave during cardiac systole. The pulse wave passed through the obex to the central canal of the spinal cord, which progressively expanded into a syrinx.3 Williams believed that pressure differentials created between the intracranial cavity and spinal canal during coughing and straining (Valsalva) drove CSF from the fourth ventricle to the spinal cord central canal, forming a syrinx.4 Both theories require the presence of a patent central canal of the spinal cord between the fourth ventricle and syrinx. However, pathologic and radiographic studies showed that the central canal is rarely patent and usually obstructed at several spinal segments in adults with or without syringomyelia. 5,6
More recently, investigators demonstrated various factors associated with syringomyelia in Chiari I malformation patients. One factor was the extent to which the cerebellar tonsillar ectopia obstructed the normal flow of cerebrospinal fluid at the foramen magnum as evaluated by phase-contrast cine-MRI. Caudal location of the medulla also significantly heightened the risk of syringomyelia. Syringomyelia was three times likelier in Chiari I malformation patients with a low-obex position (54%) than a normal position (18%).7 Other factors were the reduced volume of the entire posterior fossa or its inferior part around the foramen magnum and abnormal tapering of the width of the adjacent upper cervical spinal canal.8–10 Syringomyelia also accompanied intradural adhesions and arachnoidopathy, which narrow the CSF pathways at the foramen magnum.11 Narrowing of the foramen of Magendie also impeded CSF flow. 12,13 Basilar invagination, if present, can compress the anterior subarachnoid CSF pathway across the craniospinal junction in patients with Chiari I malformation. In short, Chiari I malformation patients with syringomyelia have severely narrowed CSF pathways in and around the foramen magnum.
Definitions
Syringomyelia is an intramedullary fluid-filled cavity that distends the spinal cord and is longer than one spinal segment. Communicating syringomyelia is an enlargement of the spinal cord central canal communicating with an enlarged fourth ventricle.14 Hydromyelia is communicating syringomyelia that may be associated with hydrocephalus. Non-communicating syringomyelia is an enlargement of the spinal cord central canal that does not communicate with an enlarged fourth ventricle.14 Extracanalicular syringomyelia is an intramedullary fluid-filled cavity within the spinal cord parenchyma that does not communicate with the fourth ventricle or spinal cord central canal. Atrophic syringomyelia is an intramedullary fluid-filled cavity associated with myelomalacia.14 Tumor-related syringomyelia is an intramedullary fluid collection often communicating with the spinal cord central canal and consisting of plasma ultrafiltrate of permeable tumor capillaries. A neoplastic cyst is a local intramedullary tumor cyst.14 The obex is a thin triangular gray matter membrane located 1 to 2 mm posterior to the apertura canalis centralis, the ostia of the central canal in the inferior fourth ventricle.15 On MRI, the location of the apertura canalis centralis is referred to as the obex. Medical personnel often refer to the apertura canalis centralis as the obex.
Background
Hydrodynamics is the branch of physics exploring the motion and forces of fluids on adjacent solid bodies.16 In spinal hydrodynamics, the subarachnoid space and the central canal of the spinal cord are the fluid sites, and the solid body is the spinal cord parenchyma. Neurosurgeons and engineers have long been intrigued by the possibility of a hydrodynamic link between the Chiari I malformation and syringomyelia. The Chiari I malformation consists of hindbrain herniation, a process in which the cerebellar tonsils and medulla extend inferior to their normal anatomical position. Hindbrain herniation into the foramen magnum reduces the area for CSF flow across the foramen magnum. Severe reduction of CSF flow area (A) within and around the foramen magnum is associated with syringomyelia. Syringes resolve after surgical procedures that expand the CSF flow area. Reduced CSF flow area is a critical hydrodynamic factor in syringomyelia development and progression. 17–19
A central formula in hydrodynamics is the Hagen-Poiseuille equation: ΔP = 8μLQ / πr4, in which ΔP is the difference in pressure between the ends of a circular pipe of a certain length (L), radius (r), volumetric laminar flow rate (Q), and dynamic viscosity (μ).20 The formula for cross-sectional area of a circle is A = πr2 so ΔP = 8μLQ / πr4 can also be stated ΔP = 8μLQπ / π2r4 or ΔP = 8μLQπ /A2. The Hagen-Poiseuille equation is better suited to predicting blood flow in a cylindrical artery than in the CSF pathway at the foramen magnum, where a CSF layer surrounds the neural elements. However, the Hagen-Poiseuille equation can be modified to predict flow in annular pathways.21 The Hagen-Poiseuille equation does not consider non-laminar CSF flow (turbulent flow), wall stress, friction, and irregular flow areas that reduce foramen magnum CSF flow. CSF flow at the foramen magnum varies from tubular laminar flow even further because the CSF flow across the foramen magnum is oscillatory, with ΔP changing from positive to negative and CSF flow (Q) changing in direction during every cardiac cycle, unlike an artery in which ΔP is always positive and flow (Q) always moves in the same direction (away from the heart). 21 To further complicate the modeling of CSF flow across the foramen magnum, the neural elements move within the foramen magnum during the cardiac cycle, although less than the surrounding CSF.22 Nonetheless, the Hagen-Poiseuille equation is a guide for estimating the effect of the Chiari I malformation in narrowing the CSF pathways. We can simplify the Hagen-Poiseuille equation to ΔP = KQ /A2 by considering 8μLπ a constant, K. Using that formula, a one-half reduction of the cross-sectional area at the foramen magnum (A) would require four times the pressure differential (ΔP) to displace the normal amount of CSF across the foramen magnum during the systolic and diastolic phases of the cardiac cycle.17
The Chiari I malformation’s reduction of the cross-sectional area (A) for CSF flow at the foramen magnum prevents dampening of the intracranial pressure pulse wave afforded by normal CSF outflow from the intracranial cavity to the spinal subarachnoid space in cardiac systole. Reduced CSF flow across the foramen magnum heightens the pressure change (ΔP) across the foramen magnum because the intracranial cavity has reduced compliance in patients with Chiari I and syringomyelia (Fig. 1). The high-pressure differential (ΔP) across the foramen magnum causes the hindbrain (cerebellar tonsils and medulla) to descend in cardiac systole and ascend in cardiac diastole in a pulsatile motion as seen on intraoperative ultrasonography after craniectomy17 (Fig. 2.A.). During cardiac systole, the hindbrain and CSF descend and transfer the enlarged intracranial pulse pressure (ΔP) to the partially-enclosed spinal subarachnoid space (Fig. 2. B).17 Craniocervical decompression, the operative treatment for Chiari I malformation, relieves dorsal compression on the cerebellar tonsils and cisterna magna. The cisterna magnum capacity expands as it fills with CSF after this procedure. The cross-sectional area (A) for CSF flow at the foramen magnum increases, and the pressure differential required for normal CSF flow across the foramen magnum during the cardiac cycle decreases. Additionally, craniocervical decompression removes the rigid boney dorsal covering of the foramen magnum, replacing it with compliant soft tissue that can dampen CSF pressure waves across it.17
Figure 1:
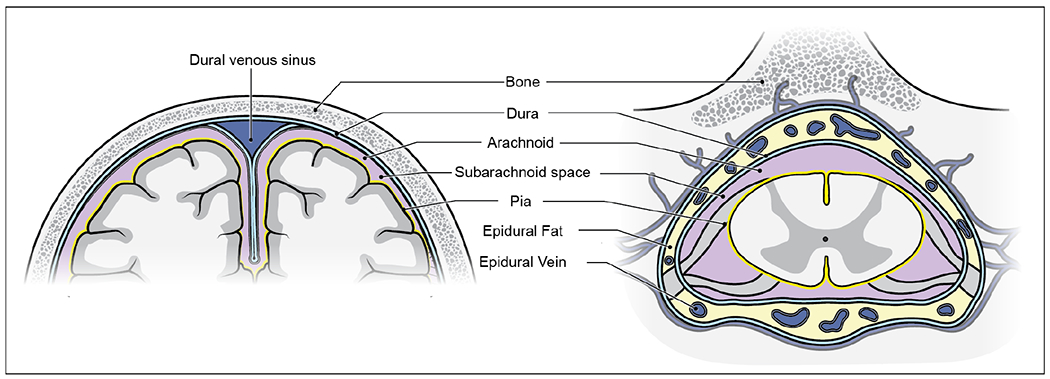
The rigid skull surrounds the intracranial cavity (left), which is much less compliant than the spinal intradural space (right). The spinal dura (right) can expand into the epidural space during cardiac systole because of the surrounding compressible fat and veins and the ligamentum flavum covering the interlaminar spaces. CSF is normally driven across from the incompliant intracranial cavity to the more compliant spinal subarachnoid space to compensate for the volumetric expansion of intracranial arteries during cardiac systole.
Figure 2:

During cardiac systole, the Chiari I malformation obstructs typical CSF displacement from the intracranial cavity to the spinal subarachnoid space. In place of CSF, the cerebellar tonsils and medulla are displaced inferiorly, creating an enlarged cervical subarachnoid pressure wave (A). The pressure wave propels syrinx fluid motion that elongates the syrinx (A). The pressure wave also drives CSF into the perivascular spaces of the spinal cord. It may oppose spinal cord interstitial fluid egress through the glymphatic system and into the spinal subarachnoid space (B). The distending force within the syrinx that expands the syrinx radially may arise from increased resistance to fluid outflow from the spinal cord central canal, syrinx, and spinal cord interstitial spaces to the spinal subarachnoid space (C).
Observations
Hydrocephalus resembles syringomyelia in many ways. For example, hydrocephalus enlarges the cerebral ventricles, the ependyma-lined internal CSF spaces of the brain, and syringomyelia enlarges the central canal, the ependyma-lined interior CSF space of the spinal cord. Hydrocephalus and syringomyelia can be communicating, obstructive, post-traumatic, chemically-induced, and post-hemorrhagic. Obstructed CSF flow in the lateral ventricles or cranial subarachnoid space and impaired CSF absorption cause hydrocephalus. Analogously, obstruction of CSF flow at the craniovertebral junction and impaired syrinx fluid absorption may cause syringomyelia. Hydrocephalus and syringomyelia distend surrounding CNS structures and create neurological deficits. Hydrocephalus can be associated with syringomyelia in patients with Chiari I malformation.23
Hydrocephalus and syringomyelia differ in several ways, primarily in the CNS structure involved. Obstructing the ventricular system causes hydrocephalus, but the spinal cord central canal is blocked in over 90% of normal adults and does not cause syringomyelia.5 In the brain, gray matter nuclei and white matter surround the ependyma of the ventricles. In the spinal cord, the central gray matter surrounds the ependyma of the central canal. The choroid plexus produces roughly one-half to two-thirds of intracranial CSF in the brain, and extrachoroidal locations make the rest. The production of CSF in the spinal cord is extrachoroidal, although CSF can enter the spinal cord from the spinal subarachnoid space.
Treatment of hydrocephalus associated with Chiari I malformation is recommended before craniocervical decompression because reducing ventricular volume may reduce hindbrain herniation and syrinx size.24 Compression of the hindbrain within the foramen magnum narrows the foramen of Magendie, bows the dorsal wall of the fourth ventricle, and impairs fourth ventricular drainage. Hydrocephalus enlargement of the fourth ventricle within the neural elements further narrows the surrounding cisterna magna and basal cisterns. It can increase the cardiac cycle-related intracranial pressure pulses transmitted to the spinal canal.
The central canal of the spinal cord expands in syringomyelia. The normal central canal is lined by ependymal cells and surrounded by a layer of tanycytes within the center of the spinal cord gray matter. The ependyma and tanycytes regulate water movement between the central canal and extracellular compartment.25 The mature ependyma is a barrier protecting neural tissue from potentially harmful substances.26 In embryonic life, the central canal extends from the apertura canalis centralis near the fourth ventricular obex to the conus medullaris. The typical adult central canal is less than one mm in diameter, and its lumen is obstructed at one or several locations, preventing communication with the apertura canalis centralis anterior of the fourth ventricle.5 Milhorat and colleagues, in an autopsy study of children and adults dying from “various causes,” found that the spinal cord central canal was stenotic in 3% of infants, 18% of children, 88% of adolescents and young adults, and 90% of middle-aged adults. Stenosis of the central canal was most prominent in the thoracic spinal cord.5 They thought that the location of the spinal cord segments affected by syringomyelia was related to the period of life in which the syrinx began, with central holocord syringes arising in childhood and central focal and paracentral syringes arising later in life. The presumed cause of central canal occlusion was intermittent inflammatory events. The central canal can be as wide as 2 to 5 mm in patients without present or future myelopathy symptoms.27 This normal variant contrasts with syringes associated with neurological deficit, typically of larger diameter, distending the spinal cord, and associated with signs and symptoms of central myelopathy.
Research
Phase-contrast cine-MRI shows the CSF pathway size, velocity, and flow (Q = velocity x A) in an individual patient with Chiari I malformation and syringomyelia. Physical models can simulate the anatomy of the Chiari I malformation and surrounding structures and the physiologic effects of cardiac pulsation and Valsalva. These models demonstrate the Chiari I malformation’s complex and myriad effects on CSF flow and physical stress on the hindbrain and spinal cord tissue. 28
Phase-contrast cine-MRI showed that patients with Chiari I malformation had greater cerebellar tonsillar movement than controls.29 However, the amount of tonsillar movement in that study could not distinguish between Chiari I malformation patients with and without syringomyelia.29 Another study showed that 150 microns of cardiac-related tissue motion during 70 ms of the cardiac cycle increased diastolic velocity by 60% and pressure by 20% at the craniocervical junction.22 The tonsils and hindbrain oscillate in response to cardiac-cycle-related intracranial volume changes and pressure differentials across the foramen magnum. Accentuated hindbrain and CSF motion across the foramen magnum creates high cervical subarachnoid pulse pressure waves in the partially enclosed spinal subarachnoid space.17 The subarachnoid pressure waves act on the outer surface of the cervical spinal cord to drive syrinx fluid motion. The pulsatile movement of the hindbrain could also oscillate fluid between obstructing septa in the central canal fluid, expand the central canal, and begin syrinx development. In phase-contrast cine-MRI CSF flow studies, syrinx fluid oscillates superiorly and inferiorly during the cardiac cycle in response to cerebral and cerebellar tonsillar pulsation.
The origin of syrinx fluid in patients with Chiari I malformation-related syringomyelia remains controversial. Syrinx fluid has the same appearance and protein concentration as spinal cord interstitial fluid and CSF.30 Fluorescently-labeled tracers injected into the cisterna magna of normal mice were distributed to the entire cross-section of the C5 level of the spinal cord within 70 minutes. They were primarily concentrated in the gray matter. The fluorescent tracer entered the spinal parenchyma through the perivascular spaces and across the pial surface. The AQP4-specific inhibitor, TGN-020, significantly reduced tracer uptake. 31 This study confirmed that it was normal for cerebrospinal fluid to enter the spinal cord from the subarachnoid space and that AQP4 mediated this process.
AQP4 is highly expressed along the entire spinal cord in the rat spinal cord, labeling astrocytic fiber bundles protruding from the gray matter to the glia limitans adjacent to the pia mater. 32 AQP4 label also encircles neurovascular units. In contrast, AQP1 labeling occurs in the dorsal gray matter’s unmyelinated neuronal fibers and endothelial cells. 32 Astrocytes around syrinx cavities expressed AQP4 in an excitotoxic model of post-traumatic syringomyelia.33 HIF-1α (hypoxia-inducible factor, 1 alpha) accompanied increased AQP4 expression in areas of CNS injury.34 Water retention in the murine brain occurs after knockout of subpial astrocytic AQP4 but not after knockout of perivascular astrocytic AQP4 or ependymal AQP4. 35
The spinal cord central canal is a sink attracting intramedullary interstitial fluid.36 The spinal cord interstitial fluid drains into small lymphatic-like perivascular spaces and empties into the surrounding spinal subarachnoid space.37 Intramedullary edema develops when intramedullary fluid production exceeds interstitial fluid drainage. Syringes form when this excess interstitial fluid enters the central canal or adjacent central gray matter, creating a cyst distending those anatomic structures (Fig. 3).38 The development of a symptomatic syrinx associated with Chiari I malformation may be preceded by spinal cord edema or a smaller syrinx. 38,39
Figure 3:

Syrinx enlargement is a slow process that proceeds from the central canal and surrounding gray matter of the spinal cord. The growing syrinx initially stretches gray matter structures and the spinothalamic crossing tracts. Syrinx pressure can injure the central spinal cord structures irreversibly.
Arterial pulsations can drive CSF through the perivascular spaces and into the extracellular space of the brain and spinal cord.40 Obstruction of CSF egress from the interstitial spaces of the CNS can also lead to fluid accumulation and hydrocephalus. 41 In patients with Chiari I malformation and syringomyelia, increased cervical subarachnoid pressure waves can increase CSF movement from the subarachnoid space to the perivascular spaces and into the spinal cord interstitial space.17
The mechanism of syrinx distension is contentious (Fig. 2C). Extrachoroidal CSF produced in the spinal cord could join subarachnoid CSF as a source of syrinx fluid. The spinal cord constantly makes extrachoroidal CSF that maintains its functional milieu.42 A unidirectional ion pump in the capillary wall moves water from plasma to the CNS interstitial (extracellular) space and creates extrachoroidal CSF. The capillary is impermeable to transcapillary back-diffusion of ions from the extracellular space and extrachoroidal CSF reuptake. Immunohistochemical techniques that preserve the meninges show that aquaporin 1 (AQP1) and Na+/K+/2Cl–cotransporter 1 (NKCC1) are present in leptomeningeal vascular endothelia of the spinal cord.43 The Chiari I malformation could promote syrinx formation by preventing normal spinal cord absorption of extrachoroidal CSF or increasing extrachoroidal CSF production. The constant output of intramedullary extrachoroidal CSF without compensatory syrinx drainage could contribute to the development of syrinx pressure and distension. Increased spinal subarachnoid CSF pressure waves could also prevent normal absorption of CSF from the spinal cord.
Extrachoroidal CSF fluid retention could be a reason Chiari I malformation-related syringes do not form immediately adjacent to the site of CSF blockage at the foramen magnum but lower in the cervical spinal cord. Gray matter comprises a more significant percentage of the cross-sectional area of the spinal cord in the lower than the upper cervical spinal cord. Extrachoroidal CSF production predominates in gray compared to white matter.44 Therefore, the lower cervical spinal cord produces more extrachoroidal CSF per spinal segment than the upper cervical spinal cord. Individual variation in the pattern of central canal occlusion also influences syrinx location.5 Aquaporin levels influence the distensibility of the ventricular ependyma and may also affect the distensibility of the central canal.45 Finally, in some patients with Chiari I malformation and syringomyelia, syrinx formation may occur inferior to narrowing of the upper cervical spinal canal that accompanies hypoplasia of the posterior fossa.8
CSF obstruction at the foramen magnum could slow CSF drainage from the spinal subarachnoid space and the exit of interstitial fluid from the syrinx and spinal cord. After surgically expanding the CSF pathways at the foramen magnum, it usually takes three to six months before the syrinx diameter becomes less than one-half its preoperative diameter.46 In rare cases, syringes regress spontaneously, presumably because of anatomical changes that improve CSF flow at the foramen magnum.47 Syrinx collapse, on the other hand, occurs immediately with syrinx shunting.48 These observations suggest that in patients with Chiari I malformation and syringomyelia, the syrinx’s pressure and distension resolve slowly after craniocervical decompression because of slow syrinx drainage through tiny interstitial pathways to the subarachnoid space.
Other observations also support that efflux of syrinx fluid from the spinal cord is a slow process. Subarachnoid space and syrinx puncture through the dura shows that Chiari I-related syringes and the spinal subarachnoid space have similar mean and pulse pressures.17 After opening the dura, Chiari-I-related syringes remain expanded and maintain a pressure greater than the atmospheric pressure in the spinal subarachnoid space.49 CT-myelography also shows that syrinx drainage is slow. The bulk flow of water-soluble myelogram dye out of syringes is much slower than the clearance of dye from the subarachnoid space, leading to the target sign on CT, in which dye is retained in the syrinx after being absorbed in the surrounding subarachnoid space. Another finding in CT-myelography is that syringes associated with Chiari I malformation or spinal subarachnoid space obstructions accumulate higher concentrations of myelogram dye than tumor-related syringes, suggesting bulk flow into the syrinx from the subarachnoid space occurs in obstruction-related syringes. 50 In Chiari I malformation patients, increased bulk flow of CSF into the syrinx from the spinal subarachnoid space and slow syrinx fluid outflow favor syrinx development and maintenance. 50
Despite its slow drainage rate, syrinx fluid associated with Chiari I malformation typically has a normal protein level as assessed by laboratory measures.30 Syrinx fluid associated with conditions causing CSF obstruction typically has the same MRI signal as CSF. FLAIR MRI sequences can detect protein in the CSF at levels as low as 95 mg/dl, somewhat above the normal CSF protein range of 20-50 mg/dl. 51
Usually, intramedullary fluid production and absorption are balanced, and syringes do not form. The cause of intramedullary fluid accumulation appears straightforward in some types of syringomyelia unassociated with Chiari I malformation. For example, there is a case report of syringomyelia associated with choroid plexus within the central canal of the spinal cord. In this case, intramedullary CSF accumulation and a syrinx appear related to intramedullary CSF produced by the choroid plexus remnant that exceeded intramedullary absorption capabilities.52 In syringes related to intramedullary tumors, plasma ultrafiltrate escapes the tumor interstitium under pressure, enters the central canal, exceeds absorption capabilities, and creates a syrinx. 50 These cases highlight the spinal cord’s limited capacity to drain excess interstitial fluid (Fig. 4).
Figure 4:

Syringes arise from diverse mechanisms that share the feature that they create a net increase in the intramedullary fluid.
Syrinx distension requires a distending force. The process of syrinx expansion must produce enough pressure to expand the central canal’s diameter until fluid production and absorption come into balance. Syringes come in various sizes, and as syringes enlarge, the spinal cord diameter increases and the spinal cord parenchyma stretches around the syrinx fluid (Fig 3.). The viscoelastic stretching of the spinal cord elements around the syrinx requires energy from the syrinx fluid transmitted by pressure, usually over a long time, to the wall of the syrinx.
The development of a syrinx associated with Chiari I malformation may be preceded by spinal cord edema.38,39 Alternatively, the syrinx may progressively enlarge over time from a net increase of fluid within the central canal due to increased interstitial CSF production or reduced absorption of extracellular fluid from the central canal. The central canal in histologic cross-sections is usually not round but irregular, with multiple fluid-filled protrusions from its center into the medial spinal cord. This shape has a larger perimeter than a circle, allowing the central canal to expand initially into a cylindrical shape without stretching its ependymal lining.53 The syrinx size will enlarge or remain stable until the obstruction at the craniocervical junction is relieved. Radial enlargement of the syrinx stretches and displaces tanycytes, neurons, astrocytes, oligodendrocytes, veins, and capillaries and softens the surrounding tissue. A gliotic capsule forms around the syrinx. The spinal cord adjusts to the syrinx, loses its inherent stiffness, and its fluid distends the spinal cord. Gravitation-related hydrostatic forces distend the syrinx’s inferior component and narrow the syrinx’s superior part, leading to phenomena known as the “collapsing cord sign” in response to raising the head of the table during myelography. 30,54,55 The pia and arachnoid maters provide much of the inherent stiffness to the spinal cord. 56 57 Loss of this stiffness demonstrates that syringomyelia weakened the pia and arachnoid. The spinal cord diameter increases with the enclosed syrinx. Permanent loss of central spinal cord structures occurs if the obstruction to CSF flow is not relieved. Improving the CSF flow at the foramen magnum reduces the size of the syrinx and arrests the neurological progression.
Current Evidence
The Chiari I malformation obstructs CSF flow at the foramen magnum and results in cervical subarachnoid CSF pressure waves. These pressure waves increase the volume of cerebrospinal fluid entering the cervical spinal cord through the perivascular space. They could also impede spinal cord extrachoroidal CSF transport from the spinal cord’s central canal and extracellular space to the spinal subarachnoid space, contributing to syrinx fluid accumulation. Extrachoroidal CSF is a potential pressure source for a growing syrinx because active transcapillary ionic gradients make it inexorably. Syringes may not form immediately adjacent to the site of CSF blockage, the foramen magnum because extrachoroidal CSF production predominates in the gray matter compared to the white matter. 44 Gray matter forms a more significant percentage of the cross-sectional area of the spinal cord in the lower cervical spinal cord, where the gray matter is larger to support upper extremity motor and sensory function. More fluid per spinal segment may be made in the lower cervical spinal cord than at higher levels. Another consideration influencing syrinx location is individual variation in the central canal occlusion pattern.5 Finally, in some patients with Chiari I malformation and syringomyelia, narrowing the upper cervical spinal canal may favor syrinx formation in the lower spinal canal.
Clinical Relevance
Syringomyelia arises because the Chiari I malformation obstructs the CSF pathway at the foramen magnum. Craniocervical decompression relieves this obstruction and restores normal CSF flow across the foramen magnum. Syringomyelia persisting or expanding after craniocervical decompression signifies that the operative procedure did not relieve the Chiari I malformation’s obstruction to CSF flow. In those cases, reoperation can usually restore CSF flow at the foramen magnum and resolve syringomyelia, except in cases of severe arachnoiditis.18 In those cases, shunting syrinx fluid can collapse the syrinx and stabilize syrinx-related central myelopathy. Further understanding of Chiari I pathophysiology and the mechanism of syrinx enlargement may lead to pharmacotherapeutic approaches for patients with syringomyelia that cannot be remediated successfully with operative therapy. 58,59
Summary
Syringomyelia develops in association with Chiari I malformation because the hindbrain is ectopic and partially obstructs the CSF pathways at the foramen magnum. Fluid accumulates within the central canal or the adjacent gray matter and forms a syrinx. The force pressurizing the syrinx fluid arises from spinal subarachnoid pressure waves generated by brain artery expansion during cardiac systole. Gliosis forming around the syrinx impairs syrinx fluid absorption. The distending force of expanding syrinx fluid compresses and injures the central gray matter and crossing fibers of the anterior spinothalamic tract and expands the spinal cord diameter. The syrinx eventually compresses the long tracts of the spinal cord, leading to long tract signs. The syrinx transforms the spinal cord slowly, usually over months to years, from a solid, viscoelastic structure to a flaccid structure whose walls are inflated by the syrinx fluid. Surgical relief of the CSF obstruction at the foramen magnum results in syrinx resolution, defined as more than a 50% decrease in syrinx diameter.46
KEY POINTS.
The Chiari I malformation narrows CSF flow pathways at the foramen magnum.
Chiari I malformation patients with critically obstructed CSF flow develop cervical syringomyelia.
Operative decompression of the Chiari I malformation usually relieves the obstruction of the CSF pathways at the foramen magnum and resolves syringomyelia.
Syringomyelia persistence after decompressive surgery indicates that the CSF pathways remain obstructed at the foramen magnum.
Pulsatile movement of the Chiari I malformation produces tonsillar gliosis and other hindbrain manifestations.
SYNOPSIS.
Anatomic MRI, MRI flow studies, and intraoperative ultrasonography demonstrate that the Chiari I malformation obstructs CSF pathways at the foramen magnum and prevents normal CSF movement through the foramen magnum. Impaired CSF displacement across the foramen magnum during the cardiac cycle increases pulsatile hindbrain motion, pressure transmission to the spinal subarachnoid space, and the amplitude of CSF subarachnoid pressure waves driving CSF into the spinal cord. Central canal septations in adults prevent syrinx formation by CSF directly transmitting its pressure wave from the fourth ventricle to the central canal. Tracer and CT-myelographic studies demonstrate perivascular channels connecting the subarachnoid and spinal interstitial spaces, spinal glymphatic system, central canal, and syrinx. Intramedullary, extrachoroidal CSF adds to the interstitial fluid. Spinal subarachnoid pressure waves oppose interstitial fluid drainage, promote syrinx expansion, drive syrinx fluid movement during the cardiac cycle, and elongate the syrinx. Craniocervical decompression relieves CSF pathway obstruction and resolves syringomyelia.
CLINICS CARE POINTS.
Syringomyelia associated with the Chiari I malformation arises because the Chiari I malformation obstructs normal CSF flow across the foramen magnum during the cardiac cycle.
Syringomyelia resolves after an operative procedure relieves the obstruction to normal CSF flow at the foramen magnum.
Syringomyelia persisting after an operative procedure indicates that the operation was unsuccessful in establishing normal CSF flow at the foramen magnum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The author has nothing to disclose.
References
- 1.Chiari H. Concerning alterations in the cerebellum resulting from cerebral hydrocephalus. 1891. Pediatr Neurosci. 1987;13(1):3–8. doi: 10.1159/000120293 [DOI] [PubMed] [Google Scholar]
- 2.Mortazavi MM, Tubbs RS, Brockerhoff MA, Loukas M, Oakes WJ. The first description of Chiari I malformation with intuitive correlation between tonsillar ectopia and syringomyelia. J Neurosurg Pediatr. Mar 2011;7(3):257–60. doi: 10.3171/2010.12.Peds10579 [DOI] [PubMed] [Google Scholar]
- 3.Gardner WJ, Angel J. The mechanism of syringomyelia and its surgical correction. Clin Neurosurg. 1958;6:131–40. doi: 10.1093/neurosurgery/6.cn_suppl_1.131 [DOI] [PubMed] [Google Scholar]
- 4.Williams B The distending force in the production of “communicating syringomyelia”. Lancet. Jul 26 1969;2(7613):189–93. doi: 10.1016/s0140-6736(69)91427-5 [DOI] [PubMed] [Google Scholar]
- 5.Milhorat TH, Kotzen RM, Anzil AP. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg. Apr 1994;80(4):716–22. [DOI] [PubMed] [Google Scholar]
- 6.Williams B. Syringomyelia. Neurosurg Clin N Am. Jul 1990;1(3):653–85. [PubMed] [Google Scholar]
- 7.Haller G, Sadler B, Kuensting T, et al. Obex position is associated with syringomyelia and use of posterior fossa decompression among patients with Chiari I malformation. J Neurosurg Pediatr. Apr 10 2020;26(1):45–52. doi: 10.3171/2020.2.PEDS19486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano M, Haughton V, Munoz del Rio A. Tapering of the cervical spinal canal in patients with Chiari I malformations. AJNR Am J Neuroradiol. Aug 2012;33(7):1326–30. doi: 10.3174/ajnr.A2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg. Jan 1997;86(1):40–7. doi: 10.3171/jns.1997.86.1.0040 [DOI] [PubMed] [Google Scholar]
- 10.Noudel R, Jovenin N, Eap C, Scherpereel B, Pierot L, Rousseaux P. Incidence of basioccipital hypoplasia in Chiari malformation type I: comparative morphometric study of the posterior cranial fossa. Clinical article. J Neurosurg. Nov 2009;111(5):1046–52. doi: 10.3171/2009.2.JNS08284 [DOI] [PubMed] [Google Scholar]
- 11.Dlouhy BJ, Dawson JD, Menezes AH. Intradural pathology and pathophysiology associated with Chiari I malformation in children and adults with and without syringomyelia. J Neurosurg Pediatr. Dec 2017;20(6):526–541. doi: 10.3171/2017.7.PEDS17224 [DOI] [PubMed] [Google Scholar]
- 12.Guan J, Yuan C, Zhang C, et al. Intradural Pathology Causing Cerebrospinal Fluid Obstruction in Syringomyelia and Effectiveness of Foramen Magnum and Foramen of Magendie Dredging Treatment. World Neurosurg. Dec 2020;144:e178–e188. doi: 10.1016/j.wneu.2020.08.068 [DOI] [PubMed] [Google Scholar]
- 13.Orakdogen M, Emon ST, Erdogan B, Somay H. Fourth Ventriculostomy in Occlusion of the Foramen of Magendie Associated with Chiari Malformation and Syringomyelia. NMC Case Rep J. Apr 2015;2(2):72–75. doi: 10.2176/nmccrj.2014-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milhorat TH. Classification of syringomyelia. Neurosurg Focus. Mar 15 2000;8(3):E1. doi: 10.3171/foc.2000.8.3.1 [DOI] [PubMed] [Google Scholar]
- 15.Longatti P, Fiorindi A, Marton E, Sala F, Feletti A. Where the central canal begins: endoscopic in vivo description. J Neurosurg. Mar 1 2022;136(3):895–904. doi: 10.3171/2020.12.Jns203649 [DOI] [PubMed] [Google Scholar]
- 16.Merriam-Webster.com. Hydrodynamics. merriam-webstercom: https://www.merriam-webster.com; 2022. [Google Scholar]
- 17.Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. Oct 1999;91(4):553–62. doi: 10.3171/jns.1999.91.4.0553 [DOI] [PubMed] [Google Scholar]
- 18.Heiss JD, Suffredini G, Smith R, et al. Pathophysiology of persistent syringomyelia after decompressive craniocervical surgery. Clinical article. J Neurosurg Spine. Dec 2010;13(6):729–42. doi: 10.3171/2010.6.SPINE10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. Jan 1994;80(1):3–15. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava A, Sood A, Joy SP, Woodcock J. Principles of physics in surgery: the laws of flow dynamics physics for surgeons - Part 1. Indian J Surg. Aug 2009;71(4):182–7. doi: 10.1007/s12262-009-0064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loth F, Yardimci MA, Alperin N. Hydrodynamic modeling of cerebrospinal fluid motion within the spinal cavity. J Biomech Eng. Feb 2001;123(1):71–9. doi: 10.1115/1.1336144 [DOI] [PubMed] [Google Scholar]
- 22.Pahlavian SH, Loth F, Luciano M, Oshinski J, Martin BA. Neural Tissue Motion Impacts Cerebrospinal Fluid Dynamics at the Cervical Medullary Junction: A Patient-Specific Moving-Boundary Computational Model. Ann Biomed Eng. Dec 2015;43(12):2911–23. doi: 10.1007/s10439-015-1355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams B, Sgouros S, Nenji E. Cerebrospinal fluid drainage for syringomyelia. Eur J Pediatr Surg. Dec 1995;5 Suppl 1:27–30. [DOI] [PubMed] [Google Scholar]
- 24.Muthukumar N Syringomyelia as a presenting feature of shunt dysfunction: Implications for the pathogenesis of syringomyelia. J Craniovertebr Junction Spine. Jan 2012;3(1):26–31. doi: 10.4103/0974-8237.110125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. May 1995;14(1):1–13. doi: 10.1002/glia.440140102 [DOI] [PubMed] [Google Scholar]
- 26.Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. Apr 1 1998;41(1):2–13. doi: [DOI] [PubMed] [Google Scholar]
- 27.Holly LT, Batzdorf U. Slitlike syrinx cavities: a persistent central canal. J Neurosurg. Sep 2002;97(2 Suppl):161–5. doi: 10.3171/spi.2002.97.2.0161 [DOI] [PubMed] [Google Scholar]
- 28.Thyagaraj S, Pahlavian SH, Sass LR, et al. An MRI-Compatible Hydrodynamic Simulator of Cerebrospinal Fluid Motion in the Cervical Spine. IEEE Trans Biomed Eng. Jul 2018;65(7):1516–1523. doi: 10.1109/tbme.2017.2756995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung V, Magnussen JS, Stoodley MA, Bilston LE. Cerebellar and hindbrain motion in Chiari malformation with and without syringomyelia. J Neurosurg Spine. Apr 2016;24(4):546–55. doi: 10.3171/2015.8.Spine15325 [DOI] [PubMed] [Google Scholar]
- 30.Ellertsson AB. Syringomyelia and other cystic spinal cord lesions. Acta Neurol Scand. 1969;45(4):403–17. [DOI] [PubMed] [Google Scholar]
- 31.Wei F, Zhang C, Xue R, et al. The pathway of subarachnoid CSF moving into the spinal parenchyma and the role of astrocytic aquaporin-4 in this process. Life Sci. Aug 1 2017;182:29–40. doi: 10.1016/j.lfs.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 32.Oklinski MK, Lim JS, Choi FIJ, Oklinska P, Skowronski MT, Kwon TH. Immunolocalization of Water Channel Proteins AQP1 and AQP4 in Rat Spinal Cord. J Histochem Cytochem. Aug 2014;62(8):598–611. doi: 10.1369/0022155414537495 [DOI] [PubMed] [Google Scholar]
- 33.Hemley SJ, Bilston LE, Cheng S, Chan JN, Stoodley MA. Aquaporin-4 expression in post-traumatic syringomyelia. J Neurotrauma. Aug 15 2013;30(16):1457–67. doi: 10.1089/neu.2012.2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong A, Li J, Xiong R, et al. Inhibition of HIF-1α-AQP4 axis ameliorates brain edema and neurological functional deficits in a rat controlled cortical injury (CCI) model. Scl Rep. Feb 17 2022;12(1):2701. doi: 10.1038/s41598-022-06773-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vindedal GF, Thoren AE, Jensen V, et al. Removal of aquaporin-4 from glial and ependymal membranes causes brain water accumulation. Mol Cell Neurosci. Dec 2016;77:47–52. doi: 10.1016/j.mcn.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storer KP, Toh J, Stoodley MA, Jones NR. The central canal of the human spinal cord: a computerised 3-D study. J Anat. May 1998;192 (Pt 4)(Pt 4):565–72. doi: 10.1046/j.1469-7580.1998.19240565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. Sep 20 2005;2:6. doi: 10.1186/1743-8454-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy EI, Heiss JD, Kent MS, Riedel CJ, Oldfield EH. Spinal cord swelling preceding syrinx development Case report. J Neurosurg. Jan 2000;92(1 Suppl):93–7. doi: 10.3171/spi.2000.92.1.0093 [DOI] [PubMed] [Google Scholar]
- 39.Takamura Y, Kawasaki T, Takahashi A, et al. A craniocervical injury-induced syringomyelia caused by central canal dilation secondary to acquired tonsillar herniation. Case report. J Neurosurg. Jul 2001;95(1 Suppl):122–7. doi: 10.3171/spi.2001.95.1.0122 [DOI] [PubMed] [Google Scholar]
- 40.Kedarasetti RT, Drew PJ, Costanzo F. Arterial vasodilation drives convective fluid flow in the brain: a poroelastic model. Fluids Barriers CNS. May 15 2022;19(1):34. doi: 10.1186/s12987-022-00326-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiol Rev. Apr 1 2022; 102(2): 1025–1151. doi: 10.1152/physrev.00031.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammock MK, Milhorat TH. The cerebrospinal fluid: current concepts of its formation. Ann Clin Lab Sci. Jan-Feb 1976;6(1):22–6. [PubMed] [Google Scholar]
- 43.Li Q, Aalling NN, Förstera B, et al. Aquaporin 1 and the Na(+)/K(+)/2Cl(−) cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system. Fluids Barriers CNS. Feb 11 2020;17(1):15. doi: 10.1186/s12987-020-0176-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA. Cerebrospinal fluid production by the choroid plexus and brain. Science. Jul 23 1971;173(3994):330–2. doi: 10.1126/science.173.3994.330 [DOI] [PubMed] [Google Scholar]
- 45.Trillo-Contreras JL, Toledo-Aral JJ, Echevarría M, Villadiego J. AQP1 and AQP4 Contribution to Cerebrospinal Fluid Homeostasis. Cells. Feb 24 2019;8(2)doi: 10.3390/cells8020197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wetjen NM, Heiss JD, Oldfield EH. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J Neurosurg Pediatr. Feb 2008;1(2):118–23. doi: 10.3171/PED/2008/l/2/118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaquero J, Ferreira E, Parajón A. Spontaneous resolution of syrinx: report of two cases in adults with Chiari malformation. Neurol Sci. Apr 2012;33(2):339–41. doi: 10.1007/s10072-011-0670-9 [DOI] [PubMed] [Google Scholar]
- 48.Rhoton ALJ. Microsurgery of syringomyelia and syringomyelic cord syndrome. In: Schmidek HH, Sweet WH, eds. Operative Neurosurgical Techniques. WB Saunders; 1988:1307–1326. [Google Scholar]
- 49.Milhorat TH, Capocelli AL Jr, Kotzen RM, Bolognese P, Heger IM, Cottrell JE. Intramedullary pressure in syringomyelia: clinical and pathophysiological correlates of syrinx distension. Neurosurgery. Nov 1997;41(5):1102–10. [DOI] [PubMed] [Google Scholar]
- 50.Heiss JD, Jarvis K, Smith RK, et al. Origin of Syrinx Fluid in Syringomyelia: A Physiological Study. Neurosurgery. Feb 1 2019;84(2):457–468. doi: 10.1093/neuros/nyy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melhem ER, Jara H, Eustace S. Fluid-attenuated inversion recovery MR imaging: identification of protein concentration thresholds for CSF hyperintensity. AJR Am J Roentgenol. Sep 1997;169(3):859–62. doi: 10.2214/ajr.169.3.9275912 [DOI] [PubMed] [Google Scholar]
- 52.Shtaya A, Sadek AR, Nicoll JAR, Nader-Sepahi A. Choroid Plexus in the Central Canal of the Spinal Cord Causing Recurrent Syringomyelia. World Neurosurg. Mar 2018;111:275–278. doi: 10.1016/j.wneu.2017.12.143 [DOI] [PubMed] [Google Scholar]
- 53.Moore SA. The Spinal Ependymal Layer in Health and Disease. Vet Pathol. Jul 2016;53(4):746–53. doi: 10.1177/0300985815618438 [DOI] [PubMed] [Google Scholar]
- 54.Merriam-Webster.com. Hydrostatics. merriam-webstercom: https://www.merriam-webster.com; 2022. [Google Scholar]
- 55.Schlesinger EB, Antunes JL, Michelsen WJ, Louis KM. Hydromyelia: clinical presentation and comparison of modalities of treatment. Neurosurgery. Oct 1981;9(4):356–65. [DOI] [PubMed] [Google Scholar]
- 56.Ramo NL, Troyer KL, Puttlitz CM. Viscoelasticity of spinal cord and meningeal tissues. Acta Biomater. Jul 15 2018;75:253–262. doi: 10.1016/j.actbio.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 57.Bilston LE, Thibault LE. The mechanical properties of the human cervical spinal cord in vitro. Ann Biomed Eng. Jan-Feb 1996;24(1):67–74. doi: 10.1007/bf02770996 [DOI] [PubMed] [Google Scholar]
- 58.Itoh T, Nishimura R, Matsunaga S, Kadosawa T, Mochizuki M, Sasaki N. Syringomyelia and hydrocephalus in a dog. J Am Vet Med Assoc. Sep 1 1996;209(5):934–6. [PubMed] [Google Scholar]
- 59.Plessas IN, Rusbridge C, Driver CJ, et al. Long-term outcome of Cavalier King Charles spaniel dogs with clinical signs associated with Chiari-like malformation and syringomyelia. Vet Rec. Nov 17 2012;171(20):501. doi: 10.1136/vr.100449 [DOI] [PubMed] [Google Scholar]


