Summary:
Wound healing problems are a major cause of morbidity for gender-affirming surgery (GAS) patients. Prior studies have shown sex differences in wound healing may exist. We hypothesized exogenous testosterone supplementation may impair post-GAS wound healing and developed a model to investigate this phenomenon. Mice were randomized by hormone regimen and gonadectomy (OVX). Gonadectomy or sham occurred on day 0 and mice were assigned to no testosterone (-T), mono- or bi-weekly (T/2T) testosterone groups. Dorsal splinted wounding occurred on day 14 and harvest on day 21. Serum testosterone levels were quantified with mass spectrometry. Tissue underwent analysis with planimetry, qPCR, ELISA, and immunofluorescence. Mean testosterone trough levels for bi-weekly regimen were higher compared to mono-weekly (397 versus 272 ng/dL; P = 0.027). At POD5, 2T injections led to 24.9% and 24.7% increases in mean wound size relative to SHAM and OVX/-T, respectively (P = 0.004; 0.001). Wounds in OVX/+2T mice demonstrated increased gene expression for inflammatory cytokines and macrophage marker F4/80 (P < 0.05). ELISA confirmed elevated wound TNFα levels (P < 0.05). Quantitative multiplex immunofluorescence with F4/80/NOS2/ARG1 showed significant increases in macrophage prevalence in OVX/+2T (P < 0.05). We developed a novel model of GAS hormonal milieu to study effects of exogenous testosterone on wound healing. Optimized twice-weekly dosing yielded serum levels comparable to clinical therapy. We showed exogenous testosterone administered to XX/OVX mice significantly impairs wound healing. A hyperinflammatory wound environment results in increased macrophage proliferation and elevated cytokines. Future efforts are directed toward mechanistic investigation and clinical validation.
Takeaways
Question: Is it possible to recreate the hormone environment of GAS in a murine model?
Findings: Exogenous testosterone administered to XX/OVX mice impairs both macroscopic and histologic wound healing.
Meaning: The hormone environment of GAS may impact subsequent clinical wound healing outcomes.
INTRODUCTION
There are nearly 25 million transgender and gender diverse (TGD) persons worldwide, and the number of patients undergoing gender-affirming surgery (GAS) is rapidly increasing.1 Exogenous hormone therapy in patients seeking GAS is typical,2 and there is an increasing trend toward continuation of hormones through the TGD patient’s perioperative course.1
Wound healing problems are a major cause of morbidity in TGD patients who undergo GAS, such as fistula and stricture formation after phalloplasty. It has been observed for decades that sex differences in wound healing may exist between men and women with the presence of sex hormones in the wound environment playing a critical role.3–5 In cutaneous wounds, androgen and androgen receptor signaling has been linked to prolonged inflammation, decreased wound re-epithelialization, and decreased collagen matrix deposition.6–8 Androgen blockade reverses many of these negative effects.6,9–11 Estrogens, by contrast, have generally positive effects on wound healing, including dampening inflammation and increasing collagen deposition via reduction in proteolysis.11,12 Treatment with both topical and systemic estrogen has been shown to increase wound healing rates in both sexes, particularly in postmenopausal women.3–5
Despite this, no animal models to date have evaluated the effect of cross-sex hormones on wound healing. We hypothesized that exogenous testosterone (T) supplementation in transgender men adversely affects cutaneous wound healing. To test this hypothesis, we developed a novel mouse model of the GAS hormonal milieu to study the effects of exogenous testosterone on cutaneous wound healing. In this pilot report, we describe our model feasibility and preliminary results.
METHODS
Experimental Model
C57BL/6J mice (n = 75) were randomized to four groups. Surgical sterilization (bilateral ovariectomies; OVX) or sham (SH) intrabdominal procedures occurred on day 0 and mice were assigned to either no testosterone (SH/-T and OVX/-T groups), mono- (OVX/T group) or bi-weekly (OVX/2T) 0.6 mg exogenous testosterone cypionate for 3 weeks (Fig. 1).
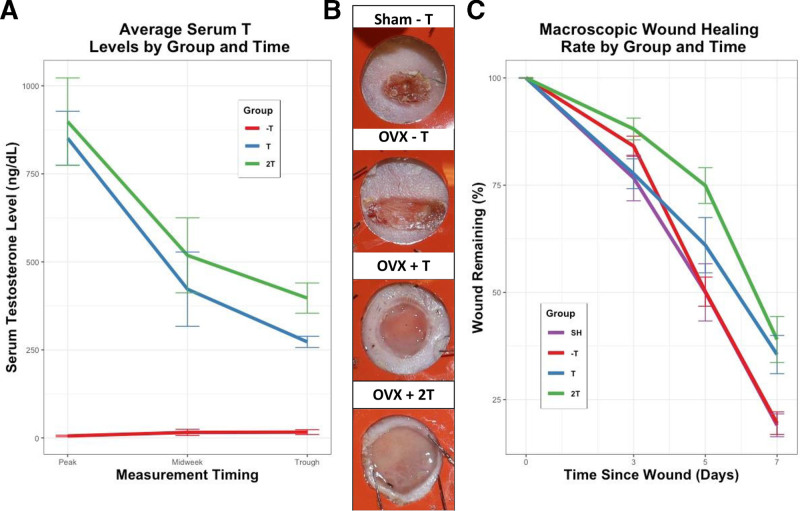
Fig. 1.
A, Average serum testosterone levels by experimental group. Groups with no exogenous T showed negligible testosterone levels, whereas groups with exogenous T showed a dose-dependent cyclic T serum level. B, Macroscopic wound pictures taken at POD5 showed a delayed wound healing rate in the mice exposed to exogenous testosterone therapy (ie, OVX/+T and OVX/+2T). C, Wound planimetry data. Pairwise comparisons revealed 2T injections led to 24.9% and 24.7% increases in macroscopic wound area relative to SHAM and OVX/-T, respectively (P = 0.004; P = 0.001).
The stented wound model of Gurtner was used.13 Bilateral dorsal wounding (6 mm punch) occurred on POD14 and wound harvest on POD21 to analyze wounds at a proliferative healing stage. All protocols were approved by Johns Hopkins ACUC.
Development of Hormone Dosing Model
Testosterone cypionate is commonly used clinically and has a half-life of approximately 7 days. Testosterone cypionate (200 mg/mL) was diluted with pharmaceutical grade sesame oil at a concentration of 1.2 mL TC in 38.8 mL of sesame oil. A total of 0.1 mL of diluted TC was injected into each mouse once- (OVX/T group) or bi-weekly (OVX/2T group).
Initially, delayed release testosterone pellets were used for delivery, but mouse serum T levels revealed that levels were highly inconsistent with this method (data not shown). Mouse serum testosterone levels were initially measured with a validated ELISA kit (IBL-America, Minneapolis, Minn.). However, measurement variation was consistently unacceptably high. It was necessary to switch to measurement using LC/MS from a CLIA-certified laboratory (Brigham Research Assay Core, Boston, Mass.).
Gene Expression Analysis with qPCR/Immunofluorescence and Multiplex Quantitative Pathology/ Enzyme-linked Immunosorbent Assay
A detailed explanation of the conducted assay techniques, such as gene expression analysis with qPCR, immunofluorescent staining, and enzyme-linked immunosorbent assay (ELISA) can be found in figure, Supplemental Digital Content 1, which shows detailed explanation of the conducted assay techniques, http://links.lww.com/PRSGO/C289.
Wound Planimetry
In vivo wound areas as determined by planimetry were quantified by image analysis using a Canon EOS Rebel T8i camera and ImageJ software.
RESULTS
Twice-weekly Cypionate Injections Achieve Clinically Relevant T Levels in Female Mice
Testosterone serum levels followed a consistent pattern with respect to dose and time (Fig. 1).
Significantly higher mean serum testosterone trough levels were seen in the bi-weekly dosing group (OVX/2T; 397.2 ng/dL) relative to the mono-weekly (OVX/T; 272.7 ng/dL; P = 0.018) and no-T regimens (OVX/-T; 16.5 ng/dL) (P < 0.0001).
Testosterone Impairs Cutaneous Wound Healing in Female Mice
Testosterone increased mean wound area at days 5 and 7 compared to both SH (+E/−T) and OVX controls (−E/−T) with a significant experimental group effect on ANOVA at POD 5 and 7 (P = 0.001). At POD5, pairwise comparison showed 2T injections led to 24.9% and 24.7% increases in mean wound size relative to sham (P = 0.004) and OVX/−T (P = 0.001) (Fig. 1).
Testosterone Generates a Proinflammatory Wound Milieu in Post-OVX Female Mice
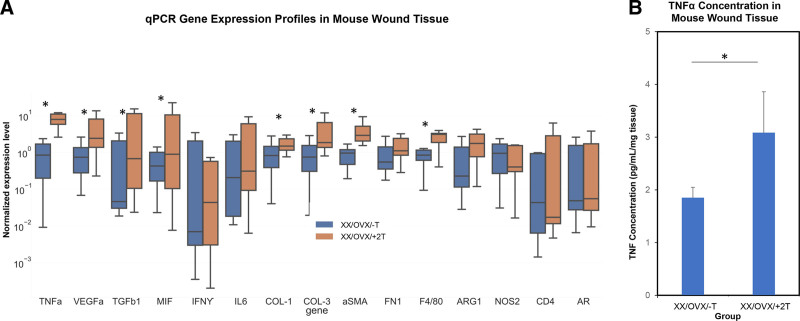
Wound tissue from OVX/+T mice demonstrated significantly increased gene expression for multiple inflammatory cytokines (Fig. 2). qPCR analysis also showed an increase in collagen 1 and collagen-3 expression and macrophage cellular markers F4/80 and arginase-1 in the OVX/+2T group compared to the OVX/-T (n = 12; P < 0.05). ELISA was used for direct protein measurement and confirmed that wound TNFα levels were elevated in the OVX/+2T experimental group (P < 0.05).
Fig. 2.
Murine wound tissue analysis. A, qPCR gene expression profiles of mouse wound tissue. Relative expression levels of cytokine (Tumor Necrosis Factor alfa, Vascular Endothelial Growth Factor A, Transforming Growth Factor beta-1, Macrophage Migration Inhibitory Factor, Interferon gamma, Interleukin 6) extracellular matrix (Collagen 1, Col 3, Smooth Muscle alpha-actin, Fibronectin-1), cell phenotype (F4/80, Arginase-1, Nitric Oxide Synthase-2, FOXP3, CD4), and androgen receptor (AR)-related genes (n = 12; P < 0.05). B, ELISA of TNFα in mouse skin wound tissue (n = 6; P < 0.05). *indicates significance of P < 0.05.
Testosterone Increases Wound Macrophage Proliferation in Post-OVX Female Mice
Quantitative multiplex immunofluorescence staining with F4/80 (pan-macrophage marker), NOS2 (M1-like macrophage; proinflammatory), and ARG1 (M2-like macrophage; regenerative) showed significant increases in both relative (versus all cells) and absolute quantity of wound macrophages in the OVX/+2T groups versus OVX/-T group (P < 0.05). (See figure, Supplemental Digital Content 2, which shows immunofluorescent staining of mouse skin wound with M1 and M2 markers, http://links.lww.com/PRSGO/C290.)
DISCUSSION
In this Ideas and Innovations report, we present a proof-of-concept animal model that recreates the hormone environment of GAS. We found that exogenous testosterone administered to XX/OVX mice impairs wound healing, both on macroscopic planimetry and on histologic evaluation. A hyperinflammatory wound environment appears to occur with recruitment of excessive macrophages and elevated cytokines such as TNFα.
Excessive wound inflammation in the first few days of wound healing has been shown to be a detriment to successful wound healing by preventing progression to the proliferative stage of wound healing and initiation of ECM repair. This mechanism is believed to contribute to many pathologies of impaired wound healing including diabetes, where inability to clear proinflammatory macrophages is a key factor.14 Another example is chronic venous ulcers, where in both humans and mice, an initial dysfunctional hyperinflammatory M1-like macrophage state fails to transition properly to a prorepair state.15 Local depletion of these macrophages fully rescues CVU wound healing rates but not in normal wounds.15 Our data showing that testosterone treatment led to a very large increase in macrophage density at the wound site, elevated proinflammatory cytokines such as TNFa, and increased proinflammatory macrophage polarization provide a mechanistic route for impairment of wound healing by T.
The most common reason for revision after chest masculinization is hypertrophic scarring, and hormones may play a role.16 Prior studies have demonstrated that the sex hormones can interact with wound healing but none have examined these effects in a transgender model (viz, cross-sex hormone administration).3,12 Interestingly, keloids have been shown to have upregulated androgen receptor levels compared to the normal skin or scar.17 The lack of a validated animal model is a significant barrier to exploration of this area.
Achieving clinically analogous testosterone exposure in our model required repeated experimentation as testosterone distribution and metabolism are different in small animals. Despite using ELISA kits validated for testosterone measurement, comparison versus control standards showed unacceptably high error, and we evolved to using LC/MS/MS by a CLIA-certified endocrinology laboratory that showed low error and high reproducibility.
Most T animal models use either slow-release pellets or daily injections of water-soluble testosterone. However, most GAS patients use once-weekly delayed release injection esters such as testosterone cypionate; therefore, we developed an optimized injection regimen for this model.
This report represents a pilot study, and many questions remain. Testosterone has negative effects on murine wound healing postovariectomy; whether estradiol may have an offsetting positive impact is not yet known. Although macrophages clearly play a central role, the androgen receptor-dependent signaling pathways most important for wound impairment are uncertain. Similarly, hormonal mechanisms are unclear: preliminary results (not shown) suggest differences in wound healing between castrated XX and XY mice treated with testosterone, suggesting not just a difference in hormone milieu but an epigenetic difference in wound healing response despite identical hormone profiles.
Human studies are essential to validate similar biology in patients. Ultimately, this work may open the opportunity for a variety of treatment approaches to offset T-induced wound healing impairment (eg, topicals). Future efforts will be directed toward mechanistic investigation of underlying pathways and validation in human clinical studies.
Supplementary Material
Footnotes
Published online 29 November 2022.
Drs Reiche and Tan contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Hontscharuk R, Alba B, Manno C, et al. Perioperative transgender hormone management: avoiding venous thromboembolism and other complications. Plast Reconst Surg. 2021;147:1008–1017. [DOI] [PubMed] [Google Scholar]
- 2.Rose AJ, Hughto JMW, Dunbar MS, et al. Trends in feminizing hormone therapy for transgender patients, 2006–2017. Transgend Health. 2021. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay M, Miller V, Ashcroft GS. Sex differences in wound healing. In Bittar E. (Ed), Principles of sex-based differences in physiology. Philadelphia: Elsevier; 2004;321–328. [Google Scholar]
- 4.Ashcroft GS, Horan MA, Schultz GS, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-~1 levels. Nat Med. 1997;3:7. [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft GS, Greenwell-Wild T, Horan MA, et al. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai J-J, Lai K-P, Chuang K-H, et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J Clin Invest. 2009;119:3739–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Simanainen U, Cheer K, et al. Androgen actions in mouse wound healing: minimal in vivo effects of local antiandrogen delivery. Wound Repair Regen. 2016;24:478–488. [DOI] [PubMed] [Google Scholar]
- 8.Gilliver S, Ruckshanthi J, Hardman M, et al. 5α-Dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. 2009;217:73–82. [DOI] [PubMed] [Google Scholar]
- 9.Gilliver SC, Ashworth JJ, Mills SJ, et al. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–732. [DOI] [PubMed] [Google Scholar]
- 10.Toraldo G, Bhasin S, Bakhit M, et al. Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes: topical blockade of β-catenin promotes wound healing. Wound Repair Regen. 2012;20:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilliver S, Wu F, Ashcroft G. Regulatory roles of androgens in cutaneous wound healing. Thromb Haemost. 2003;90:978–985. [DOI] [PubMed] [Google Scholar]
- 12.Gilliver SC, Ashcroft GS. Sex steroids and cutaneous wound healing: the contrasting influences of estrogens and androgens. Climacteric 2007;10:276–288. [DOI] [PubMed] [Google Scholar]
- 13.Galiano RD, V JM, Dobryansky M, et al. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. [DOI] [PubMed] [Google Scholar]
- 14.Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oles N, Darrach H, Landford W, et al. Gender affirming surgery: a comprehensive, systematic review of all peer-reviewed literature and methods of assessing patient-centered outcomes (part 1: breast/chest, face, and voice). Ann Surg. 2022;275:e52–e66. [DOI] [PubMed] [Google Scholar]
- 17.Schierle HP, Scholz D, Lemperle G. Elevated levels of testosterone receptors in keloid tissue: an experimental investigation. Plast Reconstr Surg. 1997;100:390–395; discussion 396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.