Abstract
Theileria parasites infect and transform bovine leukocytes. We have analyzed laboratory-established Theileria sp.-infected leukocyte lines and observed that transformed macrophages express CD5. Low-level expression of CD5 by macrophages was further confirmed on three independent Theileria annulata clinical isolates from Tunisia. Interestingly, the fourth CD5+ clinical isolate (MB2) was morphologically different, expressed surface immunoglobulin M (IgM) and BoLA class II, and had rearranged Ig light-chain genes. To demonstrate that MB2 did indeed contain CD5+ B cells, individual clonal lines were obtained by limiting dilution, and CD5 expression and Ig gene rearrangement were confirmed. This suggests that in natural infections T. annulata can invade and transform CD5+ B cells.
Theileria spp. are tick-transmitted parasites that are the causative agents of tropical theileriosis (Theileria annulata) and East Coast fever (Theileria parva), cattle diseases widespread in North Africa, the Middle East, India, China, and East Africa. T. annulata sporozoites preferentially invade macrophage types cells in vivo, but in vitro B lymphocytes can also be infected (10). T. parva sporozoites mostly invade T cells in vivo, but again, in vitro parasitized B lymphocytes are seen (1, 27). The infection of T cells, rather than B cells, by T. parva is thought to contribute to the pathogenicity of East Coast fever (19), which is a more pernicious disease than tropical theileriosis. In several respects the infected leukocytes behave like fully transformed cells, since they proliferate without the addition of cytokines or growth factors (7), are capable of forming tumors in irradiated athymic and SCID mice (9, 15), and can be cloned in soft agar (23). A reflection of the transformed state of the infected host cell is the modulation observed in leukocyte surface markers. B lymphocytes infected by T. parva lose surface IgM, but, like transformed T cells, express interleukin 2 receptor (1, 8). In addition, infection by T. annulata also leads to the down-regulation of surface immunoglobulin M (IgM) on B lymphocytes and the loss of certain surface markers on macrophages (29).
The surface antigen CD5 typically expressed on T cells is also found on a subset of B lymphocytes called B1 cells (12, 14). B1 lymphocytes differ from conventional B2 B cells in a number of characteristics (for a recent review, see reference 31). In particular, their ability to produce multireactive IgM, IgG3, and IgA in large amounts has lead to the consideration that B1 cells might be mediators of “natural” immunity (11). However, the expansion of autoreactive B1 cells can be injurious, as they are associated with the development of autoimmune disease and some parasitic infections in mice and humans (13, 17). Interestingly, CD5+ B lymphomas expressing macrophage surface markers have been described and termed the B/macrophage cell (5). This nomenclature stems from the observation that certain CD5+ B lymphomas can be induced to differentiate into macrophage-like cells and implies that the two cell types have a common lineage (2).
Given that a high percentage of B cells in bovine peripheral blood bear the CD5 marker (22) and given that Theileria parasites can invade B cells in vitro, we asked whether in natural infections Theileria parasites might be found in CD5+ cells. To test this hypothesis we examined a number of Tunisian T. annulata clinical isolates for CD5 expression.
Reverse transcriptase PCR (RT-PCR) analysis of leukocyte gene expression.
Total cellular RNA from 4 × 106 cells was obtained by disruption in lysis buffer containing 4 M guanidinium thiocyanate, and first-strand cDNA was synthesized from RNA samples by using Moloney murine leukemia virus reverse transcriptase (Boehringer Mannheim) in the presence of oligo(dT) (Pharmacia Fine Chemicals; Piscataway, N.J.), as described elsewhere (18). All cDNA samples were stored at −20°C until use. Specific amplification of the different cDNAs was achieved by using synthetic oligonucleotides based on conserved sequences in the variable (V) and conserved (C) gene segments of the Igλ chain. Primers for CD5, the T-cell receptor ζ chain, and CD4 were derived from the corresponding bovine cDNA sequence in the database (accession no. X53061, U25688, and U48356, respectively). Bovine specific oligonucleotides for CD44 were derived from exons 4 and 5 (accession no. S64418). PCRs were performed with 5 to 10 μl of cDNA samples and 2 μM each (sense and antisense) primer mixture by using a GeneAmp 9600 PCR system (Perkin-Elmer Cetus) in the presence of thermalase DNA polymerase. Products had the predicted sizes after electrophoresis in 1.3% agarose gels when compared to either φX174, HaeIII, or the 100-bp marker (Gibco BRL).
Fluorescence-activated cell sorter (FACS) analysis of membrane surface markers.
The different cell lines were used for immunofluorescence staining with the following unlabelled monoclonal antibodies directed to bovine differentiation cell markers: mouse IgG1 anti-bovine CD5 (clone CC17), mouse IgG1 anti-bovine IgM (clone ILA-30), mouse anti-CD45 (clone CC31), mouse anti-transferrin receptor (TfR, ILA-77), and mouse anti-BoLA class II (clone D112), kindly provided by Jan Naessens (20, 30). The anti-CD44 antibody (clone BAG40A) has been described elsewhere (26). To block nonspecific binding of antibodies through cellular FcγR II/III receptor, cells were previously incubated with anti-CD32/16, clone 2.4.G2 (33). Further incubations were done with rat anti-mouse IgG1 antibodies labelled with fluorescein isothiocyanate (FITC) (Tebu, Paris, France). Appropriate controls were performed and consisted of incubating the different cell lines with FITC-labelled anti-IgG1 antibodies alone to determine nonspecific staining. Fresh, uninfected bovine peripheral blood lymphocytes were also used as controls in all experiments (data not shown). After washes, 1 × 104 to 2 × 104 cells were acquired in a FACScan cytofluorometer (Becton Dickinson & Co., Mountain View, Calif.). Except during analysis of the Thei macrophage cell line, polymorphonuclear cells and macrophages were excluded from the analysis by a combined light scatter (forward and side scatter) gate in the acquisition. Dead cells were excluded in all samples by propidium iodide labelling, and fluorescence was evaluated by using the CELLQuest 3.1 program.
Characterization of Theileria-transformed laboratory-established lines: identification of CD5+ macrophages.
Since the loss of specific surface markers on T. annulata-transformed macrophages has been proposed to be due to cellular dedifferentiation associated with the transformed phenotype (26), we decided to examine whether laboratory-established Theileria-infected lines also expressed CD5 and, if so, to what degree. The TBL3 cell line was derived by in vitro infection of the spontaneous bovine B-lymphosarcoma cell line BL3 (32) with the Hissar stock of T. annulata (3). As a positive control for CD5 expression, we used a T. parva-infected T-cell line (TpM803). As a negative control, we used the B-cell line TpMD409 clone B2 (referred to below, for simplicity, as TpM409), also infected with T. parva muguga (7, 21). We have previously described the T. annulata-infected macrophage-like line Thei (6).
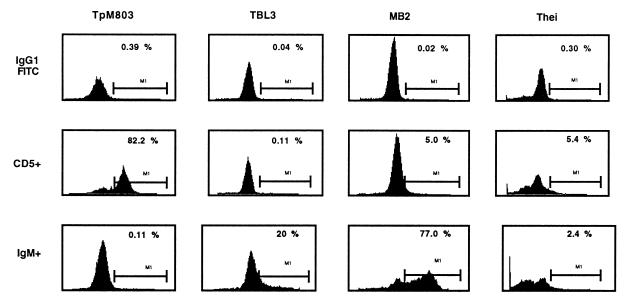
Confirmation of the B-cell origin of the laboratory lines was obtained by RT-PCR amplification of rearranged Ig light-chain transcripts (Table 1 and Fig. 1) and FACS analysis of surface IgM (Table 2). As a positive control, the noninfected B-sarcoma cell line BL3 was used, and as expected, no Igλ transcripts were amplified from the infected T-cell line TpM803 (Fig. 1). Consistent with a macrophage origin for Thei, no Igλ transcripts were detected (Fig. 1), nor did Thei express the ζ chain of the T-cell receptor (data not shown). As has been reported previously (26), all lines express CD44 (Fig. 1 and Table 2), and the transformed macrophage line Thei had markedly down-regulated expression of CD14 and reduced sensitivity to lipopolysaccharide stimulation (data not shown). Interestingly for a transformed macrophage, Thei was found to transcribe the CD5 gene (Fig. 1), and some cells expressed surface CD5 (see Fig. 3). The low percentage of CD5+ cells in Thei (5%) contrasted with the high percentage (82%) of cells expressing CD5 in the classical T-cell line TpM803 (see Fig. 3).
TABLE 1.
Oligonucleotides used in RT-PCR analysis of leukocyte gene expression
| Leukocyte marker | Sense oligonucleotide (5′ to 3′) | Antisense oligonucleotide (5′ to 3′) | Annealing temp (°C) | PCR product length (bp) |
|---|---|---|---|---|
| Igλ | TGTGCTGACTCAGCCG | GACACACACCAGGGTG | 52 | 412 |
| CD5 | GTGTGGTCCTCTGGTCTACAAGAAG | GCAGGTCATAGTCACTGT | 58 | 280 |
| ζ Chain | CAGCACATGTTATTGTGGCC | GACCATCATGCCCCTTGCC | 60 | 419 |
| CD14 | AAGCACACTCGCTTGCC | CACATCGGGTAGCACCC | 54 | 279 |
| CD44 | TGGGGAAGACTGTACATCGG | GGCCGTCTTGGTCTGGACGG | 59 | 250 |
| CKIIα | GTTAATACACACAGACCCCGA | GCTATGGCAGTAATCAAGGGCC | 49 | 384 |
FIG. 1.
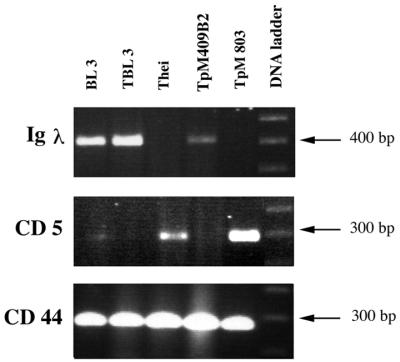
Identification by RT-PCR of transcripts for leukocyte cell surface markers on laboratory-established lines. Amplified cDNA was separated on a 1.3% agarose gel, and the sizes of the products were estimated by comparison with the 100-bp ladder. (Top) Igλ transcripts of the expected size were detected in the B-cell lines (BL3, TBL3, and TpMD409), and no message was detectable in the macrophage line (Thei) or the T-cell line (TpM803). (Center) CD5 transcripts were detected in the macrophage (Thei) and T-cell (TpM803) lines. The less-abundant mRNA expression in Thei compared to TpM803 is consistent with its reduced surface expression of CD5 (see Fig. 3). (Bottom) All lines readily express CD44 transcripts.
TABLE 2.
Relative percent expression of membrane surface markers on MB2 and established cell lines
| Marker | % Expression on the following cell line:
|
|||||
|---|---|---|---|---|---|---|
| BL3 | TBL3 | Thei | TpMD409 | TpM803 | MB2 | |
| IgM | 95 | 20 | 2 | 42 | <1 | 77 |
| CD44 | 92 | 68 | 48 | 51 | 37 | 8 |
| CD45 | 83 | 76 | NDa | 85 | 100 | 99 |
| BoLA class II | 98 | 12 | ND | 30 | 93 | 100 |
| TfR | 99 | 99 | ND | 99 | 75 | 100 |
ND, not determined.
FIG. 3.
Expression of CD5 and IgM molecules by Theileria-transformed cell lines. Cells were incubated with mouse anti-bovine CD5 or anti-IgM antibodies and revealed by a secondary goat anti-mouse IgG1–FITC antibody. Upper panels show the nonspecific staining of the secondary antibody on the different cell lines. Middle panels show the expression of CD5 molecules. Five percent of MB2 cells are CD5+. The macrophage line (Thei) shows 5.4% positivity for CD5 surface expression, and this does not reflect binding of antibody by macrophage Fc receptors, as previous saturation of cells with anti-CD32/16 antibodies was used to prevent a nonspecific reaction via FcγRII/III receptors. Note the high specificity (82.2%) of the anti-CD5 antibody on the typical T-cell line TpM803 and the low level of binding of the second antibody on all cell lines. In the bottom panels, modulation of surface IgM expression is clearly observed for the B-cell lines (compare TBL3 with MB2).
CD5+ macrophages and CD5+ B cells in Tunisian clinical isolates.
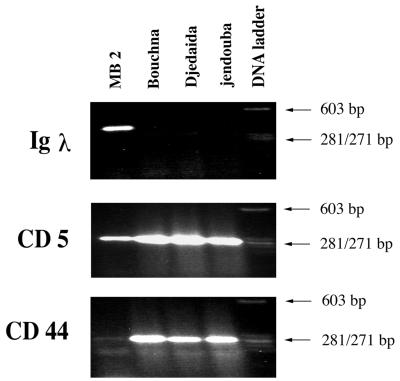
We next asked if CD5 expression on T. annulata-transformed macrophages was common or was specific to the laboratory-established line Thei. To this end, we examined a further four independent Tunisian clinical isolates whose geographical location has been described, since MB2 corresponds to isolate 1, Djedaida to isolate 3, Bouchna to isolate 4, and Jendouba to isolate 13 on the map presented in reference 4. As can be seen in Fig. 2, three of the four clinical isolates behave like Thei in that they express no Igλ transcripts but transcribe both CD5 and CD44. Interestingly, the fourth isolate (MB2) was phenotypically different, not only from Thei but also from the laboratory-established B-cell lines TBL3 and TpMD409 (data not shown), and also differed in its pattern of expression of the markers tested. The expression of Igλ transcripts and CD5 suggested that MB2 could be a B1-type B-cell (Fig. 2). The B-cell character of MB2 was confirmed by the expression of surface IgM on 77% of the cells (Fig. 3). Furthermore, a reasonable, but limited percentage (5%) of MB2 cells presented surface CD5 expression (Fig. 3 and 4). Interestingly, MB2 has down-regulated both CD44 transcripts (Fig. 2) and the number of cells positive for CD44 surface expression (Table 2).
FIG. 2.
Identification by RT-PCR of transcripts for leukocyte surface markers in T. annulata Tunisian clinical isolates. (Top) Igλ transcripts can be detected only in the MB2 isolate. (Center) All isolates express CD5, with MB2 showing reduced levels of transcript and cell surface expression (see Fig. 3). (Bottom) All isolates transcribe CD44, with MB2 showing reduced levels consistent with CD44 surface expression on a low number of cells (see Fig. 3). The sizes of the amplified products were estimated by comparison with φX size markers. Three of four Tunisian clinical isolates are Igλ−, CD5+, and CD44+ and as such resemble the CD5+ macrophage line Thei.
FIG. 4.
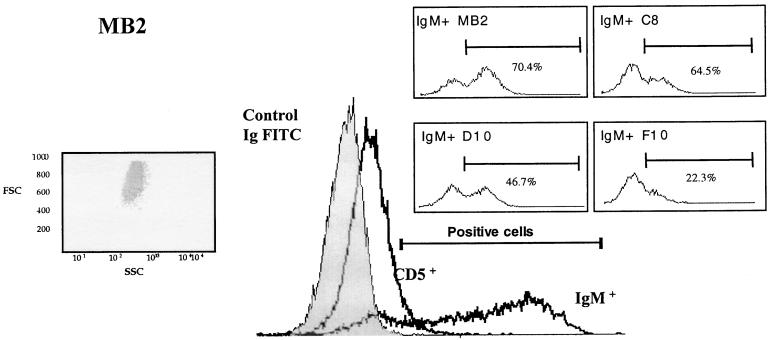
Overlay histogram of membrane differentiation markers on the T. annulata-infected MB2 line. MB2 cells were labelled with mouse IgG1 anti-bovine CD5 or IgG1 anti-bovine IgM antibodies revealed by goat anti-mouse IgG1–FITC. The lymphocytes were acquired in a FACScan apparatus using a forward scatter (FSC)–side scatter (SSC) combined gate. The light scatter distribution of MB2 cells inside the lymphocyte gate (left inset) excluded the possibility that the IgM+ CD5+ population was contaminated by macrophages. The figure shows a shift to the right after staining with IgM or CD5-specific antibodies, and a number of IgM+ cells bear the CD5 molecule, consistent with their being B1a-type B lymphocytes. (Right insets) Modulation of IgM expression on the surfaces of MB2-derived clones C8, D10, and F10 compared to the original MB2 line.
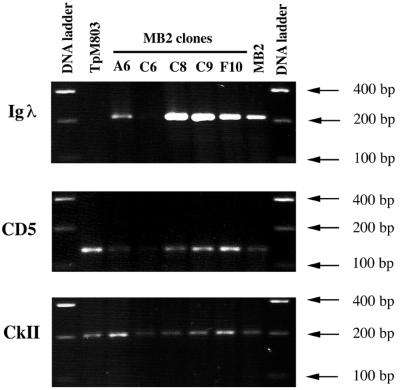
To confirm that MB2 does indeed contain CD5+ B cells with the Ig gene rearranged, individual clonal lines were obtained by limiting dilution. This was achieved by seeding two 96-well plates with less than 1 infected leukocyte per well; of the 25 different clonal lines obtained, 10 were further characterized. RT-PCR was then performed, and the results for five representative clonal lines are presented in Fig. 5. Because the MB2 isolate was low in CD44 (see Table 1 and Fig. 2), casein kinase II alpha (CKII) was used as a positive control, since this kinase is known to be expressed in Theileria-infected cells (24, 28). The MB2 isolate is composed predominantly of IgM+ cells, since four of five clones had their Ig genes rearranged (Fig. 5, top panel). Double-labelling (IgM plus CD5) FACS analysis performed on independent clones indicated that CD5 expression could be detected at the surfaces of infected cells. For example, clones C8 and F10 displayed 1.8 and 1.6% CD5 positivity, respectively, on gated IgM+ cells, compared with MB2, which in this experiment displayed 3.4% CD5 positivity (data not shown). Importantly, individual clones derived from MB2 also reflected modulation in the degree of IgM surface positivity, varying between 65 and 22% (see Fig. 4, insets).
FIG. 5.
CD5 expression on clonal lines derived from the MB2 isolate. Five representative clones derived from MB2 were analyzed for Igλ (top), CD5 (middle), and CkII (bottom) transcripts. The T. parva-infected T-cell line (TpM803) is shown as a positive control for CD5 expression (second lane from the left), and the uncloned isolate MB2 is included for comparison (second lane from the right). Specific fragments of the expected sizes were amplified as judged by comparison with the 100-bp DNA ladder.
By the combined use of RT-PCR amplification of target gene transcripts and FACS analysis of leukocyte marker cell surface expression, we have characterized a number of Theileria-infected laboratory-established lines and T. annulata-infected Tunisian clinical isolates. Although CD5+ B-cell lymphomas expressing macrophage markers have been described, the detection of CD5 on macrophage tumors appears novel. If one accepts that macrophages and CD5+ B cells have a common lineage (5), this finding could be considered consistent with the reported dedifferentiation that occurs upon T. annulata-induced macrophage transformation (26). The observation that TBL3 and TpMD409 transcribe Ig light-chain genes, are positive for surface IgM and BoLA class II, and appear CD5− is also consistent with their being classical B2-type B cells (16). Moreover, the complete lack of CD5 expression on TBL3 and TpMD409 B2-type B-cell lines supports the view that CD5 expression on B2 cells is not linked to parasite-induced dedifferentiation (25).
In contrast, the MB2 isolate contained a low number of cells expressing CD5, and this had three possible interpretations. First, the majority of cells constituting the isolate could be IgM+ CD5− B2 B cells, but MB2 also contained some CD5+ macrophages. We consider this hypothesis unlikely, because we eliminated macrophages from our FACS analysis by a combined light scatter (forward and side scatter) gate. Due to the known modulation in surface IgM positivity of Theileria-transformed leukocytes, a second explanation was that the majority of cells were CD5− B2 B cells, with a minor population of CD5+ B1 lymphocytes. Finally, due to the marked modulation of virtually all surface markers tested (see Table 2), a more likely possibility was that MB2 represents T. annulata-infected B1 B cells which have down-regulated the level of surface expression of CD5. To distinguish between these possibilities, we analyzed a number of individual clones derived from MB2, and all but one were found to be IgM+ and weakly CD5+. By RT-PCR or FACS analysis no clones were found to be either highly CD5+ or completely CD5−, which would have explained a mixed B1–B2 population. We conclude, therefore, that when T. annulata originally infected MB2, it was a B1 B cell which subsequently has down-regulated the level of CD5 expression, not unlike the down-regulation also observed for surface IgM and CD44.
The observation that T. annulata can infect CD5+ B cells should not have been unexpected, since B1 cells are common in bovine peripheral blood and T. annulata can infect B cells in vitro. Importantly, infection and transformation by Theileria parasites would result in uncontrolled proliferation of CD5+ cells, a situation already reported to be associated with infection by African trypanosomes (22). The sustained parasite-induced proliferation of B1 cells could result in altered cell differentiation and growth patterns of these cells, contributing to their neoplastic transformation. In this context, it would be of interest to examine T. parva clinical isolates for the presence of CD5+ B cells, as in other organisms and diseases the expansion of B1 lymphocytes can influence the resulting pathology.
Acknowledgments
This work received financial support from the Pasteur Institute and the CNRS, as well as a grant (no. 1708) to G.L. from l'ARC. L.B.M. was supported by an exchange fellowship between the Pasteur Institutes of Tunis and Paris, M.C. is a recipient of a MENERS fellowship from the French Ministry of Education, and M.B. is supported by a fellowship from the Swiss National Science Foundation.
We thank C. D. G. Brown, J. Naessens, and D. Dobbelaere for the gift of cell lines. J. Naessens for the gift of antibodies, B. Osborne for the Igλ sequences, and T. Jungi for communication of results prior to publication.
REFERENCES
- 1.Baldwin C L, Black S J, Brown W C, Conrad P A, Goddeeris B M, Kinuthia S W, Lalor P A, MacHugh N D, Morrison W I, Morzaria S P, Naessens J, Newson J. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun. 1988;56:462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer S R, Holmes K L, Morse III H C, Potter M. Clonal relationship of the lymphoblastic cell line P388 to the macrophage cell line P388D1 by immunoglobulin gene rearrangements and expression of cell surface antigens. J Immunol. 1986;136:4695–4699. [PubMed] [Google Scholar]
- 3.Baylis H A, Megson A, Brown C G D, Wilkie G F, Hall R. Theileria annulata-infected cells produce abundant proteases whose activity is reduced by long term culture. Parasitology. 1992;105:417–423. doi: 10.1017/s003118200007459x. [DOI] [PubMed] [Google Scholar]
- 4.Ben Miled L, Dellagi K, Bernardi G, Melrose T R, Darghouth M, Bouattour A, Kinnaird J, Shiels B, Tait A, Brown C G. Genomic and phenotypic diversity of Tunisian Theileria annulataisolates. Parasitology. 1994;108:51–60. doi: 10.1017/s0031182000078513. [DOI] [PubMed] [Google Scholar]
- 5.Borrello M A, Phipps R P. The B/macrophage cell: an elusive link between CD5+B lymphocytes and macrophages. Immunol Today. 1996;17:471–475. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- 6.Chaussepied M, Lallemand D, Moreau M-F, Adamson R, Hall R, Langsley G. Upregulation of Jun and Fos family members and permanent JNK activity lead to constitutive AP-1 activation in Theileria-transformed leukocytes. Mol Biochem Parasitol. 1998;94:215–226. doi: 10.1016/s0166-6851(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 7.Dobbelaere D A, Coquerelle T M, Roditi I J, Eichhorn M, Williams R O. Theileria parvainfection induces autocrine growth of bovine lymphocytes. Proc Natl Acad Sci USA. 1988;85:4730–4734. doi: 10.1073/pnas.85.13.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobbelaere D A, Prospero T D, Roditi I J, Kelke C, Baumann I, Eichhorn M, Williams R O, Ahmed J S, Baldwin C L, Clevers H, Morrison W I. Expression of Tac antigen component of bovine interleukin-2 receptor in different leukocyte populations infected with Theileria parva or Theileria annulata. Infect Immun. 1990;58:3847–3855. doi: 10.1128/iai.58.12.3847-3855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fell A H, Preston P M, Ansell J D. Establishment of Theileria-infected bovine cell lines in scid mice. Parasite Immunol. 1990;12:335–339. doi: 10.1111/j.1365-3024.1990.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 10.Glass E J, Innes E A, Spooner R L, Brown C G. Infection of bovine monocyte/macrophage populations with Theileria annulata and Theileria parva. Vet Immunol Immunopathol. 1989;22:355–368. doi: 10.1016/0165-2427(89)90171-2. [DOI] [PubMed] [Google Scholar]
- 11.Hardy R R. Variable gene usage, physiology and development of Ly-1+ (CD5+) B cells. Curr Opin Immunol. 1992;4:181–185. doi: 10.1016/0952-7915(92)90010-c. [DOI] [PubMed] [Google Scholar]
- 12.Hardy R R, Hayakawa K. Development and physiology of LY-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 13.Herzenberg L A, Lalor P A, Stall A M. Are Ly-1 B cells important in autoimmune disease? J Autoimmunity. 1989;2:225–231. doi: 10.1016/0896-8411(89)90134-0. [DOI] [PubMed] [Google Scholar]
- 14.Herzenberg L A, Stall A M, Lalor P A, Sidman C, Moore W A, Parks D R, Herzenberg L A. The LY-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 15.Irvin A D, Brown C G, Kanhai G K, Stagg D A. Comparative growth of bovine lymphosarcoma cells and lymphoid cells infected with Theileria parvain athymic (nude) mice. Nature. 1975;255:713–714. doi: 10.1038/255713a0. [DOI] [PubMed] [Google Scholar]
- 16.Li Y S, Hayakawa K, Hardy R R. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minoprio P, Bandeira P, Pereira P, Santos M, Coutinho A. Preferential expansion of Ly-1 B and CD4− CD8− T cells in the polyclonal lymphocyte responses to murine T. cruziinfection. Int Immunol. 1989;1:176–183. doi: 10.1093/intimm/1.2.176. [DOI] [PubMed] [Google Scholar]
- 18.Minoprio P, Cury-El-Cheikh M, Murphy E, Hontebeyrie-Joskowicz M, Coffman R, Coutinho A, O'Garra A. Xid-associated resistance to experimental Chagas' disease is IFN-γ-dependent. J Immunol. 1993;151:4200–4208. [PubMed] [Google Scholar]
- 19.Morrison W I, MacHugh N D, Lalor P A. Pathogenicity of Theileria parvais influenced by the host cell type infected by the parasite. Infect Immun. 1996;64:557–562. doi: 10.1128/iai.64.2.557-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naessens J, Grab D J, Fritsch G. Characterisation of bovine transferrin receptor on normal activated and Theileria parva-transformed lymphocytes by a new monoclonal antibody. Vet Immunol Immunopathol. 1996;52:65–76. doi: 10.1016/0165-2427(95)05537-1. [DOI] [PubMed] [Google Scholar]
- 21.Naessens J, Newson J, Bensaid A, Teale A J, Magondu J G, Black S J. De novo expression of T cell markers in Theileria parva-transformed lymphoblasts in cattle. J Immunol. 1985;135:4183–4188. [PubMed] [Google Scholar]
- 22.Naessens J, Williams D. Characterization and measurement of CD5+ B cells in normal and Trypanosoma congolense-infected cattle. Eur J Immunol. 1992;22:1713–1718. doi: 10.1002/eji.1830220708. [DOI] [PubMed] [Google Scholar]
- 23.Nelson R T, Hirumi H. In vitro cloning of Theileria-infected bovine lymphoblastoid cells: standardisation and characterisation. In: Irvin A D, Cunningham M P, Young A S, editors. Advances in the control of theileriosis. The Hague, The Netherlands: Martinus Nijhoff; 1981. p. 120. [Google Scholar]
- 24.ole MoiYoi O K, Brown W C, Iams K P, Nayar A, Tsukamoto T, Macklin M D. Evidence for the induction of casein kinase II in bovine lymphocytes transformed by the intracellular protozoan parasite Theileria parva. EMBO J. 1993;12:1621–1631. doi: 10.1002/j.1460-2075.1993.tb05807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sager H, Bertoni G, Jungi T W. Differences between B cell and macrophage transformation by the bovine parasite, Theileria annulata. A clonal approach. J Immunol. 1998;161:335–341. [PubMed] [Google Scholar]
- 26.Sager H, Davis W C, Dobbelaere D A E, Jungi T W. Macrophage-parasite relationship in theileriosis. Reversible phenotypic and functional dedifferentiation of macrophages infected with Theileria annulata. J Leukoc Biol. 1997;61:459–468. doi: 10.1002/jlb.61.4.459. [DOI] [PubMed] [Google Scholar]
- 27.Shaw M K, Tilney L G, Musoke A J. The entry of Theileria parvasporozoites into bovine lymphocytes: evidence for MHC class I involvement. J Cell Biol. 1991;113:87–101. doi: 10.1083/jcb.113.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shayan P, Ahmed A J S. Theileria-mediated constitutive expression of the casein kinase II-alpha subunit in bovine lymphoblastoid cells. Parasitol Res. 1997;83:526–532. doi: 10.1007/s004360050293. [DOI] [PubMed] [Google Scholar]
- 29.Spooner R L, Innes E A, Glass E J, Brown C G. Theileria annulata and T. parvainfect and transform different bovine mononuclear cells. Immunology. 1989;66:284–288. [PMC free article] [PubMed] [Google Scholar]
- 30.Syfrig J, Wells C, Daubenberger C, Musole A J, Naessens J. Proteolytic cleavage of surface proteins enhances susceptibility of lymphocytes to invasion by Theileria parvasporozoites. Eur J Cell Biol. 1998;76:125–132. doi: 10.1016/S0171-9335(98)80025-3. [DOI] [PubMed] [Google Scholar]
- 31.Tarakhovsky A. Bar Mitzvah for B-1 cells: how will they grow up? J Exp Med. 1997;185:981–984. doi: 10.1084/jem.185.6.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theilen G H, Rush J D, Nelson-Rees W A, Dungworth D L, Munn R J, Switzer J W. Bovine leukemia: establishment and morphological characterization of continuous cell suspension culture, BL-1. J Natl Cancer Inst. 1968;40:737–749. [PubMed] [Google Scholar]
- 33.Unkless J. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]