Abstract
OBJECTIVES:
To investigate racial and ethnic differences in unexpected, term newborn morbidity and the influence of hospital quality on disparities.
METHODS:
We used 2010–2014 birth certificate and discharge abstract data from 40 New York City hospitals in a retrospective cohort study of 483 834 low-risk (term, singleton, birth weight ≥2500 g, without preexisting fetal conditions) neonates. We classified morbidity according to The Joint Commission’s unexpected newborn complications metric and used multivariable logistic regression to compare morbidity risk among racial and ethnic groups. We generated risk-standardized complication rates for each hospital using mixed-effects logistic regression to evaluate quality, ranked hospitals on this measure, and assessed differences in the racial and ethnic distribution of births across facilities.
RESULTS:
The unexpected complications rate was 48.0 per1000 births. Adjusted for patient characteristics, morbidity risk was higher among Black and Hispanic infants compared with white infants (odds ratio: 1.5 [95% confidence interval 1.3–1.9]; odds ratio: 1.2 [95% confidence interval 1.1–1.4], respectively). Among the 40 hospitals, risk-standardized complications ranged from25.3 to 162.8 per 1000 births. One-third of Black and Hispanic women gave birth in hospitals ranking in the highest-morbidity tertile, compared with10% of white and Asian American women (P < .001).
CONCLUSIONS:
Black and Hispanic women were more likely to deliver in hospitals with high complication rates than were white or Asian American women. Findings implicate hospital quality in contributing to preventable newborn health disparities among low-risk, term births. Quality improvement targeting routine obstetric and neonatal care is critical for equity in perinatal outcomes.
Racial and ethnic disparities in infant health outcomes are well documented. The risk of neonatal death is more than twice as high among non-Hispanic Black infants than it is among non-Hispanic white infants,1 and the mortality risk is elevated in Hispanic subgroups, including Puerto Rican and very low birth weight Hispanic neonates.1–3 In addition, although overall infant mortality has declined over the past 50 years, racial gaps persist.3,4 Black2,5–9 and Hispanic2,6,10 infants have higher risks than white infants of severe morbidities, including necrotizing enterocolitis, intraventricular hemorrhage, bronchopulmonary dysplasia, and retinopathy of prematurity, complications that have consequences for immediate and long-term wellbeing.
Most infant disparities research has addressed racial and ethnic differences in preterm birth and the neonatal risks that accompany early delivery. Non-Hispanic Black infants are twice as likely as non-Hispanic white infants to be born before term.11 In addition, Black preterm infants have increased morbidity and mortality risks compared with white infants of a similar gestational age.2,6,8 Racial segregation and inequality within and across NICUs contribute to disparities,2,12–14 making quality of care a critical modifiable lever to improve high-risk outcomes. We know little about disparities outside of the NICU and preterm context, however, despite the fact that term deliveries constitute >90% of births.
The objective of this study was to understand whether racial and ethnic disparities exist among the lower-risk population of term neonates and, if so, estimate the influence of hospital quality on these disparities. We evaluated term neonatal morbidity with the “unexpected newborn complications” quality measure used by The Joint Commission. Specifically, we quantified potentially avoidable severe and moderate complications among otherwise healthy, term births,15 determined if complication rates varied by race and ethnicity, and identified whether differences in delivery hospital explained patterns in morbidity risk.
METHODS
Study Population
We conducted a population-based study of live births in New York City (NYC). We used vital statistics infant birth records linked to New York State hospital discharge abstract data for all NYC delivery hospitalizations from 2010 to 2014. The Statewide Planning and Research Cooperative System (SPARCS) provided discharge data. The New York State Department of Health conducted data linkage using a probabilistic linking method, with 99.9% of birth certificates matched to infant discharge data. The subsequent match rate with maternal discharge records was 96% (n = 573 846). The institutional review boards of the NYC Department of Health and Mental Hygiene, New York State Department of Health, and Icahn School of Medicine at Mount Sinai approved the study protocol.
Following the unexpected newborn complications measure,15 the study was restricted to term (≥37 completed weeks’ gestation), singleton neonates, birth weight ≥2500 g, without congenital anomalies, genetic disorders, preexisting fetal or placental conditions, or exposure to maternal drug use. We identified gestational age and birth weight from birth certificates. Plurality, congenital disorders, fetal-placental conditions, and maternal drug use were identified from International Classification of Diseases, Ninth Revision (ICD-9), diagnosis and procedure codes in discharge records. We excluded births in 4 hospitals with annual delivery volumes <5 births and 1 hospital that was closed for most of the study period, for a final study population of 483 834 births in 40 hospitals (Supplemental Fig 3).
Unexpected Newborn Complications Measure
We classified complications by type (neonatal death within 28 days of birth, shock and resuscitation, transfer to a higher-level facility, birth trauma or neurologic complications, respiratory complications, or infection) and severity (severe versus moderate) using ICD-9 discharge codes (Supplemental Table 4).15 All cases of neonatal death, shock and resuscitation, and transfer were classified as severe complications, whereas the remaining categories (birth trauma and neurologic, infection, and respiratory complications) were further subdivided by severe versus moderate conditions. The ICD-9 coding algorithm includes protections against undercoding (eg, the use of both procedure and diagnosis codes to increase ascertainment) and overcoding (eg, the inclusion of length of stay modifiers, >4 days after cesarean delivery and 2 days after vaginal birth, to distinguish the seriousness of morbidity) and has shown strong face validity and reliability across hospitals.16 In cases in which the infant had a length of stay >5 days without an accompanying morbidity, we screened the record for codes for jaundice, phototherapy, and social problems. If none of these were identified, we assigned “long length of stay with no listed complication” as a moderate morbidity.15 Severe and moderate classifications were mutually exclusive and applied hierarchically so that cases with both severe and moderate morbidity were classified only as severe.
Covariates
We included maternal sociodemographic characteristics (self-identified race and ethnicity, age, educational attainment, and insurance coverage) and clinical and obstetric factors (parity, previous cesarean delivery, prenatal care use, prepregnancy BMI, gestational age at birth, mode of delivery, and maternal comorbidities) in a series of risk-adjusted models of complication rates. Race and ethnicity were categorized as non-Hispanic Black (referred to throughout as Black), Hispanic, and non-Hispanic Asian American (referred to throughout as Asian American), compared with non-Hispanic white (referred to throughout as white). We ascertained covariates from birth certificates, with the exception of insurance status (obtained from SPARCS) and mode of delivery (procedure codes: 74.0, 74.1, 74.2, 74.4, and 74.99). BMI was identified from height and prepregnancy weight from maternal report on the birth certificate during delivery hospitalization. We used an established algorithm to classify the adequacy of prenatal care based on the trimester of initiation and number of visits.17
We used ICD-9 codes to identify maternal health conditions that may influence morbidity risk and were likely to have been present on admission for delivery, including diabetes (preexisting and gestational), hypertensive disorders (preexisting and gestational; preeclampsia), and chronic cardiac, circulatory, respiratory, pulmonary, renal, liver, biliary, skin, musculoskeletal, digestive, autoimmune, and central nervous system disorders. These comorbidities have been used in risk-adjustments for maternal18–21 and neonatal2,6,20,22 outcomes, including a Florida study of hospital variation in this newborn complications measure.23 The identification of diabetes and hypertension came from both birth certificates and discharge data, given previous research showing improved ascertainment by using both, as opposed to either source alone, when compared with medical record abstraction.24
We examined hospital-level characteristics including teaching status (obtained from the American Hospital Association), hospital ownership and nursery level (New York State Department of Health), and hospital delivery volume (SPARCS). We dichotomized nursery level (level 1 or 2 versus level 3 or 4) and classified the annual delivery volume by quartile. We calculated the percentage of deliveries covered by Medicaid in each hospital and divided hospitals into quartiles ranked by the percentage of Medicaid births.
Statistical Analyses
We examined the incidence of total, severe, and moderate complications over the study period by patient and hospital characteristics. We calculated unadjusted odds ratios for associations between race and ethnicity and unexpected newborn complications. Although our primary hypothesis did not address Asian American infants, we included comparisons of Asian American versus white births, given previous research finding higher risk of some morbidities among Asian American very preterm neonates.6 We adjusted models incrementally for maternal sociodemographic and clinical covariates to identify the extent to which disparities persisted beyond adjustment for patient-level risk factors not included in the unexpected newborn complications metric restrictions. We then added a fixed effect for each hospital to account for structural differences between hospitals that may influence effect estimates and identify disparities that exist within hospitals.25,26 In all models, we used logistic regression with a robust SE clustered at the hospital level.
To explore the role of the delivery hospital on neonatal risk by race and ethnicity, we examined whether Black, Hispanic, and Asian American women delivered in different and lower quality hospitals than white women did.27 We measured quality by calculating risk-standardized unexpected newborn complication rates for each hospital using mixed-effects logistic regression with a random hospital-specific intercept. These risk-standardized rates were computed by using the method recommended by the Centers for Medicare and Medicaid Services Hospital Compare and are the ratio of predicted to expected neonatal morbidity rates multiplied by the sample mean neonatal morbidity rate.20,28 For each hospital, the numerator of the ratio is the predicted number of morbidity cases based on the hospital’s performance with its case mix. The denominator is the expected number of cases based on NYC performance with that hospital’s case mix. Risk-standardization included patient-level but not hospital-level variables. We ranked hospitals from the lowest to highest risk-standardized newborn morbidity and used the Kolmogorov-Smirnov test to compare the cumulative distribution of births between racial and ethnic groups across hospitals ranked by risk-standardized morbidity. We also categorized hospitals into tertiles of risk-standardized morbidity and used χ2 tests to identify racial and ethnic differences in the percentage of births by tertile.
We conducted a sensitivity analysis to examine whether hospital rankings were different for severe complications only. We re-ran our models with this outcome and compared the rankings with those derived from our main model using a Spearman’s rank test. Finally, in a secondary analysis of outcomes related to hospital ranks, we assessed the racial and ethnic differences in newborn complication types among hospitals in the lowest-, middle-, and highest-morbidity tertiles. All analyses were conducted in SAS version 9.4 (SAS Institute, Inc, Cary, NC).
RESULTS
Among 483 834 neonates, the incidence of unexpected newborn complications was 48.0 per 1000 births (Table 1). The rates of severe and moderate complications were 16.8 and 31.2 per 1000 births, respectively.
TABLE 1.
Incidence of Unexpected Complications Among 483 834 Term Neonates Delivered in NYC Hospitals, 2010–2014, by Sociodemographic, Clinical, and Hospital Factors
| Unexpected Neonatal Complications (per 1000 Births) | ||||
|---|---|---|---|---|
| No. Births | Severe | Moderate | Total | |
| Overall incidence | 483 834 | 16.8 | 31.2 | 48.0 |
| Incidence by maternal sociodemographic characteristics | ||||
| Race and ethnicity | ||||
| Non-Hispanic white | 158 117 | 11.8 | 22.9 | 34.7 |
| Non-Hispanic Black | 93 766 | 24.6 | 47.0 | 71.6 |
| Hispanic | 147 827 | 19.9 | 34.1 | 54.0 |
| Asian American | 82 582 | 12.1 | 23.7 | 35.9 |
| Other | 1098 | 18.2 | 41.9 | 60.1 |
| Maternal age, y | ||||
| <20 | 22 791 | 27.0 | 41.0 | 68.0 |
| 20–34 | 354 490 | 16.9 | 30.7 | 47.6 |
| 35–39 | 83 145 | 14.1 | 29.7 | 43.9 |
| 40–44 | 21 817 | 15.7 | 33.7 | 49.4 |
| ≥45 | 1591 | 20.1 | 45.3 | 65.4 |
| Maternal education | ||||
| Less than high school | 104 023 | 19.6 | 35.0 | 54.6 |
| High school complete | 106 690 | 18.5 | 33.3 | 51.8 |
| More than high school | 271 740 | 15.1 | 28.8 | 43.9 |
| Insurance coverage | ||||
| Medicaid | 289 968 | 19.4 | 35.4 | 54.8 |
| Private insurance | 180 406 | 12.6 | 24.2 | 36.8 |
| Other coverage | 3553 | 11.5 | 27.9 | 39.4 |
| Uninsured | 9906 | 19.3 | 38.1 | 57.3 |
| Incidence by medical and obstetric characteristics | ||||
| Parity | ||||
| Nulliparous | 217 765 | 22.6 | 37.7 | 60.2 |
| Multiparous | 265 718 | 12.1 | 25.9 | 38.0 |
| Previous cesarean | 76 210 | 14.2 | 36.0 | 50.2 |
| Prenatal care usea | ||||
| Intensive | 31 337 | 16.8 | 36.8 | 53.5 |
| Adequate | 145 967 | 15.1 | 30.5 | 45.6 |
| Intermediate | 244 879 | 16.6 | 28.6 | 45.1 |
| Inadequate | 50 772 | 21.5 | 38.7 | 60.3 |
| None | 2193 | 28.7 | 62.9 | 91.7 |
| Prepregnancy BMIb | ||||
| Underweight | 26 416 | 11.3 | 22.1 | 33.4 |
| Normal wt | 263 501 | 14.3 | 26.5 | 40.8 |
| Overweight | 113 869 | 19.2 | 34.7 | 53.9 |
| Obese class I/II | 66 548 | 22.8 | 43.1 | 65.9 |
| Obese class III | 9679 | 31.5 | 56.8 | 88.3 |
| Hypertensive disorderc | 36 596 | 27.3 | 59.8 | 87.1 |
| Diabetic disorderd | 27 633 | 25.9 | 51.4 | 77.4 |
| Other maternal comorbiditye | 45 029 | 23.2 | 49.2 | 72.4 |
| Gestational age, wk | ||||
| Early term (37 + 0/7 to 38 + 6/7) | 115 382 | 17.3 | 35.4 | 52.6 |
| Term (39 + 0/7 to 40 + 6/7) | 319 319 | 15.9 | 28.8 | 44.7 |
| Late term (41 + 0/7 to 41 + 6/7) | 46 210 | 21.3 | 36.9 | 58.2 |
| Post term (≥42 + 0/7) | 2923 | 27.0 | 40.7 | 67.7 |
| Mode of delivery | ||||
| Vaginal | 337 987 | 14.0 | 24.2 | 38.3 |
| Cesarean | 145 847 | 23.4 | 47.3 | 70.6 |
| Incidence by characteristics of the delivery hospital (40 hospitals) | ||||
| Hospital ownership | ||||
| Private (29 hospitals) | 403 169 | 14.9 | 28.4 | 43.3 |
| Public (11 hospitals) | 80 665 | 26.4 | 45.3 | 71.7 |
| Delivery vol, annual, by quartile | ||||
| Low (<1389 deliveries) | 44 136 | 33.8 | 45.3 | 79.2 |
| Medium (1389 to <2317) | 84 897 | 26.1 | 42.8 | 68.9 |
| High (2317 to <3982) | 131 322 | 14.9 | 30.8 | 45.7 |
| Very high (≥3982) | 223 479 | 11.1 | 24.2 | 35.3 |
| Medicaid deliveries by quartile, % | ||||
| First (<52.6) | 187 342 | 13.0 | 26.2 | 39.2 |
| Second (52.6 to 81.6) | 143 495 | 11.1 | 25.2 | 36.3 |
| Third (81.6 to <88.3) | 75 863 | 25.5 | 35.2 | 60.7 |
| Fourth (≥88.3) | 77 134 | 28.1 | 50.4 | 78.6 |
The length of stay modifier for moderate complications requires >2 d stay for vaginal deliveries and >4 d for cesarean deliveries; septicemia with a length of stay ≤4 d was coded as moderate complication. Severe and moderate complications are identified in Supplemental Table 4.
Based on the trimester of prenatal care initiation and number of prenatal visits, by using the Adequacy of Prenatal Care Index (Kotelchuck17): inadequate (prenatal care initiated after the fourth month or <50% of recommended visits received); intermediate (prenatal care initiated by fourth month and 50% to 90% of recommended visits received); adequate (prenatal care initiated by fourth month and 80% to 109% of visits received); intensive (prenatal care initiated by fourth month and ≥110% of visits received).
BMI (underweight: <18.5; normal wt: 18.5–24.9; overweight: 25.0–29.9; obese class I or II: 30.0–39.9; obese class III: ≥40).
Includes preeclampsia, preexisting hypertension, and gestational hypertension.
Includes preexisting and gestational diabetes.
Includes diagnoses in the following categories: cardiac, pulmonary, renal, skin and/or subcutaneous, musculoskeletal, digestive, and central nervous system disorders, rheumatic heart disease, arterial and venous disorders, lupus, collagen vascular disorders, rheumatoid arthritis, anemia, asthma, chronic bronchitis, and live and biliary tract disorders. Complication rates by NICU level are not provided to protect confidentiality, given the small cell size (<10 hospitals with a level 1 or 2 NICU).
Racial and Ethnic Variation in Unexpected Newborn Complications
Total newborn complication rates were higher among Black (71.6 per 1000 uncomplicated term births) and Hispanic (54.0 per 1000) infants than among white (34.7.9 per 1000) and Asian American (35.9 per 1000) infants (Table 1). Table 2 presents associations between race and ethnicity and adjusted risk of unexpected newborn complications. The higher risk observed among Black and Hispanic than among white infants was attenuated with an adjustment for maternal sociodemographic (model 1) and clinical and obstetric (model 2) characteristics. The odds of complications were 1.5 (95% confidence interval [CI] 1.3–1.9) times higher among Black infants and 1.2 (95% CI 1.1.–1.4) times higher among Hispanic infants, after adjustment for patient-level characteristics. The morbidity risk did not differ between Asian American and white women in either unadjusted or adjusted analyses. The inclusion of hospital fixed effects eliminated the increased risk among Hispanic infants, and only a slightly elevated risk remained among Black infants. We conducted a sensitivity analysis, additionally adjusting for nativity, and it did not alter effect estimates (results not shown).
TABLE 2.
Associations Between Race and Ethnicity and Total (Severe or Moderate) Unexpected Neonatal Complications (N = 483 834)
| Race and Ethnicity | Unadjusted OR (95% CI) | Model 1 (Adjusted for Sociodemographic Characteristics),a aOR (95% CI) | Model 2 (Model 1 and Clinical and Obstetric Characteristics),b aOR (95% CI) | Model 3 (Model 2 and Delivery Hospital),c aOR (95% CI) |
|---|---|---|---|---|
| Non-Hispanic white | Reference | Reference | Reference | Reference |
| Non-Hispanic Black | 2.1 (2.1–2.2) | 1.9 (1.5–2.5) | 1.5 (1.3–1.9) | 1.1 (1.1–1.2) |
| Hispanic | 1.6 (1.5–1.6) | 1.4 (1.2–1.7) | 1.2 (1.1–1.4) | 1.0 (1.0–1.1) |
| Asian American | 1.0 (1.0–1.1) | 1.0 (0.8–1.1) | 0.9 (0.8–1.0) | 0.9 (0.9–1.0) |
All models used logistic regression, with a robust SE clustered at the hospital level. aOR, adjusted odds ratio; OR, odds ratio.
Adjusted for maternal age, educational attainment, and insurance coverage.
Adjusted for covariates in Model 1 as well as parity, previous cesarean delivery, prenatal care use, prepregnancy BMI, hypertensive disorders, diabetic disorders, other maternal comorbidities, gestational age, and delivery mode.
Adjusted for covariates in Model 2 as well as a fixed effect for each delivery hospital.
Influence of the Delivery Hospital
Most hospitals were private facilities with level 3 or 4 nurseries (Table 1). Hospitals had a median delivery volume of 2317 (interquartile range 1389–3928) deliveries per year and an 81.6% (interquartile range 52.6%–88.3%) median percentage of births covered by Medicaid. Unadjusted newborn complication rates ranged from 25.3 to 162.8 per 1000 births across the 40 hospitals (Fig 1). Hospital risk-standardized morbidity rates ranged from 24.5 to 138.1 per 1000 births. Hospital risk-standardized rates of severe morbidity ranged from 5.7 to 85.9 per 1000 births, and hospital rankings remained similar when limited to severe morbidity only (rs = 0.9, P < .001).
FIGURE 1.
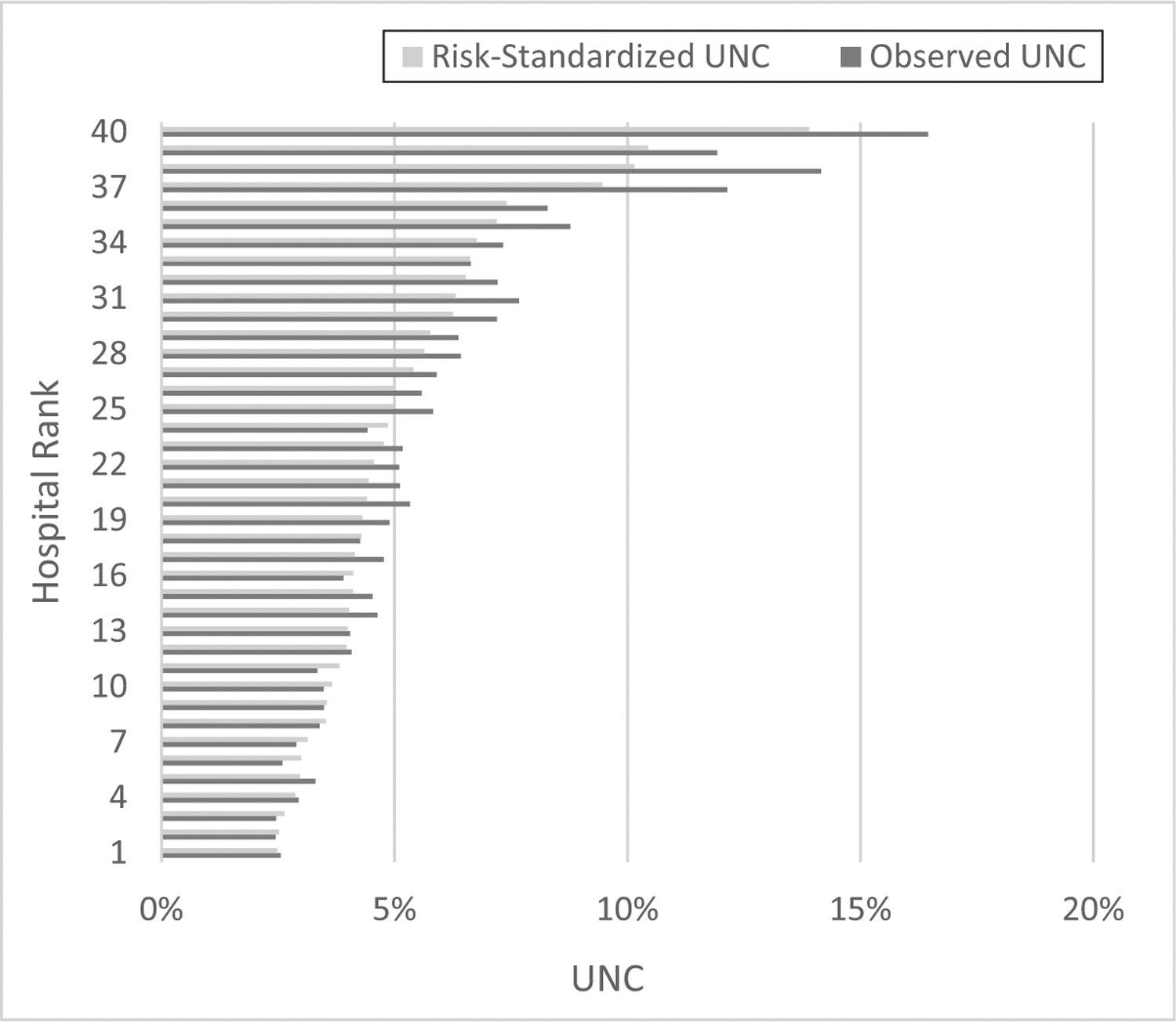
Observed and risk-standardized unexpected neonatal complications among 40 NYC hospitals, 2010–2014. Risk-standardized rates are adjusted for maternal race and ethnicity, age, educational attainment, insurance coverage, parity, previous cesarean delivery, prenatal care use, prepregnancy BMI, hypertensive disorders, diabetic disorders, other maternal comorbidities, gestational age, and delivery mode. UNC, unexpected newborn complications.
The cumulative distribution of deliveries according to hospital ranking differed among racial and ethnic groups (P < .001; Fig 2). Approximately one-third of Black and Hispanic women (33.1% and 34.3%, respectively) gave birth in hospitals in the highest tertile of risk-standardized neonatal morbidity, compared with only 10% of white and Asian American women (P < .001). Conversely, the majority of white (69.8%) and Asian American (62.4%) deliveries occurred in the lowest morbidity hospitals, compared with 35.8% of Black and 34.4% of Hispanic births (Supplemental Table 5).
FIGURE 2.
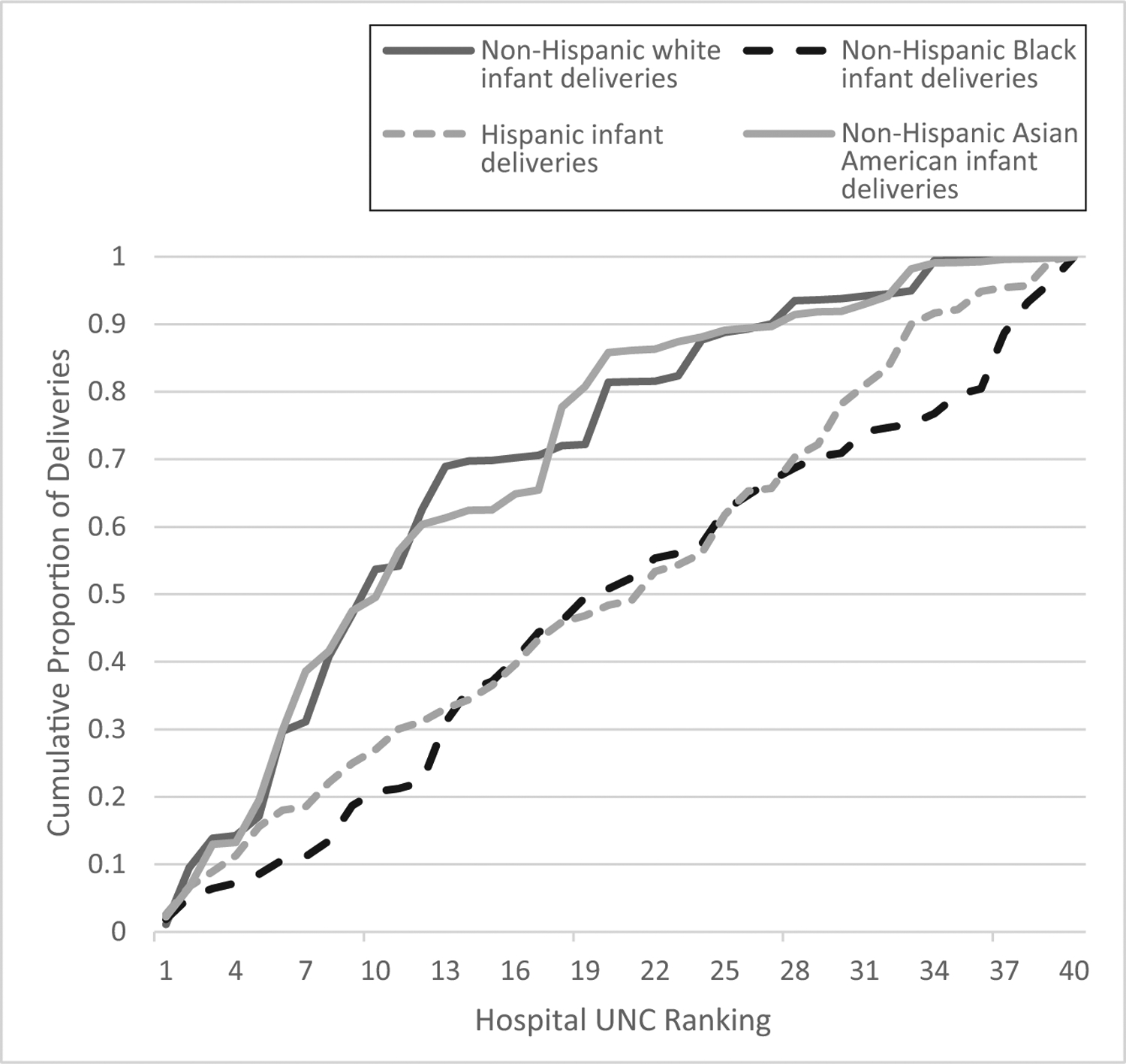
Cumulative distribution of births according to delivery hospital, ranked from the lowest (1) to highest (40) risk-standardized unexpected neonatal complications rate, by maternal race and ethnicity. UNC, unexpected newborn complications.
Complication Types
Respiratory complications were the most prevalent morbidity (29.9 per 1000 births), followed by infection (9.3 per 1000). The incidence of specific complications differed by race and ethnicity and hospital ranking, with particularly pronounced rates of infection and respiratory complications among Black infants and in the highest-morbidity hospitals (Table 3). Infection rates showed the widest variation. Severe infection, specifically, was elevated among Black and Hispanic infants (11.8 and 9.0 per 1000 births, respectively) compared with Asian American and white infants (5.2 and 3.9 per 1000) and was roughly sixfold higher for hospitals in the highest versus lowest morbidity tertile (18.5 vs 3.1 cases per 1000 births; not shown in the table). Notably, racial and ethnic disparities were present even in low-morbidity hospitals and were similar in magnitude across tertiles for some morbidities, such as respiratory complications.
TABLE 3.
Newborn Complication Types by Race and Ethnicity and Morbidity Ranking of the Delivery Hospitals
| All Complications, by Race and Ethnicity and Risk-Standardized Morbidity Rate of the Delivery Hospital, Rate per 1000 Births | |||||||
|---|---|---|---|---|---|---|---|
| Race and Ethnicity | Neonatal Deatha | Respiratory | Neurologic and/or Birth Injury | Infection | Neonatal Transferb | Shock and/or Resuscitation | Length of Stay >5 d With No Listed Complicationc |
| Tertile 1 (lowest risk-standardized hospital neonatal morbidity rates) | |||||||
| Non-Hispanic white | 0.1 | 19.7 | 4.1 | 3.6 | 0.8 | 0.2 | 1.0 |
| Non-Hispanic Black | 0.3 | 27.9 | 5.7 | 5.8 | 1.0 | 0.1 | 8.7 |
| Hispanic | 0.1 | 22.8 | 4.8 | 5.8 | 0.9 | 0.1 | 4.8 |
| Non-Hispanic Asian American | 0.1 | 16.0 | 3.9 | 4.7 | 1.1 | 0.1 | 2.8 |
| Tertile 2 | |||||||
| Non-Hispanic white | 0.0 | 30.6 | 4.0 | 6.3 | 0.5 | 0.1 | 5.9 |
| Non-Hispanic Black | 0.2 | 33.8 | 5.3 | 10.0 | 1.9 | 0.0 | 13.0 |
| Hispanic | 0.4 | 29.6 | 6.9 | 7.8 | 1.3 | 0.2 | 9.6 |
| Non-Hispanic Asian American | 0.0 | 25.3 | 4.3 | 5.9 | 1.5 | 0.1 | 10.2 |
| Tertile 3 (highest risk-standardized hospital neonatal morbidity rates) | |||||||
| Non-Hispanic white | 0.1 | 45.3 | 5.5 | 11.4 | 0.8 | 0.1 | 8.2 |
| Non-Hispanic Black | 0.2 | 66.7 | 8.4 | 33.5 | 1.6 | 0.3 | 17.8 |
| Hispanic | 0.2 | 43.4 | 9.6 | 19.4 | 1.4 | 0.2 | 11.4 |
| Non-Hispanic Asian American | 0.1 | 47.5 | 7.0 | 19.7 | 1.9 | 0.1 | 12.1 |
Complications were classified by using the unexpected complications in term newborns algorithm, available from the California Maternal Quality Care Collaborative at https://www.cmqcc.org/focus-areas/quality-metrics/unexpected-complications-term-newborns/.
Death within 28 d of birth.
Transfer to a higher level of care.
Infant length of stay >5 days, no neonatal complication coded on discharge record, and no codes for jaundice and phototherapy or social reasons for extended length of stay.
DISCUSSION
We observed racial and ethnic disparities in unexpected complications among otherwise healthy term neonates born in NYC hospitals. Black and Hispanic infants were at a higher risk for unexpected morbidity than were white or Asian American infants. In addition, we found substantial variation in delivery distributions among hospitals with low versus high morbidity, with Black and Hispanic women more likely than white or Asian American women to deliver in hospitals with high risk-adjusted complication rates. These high morbidity hospitals had high rates of infectious and respiratory morbidity, especially among Black infants.
Our findings are consistent with previous research on disparities among high-risk preterm and low birth weight deliveries2,5,6,13,14 and for other obstetric outcomes.6,29–35 The incremental adjustment in our regression models provides information on potential causal mechanisms. By adjusting for sociodemographic and medical characteristics, we attenuated the excess morbidity risk among Black and Hispanic births, implicating patient-level risk factors such as maternal age and prepregnancy health in neonatal disparities. The remaining variation was explained almost entirely by differences between delivery hospitals independent of their case mix. This finding suggests that structural and organizational characteristics among hospitals where minority women deliver are associated with lower quality of care than those with predominantly white obstetric populations.27 Although intervening on social determinants of health and intermediary health behaviors is critical for addressing health inequity, hospital quality can be addressed directly by improvements to the health care system.36
The wide variation in hospital-level morbidity in our study corroborates findings from other US locations.23,37,38 Respiratory morbidity was similarly the most common type of unexpected newborn complication among California hospitals.15 In previous analyses,38,39 researchers found that neonatal transfers were the primary driver of hospital severe complication rates, but these studies included hospitals without a NICU or with level 1 facilities. The majority of nurseries in our study (33 of 40) were level 3 or 4 NICUs, and none were classified as level 1, which may explain the lower transfer rate observed in our data.
We explored the influence of the delivery hospital on morbidity risk and found greater proportions of Black and Hispanic infant births, compared with white infant births, in hospitals with high rates of risk-standardized neonatal complications. Similar patterns have been observed for other health outcomes,40 maternal health,33,34 and very low birth weight (<1500 g) or very preterm (<32 weeks’ gestation) infants.2,5 Significant racial and ethnic variation in NICU quality exists across2,13,14 and, in some cases, within13 US hospitals. Importantly, the quality disadvantage may be concentrated in modifiable health care practices, with Black and Hispanic infants scoring worse than white infants on process indicators of NICU quality.13 Our findings show that considerable disparities exist not only among the vulnerable neonatal populations who require complex care, but also among low-risk, term births. In addition, we demonstrate the capacity of The Joint Commission unexpected newborn complications measure to capture disparities, which is a shortcoming identified for other perinatal quality metrics.41
Although this population was selected to be at minimal risk of complications, one US study found that nearly 30% of births considered low-risk had an unexpected complication that would necessitate additional obstetric or neonatal intervention.42 Our results reinforce the difficulty of classifying low-risk pregnancies and suggest that improving the recognition and management of acute complications during delivery care could both reduce morbidity overall and lessen racial and ethnic gaps in infant outcomes. Our finding of especially high rates of infectious and respiratory morbidity among Black women delivering in the highest-morbidity hospitals isolates a specific set of conditions and subgroup of patients for whom targeted quality improvements may be especially beneficial. Scrutiny of patient safety practices in these cases may be a warranted first-step in identifying clinical leverage points.43 Expert consensus from the maternal health arena provides steps for hospitals and health systems to improve safety and quality, such as standardizing labor protocols for obstetric emergencies and implementing disparities dashboards and implicit bias interventions.44,45 These strategies may provide lessons for the reduction of avoidable neonatal disparities. Finally, disparities in low-morbidity hospitals suggest that the contribution of quality may not be uniform across newborn complication types.
Our study may be limited by the accuracy of diagnosis and procedure codes in SPARCS data. We used both birth certificates and discharge abstracts for conditions such as maternal comorbidities to improve validity.24 Variation in hospital coding may have biased results if practices are related to patient race or ethnicity. We were unable to determine if neonatal transfer was to a higher level of care, but transfer frequency decreased with increasing level of care. In addition, when we excluded neonatal transfer the rate of severe complications decreased slightly from 16.8 to 16.2 cases per 1000 births and the distribution of complications by patient and hospital characteristics did not change, so it is unlikely to have biased our results. We could not identify whether the contribution of delivery hospital was in part due to unmeasured patient social or medical factors, such as neighborhood environment and income. Our results represent a racially and ethnically diverse population of low-risk births in NYC and may not generalize to other areas of the country. Further research should evaluate whether associations differ with more finely classified racial and ethnic backgrounds, such as Asian American and Hispanic subgroups. Finally, we were not able to identify cases of fetal death in our data set, and findings generalize to live births.
CONCLUSIONS
Low-risk Black and Hispanic infants born at term were at increased risk of complications, compared with white infants. We observed variation in morbidity independent of maternal and neonatal risk factors known to be socially patterned, including maternal comorbidities, length of gestation, and fetal growth. Our findings implicate hospital quality as a contributing factor to racial and ethnic inequalities in unexpected newborn complications. Term births constitute the vast majority of deliveries, and minimizing potentially preventable complications at term would have a substantial population-level impact. By identifying disparities among otherwise healthy, low-risk infants, we emphasize patient safety and quality improvement targeting routine obstetric and neonatal care as a critical but underused approach to disparity reduction.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
Hospital quality contributes to health disparities among high-risk neonates, but little is known about the role of quality outside the NICU context. The Joint Commission’s unexpected newborn complications measure may help to identify potentially avoidable disparities among low-risk, term births.
WHAT THIS STUDY ADDS:
Morbidity among low-risk, term newborns varied widely across hospitals. Greater proportions of Black and Hispanic infant births, compared with white infant births, occurred in hospitals with the highest risk-standardized complication rates, suggesting hospital quality is a lever for disparity reduction.
FUNDING:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD078565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder did not participate in the work. Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- CI
confidence interval
- ICD-9
International Classification of Diseases, Ninth Revision
- NYC
New York City
- SPARCS
Statewide Planning and Research Cooperative System
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have no potential conflicts of interest relevant to this article to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2021-050768.
REFERENCES
- 1.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep. 2015;64(9):1–30 [PubMed] [Google Scholar]
- 2.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in morbidity and mortality rates in Black, white, and Hispanic very preterm infants among New York City hospitals. JAMA Pediatr. 2018;172(3):269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willis E, McManus P, Magallanes N, Johnson S, Majnik A. Conquering racial disparities in perinatal outcomes. Clin Perinatol. 2014;41(4):847–875 [DOI] [PubMed] [Google Scholar]
- 4.Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144(2):e20183114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell EA, Hebert P, Chatterjee S, Kleinman LC, Chassin MR. Black/white differences in very low birth weight neonatal mortality rates among New York City hospitals. Pediatrics. 2008;121(3):e407–e415 [DOI] [PubMed] [Google Scholar]
- 6.Janevic T, Zeitlin J, Auger N, et al. Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr. 2018;172(11): 1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Lin A, Maller-Kesselman J, et al. ; Gene Targets for Intraventricular Hemorrhage Study. Maternal race, demography, and health care disparities impact risk for intraventricular hemorrhage in preterm neonates. J Pediatr. 2014;164(5):1005.e3–1011.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace ME, Mendola P, Kim SS, et al. Racial/ethnic differences in preterm perinatal outcomes. Am J Obstet Gynecol. 2017;216(3):306.e1–306.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30(11): 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lad EM, Nguyen TC, Morton JM, Moshfeghi DM. Retinopathy of prematurity in the United States. Br J Ophthalmol. 2008;92(3):320–325 [DOI] [PubMed] [Google Scholar]
- 11.Schaaf JM, Liem SM, Mol BW, Abu-Hanna A, Ravelli AC. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol. 2013;30(6): 433–450 [DOI] [PubMed] [Google Scholar]
- 12.Sigurdson K, Morton C, Mitchell B, Profit J. Disparities in NICU quality of care: a qualitative study of family and clinician accounts. J Perinatol. 2018;38(5):600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Profit J, Gould JB, Bennett M, et al. Racial/ethnic disparity in NICU quality of care delivery. Pediatrics. 2017; 140(3):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horbar JD, Edwards EM, Greenberg LT, et al. Racial segregation and inequality in the neonatal intensive care unit for very low-birth-weight and very preterm infants. JAMA Pediatr. 2019;173(5): 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.California Maternal Quality Care Collaborative. Unexpected Complications in Term Newborns. Stanford, CA: California Maternal Quality Care Collaborative; 2007 [Google Scholar]
- 16.Main E Unexpected newborn complications: understanding the new measure (PC-06) slideset. California Maternal Quality Care Collaborative. Available at: https://www.cmqcc.org/resource/3441/download. Accessed October 26, 2019 [Google Scholar]
- 17.Kotelchuck M An evaluation of the adequacy of prenatal care index and a proposed adequacy of prenatal care utilization index. Am J Public Health. 1994;84(9):1414–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray KE, Wallace ER, Nelson KR, Reed SD, Schiff MA. Population-based study of risk factors for severe maternal morbidity. Paediatr Perinat Epidemiol. 2012;26(6):506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell EA, Zeitlin J, Hebert PL, Balbierz A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12(4):469–477 [DOI] [PubMed] [Google Scholar]
- 22.Main EK, Chang SC, Cape V, Sakowski C, Smith H, Vasher J. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133(4):613–623 [DOI] [PubMed] [Google Scholar]
- 23.Sebastião YV, Womack LS, López Castillo H, et al. Hospital variations in unexpected complications among term newborns. Pediatrics. 2017;139(3): e20162364. [DOI] [PubMed] [Google Scholar]
- 24.Lain SJ, Hadfield RM, Raynes-Greenow CH, et al. Quality of data in perinatal population health databases: a systematic review. Med Care. 2012; 50(4):e7–e20 [DOI] [PubMed] [Google Scholar]
- 25.Howell EA, Egorova NN, Janevic T, et al. Race and ethnicity, medical insurance, and within-hospital severe maternal morbidity disparities. Obstet Gynecol. 2020;135(2):285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebert PL, Howell EA, Wong ES, et al. Methods for measuring racial differences in hospitals outcomes attributable to disparities in use of high-quality hospital care. Health Serv Res. 2017;52(2):826–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howell EA, Zeitlin J. Improving hospital quality to reduce disparities in severe maternal morbidity and mortality. Semin Perinatol. 2017;41(5):266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ash AS, Feinberg SE, Louis TA, Normand S-L, Stukel TA, Utts J. Statistical Issues in Assessing Hospital Performance. Baltimore, MD: Centers for Medicare and Medicaid Services; 2012 [Google Scholar]
- 29.Borrell LN, Rodriguez-Alvarez E, Savitz DA, Baquero MC. Parental race/ethnicity and adverse birth outcomes in New York City: 2000–2010. Am J Public Health. 2016;106(8):1491–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant AS, Washington S, Kuppermann M, Cheng YW, Caughey AB. Quality and equality in obstetric care: racial and ethnic differences in caesarean section delivery rates. Paediatr Perinat Epidemiol. 2009;23(5):454–462 [DOI] [PubMed] [Google Scholar]
- 31.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol. 2014;210(5): 435.e1–435.e8 [DOI] [PubMed] [Google Scholar]
- 32.Gyamfi-Bannerman C, Srinivas SK, Wright JD, et al. Postpartum hemorrhage outcomes and race. Am J Obstet Gynecol. 2018;219(2):185.e1–185.e10 [DOI] [PubMed] [Google Scholar]
- 33.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howell EA, Egorova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe maternal morbidity among Hispanic women in New York City. Obstet Gynecol. 2017;129(2):285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsubara S, Takahashi H, Ohkuchi A. Racial difference in postpartum hemorrhage outcome: pathophysiologic, clinical, and social significance? Am J Obstet Gynecol. 2018;219(5):515–516 [DOI] [PubMed] [Google Scholar]
- 36.Howell EA, Zeitlin J. Quality of care and disparities in obstetrics. Obstet Gynecol Clin North Am. 2017;44(1):13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Perinatal Information Center. Unexpected newborn complications update (July 2013). Available at: https://www.cmqcc.org/resource/2932/download. Accessed January 30, 2020
- 38.Clapp MA, James KE, Bates SV, Kaimal AJ. Patient and hospital factors associated with unexpected newborn complications among term neonates in US hospitals. JAMA Netw Open. 2020; 3(2):e1919498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sebastiao YV, Womack L, Vamos CA, et al. Hospital variation in cesarean delivery rates: contribution of individual and hospital factors in Florida. Am J Obstet Gynecol. 2016;214(1): 123.e1–123.e8 [DOI] [PubMed] [Google Scholar]
- 40.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43(4):308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janevic T, Egorova NN, Zeitlin J, Balbierz A, Hebert PL, Howell EA. Examining trends in obstetric quality measures for monitoring health care disparities. Med Care. 2018;56(6):470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danilack VA, Nunes AP, Phipps MG. Unexpected complications of low-risk pregnancies in the United States. Am J Obstet Gynecol. 2015;212(6): 809.e1–809.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020; 87(2):227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities: a conceptual framework and maternal safety consensus bundle. Obstet Gynecol. 2018;131(5):770–782 [DOI] [PubMed] [Google Scholar]
- 45.Main EK, Goffman D, Scavone BM, et al. ; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126(1):155–162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


