Abstract
To identify novel putative staphylococcal adhesins, lithium chloride extraction (an established method for selective surface molecule solubilization) was employed. N-terminal sequencing and functional assays identified a 42-kDa fibronectin-binding protein from Staphylococcus epidermidis as ornithine carbamoyltransferase (OCTase). However, OCTase was not recognizable extracellularly, and this fact together with the fact that LiCl induced DNA release and a decrease in viability suggests that LiCl extraction may not be the method of choice for selective surface molecule extraction from staphylococci.
Attachment to artificial or biological surfaces is prerequisite for the commensal coagulase-negative staphylococci (CoNS) to cause invasive disease (1). Staphylococci may interact with adhesive surface sites consisting of exposed or immobilized extracellular matrix (ECM) proteins such as fibronectin (FN), fibrinogen (FG), vitronectin (VN), collagen, elastin, and several other proteins (16), and evidence from in vitro and ex vivo studies has suggested a role for this interaction in clinical disease (9, 13, 18). Evaluation of the role of the staphylococcus-ECM protein interaction led to the identification and the molecular and functional characterization of staphylococcal adhesins, the so-called microbial surface components recognizing adhesive matrix molecules of the staphylococcal adhesin superfamily (for a review, see reference 2). Most of this information has been obtained with Staphylococcus aureus, and in spite of the ability of CoNS to avidly adhere to ECM molecules (7), the information on adhesive molecules in CoNS is relatively scant (15, 17).
In order to study their functional and biological roles, putative adhesins have to be extracted from microorganisms and isolated in a pure form. Although many other methods are available (5, 8, 11, 21), LiCl extraction has been employed in a number of studies to specifically extract and solubilize surface components of staphylococci while maintaining cell integrity (10, 11, 19). In this study we intended to use this method for identification of such adhesins recognizing FN in CoNS, and we report our results with respect to the suitability of the LiCl extraction method for CoNS and the nature of a FN-binding Staphylococcus epidermidis protein.
Extraction with LiCl.
Thirty blood isolates of S. epidermidis were obtained from patients with indwelling-device infections admitted at the University Hospital of Muenster, Germany. Isolates were maintained on blood agar plates and subcultured prior to experimentation with brain heart infusion medium. Cell surface proteins were released by LiCl extraction according to published protocols (10). Briefly, bacteria were grown overnight at 37°C with shaking (150 rpm) and cells were harvested by centrifugation and washed twice with phosphate-buffered saline. Washed cells were suspended in 1 M LiCl (Sigma-Aldrich Chemie, Deisenhofen, Germany) and pelleted after incubation at 42°C with shaking (150 rpm) for 2 h. The supernatant was extensively dialyzed against distilled water (4°C) and was freeze-dried. For identification of putative staphylococcal adhesins, either FG, FN (Chemicon, Temecula, Calif.), or VN was used. VN was affinity purified in a two-step purification procedure with urea-activated VN and a heparin column as described previously (20). FN, FG, and VN were labeled with biotin (Boehringer Mannheim GmbH, Mannheim, Germany). For Western ligand experiments, LiCl extracts were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membranes, and probed with either biotin-labeled FN, FG, or VN. With biotin-labeled FN, a protein of 42 kDa was observed in LiCl extracts of several tested strains (Fig. 1, lane A). In extracts of strain AB9, this protein was found in larger quantity than in the other strains tested. Modification of standard conditions by shaking of bacteria in 1 M LiCl for 30 min and 1 h, and at 25 and 37°C, yielded similar results, i.e., the presence of the 42-kDa protein by SDS-PAGE as well as in Western ligand blots probed with biotinylated VN and FN.
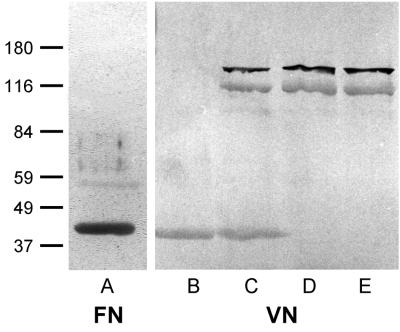
FIG. 1.
Western ligand blot of S. epidermidis AB9 extracts. Lanes: A and B, LiCl extract; C, lysostaphin plus lysozyme extract; D, SDS extract; E, supernatant of protoplast preparation. Molecular mass markers (in kilodaltons) are noted at the left.
Extraction by other methods.
For comparison with the LiCl extraction method, whole-cell extracts of bacteria (1 g [wet weight]/20 ml) were obtained with recombinant lysostaphin (50 μg/ml; Applied Micro, New York, N.Y.) along with lysozyme (1 mg/ml; Merck, Darmstadt, Germany) in Tris-HCl buffer (50 mM Tris, 150 mM NaCl [pH 8.0]) containing a protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride [Sigma], 2 mM N-ethylmaleimide [Sigma], and 1 mM EDTA [Sigma], final concentrations). Western ligand analysis of lysostaphin-lysozyme extracts revealed the 42-, a 160-, and a 112-kDa bacterial protein band recognized by labeled FN. In contrast, extracts of S. epidermidis surface molecules with 2% SDS (95°C, 10 min) yielded the 160- and 112-kDa proteins but not the 42-kDa protein. In addition, cell-free supernatants after protoplast preparation of S. epidermidis AB9 cell wall molecules obtained by digesting microorganisms with lysostaphin plus lysozyme in a hypertonic milieu (50 mM Tris, 0.45 M sucrose, 8 mM EDTA [pH 8.0]) contained only the 160- and the 112-kDa protein bands recognized by labeled FN. Parallel Western ligand assays performed with labeled VN (Fig. 1, lanes B to E) or FG (not shown) demonstrated recognition by these ligands of proteins in all extraction procedures identical to the proteins recognized by FN, suggesting broad-spectrum specificities of the putative adhesins. These findings raised the possibility that LiCl extraction releases a 42-kDa intracellular molecule, whereas the lack of the 42-kDa protein and the presence of the 160- and 112-kDa bands in either SDS extracts or supernatants from protoplast preparations suggest the latter to be of cell wall or extracellular localization.
Localization of the 42-kDa antigen.
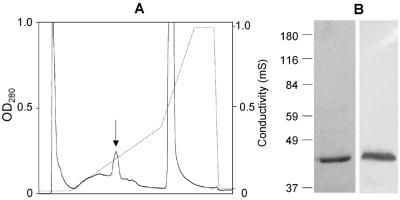
Intracellular location was further confirmed by failure to recognize the 42-kDa antigen immunologically on the cell surface. The 42-kDa protein was purified by medium-pressure chromatography (Biologic; Bio-Rad, Munich, Germany) over an anion-exchange column (Bio-Q10; Bio-Rad). Bound protein was eluted in fractions 48 to 57 with 0.15 to 0.25 M NaCl in loading buffer (Fig. 2A). These fractions contained the 42-kDa protein at high purity (99%) as analyzed by SDS-PAGE and staining with Coomassie brilliant blue, and after purification, the 42-kDa protein was recognizable by labeled FN in Western ligand assays (Fig. 2B). An anti-42 kDa antiserum was raised by one injection with the purified 42-kDa antigen followed by two booster injections each in two rabbits according to standard procedures (4). For a control, antiserum against whole S. epidermidis AB9 cells was prepared by injecting formalin-fixed cells by an analogous procedure. The anti-42 kDa antiserum specifically and avidly recognized the 42-kDa protein as demonstrated in immunoblots both of LiCl extracts and of the purified 42-kDa antigen incubated with the anti-42-kDa antibodies at dilutions of up to 1:10,000. For detection of the 42-kDa protein on surfaces on intact cells, S. epidermidis AB9 cells (37°C, 30 min) were incubated with antiserum (dilution, 1:200; 37°C, 30 min), washed three times, and then incubated with secondary fluorescein-conjugated anti-rabbit immunoglobulin G (Sigma). No immunofluorescence was observed if whole S. epidermidis AB9 cells were treated with the antiserum specific to the 42-kDa protein, while strong immunofluorescence was observed when S. epidermidis AB9 cells were exposed to anti-whole-cell antibodies.
FIG. 2.
Medium-pressure chromatography of the 42-kDa protein from S. epidermidis AB9. (A) Eluate characteristics. Arrow, fractions 48 to 57 containing the 42-kDa protein. (B) SDS-PAGE (left lane) and Western ligand blot with labeled FN (right lane) of the purified 42-kDa protein from pooled fractions 48 to 57. Molecular mass markers (in kilodaltons) are noted at the left.
Nucleic acid release after LiCl extraction.
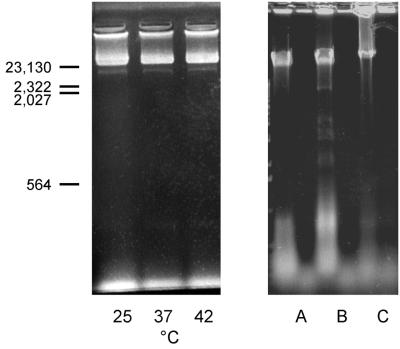
Additional evidence of release of intracellular molecules after LiCl extraction was obtained by determination of nucleic acid release. LiCl extracts were analyzed in a scanning spectrophotometer yielding a maximum absorbance peak at 260 nm and an optical density at 260 nm (OD260)/OD280 ratio of 1.75. Ethidium bromide-stained agarose gels of LiCl extracts yielded significant amounts of nucleic acids of S. epidermidis as well as of S. aureus bacteria. The nucleic acids were found to be sensitive to DNase I treatment (Fig. 3). Finally, LiCl extraction affected bacterial viability and cell morphology. Viability of bacteria from fresh overnight cultures of S. epidermidis KH11 and of S. aureus Newman was determined by CFU determination after brief sonication (10 cycles of 1 s, 40-W output, model 250 sonifier; Branson, Danbury, Conn.), and viable counts were found to be 9.5 × 1010 and 3.37 × 1010 CFU/ml, respectively. After treatment with 1 M LiCl (2 h, 42°C), viable counts of sonicated S. epidermidis and S. aureus microorganisms were reduced to 4.3 × 1010 CFU/ml (54.7% decrease) and 2.37 × 1010 CFU/ml (29.7% decrease), respectively. Gram staining of LiCl-extracted microorganisms revealed >50% of cells to be disintegrated and/or decolorized.
FIG. 3.
Ethidium bromide-stained agarose gel of LiCl extracts of staphylococci. (Left) LiCl extracts of S. epidermidis AB9 prepared at 25, 37, and 42°C. Size standards (in base pairs) are noted at the left. (Right) LiCl extracts of S. epidermidis AB9 (lanes A) and KH11 (lanes B) and of S. aureus Newman (lanes C) before (left lanes) and after (right lanes) DNase I treatment.
N-terminal sequencing.
After purification by medium-pressure chromatography, both the 42- and the 112-kDa protein were subjected to N-terminal amino acid sequence analysis with an automated 473A sequencer (Perkin-Elmer, Weiterstadt, Germany). For the 42-kDa protein, the following sequence was obtained: MKNLR NRSFL TLLDF SRQEV EFLLT LSEDL. This N-terminal amino acid sequence exhibited 61% identity to that of ornithine carbamoyl transferase (OCTase) of Aeromonas formicans (SwissProt data base accession no. P11726), an enzyme involved in the synthesis of arginine. Since the function of OCTase is to convert ornithine to citrulline, the reaction can be monitored by detection of citrulline by a colorimetric assay (22). Fractions from an anion-exchange column containing only the 42-kDa protein were positive in this test (OD490, 0.307 to 2.192, as fractions contained increasing protein concentrations). In contrast, other fractions that did not contain the 42-kDa protein were negative for OCTase. Thus, the purified protein was found to catalyze the conversion of ornithine to citrulline, demonstrating for the first time a functional characterization of OCTase activity in S. epidermidis. OCTases with a similar molecular mass (44 kDa) in Bacillus subtilis (14) and S. aureus (22) have been reported previously. The N-terminal amino acid sequence of the 112-kDa protein extracted with 2% SDS reads as follows: A(V)GPQK TLG(S)LV KYTDK VNXXI. This N-terminal amino acid sequence showed 80% homology with the N terminus of the amidase domain of the S. epidermidis AtlE protein (6), thus representing the mature protein. Unpublished evidence from our laboratory indicates that the AtlE molecule extracted by SDS treatment does not contribute substantially to the adhesion of S. epidermidis to FN. Sequence information on the 160-kDa protein remained inconclusive; the nature of this protein needs to be further characterized.
Enzymes with cell metabolic functions may be present on the cell surface, as reported for the glyceraldehyde-3-phosphate dehydrogenases of S. aureus and S. epidermidis (12) and Candida albicans (3). However, the lack of OCTase supernatant from extracts in which the integrity of the cell was preserved, such as after SDS treatment or protoplast preparation, the failure to demonstrate the OCTase antigen on the bacterial surface, the release of nucleic acids, and the alteration of cell morphology and viability all strongly suggest release of intracellular molecules by LiCl extraction. Therefore, extraction methods used for cell surface protein solubilization may not reliably preserve the cell integrity of different staphylococcal species, and LiCl extraction appears not to be a method of choice for identification of candidate adhesins in S. epidermidis. Care must be taken to ensure the cell wall location of any staphylococcal protein presumably recognizing adhesive matrix molecules.
Acknowledgments
This work was supported by a grant from the German Ministry for Education and Research (grant 01KI9750/9) and a grant from the Medical Faculty of the University of Muenster (grant HE119840).
We thank R. A. Proctor for critically reviewing the manuscript.
REFERENCES
- 1.Christensen G D, Baldassari L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C: American Society for Microbiology; 1994. pp. 45–78. [Google Scholar]
- 2.Foster T J, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Navarro I, Gil M L, Casanova M, O'Connor J E, Martinez J P, Gozalbo D. The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicansis a surface antigen. J Bacteriol. 1997;179:4992–4999. doi: 10.1128/jb.179.16.4992-4999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 53–138. [Google Scholar]
- 5.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidisaffected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidisto a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R, Lew P D, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 8.Jahreis A, Yousif Y, Rump J A, Drager R, Vogt A, Peter H H, Schlesier M. Two novel cationic staphylococcal proteins induce IL-2 secretion, proliferation and immunoglobulin synthesis in peripheral blood mononuclear cells (PBMC) of both healthy controls and patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1995;100:406–411. doi: 10.1111/j.1365-2249.1995.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuypers J M, Proctor R A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang O D, Flock J-I, Wadström T. Isolation and characterisation of a vitronectin-binding surface protein from Staphylococcus aureus. Biochim Biophys Acta. 1995;1250:110–116. doi: 10.1016/0167-4838(95)00076-7. [DOI] [PubMed] [Google Scholar]
- 11.McGavin M H, Krajewska Pietrasik D, Rydén C, Höök M. Identification of a Staphylococcus aureusextracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modun B, Williams P. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. 1999;67:1086–1092. doi: 10.1128/iai.67.3.1086-1092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreillon P, Entenza J M, Francioli P, McDevitt D, Foster T J, François P, Vaudaux P. Role of Staphylococcus aureuscoagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neway J O, Switzer R L. Purification, characterization, and physiological function of Bacillus subtilisornithine transcarbamylase. J Bacteriol. 1983;155:512–521. doi: 10.1128/jb.155.2.512-521.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson M, Frykberg L, Flock J I, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patti J M, Allen B L, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 17.Paulsson M, Ljungh A, Wadström T. Rapid identification of fibronectin, vitronectin, laminin, and collagen cell surface binding proteins on coagulase-negative staphylococci by particle agglutination assays. J Clin Microbiol. 1992;30:2006–2012. doi: 10.1128/jcm.30.8.2006-2012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proctor R A, Christman G, Mosher D F. Fibronectin-induced agglutination of Staphylococcus aureuscorrelates with invasiveness. J Lab Clin Med. 1984;104:455–469. [PubMed] [Google Scholar]
- 19.Yacoub A, Lindahl P, Rubin K, Wendel M, Heingard D, Rydén C. Purification of a bone sialoprotein-binding protein from Staphylococcus aureus. Eur J Biochem. 1994;222:919–925. doi: 10.1111/j.1432-1033.1994.tb18940.x. [DOI] [PubMed] [Google Scholar]
- 20.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 21.Yousi Y A, Mertz A, Bastford S R, Vogt A. Cationic staphylococcal antigens have affinity for glomerular structure: possible pathogenetic role in glomerulonephritis. In: Jeljaszewicz J, Ciborowski P, editors. The staphylococci. Stuttgart, Germany: Gustav Fischer; 1991. pp. 168–169. [Google Scholar]
- 22.Zaharia O, Soru E. Staphylococcal ornithine carbamoyltransferase. Purification and some properties. Eur J Biochem. 1971;18:28–34. doi: 10.1111/j.1432-1033.1971.tb01210.x. [DOI] [PubMed] [Google Scholar]